Abstract
Researchers frequently study the fruit fly Drosophila melanogaster as a model system for mammalian development and behavior. Drosophila appear resistant to alcohol’s toxic effects and display many behaviors resembling intoxication (e.g., impaired motor control) when exposed to alcohol vapors. Accordingly, investigators have begun to measure alcohol sensitivity in Drosophila and to identify genetic mutations associated with increased or decreased sensitivity. One mutant called cheapdate affects a signaling system that plays a role in many regulatory processes in a cell and which involves the compound cyclic adenosine monophosphate (cAMP). Additional Drosophila mutants with altered alcohol sensitivity carry mutations in other components of the cAMP signaling system. Because the cAMP system also is affected in human alcoholics, these results indicate that studies using Drosophila as a model system may identify genetic changes relevant to human alcoholism.
Keywords: animal model, Drosophila melanogaster, AOD sensitivity, cAMP, mutation, adenylate cyclase, genetic trait, animal strains
The fruit fly Drosophila melanogaster is one of the most widely used and successful genetic model systems for studying development and behavior. The usefulness of this model system is based on the fact that the genes and biochemical pathways underlying development and behavior have largely been conserved during evolution. As a result, many genes first identified in Drosophila have provided major insights into human and other vertebrate development and disease. Drosophila has a relatively sophisticated nervous system and is capable of many complex behaviors. For example, the flies can learn to associate certain events and to remember that association (Davis 1996; Dubnau and Tully 1998). Furthermore, they have sophisticated courtship behaviors (O’Dell and Kaiser 1997). Another advantage of Drosophila is that they are easy to rear and have a generation time of only approximately 2 weeks, allowing researchers to explore the heritability of certain traits or behaviors over many generations in a short period of time.
Nearly a century of intense genetic research on Drosophila has generated innumerable and sophisticated genetic tools, such as chromosomes that carry mutations resulting in visible traits (i.e., phenotypes) that can be used to map novel mutations. Subsequently, such genetic techniques as germ-line transformation1 (Rubin and Spradling 1982), use of transposable elements as mutagens (Engels 1983), transposon tagging (Bingham et al. 1981), and targeted gene disruption (Rong and Golic 2000) have allowed researchers to conduct molecular and reverse genetic analyses. Finally, the sequence of the Drosophila genome has recently been published, and its analysis has revealed further similarities between flies and mammals (Adams et al. 2000).
Drosophila have been used extensively to investigate developmental processes and to study nervous system function. In doing so, researchers have identified many Drosophila gene products for whom the corresponding mammalian gene products (i.e., the mammalian homologs) have been implicated as potential targets for alcohol. For example, flies carry homologs of the mammalian receptors for the brain chemicals (i.e., neurotransmitters) gamma-aminobutyric acid, serotonin, dopamine, and glutamate (Littleton and Ganetzky 2000), all of which have been implicated in alcohol’s actions (Tabakoff and Hoffman 1996). Consequently, Drosophila may be a suitable animal model to study alcohol’s effects on brain function and alcohol-related behaviors, such as sensitivity and tolerance to alcohol’s effects. This article describes experimental designs to measure alcohol sensitivity and reviews the initial results of alcohol-related genetic research in Drosophila.
Measuring Alcohol Sensitivity in Drosophila
The natural habitat of Drosophila includes fermenting plants, which often contain high alcohol levels (i.e., 3 or more percent). Accordingly, fruit flies are resistant to alcohol’s toxic effects and can metabolize alcohol efficiently for use as an energy source or as a starting material (i.e., substrate) for the production of lipids (Geer et al. 1993). To test whether a particular Drosophila strain is resistant to alcohol’s toxic effects, researchers add alcohol to the culture medium serving as the flies’ food (Geer et al. 1993). Such analyses found that Drosophila strains isolated from the wild differ in their resistance to alcohol (Kamping and van Delden 1978; David and Van Herrewege 1993). In addition, researchers found that they could quickly and substantially increase a Drosophila population’s resistance to alcohol in the laboratory. For example, resistant strains were obtained by selectively breeding flies that survived exposure to high alcohol levels in their food (Chambers 1991) or were resistant to the effects of alcohol vapor (Cohan and Hoffman 1986; Weber and Diggins 1990).
When exposed to alcohol vapor, adult Drosophila display many behaviors resembling acute intoxication in mammals, such as impaired motor control. To measure alcohol’s effects on locomotion, researchers place flies into a small chamber that is covered with grid lines. Locomotor behavior is measured by counting the number of lines of the grid crossed as a function of time (Bainton et al. 2000; Singh and Heberlein in press). Within a few minutes of exposure, the flies become hyperactive and disoriented and then uncoordinated and sedated. After approximately 20 minutes of exposure, they become immobile, but recover 5 to 10 minutes after the alcohol is withdrawn (Singh and Heberlein in press).
Studies found that in rodents, alcohol’s locomotor-stimulating effects are modulated by nerve-cell systems using the neurotransmitter dopamine (i.e., dopaminergic systems) (Phillips and Shen 1996). To explore a potential role for dopamine in alcohol responses in Drosophila, researchers tested flies with severely reduced dopamine levels for alcohol-induced changes in locomotor behavior using the test described above. In these flies, alcohol’s ability to induce locomotor activation was significantly reduced; however, alcohol-induced sedation was normal (Bainton et al. 2000). These data suggest that dopaminergic systems play a similar role in the responses of rodents and Drosophila to acute alcohol exposure.
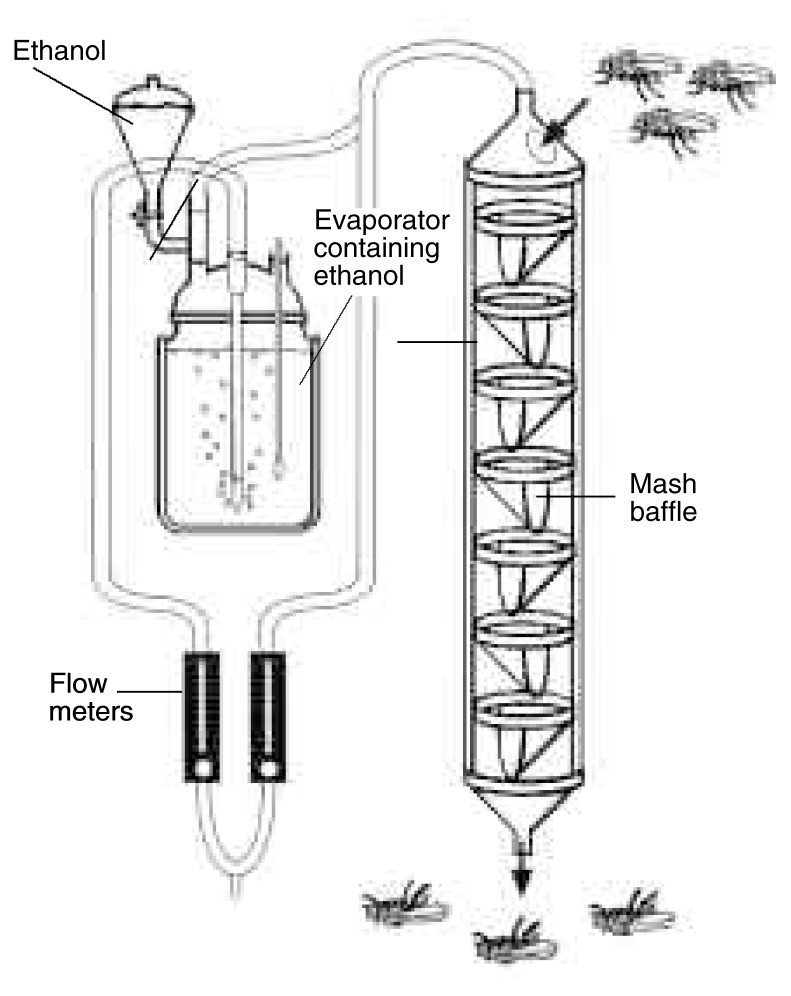
Another approach to measuring alcohol’s effects on Drosophila is to determine their inability to stand (i.e., loss of postural control) after alcohol intake using an inebriometer. This instrument, which was designed originally to selectively breed alcohol-resistant flies (Weber and Diggins 1990), consists of a 125-cm long vertical tube containing a series of sloping mesh baffles on which flies can stand (see figure). The flies are placed at the top of the tube. Then, alcohol vapor is circulated through the tube, causing the flies to become intoxicated. As a result, the flies lose postural control and begin to fall through the tube. Their sensitivity to alcohol intoxication is measured by the time required for them to emerge from the bottom of the tube at a particular alcohol concentration. Thus, flies that are more sensitive to alcohol-induced loss of postural control emerge from the tube after a shorter time than do flies that are more resistant to alcohol’s effects.
The inebriometer is an apparatus that is used to measure the sensitivity of Drosophila to alcohol vapor. Approximately 100 flies are introduced into the top of a 4-foot glass column through which a controlled concentration of alcohol vapor circulates. As they become intoxicated, the flies progressively lose postural control and tumble downwards; their fall is impeded by their ability to cling to oblique mesh baffles distributed along the length of the column. The time required for the flies to emerge at the bottom of the column is a measure of their alcohol sensitivity.
Genetic Analyses of Alcohol-Related Traits
Researchers have conducted genetic analyses in Drosophila to identify mutations that alter sensitivity to alcohol intoxication (Singh and Heberlein in press). To this end, flies were treated with a chemical (i.e., ethyl methane sulfonate) that induces lesions in the DNA. The researchers then tested many thousands of potentially mutant flies in the inebriometer to identify those strains that exhibited altered alcohol-induced behaviors. This approach identified mutants that displayed either increased or decreased sensitivity to a single alcohol exposure (Singh and Heberlein in press).
Two of these mutant strains were named tipsy and barfly to reflect their increased and reduced sensitivity to alcohol, respectively. Both these mutant strains responded normally when tested for alcohol-induced locomotor activation. However, tipsy flies became sedated at alcohol concentrations that were lower than those needed to sedate normal (i.e., wild-type) flies. Conversely, barfly mutants required higher alcohol doses than did wild-type flies to achieve sedation (Singh and Heberlein in press). These results suggest that the genes that control alcohol’s activating effects can differ from the genes regulating alcohol’s sedative effects. Analyses of well-established rodent models for alcohol-induced behaviors have led to similar conclusions (Shen et al. 1996). The identification of the genes disrupted by these mutations in flies should help shed light onto the mechanisms by which acute alcohol exposure modulates behavior.
An alternative approach to generate mutations in Drosophila involves the use of a transposable element (i.e., a short piece of DNA named P-element) that integrates randomly into the flies’ DNA and inactivates any genes located near the integration site (Engels 1983). One mutant generated using this method that shows increased sensitivity to alcohol was called cheapdate (Moore et al. 1998). Further analysis demonstrated that cheapdate flies carry a mutation in a gene called amnesiac, which was identified originally because of its role in learning and memory (Quinn et al. 1979). The amnesiac gene is believed to encode a neuropeptide that shows some similarity to a vertebrate peptide that activates the enzyme adenylate cyclase (AC) (Feany and Quinn 1995). AC promotes the formation of a compound called cyclic adenosine monophosphate (cAMP), which is involved in numerous regulatory and signaling pathways in the cell.
In addition to the cheapdate mutant, other findings from Drosophila studies indicate that the cAMP signaling system appears to play a role in mediating alcohol’s effects. Thus, Drosophila mutants with disruptions in the gene encoding a certain type of AC also display increased sensitivity to alcohol (Moore et al. 1998). Furthermore, researchers recently reported that a Drosophila strain carrying a mutation in a gene that encodes part of an enzyme called cAMP-regulated protein kinase (PKA–RII) is more resistant to the acute effects of alcohol than are wild-type flies (Park et al. 2000). Interestingly, mice carrying a mutation in the homologous PKA–RII gene also show increased alcohol resistance and voluntarily consume greater amounts of alcohol (Thiele et al. 2000).
Taken together, these findings indicate that mutations that disrupt the flies’ ability to properly regulate cAMP levels also affect their sensitivity to alcohol. This observation is particularly interesting, because studies in humans found that alcoholics exhibit reduced levels of AC activity (Diamond et al. 1987; Tabakoff et al. 1988) and that the cAMP signaling pathway is sensitive to alcohol’s effects (Diamond and Gordon 1997). Finally, the finding that disruption of a single gene (i.e., PKA–RII) leads to similar alterations in the alcohol responses of mice and flies provides provocative evidence for evolutionary conservation of the underlying molecular mechanisms.
Conclusions
The particular forward genetic approach described in this article can potentially result in the isolation of all genes that influence alcohol-related behaviors. Therefore, this approach is a powerful means of obtaining unbiased information about the mechanisms underlying those behaviors. Initial analyses suggest that this approach can indeed identify genes contributing to alcohol-related biochemical phenotypes (e.g., genes influencing the AC pathway) and, therefore, is viable and relevant. However, it is too soon to speculate whether these genes will provide clues to alcohol addiction in humans.
The identification of Drosophila genes that disrupt alcohol-related behaviors provides at least two potential benefits. First, identifying these genes could facilitate studies in vertebrates, particularly humans, because the vertebrate homologs could be investigated as potential candidate genes. Second, such analyses could provide researchers with tools to investigate in Drosophila the mechanisms underlying alcohol-induced behaviors in higher organisms. For example, identifying novel genes associated with alcohol-related phenotypes in mammals or humans may provide few clues for the development of therapy because of the difficulties of studying these genes in complex mammalian systems. The introduction of such genes into Drosophila, however, might allow researchers to identify the mechanism through which the novel gene acts in a simpler model system.
Footnotes
For a definition of this and other technical terms used in this article, please see the central glossary, pp. 193–195.
References
- Adams MD, Celniker SE, Holt RA, et al. The genome sequence of Drosophilamelanogaster. Science. 2000;287:2185–2195. doi: 10.1126/science.287.5461.2185. [DOI] [PubMed] [Google Scholar]
- Bainton RJ, Tsai LT-Y, Singh CM, et al. Dopamine modulates acute responses to cocaine, nicotine, and ethanol in Drosophila. Current Biology. 2000;10:187–194. doi: 10.1016/s0960-9822(00)00336-5. [DOI] [PubMed] [Google Scholar]
- Bingham PM, Lewis R, Rubin GM. Cloning of DNA sequences from the white locus of D. melanogaster by a novel and general method. Cell. 1981;25:693–704. doi: 10.1016/0092-8674(81)90176-8. [DOI] [PubMed] [Google Scholar]
- Chambers GK. Gene expression, adaptation and evolution in higher organisms. Evidence from studies of Drosophila alcohol dehydrogenases. Comparative Biochemistry and Physiology. 1991;99B:723–730. doi: 10.1016/0305-0491(91)90135-z. [DOI] [PubMed] [Google Scholar]
- Cohan FM, Hoffman AA. Genetic divergence under uniform selection. II. Different responses to selection for knockdown resistance to ethanol among Drosophila melanogaster populations and their replicate lines. Genetics. 1986;114:145–163. doi: 10.1093/genetics/114.1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David JR, Van Herrewege J. Adaptation to alcoholic fermentation in Drosophila species: Relationship between alcohol tolerance and larval habitat. Comparative Biochemistry and Physiology. 1993;74A:283–288. [Google Scholar]
- Davis RL. Physiology and biochemistry of Drosophila learning mutants. Physiological Reviews. 1996;76:299–317. doi: 10.1152/physrev.1996.76.2.299. [DOI] [PubMed] [Google Scholar]
- Diamond I, Gordon AS. Cellular and molecular neuroscience of alcoholism. Physiological Reviews. 1997;77:1–20. doi: 10.1152/physrev.1997.77.1.1. [DOI] [PubMed] [Google Scholar]
- Diamond I, Wrubel B, Estrin E, Gordon AS. Basal and adenosine receptor-stimulated levels of cAMP are reduced in lymphocytes from alcoholic patients. Proceedings of the National Academy of Sciences of the USA. 1987;84:1413–1416. doi: 10.1073/pnas.84.5.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubnau J, Tully T. Gene discovery in Drosophila: New insights for learning and memory. Annual Review of Neuroscience. 1998;21:407–444. doi: 10.1146/annurev.neuro.21.1.407. [DOI] [PubMed] [Google Scholar]
- Engels WR. The P family of transposable elements in Drosophila. Annual Review of Genetics. 1983;17:315–344. doi: 10.1146/annurev.ge.17.120183.001531. [DOI] [PubMed] [Google Scholar]
- Feany MB, Quinn WG. A neuropeptide gene defined by the Drosophila memory mutant amnesiac. Science. 1995;268:869–873. doi: 10.1126/science.7754370. [DOI] [PubMed] [Google Scholar]
- Geer BW, Heinstra PWH, McKechnie SW. The biological basis of ethanol tolerance in Drosophila. Comparative Biochemistry and Physiology. 1993;105B:203–229. doi: 10.1016/0305-0491(93)90221-p. [DOI] [PubMed] [Google Scholar]
- Kamping A, van Delden DW. Alcohol dehydrogenase polymorphism in populations of Drosophilamelanogaster. II. Relation between ADH activity and adult mortality. Biochemical Genetics. 1978;16:541–551. doi: 10.1007/BF00484218. [DOI] [PubMed] [Google Scholar]
- Littleton JT, Ganetzky B. Ion channels and synaptic organization: Analysis of the Drosophila genome. Neuron. 2000;26:35–43. doi: 10.1016/s0896-6273(00)81135-6. [DOI] [PubMed] [Google Scholar]
- Moore MS, DeZazzo J, Luk AY, et al. Ethanol intoxication in Drosophila: Genetic and pharmacological evidence for regulation by the cAMP signaling pathway. Cell. 1998;93:997–1007. doi: 10.1016/s0092-8674(00)81205-2. [DOI] [PubMed] [Google Scholar]
- O’Dell KM, Kaiser K. Sexual behaviour: Secrets and flies. Current Biology. 1997;7:R345–R347. doi: 10.1016/s0960-9822(06)00170-9. [DOI] [PubMed] [Google Scholar]
- Park SK, Sedore SA, Cronmiller C, Hirsh J. PKA-RII-deficient Drosophila are viable but show developmental, circadian and drug response phenotypes. Journal of Biological Chemistry. 2000;275:20588–20596. doi: 10.1074/jbc.M002460200. [DOI] [PubMed] [Google Scholar]
- Phillips TJ, Shen EH. Neurochemical bases of locomotion and ethanol stimulant effects. International Reviews of Neurobiology. 1996;39:243–282. doi: 10.1016/s0074-7742(08)60669-8. [DOI] [PubMed] [Google Scholar]
- Quinn WG, Sziber PP, Booker R. The Drosophila memory mutant amnesiac. Nature. 1979;277:212–214. doi: 10.1038/277212a0. [DOI] [PubMed] [Google Scholar]
- Rong YS, Golic KG. Gene targeting by homologous recombination in Drosophila [see comments] Science. 2000;288:2013–2018. doi: 10.1126/science.288.5473.2013. [DOI] [PubMed] [Google Scholar]
- Rubin GM, Spradling AC. Genetic transformation of Drosophila with transposable element vectors. Science. 1982;218:348–353. doi: 10.1126/science.6289436. [DOI] [PubMed] [Google Scholar]
- Shen EH, Dorow JD, Huson M, Phillips TJ. Correlated responses to selection in FAST and SLOW mice: Effects of ethanol on ataxia, temperature, sedation, and withdrawal. Alcoholism: Clinical and Experimental Research. 1996;20:688–696. doi: 10.1111/j.1530-0277.1996.tb01673.x. [DOI] [PubMed] [Google Scholar]
- Singh CM, Heberlein U. Genetic control of acute ethanol-induced behaviors in Drosophila. Alcoholism: Clinical and Experimental Research. In press. [PubMed] [Google Scholar]
- Tabakoff B, Hoffman PL. Alcohol addiction: An enigma among us. Neuron. 1996;16:909–912. doi: 10.1016/s0896-6273(00)80113-0. [DOI] [PubMed] [Google Scholar]
- Tabakoff B, Hoffman PL, Lee JM, et al. Differences in platelet enzyme activity between alcoholics and nonalcoholics. New England of Journal of Medicine. 1988;318:134–139. doi: 10.1056/NEJM198801213180302. [DOI] [PubMed] [Google Scholar]
- Thiele TE, Willis B, Stadler J, et al. High ethanol consumption and low sensitivity to ethanol-induced sedation in protein kinase A-mutant mice. Journal of Neuroscience Online. 2000;20:RC75. doi: 10.1523/JNEUROSCI.20-10-j0003.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber KE, Diggins LT. Increased selection response in larger populations. II: Selection for ethanol vapor resistance in Dropsophia melanogaster at two population sizes. Gentics. 1990;125:585–597. doi: 10.1093/genetics/125.3.585. [DOI] [PMC free article] [PubMed] [Google Scholar]