Abstract
Background
Implantable cardiac defibrillators (ICDs) are used to prevent sudden cardiac death in patients with cardiac sarcoidosis. The most recent recommendations for ICD implantation in these patients are in the 2017 American Heart Association (AHA)/American College of Cardiology (ACC)/Heart Rhythm Society (HRS) Guideline for Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death. These recommendations – based on observational studies or expert opinion – have not been assessed. We aimed to assess them.
Methods
We performed a large retrospective cohort study of patients with biopsy-proven sarcoidosis and known or suspected cardiac sarcoidosis that underwent cardiovascular magnetic resonance imaging (CMR). Patients were followed for a composite endpoint of significant ventricular arrhythmia or sudden cardiac death. The discriminatory performance of the Guideline recommendations was tested using time-dependent receiver operating characteristics analyses. The optimal cutoff for the extent of late gadolinium enhancement (LGE) predictive of the composite endpoint was determined using the Youden index.
Results
In 290 patients, the class I and IIa recommendations identified all patients who experienced the composite endpoint over a median follow up of 3.0 years. Patients meeting class I recommendations had a significantly higher incidence of the composite endpoint than those meeting class IIa recommendations. Left ventricular ejection fraction (LVEF) >35% with >5.7% LGE on CMR was as sensitive as and significantly more specific than LVEF >35% with any LGE. Patients meeting 2 class IIa recommendations – LVEF >35% with need for a permanent pacemaker, and LVEF >35% with LGE >5.7% – had high annualized event rates. Excluding 2 class IIa recommendations – LVEF >35% with syncope, and LVEF >35% with inducible ventricular arrhythmia – resulted in improved discrimination for the composite endpoint.
Conclusions
We assessed the Guideline recommendations for ICD implantation in patients with known or suspected cardiac sarcoidosis and identified topics for future research.
Keywords: cardiac sarcoidosis, guideline, sarcoidosis, cardiac magnetic resonance imaging, implanted cardioverter defibrillator
Journal Subject Terms: Magnetic Resonance Imaging (MRI), Prognosis, Sudden Cardiac Death, Arrhythmias, Catheter Ablation and Implantable Cardioverter-Defibrillator
Introduction
Sarcoidosis is a multisystem granulomatous disease of unknown etiology. An estimated 200,000 Americans live with sarcoidosis, of which up to 25% have cardiac sarcoidosis1. Cardiac sarcoidosis is the second leading cause of sarcoidosis-related mortality, pulmonary sarcoidosis being the first2. Most deaths from cardiac sarcoidosis are sudden cardiac deaths due to cardiac arrhythmias3. Implantable cardiac defibrillators (ICDs) are used for primary and secondary prevention of sudden cardiac death in patients with cardiac sarcoidosis4.
The first recommendations for ICD implantation in patients with cardiac sarcoidosis were included in the Heart Rhythm Society (HRS) Expert Consensus Statement on the Diagnosis and Management of Arrhythmias Associated with Cardiac Sarcoidosis5. The latest recommendations are from the 2017 American Heart Association (AHA)/American College of Cardiology (ACC)/HRS Guideline for Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death6 (hereafter referred to as the Guideline), listed in Table 1. These recommendations are based on observational studies or expert opinion, and they have not been validated. Since ICDs may be associated with complications, which are increased in patients with cardiac sarcoidosis4, 7, it is critical to assess the current recommendations for ICD implantation in patients with cardiac sarcoidosis.
Table 1.
2017 AHA/ACC/HRS Guideline for Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death Recommendations for ICD Implantation in Cardiac Sarcoidosis
| Class I | Class IIa |
|---|---|
| Sustained VT or cardiac arrest | LVEF >35% with syncope |
| LVEF ≤35% | LVEF >35% with scar by CMR or PET |
| LVEF >35% with an indication for permanent pacing | |
| LVEF >35% with inducible ventricular arrhythmia | |
Recommendations apply to patients that are ICD candidates as determined by functional status, life expectancy of >1 year, and patient preference.
Further, 1 of the class IIa recommendations for ICD implantation in patients with cardiac sarcoidosis is in patients with left ventricular ejection fraction (LVEF) greater than 35% with scar by cardiovascular magnetic resonance imaging (CMR). Figure 8 of the Guideline clarifies this as “extensive scar”; however, this is not defined6.
We conducted this study with 3 objectives: first, to assess the Guideline recommendations for ICD implantation in a large cohort of patients with known or suspected cardiac sarcoidosis who underwent CMR; second, to clarify the recommendation involving the extent of scar on CMR; and third, to identify topics for future research to improve the recommendations.
Methods
Patients and Data Collection
The authors declare that all supporting data are available within the article. We studied consecutive patients with biopsy-proven (extra-cardiac or cardiac) sarcoidosis who underwent CMR with late gadolinium enhancement (LGE) imaging for the evaluation of cardiac sarcoidosis at the University of Minnesota. Eligible patients were identified from the University of Minnesota’s Cardiovascular Magnetic Resonance Registry8–10.
Demographic data, medical history, co-morbidities, medications, and outcome data were collected blinded to CMR data. Syncope was defined according to the 2017 ACC/AHA/HRS Guideline for the Evaluation and Management of Patients with Syncope as “abrupt, transient, complete loss of consciousness, associated with an inability to maintain postural tone, with rapid and spontaneous recovery. The presumed mechanism is cerebral hypoperfusion. There should not be clinical features of other non-syncope causes of loss of consciousness, such as seizure, antecedent head trauma, or apparent loss of consciousness (i.e., pseudo-syncope)”11. CMRs were interpreted and analyzed blinded to all other patient data by the consensus of 2 investigators with expertise in CMR (FK and CS). We did not include data from echocardiography or positron emission tomography in our current analyses. This retrospective cohort study was approved by University of Minnesota’s Institutional Review Board with a waiver of informed consent.
CMR Protocol
CMR was performed on clinical 1.5T Siemens scanners (Avanto and Aera) using phased-array receiver coils according to standard recommendations12, 13. A typical protocol was as follows: First, localizers were acquired to identify the cardiac position. Next, cine CMR images were acquired in many short-axis (every 10 mm to cover the entire LV from the mitral valve plane through the apex) and 3 long-axis views (2-, 3-, and 4-chamber) using a steady-state free-precession sequence. Standard LGE CMR imaging was performed 10–15 minutes after administration of gadolinium contrast (0.15 mmol/kg), using a 2-dimensional segmented inversion-recovery gradient-echo sequence in identical views as cine CMR imaging. Typical inversion delay times were 280 to 360 ms.
CMR Analyses
CMR analyses were performed by the consensus of 2 investigators with expertise in CMR (FK and CS), blinded to all clinical information. LVEF and right ventricular ejection fraction (RVEF) were determined by quantitative analysis according to standard recommendations14. In patients with LGE, the extent was quantified using the signal threshold versus reference myocardium approach15. First, the endocardial and epicardial borders were traced and a reference region of interest was placed over the largest contiguous region of homogeneously nulled (i.e., normal) myocardium. Next, a signal threshold of >5 standard deviations (SD) above the mean signal of the reference myocardium was applied to derive the total LGE mass, which was then divided by total LV mass to obtain LGE extent as a percentage. The >5SD threshold was chosen because it is the best predictor of cardiovascular events in non-ischemic cardiomyopathy when compared to expert visual scoring and the >2SD and >3SD thresholds16. Since LGE could include scar (chronic) or necrosis (acute), we use the term “LGE” rather than “scar” as used in the Guideline.
Clinical Follow-up and Endpoints
Follow-up data were collected through a review of electronic medical records from all hospitals and clinics within the University of Minnesota Health system. The pre-specified endpoint was a composite of sudden cardiac death, resuscitated cardiac arrest with documented ventricular tachycardia (VT), or significant ventricular arrhythmia, including sustained VT (duration >30 seconds) and appropriate ICD therapy (shock or anti-tachycardia pacing). Sudden cardiac death was defined according to the 2017 Cardiovascular and Stroke Endpoint Definitions for Clinical Trials17. ICD therapies were adjudicated by board-certified cardiac electrophysiologists as part of the patients’ clinical care. Appropriateness of ICD therapy was determined from the intracardiac electrograms recorded by the ICD based on the tachycardia rate, onset, stability, atrioventricular association, and the QRS morphology. Mortality status and death dates were obtained from the electronic medical records and the Minnesota State Department of Health’s Office of Vital Records. For patients who died outside the hospital, death certificates were reviewed to determine the cause of death.
Statistical Analysis
Normally-distributed continuous variables were expressed as mean ± SD, and non-normally-distributed continuous variables were presented as medians with interquartile range (IQR). Categorical variables were expressed as counts with percentages. Comparison between groups was performed with a 2-sample Student t test for continuous, normally-distributed variables, and Wilcoxon rank sum test for continuous, non-normally distributed data. Chi-square tests were used to compare discrete data between groups; in those cases where the expected cell count was <5, Fisher exact test was used. The cumulative probability for the occurrence of an endpoint was estimated using the Kaplan-Meier method, and hazard ratios (HR) were calculated using Cox regression and presented with their associated 95% confidence intervals (CIs). Censoring events were orthotopic heart transplantation, left ventricular assist device implantation, and death. The discriminating performances of Guideline recommendations for ICD implantation were tested using time-dependent receiver operating characteristic (ROC) curve analyses using the inverse probability of censoring weighting estimation method18–20. Annual event rates were calculated by dividing the number of patients with events by the time to the first event or the end of follow-up across all patients and expressed as a percentage. The optimal cutoff (i.e., the best compromise between sensitivity and specificity) for the extent of LGE predictive of the endpoint was determined using the Youden index21. By assigning equal weight to both sensitivity and specificity, the index provides an optimal tradeoff between the two. Statistical analyses were performed using R version 3.3.3 (The R Foundation; https://www.r-project.org/). All statistical tests were 2-tailed, and a p value of <0.05 was considered statistically significant.
Results
Patient characteristics
Two hundred and ninety consecutive patients with biopsy-proven sarcoidosis underwent CMR and were included in the study (Table 2). Of the 290 patients, 284 (98%) had biopsy-proven extra-cardiac sarcoidosis including 2 with concomitant biopsy-proven cardiac sarcoidosis, whereas 6 had biopsy-proven cardiac sarcoidosis without clinical findings characteristic of sarcoidosis in any other organs.
Table 2.
Patient Characteristics at the Time of CMR (n = 290)
| Age, years (SD) | 53.1 (12.2) |
| Women, n (%) | 141 (48.6) |
| Race | |
| Caucasian, n (%) | 233 (80.3) |
| African-American, n (%) | 50 (17.2) |
| Other, n (%) | 7 (2.4) |
| Extra-cardiac sarcoidosis Involvement (may be more than one) | |
| Lung, n (%) | 260 (89.7) |
| Skin, n (%) | 27 (9.3) |
| Liver, n (%) | 23 (7.9) |
| Eye, n (%) | 19 (6.6) |
| Nervous system, n (%) | 11 (3.8) |
| Other, n (%) | 96 (33.6) |
| Cardiac symptoms | |
| Palpitations, n (%) | 97 (33.4) |
| Chest pain, n (%) | 100 (34.5) |
| Syncope, n (%) | 14 (4.8) |
| NYHA functional class (I/II/III/IV), n (%) | 92/43/22/13 (31.7/14.8/7.6/4.5) |
| Comorbidities | |
| Hypertension, n (%) | 153 (52.8) |
| Dyslipidemia, n (%) | 136 (46.9) |
| Diabetes mellitus, n (%) | 69 (23.8) |
| Former tobacco use, n (%) | 120 (41.4) |
| Tobacco use, n (%) | 28 (9.7) |
| Coronary artery disease, n (%) | 31 (10.7) |
| Pulmonary hypertension, n (%) | 48 (16.6) |
| Medications | |
| Aspirin, n (%) | 90 (31.0) |
| Beta-blockers, n (%) | 87 (30.0) |
| ACE-inhibitor/ Angiotensin receptor blocker, n (%) | 87 (30.0) |
| Statin, n (%) | 91 (31.4) |
| Steroids, n (%) | 98 (33.8) |
| Non-steroidal immune modulatory agents, n (%) | 56 (19.3) |
| Arrhythmia prior to CMR | |
| PVCs, n (%) | 70 (24.1) |
| Ventricular arrhythmia (sustained or non-sustained), n (%) | 20 (6.9) |
| Sustained VT | 7 (2.4) |
| Aborted cardiac arrest | 1 (0.3) |
| Supraventricular tachycardia, n (%) | 27 (9.3) |
| Atrial fibrillation/Flutter, n (%) | 33 (11.3) |
| Advanced AVB (2nd degree Type 2 or 3rd degree), n (%) | 14 (4.8) |
| CMR findings | |
| LVEDVI, ml/m2 (IQR) | 59.9 (50.3 – 71.9) |
| LVESVI, ml/m2 (IQR) | 26.3 (20.8 – 32.5) |
| LVEF, % (IQR) | 56.6 (53.0 – 60.3) |
| LVEF ≤35%, n (%) | 20 (6.9) |
| RVEDVI, ml/m2 (IQR) | 62.7 (53.3 – 72.8) |
| RVESVI, ml/m2 (IQR) | 28.9 (23.7 – 36.8) |
| RVEF, % (IQR) | 52.5 (49.1 – 56.9) |
| LV LGE, n (%) | 87 (30.0) |
Values are n (%), mean ± SD or median (interquartile range).
ACE = angiotensin converting enzyme, AVB = atrioventricular block; CMR = cardiovascular magnetic resonance; EDVI = end-diastolic volume index; EF = ejection fraction; ESVI = end-systolic volume index; LV = left ventricle; LGE = late gadolinium enhancement; NYHA = New York Heart Association; PVC = premature ventricular complexes; RV = right ventricle; VT = ventricular tachycardia
Follow-up
The median follow-up time was 3.0 years (IQR 1.4 to 5.5 years) with a total of 1,019 patient-years of follow-up. No patients were lost to follow-up. During follow-up, 17 patients had significant ventricular arrhythmia and 1 had sudden cardiac death. Thus, 18 patients reached the composite outcome. Of the 17 with significant ventricular arrhythmia, the details of the ventricular arrhythmia that required ICD therapy (shock or anti-tachycardia pacing) were available for review in 16. The patient whose arrhythmia details could not be reviewed had recurrent ICD shocks and subsequently expired due to cardiogenic shock after being listed for heart transplantation. Of the 16 patients with available data, 12 (75%) had sustained VT lasting from 40 seconds to >24 hours in duration. The other 4 received ICD therapy for VT/ventricular fibrillation (VF) at rates greater than 190 beats/min. Interestingly, 15/17 (88%) patients with significant ventricular arrhythmia had >1 distinct episode of care (hospitalization or outpatient visit) due to recurrent significant ventricular arrhythmia.
Assessment of class I recommendations (Table 3)
Table 3.
Patients Meeting Guideline Recommendations for ICD Implantation
| Indication | Eligible patients | Patients with ICDs | Patients with PPMs | Patients reaching endpoint | Annualized Event Rate | Time dependent AUC |
|---|---|---|---|---|---|---|
| Class I recommendations | ||||||
| Spontaneous sustained VT or sudden cardiac arrest | 8 | 6 | 0 | 6 | 81.7% | - |
| LVEF ≤35% | 20 | 13 | 0 | 9 | 19.4% | - |
| Class IIa recommendations | ||||||
| LVEF >35% with syncope | 12 | 3 | 0 | 1 | 2.7% | - |
| LVEF >35% with need for PPM | 14 | 9 | 3 | 5 | 19.6% | - |
| LVEF >35% with inducible sustained ventricular arrhythmias | 1 | 1 | 0 | 0 | 0% | - |
| LVEF >35% with any LGE by CMR | 70 | 16 | 1 | 5 | 2.1% | - |
| LVEF >35% with >5.7% LGE by CMR | 19 | 12 | 1 | 5 | 12.0% | - |
| Either class I recommendation only | 25 | 17 | 0 | 12 | 22.4% | 0.82 |
| Any class I or IIa recommendation; includes any LGE by CMR | 110 | 36 | 3 | 18 | 5.3% | 0.80 |
| Any class I or IIa recommendation; includes >5.7% LGE by CMR | 60 | 32 | 3 | 18 | 12.3% | 0.94 |
| Any class I or select IIa recommendations: LVEF >35% with >5.7% LGE by CMR and/or need for PPM | 50 | 31 | 3 | 18 | 16.4% | 0.97 |
AUC = area under the curve; CMR = cardiovascular magnetic resonance; ICD = implantable cardioverter-defibrillator; LVEF = left ventricular ejection fraction; PPM = permanent pacemaker; VT = ventricular tachycardia
Sustained VT or sudden cardiac arrest: Eight patients met the recommendation for an ICD due to spontaneous sustained VT or sudden cardiac arrest, of which 6 reached the composite endpoint, with an annualized event rate of 81.7%.
LVEF ≤35%: Twenty patients met the recommendation due to LVEF of ≤35%, of which nine reached the composite endpoint, with an annualized event rate of 19.4%. Three patients met both class I recommendations (sustained VT or sudden cardiac arrest, and LVEF ≤35%), and thus, 25 were eligible for ICDs, of which, 12 met the composite endpoint, with an annualized event rate of 22.4%.
Assessment of class IIa recommendations (Table 3)
LVEF >35% with syncope: Twelve patients had LVEF >35% with syncope, of which only 1 reached the composite endpoint, with an annualized event rate of 2.7%. This patient also met another class IIa recommendation – need for a permanent pacemaker.
LVEF >35% with need for a permanent pacemaker: Fourteen patients had LVEF >35% with need for a permanent pacemaker, of which, 5 reached the composite endpoint, with an annualized event rate of 19.6%.
LVEF >35% with inducible VT: Only 1 patient was eligible for an ICD on this basis, and this patient did not reach the composite endpoint.
LVEF >35% with any LGE by CMR: Seventy patients were eligible for an ICD on this basis, of which 5 reached the composite endpoint, with an annualized event rate of 2.1%. The sensitivity of LVEF >35% with any LGE for the composite endpoint was 83.3%, while the specificity was 74.9%. Of the 5 patients with LVEF >35% with any LGE that reached the endpoint, none had ICDs at the time of their CMR; one had a permanent pacemaker.
Optimal cutoff of LGE extent by CMR
Using ROC analyses, the optimal cutoff of LGE extent for the prediction of the composite endpoint in patients with LGE and without a class I indication for an ICD was >5.7%. This cutoff had a sensitivity of 83.3% and a specificity of 94.6%.
LVEF >35% with >5.7% LGE by CMR: Nineteen patients were eligible for an ICD based on LVEF >35% with >5.7% LGE by CMR, of which 5 reached the composite endpoint with an annualized event rate of 12.0%. Thus, a cutoff of >5.7% for LGE extent significantly improved the specificity over any LGE (p<0.0001), while reducing the number of patients eligible for an ICD by 73%.
Comparison between the prognostic value of class I and IIa recommendations (Figure 1)
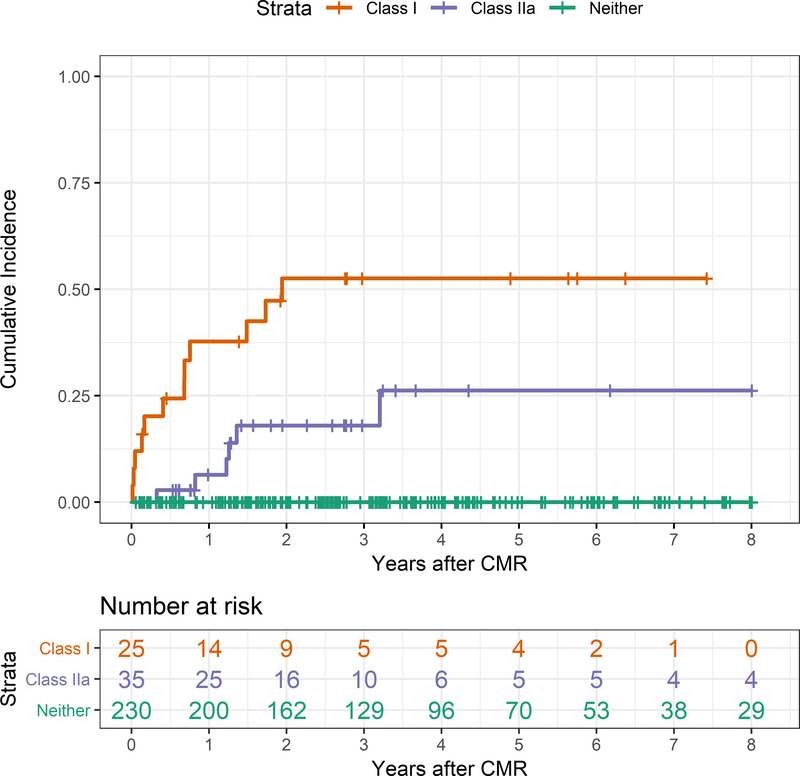
Figure 1.
Kaplan-Meier curves comparing the cumulative incidence of the composite endpoint in patients meeting class I, class IIa (includes LVEF >35% with >5.7% LGE by CMR), or no recommendations from the Guideline.
Patients with a class I recommendation for an ICD had a significantly higher incidence of the composite endpoint compared with patients with no indication for ICD (log rank p<0.0001). Similarly, patients with a class IIa recommendation (using >5.7% LGE) for an ICD had a significantly higher incidence of the composite endpoint compared with patients with no indication for ICD (log rank p<0.0001). Finally, patients with a class I recommendation for an ICD also had a significantly higher incidence of the composite endpoint compared with patients with a class IIa recommendation for ICD (log rank p=0.01). The Kaplan-Meier estimate of the cumulative incidence of the composite endpoint in patients meeting any class I recommendation was 52.6% at 1.9 years and in those meeting any class IIa recommendation was 18.0% at the same time. On Cox proportional hazards regression analysis, patients with a class I recommendation for an ICD had a hazard ratio (HR) of 3.4 (95% confidence interval 1.2–9.0; p=0.01) for the composite endpoint compared with patients with a class IIa recommendation (using >5.7% LGE by CMR) for an ICD.
Assessment of class I and IIa recommendations combined (Figure 2)
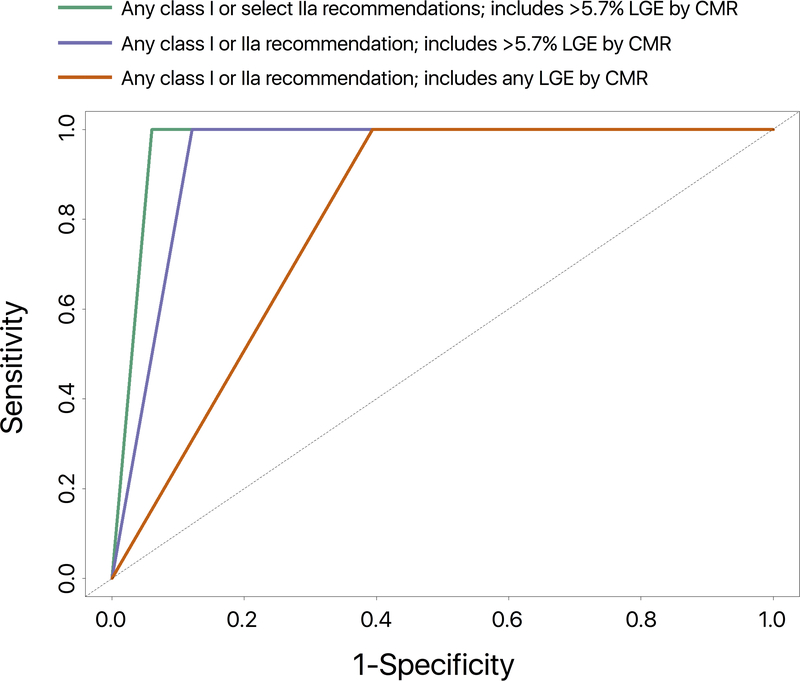
Figure 2.
Time-dependent ROC curves for the prediction of the composite endpoint in patients meeting any class I or IIa recommendation; includes any LGE by CMR (red; AUC = 0.80), any class I or IIa recommendation; includes LVEF >35% with >5.7% LGE by CMR (blue; AUC = 0.94), any class I or select IIa recommendations: LVEF>35% with >5.7% LGE by CMR and/or need for permanent pacemaker (green; AUC = 0.97).
When class I and class IIa recommendations were evaluated together using >5.7% LGE by CMR, 60 patients were eligible for ICDs; this group included all 18 patients who experienced the composite endpoint. The area under the curve (AUC) on time-dependent ROC analyses was 0.94.
Select class IIa recommendations (Figure 2)
Since patients with LVEF >35% with syncope had a low incidence of the composite endpoint, and LVEF >35% with inducible ventricular arrhythmia was noted in only 1 patient, we tested the AUC for class IIa recommendations by only including 2 indications – LVEF >35% with >5.7% LGE, or with the need for a permanent pacemaker. Combining these 2 class IIa indications with class I indications resulted in 50 patients being eligible for ICDs, including all 18 patients that experienced the composite endpoint. The AUC on time-dependent ROC analyses was 0.97. However, this AUC was not statistically higher than the AUC of 0.94 with all class I and IIa recommendations (using >5.7% LGE) combined (p = 0.15).
Discussion
Using a large cohort of 290 patients with known or suspected cardiac sarcoidosis, we assessed the recommendations for ICD implantation in patients with cardiac sarcoidosis from the 2017 AHA/ACC/HRS Guideline for Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death. We found that the class I and IIa recommendations identified all patients who experienced either significant ventricular arrhythmia or sudden cardiac death. To assess the recommendations, we included clinical data from their presentation, and CMR LVEF and LGE data, but we did not include data from echocardiography or positron emission tomography. Patients with 1 or both class I indications had a significantly higher incidence of the composite endpoint than patients with 1 or more class IIa indications. The presence of >5.7% LGE on CMR was as sensitive as and significantly more specific than the presence of any LGE for the identification of patients at risk for the composite endpoint. Finally, excluding 2 of the 4 class IIa recommendations (LVEF >35% with syncope, LVEF >35% with inducible ventricular arrhythmia) resulted in improved discrimination for the composite endpoint.
The HRS Expert Consensus Statement on the Diagnosis and Management of Arrhythmias Associated with Cardiac Sarcoidosis was written by a committee with specific expertise in cardiac sarcoidosis5. The recommendations in the 2017 ACC/AHA/HRS Guideline are very similar to those in the HRS Expert Consensus Statement, and the key difference between the 2 documents pertains to patients with LVEF >35%22.
Prior literature
Class I recommendations
In patients with sustained VT or sudden cardiac arrest, previous studies have found high rates of ICD therapies in patients with ICDs implanted for secondary prevention4, 7, 23–25. In patients with LVEF ≤35%, previous studies have also demonstrated high rates of ICD therapies in patients with ICDs and reduced LVEF4, 7, 23, 24, 26, 27.
The annualized rates of appropriate therapies in these studies ranged from 5%27 to 28%24. In our study, patients meeting class I recommendations for an ICD had an annualized event rate of 22.4%, which is in line with estimates from these prior studies and is well above the threshold event rate for which ICD implantation has been recommended in guidelines for other cardiomyopathies28.
Class IIa recommendations
Class IIa recommendations pertain to patients with LVEF >35%. Recently, in a post-hoc analysis of the Sudden Cardiac Death in Heart Failure Trial (SCD-HeFT) trial, ICDs were shown to be associated with reduced mortality even among patients with LVEF of ≤35% who had an improvement to >35% during follow-up29. Thus, ICDs may have a role in the prevention of sudden cardiac death in certain subgroups of patients with LVEF >35%.
No studies have investigated the prognostic role of syncope in patients with known or suspected cardiac sarcoidosis. Syncope in patients with known or suspected cardiac sarcoidosis may occur due to arrhythmias such as atrioventricular block or VT, pulmonary hypertension related to pulmonary sarcoidosis30, vasovagal syncope, or other causes. Patients with syncope with LVEF ≤35%, documented atrioventricular block, LGE >5.7%, or VT should be recommended ICDs. In our study, none of the 11 patients with syncope who did not also meet other class I or IIa indications experienced significant ventricular arrhythmia or sudden cardiac death during follow up. Thus, this is a topic that could benefit from future research. If our findings are replicated, in the absence of other indications, syncope alone may not warrant ICD implantation. In the absence of other indications, syncope could be evaluated carefully to identify its likely etiology – vasovagal, arrhythmic, or pulmonary hypertension-related. In those with unexplained syncope of suspected arrhythmic etiology, further evaluation with a tilt-table test, an insertable cardiac monitor, or programmed electrical stimulation11 may be reasonable to guide decision-making regarding the implantation of an ICD.
Several studies have documented a high rate of ventricular arrhythmias in patients presenting with an advanced atrioventricular block from cardiac sarcoidosis. Of 18 patients with advanced atrioventricular block and either cardiac sarcoidosis or giant cell myocarditis in a study by Kandolin et al., 7 (39%) experienced cardiac death, cardiac transplantation, ventricular fibrillation, or treated sustained VT at a mean follow-up of 48 months31. Nery et al, studied 11 patients with advanced atrioventricular block and cardiac sarcoidosis, 2 (18%) had recurrent VT resulting in ICD shocks at a mean follow-up of 21 months32. In a study of 22 cardiac sarcoidosis patients with advanced atrioventricular block, over a median follow-up of 45 months, 2 patients suffered aborted sudden cardiac death and 9 had sustained VT33. More recently, Nordenswan et al. reported on the arrhythmic outcomes of 143 cardiac sarcoidosis patients who presented with advanced atrioventricular block34. Over a median follow-up period of 4.1 years, 16.1% (23/143) patients suffered either fatal (n = 13) or aborted (n = 10) sudden cardiac death. An additional 21 patients had sustained VT, and the composite endpoint of sudden cardiac death or VT occurred in 44/143 (30.8%) patients34. In the present study, we found a 19.6% annualized rate of significant ventricular arrhythmia or sudden cardiac death, strongly validating this Guideline recommendation.
Three studies have investigated the role of programmed electrical stimulation in patients with known or suspected cardiac sarcoidosis35–37; however, in 2 of these studies, patients were not stratified by LVEF35, 37. Aizer et al. studied 32 patients with cardiac sarcoidosis who underwent programmed electrical stimulation and found a positive predictive value for the prediction of sudden cardiac death or ventricular arrhythmias of 75% and a negative predictive value of 90%35. Of these, only 6 had no spontaneous sustained ventricular arrhythmias on presentation but had inducible sustained ventricular arrhythmias, and their LVEFs were not provided. Mehta et al. subsequently studied 76 patients with suspected cardiac sarcoidosis who underwent programmed electrical stimulation and found a positive predictive value of 75% and a negative predictive value of 98.5% for the prediction of ventricular arrhythmias36. Eight had inducible sustained ventricular arrhythmias, of which only 3 had LVEF of >40%. Smedema et al. studied 19 patients with suspected cardiac sarcoidosis who underwent programmed electrical stimulation and found a positive predictive value for the prediction of ventricular arrhythmias of 75% and a negative predictive value of 91%. In this study, the median LVEF for patients with a positive programmed electrical stimulation study was 38% while that for a negative study was 61%. Due to the small numbers of patients with inducible sustained ventricular tachyarrhythmias in these studies, and the lack of stratification by LVEF in 2, the true prognostic value of programmed electrical stimulation for patients with LVEF >35% remains unknown. In our study, only 1 such patient underwent programmed electrical stimulation, and the patient did not reach the composite endpoint.
Several studies have investigated the prognostic role of LGE on CMR to predict adverse outcomes. Two meta-analyses on this topic have been performed; Hulten et al.’s meta-analysis included 7 studies with a total of 694 subjects38, while Coleman et al.’s meta-analysis included 10 studies of a total of 760 patients39. Both meta-analyses and almost all of the studies included in them reached the same conclusion – the presence of LGE on CMR imaging is associated with increased odds of arrhythmic events and sudden cardiac death. Combining data from 7 studies reporting ventricular arrhythmia outcomes, Hulten et al. found an annualized event rate of 5.9% for patients with LGE vs. 0% for those without. In concordance with the findings of these 2 meta-analyses, we found that the presence of LGE was associated with ventricular arrhythmia or sudden cardiac death.
Seven studies have examined the prognostic value of LGE extent and have identified various LGE cut-offs for various composite endpoints including outcomes such as heart failure hospitalization and all-cause death25, 37, 40–44. Only Crawford et al. have identified an optimal LGE cutoff to predict ventricular arrhythmias; using the full width at half maximum thresholding method to quantify LGE, they identified that LGE >6% was associated with a sensitivity of 75% and a specificity of 82% to identify patients with ventricular arrhythmias (the area under the curve was 0.79)25. Similarly, we found >5.7% to be the optimal LGE cutoff for the prediction of sudden cardiac death or ventricular arrhythmias.
Of note, no prior study has examined the associations between clinical markers and sudden cardiac death or ventricular arrhythmias using time-dependent ROC analyses. While conventional ROC analyses are used to evaluate the discrimination power of a variable for a binary outcome, they do not account for the possibility that the outcome status could change with time. Time-dependent ROC analyses - using ROC curves that vary as a function of time - are more appropriate in this setting where patients may develop the disease later during longer study follow-up45, 46.
Clinical Implications
Comparing the class I and IIa recommendations, we found that patients meeting class I recommendations for an ICD had a significantly higher incidence of the composite endpoint than patients meeting class IIa recommendations, as would be expected. While LVEF >35% with any LGE captured all patients without a class I indication that experienced the composite endpoint, an LGE cutoff of >5.7% had a significantly improved specificity while maintaining the same sensitivity. Patients with LVEF >35% with LGE <5.7% may not benefit from an ICD. Future studies are needed to validate our cutoff. Patients meeting 1 of 2 class IIa recommendations – LVEF >35% with the need for a permanent pacemaker, or LVEF>35% with LGE >5.7% – had high annualized event rates of 20% and 12% respectively. If future studies replicate our findings, these 2 recommendations may need to be upgraded to class I. Similarly, the class IIa recommendations for an ICD in patients with LVEF >35% with syncope, or in patients with LVEF >35% with inducible sustained ventricular arrhythmias, would benefit from further research. If future studies replicate our findings in patients with LVEF >35% with syncope, the recommendation may benefit from either clarification to first consider evaluation with a tilt-table test, an insertable cardiac monitor, or programmed electrical stimulation47 to determine the cause of the unexplained syncope before implantation of an ICD, or a downgrade to class IIb.
Limitations
Our cohort is a single-center cohort of predominantly white patients who underwent CMR. Although it is 1 of the largest cohorts of patients with known or suspected cardiac sarcoidosis studied thus far, it may be prone to referral bias and exclusion of patients with contraindications for CMR. Since LGE quantification could be time-consuming for routine clinical implementation, further studies are warranted comparing it to quick visual LGE assessment focusing on patterns48. We do not have an adequate number of patients with a positive programmed electrical stimulation to study its prognostic value. We also recognize the modest number of events in the study, due to our inclusion of only clinical events that are relevant to ICD implantation. Therefore, our findings need to be replicated in a larger, more racially-diverse, multicenter cohort. It is well recognized that appropriate ICD therapy is an imperfect surrogate for sudden cardiac death and may overestimate risk; some ventricular arrhythmias that lead to ICD therapies may have terminated spontaneously without resulting in death49. Finally, while guidelines provide guidance, recommending ICD implantation requires nuanced decision-making, often incorporating variables not discussed in the guidelines.
Conclusions
Using a cohort of 290 patients with known or suspected cardiac sarcoidosis who underwent CMR, we assessed the 2017 AHA/ACC/HRS Guideline for Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death. The class I and IIa recommendations in the Guideline identified all patients who experienced significant ventricular arrhythmia or sudden cardiac death. Concerning the class IIa recommendation for ICD implantation in patients with LVEF>35% with LGE, we identified an optimal cutoff of 5.7% that provided the highest discriminating performance for the composite endpoint.
Supplementary Material
What Is Known.
Implantable cardiac defibrillators (ICDs) are used to prevent sudden cardiac death in patients with cardiac sarcoidosis.
The most recent recommendations for ICD implantation in these patients are in the 2017 American Heart Association (AHA)/American College of Cardiology (ACC)/Heart Rhythm Society (HRS) Guideline for Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death.
What the Study Adds.
In 290 patients with sarcoidosis, the class I and IIa recommendations identified all who experienced significant ventricular arrhythmia or sudden cardiac death over a median follow up of 3.0 years.
Left ventricular ejection fraction (LVEF) >35% with >5.7% late gadolinium enhancement (LGE) on cardiovascular magnetic resonance imaging (CMR) was as sensitive as and significantly more specific than LVEF >35% with any LGE.
Patients meeting 2 class IIa recommendations – LVEF >35% with need for a permanent pacemaker, and LVEF >35% with LGE >5.7% – had high annualized event rates.
Acknowledgments
Sources of Funding: Mehmet Akçakaya was supported by NIH grant R00HL111410. Chetan Shenoy was supported by NIH grant K23HL132011, University of Minnesota Clinical and Translational Science Institute KL2 Scholars Career Development Program Award (NIH grant KL2TR000113-05) and NIH grant UL1TR000114.
Non-standard Abbreviations and Acronyms
- ACC
American College of Cardiology
- AHA
American Heart Association
- AUC
area under the curve
- CI
confidence interval
- CMR
cardiovascular magnetic resonance imaging
- EF
ejection fraction
- HR
hazard ratio
- HRS
Heart Rhythm Society
- ICD
implantable cardiac defibrillator
- IQR
interquartile range
- LGE
late gadolinium enhancement
- LV
left ventricle
- ROC
receiver operating characteristic
- RV
right ventricle
- SD
standard deviation
- VF
ventricular fibrillation
- VT
ventricular tachycardia
Footnotes
Disclosures: None
References:
- 1.Baughman RP, Field S, Costabel U, Crystal RG, Culver DA, Drent M, Judson MA, Wolff G. Sarcoidosis in America. Analysis Based on Health Care Use. Ann Am Thorac Soc. 2016;13:1244–52. [DOI] [PubMed] [Google Scholar]
- 2.Sauer WH, Stern BJ, Baughman RP, Culver DA, Royal W. High-Risk Sarcoidosis. Current Concepts and Research Imperatives. Ann Am Thorac Soc. 2017;14:S437–S444. [DOI] [PubMed] [Google Scholar]
- 3.Tavora F, Cresswell N, Li L, Ripple M, Solomon C, Burke A. Comparison of necropsy findings in patients with sarcoidosis dying suddenly from cardiac sarcoidosis versus dying suddenly from other causes. The American journal of cardiology. 2009;104:571–7. [DOI] [PubMed] [Google Scholar]
- 4.Kron J, Sauer W, Schuller J, Bogun F, Crawford T, Sarsam S, Rosenfeld L, Mitiku TY, Cooper JM, Mehta D, et al. Efficacy and safety of implantable cardiac defibrillators for treatment of ventricular arrhythmias in patients with cardiac sarcoidosis. Europace. 2013;15:347–54. [DOI] [PubMed] [Google Scholar]
- 5.Birnie DH, Sauer WH, Bogun F, Cooper JM, Culver DA, Duvernoy CS, Judson MA, Kron J, Mehta D, Cosedis Nielsen J, et al. HRS expert consensus statement on the diagnosis and management of arrhythmias associated with cardiac sarcoidosis. Heart rhythm : the official journal of the Heart Rhythm Society. 2014;11:1305–23. [DOI] [PubMed] [Google Scholar]
- 6.Al-Khatib SM, Stevenson WG, Ackerman MJ, Bryant WJ, Callans DJ, Curtis AB, Deal BJ, Dickfeld T, Field ME, Fonarow GC, et al. 2017 AHA/ACC/HRS Guideline for Management of Patients With Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death. Circulation. 2018;138:e272–e391. [DOI] [PubMed] [Google Scholar]
- 7.Betensky BP, Tschabrunn CM, Zado ES, Goldberg LR, Marchlinski FE, Garcia FC, Cooper JM. Long-term follow-up of patients with cardiac sarcoidosis and implantable cardioverter-defibrillators. Heart rhythm : the official journal of the Heart Rhythm Society. 2012;9:884–91. [DOI] [PubMed] [Google Scholar]
- 8.Huang H, Nijjar PS, Misialek JR, Blaes A, Derrico NP, Kazmirczak F, Klem I, Farzaneh-Far A, Shenoy C. Accuracy of left ventricular ejection fraction by contemporary multiple gated acquisition scanning in patients with cancer: comparison with cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2017;19:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lin LQ, Kazmirczak F, Chen KA, Okasha O, Nijjar PS, Martin CM, Akcakaya M, Farzaneh-Far A, Shenoy C. Impact of Cardiovascular Magnetic Resonance Imaging on Identifying the Etiology of Cardiomyopathy in Patients Undergoing Cardiac Transplantation. Sci Rep. 2018;8:16212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kazmirczak F, Nijjar PS, Zhang L, Hughes A, Chen KA, Okasha O, Martin CM, Akcakaya M, Farzaneh-Far A, Shenoy C. Safety and prognostic value of regadenoson stress cardiovascular magnetic resonance imaging in heart transplant recipients. J Cardiovasc Magn Reson. 2019;21:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shen WK, Sheldon RS, Benditt DG, Cohen MI, Forman DE, Goldberger ZD, Grubb BP, Hamdan MH, Krahn AD, Link MS, et al. 2017 ACC/AHA/HRS Guideline for the Evaluation and Management of Patients With Syncope: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Circulation. 2017;136:e60–e122. [DOI] [PubMed] [Google Scholar]
- 12.Kramer CM, Barkhausen J, Flamm SD, Kim RJ, Nagel E, Society for Cardiovascular Magnetic Resonance Board of Trustees Task Force on Standardized P. Standardized cardiovascular magnetic resonance imaging (CMR) protocols, society for cardiovascular magnetic resonance: board of trustees task force on standardized protocols. J Cardiovasc Magn Reson. 2008;10:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kramer CM, Barkhausen J, Flamm SD, Kim RJ, Nagel E, Society for Cardiovascular Magnetic Resonance Board of Trustees Task Force on Standardized P. Standardized cardiovascular magnetic resonance (CMR) protocols 2013 update. J Cardiovasc Magn Reson. 2013;15:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schulz-Menger J, Bluemke DA, Bremerich J, Flamm SD, Fogel MA, Friedrich MG, Kim RJ, von Knobelsdorff-Brenkenhoff F, Kramer CM, Pennell DJ, et al. Standardized image interpretation and post processing in cardiovascular magnetic resonance: Society for Cardiovascular Magnetic Resonance (SCMR) board of trustees task force on standardized post processing. J Cardiovasc Magn Reson. 2013;15:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bondarenko O, Beek AM, Hofman MB, Kuhl HP, Twisk JW, van Dockum WG, Visser CA, van Rossum AC. Standardizing the definition of hyperenhancement in the quantitative assessment of infarct size and myocardial viability using delayed contrast-enhanced CMR. J Cardiovasc Magn Reson. 2005;7:481–5. [DOI] [PubMed] [Google Scholar]
- 16.Mikami Y, Cornhill A, Heydari B, Joncas SX, Almehmadi F, Zahrani M, Bokhari M, Stirrat J, Yee R, Merchant N, et al. Objective criteria for septal fibrosis in non-ischemic dilated cardiomyopathy: validation for the prediction of future cardiovascular events. J Cardiovasc Magn Reson. 2016;18:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hicks KA, Mahaffey KW, Mehran R, Nissen SE, Wiviott SD, Dunn B, Solomon SD, Marler JR, Teerlink JR, Farb A, et al. 2017 Cardiovascular and Stroke Endpoint Definitions for Clinical Trials. Circulation. 2018;137:961–972. [DOI] [PubMed] [Google Scholar]
- 18.Uno H, Cai T, Tian L, Wei L. Evaluating prediction rules for t-year survivors with censored regression models. Journal of the American Statistical Association. 2007;102:527–537. [Google Scholar]
- 19.Hung H, Chiang CT. Estimation methods for time‐dependent AUC models with survival data. Canadian Journal of Statistics. 2010;38:8–26. [Google Scholar]
- 20.Blanche P, Dartigues JF, Jacqmin-Gadda H. Estimating and comparing time-dependent areas under receiver operating characteristic curves for censored event times with competing risks. Stat Med. 2013;32:5381–97. [DOI] [PubMed] [Google Scholar]
- 21.Youden WJ. Index for rating diagnostic tests. Cancer. 1950;3:32–5. [DOI] [PubMed] [Google Scholar]
- 22.Birnie D, Ha A, Kron J. Which Patients With Cardiac Sarcoidosis Should Receive Implantable Cardioverter-Defibrillators. Circulation Arrhythmia and electrophysiology. 2018;11:e006685. [DOI] [PubMed] [Google Scholar]
- 23.Schuller JL, Zipse M, Crawford T, Bogun F, Beshai J, Patel AR, Sweiss NJ, Nguyen DT, Aleong RG, Varosy PD, et al. Implantable cardioverter defibrillator therapy in patients with cardiac sarcoidosis. Journal of cardiovascular electrophysiology. 2012;23:925–9. [DOI] [PubMed] [Google Scholar]
- 24.Takaya Y, Kusano K, Nishii N, Nakamura K, Ito H. Early and frequent defibrillator discharge in patients with cardiac sarcoidosis compared with patients with idiopathic dilated cardiomyopathy. Int J Cardiol. 2017;240:302–306. [DOI] [PubMed] [Google Scholar]
- 25.Crawford T, Mueller G, Sarsam S, Prasitdumrong H, Chaiyen N, Gu X, Schuller J, Kron J, Nour KA, Cheng A, et al. Magnetic resonance imaging for identifying patients with cardiac sarcoidosis and preserved or mildly reduced left ventricular function at risk of ventricular arrhythmias. Circulation Arrhythmia and electrophysiology. 2014;7:1109–15. [DOI] [PubMed] [Google Scholar]
- 26.Mohsen A, Jimenez A, Hood RE, Dickfeld T, Saliaris A, Shorofsky S, Saba MM. Cardiac sarcoidosis: electrophysiological outcomes on long-term follow-up and the role of the implantable cardioverter-defibrillator. Journal of cardiovascular electrophysiology. 2014;25:171–6. [DOI] [PubMed] [Google Scholar]
- 27.Segawa M, Fukuda K, Nakano M, Kondo M, Satake H, Hirano M, Shimokawa H. Time Course and Factors Correlating With Ventricular Tachyarrhythmias After Introduction of Steroid Therapy in Cardiac Sarcoidosis. Circulation Arrhythmia and electrophysiology. 2016;9. [DOI] [PubMed] [Google Scholar]
- 28.Authors/Task Force m, Elliott PM, Anastasakis A, Borger MA, Borggrefe M, Cecchi F, Charron P, Hagege AA, Lafont A, Limongelli G, et al. 2014 ESC Guidelines on diagnosis and management of hypertrophic cardiomyopathy: the Task Force for the Diagnosis and Management of Hypertrophic Cardiomyopathy of the European Society of Cardiology (ESC). Eur Heart J. 2014;35:2733–79. [DOI] [PubMed] [Google Scholar]
- 29.Adabag S, Patton KK, Buxton AE, Rector TS, Ensrud KE, Vakil K, Levy WC, Poole JE. Association of Implantable Cardioverter Defibrillators With Survival in Patients With and Without Improved Ejection Fraction: Secondary Analysis of the Sudden Cardiac Death in Heart Failure Trial. JAMA Cardiol. 2017;2:767–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Patterson KC, Strek ME. Pulmonary fibrosis in sarcoidosis. Clinical features and outcomes. Ann Am Thorac Soc. 2013;10:362–70. [DOI] [PubMed] [Google Scholar]
- 31.Kandolin R, Lehtonen J, Kupari M. Cardiac sarcoidosis and giant cell myocarditis as causes of atrioventricular block in young and middle-aged adults. Circulation Arrhythmia and electrophysiology. 2011;4:303–9. [DOI] [PubMed] [Google Scholar]
- 32.Nery PB, Beanlands RS, Nair GM, Green M, Yang J, McArdle BA, Davis D, Ohira H, Gollob MH, Leung E, et al. Atrioventricular block as the initial manifestation of cardiac sarcoidosis in middle-aged adults. Journal of cardiovascular electrophysiology. 2014;25:875–881. [DOI] [PubMed] [Google Scholar]
- 33.Takaya Y, Kusano KF, Nakamura K, Ito H. Outcomes in patients with high-degree atrioventricular block as the initial manifestation of cardiac sarcoidosis. The American journal of cardiology. 2015;115:505–9. [DOI] [PubMed] [Google Scholar]
- 34.Nordenswan HK, Lehtonen J, Ekstrom K, Kandolin R, Simonen P, Mayranpaa M, Vihinen T, Miettinen H, Kaikkonen K, Haataja P, et al. Outcome of Cardiac Sarcoidosis Presenting With High-Grade Atrioventricular Block. Circulation Arrhythmia and electrophysiology. 2018;11:e006145. [DOI] [PubMed] [Google Scholar]
- 35.Aizer A, Stern EH, Gomes JA, Teirstein AS, Eckart RE, Mehta D. Usefulness of programmed ventricular stimulation in predicting future arrhythmic events in patients with cardiac sarcoidosis. The American journal of cardiology. 2005;96:276–82. [DOI] [PubMed] [Google Scholar]
- 36.Mehta D, Mori N, Goldbarg SH, Lubitz S, Wisnivesky JP, Teirstein A. Primary prevention of sudden cardiac death in silent cardiac sarcoidosis: role of programmed ventricular stimulation. Circulation Arrhythmia and electrophysiology. 2011;4:43–8. [DOI] [PubMed] [Google Scholar]
- 37.Smedema JP, van Geuns RJ, Ector J, Heidbuchel H, Ainslie G, Crijns H. Right ventricular involvement and the extent of left ventricular enhancement with magnetic resonance predict adverse outcome in pulmonary sarcoidosis. ESC Heart Fail. 2018;5:157–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hulten E, Agarwal V, Cahill M, Cole G, Vita T, Parrish S, Bittencourt MS, Murthy VL, Kwong R, Di Carli MF, et al. Presence of Late Gadolinium Enhancement by Cardiac Magnetic Resonance Among Patients With Suspected Cardiac Sarcoidosis Is Associated With Adverse Cardiovascular Prognosis: A Systematic Review and Meta-Analysis. Circulation Cardiovascular imaging. 2016;9:e005001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Coleman GC, Shaw PW, Balfour PC Jr., Gonzalez JA, Kramer CM, Patel AR, Salerno M Prognostic Value of Myocardial Scarring on CMR in Patients With Cardiac Sarcoidosis. JACC Cardiovascular imaging. 2017;10:411–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ise T, Hasegawa T, Morita Y, Yamada N, Funada A, Takahama H, Amaki M, Kanzaki H, Okamura H, Kamakura S, et al. Extensive late gadolinium enhancement on cardiovascular magnetic resonance predicts adverse outcomes and lack of improvement in LV function after steroid therapy in cardiac sarcoidosis. Heart. 2014;100:1165–72. [DOI] [PubMed] [Google Scholar]
- 41.Murtagh G, Laffin LJ, Beshai JF, Maffessanti F, Bonham CA, Patel AV, Yu Z, Addetia K, Mor-Avi V, Moss JD, et al. Prognosis of Myocardial Damage in Sarcoidosis Patients With Preserved Left Ventricular Ejection Fraction: Risk Stratification Using Cardiovascular Magnetic Resonance. Circulation Cardiovascular imaging. 2016;9:e003738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Agoston-Coldea L, Kouaho S, Sacre K, Dossier A, Escoubet B, Chillon S, Laissy JP, Rouzet F, Kutty S, Extramiana F, et al. High mass (>18g) of late gadolinium enhancement on CMR imaging is associated with major cardiac events on long-term outcome in patients with biopsy-proven extracardiac sarcoidosis. Int J Cardiol. 2016;222:950–956. [DOI] [PubMed] [Google Scholar]
- 43.Ekstrom K, Lehtonen J, Hanninen H, Kandolin R, Kivisto S, Kupari M. Magnetic Resonance Imaging as a Predictor of Survival Free of Life-Threatening Arrhythmias and Transplantation in Cardiac Sarcoidosis. Journal of the American Heart Association. 2016;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yasuda M, Iwanaga Y, Kato T, Izumi T, Inuzuka Y, Nakamura T, Miyaji Y, Kawamura T, Ikeguchi S, Inoko M, et al. Risk stratification for major adverse cardiac events and ventricular tachyarrhythmias by cardiac MRI in patients with cardiac sarcoidosis. Open Heart. 2016;3:e000437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Blanche P, Dartigues JF, Jacqmin-Gadda H. Review and comparison of ROC curve estimators for a time-dependent outcome with marker-dependent censoring. Biom J. 2013;55:687–704. [DOI] [PubMed] [Google Scholar]
- 46.Kamarudin AN, Cox T, Kolamunnage-Dona R. Time-dependent ROC curve analysis in medical research: current methods and applications. BMC Med Res Methodol. 2017;17:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shen WK, Sheldon RS, Benditt DG, Cohen MI, Forman DE, Goldberger ZD, Grubb BP, Hamdan MH, Krahn AD, Link MS, et al. 2017 ACC/AHA/HRS Guideline for the Evaluation and Management of Patients With Syncope: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Circulation. 2017;136:e25–e59. [DOI] [PubMed] [Google Scholar]
- 48.Okasha O, Kazmirczak F, Chen KA, Farzaneh-Far A, Shenoy C. Myocardial Involvement in Patients With Histologically Diagnosed Cardiac Sarcoidosis: A Systematic Review and Meta-Analysis of Gross Pathological Images From Autopsy or Cardiac Transplantation Cases. Journal of the American Heart Association. 2019;8:e011253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ellenbogen KA, Levine JH, Berger RD, Daubert JP, Winters SL, Greenstein E, Shalaby A, Schaechter A, Subacius H, Kadish A, et al. Are implantable cardioverter defibrillator shocks a surrogate for sudden cardiac death in patients with nonischemic cardiomyopathy? Circulation. 2006;113:776–82. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.