Abstract
Cross-sectional approaches to outcome assessment may not adequately capture heterogeneity in recovery after traumatic brain injury (TBI). Using latent class mixed models (LCMM), a data-driven analytic that identifies groups of patients with similar trajectories, we identified distinct 6 month functional recovery trajectories in a large cohort (n = 1046) of adults 18–70 years of age with complicated mild to severe TBI who participated in the Citicoline Brain Injury Treatment Trial (COBRIT). We used multinomial logistic fixed effect models and backward elimination, forward selection, and forward stepwise selection with several stopping rules to explore baseline predictors of functional recovery trajectory. Based on statistical and clinical considerations, the seven-class model was deemed superior. Visualization of these seven functional recovery trajectories revealed that each trajectory class started at one of three recovery levels at 1 month, which, for ease of reference we labeled groups A–C: Group A, good recovery (two classes; A1 and A2); Group B, moderate disability (two classes; B1 and B2); and Group C, severe disability (three classes; C1, C2, and C3). By 6 months, these three groups experienced dramatically divergent trajectories. Group A experienced stable good recovery (A1, n = 115) or dramatic decline (A2, n = 4); Group B experienced rapid complete recovery (B1, n = 71) or gradual recovery (B2, n = 742); Group C experienced dramatic rapid recovery (C1, n = 12), no recovery (C2, n = 91), or death (C3, n = 11). Trajectory class membership was not predicted by citicoline treatment (p = 0.57). The models identified demographic, pre-injury, and injury-related predictors of functional recovery trajectory, including: age, race, education, pre-injury employment, pre-injury diabetes, pre-injury psychiatric disorder, site, Glasgow Coma Scale (GCS) score, post-traumatic amnesia, TBI mechanism, major extracranial injury, hemoglobin, and acute computed tomographic (CT) findings. GCS was the most consistently selected predictor across all models. All models also selected at least one demographic or pre-injury medical predictor. LCMM successfully identified dramatically divergent, clinically meaningful 6 month recovery trajectories with utility to inform clinical trial design.
Keywords: functional outcome, predictors, TBI, trajectory
Introduction
Traumatic brain injury (TBI) is one of the most challenging public health issues today: 2,800,000 people in the United States seek hospital-based care for TBI annually,1 leading to an estimated annual cost of > $75 billion.2 Despite progress in pre-clinical TBI drug development, results of clinical trials have been disappointing.3–5 There is growing consensus that a major barrier to progress in the treatment and rehabilitation of TBI is that current approaches to outcome assessment, such as 3 or 6 month binary functional outcomes (e.g., good vs. poor recovery), are inadequate to capture the heterogeneous and evolving nature of TBI.3,6,7 To improve evidence-based clinical trial design and targeted intervention, careful characterization of the heterogeneous and evolving nature of functional recovery trajectories after TBI, across the spectrum of age and TBI severity, is critical. Additionally, reframing TBI outcome assessment around functional recovery trajectories over time, rather than an arbitrary cross-sectional time point (e.g., 6 months), may have more clinical relevance to individual patients who may wish to know whether or not they should expect continued recovery, and at what pace.
Additionally, current TBI stratification methods – such as those based on the Glasgow Coma Scale (GCS) – are increasingly recognized as insufficient for precision diagnosis and clinical trial stratification.6,7 Trajectory analysis may facilitate identification of clinically and biologically relevant subgroups of patients. Surprisingly, trajectory analysis has only rarely been applied to TBI recovery, typically to model group-level outcome trajectories according to pre-specified strata using conventional trajectory models.8–15 In most conventional trajectory models, the average group-level trajectory is modelled. In order to study subgroups of patients, the group must be manually divided according to pre-specified features, and then the average of each pre-specified subgroup is modeled. This approach, however, may lump together individual patients with very different trajectories, thereby providing an incomplete view of recovery patterns.
Data-driven trajectory analytics, on the other hand, hold great promise for capturing the multiple distinct clinical trajectories that coexist within a single TBI population without imposing a pre-conceived, and possibly inadequate, stratification system.16–18 Latent class mixed modelling (LCMM),19 and other similar analytics such as latent class growth analysis (LCGA) or latent growth mixture modelling (LGMM), are data-driven analytics that identify groups of patients with similar trajectories, thus facilitating identification and visualization of multiple distinct outcome trajectories within a single population. In the past 10 years, this approach has been applied to TBI populations with compelling results, identifying distinct 1 year or multi-year trajectories of cognitive, motor, mental health, post-concussive, life-satisfaction, or global function after TBI in a variety of subpopulations.14,15,20–30 To date, however, no study has applied latent class trajectory analytics to identify granular 6 month global functional outcome trajectories (Glasgow Outcome Scale Extended [GOSE]), and associated baseline predictors of these trajectories, in a very large clinical trial cohort of patients with TBI.
We hypothesized that LCMM may help elucidate the distinct functional recovery trajectories experienced by patients in the first few months following TBI. Additionally, LCMM may offer insights into baseline predictors of recovery that may aid in the discovery of heterogeneous biological mechanisms of recovery. Finally, LCMM may inform the design of future acute TBI clinical trials with refined population enrichment, participant stratification criteria, end-points, and analytic strategies. Our primary aim in this study was to apply LCMM to identify distinct functional recovery trajectories in a large cohort of well-characterized adults with complicated mild to severe TBI who participated in the negative Citicoline Brain Injury Treatment Trial (COBRIT). Our secondary aim was to describe the baseline characteristics of patients with various functional recovery trajectories and then to perform exploratory analyses of key baseline clinical predictors of functional recovery trajectory in order to simultaneously assess face validity of the trajectories identified via LCMM and also to offer novel insights into baseline predictors of 6 month recovery patterns.
Methods
Study design and ethics approval
This retrospective cohort study used existing longitudinal clinical trial data which is available on the Federal Interagency TBI Research (FITBIR) platform.31 This study was approved by the University of California at San Francisco (UCSF) Committee for Human Research. The need for informed consent was waived, as these data are de-identified.
Data source
De-identified data from the COBRIT were obtained from the TBI Endpoints Development (TED) Metadataset7 upon execution of a data use agreement. The COBRIT study was a negative phase 3, double-blind randomized clinical trial of citicoline versus placebo to improve 3 month (primary) and 6 month (secondary) outcome in 1213 adults presenting between July 20, 2007 and February 4, 2011 to one of eight participating United States level 1 trauma centers within 24 h of complicated mild to severe TBI.4 COBRIT inclusion criteria included: being 18–70 years of age and having had an inpatient acute hospitalization for a non-penetrating, complicated mild to severe TBI (defined subsequently) at one of eight participating sites. Exclusion criteria were: bilaterally fixed and dilated pupils, imminent death, current life-threatening illness, being a prisoner/patient in custody, pregnancy, current participation in another study, acetylcholinesterase inhibitor use within the past 2 weeks, or any pre-existing disabling neurological or psychiatric condition that would impair the ability to complete outcome assessments. For the present secondary data analysis, we included all patients with at least one recorded functional outcome assessment at 1 month, 3 months, or 6 months post-injury (n = 1046). We did not include those who died or were lost to follow-up before the first COBRIT outcome assessment at 1 month (n = 167).
Classification of TBI
COBRIT classified TBI using a combination of GCS score and specified computed tomographic (CT) findings. CT criteria were: ≥10 mm total diameter of all intraparenchwoymal hemorrhages, acute extra-axial hematoma ≥5 mm thick, subarachnoid hemorrhage visible on at least contiguous 5mm slices or at least three contiguous 3 mm slices, intraventricular hemorrhage present on two slices, or ≥5 mm midline shift.4 Complicated mild TBI was defined as GCS 13–15 meeting the abovementioned CT criteria. Moderate to severe TBI were defined as pre-paralytic GCS 3–12 with motor score ≤5 or GCS 3–12 with motor score 6 (and meeting any of the CT criteria), or post-paralytic GCS 3T–11T (and meeting any of the CT criteria). Patients with mild, uncomplicated TBI (e.g., GCS 13–15 not meeting any CT criteria) were excluded from COBRIT and, therefore, this analysis does not apply to those with these milder forms of TBI.
Outcome
Although COBRIT's primary outcome was a 3 month binary composite metric of function and cognition, the primary outcome in this analysis is functional recovery as measured at 1 month, 3 months, and 6 months after TBI according to the GOSE, the most widely used functional outcome assessment in TBI research.32,33 The GOSE is an ordinal scale ranging from 1 to 8 as follows: 1-dead, 2-in a vegetative state, 3-lower severe disability, 4-upper severe disability, 5-lower moderate disability, 6-upper moderate disability, 7-lower good recovery and 8-upper good recovery. A total of 76.3% of patients had complete GOSE data at all three time points; 15.8% of patients had one missing time point and 7.9% of patients had two missing time points.
Baseline predictors
Details of baseline data collection in COBRIT have been previously reported.4,34 For the present analysis, given our dual goals of describing the baseline phenotype of patients with different distinct recovery trajectories and demonstrating face validity and key baseline predictors of LCMM-based trajectories, we studied demographics, all coded comorbidities with at least a 5% overall prevalence in the study cohort, and all coded baseline TBI and injury variables that have been previously shown to be predictive of cross-sectional functional outcome after TBI.35–38 These included the following baseline demographics and pre-existing medical conditions: age, sex, race, ethnicity, living situation, educational attainment, employment status, cardiovascular disease, hypertension, psychiatric condition, and diabetes; the following TBI and acute medical characteristics upon presentation: GCS score (< 9, 9–12, 13–15), post-traumatic amnesia (PTA), TBI mechanism, international normalized ratio (INR), hypotension (lowest systolic blood pressure <90 mm Hg), hypoxia (lowest oxygen saturation <90), highest glucose, hemoglobin, and presence of major extra cranial trauma (non-head Abbreviated Injury Scale [AIS] score ≥3); and the following pathoanatomical features on initial head CT: midline shift, subarachnoid hemorrhage (SAH), intraparenchymal hemorrhage (IPH), subdural hemorrhage (SDH), epidural hemorrhage (EDH), and compression of mesencephalic cisterns. We additionally assessed for an effect of study site and treatment arm (citicoline vs. placebo). It is of note that less than 1% of the study population was coded as having a history of prior TBI, indicating substantial under-reporting,39 and, therefore, this baseline predictor was not included in the analysis.
Statistical analysis
All analyses were conducted using Stata 15,40 SAS 9.4,41 R 3.4,42 or RStudio 1.0 statistical software.
Data-driven discovery of trajectory classes and approach to model selection
Using the LCMM 1.7.8 package in R (https://arxiv.org/pdf/1503.00890.pdf),19 we used unconditional LCMM19 for ordinal data to model GOSE functional recovery trajectories over time and to classify patients into distinct latent trajectory classes. The only variables used to infer latent class were subject, GOSE, and time. We chose to use unconditional LCMM (instead of conditional LCMM), because our primary aim was to describe the “raw” latent GOSE trajectories in the population without imposing any conditions/predictors on the model, whereas our secondary aim was to then explore predictors of these unconditional trajectories. LCMM assumes that the population is heterogeneous and is divided into distinct groups, with each group having its own trajectory of GOSE versus time. LCMM, like other likelihood-based methods, can analyze data with missing observations. As long as missing observations are missing in a way that depends only on observed values, then the estimates will be unbiased.43 Starting with a one-class model, we fitted models with increasing numbers of classes until we reached the inflection point of the Akaike information criterion (AIC). The AIC is a way to identify the point at which the benefits of improved model fit are outweighed by the cost of model complexity. We additionally examined the log likelihood, a measure of goodness of model fit regardless of model complexity, and the Bayesian information criteria (BIC). The BIC is similar to the AIC but has a slightly different threshold such that increased model complexity is penalized more heavily than it is in the AIC, generally resulting in an inflection point at a less complex model. Next, we estimated the posterior probabilities of membership for each patient in each trajectory class using the maximum probability assignment rule of Strauss and coworkers,44 and then assigned each individual patient to the class with their highest predicted probability of membership. We plotted the mean GOSE trajectory of all patients assigned to each class for each of the models. We visualized the flow of patients between classes in each successive model using a river plot.45 The final model was then selected based on both statistical (log likelihood, AIC, BIC) and clinical (class size, distinctness of class-specific trajectories, likelihood of class membership based on posterior probabilities, impact of missing GOSE time points) considerations.
Model-based validation and identification of predictors of trajectory classes
After selecting the final model and classifying each patient into a specific latent class, we used χ2 tests for categorical variables and Kruskal–Wallis tests for continuous variables to examine baseline characteristics by trajectory class including treatment effects and the interaction of treatment with TBI severity (GCS <13 vs. 13–15). Next, multinomial logistic models were used to investigate the role of different baseline predictors in predicting the heterogeneous trajectories (i.e., latent class membership). Because there is no clear consensus regarding the optimal approach or stopping rule for variable selection, we used three different variable selection methods and three different stopping rules to achieve our exploratory aim of identifying significant independent baseline predictors of recovery trajectories, as follows:
-
1.
Backward elimination:46 we started with the maximum model with all available baseline predictors and performed backward elimination, omitting predictors with the largest p value first. We ran the model three times with the following stopping rules: (1) p < 0.05 and then best model selected using AIC, (2) p < 0.05 only, and (3) p < 0.1 only. The advantage of backward selection is that all variables are initially allowed to compete or work all together in the model, thus estimating their joint predictive capability.
-
2.
Forward selection: we started with an intercept-only model and performed forward selection, adding predictors with the smallest p value (based on the score test47,48) first, and stopping using the same three stopping rules described previously. The advantage of forward selection is that it does not depend on possibly ill-conditioned models with many variables, and adding one variable at a time does not reduce the sample size because of missing values of variables not ultimately included in the model (which is not the case for backward elimination).
-
3.
Forward stepwise selection: This approach is similar to forward selection except that variables may be dropped as others are added. This model was run three times with the same three stopping rules described previously.
Each variable selection model was run to select predictors of membership across all trajectory classes and again to specifically select predictors of a pairwise comparison between the largest trajectory class and one other (determined based on clinical considerations), so as to more granularly investigate the specific magnitude and direction of the effect of the selected variables. Lastly, variables selected via any of the selection models or stopping rules were then included in a single final multivariable logistic regression model, and odds ratios were estimated to illustrate the clinical impact of each selected predictor on recovery trajectory.
Results
Model selection: Statistical considerations
After fitting a one- to eight-class LCMM model, the AIC identified an inflection point at a seven-class model, suggesting that the added complexity cost of an eight-class model outweighed the added benefit in model fit (Table S1). Model fit (log likelihood) continued to improve with increasing numbers of classes. BIC identified an inflection point at a five-class model, suggesting that at this more stringent threshold, the added complexity cost of a six-, or seven-, or eight-class model outweighed the added benefit in model fit (Table S1). The river plot of the flow of patients between classes in each successive model identified a major split at the six-class model in which the single largest class was split into two smaller classes, suggesting that the five-class model chosen by the BIC may be overly simplistic (Fig. S1). Substantial recategorization again occurred on the river plot between the six-class and the seven-class model chosen by the AIC, whereas only minimal recategorization occurred from the seven-class to the eight-class model (Fig S1). Overall, based on statistical parameters, the seven-class model was considered the best model.
Model selection: Clinical considerations
After assigning each patient to a trajectory class based on maximum predicted probability of class membership, we graphed average GOSE trajectories for patients assigned to each trajectory class for the two-class through eight-class models to better visualize the clinical value of each model (Fig. S2). Deaths (GOSE = 1; n = 12) were categorized into their own distinct and very small class quite early (rudimentary three-class model); therefore, we were unable to choose a model based on a class-size cutoff, as proposed by Strauss and coworkers.44 As expected by the visualization of the river plot described previously, the five-class model selected by the BIC clustered nearly all of the patients in the entire cohort into a single gradually improving class (n = 917), whereas the six-class and seven-class models clearly identified additional distinct trajectory classes and grouped patients into more distributed classes. Comparison of the six-class, seven-class, and eight-class models revealed substantial clinical differences from the six-class model to the seven-class model and only minimal clinical differences from the seven-class model to the eight-class model. The mean of the posterior probabilities of class membership of patients assigned to each class for the seven-class model ranged from 0.65 to 0.99 (Table S2),suggesting a high confidence for most class assignments in the seven-class model. Because 7.9% of patients in the cohort only had a single GOSE time point, we re-ran the seven class model after excluding these patients. We assigned each patient to the class with their highest predicted probability of membership and plotted the mean GOSE trajectory of all patients assigned to each class, as described previously. The resultant seven GOSE trajectories of patients with at least two GOSE time points were virtually identical to the original seven-class model that included all patients. Specifically, excluding patients with only one time point did not substantially change either the appearance or classification of the trajectories, and >96% of patients were categorized into the same trajectory class as in the original model. It is of note that the model excluding patients with only one time point assumes that data are missing “completely at random” (e.g., not depending on anything), whereas the model including patients with only one time point assumes that data are missing “at random” (e.g., depending on observed data but not on unobserved data), a less stringent assumption. Our findings indicate that the models are not sensitive to different assumptions about missing data. Therefore, based on both statistical and clinical parameters, the seven-class model was chosen as the final model.
Characterization of 6 month functional recovery trajectories
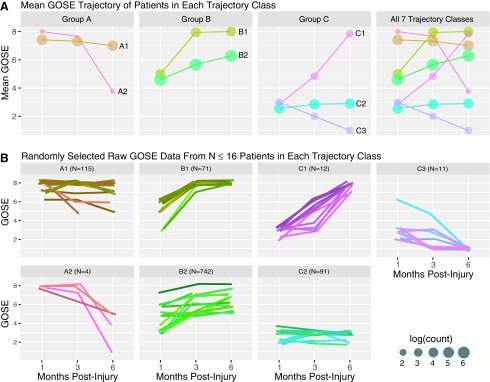
Using the seven-class model, each patient was assigned to a latent trajectory class based on their maximum posterior probability of class membership. Average GOSE at each time point for patients assigned to each class was calculated (Table 1)and graphed in order to visualize distinct latent functional recovery trajectories (Fig. 1). At 1 month post-injury, each of the seven trajectories started out at one of three initial levels of recovery, which, for ease of reference we labeled groups A-C: Group A had largely achieved good recovery (two trajectory classes, A1 and A2); group B had moderate residual disability (two trajectory classes, B1 and B2); and group C had severe residual disability (three trajectory classes, C1–C3). By the 6 month time point, however, patients in each of these three 1 month groupings demonstrated dramatically divergent functional trajectories (Fig. 1). Specifically, those in group A either experienced stable recovery to slight decline (A1) or dramatic decline culminating in moderate-severe disability or death (A2); those in group B either experienced rapid complete recovery (B1) or more gradual recovery to varying levels of disability or complete recovery (B2); and those in group C either experienced dramatic rapid good recovery (C1), no recovery (C2), or death (C3).
Table 1.
Glasgow Outcome Scale Extended (GOSE) at Each Time Point by Recovery Trajectory Class
| Trajectory class | Description | Time point | n | Mean (SD) GOSE | Range GOSE |
|---|---|---|---|---|---|
| A1: Stable good recovery to slight decline (n = 115; 11.0%) | Good recovery → good recovery ± slight decline | 1 month | 111 | 7.41 (0.71) | 5–8 |
| 3 month | 110 | 7.31 (0.82) | 4–8 | ||
| 6 month | 101 | 7.00 (1.04) | 4–8 | ||
| A2: Dramatic unexpected decline (n = 4; 0.4%) | Complete recovery → severe disability/death | 1 month | 4 | 8.00 (0.00) | 8–8 |
| 3 month | 3 | 7.67 (0.58) | 7–8 | ||
| 6 month | 4 | 3.75 (1.89) | 1–5 | ||
| B1: Rapid complete recovery (n = 71; 6.8%) | Moderate disability → complete recovery | 1 month | 64 | 5.00 (1.14) | 2–6 |
| 3 month | 70 | 7.94 (0.23) | 7–8 | ||
| 6 month | 57 | 8.00 (0.00) | 8–8 | ||
| B2: Gradual variable recovery (n = 742; 70.9%) | Moderate disability → gradual recovery | 1 month | 717 | 4.59 (1.36) | 2–7 |
| 3 month | 662 | 5.66 (1.33) | 3–8 | ||
| 6 month | 577 | 6.28 (1.19) | 3–8 | ||
| C1: Dramatic rapid good recovery (n = 12; 1.2%) | Severe disability → good recovery | 1 month | 12 | 2.83 (0.39) | 2–3 |
| 3 month | 12 | 4.83 (1.27) | 3–6 | ||
| 6 month | 12 | 7.83 (0.39) | 7–8 | ||
| C2: Marginal to no recovery (n = 91; 8.7%) | Severe disability → minimal change | 1 month | 91 | 2.55 (0.52) | 2–4 |
| 3 month | 88 | 2.85 (0.44) | 2–4 | ||
| 6 month | 80 | 2.90 (0.38) | 2–4 | ||
| C3: Death (n = 11; 1.1%) | Severe disability → death | 1 month | 10 | 3.00 (1.15) | 2–6 |
| 3 month | 11 | 2.00 (1.34) | 1–5 | ||
| 6 month | 11 | 1.00 (0.00) | 1–1 |
FIG. 1.
Functional trajectories. (A) Mean Glasgow Outcome Scale Extended (GOSE) is shown at each time point for all patients assigned to each trajectory class. Dot size represents log count of sample size at each time point, with larger dots representing larger sample size. At 1 month post-injury, the top two (Group A: red and orange), middle two (Group B: khaki and green), and lower three (Group C: pink, teal, and purple) classes all start at similar levels of recovery or disability, but then experience dramatically divergent recovery trajectories by 6 months. (B) Randomly selected raw GOSE data are plotted for up to 16 patients in each trajectory class. Because these are raw GOSE data, all GOSE values are whole numbers ranging from 1 to 8. However, slight random jitter in GOSE (and color) was introduced to improve visualization of each individual trajectory. For example, all patients in B1 actually achieved complete recovery (GOSE = 8) by 6 months.
Bivariate associations with 6 month functional recovery trajectory
Baseline characteristics of patients by functional recovery trajectory are shown in Table 2. It is of note that patients in each functional recovery trajectory class significantly differed on race, education, employment, diabetes, and nearly every measure of TBI severity assessed. Citicoline treatment did not have statically significant effects on functional recovery trajectory. We also did not identify an effect of citicoline on functional recovery trajectory among patients specifically with complicated mild TBI (p = 0.318), moderate TBI (p = 0.514), severe TBI (p = 0.455), or combined moderate-severe TBI (p = 0.698), which was investigated because of the non-significant trend toward a differential treatment effect by severity in the COBRIT study.4
Table 2.
Characteristics of Patients by Recovery Trajectory Class
| Baseline predictor column n(%) or mean ± SD | A1 Stable recovery (n = 115) | A2 Dramatic decline (n = 4) | B1 Rapid complete recovery (n = 71) | B2 Gradual variable recovery (n = 742) | C1 Dramatic rapid good recovery (n = 12) | C2 No recovery (n = 91) | C3 Death (n = 11) | p |
|---|---|---|---|---|---|---|---|---|
| Demographics | ||||||||
| Age, y | 42.0 ± 17.2 | 49.8 ± 14.9 | 37.4 ± 16.0 | 39.6 ± 15.5 | 35.4 ± 18.7 | 39.0 ± 15.9 | 50.4 ± 13.6 | 0.080a |
| Female | 32 (27.8%) | 0 (0.0%) | 17 (23.9%) | 197 (26.5%) | 3 (25.0%) | 23 (25.3%) | 2 (18.2%) | 0.90b |
| Race | 0.039b | |||||||
| White | 101 (87.8%) | 2 (50.0%) | 59 (83.1%) | 609 (82.1%) | 11 (91.7%) | 63 (69.2%) | 8 (72.7%) | |
| Black | 11 (9.6%) | 1 (25.0%) | 10 (14.1%) | 105 (14.2%) | 1 (8.3%) | 20 (22.0%) | 3 (27.3%) | |
| Other | 3 (2.6%) | 1 (25.0%) | 2 (2.8%) | 28 (3.8%) | 0 (0.0%) | 8 (8.8%) | 0 (0.0%) | |
| Hispanic | 2 (1.7%) | 1 (25.0%) | 7 (9.9%) | 32 (4.3%) | 1 (8.3%) | 3 (3.3%) | 0 (0.0%) | 0.057b |
| Pre-injury housing | 0.88b | |||||||
| Alone | 15 (13.0%) | 0 (0.0%) | 12 (16.9%) | 123 (16.6%) | 2 (16.7%) | 13 (14.3%) | 3 (27.3%) | |
| With family/friends | 98 (85.2%) | 4 (100.0%) | 59 (83.1%) | 615 (82.9%) | 10 (83.3%) | 78 (85.7%) | 8 (72.7%) | |
| Other | 2 (1.7%) | 0 (0.0%) | 0 (0.0%) | 4 (0.5%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |
| Education | 0.004b | |||||||
| <High school | 8 (7.0%) | 0 (0.0%) | 8 (11.3%) | 98 (13.3%) | 1 (8.3%) | 23 (26.4%) | 1 (10.0%) | |
| High school grad/GED | 30 (26.1%) | 2 (50.0%) | 23 (32.4%) | 256 (34.6%) | 5 (41.7%) | 36 (41.4%) | 6 (60.0%) | |
| Some college/technical | 49 (42.6%) | 2 (50.0%) | 24 (33.8%) | 260 (35.2%) | 5 (41.7%) | 19 (21.8%) | 1 (10.0%) | |
| College grad or greater | 28 (24.3%) | 0 (0.0%) | 16 (22.5%) | 125 (16.9%) | 1 (8.3%) | 9 (10.3%) | 2 (20.0%) | |
| Pre-injury employment | 0.006b | |||||||
| FT employed/student | 77 (67.5%) | 2 (50.0%) | 49 (69.0%) | 522 (71.1%) | 9 (75.0%) | 57 (64.0%) | 6 (60.0%) | |
| PT employed/student | 5 (4.4%) | 0 (0.0%) | 7 (9.9%) | 61 (8.3%) | 3 (25.0%) | 9 (10.1%) | 0 (0.0%) | |
| Retired | 12 (10.5%) | 2 (50.0%) | 7 (9.9%) | 37 (5.0%) | 0 (0.0%) | 5 (5.6%) | 2 (20.0%) | |
| Unemployed/disabled | 20 (17.5%) | 0 (0.0%) | 8 (11.3%) | 114 (15.5%) | 0 (0.0%) | 18 (20.2%) | 2 (20.0%) | |
| Pre-existing conditions | ||||||||
| Cardiovascular disease | 11 (9.6%) | 0 (0.0%) | 6 (8.5%) | 45 (6.1%) | 0 (0.0%) | 6 (6.6%) | 3 (27.3%) | 0.095b |
| Hypertension | 24 (20.9%) | 1 (25.0%) | 10 (14.1%) | 135 (18.2%) | 1 (8.3%) | 18 (19.8%) | 5 (45.5%) | 0.26b |
| Diabetes | 8 (7.0%) | 0 (0.0%) | 6 (8.5%) | 53 (7.1%) | 1 (8.3%) | 8 (8.8%) | 4 (36.4%) | 0.032b |
| Psychiatric disorder | 14 (12.3%) | 1 (25.0%) | 4 (5.6%) | 64 (8.6%) | 0 (0.0%) | 8 (8.8%) | 3 (27.3%) | 0.15b |
| Treatment | ||||||||
| Citicoline treatment | 61 (53.0%) | 4 (100.0%) | 38 (53.5%) | 368 (49.6%) | 6 (50.0%) | 46 (50.5%) | 5 (45.5%) | 0.57b |
| Site | NS | NS | NS | NS | NS | NS | NS | 0.36b |
| TBI and acute medical characteristics | ||||||||
| GCS | <0.001b | |||||||
| 13–15 (mild) | 96 (83.5%) | 3 (75.0%) | 52 (73.2%) | 401 (54.0%) | 2 (16.7%) | 7 (7.7%) | 5 (45.5%) | |
| 9–12 (moderate) | 8 (7.0%) | 1 (25.0%) | 7 (9.9%) | 122 (16.4%) | 1 (8.3%) | 9 (9.9%) | 2 (18.2%) | |
| <9 (severe) | 11 (9.6%) | 0 (0.0%) | 12 (16.9%) | 219 (29.5%) | 9 (75.0%) | 75 (82.4%) | 4 (36.4%) | |
| PTA days | <0.001b | |||||||
| 0 | 49 (42.6%) | 3 (75.0%) | 14 (19.7%) | 87 (11.7%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |
| 1 | 16 (13.9%) | 0 (0.0%) | 9 (12.7%) | 51 (6.9%) | 0 (0.0%) | 0 (0.0%) | 1 (9.1%) | |
| >1 | 45 (39.1%) | 1 (25.0%) | 37 (52.1%) | 384 (51.8%) | 4 (33.3%) | 6 (6.6%) | 0 (0.0%) | |
| unknown | 5 (4.3%) | 0 (0.0%) | 11 (15.5%) | 220 (29.6%) | 8 (66.7%) | 85 (93.4%) | 10 (90.9%) | |
| TBI mechanism | <0.001b | |||||||
| Motor vehicle accident | 34 (29.6%) | 2 (50.0%) | 43 (60.6%) | 427 (57.5%) | 7 (58.3%) | 67 (73.6%) | 5 (45.5%) | |
| Fall from moving | 19 (16.5%) | 0 (0.0%) | 8 (11.3%) | 60 (8.1%) | 1 (8.3%) | 7 (7.7%) | 2 (18.2%) | |
| Fall from stationary | 38 (33.0%) | 1 (25.0%) | 14 (19.7%) | 151 (20.4%) | 2 (16.7%) | 11 (12.1%) | 3 (27.3%) | |
| Assault | 18 (15.7%) | 1 (25.0%) | 4 (5.6%) | 74 (10.0%) | 1 (8.3%) | 1 (1.1%) | 1 (9.1%) | |
| Other | 6 (5.2%) | 0 (0.0%) | 2 (2.8%) | 30 (4.0%) | 1 (8.3%) | 5 (5.5%) | 0 (0.0%) | |
| Major extracranial injury | 18 (15.7%) | 3 (75.0%) | 32 (45.1%) | 313 (42.2%) | 9 (75.0%) | 54 (59.3%) | 4 (36.4%) | <0.001b |
| Hypotension | 11 (9.6%) | 0 (0.0%) | 8 (11.3%) | 114 (15.4%) | 3 (25.0%) | 26 (28.6%) | 3 (27.3%) | 0.005b |
| Hypoxia | 8 (7.0%) | 0 (0.0%) | 10 (14.1%) | 72 (9.7%) | 1 (8.3%) | 13 (14.3%) | 2 (18.2%) | 0.46b |
| INR | <0.001b | |||||||
| ≤1.2 | 109 (94.8%) | 4 (100.0%) | 58 (81.7%) | 606 (81.7%) | 5 (41.7%) | 57 (62.6%) | 8 (72.7%) | |
| 1.3-2 | 4 (3.5%) | 0 (0.0%) | 11 (15.5%) | 124 (16.7%) | 7 (58.3%) | 30 (33.0%) | 3 (27.3%) | |
| >2 | 2 (1.7%) | 0 (0.0%) | 2 (2.8%) | 12 (1.6%) | 0 (0.0%) | 4 (4.4%) | 0 (0.0%) | |
| Hemoglobin | 13.3 ± 1.8 | 11.9 ± 1.1 | 12.4 ± 1.5 | 12.5 ± 2.2 | 11.8 ± 2.5 | 11.1 ± 2.3 | 12.4 ± 2.0 | <0.001a |
| Glucose | 134.0 ± 43.2 | 122.5 ± 82.4 | 147.7 ± 42.1 | 159.0 ± 53.7 | 166.3 ± 29.2 | 187.7 ± 59.7 | 184.6 ± 98.6 | <0.001a |
| Baseline head CT | ||||||||
| Midline shift | <0.001b | |||||||
| None | 100 (87.0%) | 4 (100.0%) | 52 (73.2%) | 586 (79.0%) | 8 (66.7%) | 55 (60.4%) | 8 (72.7%) | |
| 0–5mm | 12 (10.4%) | 0 (0.0%) | 18 (25.4%) | 108 (14.6%) | 2 (16.7%) | 17 (18.7%) | 2 (18.2%) | |
| >5mm | 3 (2.6%) | 0 (0.0%) | 1 (1.4%) | 48 (6.5%) | 2 (16.7%) | 19 (20.9%) | 1 (9.1%) | |
| Mesencephalic cisterns | <0.001b | |||||||
| No compression | 103 (89.6%) | 4 (100.0%) | 53 (74.6%) | 557 (75.3%) | 7 (58.3%) | 46 (50.5%) | 9 (81.8%) | |
| Effaced/compressed | 12 (10.4%) | 0 (0.0%) | 15 (21.1%) | 157 (21.2%) | 3 (25.0%) | 33 (36.3%) | 1 (9.1%) | |
| Obliterated | 0 (0.0%) | 0 (0.0%) | 3 (4.2%) | 26 (3.5%) | 2 (16.7%) | 12 (13.2%) | 1 (9.1%) | |
| SAH | <0.001b | |||||||
| None | 24 (20.9%) | 2 (25.0%) | 18 (25.4%) | 190 (25.6%) | 2 (16.7%) | 17 (18.7%) | 4 (36.4%) | |
| Sulci or cisterns only | 80 (69.6%) | 3 (75.0%) | 42 (59.2%) | 437 (58.9%) | 9 (75.0%) | 39 (42.9%) | 3 (27.3%) | |
| Sulci and cisterns | 11 (9.6%) | 0 (0.0%) | 11 (15.5%) | 115 (15.5%) | 1 (8.3%) | 35 (38.5%) | 4 (36.4%) | |
| IPH | 0.011b | |||||||
| None | 73 (63.5%) | 1 (25.0%) | 35 (49.3%) | 366 (49.3%) | 6 (50.0%) | 38 (41.8%) | 7 (63.6%) | |
| ≤25cc | 41 (35.7%) | 3 (75.0%) | 36 (50.7%) | 351 (47.3%) | 4 (33.3%) | 50 (54.9%) | 3 (27.3%) | |
| >25cc | 1 (0.9%) | 0 (0.0%) | 0 (0.0%) | 25 (3.4%) | 2 (16.7%) | 3 (3.3%) | 1 (9.1%) | |
| SDH | <0.001b | |||||||
| None | 91 (79.1%) | 3 (75.0%) | 48 (67.6%) | 516 (69.5%) | 6 (50.0%) | 45 (49.5%) | 5 (45.5%) | |
| ≤25cc | 22 (19.1%) | 1 (25.0%) | 20 (28.2%) | 195 (26.3%) | 5 (41.7%) | 32 (35.2%) | 4 (36.4%) | |
| >25cc or evacuated | 2 (1.7%) | 0 (0.0%) | 3 (4.2%) | 31 (4.2%) | 1 (8.3%) | 14 (15.4%) | 2 (18.2%) | |
| EDH | 0.75b | |||||||
| None | 103 (89.6%) | 4 (100.0%) | 61 (85.9%) | 637 (85.8%) | 11 (91.7%) | 79 (86.8%) | 11 (100.0%) | |
| ≤ 25cc | 8 (7.0%) | 0 (0.0%) | 10 (14.1%) | 78 (10.5%) | 1 (8.3%) | 8 (8.8%) | 0 (0.0%) | |
| >25cc or evacuated | 4 (3.5%) | 0 (0.0%) | 0 (0.0%) | 27 (3.6%) | 0 (0.0%) | 4 (4.4%) | 0 (0.0%) | |
Based on Kruskal–Wallis test.
Based on χ2 test.
SD, standard deviation; GED, general educational development test; TBI, traumatic brain injury; FT, full time; PT, part time; GCS, Glasgow Coma Scale; PTA, post-traumatic amnesia; INR, international normalized ratio; CT, computed tomography; SAH, subarachnoid hemorrhage; IPH, intraparenchymal hemorrhage; SDH, subdural hemorrhage; EDH, epidural hemorrhage.
Independent predictors of 6 month functional recovery trajectory in multivariable models
Backward elimination, forward selection, and forward stepwise selection with various stopping rules identified different but overlapping independent predictors of seven-class functional recovery trajectory (Table 3), including age, race, education, pre-injury employment, pre-injury diabetes, pre-injury psychiatric disorder, site, GCS, PTA, TBI mechanism, major extracranial injury, hemoglobin, mesencephalic cistern compression, SAH, IPH, and SDH. To more granularly assess the direction of the effects of baseline predictors on the most common functional recovery trajectories, we re-ran variable selection for models comparing the rapid recoverers (B1) to the gradual recoverers (B2; the largest class). Backward elimination, forward selection, and/or forward stepwise selection identified age, ethnicity, pre-injury employment, GCS, and major extracranial injury as independent predictors of rapid (B1) versus gradual (B2) 6 month recovery among patients starting out with a similar 1 month GOSE score. Logistic regression was then used to determine the direction of the effects of these predictors of rapid (B1) versus gradual (B2) recovery (Table 4). This demonstrated that older age, pre-injury unemployment/disability, and more severe TBI by GCS were associated with slower recovery, whereas Hispanic ethnicity, pre-injury retirement, and presence of extracranial injury were associated with more rapid recovery. It is of note that GCS was the most consistently selected predictor of recovery trajectory across all models, although all models also selected at least one baseline demographic or pre-injury medical predictor.
Table 3.
Independent Predictors of Recovery Trajectory Selected with Each Variable Selection Model
| Predictors of recovery trajectory: All 7 classes | Predictors of recovery trajectory: B1 (rapid recovery) vs. B2 (gradual recovery) only | |||||
|---|---|---|---|---|---|---|
| Backward | Forward | Step | Backward | Forward | Step | |
| Demographics | ||||||
| Age | A | *A | ||||
| Sex | ||||||
| Race | *A | |||||
| Ethnicity | A | * | * | |||
| Living situation | ||||||
| Education | A | |||||
| Employment | ** | A | ||||
| Pre-existing canditions | ||||||
| Cardiovascular disease | ||||||
| Hypertension | ||||||
| Diabetes | **A | **A | **A | |||
| Psychiatric disorder | *A | * | ||||
| Treatment | ||||||
| Citicoline treatment | ||||||
| Site | ** | |||||
| TBI/acute medical characteristics | ||||||
| GCS | A | **A | **A | **A | **A | **A |
| PTA | **A | **A | **A | |||
| TBI mechanism | A | |||||
| Major extracranial injury | **A | **A | **A | A | ||
| Hypotension | ||||||
| Hypoxia | ||||||
| INR | ||||||
| Hemoglobin | ** | |||||
| Glucose | ||||||
| Baseline head CT | ||||||
| Midline shift | ||||||
| Mesencephalic cisterns | *A | |||||
| SAH | A | |||||
| IPH | A | |||||
| SDH | *A | ** | ||||
| EDH | ||||||
Stopping rules for each model are designated as follows: *p < 0.1; **p < 0.05 (all variables selected with stopping rule of p < 0.05 were also selected with stopping rule of p < 0.1); A, Akaike information criterion (AIC).
PMH, past medical history; GCS, Glasgow Coma Scale; PTA, post-traumatic amnesia; TBI, traumatic brain injury; INR, international normalized ratio; SAH, subarachnoid hemorrhage; IPH, intraparenchymal hemorrhage; SDH, subdural hemorrhage; EDH, epidural hemorrhage.
Table 4.
Final Multivariable Predictor Model of Odds of Slower (“Gradual” B2) versus Faster (“Rapid” B1) Recovery
| Predictor | Level | OR (95% CI) Odds of gradual recovery | p |
|---|---|---|---|
| Age in decades | - | 1.3 (1.1–1.6) | 0.011 |
| Ethnicity | Non-Hispanic (Ref) | ||
| Hispanic | 0.5 (0.2–1.2) | 0.120 | |
| Employment | Full time (Ref) | ||
| Part time | 0.8 (0.3–1.8) | 0.910 | |
| Retired | 0.3 (0.1–0.8) | 0.020 | |
| Unemployed/disabled | 1.3 (0.6–2.9) | 0.070 | |
| GCS | 13–15 (Ref) | ||
| 9–12 | 2.6 (1.1–6.1) | 0.339 | |
| <9 | 3.1 (1.5–6.3) | 0.085 | |
| Major extracranial injury | No major extracranial injury (Ref) | ||
| Major extracranial injury | 0.6 (0.4–1.1) | 0.081 |
OR, odds ratio; CI, confidence interval; Ref, reference value; GCS, Glasgow Coma Scale.
Discussion
We successfully applied LCMM to model functional outcome trajectories in 1046 patients 18–70 years of age who presented acutely with complicated mild to severe TBI and participated in the negative COBRIT study. Given our large sample size across the spectrum of TBI severity, we were able to identify seven clinically meaningful classes of patients with distinct, and surprisingly divergent, functional recovery trajectories from 1 to 6 months post-injury, including two classes of extreme outliers with dramatic unexpected decline or dramatic rapid good recovery, which warrant further study. Our predictor selection models further identified several well-established baseline predictors of functional recovery including both pre-injury and injury characteristics, lending face validity to this approach. It is of note that although the COBRIT study's primary end-point defined good functional outcome as a 3 month GOSE of 7–8, and then incorporated this dichotomized outcome into a composite outcome metric that also incorporated dichotomized neuropsychological test results, we observed that many patients achieved substantial recovery, but did not necessarily reach a GOSE of 7 by 3 or 6 months, whereas others experienced slight decline (e.g., from 8 to 7) by 6 months. Therefore, analyzing GOSE as a cross-sectional dichotomous outcome may miscategorize clinically relevant groups of improving or declining patients.49 Our findings support the importance of conceptualizing TBI as a highly heterogeneous and evolving condition.
Data-driven latent class trajectory analysis (e.g., LCMM, LCGA, LGMM)16–18 has been applied to model TBI outcomes a handful of times over the past 10 years. Three studies have applied latent class trajectory analytics to relatively small samples of children with moderate to severe TBI (n < 100)20,28 or mild TBI (n = 186)21 to model functional, post-concussive, or cognitive trajectories over ≥1 or year. These small studies generally identified three or four latent trajectory classes, with one study reporting that worse TBI severity was a significant predictor of worse functional outcome trajectory.20 Some of the earliest studies to model individual functional outcome trajectories in adults14,15 leveraged the TBI Model Systems (TBIMS) database and used individual growth curve analysis to model GOSE and Disability Rating Scale (DRS) trajectories in a very large sample of adults with TBI admitted to acute rehabilitation hospitals. This approach, however, is different from latent class trajectory analysis, as growth models provide a group-level trajectory for the entire cohort and trajectories of subsets of patients may only be modelled by incorporating pre-specified covariates. Therefore, this approach does not allow for the “agnostic” identification and visualization of a comprehensive set of latent outcome trajectories present in the cohort.
More recently, several small (n < 250)23,25,29 and large (n = ≥ 500)24,26,27,30 studies have applied latent class trajectory analytics to model 1 year or multi-year outcome trajectories in subsets of adult TBI patients. Each of these studies identified between three and five latent classes of either functional outcome or other neurobehavioral outcome (e.g., post-concussive symptoms, headache, post-traumatic stress disorder, depression, quality of life, life satisfaction) trajectories. All of these studies investigated baseline predictors of trajectories, with several identifying demographic/pre-injury features (age, sex, race/ethnicity, employment status, Medicare/Medicaid insurance, pre-injury medical comorbidities, pre-injury headache, pre-injury psychiatric disorder, pre-injury alcohol dependence) and injury features (more severe TBI, open head injury, positive toxicology screen, hospital length of stay) as significant predictors of outcome trajectory.
Our study expands the evidence base by applying LCMM to a large clinical trial cohort with repeated assessment of GOSE just within the first 6 months post-injury, thereby allowing for highly granular delineation of 6 month functional outcome trajectories among patients with complicated mild to severe TBI. We identified seven distinct, clinically meaningful, trajectories, which is more than were identified by any prior latent class trajectory study of TBI outcomes. We identified some of the same baseline demographic/pre-injury predictors of outcome trajectory that had been identified in prior studies with longer follow-up periods, including age, race, employment status, medical comorbidities (specifically, diabetes), and pre-injury psychiatric disorder. Similar to the prior study of individual growth curve analysis of DRS from the TBIMS, we identified education as an important baseline predictor of functional outcome trajectory.14 Consonant with prior studies, we also identified a number of baseline injury features that were predictive of functional outcome trajectory including study site, GCS, PTA, TBI mechanism, major extracranial injury, hemoglobin, mesencephalic cistern compression, SAH, IPH, and SDH. The identification of site as a predictor suggests that there remains substantial heterogeneity in acute management practices across level 1 trauma centers, which may impact recovery up to 6 months post-injury.
Our logistic regression model, which allowed for determination of the direction of the effect of each predictor on functional recovery rate in our largest identified group of patients (group B), found that older age, pre-injury unemployment/disability, and lower GCS were associated with a slower recovery trajectory, lending face validity to our findings.30,35–38,50 Surprisingly, however, extracranial injury, pre-injury retirement, and Hispanic ethnicity were associated with a more rapid functional recovery trajectory. We hypothesize that the appearance of a protective effect of extracranial injury may reflect rapid improvements in global disability from extracranial injury – which may be captured by the GOSE, a global disability measure – rather than from TBI. The protective effect of retirement versus the deleterious effect of unemployment/disability highlights the potential pitfalls of examining employment as a binary variable (employed vs. unemployed). This finding is consistent with a prior study that used LCGA to characterize multi-year life satisfaction trajectories and predictors after moderate-severe TBI in TBIMS, and identified a protective effect of both worker status and leisure activities.30 Regarding the role of ethnicity, at least one prior small study has identified worse 6 month functional outcomes among Hispanic patients versus non-Hispanic white patients,51 although another small study identified lack of health insurance as the primary driver of this association.52 A large population-based study of older adults with TBI admitted to inpatient rehabilitation hospitals, however, found that Hispanic patients were more likely than non-Hispanic white or black patients to be discharged to home (rather than to an assisted living facility or other institution),53 a large TBIMS study identified lower rates of depression and higher rates of life satisfaction among Hispanic patients with TBI compared with other racial/ethnic minorities up to 2 years post-injury,54 and a single center study of nearly 400 adults with TBI identified slightly better outcomes among Hispanic versus non-Hispanic patients at hospital discharge.55 These latter studies, as well as our study, suggest a potential protective effect of Hispanic ethnicity on functional outcome after TBI, which deserves further research to determine mechanisms. Interestingly, one of the two predictors that retained statistical significance in the multivariable logistic regression model was age, such that with every 10 year increase in age, patients had 30% higher odds of having a slower (rather than rapid) recovery, even despite adjustment for ethnicity, employment, GCS, and major extracranial injury. Although this finding is consistent with prior studies from the TBIMS showing slower daily functional gains in older versus younger patients with TBI admitted to acute rehabilitation centers,56,57 mechanisms of this age-related difference in recovery trajectory deserve further study to determine whether any modifiable or targetable mechanisms exist.
Strengths of this study include the large size, generalizability to acute TBI clinical trials cohorts that may have similar inclusion criteria as the COBRIT study, and identification of seven distinct functional outcome trajectories including extreme outliers that may benefit from further intensive study of biological mechanisms. However, we recognize several limitations. The age cutoff of 70 years limits generalizability to most older adults, who now have the highest and fastest rising incidence of TBI in the United States and several other countries worldwide.58 Three of the seven trajectories identified had very small sample sizes, limiting the reliability of these estimates, and highlighting the need for replication in other studies. Injury severity as defined by the GCS was identified as an important predictor of GOSE trajectory; the construct used by GOSE to define recovery, however, may be biased toward injury severity and may not adequately capture important patient-centered metrics of recovery such as adaptive strategies and social support. A related limitation is the lack of detailed information about several potentially important predictors of 6 month outcome such as socioeconomic status, access to care, comprehensive past medical history, and pre-injury exposures. For example, <1% of the cohort reported a prior history of TBI (therefore, this predictor was not included in our analyses), suggesting likely incomplete exposure ascertainment.39 Lastly, this study is limited by lack of follow-up beyond 6 months and lack of more frequent time points, with the earliest time point being 1 month post-injury. Recently completed and ongoing cohort studies, such as the Transforming Research and Clinical Knowledge in TBI (TRACK-TBI) study that includes 2 week, 3 month, 6 month, and 12 month time points,59 will provide an opportunity for more granular, longer-term trajectory analysis across multiple outcome domains.
Conclusion
Identification of dramatically divergent trajectories among patients who had similar GOSE scores at 1 month post-injury highlights the critical importance of conceptualizing of TBI as a highly heterogeneous evolving condition. Functional end-points for TBI clinical trials that take these heterogeneous trajectories into account should be developed. For example, ordinal analysis, such as proportional odds analysis or the sliding dichotomy approach, may capture clinically relevant changes in highly heterogeneous TBI populations better than dichotomized end-points.60 Identification of several pre-injury predictors of recovery trajectory, including pre-injury employment, diabetes, and psychiatric disorders, highlight the critical importance of incorporating pre-injury health status into TBI outcome prediction models. Identification of race, ethnicity, and site as predictors of recovery trajectory warrant further research to determine underlying mechanisms of these associations such as guideline adherence variability across centers, access to care, financial resources, or social or cultural practices and preferences (both of patients/families and providers). LCMM may be a valuable tool for identifying extreme outliers that warrant further research into mechanisms of unexpectedly good or poor outcomes. Based on our findings, we therefore recommend LCMM, and other similar approaches to latent-class trajectory modeling, as powerful analytical tools to resolve heterogeneity of longitudinal outcomes and predictors in TBI populations. Given the data-driven and somewhat exploratory nature of these analytics, however, we do not recommend that they be used in prospective clinical trials to define end-points without further validation. Rather, findings from observational studies that apply these data-driven analytics to rich, multimodal, longitudinal TBI data sets may accelerate identification of important predictors, biological mechanisms, and outcomes that may then be applied to enrich, stratify, and design improved end-points for TBI clinical trials.
Citicoline Brain Injury Treatment Investigators
Ross D. Zafonte, Department of Physical Medicine and Rehabilitation, Harvard Medical School, Charlestown, MA; Emilia Bagiella, Department of Population health Science and Policy, Mount Sinai School of Medicine, New York, NY; Beth M. Ansel, Traumatic Brain Injury and Stroke Rehabilitation Program, The Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Bethesda, MD; Thomas A. Novack, Department of Physical Medicine and Rehabilitation, University of Alabama at Birmingham, Birmingham, AL; William T. Friedewald, Departments of Biostatistics and Epidemiology and Medicine, Columbia University Medical Center, New York, NY; Dale C. Hesdorffer, Department of Epidemiology, Columbia University Medical Center, New York, NY; Shelly D. Timmons, Department of Neurosurgery, Penn State University Milton S. Hershey Medical Center, Hershey, PA; Jack Jallo, Department of Neurosurgery, Division of Neurotrauma and Critical Care, Thomas Jefferson University, Jefferson Medical College, Philadelphia, PA; Howard Eisenberg, Department of Neurosurgery, University of Maryland School of Medicine, Baltimore, MD; Tessa Hart, Moss Rehabilitation Research Institute, Department of Rehabilitation Medicine, Sidney Kimmel Medical College at Thomas Jefferson University, Elkins Park, PA; Joseph H. Ricker, Departments of Rehabilitation Medicine, Psychiatry, and Radiology, New York University, Division of Psychology, Rusk Institute of Rehabilitation, New York, NY; Ramon Diaz-Arrastia, Department of Neurology, University of Pennsylvania Perelman School of Medicine, Penn Presbyterian Medical Center, Philadelphia, PA; Randall E. Merchant, Department of Anatomy and Neurobiology, Center for Rehabilitation Science and Engineering, Virginia Commonwealth University School of Medicine, Richmond, VA; Nancy R. Temkin, Departments of Neurological Surgery, Biostatistics, and Rehabilitation Medicine, University of Washington, School of Public Health; Sherry Melton, Department of Surgery, Lincoln Hospital, Bronx, NY; Sureyya S. Dikmen, Department of Rehabilitation Medicine, University of Washington, Seattle, WA; David O. Okonkwo, Department of Neurological Surgery, University of Pittsburgh Medical Center, Pittsburgh, PA
Supplementary Material
Acknowledgments
This study was supported by the National Institutes of Neurological Disorders and Stroke (Beeson K23NS095755 to R.C.G. and U01NS086090 to G.T.M.), the American Federation for Aging Research (to R.C.G.), the Weill Institute for Neurosciences (Scholar Award to R.C.G.), and the Department of Defense (W81XWH-14-2-0176 to G.T.M.). The COBRIT study was supported by the National Institute of Child Health and Human Development grants at the following institutions: Temple University (U01HD042738), University of Alabama at Birmingham (U01HD042687), University of Maryland (U01HD042736), University of Pittsburgh (U01HD042678), University of Tennessee Health Sciences Center (U01HD042686), University of Texas Southwestern (U01HD042652), University of Washington (U01HD042653), Virginia Commonwealth University (U01HD042689), and Columbia University (U01HD042823).
Contributor Information
Collaborators: the Citicoline Brain Injury Treatment Trial Investigators, Ross D. Zafonte, Emilia Bagiella, Beth M. Ansel, Thomas A. Novack, William T. Friedewald, Dale C. Hesdorffer, Shelly D. Timmons, Jack Jallo, Howard Eisenberg, Tessa Hart, Joseph H. Ricker, Ramon Diaz-Arrastia, Randall E. Merchant, Nancy R. Temkin, Sherry Melton, Sureyya S. Dikmen, and David O. Okonkwo
Author Disclosure Statement
No competing financial interests exist.
Supplementary Material
References
- 1. Taylor C.A., Bell J.M., Breiding M.J., and Xu L. (2017). Traumatic brain injury-related emergency department visits, hospitalizations, and deaths – United States, 2007 and 2013. MMWR Surveill. Summ. 66, 1–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Finkelstein E., Corso P., and Miller T. (2006). The Incidence and Economic Burden of Inuries in the United States. Oxford University Press: New York [Google Scholar]
- 3. Maas A.I., Roozenbeek B., and Manley G.T. (2010). Clinical trials in traumatic brain injury: past experience and current developments. Neurotherapeutics 7, 115–126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zafonte R.D., Bagiella E., Ansel B.M., Novack T.A., Friedewald W.T., Hesdorffer D.C., Timmons S.D., Jallo J., Eisenberg H., Hart T., Ricker J.H., Diaz-Arrastia R., Merchant R.E., Temkin N.R., Melton S., and Dikmen S.S. (2012). Effect of citicoline on functional and cognitive status among patients with traumatic brain injury: Citicoline Brain Injury Treatment Trial (COBRIT). JAMA 308, 1993–2000 [DOI] [PubMed] [Google Scholar]
- 5. Wright D.W., Yeatts S.D., Silbergleit R., Palesch Y.Y., Hertzberg V.S., Frankel M., Goldstein F.C., Caveney A.F., Howlett-Smith H., Bengelink E.M., Manley G.T., Merck L.H., Janis L.S., Barsan W.G., and Investigators N. (2014). Very early administration of progesterone for acute traumatic brain injury. N. Engl. J. Med. 371, 2457–2466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Manley G.T., and Maas A.I. (2013). Traumatic brain injury: an international knowledge-based approach. JAMA 310, 473–474 [DOI] [PubMed] [Google Scholar]
- 7. Manley G.T., MacDonald C.L., Markowitz A., Stephenson D., Robbins A., Gardner R.C., Winkler E.A., Bodien Y., Taylor S., Yue J.K., Kannan L., Kumar A., McCrea M., and Wang K. (2017). The Traumatic Brain Injury Endpoints Development (TED) Initiative: progress on a public–private regulatory collaboration to accelerate diagnosis and treatment of traumatic brain injury. J. Neurotrauma [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chu B.C., Millis S., Arango-Lasprilla J.C., Hanks R., Novack T., and Hart T. (2007). Measuring recovery in new learning and memory following traumatic brain injury: a mixed-effects modeling approach. J. Clin. Exp. Neuropsychol. 29, 617–625 [DOI] [PubMed] [Google Scholar]
- 9. Alexander S., Kerr M.E., Kim Y., Kamboh M.I., Beers S.R., and Conley Y.P. (2007). Apolipoprotein E4 allele presence and functional outcome after severe traumatic brain injury. J. Neurotrauma 24, 790–797 [DOI] [PubMed] [Google Scholar]
- 10. Christensen B.K., Colella B., Inness E., Hebert D., Monette G., Bayley M., and Green R.E. (2008). Recovery of cognitive function after traumatic brain injury: a multilevel modeling analysis of Canadian outcomes. Arch. Phys. Med. Rehabil. 89, S3–15 [DOI] [PubMed] [Google Scholar]
- 11. Noe E., Ferri J., Colomer C., Moliner B., and Chirivella J. (2010). APOE genotype and verbal memory recovery during and after emergence from post-traumatic amnesia. Brain Inj. 24, 886–892 [DOI] [PubMed] [Google Scholar]
- 12. Gould K.R., Ponsford J.L., Johnston L., and Schonberger M. (2011). The nature, frequency and course of psychiatric disorders in the first year after traumatic brain injury: a prospective study. Psychol. Med. 41, 2099–2109 [DOI] [PubMed] [Google Scholar]
- 13. Hanten G., Li X., Ibarra A., Wilde E.A., Barnes A., McCauley S.R., McCarthy J., Hoxhaj S., Mendez D., Hunter J.V., Levin H.S., and Smith D.H. (2013). Updating memory after mild traumatic brain injury and orthopedic injuries. J. Neurotrauma 30, 618–624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pretz C.R., Malec J.F., and Hammond F.M. (2013). Longitudinal description of the disability rating scale for individuals in the National Institute on Disability and Rehabilitation Research traumatic brain injury model systems national database. Arch. Phys. Med. Rehabil. 94, 2478–2485 [DOI] [PubMed] [Google Scholar]
- 15. Pretz C.R., and Dams-O'Connor K. (2013). Longitudinal description of the glasgow outcome scale-extended for individuals in the traumatic brain injury model systems national database: a National Institute on Disability and Rehabilitation Research traumatic brain injury model systems study. Arch. Phys. Med. Rehabil. 94, 2486–2493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. McArdle J.J. (2009). Latent variable modeling of differences and changes with longitudinal data. Annu. Rev. Psychol. 60, 577–605 [DOI] [PubMed] [Google Scholar]
- 17. Nagin D.S., and Odgers C.L. (2010). Group-based trajectory modeling in clinical research. Annu. Rev. Clin. Psychol. 6, 109–138 [DOI] [PubMed] [Google Scholar]
- 18. Duncan T.E., Duncan S.C., and Strycker L.A. (2006). An Introduction to Latent Variable Growth Curve Modeling: Concepts, Issues, and Application, 2nd ed. Routledge: New York [Google Scholar]
- 19. Proust-Lima C., Philipps V., and Liquet B. (2017). Estimation of extended mixed models using latent classes and latent processes: the R package lcmm. J. Stat. Soft. 78 [Google Scholar]
- 20. Fay T.B., Yeates K.O., Wade S.L., Drotar D., Stancin T., and Taylor H.G. (2009). Predicting longitudinal patterns of functional deficits in children with traumatic brain injury. Neuropsychology 23, 271–282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yeates K.O., Taylor H.G., Rusin J., Bangert B., Dietrich A., Nuss K., Wright M., Nagin D.S., and Jones B.L. (2009). Longitudinal trajectories of postconcussive symptoms in children with mild traumatic brain injuries and their relationship to acute clinical status. Pediatrics 123, 735–743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bryant R.A., Nickerson A., Creamer M., O'Donnell M., Forbes D., Galatzer-Levy I., McFarlane A.C., and Silove D. (2015). Trajectory of post-traumatic stress following traumatic injury: 6-year follow-up. Br. J. Psychiatry 206, 417–423 [DOI] [PubMed] [Google Scholar]
- 23. Sawyer K., Bell K.R., Ehde D.M., Temkin N., Dikmen S., Williams R.M., Dillworth T., and Hoffman J.M. (2015). Longitudinal study of headache trajectories in the year after mild traumatic brain injury: relation to posttraumatic stress disorder symptoms. Arch. Phys. Med. Rehabil. 96, 2000–2006 [DOI] [PubMed] [Google Scholar]
- 24. Bombardier C.H., Hoekstra T., Dikmen S., and Fann J.R. (2016). Depression trajectories during the first year after traumatic brain injury. J. Neurotrauma 33, 2115–2124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chiang C.C., Guo S.E., Huang K.C., Lee B.O., and Fan J.Y. (2016). Trajectories and associated factors of quality of life, global outcome, and post-concussion symptoms in the first year following mild traumatic brain injury. Qual. Life Res. 25, 2009–2019 [DOI] [PubMed] [Google Scholar]
- 26. Gomez R., Skilbeck C., Thomas M., and Slatyer M. (2017). Growth mixture modeling of depression symptoms following traumatic brain injury. Front. Psychol. 8, 1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Howrey B.T., Graham J.E., Pappadis M.R., Granger C.V., and Ottenbacher K.J. (2017). Trajectories of functional change after inpatient rehabilitation for traumatic brain injury. Arch. Phys. Med. Rehabil. 98, 1606–1613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Narad M.E., Treble–Barna A., Peugh J., Yeates K.O., Taylor H.G., Stancin T., and Wade S.L. (2017). Recovery trajectories of executive functioning after pediatric TBI: a latent class growth modeling analysis. J. Head Trauma Rehabil. 32, 98–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lu J., Roe C., Sigurdardottir S., Andelic N., and Forslund M.V. (2018). Trajectory of functional independent measurements during first 5 years after moderate and severe traumatic brain injury. J. Neurotrauma. 35, 1596–1603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Juengst S.B., Adams L.M., Bogner J.A., Arenth P.M., O'Neil–Pirozzi T.M., Dreer L.E., Hart T., Bergquist T.F., Bombardier C.H., Dijkers M.P., and Wagner A.K. (2015). Trajectories of life satisfaction after traumatic brain injury: Influence of life roles, age, cognitive disability, and depressive symptoms. Rehabil. Psychol 60, 353–364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Federal Interagency Traumatic Brain Injury Research (FITBIR) Informatics System National Institutes of Health, Center for Information Technology. https://fitbit.nih.gov (last accessed August24, 2018)
- 32. Wilson J.T., Pettigrew L.E., and Teasdale G.M. (1998). Structured interviews for the Glasgow Outcome Scale and the extended Glasgow Outcome Scale: guidelines for their use. J. Neurotrauma 15, 573–585 [DOI] [PubMed] [Google Scholar]
- 33. McMillan T., Wilson L., Ponsford J., Levin H., Teasdale G., and Bond M. (2016). The Glasgow Outcome Scale – 40 years of application and refinement. Nat. Rev. Neurol. 12, 477–485 [DOI] [PubMed] [Google Scholar]
- 34. Zafonte R., Friedewald W.T., Lee S.M., Levin B., Diaz–Arrastia R., Ansel B., Eisenberg H., Timmons S.D., Temkin N., Novack T., Ricker J., Merchant R., and Jallo J. (2009). The citicoline brain injury treatment (COBRIT) trial: design and methods. J. Neurotrauma 26, 2207–2216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Steyerberg E.W., Mushkudiani N., Perel P., Butcher I., Lu J., McHugh G.S., Murray G.D., Marmarou A., Roberts I., Habbema J.D., and Maas A.I. (2008). Predicting outcome after traumatic brain injury: development and international validation of prognostic scores based on admission characteristics. PLoS Med. 5, e165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Roozenbeek B., Lingsma H.F., Lecky F.E., Lu J., Weir J., Butcher I., McHugh G.S., Murray G.D., Perel P., Maas A.I., and Steyerberg E.W. International Mission on Prognosis Analysis of Clinical Trials in Traumatic Brain Injury (IMPACT) Study Group, Corticosteroid Randomisation After Significant Head Injury (CRASH) Trial Collaborators, Tranma Audit and Research Network (TARN). (2012). Prediction of outcome after moderate and severe traumatic brain injury: external validation of the International Mission on Prognosis and Analysis of Clinical Trials (IMPACT) and Corticoid Randomisation After Significant Head injury (CRASH) prognostic models. Crit. Care Med. 40, 1609–1617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. MRC CRASH Trial Collaborators, Perel P., Arango M., Clayton T., Edwards P., Komolafe E., Poccock S., Roberts I., Shakur H., Steyerberg E. and Yutthakasemsunt S. (2008). Predicting outcome after traumatic brain injury: practical prognostic models based on large cohort of international patients. BMJ 336, 425–429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lingsma H.F., Yue J.K., Maas A.I., Steyerberg E.W., Manley G.T., and TRACK-TBI Investigators (2015). Outcome prediction after mild and complicated mild traumatic brain injury: external validation of existing models and identification of new predictors using the TRACK–TBI pilot study. J. Neurotrauma 32, 83–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Whiteneck G.G., Cuthbert J.P., Corrigan J.D., and Bogner J.A. (2016). Prevalence of self-reported lifetime history of traumatic brain injury and associated disability: a statewide population-based survey. J. Head Trauma Rehabil. 31, E55–62 [DOI] [PubMed] [Google Scholar]
- 40. StataCorp (2017). Stata Statistical Software: Release 15. StataCorp LLC: College Station, TX [Google Scholar]
- 41. SAS Institute., S (2017). SAS Software. SAS Institute Inc.: Cary, NC [Google Scholar]
- 42. R Development Care Team. (2010). R: A language and environment for statistical computing. R Foundation for Statistical Computing: Vienna, Austria [Google Scholar]
- 43. Little R.J.A. (1995). Modeling the drop–out mechanism in repeated–measures studies. J. Am. Stat. Assoc. 90, 1112 [Google Scholar]
- 44. Strauss V.Y., Jones P.W., Kadam U.T., and Jordan K.P. (2014). Distinct trajectories of multimorbidity in primary care were identified using latent class growth analysis. J. Clin. Epidemiol. 67, 1163–1171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Weiner J. (2017). Riverplot: Sankey or Ribbon Plots. R package version 0.6. https://CRAN.R-projects.org/package=riverplot (last accessed March13, 2018)
- 46. Cheng J., Edwards L.J., Maldonado–Molina M.M., Komro K.A., and Muller K.E. (2010). Real longitudinal data analysis for real people: building a good enough mixed model. Stat. Med. 29, 504–520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Nuamah I.F., Qu Y., and Amini S.B. (1996). A SAS macro for stepwise correlated binary regression. Comput. Methods Programs Biomed. 49, 199–210 [DOI] [PubMed] [Google Scholar]
- 48. The LOGISTIC Procedure, in: SAS OnlineDoc: Version 8. http://www.math.wpi.edu/sos.pdf/stat/chap39.pdf (last accessed December18, 2018)
- 49. Nelson L.D., Ranson J., Ferguson A.R., Giacino J., Okonkwo D.O., Valadka A., Manley G., and McCrea M. (2017). Validating multidimensional outcome assessment using the TBI Common Data Elements: An analysis of the TRACK–TBI pilot sample. J. Neurotrauma [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Yue J.K., Rick J.W., Morrissey M.R., Taylor S.R., Deng H., Suen C.G., Vassar M.J., Cnossen M.C., Lingsma H.F., Yuh E.L., Mukherjee P., Gardner R.C., Valadka A.B., Okonkwo D.O., Cage T.A., Manley G.T., and TRACK–TBI Investigators (2018). Preinjury employment status as a risk factor for symptomatology and disability in mild traumatic brain injury: a TRACK–TBI analysis. NeuroRehabilitation 43, 169–182 [DOI] [PubMed] [Google Scholar]
- 51. Staudenmayer K.L., Diaz–Arrastia R., de Oliveira A., Gentilello L.M., and Shafi S. (2007). Ethnic disparities in long–term functional outcomes after traumatic brain injury. J. Trauma 63, 1364–1369 [DOI] [PubMed] [Google Scholar]
- 52. Shafi S., Marquez de la Plata C., Diaz–Arrastia R., Shipman K., Carlile M., Frankel H., Parks J., and Gentilello L.M. (2007). Racial disparities in long–term functional outcome after traumatic brain injury. J. Trauma 63, 1263–1270 [DOI] [PubMed] [Google Scholar]
- 53. Chang P.F., Ostir G.V., Kuo Y.F., Granger C.V., and Ottenbacher K.J. (2008). Ethnic differences in discharge destination among older patients with traumatic brain injury. Arch. Phys. Med. Rehabil. 89, 231–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Perrin P.B., Krch D., Sutter M., Snipes D.J., Arango–Lasprilla J.C., Kolakowsky–Hayner S.A., Wright J., and Lequerica A. (2014). Racial/ethnic disparities in mental health over the first 2 years after traumatic brain injury: a model systems study. Arch. Phys. Med. Rehabil. 95, 2288–2295 [DOI] [PubMed] [Google Scholar]
- 55. Sorani M.D., Lee M., Kim H., Meeker M. and Manley G.T. (2009). Race\ethnicity and outcome after traumatic brain injury at a single, diverse center. J. Trauma 67, 75–80 [DOI] [PubMed] [Google Scholar]
- 56. Cifu D.X., Kreutzer J.S., Marwitz J.H., Rosenthal M., Englander J., and High W. (1996). Functional outcomes of older adults with traumatic brain injury: a prospective, multicenter analysis. Arch. Phys. Med. Rehabil. 77, 883–888 [DOI] [PubMed] [Google Scholar]
- 57. Frankel J.E., Marwitz J.H., Cifu D.X., Kreutzer J.S., Englander J., and Rosenthal M. (2006). A follow–up study of older adults with traumatic brain injury: Taking into account decreasing length of stay. Arch. Phys. Med. Rehabil. 87, 57–62 [DOI] [PubMed] [Google Scholar]
- 58. Gardner R.C., Dams–O'Connor K., Morrissey M.R., and Manley G.T. (2018). Geriatric traumatic brain injury: epidemiology, outcomes, knowledge gaps, and future directions. J. Neurotrauma [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Transforming Research and Clinical Knowledge in Traumatic Brain Injury (TRACK–TBI). https://tracktbi.ucsf.edu/transforming-research-and-clinical-knowledge-tbi (last accessed August24, 2018)
- 60. Roozenbeek B., Lingsma H.F., Perel P., Edwards P., Roberts I., Murray G.D., Maas A.I., Steyerberg E.W., Impact Study Group, and CRASH Trial Collaborators. (2011). The added value of ordinal analysis in clinical trials: an example in traumatic brain injury. Crit. Care 15, R127. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.