Abstract
It is well known that sex steroids, particularly estrogen, play a crucial role in the attainment and maintenance of peak bone density in all people. Transgender (trans) women have been frequently observed to have low bone density prior to initiation of gender-affirming hormone therapy, while trans men generally do not. With pharmacologic estrogen, many studies show improving bone density in trans women. With pharmacologic testosterone, bone density in trans men remains largely unchanged although androgens have indirect effects on bone health via changes in fat and lean mass. Much remains unknown about best practices to optimize bone health, interpret DXA scans and assess fracture risk in trans adults.
Keywords: Osteoporosis, Transgender, Sex steroids, Body composition, DXA, Puberty blockade
Introduction
An estimated 1.4 million people (0.6% of the adult US population) identify as transgender (trans), meaning their gender identity does not align with the sex they were assigned at birth [1]. Despite increasing media attention, trans individuals frequently report negative experiences with the health-care system, fears of discrimination and mistreatment, and lack of health-care coverage for gender-affirming hormone therapy (GAHT) and surgery (GAS) [2]. Providers report limited to no training in trans health and many desire more educational opportunities [3–5]. There is growing awareness of health disparities that need to be addressed [6] and this review will focus on the current data available regarding bone health in adult transgender men and women as well as adolescents. Table 1 provides definitions for terms that will be used in this paper.
Table 1.
Terminology used in this paper
| Term/phrase | Definition |
|---|---|
| Cisgender or “cis” (e.g., cis women, cis men) | Those whose gender identity and/or gender expression align with their sex assigned at birth (i.e., not transgender). |
| Transgender or “trans” | Encompasses those whose gender identity and/or gender expression differs from their sex assigned at birth; independent of the decision whether to use gender-affirming hormone therapy or undergo surgery, and inclusive of gender non-binary people. |
| Trans man | Sex assigned at birth was female but gender identity is male or more masculine. |
| Trans woman | Sex assigned at birth was male but gender identity is female or more feminine. |
| Gender-affirming hormone therapy (GAHT) | Treatment for those who want to adapt their bodies in line with their gender identity by means of hormones. |
| Gender-affirming surgery (GAS) | Treatment for those who want to adapt their bodies in line with their gender identity by means of surgery. |
| Sex assigned at birth (SAB) | Refers to sex assigned/designated at birth which in most countries is restricted to either female (assigned female at birth, AFAB) or male (assigned male at birth, AMAB) |
| Gender non-binary | Those who may identify and present themselves as both or alternatively male and female, as neither male nor female, or as a gender outside the male/female binary. Inclusive of gender fluid, pangender, polygender, agender. |
Much has been learned in the last 25 years about the role of sex steroids in the attainment of peak bone mass and maintenance thereafter. Estrogen is a key regulator of bone health, and estrogen deficiency plays an important role in both the rapid decline in bone mineral density (BMD) seen in postmenopausal cisgender (cis) women as well as the more gradual loss seen with aging in cis men [7, 8]. For trans people who desire medical transition, GAHT can include estrogen with or without anti-androgen therapy for trans women and testosterone therapy for trans men. As we examine the current available data on bone health in trans people, it is clear that exogenous estrogen benefits BMD. However, many of the studies are small and from European centers of excellence, where the population has different age at initiation of GAHT, type of GAHT, race, ethnicity, body mass index (BMI), current tobacco use rates, and gonadectomy status, compared to other trans populations around the world. New guidelines from the International Society of Clinical Densitometry (ISCD) will be available in 2019 to address some of the issues regarding screening recommendations and dual-energy X-ray absorptiometry (DXA) interpretation in trans people. However, at this time, many questions remain as to the overall prevalence of low BMD, fracture risk and best screening practices in the trans population. Also unknown are the long-term effects of puberty blockade, the effect of changes in body composition and the optimal type, timing, dosage, and route of administration of GAHT for bone outcomes. We also recognize that many individuals identify as gender non-binary (i.e., not singularly male or female), and may seek GAHT in different doses than traditionally prescribed or recommended by clinical practice guidelines. However, the use of hormones in non-binary people falls outside the scope of this review, as available studies have been restricted to trans men, those whose sex assigned at birth was female but gender identity is male or more masculine, and trans women, those whose sex assigned at birth was male but gender identity is female or more feminine.
The Effect of Sex Steroids on the Bone in Natal Puberty
During puberty, both linear bone growth and radial expansion occur as bone length, width, and density increase. Growth hormone and IGF-1 are crucial for linear bone growth but androgens and estrogens contribute via indirect effects on these hormones [9]. At the growth plate, osteoclasts resorb calcified cartilage and osteoblasts replace mineralized bone via endo-chondral bone formation. Appositional growth or expansion occurs with increased resorption at the endosteal surface of the bone and increased bone formation at the periosteum [10]. During puberty, net cortical bone thickness increases as periosteal apposition is greater than endocortical resorption. Although puberty starts earlier in girls, it lasts longer in boys and their marrow cavity enlarges more. With increased periosteal bone formation and a greater marrow cavity enlargement in boys, the cortex is further away from the neutral axis and adult men end up with larger bones and greater bending strength [10, 11].
Case reports and animal models have helped us understand the key role estrogen plays on peak bone mass attainment in all people. Estrogen has effects on both bone formation and resorption as it inhibits sclerostin, decreases osteoblast and osteocyte apoptosis, suppresses RANK-L, and induces apoptosis in osteoclasts [12]. In males and females with aromatase mutations and high endogenous levels of testosterone, growth spurt and subsequent fusion do not occur until treatment with estrogen [13]. In males and females described with an estrogen alpha receptor mutation, BMD was strikingly low and not responsive to estrogen administration despite increases in serum estradiol (E2) [14, 15].
The role of testosterone during puberty in humans is less clear cut. At the end of puberty, cisgender boys have stronger bones thought to be related to increased strength to load ratio and increased trabecular volume [16]. How much of this is a direct effect of androgens on bone cells is harder to elucidate, partially because data from animal models and humans have differing results [9]. Androgen receptor knockout mice show reduced periosteal bone formation, but BMD is mainly reported as normal in patients with complete androgen insensitivity syndrome (CAIS) who have non-functional androgen receptors. The studies in CAIS patients that do show lower BMD are confounded by varying degrees of estrogen replacement as well as timing of gonadectomy [17]. In a male with aromatase deficiency, E2 treatment led to increased longitudinal bone growth as well as increased cross-sectional area and cortical thickness, measured by peripheral quantitative computed tomography (pQCT) [18]. It may be that both androgens and estrogens are required for optimal bone expansion [19]. During puberty, young men are reported to have greater cortical porosity, which does correlate with a time of increased fracture. Toward the end of puberty, trabecular bone volume is increased and cortical porosity decreases [9, 16, 20]. Additionally testosterone is known to increase muscle mass and decrease body fat which relate to observed changes in BMD [16, 19]. (Fig. 1).
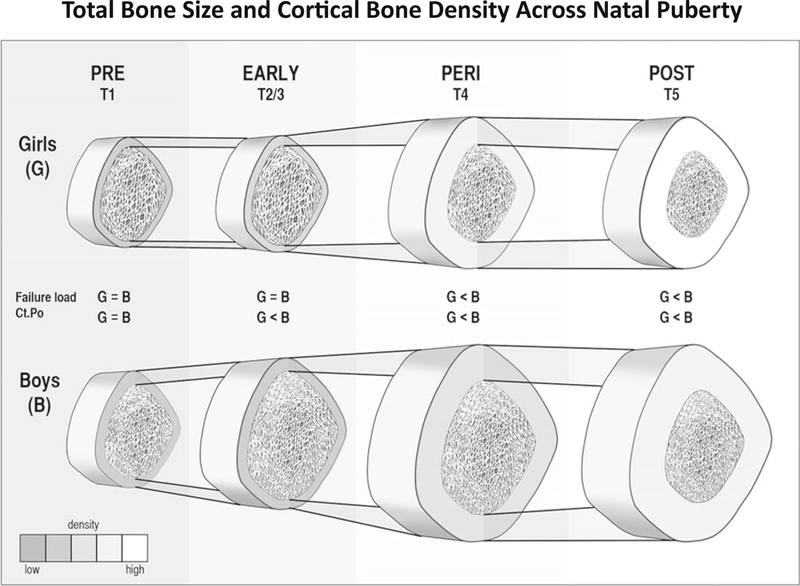
Fig. 1.
A schematic representation of differences in total bone size and cortical bone density for girls (G) and boys (B) across puberty (assessed using the method of Tanner [T]). For our purposes, we defined Tanner stage 1 as prepuberty (PRE), Tanner stages 2 and 3 as early puberty (EARLY), Tanner stage 4 as peripuberty (PERI), and Tanner stage 5 as postpuberty (POST). Significant differences between girls and boys are shown for finite element estimated failure load, where boys’ values exceed girls’ after early puberty, and cortical porosity (Ct.Po), where boys’ values exceed girls’ after prepuberty. (Diagram not exact scale.). With permission from: Nishiyama et al Journal of Bone and Mineral Research, Volume: 27, Issue: 2, Pages: 273–282, First published: 25 October 2011
We also understand that E2 plays a key role in bone maintenance in adult cisgender men. In an elegant study of mechanism [21], Finkelstein and colleagues administered goserelin acetate (a GnRH agonist) and then added back testosterone in varying doses (0/1.25/2.5/5/10 g daily topical gel) to one group and testosterone in the same doses with the addition of an aromatase inhibitor to another group. They had a third control group of men, untreated, reflecting endogenous testosterone, and E2 production. In the group with add back testosterone, areal bone mineral density (aBMD) trended down in concordance with lower testosterone levels but did not significantly differ from controls. However, in the group with testosterone plus aromatase inhibition, aBMD declined 1–2% in all groups independent of testosterone level. Changes were more apparent by qCT measurements where E2 deficiency reduced trabecular BMD independent of testosterone dose as well as cortical and trabecular volumetric BMD (vBMD) at the tibia and radius. Serum C-telopeptide (CTX) increased significantly when testosterone was < 200 ng/dl or serum E2 was < 10 pg/ml. Other studies have also shown correlations with E2 levels and osteoporosis in cisgender men. Data from MrOS showed a higher prevalence of osteoporosis among men with deficient levels of E2 [22]. Another study looking at the effect of DHEA treatment on BMD showed an association with E2 levels [23]. A more recent trial showed that in hypogonadal men treated with testosterone, a 15 pg/mL increase in E2 was associated with a 6.3% increase in spine trabecular vBMD [24].
The understanding of the role estrogen plays at trabecular versus cortical bone is evolving. Historically, estrogen was thought to affect mainly trabecular bone, but data show trabecular bone loss occurs during the third decade of life, prior to sex steroid deficiency [3, 12]. Cortical bone is maintained through much of adulthood but then decreases linearly in older adults, although cortical bone is lower in post menopausal cis women vs age-matched cis men. This is thought to be related to rapid bone loss during menopause [25]. It may also be that the effects of E2 at low levels are different than at pharmacologic levels. At high levels of E2, all bone turnover is suppressed but at lower levels the effects on cortical bone may be more apparent. Androgen levels may need to be higher to act on trabecular bone and may not have as dominant a role in cortical bone. Additionally, cellular senescence and changes related to aging need to be taken into consideration as they are inexorably linked with bone loss [16, 26].
Bone Health in Trans Women
Studies have shown a high prevalence of low bone mass in trans women when compared with reference men both prior to GAHT initiation and while on GAHT. A study of 50 trans women in Ghent, Belgium, post-orchiectomy and on GAHT for 3 years, found Z-scores < −2.0 in 26% of subjects at the lumbar spine and 2% of subjects at the hip, while no subjects in the reference population had low bone mass [27]. An additional study of 50 trans women, average age of 43 ± 10 years and all post-orchiectomy on a variety of types of estrogen, found that 23.4% had T-scores of < −2.5 at the lumbar spine and overall average Z-scores were − 1.0 ± 1.4 [28]. A recent study of 142 trans women in Brazil on GAHT for a variable amount of time found 18% had low bone mass for age while none of the reference men and women did [29].
It was initially thought that the lower BMD was related to inadequate estrogen treatment or loss of testosterone, but interestingly, multiple studies have shown alterations in BMD measurements in trans women prior to any hormonal treatment. In one study, 25 treatment-naïve trans women aged 28–42 years had lower aBMD compared to age-matched control men [30]. Additionally, the trans women had thinner radial cortices and lower cortical area at the radius and tibia as measured by pQCT as well as lower muscle mass and grip strength measured by dynamometry. In this cohort, baseline physical activity questionnaires did not differ significantly, but vitamin D levels were lower in the trans women when compared with both age-matched cis men and the larger male reference population.
A later study of 49 trans women also utilized pQCT to examine cortical volume at the dominant mid-radius and tibia and trabecular parameters at the metaphysis of the dominant radius prior to GAHT and matched to 49 cis men controls [31]. At baseline, the outer circumference and cortical bone area and thickness of the trans women were significantly smaller than that of the control men. Trabecular vBMD at the radius in the trans women was lower (p = 0.013), as was aBMD at the lumbar spine, hip, femoral neck and radial forearm. Additionally, trans women had lower grip strength and muscle mass as reflected by muscle cross-sectional area. These differences may be explained in part by lifestyle as the trans women had lower vitamin D and higher parathyroid hormone (PTH) and reported lower weekly sports activity. FSH was also slightly higher in the trans women at baseline. Some authors have argued that FSH may have effects on the bone independent of sex steroids, although this is disputed [12, 32]. Early studies of trans women treated with estrogen also postulated a correlation between higher LH and lower BMD after GAS [33], although more recent studies have not confirmed this [34, 35]. As mentioned above, there may also be differing response to estrogen at cortical vs trabecular bone. Genetics is thought to play a large role in attainment of peak bone mass, but dietary composition, exercise, puberty timing and lifestyle factors such as smoking and alcohol use are influential in determining peak bone mass and likely play a role in these baseline differences.
With the initiation of estrogen, many studies report a positive change in BMD in trans women [36] (Fig. 2B). After 1 year of GAHT, a study of 231 trans women from Ghent and Amsterdam reported BMD increases at the lumbar spine (+ 3.67%, 95% CI 3.20–4.13%, p < 0.001), total hip (+ 0.97%, 95% CI 0.62–1.31%, p < 0.0001), and femoral neck (+ 3.67%, 95% CI 3.20–4.13, p < 0.001) [37]. A recent meta-analysis [38] reviewed 13 studies with 392 total trans women and showed significant gains overall in lumbar spine BMD at both 12 months (+ 0.04 g/cm2; 95% CI + 0.03 to + 0.060 g/cm2) and 24 months (+ 0.06 g/cm2; 95% CI + 0.04 to + 0.08 g/cm2), which is within a range often considered clinically significant. Changes at the hip were not significant. A larger cohort of 711 trans women from Amsterdam has now been studied for 10 years [35]. At baseline, they were without GAHT or orchiectomy, median age was 35 years, 97.2% reported White ethnicity and 34.9% reported current tobacco use. Absolute BMD was 0.976 ± 0.140 g/cm2 with 21.9% having low BMD (defined as Z-score < −2.0 using reference male population). After 10 years of GAHT, DXA was reassessed in 102 trans women (14%) and, although lumbar spine BMD did not show a significant change (+ 0.006; 95% CI-0.005 to + 0.017) there was a significant increase in Z-score. There was also an association between serum E2 and lumbar spine BMD where the trans women in the highest tertile of E2 (mean 443 pmol/L or 121 pg/ml) had a significant increase in lumbar spine BMD (+ 0.044 g/cm2; 95% CI + 0.025 to + 0.063) (Fig. 2C) while those in the lowest tertile of estradiol (mean 118 pmol/L or 32 pg/ml) had a significant decrease in lumbar spine BMD (− 0.036 g/cm2; 95% CI − 0.044 to − 0.009 g/cm2) [Fig. 2C). There was no association with LH or degree of testosterone suppression. This is the longest study to date although there are several limitations; follow-up visits did not address lifestyle measures, and there were variations in gonadectomy timing from initiation of GAHT, type of hormone therapy as well as a change in the densitometer used during the study, although calibration measures were performed [35]. None of these studies have been powered to look at fracture outcomes. However, these data support the concept that pharmacologic estrogen, even in the setting of testosterone suppression, can increase bone density.
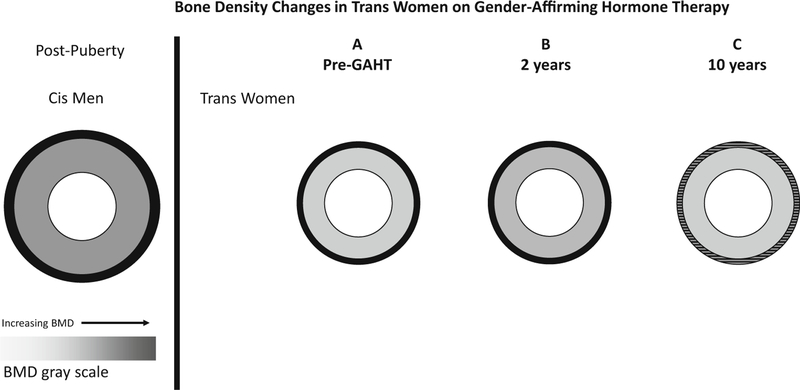
Fig. 2.
Prior to GAHT, many trans women have lower aBMD, smaller periosteal circumference, cortical bone area and thickness when compared to cis men (panel A). After 2 years, there were not significant changes in bone geometry by qCT but many studies show a gain in aBMD (panel B). There does not appear to be further improvement after 10 years of GAHT and there may in fact be a decline toward baseline (panel C). There was an association seen between serum E2 and lumbar spine BMD (not pictured). We do not have qCT data at 10 years (speculation/extrapolation represented by horizontal lines in panel C). Adapted with permission: Van Caenegem E, T’Sjoen G. Bone in trans persons. Curr Opin Endocrinol Diabetes Obes. 2015;22(6):459–66.
Body composition changes also occur with GAHT that likely influence bone health. A study of 142 trans women in Brazil assessed appendicular lean mass, as an approximation of muscle mass, derived by DXA measurements [29]. The mean age in the study was 33.7 ± 10.3 years with mean BMI 25.4 ± 4.6 kg/m2. The study was done 3 months into a care program, but 86% had been on GAHT before for variable amounts of time and 33% had undergone GAS. Appendicular lean mass in trans women was similar to reference women but lower than in reference men at the baseline measurement. Trans women had significantly lower lumbar spine BMD, femoral neck BMD, femoral neck Z-score, total femur BMD and total femur Z-score in comparison with reference men. There was a positive correlation between appendicular lean mass and total fat mass with lumbar spine BMD which explained 14.9% of BMD variation. In the previously described cohort of 49 trans women [31], after 1 and 2 years of GAHT, there were decreases in muscle mass, lean body mass, and strength with increases in fat mass with improvements in aBMD and decreases in bone turnover markers (both resorption and formation). IGF-1 levels did increase in the first year of treatment. However, there were no changes in cortical bone parameters. The 2-year time frame may not have been long enough to see the full effect of GAHT on pQCT parameters and perhaps estrogen-independent factors may also be playing a role in BMD changes. There were variations in type of estrogen used and rates of gonadectomy in this group as well. The question has been raised as to whether some of the changes in DXA may be artifactual as DXA is affected by fat mass. However, the correlation between markers of bone turnover and BMD changes would dispute this. Future studies using HRpQCT and larger groups with controlled dosing and type of estrogen should help to delineate these changes further. (Fig. 2).
Bone Health in Trans Men
Several small studies show more reassuring baseline bone health in trans men than trans women [36]. Prior to the initiation of GAHT, a study of 23 trans men and 23 control women showed similar areal BMD, trabecular, and cortical vBMD by QCT and bone turnover markers despite higher rates of smoking and lower vitamin D levels in the trans men [39]. Another study of 16 trans men prior to hormonal treatment were compared to age-matched cis women and again DXA and body composition measures were similar, as was reported physical activity [40].
Gains in BMD with testosterone treatment are reported less consistently in trans men compared to trans women receiving E2 [30]. After 1 year of treatment with testosterone, undecanoate therapy in 23 trans men mentioned above, bone formation and resorption markers both increased; however, aBMD measures did not change significantly [39]. An early study of 15 trans men showed a 7.8% increase in BMD at the femoral neck but no change at the spine over 2 years. One-third had undergone oophorectomy. The findings were similar among the testosterone-naïve patients as well as those previously treated [41]. A larger study of 199 trans men studied at baseline and then 1 year after testosterone treatment showed statistically significant increases in the total hip BMD (+ 1.04%, 95% CI 0.64–1.44%, p < 0.001) but not the femoral neck. The lumbar spine BMD changes were the most pronounced in trans men over age 50 years (+ 4.32%, CI 2.28–6.36%, p = 0.001) compared to younger trans men (+ 0.68%, 95% CI 0.19–1.17%, p = 0.007) which led the authors to postulate a role for increased estradiol action via aromatization. A 2017 meta-analysis examining 247 trans men showed no significant difference seen in BMD at 12 and 24 months compared with baseline prior to hormone initiation at the at the lumbar spine, femoral neck, or total femur [38].
Recently, 10-year data was published on a group of 543 trans men followed with serial DXA [35] (Fig. 3C). Again, low BMD was not seen at the start in trans men with overall Z-scores of 0.01 ± 1.14 g/cm2 although 4.3% of subjects had low BMD for age (defined as Z-score < −2.0). In the group that had DXA repeated at 10 years (n = 70), BMD was similar but L-spine Z-score had increased by 0.34. Again, the improvement was largely driven by a change in the oldest age group (over age 40 years at the time of GAHT initiation) of + 0.054 g/cm2 in LS BMD (95% CI 0.032– 0.076 g/cm2). This older group had lower baseline E2 levels and the group with the highest tertile of E2 had significant change in LS BMD although this did not remain significant after multivariable analysis. There was not an association with testosterone although there were larger gains seen in trans men with lower LH levels. Low LH did correlate with change in BMD and may be a marker of adequate testosterone treatment. As mentioned above, this is the longest study to date although there are limitations and these studies were not powered to look at fracture outcomes.
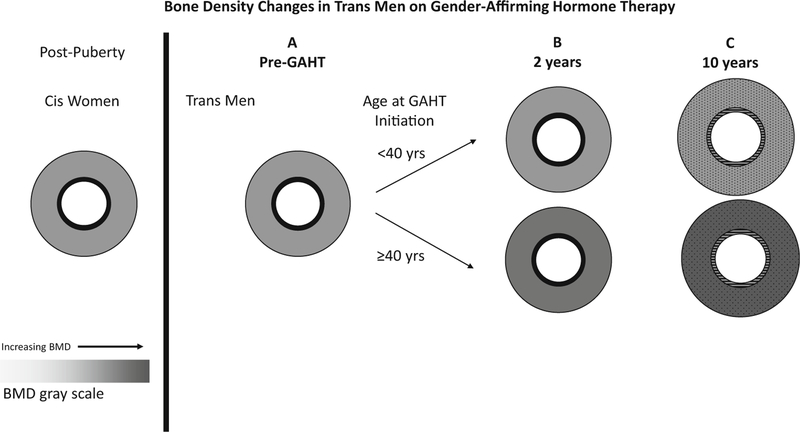
Fig. 3.
Prior to GAHT, trans men have a similar aBMD and bone geometry to cis women controls (Panel A). After 2 years, most studies showed aBMD to be stable and qCT data did not show differences compared with baseline. Recent data suggest an increase in aBMD in those who initiated GAHT at or after age 40 years. (Panel B). These data also showed aBMD continues to be stable at 10 years in those under 40 years but those over 40 years at the time of GAHT initiation had ongoing gains (Panel C). Some data suggest a larger cortical size in trans men on testosterone versus cis women and again qCT parameters at this time are unknown (speculations/extrapolations are represented by dots and lines in panel C). Adapted with permission: Van Caenegem E, T’Sjoen G. Bone in trans persons. Curr Opin Endocrinol Diabetes Obes. 2015;22(6):459–66
Although the overall increases in BMD in trans men are not significant across studies, it is reassuring that there is not a decline in BMD despite the relative reduction in E2 levels. This may relate to some the effects of testosterone on body composition as well as direct effects on the bone. Several studies have reported body composition changes with testosterone that include an increase in muscle mass and decrease in fat mass [39, 40, 42]. A study of 50 Belgian trans men (aged 37 ± 8 years) treated with testosterone for an average of 9 years after GAS were compared to 50 cis women (aged 38 ± 8 years) [40]. The trans men had 9% more lean body mass and almost 30% lower fat mass with a higher waist:hip ratio and higher grip strength [40]. LH, but not serum testosterone level, was associated with fat mass (p = 0.010) and inversely associated with total body lean mass (p = 0.007) in this group. The authors postulate that variations in levels and timing of testosterone injections may explain this discrepancy. However, other studies have reported an increase in visceral fat mass [43]. There may be other additional hormonal factors; a 1996 paper from the Netherlands examined 35 trans men and found significant increases in IGF-1 after testosterone administration for a year despite no change in BMD [44].
There may also be differences in gain in cortical vs trabecular bone where aBMD by DXA cannot discriminate. Although several studies have shown higher BMD at cortical sites by DXA [41, 45, 46], peripheral QCT allows us to look at changes in cortical and trabecular bone more precisely. Statistical models were used to compare trans men before and after GAHT with female controls and found a positive association with bone size and endosteal circumference at the radius even after adjusting for grip strength suggesting a direct effect of testosterone on the bone (p = 0.003) [40]. There was a larger cortical bone size but a lower cortical vBMD. This may be related to higher cortical porosity as described during puberty in cis men [20]. Further studies using high resolution pQCT may help delineate changes in different bone types and the roles of androgens versus estrogens (Fig. 3).
The Effect of Pubertal Blockade on Bone Health in Trans Adolescents
GnRH analogues are frequently employed to provide puberty blockade in adolescents with gender incongruence or gender dysphoria. From their use in other medical conditions such as prostate cancer, their deleterious effects on the bone are well known, although these have the potential to be reversible if treatments are stopped or add back therapies can be given [47, 48].
Thirty-four adolescents were treated with GnRH analogues followed by GAHT and studied with serial bone densities until age 22 [49]. Many were already in later stages of puberty as the average age of GnRHa initiation was 14.9 ± 1.9 years for the 15 trans girls and 15.0 ± 2.0 for the 19 trans boys. Low-dose GAHT began at 16.6 ± 1.4 years for the trans girls and 16.4 for the trans boys. Gonadectomy was considered at a minimum age of 18 with continuation of GAHT afterwards. Similar to adult trans women, the trans girls had lower baseline Z-scores than the population mean prior to initiation of any therapy (areal BMD Z-score − 0.77 ± 0.89, bone mineral apparent density (BMAD) Z-scores − 0.44 ± 1.10). During GnRH monotherapy in the trans girls, Z-scores did show a non-significant decrease but after initiation of GAHT, despite an increase in absolute aBMD, the Z-score at age 22 was lower than at the start of treatment, with 6 subjects (40%) with a LS a BMD Z-score of < −2.0. The baseline Z-scores in the trans boys were better with average areal BMD Z-score 0.17 ± 1.18, BMAD Z-score 0.28 ± 0.90). However, Z-scores in the trans boys also showed an expected drop during GnRHa treatment. Similarly, they did not fully Bmake up^ their bone loss as Z-scores at age 22 were still lower than baseline (aBMD Z-score − 0.33 ± 1.12 and BMAD Z-score average − 0.033 ± 0.95), despitea small increase in absolute aBMD. One transman at age 22 had a Z-score of < −2.0.
This group also reported on bone density and turnover markers in a trial of adolescents/young adults [50] (median age 13.5 range 11.5–18.3) which included some overlapping study participants treated with GnRHa followed by GAHT. In both the young trans men and trans women BMAD Z-scores decreased during treatment with GnRHa and increased after initiation of GAHT but remained below the population average and below baseline levels even after 24 months of GAHT. They also looked at bone turnover markers, which did not completely correlate with DXA findings. GnRHa resulted in lower formation and resorption markers, as measured by P1NP and ICTP, which is consistent with GnRHa use in cis adolescents. Despite initiation of GAHT, these markers continued to decrease although BMAD increased as described above. This may be in line with findings at the end of puberty, but many require further study as to the impact of other hormones or lifestyle on bone health in young trans adults.
Another recent study looked at the use of pro-androgenic and anti-androgenic progestins (lynestrenol and cyproterone acetate) as a potential way to avoid the negative bone effects of GnRHa [51]. Twenty-one late pubertal trans girls (Tanner 4 with mean bone age at the start of 17.1 ± 1.28) and 44 trans boys (mean bone age of 16.4 ± 1.08) were treated with cyproterone acetate and lynestrenol respectively. After 1 year of therapy, the trans girls treated with cyproterone acetate had a loss of lean mass and gain of fat mass as well as a decrease in Z-scores seen most profoundly at the lumbar spine (before − 0.765 ± 1.083, after − 1.145 ± 0.936; p = 0.002) but also at the femoral neck and total hip. Areal BMD changes decreased at the total hip, but stayed stable at the spine and femoral neck. This indicates that some of the changes seen in Z-score may be related to comparing trans girls to cis boys during a typical age of rapid BMD gain. In the trans boys lean mass and grip strength increased significantly and there were gains in areal BMD and Z-scores (although they remained less than 0 at all sites). Serum testosterone levels did not change although the testosterone/estradiol ratio was increased with lynestrenol. This is an area that requires further study, particularly since youth today are starting pubertal blockade earlier than in these studies, which may have varying effects on skeletal development. Additionally, the length of time on GnRH agonists or other agents may differ between different centers and could impact peak bone density as well.
Lifestyle and Clinical Recommendations for Screening
Given concerns about health disparities and access to care, transgender individuals may not have optimal lifestyle measures to obtain and maintain peak bone mass. A small US survey of 31 trans men and women found their reported calcium intake to be an average of 800 mg daily which is below the recommended adult guidelines [52, 53]). Four (12.9%) of the participants were taking supplemental calcium and seven (22.6%) were taking vitamin D. Participants reported average daily walking activity of 16.67 min per day (SD 14.46) without differences between trans men vs trans women. Seven (22.6%) respondents were smoking cigarettes and 42% reported alcohol use with a mean intake of 0.46 glasses/day [52].
The Endocrine Society clinical practice guidelines for gender dysphoric/incongruent people suggest checking bone density in patients who have traditional risk factors for osteoporosis and specifically in those who stop hormones after gonadectomy [54]. However, given the high prevalence of low BMD even prior to the initiation of GAHT or GAS in trans women, they and others note it may be reasonable to assess a baseline DXA earlier in trans women [36, 54]. Most current adult trans patients went through puberty with the hormones of their sex assigned at birth; however, this is changing as more younger trans patients receive GAHT with or without puberty blockade. At this time, there are no guidelines as to what database to use for interpretation although some have suggested utilizing both male and female databases for reference in DXA reports [55]. For monitoring, trans patients should be compared to themselves with changes reported in g/cm2. For youth, BMD and BMAD have been reported with the use of Z-scores, typically compared with children in the database consistent with sex assigned at birth. There are no data on how to utilize FRAX or other risk assignment calculators in the trans population. Updated guidelines expected this year from the ISCD as well as the World Professional Association of Transgender Health should give us further guidance in this area.
Conclusion
In summary, bone density changes seen in trans people on GAHT largely relate to the known effects of sex steroids on the bone. However, BMD in trans women runs low even prior to initiation of GAHT. Lifestyle factors likely contribute to this. Studies to date show the baseline bone density in trans men is similar to the general population. When estrogen is initiated in trans women, there are positive changes in BMD and some measures of bone quality; however, the effect on fracture rates is not fully known as studies have not been powered to examine this end point. When testosterone is initiated in trans men, the changes in BMD are not as robust, but body composition changes and direct effects of testosterone on the bone likely protect BMD. Low levels of estradiol likely still offer bone protection in trans men as in cis men.
Questions remain as to the effect of type and route of estrogen and testosterone utilized for GAHT as well as the effect of GnRH agonists, gonadectomy and anti-androgens. The US trans population, which may have differences in ethnicity, average BMI and lifestyle habits as well as types of GAHT regimens used and rates of gonadectomy, has not been well studied and certain measures bear repeating. Future studies using estradiol measurements via LCMS/MS and newer HRpQCT may offer further insights. Updates in guidelines from professional societies are expected to address some of the clinical scenarios regarding screening and DXA interpretation in trans people. We should continue to emphasize the role of nutrition and weight-bearing exercise, particularly in young people who have not yet attained peak bone mass.
Acknowledgements
The authors would like to thank Prof. Dr. Guy T’Sjoen and Dr. Chantal Wiepjes for their assistance in reviewing the figures. We would also like to thank Dr. Carol Zapalowski for reviewing the manuscript.
Funding Advanced Fellowship in Geriatrics from the Geriatric Research, Education, and Clinical Center, VA Eastern Colorado Health Care System, Rocky Mountain Regional VA Medical Center (SJI).
Footnotes
Compliance with Ethical Standards
Conflict of Interest The authors declare that they no conflict of interest.
Ethical Approval N/A
Informed Consent N/A
Publisher’s Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Williams Institute. How many adults identify as transgender in the United States 2016. Website: https://williamsinstitute.law.ucla.edu/wp-content/uploads/How-Many-Adults-Identify-as-Transgender-in-the-United-States.pdf.
- 2.U.S. Transgender Survey. 2015. Website: http://www.ustranssurvey.org/reports/.
- 3.Shires DA, Stroumsa D, Jaffee KD, Woodford MR. Primary care providers’ willingness to continue gender-affirming hormone therapy for transgender patients. Fam Pract 2018;35(5):576–81. [DOI] [PubMed] [Google Scholar]
- 4.Davidge-Pitts CJ, Nippoldt TB, Natt N. Endocrinology fellows’ perception of their confidence and skill level in providing transgender healthcare. Endocr Pract 2018;24(12):1038–42. [DOI] [PubMed] [Google Scholar]
- 5.Paradiso C, Lally RM. Nurse practitioner knowledge, attitudes, and beliefs when caring for transgender people. Transgend Health 2018;3(1):47–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feldman J, Brown GR, Deutsch MB, Hembree W, Meyer W, Meyer-Bahlburg HF, et al. Priorities for transgender medical and healthcare research. Curr Opin Endocrinol Diabetes Obes 2016;23(2):180–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Riggs BL, Khosla S, Melton LJ 3rd. A unitary model for involutional osteoporosis: estrogen deficiency causes both type I and type II osteoporosis in postmenopausal women and contributes to bone loss in aging men. J Bone Miner Res 1998;13(5):763–73. [DOI] [PubMed] [Google Scholar]
- 8.Cauley JA. Estrogen and bone health in men and women. Steroids 2015;99(Pt A):11–5. [DOI] [PubMed] [Google Scholar]
- 9.Vanderschueren D, Laurent MR, Claessens F, Gielen E, Lagerquist MK, Vandenput L, et al. Sex steroid actions in male bone. Endocr Rev 2014;35(6):906–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Manolagas SC, Almeida M. Gonadal Steroids. In: Bilezikian J, editor. Primer on the metabolic bone diseases and disorders of mineral metabolism 9th ed. Hoboken: Wiley; 2019. [Google Scholar]
- 11.Duan Y, Beck TJ, Wang XF, Seeman E. Structural and biomechanical basis of sexual dimorphism in femoral neck fragility has its origins in growth and aging. J Bone Miner Res 2003;18(10): 1766–74. [DOI] [PubMed] [Google Scholar]
- 12.Khosla S, Melton LJ 3rd, Riggs BL. The unitary model for estrogen deficiency and the pathogenesis of osteoporosis: is a revision needed? J Bone Miner Res 2011;26(3):441–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morishima A, Grumbach MM, Simpson ER, Fisher C, Qin K. Aromatase deficiency in male and female siblings caused by a novel mutation and the physiological role of estrogens. J Clin Endocrinol Metab 1995;80(12):3689–98. [DOI] [PubMed] [Google Scholar]
- 14.Smith EP, Boyd J, Frank GR, Takahashi H, Cohen RM, Specker B, et al. Estrogen resistance caused by a mutation in the estrogen-receptor gene in a man. N Engl J Med 1994;331(16):1056–61. [DOI] [PubMed] [Google Scholar]
- 15.Quaynor SD, Stradtman EW Jr, Kim HG, Shen Y, Chorich LP, Schreihofer DA, et al. Delayed puberty and estrogen resistance in a woman with estrogen receptor alpha variant. N Engl J Med 2013;369(2):164–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Almeida M, Laurent MR, Dubois V, Claessens F, O’Brien CA, Bouillon R, et al. Estrogens and androgens in skeletal physiology and pathophysiology. Physiol Rev 2017;97(1):135–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.King TFJ, Wat WZM, Creighton SM, Conway GS. Bone mineral density in complete androgen insensitivity syndrome and the timing of gonadectomy. Clin Endocrinol 2017;87(2):136–40. [DOI] [PubMed] [Google Scholar]
- 18.Bouillon R, Bex M, Vanderschueren D, Boonen S. Estrogens are essential for male pubertal periosteal bone expansion. J Clin Endocrinol Metab 2004;89(12):6025–9. [DOI] [PubMed] [Google Scholar]
- 19.Laurent M, Antonio L, Sinnesael M, Dubois V, Gielen E, Classens F, et al. Androgens and estrogens in skeletal sexual dimorphism. Asian J Androl 2014;16(2):213–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ego S Skeletal growth: a major determinant of Bone’s structural diversity in women and men. In: Bilezikian J, editor. Primer on the metabolic bone diseases and disorders of mineral metabolism 9th ed. Hoboken: Wiley; 2019. [Google Scholar]
- 21.Finkelstein JS, Lee H, Leder BZ, Burnett-Bowie SA, Goldstein DW, Hahn CW, et al. Gonadal steroid-dependent effects on bone turnover and bone mineral density in men. J Clin Invest 2016;126(3):1114–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fink HA, Ewing SK, Ensrud KE, Barrett-Connor E, Taylor BC, Cauley JA, et al. Association of testosterone and estradiol deficiency with osteoporosis and rapid bone loss in older men. J Clin Endocrinol Metab 2006;91(10):3908–15. [DOI] [PubMed] [Google Scholar]
- 23.Jankowski CM, Gozansky WS, Kittelson JM, Van Pelt RE, Schwartz RS, Kohrt WM. Increases in bone mineral density in response to oral dehydroepiandrosterone replacement in older adults appear to be mediated by serum estrogens. J Clin Endocrinol Metab 2008;93(12):4767–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Snyder PJ, Kopperdahl DL, Stephens-Shields AJ, Ellenberg SS, Cauley JA, Ensrud KE, et al. Effect of testosterone treatment on volumetric bone density and strength in older men with low testosterone: a controlled clinical trial. JAMA Intern Med 2017;177(4): 471–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Drake M, Khosla S. Sex steroids and the pathogenesis of osteoporosis. In: Bilezikian J, editor. Primer on the metabolic bone diseases and disorders of mineral metabolism 9th ed. Hoboken: Wiley; 2019. [Google Scholar]
- 26.Manolagas SC. The quest for osteoporosis mechanisms and rational therapies: how far we’ve come, how much further we need to go. J Bone Miner Res 2018;33(3):371–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.T’Sjoen G, Weyers S, Taes Y, Lapauw B, Toye K, Goemaere S, et al. Prevalence of low bone mass in relation to estrogen treatment and body composition in male-to-female transsexual persons. J Clin Densitom 2009;12(3):306–13. [DOI] [PubMed] [Google Scholar]
- 28.Wierckx K, Mueller S, Weyers S, Van Caenegem E, Roef G, Heylens G, et al. Long-term evaluation of cross-sex hormone treatment in transsexual persons. J Sex Med 2012;9(10):2641–51. [DOI] [PubMed] [Google Scholar]
- 29.Fighera TM, da Silva E, Lindenau JD, Spritzer PM. Impact of cross-sex hormone therapy on bone mineral density and body composition in transwomen. Clin Endocrinol 2018;88(6):856–62. [DOI] [PubMed] [Google Scholar]
- 30.Van Caenegem E, Taes Y, Wierckx K, Vandewalle S, Toye K, Kaufman JM, et al. Low bone mass is prevalent in male-to-female transsexual persons before the start of cross-sex hormonal therapy and gonadectomy. Bone 2013;54(1):92–7. [DOI] [PubMed] [Google Scholar]
- 31.Van Caenegem E, Wierckx K, Taes Y, Schreiner T, Vandewalle S, Toye K, et al. Preservation of volumetric bone density and geometry in trans women during cross-sex hormonal therapy: a prospective observational study. Osteoporos Int 2015;26(1):35–47. [DOI] [PubMed] [Google Scholar]
- 32.Zhu LL, Blair H, Cao J, Yuen T, Latif R, Guo L, et al. Blocking antibody to the beta-subunit of FSH prevents bone loss by inhibiting bone resorption and stimulating bone synthesis. Proc Natl Acad Sci U S A 2012;109(36):14574–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van Kesteren P, Lips P, Gooren LJ, Asscheman H, Megens J. Long-term follow-up of bone mineral density and bone metabolism in transsexuals treated with cross-sex hormones. Clin Endocrinol 1998;48(3):347–54. [DOI] [PubMed] [Google Scholar]
- 34.Haraldsen IR, Haug E, Falch J, Egeland T, Opjordsmoen S. Cross-sex pattern of bone mineral density in early onset gender identity disorder. Horm Behav 2007;52(3):334–43. [DOI] [PubMed] [Google Scholar]
- 35.Wiepjes CM, de Jongh RT, de Blok CJ, Vlot MC, Lips P, Twisk JW, et al. Bone safety during the first ten years of gender-affirming hormonal treatment in transwomen and transmen. J Bone Miner Res 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Van Caenegem E, T’Sjoen G. Bone in trans persons. Curr Opin Endocrinol Diabetes Obes 2015;22(6):459–66. [DOI] [PubMed] [Google Scholar]
- 37.Wiepjes CM, Vlot MC, Klaver M, Nota NM, de Blok CJ, de Jongh RT, et al. Bone mineral density increases in trans persons after 1 year of hormonal treatment: a multicenter prospective observational study. J Bone Miner Res 2017;32(6):1252–60. [DOI] [PubMed] [Google Scholar]
- 38.Singh-Ospina N, Maraka S, Rodriguez-Gutierrez R, Davidge-Pitts C, Nippoldt TB, Prokop LJ, et al. Effect of sex steroids on the bone health of transgender individuals: a systematic review and meta-analysis. J Clin Endocrinol Metab 2017;102(11):3904–13. [DOI] [PubMed] [Google Scholar]
- 39.Van Caenegem E, Wierckx K, Taes Y, Schreiner T, Vandewalle S, Toye K, et al. Body composition, bone turnover, and bone mass in trans men during testosterone treatment: 1-year follow-up data from a prospective case-controlled study (ENIGI). Eur J Endocrinol 2015;172(2):163–71. [DOI] [PubMed] [Google Scholar]
- 40.Van Caenegem E, Wierckx K, Taes Y, Dedecker D, Van de Peer F, Toye K, et al. Bone mass, bone geometry, and body composition in female-to-male transsexual persons after long-term cross-sex hormonal therapy. J Clin Endocrinol Metab 2012;97(7):2503–11. [DOI] [PubMed] [Google Scholar]
- 41.Turner A, Chen TC, Barber TW, Malabanan AO, Holick MF, Tangpricha V. Testosterone increases bone mineral density in female-to-male transsexuals: a case series of 15 subjects. Clin Endocrinol 2004;61(5):560–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mueller A, Haeberle L, Zollver H, Claassen T, Kronawitter D, Oppelt PG, et al. Effects of intramuscular testosterone undecanoate on body composition and bone mineral density in female-to-male transsexuals. J Sex Med 2010;7(9):3190–8. [DOI] [PubMed] [Google Scholar]
- 43.Elbers JM, Asscheman H, Seidell JC, Megens JA, Gooren LJ. Long-term testosterone administration increases visceral fat in female to male transsexuals. J Clin Endocrinol Metab 1997;82(7): 2044–7. [DOI] [PubMed] [Google Scholar]
- 44.van Kesteren P, Lips P, Deville W, Popp-Snijders C, Asscheman H, Megens J, et al. The effect of one-year cross-sex hormonal treatment on bone metabolism and serum insulin-like growth factor-1 in transsexuals. J Clin Endocrinol Metab 1996;81(6):2227–32. [DOI] [PubMed] [Google Scholar]
- 45.Broulik PD, Urbanek V, Libansky P. Eighteen-year effect of androgen therapy on bone mineral density in trans(gender) men. Horm Metab Res 2018;50(2):133–7. [DOI] [PubMed] [Google Scholar]
- 46.Ruetsche AG, Kneubuehl R, Birkhaeuser MH, Lippuner K. Cortical and trabecular bone mineral density in transsexuals after long-term cross-sex hormonal treatment: a cross-sectional study. Osteoporos Int 2005;16(7):791–8. [DOI] [PubMed] [Google Scholar]
- 47.Bruder JM, Ma JZ, Basler JW, Welch MD. Prevalence of osteopenia and osteoporosis by central and peripheral bone mineral density in men with prostate cancer during androgen-deprivation therapy. Urology 2006;67(1):152–5. [DOI] [PubMed] [Google Scholar]
- 48.Panagiotakopoulos L Transgender medicine - puberty suppression. Rev Endocr Metab Disord 2018;19(3):221–5. [DOI] [PubMed] [Google Scholar]
- 49.Klink D, Caris M, Heijboer A, van Trotsenburg M, Rotteveel J. Bone mass in young adulthood following gonadotropin-releasing hormone analog treatment and cross-sex hormone treatment in adolescents with gender dysphoria. J Clin Endocrinol Metab 2015;100(2):E270–5. [DOI] [PubMed] [Google Scholar]
- 50.Vlot MC, Klink DT, den Heijer M, Blankenstein MA, Rotteveel J, Heijboer AC. Effect of pubertal suppression and cross-sex hormone therapy on bone turnover markers and bone mineral apparent density (BMAD) in transgender adolescents. Bone 2017;95:11–9. [DOI] [PubMed] [Google Scholar]
- 51.Tack LJW, Craen M, Lapauw B, Goemaere S, Toye K, Kaufman JM, et al. Proandrogenic and antiandrogenic progestins in transgender youth: differential effects on body composition and bone metabolism. J Clin Endocrinol Metab 2018;103(6):2147–56. [DOI] [PubMed] [Google Scholar]
- 52.Sedlak CA, Roller CG, van Dulmen M, Alharbi HA, Sanata JD, Leifson MA, et al. Transgender individuals and osteoporosis prevention. Orthop Nurs 2017;36(4):259–68. [DOI] [PubMed] [Google Scholar]
- 53.www.nof.org. Accessed 2/26, 2019.
- 54.Hembree WC, Cohen-Kettenis PT, Gooren L, Hannema SE, Meyer WJ, Murad MH, et al. Endocrine treatment of gender-dysphoric/gender-incongruent persons: an Endocrine Society clinical practice guideline. Endocr Pract 2017;23:1437. [DOI] [PubMed] [Google Scholar]
- 55.Hammond I, Lentle B, van den Berg L, Vitols-McKay M. Gender identity and bone densitometry. Can Assoc Radiol J 2017;68(3): 267–9. [DOI] [PubMed] [Google Scholar]