Abstract
A novel Dynamic Nuclear Polarization (DNP) NMR polarizing agent ToSMTSL-PTE representing a phospholipid with a biradical TOTAPOL tethered to the polar head group has been synthesized, characterized, and employed to enhance solid-state Nuclear Magnetic Resonance (SSNMR) signal of a lipid-reconstituted integral membrane protein proteorhodopsin (PR). A matrix-free PR formulation for DNP improved the absolute sensitivity of NMR signal by a factor of ca. 4 compared to a conventional preparation with TOTAPOL dispersed in a glassy glycerol/water matrix. DNP enhancements measured at 400 MHz/263 GHz and 600 MHz/395 GHz showed a strong field dependence but remained moderate at both fields, and comparable to those obtained for PR covalently modified with ToSMTSL. Additional continuous wave (CW) X-band electron paramagnetic resonance (EPR) experiments with ToSMTSL-PTE in solutions and in lipid bilayers revealed that an unfavorable conformational change of the linker connecting mononitroxides could be one of the reasons for moderate DNP enhancements. Further, differential scanning calorimetry (DSC) and CW EPR experiments indicated an inhomogeneous distribution and/or a possibility of a partial aggregation of ToSMTSL-PTE in DMPC:DMPA bilayers when the concentration of the polarizing agent was increased to 20 mol% to maximize the DNP enhancement. Thus, conformational changes and inhomogeneous distribution of the lipid-based biradicals in lipid bilayers emerged as important factors to consider for further development of this matrix-free approach for DNP of membrane proteins.
Keywords: Nuclear Magnetic Resonance, Dynamic Nuclear Polarization, Electron Spin Resonance, biradical, signal enhancement, membrane protein, lipid, bilayer
Introduction
Solid-state Nuclear Magnetic Resonance (SSNMR) is a powerful technique for the characterization of molecular structure and interactions. However, poor sensitivity remains the main obstacle for broader applications of SSNMR to many chemical and biological systems, and in particular to membrane proteins. NMR signals can be enhanced by Dynamic Nuclear Polarization (DNP), a rapidly developing method to generate a hyperpolarized state of nuclear spins by transferring the significantly larger spin polarization of unpaired electrons, thereby permitting structural investigation of samples that would otherwise have insufficiently strong NMR signals [1]. While many endogenous and exogenous molecular species possessing unpaired electronic spins can act as polarization sources for DNP, the vast majority of current DNP experiments rely on exogenous persistent biradicals [2–6]. For protein applications, the nitroxide biradicals TOTAPOL [2] and AMUPol [7] have been the most popular polarizing agents, as they are soluble in water in high concentrations and are readily compatible with biological samples. Since the optimal conditions for DNP are obtained with uniform distributions of the polarizing agents, a protein and a biradical are typically mixed/co-dissolved with large amounts of glycerol and/or dimethyl sulfoxide (DMSO) (20–75 v%) in water [1] to promote formation of a glassy matrix at low temperatures, thus, avoiding phase separation between the biradical and both the protein and the aqueous phases. Good solubility of the polarizing agents in biocompatible solvents is important because typically for the optimal DNP enhancement either AMUPol or TOTAPOL is dissolved in water-glycerol (40:60 w:w) mixtures at 5–10 mM [7, 8] or higher concentrations [7] [9, 10] [11]. While the optimal sample preparation protocols for DNP MAS NMR of water soluble proteins are well established, we note that DNP enhancements for membrane proteins vary strongly depending on the sample formulation and the type of the biradical [12–15], and the reasons for such a high variability are not well understood.
While formation of a glassy matrix with uniformly distributed biradicals represents a versatile approach for preparing biological macromolecules for DNP, the matrix itself occupies a large – up to 75% – fraction of the sample volume, thus, significantly decreasing the absolute NMR signal intensity. Furthermore, some of biological samples and lipid bilayers in particular may not be fully compatible with glycerol and DMSO, especially at high concentrations when these cryoprotectants may alter hydrogen bonding at the surface of biomolecules [16–18]. For these reasons, “matrix-free” DNP represents a promising alternative for enhancing NMR signals of lipid membranes and membrane protein systems. Specific examples include the use of paramagnetic tags exhibiting a high affinity to the molecules of interest [19–22] and covalent attachment of the tags directly to the target molecule(s) [23–26]. For membrane proteins the polarizing agents can also be introduced directly into lipid bilayers. For example, Long and co-workers employed lipid bilayers doped with two types of unnatural lipids labeled with mono-nitroxides which form pseudo-biradicals when in close proximity [27]. DNP enhancements of up to ε=8.9 were observed at magnetic fields corresponding to 600 MHz1H frequency for a short hydrophobic peptide reconstituted into such bilayers with concentration of electronic spins reaching 6 mol% vs. lipids [27]. By comparison, a conventional DNP preparation of a lipid-reconstituted peptide and TOTAPOL dispersed in a glassy matrix yielded an enhancement of only ε=3.7 under similar experimental conditions. De Paëpe and co-workers reported on N-propyl-PALMIPOL – a TOTAPOL molecule covalently modified with a palmitate chain to effectively partition in the lipid phase of a bilayer [28]. The enhancements of ε=3.1 – 8.1 were observed for the lipid NMR signals at 400 MHz1H Larmor frequency [28].
We have previously reported the synthesis and characterization of a novel biradical ToSMTSL which is based on the well-known DNP polarizing agent TOTAPOL and contains a thiol-specific methanethiosulfonate group that can be covalently attached to a sulfhydryl group of cysteines [24]. For a lipid-reconstituted heptahelical transmembrane protein Anabaena sensory rhodopsin (ASR) covalently modified with ToSMTSL at a solvent-exposed cysteine, an enhancement of up to ε=15 was observed in a deuterated buffer at 400 MHz/263 GHz NMR/EPR frequencies [24]. Here, we conjugate ToSMTSL with the sulfhydryl group of 1,2-dipalmitoyl-sn-glycero-3-phosphothioethanol (PTE) to obtain the first lipid with a biradical-modified head group (ToSMTSL-PTE, Figure 1). We reconstitute ToSMTSL-PTE in lipid bilayers and measure DNP enhancements of NMR signals from a lipid-embedded heptahelical membrane protein proteorhodopsin (PR) that acts as a light-driven proton pump [29]. Depending on the lipid composition, reconstituted PR can be found in a number of oligomeric states ranging from monomer to hexamer [30]. Here we investigate the effects of paramagnetic lipid concentration, buffer deuteration and magnetic field strength on the DNP enhancement, and compare with enhancements obtained with both (i) conventional DNP sample preparations containing TOTAPOL in a glassy water-glycerol matrix, and (ii) matrix-free DNP samples obtained by covalently linking PR with ToSMTSL. DNP enhancements of ε=1.7–2.4 at 600 MHz and ε=4.6–5.5 at 400 MHz1H frequency were observed for PR samples prepared with paramagnetic ToSMTSL-PTE lipids. Continuous wave (CW) X-band electron paramagnetic resonance (EPR) experiments with the biradical in solution and in lipid bilayers indicated that ToSMTSL-PTE may undergo conformational changes which could be unfavorable for the DNP. Differential scanning calorimetry (DSC) and CW EPR experiments as a function of ToSMTSL-PTE concentration in the DMPC:DMPA lipid bilayers were employed to investigate a possibility of phase separation and/or aggregation that would cause inhomogeneous distribution of biradicals in the membrane preparation and a suboptimal DNP enhancement.
Figure 1.
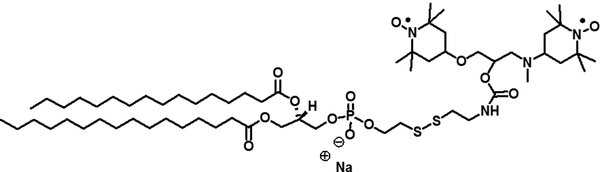
Chemical structure of ToSMTSL-PTE – an unnatural phospholipid with a biradical tethered to the lipid polar head synthesized and characterized in this work.
2. Materials and Methods
2.1. Materials
Unless otherwise indicated, all chemicals and solvents for biradical synthesis were purchased from VWR International (Radnor, PA) or Sigma-Aldrich (St. Louis, MO), and used without additional purification. Common chemicals of a reagent grade for protein expression, isolation and reconstitution were purchased from either Fisher Scientific (Unionville, Ontario, Canada) or Sigma-Aldrich (Oakville, Ontario, Canada). 15N-labeled ammonium chloride was purchased from Cambridge Isotope Laboratories (Andover, MA, USA). 1,2-Dipalmitoyl-sn-glycero-3-phosphothioethanol (PTE), 1,2-dimyristoyl-sn-glycero-3-phosphocholine (DMPC), and 1,2-dimyristoyl-sn-glycero-3-phosphate (DMPA) were purchased from Avanti Polar Lipids (Alabaster, AL) as chloroform solutions (>99% purity) and used without further purification. The Ni2+-NTA (nitrilotriacetic acid) agarose resin was purchased from Qiagen (Mississauga, Ontario, Canada). TOTAPOL was either purchased from Dynupol (Cambridge, MA, USA) or synthesized at NCSU according to the published procedures [2].
2.2. Synthesis of ToSMTSL-PTE.
4 ml of a chloroform solution, containing 0.1 g (1.37×10−4 mol) of the PTE phospholipid, were mixed with 4 ml of 50 mM phosphate buffer solution, pH=6.86, and ToSMTSL (0.106 g, 1.78×10−4 mol) in 1 ml of chloroform was added. The resulting two-phase mixture was vigorously stirred on a magnetic stirrer (room temperature, 48 h); the organic layer was separated from the aqueous phase, concentrated under a reduced pressure, and the residue was purified on a column packed with silica gel using a mixture of CHCl3:CH3OH:H2O (70:12:0.5 v/v) as an eluent to give ToSMTSL-PTE as a dark red oil, yield 0.128 g, 63%. ESI HRMS [M‒Na+H]+: calcd for C62H118N4NaO13PS2, 1222.7953; found, 1222.8088.
The chemical structure of ToSMTSL-PTE is shown in Figure 1. Mass spectrometry analysis was carried out using a high resolution Thermo Fisher Scientific Extractive Plus MS (Waltham, MA) and a benchtop full-scan Orbitrap™ mass spectrometer, using Heated Electrospray Ionization (HESI) method. The mass spectrometer was operated in a positive mode.
2.3. Expression of isotopically labeled proteorhodopsin.
[U-15N]–labeled (UN) His-tagged, wild-type green proteorhodopsin (PR) and its double C107S/C156S mutant (the DNA kindly provided by Drs. A. R. Choi and K.-H. Jung, Sogang University, Korea) were produced according to a previously published protocol [31]. Protein was expressed in BL21-Codonplus-RIL E. coli grown on M9 minimal medium at 30 °C, using 4 g of natural abundance glucose and 1 g of 15N-labeled ammonium chloride per liter of culture as the sole carbon and nitrogen sources, respectively. When cultures reached a target cell density of A600=0.4 OD, the protein expression was induced by an addition of IPTG to a final concentration of 1 mM and retinal to a final concentration of 7.5 μM. Cells were incubated for 21 h after induction, collected by centrifugation, pre-treated with lysozyme (12 mg/L of culture) and DNAse I (600 units per liter of culture), and then lysed by sonication. Membranes were solubilized in 1% Triton X-100 overnight at 4 °C, and insoluble debris were removed by ultracentrifugation (150,000×g). The supernatant was mixed with Ni2+-NTA resin for 18 h allowing PR to bind to the resin. Purification was carried out using batch procedure described in the Qiagen Ni2+-NTA resin manual. The washing buffer had Triton X-100 as the solubilizing detergent for the first five washes; the final three washes were done with n-dodecyl β-D-maltoside (DDM) as the detergent. Approximately 15 mg of UN PR was purified from one liter of culture, as determined from the absorbance of the opsin-bound retinal, using an extinction coefficient of 44,000 M−1cm−1 [32]. Purified proteins were buffer-exchanged using an Amicon Ultra-15 10K centrifugal filter (Millipore, Massachusetts, MA, USA) into pH 8.0 buffer (5 mM Tris, 10 mM NaCl, 0.05 v% DDM), and concentrated to ~1 mg/ml (36 μM).
2.4. Lipid samples for DNP, EPR, and DSC experiments.
For samples containing ToSMTSL-PTE, partially deuterated liposomes were prepared by mixing DMPC, DMPC with deuterated acyl chains (d54-DMPC) and DMPA at DMPC:DMPA weight ratio of 9:1. Three samples for DNP experiments were prepared by adding ToSMTSL-PTE to the lipid mixture at 5 mol% (7.94 mM), 10 mol% (15.36 mM), and 20 mol% (28.33 mM) (samples S1–S3, Table 1). The molar ratios of ToSMTSL-PTE to the combined DMPC and DMPA were ca. 1:19, 1:9, 1:4, respectively. The amount of d54-DMPC in liposomes for DNP samples was chosen such that the total level of acyl chain deuteration was 80 mol%. DDM solution of 15N PR was then mixed with these liposomes at a protein:lipid 1:80 molar ratio and incubated for 6 h at 4 °C before Bio-Beads SM (Bio-Rad Laboratories) were added for the detergent removal. For DNP experiments the ToSMTSL-PTE concentrations correspond to ca. 4, 8, and 16 biradical molecules per PR molecule. The PR functionality and the protein-to-lipid ratio of the lipid reconstituted samples were confirmed by visible and FTIR spectroscopy, as described previously [33, 34]. The DNP samples were packed in 3.2 mm sapphire Bruker Biospin NMR rotors.
Table 1.
Sample compositions, bulk 1H DNP buildup times τDNP measured at the magnetic field corresponding to 600 MHz proton Larmor frequency, and the observed DNP enhancements, ε, defined as a ratio of 15N NMR signal intensities obtained with and without mm-wave irradiation, , at magnetic fields corresponding to 600 MHz and 400 MHz proton Larmor frequencies.
| Description1 | Solvent | τDNP (ms) | ε, 400 MHz | ε, 600 MHz | |
|---|---|---|---|---|---|
| S1 | 15N PR + 5mol% ToSMTSL-PTE2 | H2O | 436±20 | - | 1.9±0.1 |
| D2O | 867±78 | - | 1.7±0.3 | ||
| S2 | 15N PR + 10mol% ToSMTSL-PTE | H2O | 475±22 | - | 2.0±0.2 |
| D2O | 889±66 | 4.6±0.1 | 2.4±0.3 | ||
| S3 | 15N PR + 20mol% ToSMTSL-PTE | H2O | 424±20 | - | 2.0±0.2 |
| D2O | 804±64 | 5.5±0.1 | 2.4±0.5 | ||
| S4 | 15N PR-ToSMTSL3 | H2O | 738±24 | - | 2.3±0.2 |
| D2O | 1420±102 | 7.0±0.1 | 2.5±0.4 | ||
| S5 | 15N PR + TOTAPOL | D2O/H2O/d8-glycerol4 | 827±42 | 15.0±0.5 | 3.3±0.8 |
For proper comparison of absolute intensities, each sample contained approximately one mg of 15N labeled protein.
This sample was lost to a spinning crash during experiments at 400 MHz.
Partially deuterated acyl side chains. See Section 2.2 for details.
D2O/H2O/d8-glycerol mixed at a volume ratio of 30:10:60.
For EPR and DSC experiments with lipid mixtures, DMPC and DMPA were co-dissolved in chloroform in 9:1 weight ratio. For experiments with ToSMTSL-PTE a series of samples were prepared by adding different amount of ToSMTSL-PTE from a chloroform stock solution. For all sample preparations the solvent was evaporated under a nitrogen stream yielding a thin lipid film on the surface of a conical glass vial. Residual solvent was removed by evacuating the vial overnight in a vacuum desiccator. Dried lipid films were hydrated by adding 24 mM CHES buffer, 10 mM NaCl, pH=9.0, and then subjected to ten consecutive freeze-thaw cycles between liquid nitrogen and a water bath at 305 K to form multilamellar lipid vesicles (MLVs). For all MLVs preparations the total lipid concentration was ~10 w %. Overall, five samples were prepared: (1) a control sample containing only DMPC:DMPA 9:1 w:w and used only for DSC and (2–5) four samples for EPR containing the same DMPC:DMPA weight ratio and a progressively increasing molar fraction of ToSMTSL-PTE with respect to the total lipid in the sample, 0.5, 2.8, 5.8, and 12.6 mol%, respectively.
2.5. Preparation of PR with covalently attached ToSMTSL.
WT PR has three cysteines at the positions 107, 156, 175. We used the double C107S/C156S mutant in which only one exposed cysteine C175 is present and the other two are replaced with serines. We refer to this protein as PR-C175 in the following. Labeling with the biradical was carried out at 20-fold molar excess of ToSMTSL. The excess label was removed through a repeated buffer exchange using Amicon Ultra-15 Centrifugal Filter Units (MilliporeSigma, Burlington, MA). After removal of the excess label, liquid chromatography-electrospray ionization-quadrupole-time of flight-mass spectrometry (LC-ESI-Q-TOF-MS) was used to confirm the nearly complete labeling of PR with ToSMTSL. PR-ToSMTSL was reconstituted in d54-DMPC:DMPA lipids at a protein to lipid molar ratio of 1:80 (Sample S4, Table 1). The sample was packed in the 3.2 mm sapphire NMR rotor.
2.6. Preparation of PR sample with TOTAPOL.
In order to compare DNP enhancements, we have also prepared lipid-reconstituted PR mixed with a solution of TOTAPOL in a glassy water-glycerol matrix (Sample S5, Table 1). Following reconstitution of PR in d54-DMCP/DMPA lipids (protein to lipid molar ratio of 1:80), PR samples were pelleted and consequently resuspended in 0.5 ml of DNP buffer (20 mM TOTAPOL, 10 v% H2O, 30 v% D2O, and 60 v% d8-glycerol). The sample was stirred at 4 °C until the pellet was completely resuspended. In order to separate the proteoliposomes from the bulk of the DNP buffer, the sample was centrifuged (900,000×g, 3 h, 4 °C); however, similar to the experiments with another heptahelical membrane protein ASR [35], it was found that the proteoliposomes had lower density than the 60% d8-glycerol DNP buffer and would not pellet. To remedy this, the sample was first diluted with a solution of 20 mM TOTAPOL in a mixture of 75 v% D2O and 25 v% H2O to achieve glycerol concentration of 50 v%, and pelleted under centrifugation, and d8-glycerol was added to the pellet to bring the total glycerol content to approximately 60 v%. The sample was homogenized with a pipette tip, and packed into a 3.2 mm sapphire rotor for MAS DNP-NMR measurements. The final TOTAPOL concentration in the sample was estimated as ~16 mM.
Based on a visual comparison of the rotors packed with equal amounts of PR with TOTAPOL in a DNP buffer (sample S5), and in a matrix free preparation, the former occupies approximately four times larger volume. Assuming that DNP buffer accounts for between 75% (lower bound) and 100% (upper bound) of the sample volume of ~ 15 μl at a TOTAPOL concentration of 16 mM, we estimated the TOTAPOL/monomer ratio to be between 5 and 7.
2.7. DNP NMR experiments.
NMR experiments were carried out on Avance III Bruker DNP-NMR spectrometers operating at 400 MHz/263 GHz at the Bruker Biospin Ltd. facility in Billerica MA, and 600 MHz/395 GHz at the University of Guelph NMR Centre. Both spectrometers were equipped with triple resonance (HCN) low temperature MAS DNP probes. All samples contained ~1 mg of 15N labeled protein and were packed into 3.2 mm sapphire rotors with zirconia caps. The experiments were performed at a spinning frequency of 8.0 kHz and at a temperature of 102 K. Temperature was calibrated using 79Br T1 measurements in KBr [36]. 1D 1H/15N CPMAS experiment was employed for the determination of 1H DNP enhancements. 1H/15N cross-polarization was optimized around the n=1 Hartman-Hahn condition [37] with 50 kHz radio frequency (r.f.) power on 15N and with the r.f. field linearly ramped around 58 kHz on 1H. 1H T1 were estimated using a saturation pulse train applied to the protons, followed by a recovery delay and 1H/15N CPMAS. Protons were decoupled during 15N acquisition using 100 kHz SPINAL64 proton decoupling [38]. DNP enhancements are defined as a ratio , where Ion and Ioff are the 15N NMR signal intensities measured with the mm-wave power on and off, respectively.
2.8. Differential Scanning Calorimetry experiments.
Differential Scanning Calorimetry (DSC) experiments were carried out using DSC Q2000 instrument (TA Instruments, New Castle, DE) in Tzero T4 heat flow calibration configuration. TA Instruments low volume hermetic alodined aluminum pellets were loaded with about 7 mg of aqueous MLV dispersion containing 10 w% of lipid. The instrument was programmed to cycle over a wide temperature range from 10 to 65 °C and zoomed in the range from 10 to 35 °C at the same rate of 1 °C/min for both the heating and cooling cycles. A total of five heating/cooling cycles were recorded for each of the samples. Experimental DSC curves were averaged out and the background drift was corrected using splines and a Matlab (MathWorks, Inc., Natick, MA) script.
2.9. Electron Paramagnetic Resonance experiments.
CW EPR spectra of solutions of biradicals and spin-labeled lipid bilayers were measured with either a Varian (Varian Associates, Palo Alto, CA, USA) Century Series E-102 X-band (9 GHz) spectrometer or a Bruker ELEXSYS E500 spectrometer (Bruker Biospin, Billerica, MA, USA). Aqueous dispersions of MLVs were drawn into a polytetrafluoroethylene tube (PTFE, 0.81×1.12 mm, Jaguar Industries, Stony Point, NY), the tube was folded twice and inserted into a standard X-band 3×4 mm (I.D. × O. D.) quartz EPR tube (Norell, Marion, NC, USA) for measurements at room temperature. For measurements at 77 K PTFE tubes were folded a few more times, as many as the diameter of the standard EPR tube would allow for, and the tube was inserted into a quartz finger Dewar (Wilmad-LabGlass, Vineland, NJ, USA) filled with liquid nitrogen. Incident microwave power was set below saturation (at 0.06 mW or lower) and the amplitude of the 100 kHz magnetic field modulation was set to a half of the linewidth or less. EPR spectra were simulated using software described earlier [39, 40].
3. Results and Discussion
3.1. Characterization of ToSMTSL-PTE by CW EPR.
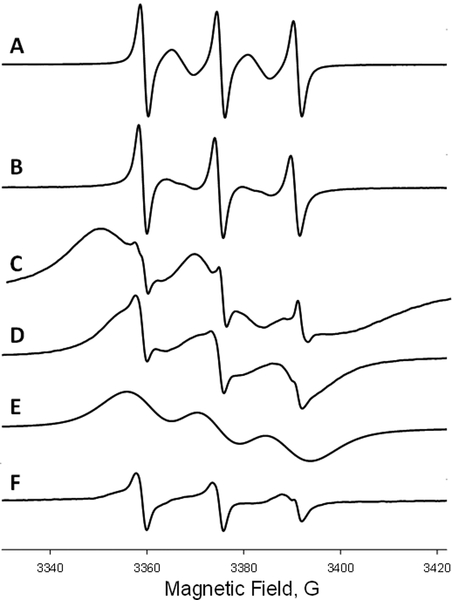
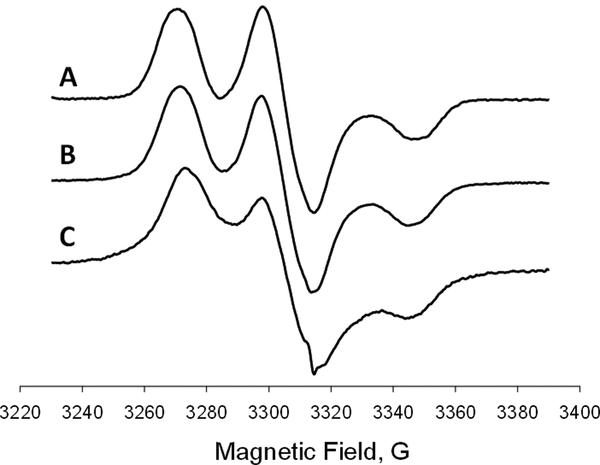
ToSMTSL-PTE in solutions and incorporated in lipid bilayers was characterized by CW EPR at X-band. Figure 2 compares room temperature CW X-band (9.5 GHz) EPR spectra of nitrogen-equilibrated chloroform solutions of ToSMTSL and ToSMTSL-PTE (Figures 2A and B, respectively). The spectra reveal a five-line pattern that is characteristic of a biradical experiencing rapid tumbling in solution which effectively averages out anisotropic magnetic interactions such as anisotropic components of hyperfine coupling interactions of electronic spins with 14N spins [41]. The flexible linker between two nitroxide moieties is expected to further modulate the electronic spin exchange and dipolar coupling interactions by an intermolecular motion [41]. The observed five-line pattern is indicative of the average exchange coupling, J, being larger than the isotropic nitrogen hyperfine coupling, AN [42]. Line shape changes in the EPR spectrum of ToSMTSL-PTE in chloroform vs. that of ToSMTSL are attributed to a slower overall molecular tumbling of the biradical attached to the phospholipid.
Figure 2.
Room temperature (23 °C) X-band (9.5 GHz) CW EPR spectra of nitrogen equilibrated 1 mM chloroform solutions of ToSMTSL (A) and ToSMTSL-PTE (B). EPR spectra of aqueous suspension of multilamellar lipid vesicles (10 w% of lipids, 24 mM CHES buffer, 10 mM NaCl, pH=9.0) prepared from the mixture of DMPC:DMPA (9:1 w:w) and ToSMTSL-PTE at 0.5 mol% measured at 23 °C (C) and 55 °C (D). (E) is a simulated broad component attributable to ToSMTSL-PTE and (F) is the difference spectrum characteristic of a nitroxide biradical partitioning between the lipid and the aqueous bilayer phases. All spectra are normalized by peak-to-peak amplitude and magnetic field positions are adjusted by g-factors.
Room temperature CW EPR spectra of the ToSMTSL-PTE incorporated into MLVs composed of DMPC:DMPA lipids (Figure 2C) were significantly broader and consistent with a slower rate of molecular tumbling in a more viscous environment of lipid bilayers. Raising sample temperature to 55 °C increased both the rate of axial rotational diffusion and intermolecular motion and improved spectral resolution (Figure 2D). Such a spectrum could be simulated as a superposition of three broad lines (Figure 2E) attributed to ToSMTSL-PTE within the lipid bilayer, and another smaller component (Figure 2F) corresponding to faster tumbling of dinitroxide partitioning between the lipid and the aqueous bilayer phases. We note that even though the latter narrow line components are clearly notable in these first derivative EPR spectra, a comparison of the double integrals reveals that the narrow line components correspond to less than 0.5% of the total nitroxide species in the sample, and can be ignored in further analysis.
Next, EPR spectra of DMPC:DMPA:ToSMTSL-PTE bilayers were studied as a function of ToSMTSL-PTE concentration from 0.5 to 12.6 mol% of total lipids. While the spectra were measured at both room temperature (cf. Figure 2C) and 55 °C (Figure 3), the spectra at the higher temperature were found to be more informative because of narrower line width due to faster uniaxial rotational diffusion and intermolecular motion of this biradical-labeled lipid.
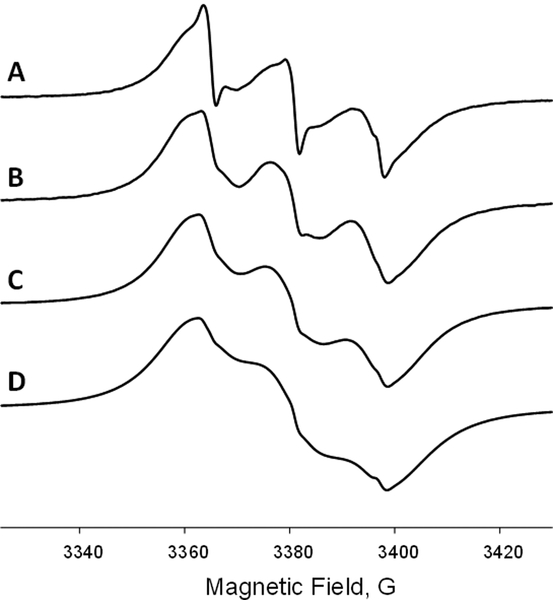
Figure 3.
X-band (9.5 GHz) EPR spectra of lipid vesicles (aqueous suspension of 10 w% of lipids in 24 mM CHES buffer, 10 mM NaCl, pH=9.0) prepared from the mixture of DMPC:DMPA (9:1 w:w) and doped with ToSMTSL-PTE at 0.5 mol% (A), 2.8 mol% (4.5 mM) (B), 5.8 mol% (8.9 mM) (C), and 12.6 mol% (17.9 mM) (D) and measured at 55 °C. All spectra are normalized by peak-to-peak amplitude and magnetic field positions are adjusted by g-factors.
EPR spectra in Figure 3 demonstrate a progressive broadening with increasing biradical concentration. The broadening is caused by intermolecular dipolar and spin exchange interactions but is expected to be negligible at the lowest 0.5 mol% ToSMTSL-PTE concentration. However, even at the lowest concentration, the EPR spectrum is excessively broad compared to that of the ToSMTSL-PTE in a chloroform solution (Figure 2B), indicating strong electron-electron coupling within biradical. The shape of the spectrum and the widths of the individual components are similar to those reported by Mchaourab et al. for a pair of spin labels tethered to T4 Lysozyme in solution with the interspin distance shorter than 9 Å [43]. This would indicate that when incorporated into lipid bilayers, the interspin distance in ToSMTSL-PTE is significantly shorter than the estimated r = 12.8 ± 0.8 Å for the parent ToSMTSL [35].
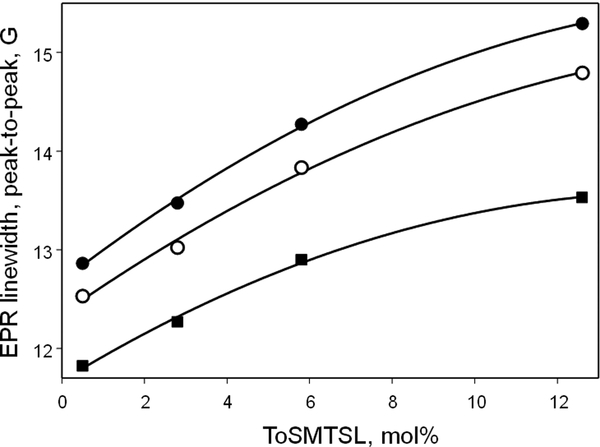
In order to analyze concentration-dependent changes in the EPR spectra, the data were least-squared simulated, similar to Figure 2D–F, as a superposition of Voigt line shapes (i.e., Gaussian-Lorentzian convolution) for each of the three nitrogen hyperfine components and an additional fast-motion nitroxide impurity signal, using software described earlier [39, 40]. The best-fit peak-to-peak linewidths of each of the main three components were plotted as a function of ToSMTSL-PTE concentration (Figure 4). Mchaourab et al. have previously investigated how room temperature EPR linewidths change with interspin distance r for nitroxides attached to biological molecules by flexible linkers [43]. They have established an approximate r−6 dependence for distances exceeding 8–9 Å. Thus, for randomly distributed nitroxides an integration procedure would yield the r−3 dependence which corresponds to linewidth changing linearly with concentration. Assuming that the onion-like structure of MLVs results in a quasi-3D distribution of ToSMTSL-PTE molecules, the EPR linewidth is also expected to change linearly with the biradical concentration. Indeed, Figure 4 shows an approximately linear dependence of peak-to-peak linewidths for each of the three nitrogen hyperfine components of EPR spectra from ToSMTSL-PTE at concentration up to 5.8 mol%, and a slight trend of saturation at 12.6 mol%. The linear dependence is a strong indication of good miscibility of ToSMTSL-PTE with DMPC:DMPA lipids at least up to 5.8 mol%.
Figure 4.
Best-fit peak-to-peak EPR linewidths of each of the three nitrogen hyperfine coupling components of X-band (9.5 GHz) CW EPR spectra of lipid vesicles (aqueous suspension of 10 w% of lipids, in 24 mM CHES buffer, 10 mM NaCl, pH=9.0) prepared from the mixture of DMPC:DMPA as a function of ToSMTSL-PTE concentration. All spectra were measured at 55 °C. The solid lines are quadratic regressions provided to guide the eye. See text for details.
The observed linear dependence of EPR peak-to-peak linewidth on ToSMTSL-PTE concentration allows for extrapolating data to 0 mol% of this biradical when the intermolecular magnetic interactions should be absent. Such an extrapolation yields 11.71±0.06 G peak-to-peak linewidth for the narrowest (mI=0) nitrogen hyperfine coupling component. This linewidth is significantly broader than ca. 2 G reported at a similar temperature of 57 °C for a lipid with a monoradical Tempo tethered to the polar head (Tempo-PC, 1,2-dioleoyl-sn-glycero-3-tempo-phosphocholine) and incorporated into DPPC (1,2-dipalmitoyl-sn-glycero-3-phosphocholine) bilayers [44]. From these values we can estimate the EPR linewidth broadening of ToSMTSL-PTE in lipid bilayers at 55–57 °C as ≈10 G or ≈28 MHz. While this value is comparable to Jdd = 25 ± 4 MHz reported from fitting rigid limit X-band EPR spectra of the parent radical ToSMTSL measured at 77K [35] the data for ToSMTSL-PTE were obtained at ca. 55 °C (328 K), the conditions when one would expect some dynamic averaging. Thus, the observed magnetic interactions of ≈28 MHz indicate that in ToSMTSL-PTE the monoradicals are placed closer than in the parent biradicals ToSMTSL and TOTAPOL.
Figure 5 compares the rigid limit CW EPR spectra of TOTAPOL in a glassy water: glycerol mixture with that of ToSMTSL-PTE incorporated into DMPC:DMPA bilayers. We note that the spectra are similar in both formulations (Figure 5A and B), thereby indicating the similar magnitudes of exchange and dipolar coupling between the nitroxide species. However, the spectrum of a frozen DMPC:DMPA lipid dispersion doped with 0.5 mol% of ToSMTSL-PTE showed a significant additional broadening compared to that of the parent biradical TOTAPOL (Figure 5A and B), as clearly evident from the shape of the wings (Figure 5C). Such broadening likely results from additional strong intermolecular spin exchange and/or dipolar interactions. We note that typical concentrations of randomly dispersed mononitroxides in EPR experiments are from 0.5 to 1.0 mol% of total lipids to avoid lineshape distortions and shortening of electronic relaxation times due to intermolecular magnetic interactions [45–47]. Thus, for a randomly dispersed biradical at a concentration of 0.5 mol% of lipids (corresponding monoradical concentration of 1 mol% of lipids), the effect of intermolecular magnetic interactions is also expected to be negligible. The additional broadening of the EPR spectrum of Figure 5C could be an indication of either some clustering of ToSMTSL-PTE within lipid bilayers or a shorter interspin distance between the mononitroxides. However, the concentration dependence of EPR spectra of ToSMTSL-PTE in DMPC:DMPA bilayers taken at 55 °C (Figures 3 and 4) provides no indication of clustering at least up to 5.8 mol%. We speculate that the main reason for the additional broadening of rigid limit EPR spectra of 0.5 mol% ToSMTSL-PTE is a conformational change in the flexible biradical moiety in the DMPC:DMPA bilayers, e.g, due to the nitroxide moieties being in a closer proximity. This hypothesis fully agrees with an excessive broadening of EPR spectra observed for ToSMTSL-PTE in DMPC:DMPA bilayers at 55 °C as discussed above. We cannot also exclude that freezing the sample for DNP experiments would trap certain biradical conformations unfavorable for DNP.
Figure 5.
Experimental rigid limit (T = 77 K) X-band (9.5 GHz) EPR spectra of (A) 0.8 mM solution of TOTAPOL in water:glycerol (80:20 v/v), (B) 0.8 mM solution of TOTAPOL in water:glycerol (80:20 v/v) when mixed with DMPC:DMPA lipid bilayers, and (C) lipid bilayers (dispersion 10 w% of total lipids in 24 mM CHES buffer, 10 mM NaCl, pH=9.0) composed of DMPC:DMPA and doped with 0.5 mol% of ToSMTSL-PTE. All spectra are normalized by peak-to-peak amplitude and magnetic field positions are adjusted by g-factors.
3.2. Characterization of ToSMTSL-PTE by DSC
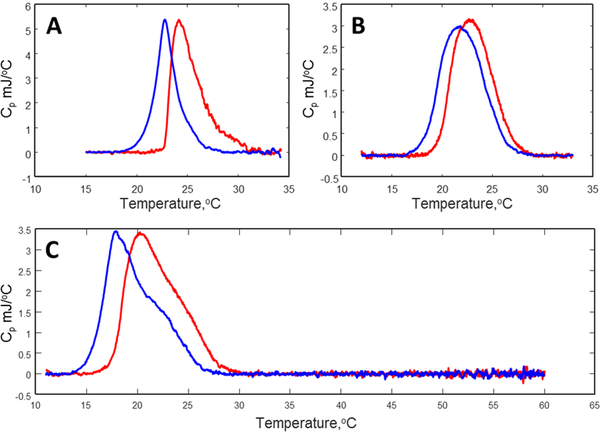
DSC was employed to investigate the miscibility of ToSMTSL-PTE, DMPC, and DMPA in lipid bilayers. The main phase transitions are expected to occur at ~24 °C for DMPC [48], and at ~55 °C for DMPA [49]. At low fraction of DMPA lipid (i.e., ≤10mol% of total lipid), the main phase transition is expected to occur closer to the phase transition of the pure DMPC provided that the two lipids are miscible in the bilayers. Perturbations to the lipid bilayer structure are generally reflected by a lower number of cooperating units undergoing transition at the same time. This is theoretically described by a lower Van’t Hoff enthalpy and a broader cp(T) transition width. Similarly, the effects of doping the DMPC:DMPA lipid bilayers with ToSMTSL-PTE can be inferred from shifts of position and width of DSC peaks compared to the pure DMPC:DMPA bilayer sample.
Figure 6 summarizes DSC data for multilamellar vesicles composed of all the lipid mixtures studied. For all the samples no main phase transition peaks corresponding to pure DMPA (~55 °C) were detected (see the wide range DSC scan for the highest ToSMTSL-PTE concentration of 12.6 mol% shown in Figure 4C). Pure DMPC:DMPA bilayers exhibit only a moderate DMPC peak broadening compared to the typically very sharp peak (<1 °C in width) observed for pure DMPC multilamellar vesicles (MLVs). We note here that the width of the DSC peak decreases with an increase in the cooperativity number N (e.g., see [46, 50]). Lower cooperativity for mixed bilayers is expected to yield broader DSC peaks. Therefore, we conclude that DMPC and DMPA lipids are fully miscible at the 9:1 w:w ratio. However, with increasing ToSMTSL-PTE content the main phase transition peak is further broadened and shifted towards lower temperatures (as expected when impurities are present). While the DSC peak at 5.8 mol% (Figure 6B) has no indication of the phase separation, the peak at 12.6 mol% (Figure 6C) becomes partially deformed so that the original peak at ~24 °C represents only a high temperature shoulder. This observation suggests an inhomogeneous distribution of ToSMTSL-PTE within the DMPC:DMPA lipid matrix when its concentration reaches 12.6 mol%.
Figure 6.
DSC data for aqueous dispersions (10% of total lipids) of multilamellar vesicles composed of pure DMPC:DMPA (A), and doped with ToSMTSL-PTE at 5.8 mol% (B) and 12.6 mol% (C) of total lipids. Red lines represent heating temperature scan while the cooling scans are colored in blue.
3.3. DNP experiments
Three samples of 15N-labeled PR reconstituted in partially deuterated DMPC:DMPA lipids (total level of acyl chain deuteration ~80%) containing 5 mol%, 10 mol%, and 20 mol% of ToSMTSL-PTE (Figure 1), were prepared for DNP experiments (samples S1, S2, S3, Table 1). These samples contained approximately 4, 8, and 16 biradicals per protein, respectively. Paramagnetic doping results in shortening of proton nuclear T1n relaxation times from 600 ms in diamagnetic PR down to 400–500 ms in all three samples. In line with this, doping lipid bilayers with paramagnetic ToSMTSL-PTE lipids induces a noticeable, ~40% signal intensity reduction in all three samples due to paramagnetic quenching and depolarization [51, 52] compared to a diamagnetic PR sample (Figure 7A). Similar magnitude of quenching and depolarization was observed in PR samples with covalently attached ToSMTSL (sample S4), and in a conventionally prepared TOTAPOL-doped PR (sample S5). As ToSMTSL is attached to C175, located in the cytoplasmic EF loop, and is spatially constrained by the covalent bond to within ~15 Å of the cytoplasmic face of the protein, we conclude that ToSMTSL-PTE lipids in samples S1–S3, as well as TOTAPOL molecules in the sample S5 are in close proximity to the protein.
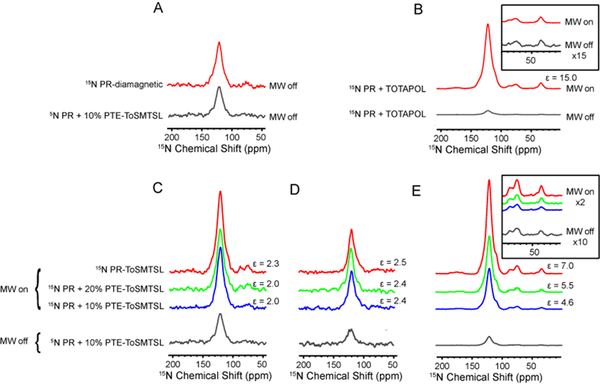
Figure 7.
Comparison of CPMAS NMR spectra of different 15N-labeled PR sample preparations measured with mm-wave on and off. (A) 15N spectra of diamagnetic PR (no biradicals present) and PR with 10% ToSMTSL-PTE collected at a 1H Larmor frequency of 600 MHz in a H2O-based buffer. (B) 15N spectra PR demonstrating DNP enhancements from TOTAPOL. Inset shows the region of arginine and lysine side chains. (C) Spectra collected on PR a 1H Larmor frequency of 600 MHz in a H2O-based buffer. (D) The same as in C, but samples were resuspended in a D2O-based buffer. Reduced absolute intensity in these samples is due to the lower CP efficiency. (E) Spectra collected at the magnetic field strength of 400 MHz on samples in a D2O-based buffer. Inset shows the region of arginine and lysine side chains. All spectra were processed with 100 Hz exponential line broadening.
Figure 7C compares 15N CPMAS NMR spectra of samples S2 and S3 with that of the control sample S4, all prepared with H2O-based buffer and measured at the magnetic field corresponding to 1H Larmor frequency of 600 MHz. Small DNP enhancements of ε≈1.9–2.0 were observed for S1, S2, S3 samples demonstrating no dependence (within the experimental error) of ε upon the concentration of the biradical-labeled lipid (Table 1). Only a slightly higher DNP enhancement of ε≈2.3 was observed for the sample S4, whereas the TOTAPOL-doped sample S5 resulted in an enhancement of ε≈3.3.
The relatively low DNP enhancement factors observed for the samples S1–S3 at 600 MHz may be due to several factors. From one hand EPR and DSC data for the pure lipid mixtures do not exclude an inhomogeneous distribution of ToSMTSL-PTE in the bilayers at lipid concentrations of 5.8 mol% and above. Such an inhomogeneity could be further amplified by an oligomeric integral membrane protein such as PR present at high concentration. On the other hand, shortening of T1n and a sizeable magnitude of the paramagnetic quenching effect argue against this and indicate that at least some biradicals are in close proximity to the protein. However, if these biradicals are still clustered, the electronic relaxation would be shortened by intermolecular dipolar and exchange interactions. Indeed, CW EPR spectra of DMPC:DMPA (9:1 w/w) lipids doped with just 0.5 mol% of ToSMTSL-PTE (Figure 5C) are indicative of stronger dipolar and/or exchange interactions when compared with ToSMTSL in a glassy water:glycerol matrix (Figures 5A and B). Shorter electronic relaxation time is expected to change the mechanism of cross-effect MAS-DNP, which is known to be dependent on electron spin relaxation rates as well as other parameters, such as hyperfine interaction, electron-electron dipolar interaction, and mm-wave field strength [53].
Clearly, the high proton concentration in samples S1–S3 prepared without any deuteration might be another factor contributing to low DNP enhancements observed in these experiments. Dilution of a proton bath through deuteration is expected to decrease nuclear spin relaxation rates and facilitate a more efficient proton-proton spin diffusion to the protein, as well as a reduction of the spin polarization transferred to the solvent [4, 54, 55]. Indeed, a replacement of H2O in a buffer by a 9:1 w/w mixture of D2O and H2O resulted in longer T1n relaxation times in the range of 800–900 ms in the three samples S1–S3. As expected, buffer deuteration resulted in a small but non-negligible increase in the DNP enhancements to ε=2.4 in samples S2 and S3 (Figure 7D and Table 1). Similarly, slightly higher enhancements were detected for the PR sample S4 with the covalently attached ToSMTSL. On the contrary, sample S1 containing 5 mol% of ToSMTSL-PTE lipid yielded a lower enhancement of ε=1.7 when prepared with an increased fraction of the deuterated lipids.
The strong dependence of the DNP enhancement on the static magnetic field strength is another factor that contributes to low DNP enhancements. Indeed, biradicals facilitate the electron-nuclear spin polarization transfer via the cross-effect [56, 57], which is strongly dependent on the magnetic field strength [53]. To test the field dependence, additional experiments were performed at a lower magnetic field corresponding to 400 MHz/263 GHz. As expected, higher enhancements of 4.6 and 5.5 were observed for the sample preparations S2 and S3 containing ToSMTSL-PTE lipid (sample S1 was lost to a spinning crash during DNP experiments), while more significant enhancements of ε=7.0 and ε=15.0 were obtained for sample S4 with the covalently attached ToSMTSL (Figure 7E and Table 1), and for the TOTAPOL-doped sample S5, respectively. Interestingly, the DNP enhancement factors were nearly uniform in all samples for the resonances representing protein signals from transmembrane residues (~110–140 ppm) and primarily exposed side chains of lysines resonating at ~30 ppm, and arginines at 80–90 ppm (Figures 7B inset, 7E inset).
Another factor that most likely contributes to a relatively low DNP enhancement is a sub-optimal relative orientation of the nitroxide rings in ToSMTSL-PTE. The DNP cross-effect is expected to be optimal when the difference between the electron Larmor frequencies is approximately equal to the nuclear Larmor frequency. For biradicals, this matching condition strongly depends on the relative orientations of g-matrices of the two nitroxide moieties. An extensive study by Emsley and co-workers [58] demonstrated, that the highest DNP enhancement is reached with rigid dinitroxides when two piperidine rings are locked in a nearly orthogonal orientation [59]. Numerical calculations predict that the cross-effect condition is satisfied when gyy components of the individual nitroxides within the biradical are oriented perpendicularly to each other [60]. However, in the TOTAPOL derivative ToSMTSL-PTE, the flexible linker between the two nitroxide moieties can sample multiple conformational states. Thus, for such a flexible biradical many mutual nitroxide orientations may not result in the required frequency separation for optimal cross-effect DNP. This effect takes place in solution, and it is most likely even more pronounced in the conformationally constraining environment of protein side chains or in the bilayer. In a contrast, biradicals containing an urea linker such as bTUrea and AMUPol are more rigid than TOTAPOL and, therefore, would yield more molecules to match the cross-effect (CE) conditions for the DNP enhancement [7]. Such molecular rigidity and longer electronic relaxation times observed for AMUPol would be beneficial to explore for the next generation of matrix-free DNP agents based on biradical-modified lipids.
We note here that in our experiments the concentration of ToSMTSL-PTE in lipid bilayers has not been fully optimized for the maximum DNP enhancement and signal-to-noise ratio when compared with the diamagnetic sample (Figure 7A). As discussed by Oschkinat and coworkers, an increase in the biradical concentration results in a considerable decrease of the NMR relaxation times (T1, , T1ρ) and also broadens the lines while providing for DNP enhancement [11]. Thus, one has to carefully decide on the optimal concentration depending on the DNP agent employed [9–11], especially when the goals are the gains in absolute sensitivity and spectral resolution [52]. While several studies have been focused on optimizing concentration of DNP agents in glassy aqueous matrix for DNP enhancement of NMR of co-dissolved molecules (e.g., [9–11]), optimization of DNP for membrane proteins has been mainly focused on sample preparation protocols (e.g., [13]). Nevertheless, optimization of concentration of a palmitate-anchored biradical derived from TOTAPOL (N-propyl-PALMIPOL) and incorporated into lipid bilayers for DNP-enhanced natural-abundance 13C-CPMAS spectra of egg PC vesicles yielded the best enhancement of ε=8.1 at only 1:125 biradical:lipid molar ratio when measured at a magnetic field corresponding to the 400 MHz 1H Larmor frequency [28]. The optimal concentration is also clearly dependent on the type of the DNP agent and the sample. For example, concentration of lipid-embedded monoradicals for DNP MAS 13C SSNMR experiments with a small membrane peptide carried out at 600 MHz/395 GHz was reported to be much higher reaching 6 mol% of the total lipid (or 3 mol% if recalculated for the biradical concentration) [27]. In our preceding experiments with ToSMTSL covalently attached to another heptahelical protein which forms trimers in lipid bilayers, the optimal enhancement was obtained at a biradical to protein ratio of 1:1 [35]. Finally, in our sample S5 containing TOTAPOL in a glassy water:glycerol matrix the estimated TOTAPOL/monomer ratio is in the range from 5–7. Thus, in order to keep biradical/monomer ratio comparable, we have chosen ToSMTSL-PTE concentrations corresponding to ca. 4, 8, and 16 biradical molecules per PR monomer. We also note that the DNP enhancement data for the samples S1, S2, and S3 do show an upward trend with increase in ToSMTSL-PTE concentration (Table 1) indicating that these concentrations are below or at the saturation point above which the DNP enhancement is expected to decrease similar to the data reported in refs. [9, 11].
4. Conclusions
We have previously described synthesis of a novel biradical ToSMTSL which originates from TOTAPOL and contains a thiol-specific methanethiosulfonate group. Here, we conjugate ToSMTSL with 1,2-dipalmitoyl-sn-glycero-3-phosphothioethanol to obtain the first example of a biradical-labeled phospholipid and report on matrix-free DNP NMR experiments with a oligomeric heptahelical membrane protein. The results are compared with conventionally dispersed TOTAPOL in a glassy matrix. While the enhancements at the 1H Larmor frequency of 600 MHz for the samples prepared with either ToSMTSL-PTE lipid or conventional TOTAPOL in a glassy matrix were found to be moderate and similar, the matrix-free ToSMTSL-PTE preparation allows for approximately fourfold more protein sample to be packed into the rotor. The latter feature results in improving the absolute sensitivity by a factor of 4 vs. the DNP experiments on PR doped with TOTAPOL.
While moderate enhancements observed for PR at 400 MHz were comparable to those reported for lipids using another lipophilic TOTAPOL derivative N-propyl-PALMIPOL, the large drop in enhancement at 600 MHz is likely attributed to the strong magnetic field dependence of the enhancement previously observed for the parent biradical TOTAPOL. Conformational changes in ToSMTSL-PTE when it is incorporated into lipid bilayers, along with potential inhomogeneity in the ToSMTSL-PTE distribution in lipid bilayers are important additional factors to consider for further development of lipid-based matrix-free membrane protein sample preparation for DNP.
Acknowledgments
This research was supported by U.S. Department of Energy Basic Biosciences Program DOE Contract DE-FG02–02ER15354 (biradical synthesis; DSC and EPR experiments) to A.I.S., NIH GM130821 to A.I.S. for the final manuscript preparation, and NSERC Discovery Grants RGPIN-2014–04547 to V.L. and RGPIN-2018–04397 to L.S.B. EPR instrumentation at NCSU was supported by grants from the National Institutes of Health (no. RR023614), the National Science Foundation (no. CHE-0840501), and North Carolina Biotechnology Center (NCBC no. 2009-IDG-1015).
The authors are grateful to A. R. Choi and K.-H. Jung (Sogang University, Seoul, Korea) for providing the DNA of the mutant PR. We thank Drs. A. Charchoglyan and D. Brewer of the University of Guelph Advanced Analysis Centre for their help with MassSpec data collection and analysis. Ms. B. Griffin (Department of Chemistry, North Carolina State University) is thanked for the help with the initial DSC experiments. D.B.G. and M.E.W. were recipients of the NSERC PGS fellowship.
Footnotes
Authors declare no competing financial interest.
References
- [1].Lilly Thankamony AS, Wittmann JJ, Kaushik M, Corzilius B, Dynamic nuclear polarization for sensitivity enhancement in modern solid-state NMR, Prog Nucl Magn Reson Spectrosc 102–103 (2017) 120–195. [DOI] [PubMed] [Google Scholar]
- [2].Song CS, Hu KN, Joo CG, Swager TM, Griffin RG, TOTAPOL: A biradical polarizing agent for dynamic nuclear polarization experiments in aqueous media, J. Am. Chem. Soc 128(35) (2006) 11385–11390. [DOI] [PubMed] [Google Scholar]
- [3].Hu KN, Polarizing agents and mechanisms for high-field dynamic nuclear polarization of frozen dielectric solids, Solid State Nucl Magn Reson 40(2) (2011) 31–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Rogawski R, McDermott AE, New NMR tools for protein structure and function: Spin tags for dynamic nuclear polarization solid state NMR, Archives of biochemistry and biophysics 628 (2017) 102–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Jagtap AP, Geiger MA, Stoppler D, Orwick-Rydmark M, Oschkinat H, Sigurdsson ST, bcTol : a highly water-soluble biradical for efficient dynamic nuclear polarization of biomolecules, Chem Commun (Camb) 52(43) (2016) 7020–3. [DOI] [PubMed] [Google Scholar]
- [6].Geiger MA, Jagtap AP, Kaushik M, Sun H, Stoppler D, Sigurdsson ST, Corzilius B, Oschkinat H, Efficiency of Water-Soluble Nitroxide Biradicals for Dynamic Nuclear Polarization in Rotating Solids at 9.4 T: bcTol-M and cyolyl-TOTAPOL as New Polarizing Agents, Chemistry 24(51) (2018) 13485–13494. [DOI] [PubMed] [Google Scholar]
- [7].Sauvee C, Rosay M, Casano G, Aussenac F, Weber RT, Ouari O, Tordo P, Highly efficient, water-soluble polarizing agents for dynamic nuclear polarization at high frequency, Angew Chem Int Ed Engl 52(41) (2013) 10858–61. [DOI] [PubMed] [Google Scholar]
- [8].Song C, Hu KN, Joo CG, Swager TM, Griffin RG, TOTAPOL: a biradical polarizing agent for dynamic nuclear polarization experiments in aqueous media, J Am Chem Soc 128(35) (2006) 11385–90. [DOI] [PubMed] [Google Scholar]
- [9].Corzilius B, Andreas LB, Smith AA, Ni QZ, Griffin RG, Paramagnet induced signal quenching in MAS-DNP experiments in frozen homogeneous solutions, Journal of Magnetic Resonance 240 (2014) 113–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Takahashi H, Fernández-de-Alba C, Lee D, Maurel V, Gambarelli S, Bardet M, Hediger S, Barra A-L, De Paëpe G, Optimization of an absolute sensitivity in a glassy matrix during DNP-enhanced multidimensional solid-state NMR experiments, Journal of Magnetic Resonance 239 (2014) 91–99. [DOI] [PubMed] [Google Scholar]
- [11].Lange S, Linden AH, Akbey U, Franks WT, Loening NM, van Rossum BJ, Oschkinat H, The effect of biradical concentration on the performance of DNP-MAS-NMR, Journal of magnetic resonance 216 (2012) 209–12. [DOI] [PubMed] [Google Scholar]
- [12].Mak-Jurkauskas ML, Bajaj VS, Hornstein MK, Belenky M, Griffin RG, Herzfeld J, Energy transformations early in the bacteriorhodopsin photocycle revealed by DNP-enhanced solid-state NMR, Proc Natl Acad Sci U S A 105(3) (2008) 883–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Liao SY, Lee M, Wang T, Sergeyev IV, Hong M, Efficient DNP NMR of membrane proteins: sample preparation protocols, sensitivity, and radical location, J Biomol NMR 64(3) (2016) 223–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Barnes AB, Corzilius B, Mak-Jurkauskas ML, Andreas LB, Bajaj VS, Matsuki Y, Belenky ML, Lugtenburg J, Sirigiri JR, Temkin RJ, Herzfeld J, Griffin RG, Resolution and polarization distribution in cryogenic DNP/MAS experiments, Phys Chem Chem Phys 12(22) (2010) 5861–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Maciejko J, Mehler M, Kaur J, Lieblein T, Morgner N, Ouari O, Tordo P, Becker-Baldus J, Glaubitz C, Visualizing Specific Cross-Protomer Interactions in the Homo-Oligomeric Membrane Protein Proteorhodopsin by Dynamic-Nuclear-Polarization-Enhanced Solid-State NMR, J Am Chem Soc 137(28) (2015) 9032–43. [DOI] [PubMed] [Google Scholar]
- [16].Anchordoguy TJ, Rudolph AS, Carpenter JF, Crowe JH, Modes of interaction of cryoprotectants with membrane phospholipids during freezing, Cryobiology 24(4) (1987) 324–31. [DOI] [PubMed] [Google Scholar]
- [17].Schrader AM, Cheng CY, Israelachvili JN, Han S, Communication: Contrasting effects of glycerol and DMSO on lipid membrane surface hydration dynamics and forces, J Chem Phys 145(4) (2016) 041101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Lee M, Hong M, Cryoprotection of lipid membranes for high-resolution solid-state NMR studies of membrane peptides and proteins at low temperature, J Biomol NMR 59(4) (2014) 263–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Takahashi H, Lee D, Dubois L, Bardet M, Hediger S, De Paepe G, Rapid natural-abundance 2D 13C-13C correlation spectroscopy using dynamic nuclear polarization enhanced solid-state NMR and matrix-free sample preparation, Angew Chem Int Ed Engl 51(47) (2012) 11766–9. [DOI] [PubMed] [Google Scholar]
- [20].Takahashi H, Ayala I, Bardet M, De Paepe G, Simorre JP, Hediger S, Solid-State NMR on Bacterial Cells: Selective Cell Wall Signal Enhancement and Resolution Improvement using Dynamic Nuclear Polarization, J. Am. Chem. Soc (2013) 5105–5110. [DOI] [PubMed] [Google Scholar]
- [21].Nagaraj M, Franks TW, Saeidpour S, Schubeis T, Oschkinat H, Ritter C, van Rossum BJ, Surface Binding of TOTAPOL Assists Structural Investigations of Amyloid Fibrils by Dynamic Nuclear Polarization NMR Spectroscopy, Chembiochem 17(14) (2016) 1308–11. [DOI] [PubMed] [Google Scholar]
- [22].Viennet T, Viegas A, Kuepper A, Arens S, Gelev V, Petrov O, Grossmann TN, Heise H, Etzkorn M, Selective Protein Hyperpolarization in Cell Lysates Using Targeted Dynamic Nuclear Polarization, Angew Chem Int Ed Engl 55(36) (2016) 10746–50. [DOI] [PubMed] [Google Scholar]
- [23].Vitzthum V, Borcard F, Jannin S, Morin M, Mieville P, Caporini MA, Sienkiewicz A, Gerber-Lemaire S, Bodenhausen G, Fractional Spin-Labeling of Polymers for Enhancing NMR Sensitivity by Solvent-Free Dynamic Nuclear Polarization, Chemphyschem 12(16) (2011) 2929–2932. [DOI] [PubMed] [Google Scholar]
- [24].Voinov MA, Good DB, Ward ME, Milikisiyants S, Marek A, Caporini MA, Rosay M, Munro RA, Ljumovic M, Brown LS, Ladizhansky V, Smirnov AI, Cysteine-Specific Labeling of Proteins with a Nitroxide Biradical for Dynamic Nuclear Polarization NMR, The journal of physical chemistry. B 119(32) (2015) 10180–90. [DOI] [PubMed] [Google Scholar]
- [25].van der Cruijsen EA, Koers EJ, Sauvee C, Hulse RE, Weingarth M, Ouari O, Perozo E, Tordo P, Baldus M, Biomolecular DNP-Supported NMR Spectroscopy using Site-Directed Spin Labeling, Chemistry 21(37) (2015) 12971–7. [DOI] [PubMed] [Google Scholar]
- [26].Wylie BJ, Dzikovski BG, Pawsey S, Caporini M, Rosay M, Freed JH, McDermott AE, Dynamic nuclear polarization of membrane proteins: covalently bound spin-labels at protein-protein interfaces, J Biomol NMR 61(3–4) (2015) 361–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Smith AN, Caporini MA, Fanucci GE, Long JR, A method for dynamic nuclear polarization enhancement of membrane proteins, Angew Chem Int Ed Engl 54(5) (2015) 1542–6. [DOI] [PubMed] [Google Scholar]
- [28].Fernandez-de-Alba C, Takahashi H, Richard A, Chenavier Y, Dubois L, Maurel V, Lee D, Hediger S, De Paepe G, Matrix-Free DNP-Enhanced NMR Spectroscopy of Liposomes Using a Lipid-Anchored Biradical, Chemistry 21(12) (2015) 4512–7. [DOI] [PubMed] [Google Scholar]
- [29].Beja O, Aravind L, Koonin EV, Suzuki MT, Hadd A, Nguyen LP, Jovanovich S, Gates CM, Feldman RA, Spudich JL, Spudich EN, DeLong EF, Bacterial rhodopsin: Evidence for a new type of phototrophy in the sea, Science 289(5486) (2000) 1902–1906. [DOI] [PubMed] [Google Scholar]
- [30].Hoffman J, Aslimovska L, Bamann C, Glaubitz C, Bamberg E, Brutschy B, Studying the stoichiometries of membrane proteins by mass spectrometry: microbial rhodopsins and a potassium ion channel, Physical Chemistry Chemical Physics 12(14) (2010) 3480–3485. [DOI] [PubMed] [Google Scholar]
- [31].Shi L, Ahmed MA, Zhang W, Whited G, Brown LS, Ladizhansky V, Three-dimensional solid-state NMR study of a seven-helical integral membrane proton pump--structural insights, J Mol Biol 386(4) (2009) 1078–93. [DOI] [PubMed] [Google Scholar]
- [32].Wada Y, Kawanabe A, Furutani Y, Kandori H, Ohtani H, Quantum yields for the light adaptations in Anabaena sensory rhodopsin and bacteriorhodopsin, Chem. Phys. Lett 453(1–3) (2008) 105–108. [Google Scholar]
- [33].daCosta CJ, Baenziger JE, A rapid method for assessing lipid:protein and detergent:protein ratios in membrane-protein crystallization, Acta Crystallogr D Biol Crystallogr 59(Pt 1) (2003) 77–83. [DOI] [PubMed] [Google Scholar]
- [34].Wang S, Munro RA, Shi L, Kawamura I, Okitsu T, Wada A, Kim SY, Jung KH, Brown LS, Ladizhansky V, Solid-state NMR spectroscopy structure determination of a lipid-embedded heptahelical membrane protein, Nature Methods 10(10) (2013) 1007–12. [DOI] [PubMed] [Google Scholar]
- [35].Voinov MA, Good DB, Ward ME, Milikisiyants S, Marek A, Caporini MA, Rosay M, Munro RA, Ljumovic M, Brown LS, Ladizhansky V, Smirnov AI, Cysteine-Specific Labeling of Proteins with a Nitroxide Biradical for Dynamic Nuclear Polarization NMR, Journal of Physical Chemistry B 119(32) (2015) 10180–10190. [DOI] [PubMed] [Google Scholar]
- [36].Thurber KR, Tycko R, Measurement of sample temperatures under magic-angle spinning from the chemical shift and spin-lattice relaxation rate of Br-79 in KBr powder, J. Magn. Reson 196(1) (2009) 84–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Hartmann SR, Hahn EL, Nuclear Double Resonance in the Rotating Frame Phys. Rev 128 (1962) 2042–2053. [Google Scholar]
- [38].Fung BM, Khitrin AK, Ermolaev K, An improved broadband decoupling sequence for liquid crystals and solids, J. Magn. Reson 142(1) (2000) 97–101. [DOI] [PubMed] [Google Scholar]
- [39].Smirnov AI, Belford RL, Rapid Quantitation from Inhomogeneously Broadened EPR Spectra by a Fast Convolution Algorithm, J. Magn. Reson. Ser. A 113(1) (1995) 65–73. [Google Scholar]
- [40].Smirnov AI, Smirnova TI, Convolution-based algorithm: from analysis of rotational dynamics to EPR oximetry and protein distance measurements, EPR: Instrumental Methods, Springer2004, pp. 277–348. [Google Scholar]
- [41].Luckhurst GR, in: Berliner LJ (Ed.), Spin Labeling: Theory and Applications, Academic Press, New York, 1976, pp. 133–181. [Google Scholar]
- [42].Luckhurst GR, Pedulli GF, Interpretation of Biradical Electron Resonance Spectra, J. Am. Chem. Soc 92(15) (1970) 4738–9. [Google Scholar]
- [43].McHaourab HS, Oh KJ, Fang CJ, Hubbell WL, Conformation of T4 Lysozyme in Solution. Hinge-Bending Motion and the Substrate-Induced Conformational Transition Studied by Site-Directed Spin Labeling, Biochemistry 36(2) (1997) 307–316. [DOI] [PubMed] [Google Scholar]
- [44].Kausik R, Han S, Dynamics and state of lipid bilayer-internal water unraveled with solution state H-1 dynamic nuclear polarization, Physical Chemistry Chemical Physics 13(17) (2011) 7732–7746. [DOI] [PubMed] [Google Scholar]
- [45].Smirnov AI, Clarkson RB, Belford RL, EPR linewidth (T-2) method to measure oxygen permeability of phospholipid bilayers and its use to study the effect of low ethanol concentrations, J. Magn. Reson. Ser. B 111(2) (1996) 149–157. [DOI] [PubMed] [Google Scholar]
- [46].Alaouie AM, Smirnov AI, Ultra-stable temperature control in EPR experiments: Thermodynamics of gel-to-liquid phase transition in spin-labeled phospholipid bilayers and bilayer perturbations by spin labels, Journal of Magnetic Resonance 182(2) (2006) 229–238. [DOI] [PubMed] [Google Scholar]
- [47].Subczynski WK, Raguz M, Widomska J, Studying Lipid Organization in Biological Membranes Using Liposomes and EPR Spin Labeling, in: Weissig V (Ed.), Liposomes: Methods and Protocols, Vol 2: Biological Membrane Models2010, pp. 247–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Mabrey S, Sturtevant JM, Investigation of phase transitions of lipids and lipid mixtures by sensitivity differential scanning calorimetry, Proc. Natl. Acad. Sci. U S A 73(11) (1976) 3862–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Silvius JR, Thermotropic Phase Transitions of Pure Lipids in Model Membranes and Their Modifications by Membrane Proteins, in: Jost PC, Griffith OH (Ed.), Lipid-Protein Interactions, John Wiley & Sons, Inc., New York, 1982, pp. 239–281. [Google Scholar]
- [50].Alaouie AM, Smirnov AI, Cooperativity and kinetics of phase transitions in nanopore-confined bilayers studied by differential scanning calorimetry, Biophysical Journal 88(2) (2005) L11–L13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Thurber KR, Tycko R, Theory for cross effect dynamic nuclear polarization under magic-angle spinning in solid state nuclear magnetic resonance: The importance of level crossings, J. Chem. Phys 137 (2012) 084508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Lee D, Hediger S, De Paepe G, Is solid-state NMR enhanced by dynamic nuclear polarization?, Solid State Nucl Magn Reson 66–67C (2015) 6–20. [DOI] [PubMed] [Google Scholar]
- [53].Mance D, Gast P, Huber M, Baldus M, Ivanov KL, The magnetic field dependence of cross-effect dynamic nuclear polarization under magic angle spinning, J. Chem. Phys 142 (2015) 234201. [DOI] [PubMed] [Google Scholar]
- [54].Rosay M, Sensitivity-enhanced Nuclear Magnetic Resonance of BIological Solids, Chemistry, Massachusetts Institute of Technology, Massachusetts Institute of Technology, 2001, p. 152. [Google Scholar]
- [55].Akbey U, Franks WT, Linden A, Lange S, Griffin RG, van Rossum BJ, Oschkinat H, Dynamic nuclear polarization of deuterated proteins, Angewandte Chemie 49(42) (2010) 7803–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Hwang CF, Hill DA, New Effect in Dynamic Polarization, Phys. Rev. Lett 18(4) (1967) 110–112. [Google Scholar]
- [57].Hu KN, Debelouchina GT, Smith AA, Griffin RG, Quantum mechanical theory of dynamic nuclear polarization in solid dielectrics, J Chem Phys 134 (2011) 125105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Kubicki DJ, Casano G, Schwarzwalder M, Abel S, Sauvee C, Ganesan K, Yulikov M, Rossini AJ, Jeschke G, Coperet C, Lesage A, Tordo P, Ouari O, Emsley L, Rational design of dinitroxide biradicals for efficient cross-effect dynamic nuclear polarization, Chem Sci 7(1) (2016) 550–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Matsuki Y, Maly T, Ouari O, Karoui H, Le Moigne F, Rizzato E, Lyubenova S, Herzfeld J, Prisner T, Tordo P, Griffin RG, Dynamic nuclear polarization with a rigid biradical, Angew Chem Int Ed Engl 48(27) (2009) 4996–5000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Perras FA, Sadow A, Pruski M, In Silico Design of DNP Polarizing Agents: Can Current Dinitroxides Be Improved?, Chemphyschem 18(16) (2017) 2279–2287. [DOI] [PubMed] [Google Scholar]