Abstract
Objective
To determine the overall effectiveness of instrument-assisted soft tissue mobilization (IASTM) in improving range of motion (ROM), pain, strength, and patient-reported function in order to provide recommendations for use. We also sought to examine the influence of IASTM on injured and healthy participants, body part treated, and product used.
Data Sources
We searched the Academic Search Premier, Alt Healthwatch, CINAHL Complete, Cochrane Library, MEDLINE with full text, NLM PubMed, Physical Education Index, Physiotherapy Evidence Database (PEDro), SPORTDiscus with full text, and Web of Science databases for articles published from 1997 through 2016. The Boolean string advantEDGE OR astym OR graston OR iastm OR “instrument assist* soft tissue mobil*” OR “augment* soft tissue mobil*” OR “myofascial release” OR “instrument assist* massage” OR “augment* massage” OR “instrument assist* cross fiber massage” was used.
Study Selection
Included articles were randomized controlled trials that measured ROM, pain, strength, or patient-reported function and compared IASTM treatment with at least 1 other group.
Data Extraction
Thirteen articles met the inclusion criteria. Four independent reviewers assessed study quality using the PEDro and Centre for Evidence-Based Medicine scales. Twelve articles were included in the effect-size analysis.
Data Synthesis
The average PEDro score for studies of uninjured participants was 5.83 (range = 5 to 7) and that for studies of injured participants was 5.86 (range = 3 to 7). Large effect sizes were found in outcomes for ROM (uninjured participants), pain (injured participants), and patient-reported function (injured participants). The different IASTM tools used in these studies revealed similar effect sizes in the various outcomes.
Conclusions
The current literature provides support for IASTM in improving ROM in uninjured individuals as well as pain and patient-reported function (or both) in injured patients. More high-quality research involving a larger variety of patients and products is needed to further substantiate and allow for generalization of these findings.
Keywords: soft tissue therapy, critical summary, clinical meaningfulness
Instrument-assisted soft tissue mobilization (IASTM) is the use of hard tools to manipulate soft tissue and was derived from the Cyriax1 cross-friction massage.2 It has recently emerged as a popular alternative to traditional manual therapy techniques, but the first controlled IASTM study3 was published in 1997. Similar to massage, the motions used during IASTM treatments vary in direction, force, and pattern and allow for pressure to be dispersed to the underlying tissues.3 Modern-day IASTM instruments vary in material (eg, stainless steel, plastic) and design2 and are used to improve a variety of musculoskeletal conditions and associated outcomes.4–6 As such, many IASTM instruments, companies, and proposed application protocols, including ASTYM (Performance Dynamics, Muncie, IN),7 Fascial Abrasion Technique (FIT Institute, Niagara Falls, ON, Canada),8 Graston Technique (Indianapolis, IN),9 and HawkGrips (Conshohocken, PA),10 to name a few, exist.
Despite instrument and protocol variability, all of these techniques and companies plus others11,12 fall under the IASTM umbrella13 and refer to the same studies that have found IASTM facilitates the healing process through increased fibroblast proliferation3,14 and increased collagen synthesis, maturation, and alignment.15,16 The IASTM literature has been inundated with successful case studies and case series (level 4 research).17 It can therefore be tedious for a clinician to sift through the vast array of published works to determine best practices. Recently, Cheatham et al18 published an IASTM systematic review; however, their search was limited in study selection, and the findings were inconclusive because of variability in study designs. Additionally, Lambert et al19 conducted a systematic review of the effects of IASTM compared with other interventions but only examined the clinical outcomes of pain and function. Given these limitations and continued additions to the literature, the purpose of our study was to conduct a comprehensive systematic review of the effects of IASTM on range of motion (ROM), pain, strength, and patient-reported function. Furthermore, because of the variability in designs reported by Cheatham et al,18 we performed an effect-size analysis to further determine IASTM's effectiveness, provide recommendations for use, and guide future research.
METHODS
Data Sources and Searches
We conducted the literature search on September 15, 2016, using the following databases: Academic Search Premier, Alt Healthwatch, CINAHL Complete, Cochrane Library, MEDLINE with full text, NLM PubMed, Physical Education Index, Physiotherapy Evidence Database (PEDro), SPORTDiscus with full text, and the Web of Science. The Boolean string advantEDGE OR astym OR graston OR iastm OR “instrument assist* soft tissue mobil*” OR “augment* soft tissue mobil*” OR “myofascial release” OR “instrument assist* massage” OR “augment* massage” OR “instrument assist* cross fiber massage” was used. We used the name brands Graston Technique, ASTYM, and AdvantEDGE (the original name of ASTYM) as search terms because they were commonly mentioned in articles used for the preliminary literature review. The remaining terms were included as they represent the many synonyms and variations of the term IASTM.
Study Selection
Articles were included if they met all of the following: (1) the study was a randomized controlled trial; (2) ROM, pain, strength, or patient-reported function was measured preintervention and postintervention; (3) the article was written in English; (4) human participants were assessed; and (5) IASTM was examined as an intervention and compared with at least 1 other group not receiving IASTM. Articles were excluded if (1) the randomization methods were not clear or (2) foam rolling or self-myofascial release was studied as the main intervention. The first controlled study3 on IASTM was published in 1997; therefore, all articles published before 1997 were excluded.
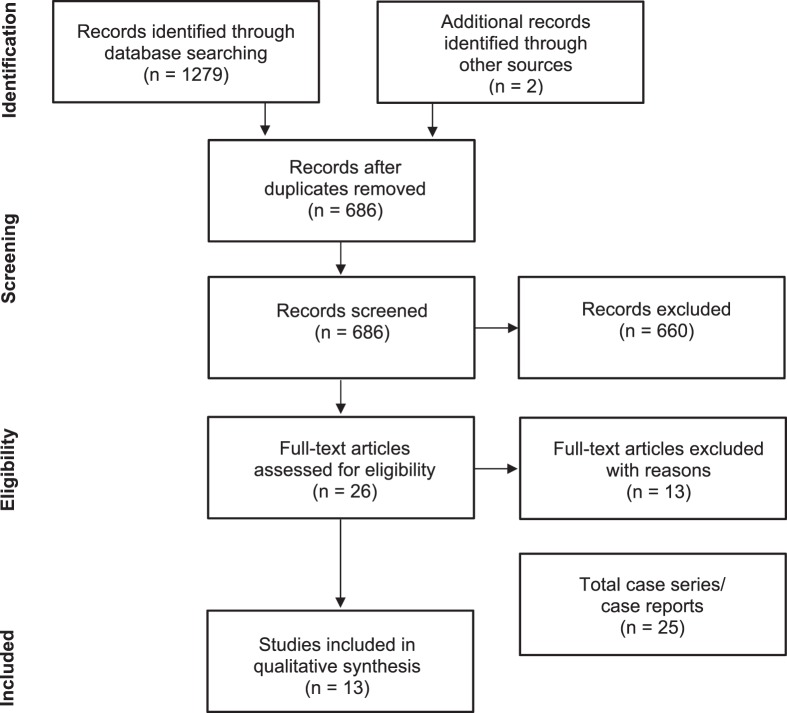
The primary reviewer (C.B.S.) conducted the comprehensive literature search. All records were exported into EndNote (version X7; Clarivate Analytics, Philadelphia, PA).20 Once all records were imported, duplicates were removed. Titles and abstracts were then screened for potential eligibility by the primary reviewer. Once screened, remaining articles were retrieved in full text and reassessed for the inclusion and exclusion criteria. The reference lists of all 26 full-text articles and 3 manufacturer Web sites were manually searched to identify any additional articles not located through the electronic database search. If the primary reviewer was unsure whether a study should be included, a second author (A.M.G.S.) was consulted. The Figure provides an overview of the study-selection process.
Figure.
Screening process shown in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow chart, with an additional section for instrument-assisted soft tissue mobilization case series and reports.
Data Extraction
Primary data extraction was performed by the lead researcher (C.B.S.) and the following characteristics were entered into a spreadsheet: author, year, pathology or body region treated, study aim, participants, study design, experimental groups, follow-up period, participant withdrawal, outcome scales, all results, effect size reported (if provided), power analysis (if conducted a priori), and product used. A second author (A.M.G.S.) confirmed the accuracy of the extracted data.
Secondary data extraction for the effect-size calculation was also performed by the lead researcher (C.B.S.) and resulted in pretreatment and posttreatment values for all outcomes at every time point measured in the IASTM groups. Author A.M.G.S. confirmed the accuracy of the extracted data.
Quality Assessment
The PEDro Scale is an objective assessment of internal validity and is the most appropriate scale for comprehensively assessing RCTs.21 Therefore, it was our primary method of quality assessment. We further rated studies using the Centre for Evidence-Based Medicine (CEBM) levels of evidence.17 The CEBM levels of evidence are meant to provide a quick appraisal of the best evidence for different outcomes.17 These were used to assist in clinical recommendations.
Four independent reviewers (C.B.S., A.M.G.S., and 2 nonauthors) assessed the quality of the included studies using the PEDro Scale. The same 4 independent reviewers then assessed each study using the CEBM levels of evidence. After independent scoring was complete, the primary reviewers (C.B.S., A.M.G.S.) met to determine a consensus score for each article. Any disputes in the independent assessment were settled by consensus of the 2 remaining authors (N.M.C., M.A.R.). Lastly, we searched the PEDro Web site22 to ensure that our scores were consistent with those formally assessed and confirmed in the database.
Data Synthesis and Analysis
After all data were extracted, a main table was created. To allow for ease of readability and comparison, we organized studies by the uninjured or injured classification and then further subdivided by body part or region. The separation based on uninjured or injured classification allowed for better readability and took into consideration the fact that healthy and injured tissues react differently to manual therapies.23 The following characteristics were then transferred from the spreadsheet: author, year, pathology or region treated, number and characterization of participants, outcomes measured, experimental groups, major results, and product used. The PEDro scores were also included for reference.
Effect sizes were calculated to examine the magnitude of treatment and comparison outcomes24 and standardize results, permitting comparisons over time across a variety of studies and outcome measures.24 The Cohen d was used to calculate the effect size for each time point reported, using the following formula25–27:
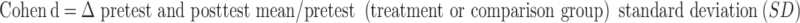 |
A 95% confidence interval (CI) for each effect size was also calculated using the following formula:
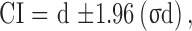 |
where σd = 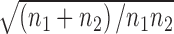 + [ES2/(2n1n2)], σ is the SD, and n is the sample size.28
+ [ES2/(2n1n2)], σ is the SD, and n is the sample size.28
Cohen created a scale to qualify effect size, in which effect sizes of 0.2 are considered to be small; 0.5, moderate; and 0.8, large.29 However, this scale was created for psychological studies in which small effects can have profound consequences.24,26 Because of the nature of the outcomes included in this study, we used Rhea categories of effect size to describe the calculated Cohen d effect sizes. Rhea26 proposed 3 variations (1 for untrained, 1 for recreationally trained, and 1 for highly trained athletes) of this scale that are meant to be applied to studies that require larger effect sizes to achieve clinically meaningful results. For qualifying the effect sizes of outcomes such as ROM, use of the middle-range scale is recommended, in which effect sizes <0.35 are trivial, 0.35 to 0.79 are small, 0.80 to 1.50 are moderate, and >1.50 are large.24,25 After calculations, comparison and treatment group categorical designations were compared by time point; when the treatment-group category value exceeded the comparison-group value (eg, trivial in comparison versus moderate in treatment), it was deemed clinically meaningful.
RESULTS
Study Selection
The initial search yielded 1279 articles, plus 2 articles found via a hand search of the major manufacturer Web sites: ASTYM, Graston Technique, and HawkGrips. After the lead author (C.B.S.) screened for duplicates, a total of 686 articles remained. Titles, key words, and abstracts were then screened for the inclusion and exclusion criteria, leaving 26 articles. Four studies30–33 were excluded because the authors did not assess IASTM. Five studies were excluded because of study design: 3 cohort studies,5,34,35 1 nonrandomized controlled trial,36 and 1 case series.37 One study38 was excluded because IASTM was used as the control rather than the intervention. Another 2 studies39,a were excluded because they did not assess any of the outcomes required for this systematic review. A final record40 was excluded because it was a presentation and therefore not a full-text article. After full-text screening, 13 articles4,6,41–51 were identified as meeting the inclusion criteria.
Study Characteristics
Studies that met the inclusion criteria in the systematic search varied in their characteristics. They are presented in Table 1 with their respective PEDro scores and elaborated on in Table 2. Publication dates ranged from 2000 to 2016. Participants in these studies varied in age (high school to middle age) and activity level (sedentary lifestyle to competitive athletics). As shown in Table 1, 5 IASTM instruments (ASTM AdvantEDGE, ASTYM, Graston Technique, Fascial Abrasion Technique, and sound-assisted soft-tissue mobilization ([SASTM]) were represented. Of the 13 studies, 6 examined the upper extremities,4,41,42,44,46,49 6 examined the lower extremities,6,45,47,48,50,51 and 1 examined the thoracic spine.43 The systematic search yielded 6 studies4,6,44,46,48,50 that assessed outcomes in uninjured participants and 7 studies41–43,45,47,49,51 involved injured participants. The 4 outcomes of interest (ROM, pain, strength, and patient-reported function) in this systematic review were assessed in part or whole depending on the study. Of the 13 included articles, 6 assessed ROM,4,6,42,44,46,48 6 assessed pain,41–43,47–49 4 assessed strength,41,42,45,49 and 6 assessed patient-reported function.41–43,47–49
Table 1.
Characteristics of Studies Involving Injured and Uninjured Participants Continued on Next Page
| Author (Year) |
Body Part/ Condition |
Participants |
Outcome Scales |
Groups |
Results |
PEDro Score |
Product Used |
|||
| Pain |
Range of Motion |
Function |
Strength |
|||||||
| Non–injury-based studies | ||||||||||
| Bailey et al4 (2015) | Shoulder | 60 collegiate baseball players | X | IASTM and self-stretching Control (self-stretching) |
↑ Glenohumeral HA, IR compared with control (P < .001) | 7 | SASTM | |||
| Heinecke et al44 (2014) | Shoulder | 15 collegiate overhead athletes | X | IASTM and strength/stretch Control (strength/stretch) |
↑ Left arm Apley scratch test compared with control (P = .045) | 5 | GT | |||
| Laudner et al46 (2014) | Shoulder | 35 collegiate baseball players | X | IASTM Rest |
↑ Glenohumeral HA and IR after treatment compared with control (P < .001) | 6 | GT | |||
| Markovic6 (2015) | Lower extremity | 20 regional-level male soccer players | X | IASTM Foam rolling |
↑ Hip flexion compared with foam rolling (P = .039) ↑ Pre-post hip and knee flexion at both follow-up points in IASTM group; ↑ pre-post immediate measurement only in foam-rolling group |
5 | FAT | |||
| Vardiman et al50 (2015) | Lower leg | 11 healthy males | X | X | X | X | IASTM Control |
↑ PFAQ in IASTM for daily living (P = .02) and pain (P = .006) | 6 | GT |
| Schaefer and Sandrey48 (2012) | Ankle | 45 physically active HS and college students | X | X | X | IASTM w/DBT Placebo w/DBT Control (DBT) |
No significant difference in any outcome between groups | 6 | GT | |
| Injury-based studies | ||||||||||
| Blanchette and Normand41 (2011) | Lateral elbow epicondylopathy | 27 adults | X | X | X | IASTM Control |
↑ IASTM for VAS and PRTEE at 6 wk and 3 mo; ↑ grip strength at 6 wk ↑ Control for VAS and PRTEE at 3 mo; ↑ strength at 6 wk |
7 | GT | |
| Sevier and Stegink-Jansen49 (2015) | Lateral elbow epicondylopathy | 107 participants (113 elbows) | X | X | X | IASTM and eccentric exercise/stretching Control (eccentric exercise/stretching) Delayed IASTM (after control) |
↑ DASH at 4 wk compared with control (P = .047) ↑ Grip strength at 4 wk compared with control (P = .008) 78.8% treated with IASTM resolved, 40.9% treated with only eccentric exercise resolved No significant differences between delayed group or initial IASTM group (P > .05) |
6 | Astym | |
| Burke et al42 (2007) | Carpal tunnel syndrome | 26 adults | X | X | X | X | IASTM SMT |
IASTM maintained ↑ in VAS and symptom severity at 3 mo (P < .05) whereas SMT group increased in symptoms | 5 | GT |
| Crothers et al43 (2016) | Nonspecific thoracic pain | 143 adults | X | X | IASTM SMT (HVLA to the thoracic spine) Placebo (detuned ultrasound) |
No significant differences between groups in VAS or ODI | 7 | GT | ||
| Kivlan et al45 (2015) | Lower extremity injury | 45 adults | X | Astym Placebo Control |
↑ % Change in IASTM group compared with control and sham (P = .001) No significant difference between sham and control (P = .68) |
6 | Astym | |||
| Wilson et al51 (2000) | Patellar tendinopathy | 20 adults | X | X | IASTM and stretching/strengthening Control (stretching/strengthening) |
↑ PFJES in IASTM compared with control (P < .05) ↑ Pain in IASTM (P < .05) whereas control did not have significant improvements 86% IASTM treated resolved, 60% of traditional resolved |
3 | ASTM AdvantEDGE | ||
| McCormack et al47 (2016) | Achilles tendinopathy | 16 adults | X | X | IASTM plus eccentric exercise Control (eccentric exercise) |
↑ VISA-A in IASTM group compared with control at 12, 26, 52 wk (P = .03) | 7 | Astym | ||
Abbreviations: ASTM, augmented soft tissue mobilization; DASH, Disability of the Arm, Shoulder, and Hand scale; DBT, dynamic balance training; FAT, Fascial Abrasion Technique; GT, Graston Technique; HA, horizontal adduction; HS, high school; HVLA, high velocity, low amplitude; IASTM, instrument-assisted soft tissue mobilization; IR, internal rotation; ODI, Oswestry Disability Index; PFAQ, perception of functional ability questionnaire; PFJES, patellofemoral joint evaluation scale; PRTEE, Patient-Rated Tennis Elbow Evaluation; SASTM, sound-assisted soft-tissue mobilization; SMT, soft tissue mobilization; VAS, visual analog scale; VISA-A, Victorian Institute of Sport Assessment Achilles-Specific Questionnaire; w/, with; X, outcome assessed; ↑ significant improvement.
Table 2.
Quality Assessment of 13 Studies Using the Physiotherapy Evidence Database (PEDro) Scale22 and Criteria Met and Centre for Evidence-Based Medicine (CEBM)17 Levels
| Author (Year) |
PEDro Criteria |
PEDro Score |
CEBM Level |
||||||||||
| 1 |
2 |
3 |
4 |
5 |
6 |
7 |
8 |
9 |
10 |
11 |
|||
| Bailey et al4 (2015) | Y | Y | N | Y | N | N | Y | Y | Y | Y | Y | 7 | 2 |
| Blanchette and Normand41 (2011) | Y | Y | Y | Y | N | N | N | Y | Y | Y | Y | 7 | 2 |
| Burke et al42 (2007) | Y | Y | N | N | N | N | Y | N | Y | Y | Y | 5 | 2 |
| Crothers et al43 (2016) | Y | Y | Y | Y | N | N | Y | N | Y | Y | Y | 7 | 2 |
| Heinecke et al44 (2014) | Y | Y | N | N | N | N | N | Y | Y | Y | Y | 5 | 2 |
| Kivlan et al45 (2015) | Y | Y | N | Y | Y | N | Y | N | N | Y | Y | 6 | 2 |
| Laudner et al46 (2014) | Y | Y | N | N | N | N | Y | Y | Y | Y | Y | 6 | 2 |
| Markovic6 (2015) | Y | Y | N | Y | N | N | N | N | Y | Y | Y | 5 | 2 |
| McCormack et al47 (2016) | Y | Y | Y | Y | N | N | N | Y | Y | Y | Y | 7 | 2 |
| Schaefer and Sandrey48 (2012) | Y | Y | Y | Y | N | N | N | Y | N | Y | Y | 6 | 2 |
| Sevier and Stegink-Jansen49 (2015) | Y | Y | N | Y | N | N | Y | N | Y | Y | Y | 6 | 2 |
| Vardiman et al50 (2015) | Y | Y | N | Y | N | N | N | Y | Y | Y | Y | 6 | 2 |
| Wilson et al51 (2000) | N | Y | N | N | N | N | Y | Y | N | N | N | 3 | 2 |
Abbreviations: N, no; Y, yes.
Studies of Uninjured Participants
The 6 studies4,6,44,46,48,50 of uninjured participants are represented in Table 1. The researchers in all 6 studies assessed ROM at various joints, and 4 groups4,6,44,46 reported between-groups improvements. Specifically, Bailey et al,4 Heinecke et al,44 and Laudner et al46 found IASTM to improve 1 or more shoulder ROMs in healthy overhead athletes and Markovic6 described increases in lower extremity ROM as compared with foam rolling. Schaefer and Sandrey48 and Vardiman et al50 examined pain and patient-reported function in the distal lower extremity; no between-groups improvements were noted in either outcome. Vardiman et al50 also investigated plantar-flexion strength but found no changes.
Studies of Injured Participants
The 7 studies41–43,45,47,49,51 of injured participants are also represented in Table 1. One group assessed ROM,42 4 groups assessed strength,41,42,47,49 and 6 groups assessed pain and patient-reported function.41–43,47,49,51 Burke et al,42 the only researchers to assess ROM in participants with carpal tunnel syndrome, did not find posttreatment changes in ROM, pain, strength, or patient-reported function. Kivlan et al45 and Sevier and Stegink-Jansen49 observed that IASTM improved isometric squat and grip strength, respectively, whereas the remaining authors41,42 reported no strength gains.
Six of the studies41–43,47,49,51 of injured participants evaluated patient-reported function. Of those, 3 groups47,49,51 examined common tendinopathies (elbow, patella, and Achilles) and noted improvements in patient-reported function versus the comparison groups. The same authors41–43,47,49,51 noted no between-groups improvements in pain. Although between-groups differences were not present, Blanchette and Normand41 reported that the IASTM participants' pain decreased earlier than that in the comparison group. Additionally, Burke et al42 demonstrated a significant time interaction: the IASTM group maintained improvements in pain for at least 3 months.
Quality Assessment
The full PEDro assessment for each article, along with the CEBM levels of evidence, can be seen in Table 2. All included articles yielded a CEBM level of 2; the studies of uninjured participants yielded an average PEDro score of 5.83 (range = 5 to 7), and the studies of injured participants yielded an average PEDro score of 5.86 (range = 3 to 7). The quality of evidence was moderate (less than 6)52 for all investigations.
Blinding of the therapist presents a considerable challenge given the nature of IASTM treatments. However, participants can be blinded if a third group is included. Although 2 sets of investigators45,48 attempted this, only 1 of them45 was able to blind the participants. Concealed allocation, met by only 4 studies,41,43,47,48 is achievable, as is blinding of assessors, which was met in only 7 studies.4,42,43,45,46,49,51 The lowest-scoring work51 was the only study that did not apply inclusion criteria, report results of group-comparison statistics, or provide measures of variability for at least 1 key outcome.
Effect-Size Comparison Over Time
Traditionally, effect sizes are calculated by comparing the treatment and control groups. However, we computed pretest-posttest effect sizes in this systematic review because of variations in study design. The formula (Cohen d = Δ pretest and posttest mean/pretest [treatment or comparison group] SD) used the pretest SD of the treatment or comparison group. Twelve of the 13 articles4,6,41–50 in this systematic review were included in the effect-size analysis. Data from 9 studies4,41–46,48,49 allowed for effect-size calculations using the data as reported. One group47 reported exact pretest and posttest CIs. These data were then used to calculate means or SDs with the following formula to find the missing variables: CI = mean ± t score(σ/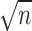 ), where σ is the SD and n is the sample size.53 We contacted 1 author to obtain missing data50 and were provided with means and standard errors of the mean for some but not all outcomes. Thus, we used the following formula to calculate the ROM and strength SDs: SEM = σ/
), where σ is the SD and n is the sample size.53 We contacted 1 author to obtain missing data50 and were provided with means and standard errors of the mean for some but not all outcomes. Thus, we used the following formula to calculate the ROM and strength SDs: SEM = σ/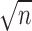 , where σ is the SD and n is the sample size.25 The authors of the remaining 2 articles6,51 were contacted to obtain missing data. One group6 was able to provide the requested information, which was therefore included in the effect-size analysis. The remaining authors51 could not provide the missing data because of the age of the study, making this the only study to not be included in the effect-size analysis.
, where σ is the SD and n is the sample size.25 The authors of the remaining 2 articles6,51 were contacted to obtain missing data. One group6 was able to provide the requested information, which was therefore included in the effect-size analysis. The remaining authors51 could not provide the missing data because of the age of the study, making this the only study to not be included in the effect-size analysis.
Effect-size calculations and 95% CIs for the treatment and comparison groups are presented in Tables 3 through 6. Positive effect sizes represented an improvement in the outcome, and negative effect sizes indicated worsened outcomes. Per Lee,28 any CI that included 0 was considered nonsignificant.
Table 3.
Effect Sizes of Range-of-Motion (ROM) Outcomes of Instrument-Assisted Soft Tissue Mobilization (IASTM) in Uninjured and Comparison Groups: Baseline to Time Point
| Author (Product) |
Body Part |
Treatment Time × No. of Treatments |
ROM |
Time-Elapsed Effect Size With 95% Confidence Intervals (Short-Term; IASTM Listed First)a |
|||||
| 0 h |
24 h |
48 h |
72 h |
2 wk |
4 wk |
||||
| Bailey et al4 (SASTM) | Shoulder | 2 min × 1 | GH ER | 0.19 (−0.32, 0.69) | |||||
| 0.11 (−0.40, 0.61) | |||||||||
| GH IR | 1.11 (0.57, 1.65)b | ||||||||
| 0.76 (0.23, 1.28) | |||||||||
| GH HA | 1.45 (0.88, 2.02)b | ||||||||
| 0.63 (0.11, 1.15) | |||||||||
| Heinecke et al44 (GT) | Shoulder | 3–5 min × 8 | GH IR right | 0.44 (−0.62, 1.50) | 0.46 (−0.60, 1.52)b | ||||
| 0.62 (−0.45, 1.69) | 0.16 (−0.89, 1.21) | ||||||||
| GH IR left | 0.51 (−0.55, 1.58)b | 0.58 (−0.49, 1.65)b | |||||||
| 0.26 (−0.79, 1.31) | −0.03 (−1.07, 1.02) | ||||||||
| GH ER right | 0.35 (−0.71, 1.40) | 0.80 (−0.29, 1.89)b | |||||||
| 0.56 (−0.50, 1.63) | 0.73 (−0.35, 1.81) | ||||||||
| GH ER left | 0.33 (−0.72, 1.36) | 0.80 (−0.29, 1.89)b | |||||||
| 0.30 (−0.75, 1.35) | 0.67 (−0.41, 1.75) | ||||||||
| GH HA right | 0.98 (−0.13, 2.09)b | 1.23 (0.09, 2.37) | |||||||
| 0.53 (−0.54, 1.60) | 1.27 (0.12, 2.42) | ||||||||
| GH HA left | 0.89 (−0.21, 1.99) | 1.24 (0.09, 2.38) | |||||||
| 0.91 (−0.19, 2.01) | 1.51 (0.33, 2.70) | ||||||||
| Laudner et al46 (GT) | Shoulder | 40 s × 1 | GH IR | 0.44 (−0.24, 1.12)b | |||||
| −0.05 (−0.70, 0.61) | |||||||||
| GH HA | 1.91 (1.10, 2.73)b | ||||||||
| −0.01 (−0.67, 0.64) | |||||||||
| Markovic6 (FAT) | Hip and knee | 2 min × 1 | Hip flex | 2.46 (1.30, 3.62)b | 1.64 (0.63, 2.65)b | ||||
| 1.13 (0.19, 2.08) | 0.16 (0.63, 2.65) | ||||||||
| Knee flex | 2.48 (1.31, 3.65)b | 1.73 (0.70, 2.76)b | |||||||
| 1.46 (0.47, 2.44) | 0.25 (−0.63, 1.13) | ||||||||
| Vardiman et al50 (GT) | Lower leg | 7–8 min × 1 | Ankle DF | 0.03 (−0.80, 0.87) | 0.05 (−0.79, 0.88) | 0.05 (−0.79, 0.88) | −0.28 (−1.12, 0.56) | ||
| −0.06 (−0.90, 0.77) | −0.23 (−1.07, 0.61) | 0.11 (−0.72, 0.95) | −0.07 (−0.90, 0.77) | ||||||
| Schaefer and Sandrey48 (GT) | Ankle | 8 min × 8 | Ankle DF | 1.62 (0.76, 2.47)b | |||||
| 1.00 (0.11, 1.89) | |||||||||
| Ankle PF | 0.92 (0.14, 1.70)b | ||||||||
| 0.14 (−0.70, 0.98) | |||||||||
| Ankle Inv | 0.75 (−0.02, 1.51) | ||||||||
| 0.36 (−0.49, 1.20) | |||||||||
| Ankle Ev | 1.0 (0.21, 1.79) | ||||||||
| 1.08 (0.19, 1.98) | |||||||||
Abbreviations: DF, dorsiflexion; ER, external rotation; Ev, eversion; FAT, Fascial Abrasion Technique; flex, flexion; GH, glenohumeral; GT, Graston Technique; HA, horizontal adduction; Inv, inversion; IR, internal rotation; PF, plantar flexion; SASTM, sound-assisted soft-tissue mobilization.
Effect size of <0.35 is considered trivial, 0.35–0.79 is considered small, 0.80–1.50 is considered moderate, >1.50 is considered large.29,31 A positive effect size indicates an improvement in ROM.
The IASTM group was at least 1 Rhea effect-size category larger than the listed comparison group.
As demonstrated in the Tables, a large number of time points were assessed. Therefore, to assist us in determining clinical inferences, short- and long-term healing descriptors were defined and included. The fibroblastic repair phase can last from 2 days to 6 weeks.54 To take into account factors that may impede healing (such as severity of injury and age) and to ensure the fibroblastic repair phase is completed,54 we set the 12-week mark as the beginning of the long-term time frame. Thus, as indicated in Tables 3 through 6, the end of the short-term measurements and start of the long-term outcomes occurred at 3 months.
Range of Motion: Uninjured Participants
The effect sizes and CIs of the 6 articles on uninjured participants4,6,44,46,48,50 that assessed ROM of IASTM and comparison groups are summarized in Table 3. In the IASTM groups, trivial to large effect sizes (0.04 to 2.48) were associated with improving ROM,4,6,44,46,48,50 with only 1 time point reflecting a small decrease (−0.28) in ROM.50 Comparison-group effect sizes ranged from −0.23 to 1.51; only 1 time point reached the large category.4,6,44,46,48,50 A summary of the 5 studies4,6,44,46,48 in which the IASTM groups were at least 1 Rhea category larger than the comparison group is provided in Table 7. Because of the variety in ROM studies, Table 7 indicates which range was higher than that of the comparison group. More than half of the IASTM and comparison-group CIs crossed zero (17 of 28 and 20 of 28, respectively).
Table 7.
Studies With Instrument-Assisted Soft Tissue Mobilization Effect Sizes Over Time at Least 1 Rhea Category Greater Than the Comparison Group in Outcome Assesseda
| Time Period |
Range of Motion: Uninjured Group (Specific) |
Pain: Injured Group |
Strength: Injured Group |
Patient-Reported Function: Injured Group |
| Short term | Bailey et al4 (IR, HA)b | Blanchette and Normand41 | Burke et al42 | Blanchette and Normand41 |
| Heinecke et al44 (IR, ER, HA) | Crothers et al43 | Kivlan et al45,b | Crothers et al43 | |
| Laudner et al46 (IR, ER, HA)b | McCormack et al47 | McCormack et al47 | ||
| Markovic6 (Hip and knee flexion)b | Sevier and Stegink-Jansen49 | Sevier and Stegink-Jansen49,b | ||
| Schaefer and Sandrey48 (dorsiflexion and plantar flexion only) | ||||
| Long term | Blanchette and Normand41 | Burke et al42 | Blanchette and Normand41 | |
| Crothers et al43 | Crothers et al43 | |||
| McCormack et al47 | McCormack et al47,b | |||
| Sevier and Stegink-Jansen49 |
Abbreviations: ER, external rotation; HA, horizontal adduction; IR, internal rotation.
See Table 1 for specifics.
Difference between groups.
Pain: Injured Participants
Table 4 displays the effect sizes and CIs of the 5 studies of injured participants41–43,47,49 that evaluated pain in the treatment and comparison groups. Four used the visual analog scale in centimeters43 or millimeters,41,42,49 and 1 group47 used the numeric pain rating scale. The IASTM treatment groups had small to large improvements in pain (0.48 to 2.08), with short-term (0–8 weeks) effect sizes ranging from small to large41–43,47,49 (range = 0.48 to 1.95) and long-term (3 months to 52 weeks) effect sizes ranging from moderate to large41–43,47,49 (range = 1.19 to 2.08). The comparison-group effect sizes ranged from trivial to large (range = 0.20 to 2.52) in the short term,41–43,47,49 with only 1 time point reaching the large category42 and moderate to large (range = 0.62 to 1.74) category in the long term.41–43,47,49 The 4 studies41,43,47,49 in which the IASTM effect sizes were at least 1 Rhea category higher than those of the comparison groups are shown in Table 7. Of note is that very few of the IASTM and comparison-group CIs crossed zero (2 of 18 and 4 of 18, respectively).
Table 4.
Effect Sizes of Pain Outcomes of Instrument-Assisted Soft Tissue Mobilization (IASTM) in Injured and Comparison Groups: Baseline to Time Point Extended on Next Page
| Author (Product) |
Condition |
Treatment Time × No. of Treatments |
Scale Used |
Time-Elapsed Effect Size With 95% Confidence Intervals (IASTM Listed First)a |
|
| Short-Term, wk | |||||
| 0 |
1 |
||||
| Blanchette and Normand41 (GT) | Lateral elbow epicondylopathy | Unspecified × 10 | VAS, mm | ||
| Sevier and Stegink-Jansen49 (Astym) | Lateral elbow epicondylopathy | Unspecified × 8 | VAS, mm | ||
| Burke et al42 (GT) | Carpal tunnel syndrome | Unspecified × 10 | VAS, mm | 1.95 (0.98, 2.92) | |
| 2.52 (1.35, 3.69) | |||||
| Crothers et al43 (GT) | Nonspecific thoracic pain | 10–15 min × max of 10 | VAS, cm | 0.48 (0.11, 0.84)b | |
| 0.20 (−0.27, 0.67) | |||||
| McCormack et al47 (Astym) | Achilles tendinopathy | 20–30 min × 12 | Numeric pain rating scale | ||
Abbreviations: GT, Graston Technique; N/R, time point measurement but no data reported; VAS, visual analog scale.
Effect size of <0.35 is considered trivial, 0.35–0.79 is considered small, 0.80–1.50 is considered moderate, >1.50 is considered large.29,31 Positive effect size indicates improvement.
The IASTM group was at least 1 Rhea effect-size category larger than the listed comparison group.
Table 4.
Extended From Previous Page
| Time-Elapsed Effect Size With 95% Confidence Intervals (IASTM Listed First)a | |||||
| Short-Term, wk |
Long-Term, wk |
||||
| 4 |
6 |
8 |
12 |
26 |
52 |
| 1.30 (0.52, 2.09)b | 1.26 (0.48, 2.04) | ||||
| 0.62 (−0.20, 1.44) | 0.62 (−0.20, 1.44) | ||||
| 0.92 (0.49, 1.34)b | 0.67 (0.07, 1.27) | N/R | 1.96 (1.42, 2.49) | 2.08 (1.53, 2.64) | |
| 0.48 (0.07, 0.89) | 0.60 (0.13, 1.07) | 1.44 (0.86, 2.02) | 1.84 (1.32, 2.36) | 1.96 (1.43, 2.49) | |
| 1.97 (0.99, 2.94) | |||||
| 1.50 (0.51, 2.49) | |||||
| 1.10 (0.71, 1.48)b | 1.19 (0.79, 1.59)b | 1.05 (0.65, 1.44) | 1.19 (0.75, 1.63) | ||
| 0.60 (0.12, 1.08) | 0.75 (0.26, 1.24) | 0.95 (0.41, 1.49) | 0.85 (0.32, 1.38) | ||
| 0.95 (−0.16, 2.05)b | 0.78 (−0.31, 1.87) | 1.19 (0.06, 2.33) | 1.56 (0.32, 2.81)b | 1.62 (0.36, 2.87) | |
| 0.62 (−0.39, 1.62) | 1.12 (0.06, 2.17) | 1.30 (0.22, 2.38) | 1.30 (0.05, 2.55) | 1.74 (0.41, 3.07) | |
Strength: Injured Participants
The effect sizes and CIs for IASTM and comparison groups in the 4 studies of injured participants41,42,45,49 that assessed strength are found in Table 5. Overall effect sizes for the treatment groups ranged from −0.06 to 0.81, indicating a decrease to a moderate increase in strength.41,42,45,49 Comparison-group effect sizes ranged from −0.11 to 0.28, indicating a decrease to a trivial improvement.41,42,45,49 Short-term (0–8 weeks) IASTM group effect sizes ranged from trivial decreases to moderate increases in strength41,42,45,49 (range = −0.06 to 0.81), and long-term (12 weeks) effect sizes ranged from small to moderate42 (range = 0.59 to 0.81). Comparison-group effect sizes demonstrated a decrease to a trivial improvement in the short term41,42,47,49 (range = −0.11 to 0.28), whereas the results were only trivial in the long-term assessment42 (range = 0.15 to 0.22). For comparison purposes, Table 7 cites the 2 studies42,45 in which the IASTM group values were categorically larger than the comparison groups in short-term assessments and 1 study42 that had larger effect sizes in the long-term assessment. Additionally, all CIs for both comparison and IASTM groups crossed zero.
Table 5.
Effect Sizes of Strength Outcomes of Instrument-Assisted Soft Tissue Mobilization (IASTM) in Injured and Comparison Groups: Baseline to Time Point Extended on Next Page
| Author (Product) |
Condition |
Treatment Time × No. of Treatments |
Scale Used |
| Blanchette and Normand41 (GT) | Lateral elbow epicondylopathy | Unspecified × 10 | Grip in kg |
| Sevier and Stegink-Jansen49 (Astym) | Lateral elbow epicondylopathy | Unspecified × 8 | Grip in pounds |
| Burke et al42 (GT) | Carpal tunnel syndrome | Unspecified × 10 | Grip in kg |
| Pinch in kg | |||
| Kivlan et al45 (Astym) | Lower extremity injury | About 12 min × 1 | Newtons |
Abbreviations: GT, Graston Technique; N/R, time point measurement but no data reported.
Effect size of <0.35 is considered trivial, 0.35–0.79 is considered small, 0.80–1.50 is considered moderate, >1.50 is considered large.29,31 A positive effect size indicates a gain in strength.
The IASTM group was at least 1 Rhea effect size category larger than the listed comparison group.
Table 5.
Extended From Previous Page
| Time-Elapsed Effect Size With 95% Confidence Intervals (IASTM Listed First)a | ||||
| Short-Term, wk |
Long-Term, wk |
|||
| 0 |
4 |
6 |
8 |
12 |
| 0.14 (−0.57, 0.86) | ||||
| 0.13 (−0.67, 0.93) | ||||
| 0.28 (−0.12, 0.68) | −0.06 (−0.66, 0.54) | N/R | ||
| 0.06 (−0.35, 0.48) | −0.01 (−0.48, 0.46) | 0.15 (−0.40, 0.70) | ||
| 0.63 (−0.19, 1.45)b | 0.59 (−0.23, 1.41)b | |||
| 0.28 (−0.60, 1.16) | 0.21 (−0.67, 1.09) | |||
| 0.81 (−0.03, 1.63)b | 0.81 (−0.03, 1.63)b | |||
| 0.22 (−0.66, 1.10) | 0.22 (−0.66, 1.10) | |||
| 0.30 (−0.42, 1.02)b | ||||
| −0.11 (−0.83, 0.61) | ||||
Patient-Reported Function: Injured Participants
The 5 studies41–43,47,49 of injured participants that assessed patient-reported function after IASTM treatments and accompanying comparison-group effect sizes and CIs are presented in Table 6. All authors used different patient-reported functional scales. Effect sizes varied from a small to a large increase (0.54 to 2.24) in the IASTM groups and from a trivial to a large increase (range = 0.13 to 1.76) in the comparison groups.41–43,47,49 Short-term IASTM group effect sizes varied from small to large improvements41–43,47,49 (range = 0.54 to 1.60), as did long-term effect sizes43,47,49 (range = 0.54 to 2.24). Comparison-groups' effect sizes were trivial to moderate (range = 0.13 to 0.82) in short-term assessments41–43,47,49 and from small to large (range = 0.58 to 1.76) in long-term assessments.43,47,49 The 4 studies41,43,47,49 that demonstrated at least 1 categorical effect-size improvement in the IASTM versus comparison groups in the short-term assessment and the 3 that did so41,43,47 in the long-term assessment are provided in Table 7.
Table 6.
Effect Sizes of Patient-Reported Function of Instrument-Assisted Soft Tissue Mobilization (IASTM) in Injured and Comparison Groups: Baseline to Time Point Extended on Next Page
| Author (Product) |
Pathology |
Treatment Time × No. of Treatments |
Scale Used |
Time-Elapsed Effect Size With 95% Confidence Intervals (IASTM Listed First)a |
|
| Short-Term, wk | |||||
| 0 |
1 |
||||
| Blanchette and Normand41 (GT) | Lateral elbow epicondylopathy | Unspecified × 10 | PRTEE | ||
| Sevier and Stegink-Jansen49 (Astym) | Lateral elbow epicondylopathy | Unspecified × 8 | DASH | ||
| VAS function | |||||
| Burke et al42 (GT) | Carpal tunnel syndrome | Unspecified × 10 | Function scale | 0.54 (−0.28, 1.35) | |
| 0.82 (−0.09, 1.74) | |||||
| Crothers et al43 (GT) | Nonspecific thoracic pain | 10–15 min × max of 10 | ODI | 0.64 (0.27, 1.00) | |
| 0.49 (0.01, 0.97) | |||||
| McCormack et al47 (Astym) | Achilles tendinopathy | 20–30 min × 12 | VISA-A | ||
Abbreviations: DASH, Disability of the Arm, Shoulder and Hand scale; GT, Graston Technique; N/R, time point measurement but no data reported; ODI, Oswestry Disability Index; PRTEE, Patient-Rated Tennis Elbow Evaluation; VAS, visual analog scale; VISA-A, Victorian Institute of Sport Assessment Achilles-Specific Questionnaire.
Effect size of <0.35 is considered trivial, 0.35–0.79 is considered small, 0.80–1.50 is considered moderate, >1.50 is considered large.29,31 A positive effect size in measurements indicates an improvement in function.
The IASTM group was at least 1 Rhea effect size category larger than the listed comparison group.
Table 6.
Extended From Previous Page
| Time-Elapsed Effect Size With 95% Confidence Intervals (IASTM Listed First)a | |||||
| Short-Term, wk |
Long-Term, wk |
||||
| 4 |
6 |
8 |
12 |
26 |
52 |
| 1.16 (0.38, 1.93)b | 1.11 (0.34, 1.87)b | ||||
| 0.28 (0.53, 1.08) | 0.72 (−0.10, 1.55) | ||||
| 0.97 (0.56, 1.38)b | 0.84 (0.26, 1.43) | N/R | 1.57 (1.08, 2.05) | 1.78 (1.26, 2.29) | |
| 0.69 (0.29, 1.10) | 0.71 (0.26, 1.17) | 1.40 (0.83, 1.98) | 1.64 (1.15, 2.12) | 1.76 (1.27, 2.25) | |
| 0.68 (0.27, 1.09) | 0.76 (0.15, 1.37) | N/R | 1.36 (0.87, 1.85) | 1.44 (0.93, 1.95) | |
| 0.44 (0.02, 0.86) | 0.44 (−0.03, 0.91) | 0.92 (0.36, 1.48) | 1.32 (0.83, 1.81) | 1.32 (0.83, 1.81) | |
| 0.54 (−0.28, 1.35) | |||||
| 0.82 (−0.09, 1.74) | |||||
| 1.05 (0.66, 1.43) | 1.22 (0.81, 1.62)b | 1.22 (0.81, 1.62) | 1.21 (0.77, 1.65)b | ||
| 0.74 (0.25, 1.23) | 0.62 (0.13, 1.11) | 0.90 (0.37, 1.43) | 0.60 (0.08, 1.12) | ||
| 1.08 (−0.04, 2.20)b | 1.57 (0.37, 2.77)b | 1.89 (0.63, 3.14) | 2.06 (0.66, 3.45)b | 2.25 (0.81, 3.70)b | |
| 0.13 (−0.85, 1.11) | 0.66 (−0.35, 1.66) | 0.58 (−0.42, 1.58) | 0.77 (−0.40, 1.95) | 1.38 (0.12, 2.63) | |
The IASTM Product Analysis
The IASTM tools used in each of the studies included in the effect-size analysis are indicated in Tables 3 through 6. The standardization offered by effect sizes allows for a comparison of the 4 tools used: Graston Technique instruments,41–44,46,48,50 Fascial Abrasion Technique,6 SASTM,4 and Astym.45,47,49 Graston Technique effect sizes41–44,46,48,50 ranged from −0.28 to 2.42 and Astym effect sizes45,47,49 from −0.06 to 2.08, indicating a decrease to a large improvement for both tools. Fascial Abrasion Technique effect sizes6 ranged from 1.52 to 2.48, indicating a large improvement. The SASTM effect sizes4 ranged from 0.19 to 1.45. As seen in Table 7, all tools studied were associated with a larger categorical difference in the IASTM groups in at least 1 observed outcome versus the comparison groups.
DISCUSSION
Our primary purpose was to conduct a comprehensive systematic review of IASTM effectiveness, given the limited scope of previous reviews18,19 and recent growth in the literature. Our systematic review consisted of 6 and 4 more studies than previously published reviews,18,19 respectively, likely because of more inclusive search terms, the criteria and databases searched, and the publication dates. We excluded 25 case reports or case series. Though case reports and case series are critical in developing evidence-based practice and are often used to assist in clinical decision making, they provide limited generalizability and carry a high risk of bias.55 Thus, moving forward, we implore researchers to consider the disproportionate number of case reports compared with randomized controlled trials.
Quality Assessment
The level 2 CEBM grade for all of the included articles indicates the current evidence is lacking full consistency in results and adequate methods, which is reflected in the PEDro scores. In particular, the 5.83 (uninjured participants') and 5.86 (injured participants') average PEDro scores may affect the generalizability of and bias in the published research for both groups.52 Because of the inadequate blinding previously mentioned, many of the included studies were susceptible to biased results, decreasing their validity. Although blinding of the therapist is impossible because of the nature of the treatment, blinding of the assessor is easily accomplished, and blinding of the participants can be done with the appropriate methods, as shown by Kivlan et al.45 Concealed allocation and adequate follow-up are other criteria that are easily met with prior consideration, yet few researchers included these in their methods.
Clinical Recommendations
Although statistical significance sets a high standard for ensuring that outcomes do not occur by chance, it does not necessarily take clinical significance into consideration.29 Traditionally, effect sizes are calculated to provide the magnitude of difference in outcomes between treatment and comparison groups. As described in the “Methods” section, we could not calculate effect sizes using a traditional approach because the studies varied greatly in their designs. As such, we calculated effect sizes using pretest-posttest results. This approach allows for a greater appreciation of IASTM's clinical ability to improve outcomes in injured and uninjured participants and in short- and long-term ranges.
Range of Motion: Uninjured Participants
The studies of uninjured participants in this review assessed ROM, with a majority examining the shoulder-joint complex.4,44,46 When we take into consideration study quality, statistical significance, the comparative effect-size analysis, and CIs, IASTM appeared to be effective in yielding short-term improvements in shoulder horizontal adduction and internal rotation among uninjured participants.4,46 The findings of Heinecke et al44 appeared to contradict those results, but this is likely due to the low quality score (PEDro score = 5) and large SDs resulting in wide CIs. Bailey et al4 credited the glenohumeral-joint ROM improvements found in their healthy overhead athletes to decreases in posterior rotator cuff muscle stiffness. For clinicians, this observation is highly relevant, as investigators56–58 have linked deficits in shoulder ROM in particular to higher incidences of injury during a season.
Only 1 author6 assessed hip ROM, yet the results are very promising. Markovic6 compared IASTM with foam rolling to determine the short-term effects (24 hours) on hip and knee ROM in regional male soccer players. The IASTM group had immediate ROM gains, which were twice as large as those of the foam-rolling group.6 Additionally, these improvements were maintained at 24 hours postintervention, whereas the foam-rolling group returned to baseline ROM.6 The ROM gains reported by Markovic6 appeared to have been retained longer than those reported in recent systematic reviews59,60 of foam rolling and proprioceptive neuromuscular facilitation (PNF) stretching. Specifically, the IASTM-treated participants in the Markovic6 study maintained their ROM gains for longer durations (up to 24 hours) compared with those in the PNF-stretching (less than 6 minutes)59 and foam-rolling (less than 30 minutes)60 studies. In support of Markovic's results,6 we found the effect sizes and strong CIs after IASTM were large and greater than those in the comparison groups. Although we recognize that this single study should not be interpreted to mean that IASTM is superior to foam rolling or PNF stretching, the substantial gains Markovic6 demonstrated indicate that further research is needed.
Pain: Injured Participants
The researchers whose work was included in this systematic review used IASTM to improve pain among participants with elbow epicondylopathy,41,49 carpal tunnel syndrome,42 thoracic back pain,43 patellar tendinopathy,51 and Achilles tendinopathy.47 Wilson et al51 were the only authors to find significant improvements in pain; however, we did not include this variable in the effect-size analysis because of a lack of data. Interestingly, although the studies included in the comparative effect-size analysis showed no differences between the IASTM and comparison groups, 4 of the 5 investigations41,43,47,49 revealed moderate to large improvements that were larger than those of the comparison groups at the short- and long-term time points. Thus, IASTM may be clinically effective in decreasing pain among populations with tendinopathy and when treating nonspecific thoracic pain in adults. Additionally, pain has adverse effects on patient compliance61–63; therefore, the use of IASTM to decrease pain may improve treatment compliance. However, more research is needed before strong recommendations can be made.
Strength: Injured Participants
Based on the inconsistent findings, small effect sizes, and wide CIs, IASTM does not yet appear to be indicated for improving strength in those with an injury.41,42,45,49 This result is unlike the other outcomes examined in this review and yet consistent with the mixed literature on other manual therapy techniques. Direct techniques such as mobilization with movement64 and general osteopathic manipulation65 show promise for improving grip and neck muscle strength, respectively, but support for indirect techniques such as foam rolling60 does not currently exist.
Patient-Reported Function: Injured Participants
Of the studies of injured participants that assessed patient-reported function, IASTM appeared to be most beneficial for treating tendinopathies. Three groups assessed participants with patellar,51 elbow,49 or Achilles47 tendinopathy and noted improvements after IASTM treatment. Blanchette and Normand,41 who examined patients with lateral epicondylopathy, observed no group differences, but the IASTM group improved more quickly than the comparison group. The researchers speculated this finding was the result of a small sample size,41 further highlighting the value of evaluating effect sizes.29 Taken together, the studies on tendinopathy had moderate to large effect sizes, larger effect sizes versus the comparison groups, and narrow CIs, indicating improved patient-reported function after IASTM treatment.41,47,49,51 Because improved patient-reported outcomes have been linked to therapy compliance,66 the magnitude of effect sizes on patient-reported function in this systematic review supports the role of patient compliance in managing tendinopathy.
Instrument-Assisted Soft Tissue Mobilization Product Choices
Once a systematic review demonstrates the effectiveness of a particular therapy, clinicians are left with the daunting task of trying to decide which product to purchase. Our effect-size analysis revealed that all products studied fell in roughly the same categories for the various outcomes assessed. The Fascial Abrasion Technique tool was the only product to not range out of the large category; however, it was used in only 1 study6 and ROM was the only outcome. Although these investigations did not involve all of the IASTM products on the market, the tools used varied greatly in material, edges, and surface texture; therefore, it may be that any tool used to assist tissue mobilization is beneficial. Wagner and Olson67 instructed clinicians on how to make their own IASTM tool and contended that the results should be similar. Though this prediction has been substantiated only by anecdotal outcomes to date, it may be an option for clinicians on a tighter budget and warrants investigation.
LIMITATIONS
The variety of treatment times, comparison groups, and populations in the included studies presented several challenges and therefore limitations to our review. Effect-size calculations were performed for only 12 of the 13 articles because we could not obtain usable data for all. The various study designs forced us to calculate effect sizes from pretreatment to posttreatment, rather than the traditional comparison with a control, because the groups were not consistent. As a result, the effect sizes we calculated should be compared only with others calculated using the same methods and cannot be compared directly with significant findings for treatment and control groups. Lastly, the inclusion of injured and uninjured populations limits the generalizability of the findings. As such, the clinical recommendations provided in this systematic review are based on the populations examined and should not be applied to other types of healthy or injured participants.
DIRECTIONS FOR FUTURE RESEARCH
It is not uncommon for a study of this nature to raise a significant number of suggestions for future research. The first and perhaps most important recommendation is related to study design and methods. Future researchers should take into consideration the variable characteristics of the studies included in this systematic review. Control groups, follow-up periods, and IASTM protocols varied greatly, which makes generalizability and comparisons difficult. Instrument-assisted soft tissue mobilization protocols differ based on the educational programs created by the manufacturers of IASTM tools; however, protocols need to be more consistent in future work if we are to determine the optimal treatment prescription. In addition to more consistent methods, limiting potential bias and dropouts should be considered before beginning a study. The current literature shows only moderate PEDro scores, which could be improved by blinding, concealment, and possibly providing incentives for participants to continue through follow-ups. Finally, authors should calculate effect sizes (including, but not limited to, Cohen d and numbers need to treat) and minimal clinically important differences and report all statistics. Effect sizes and corresponding CIs, as seen in this review, can assist in determining meaningfulness beyond statistical significance and should be included in published articles.24,26,29 For studies assessing injured participants, resolution rates (see Sevier and Stegink-Jansen49 and Wilson et al51) should be reported, as these can have large influences on clinical decision making.
The next suggestion for future research concerns the ability to effectively search for and obtain IASTM literature. The multiple synonyms and names used for IASTM, in conjunction with the length and complexity of the Boolean string used for this systematic review, made the search process difficult and time consuming. Therefore, to assist authors and readers, it would be beneficial to include 1 key term in all IASTM articles. For indexing in Medical Subject Heading terms, IASTM is most appropriate under the descriptor therapy, soft tissue.68 This term should be indexed, and IASTM should be in the title of the article or abstract.
Lastly, as this therapy continues to be used in different ways clinically, future researchers should consider studying different patient populations, such as after surgery, and a wider variety of body regions and tissue types. This will allow for a greater understanding of the mechanisms by which IASTM works. To that end, although we focused on 4 main outcomes—ROM, pain, strength, and patient-reported function—future researchers should examine IASTM's ability to alter performance outcomes, such as sprints, vertical jumps, and throwing velocity.
CONCLUSIONS
Moderate evidence supports the use of IASTM in injured and uninjured participants. Specifically, it is recommended for improving ROM in uninjured participants and for improving pain and patient-reported function in select injured patients. However, because of limited and conflicting research, it is not yet recommended for enhancing strength. Though the specific IASTM products examined in this study did not seem to generate a profound difference in treatment effects, more direct product comparisons are warranted.
ACKNOWLEDGMENTS
We thank Lindsay E. Bodine, MS, LAT, ATC, and Jennifer K. Cauley, MS, LAT, ATC, for their assistance in the quality-assessment process of this study.
Footnotes
Authors of the current study would like to cite the following article as well: Portillo-Soto A, Eberman LE, Demchak TJ, Peebles C. Comparison of blood flow changes with soft tissue mobilization and massage therapy. J Altern Complement Med. 2014;20(12):932–936.
REFERENCES
- 1.Cyriax J. Textbook of Orthopaedic Medicine. London, United Kingdom: Bailliere-Tindal;; 1984. [Google Scholar]
- 2.Hammer WI. The effect of mechanical load on degenerated soft tissue. J Bodyw Mov Ther. 2008;12(3):246–256. doi: 10.1016/j.jbmt.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 3.Davidson CJ, Ganion LR, Gehlsen GM, Verhoestra B, Roepke JE, Sevier TL. Rat tendon morphologic and functional changes resulting from soft tissue mobilization. Med Sci Sports Exerc. 1997;29(3):313–319. doi: 10.1097/00005768-199703000-00005. [DOI] [PubMed] [Google Scholar]
- 4.Bailey LB, Shanley E, Hawkins R, et al. Mechanisms of shoulder range of motion deficits in asymptomatic baseball players. Am J Sports Med. 2015;43(11):2783–2793. doi: 10.1177/0363546515602446. [DOI] [PubMed] [Google Scholar]
- 5.Chughtai M, Mont MA, Cherian C, et al. A novel, nonoperative treatment demonstrates success for stiff total knee arthroplasty after failure of conventional therapy. J Knee Surg. 2016;29(3):188–193. doi: 10.1055/s-0035-1569482. [DOI] [PubMed] [Google Scholar]
- 6.Markovic G. Acute effects of instrument assisted soft tissue mobilization vs. foam rolling on knee and hip range of motion in soccer players. J Bodyw Mov Ther. 2015;19(4):690–696. doi: 10.1016/j.jbmt.2015.04.010. [DOI] [PubMed] [Google Scholar]
- 7.Astym Web site. https://astym.com/Main Accessed July 15, 2017.
- 8.Fascial abrasion technique. FIT Institute Web site. https://www.thefitinstitute.com/therapies/soft-tissue-therapy/fascial-abrasion-technique/ Accessed July 15, 2017.
- 9.Graston Technique Web site. http://www.grastontechnique.com/home Accessed July 15, 2017.
- 10.HawkGrips Web site. http://hawkgrips.com/ Accessed July 15, 2017.
- 11.Sound Assisted Soft Tissue Mobilization Web site. http://www.sastm.com/ Accessed July 15, 2017.
- 12.Adhesion Breakers Web site. http://www.adhesionbreakers.com/default.asp Accessed July 15, 2017.
- 13.Kim J, Sung DJ, Lee J. Therapeutic effectiveness of instrument-assisted soft tissue mobilization for soft tissue injury: mechanisms and practical application. J Exerc Rehabil. 2017;13(1):12–22. doi: 10.12965/jer.1732824.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gehlsen GM, Ganion LR, Helfst R. Fibroblast responses to variation in soft tissue mobilization pressure. Med Sci Sports Exerc. 1999;31(4):531–535. doi: 10.1097/00005768-199904000-00006. [DOI] [PubMed] [Google Scholar]
- 15.Imai K, Ikoma K, Chen Q, Zhao C, An KN, Gay RE. Biomechanical and histological effects of augmented soft tissue mobilization therapy on Achilles tendinopathy in a rabbit model. J Manipulative Physiol Ther. 2015;38(2):112–118. doi: 10.1016/j.jmpt.2014.12.003. [DOI] [PubMed] [Google Scholar]
- 16.Loghmani MT, Warden SJ. Instrument-assisted cross-fiber massage accelerates knee ligament healing. J Orthop Sports Phys Ther. 2009;39(7):506–514. doi: 10.2519/jospt.2009.2997. [DOI] [PubMed] [Google Scholar]
- 17.Howick J, Chalmers I, Galsziou P, et al. Levels of evidence working group: the Oxford levels of evidence 2. Oxford Centre for Evidence-Based Medicine Web site. http://www.cebm.net/index.aspx?o=5653 Accessed January 15, 2017.
- 18.Cheatham SW, Lee M, Cain M, Baker R. The efficacy of instrument assisted soft tissue mobilization: a systematic review. J Can Chiropr Assoc. 2016;60(3):200–211. [PMC free article] [PubMed] [Google Scholar]
- 19.Lambert M, Hitchcock R, Lavallee K, et al. The effects of instrument-assisted soft tissue mobilization compared to other interventions on pain and function: a systematic review. Phys Ther Review. 2017;22(1–2):76–85. [Google Scholar]
- 20.EndNote X7 for Mac. Philadelphia, PA: Clarivate Analytics; 2016. [computer program]. Version 17.5.1.11194. [Google Scholar]
- 21.Maher CG, Sherrington C, Herbert RD, Moseley AM, Elkins M. Reliability of the PEDro scale for rating quality of randomized controlled trials. Phys Ther. 2003;83(8) 71317.5.1.11194721. [PubMed] [Google Scholar]
- 22.Physiotherapy Evidence Database Web site https://www.pedro.org.au/ 2017
- 23.Waters-Banker C, Butterfield TA, Dupont-Versteegden EE. Immunomodulatory effects of massage on nonperturbed skeletal muscle in rats. J Appl Physiol (1985) 2014;116(2):164–175. doi: 10.1152/japplphysiol.00573.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lininger M, Riemann BL. Statistical primer for athletic trainers: using confidence intervals and effect sizes to evaluate clinical meaningfulness. J Athl Train. 2016;51(12):1045–1048. doi: 10.4085/1062-6050-51.12.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thomas J, Nelson J, Silverman S. Research Methods in Physical Activity 6th ed. Champaign, IL: Human Kinetics;; 2011. [Google Scholar]
- 26.Rhea MR. Determining the magnitude of treatment effects in strength training research through the use of the effect size. J Strength Cond Res. 2004;18(4):918–920. doi: 10.1519/14403.1. [DOI] [PubMed] [Google Scholar]
- 27.Cohen J. Statistical Power Analysis for the Behavioral Sciences 2nd ed. Hillsdale, NJ: L. Erlbaum Associates;; 1988. [Google Scholar]
- 28.Lee DK. Alternatives to P value: confidence interval and effect size. Korean J Anesthesiol. 2016;69(6):555–562. doi: 10.4097/kjae.2016.69.6.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McGough JJ, Faraone SV. Estimating the size of treatment effects: moving beyond P values. Psychiatry (Edgmont) 2009;6(10):21–29. [PMC free article] [PubMed] [Google Scholar]
- 30.Castro-Sánchez AM, Matarán-Peñarrocha GA, Arroyo-Morales M, Saavedra-Hernández M, Fernández-Sola C, Moreno-Lorenzo C. Effects of myofascial release techniques on pain, physical function, and postural stability in patients with fibromyalgia: a randomized controlled trial. Clin Rehabil. 2011;25(9):800–813. doi: 10.1177/0269215511399476. [DOI] [PubMed] [Google Scholar]
- 31.Harlapur AM, Kage Vijay B, Basavaraj C. Comparison of myofascial release and positional release therapy in plantar fasciitis: a clinical trial. Indian J Physiother Occup Ther. 2010;4(4):8–11. [Google Scholar]
- 32.Kuhar S, Subhash K, Chitra J. Effectiveness of myofascial release in treatment of plantar fasciitis: a randomized controlled trial. Indian J Physiother Occup Ther. 2007;1(3):3–9. [Google Scholar]
- 33.Ramos-González E, Moreno-Lorenzo C, Matarán-Peñarrocha GA, Guisado-Barrilao R, Aguilar-Ferrándiz ME, Castro-Sánchez AM. Comparative study on the effectiveness of myofascial release manual therapy and physical therapy for venous insufficiency in postmenopausal women. Complement Ther Med. 2012;20(5):291–298. doi: 10.1016/j.ctim.2012.03.005. [DOI] [PubMed] [Google Scholar]
- 34.Davies CC, Brockopp D, Moe K. Astym therapy improves function and range of motion following mastectomy. Breast Cancer (Dove Med Press) 2016;8:39–45. doi: 10.2147/BCTT.S102598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Davies CC, Brockopp DY. Use of ASTYM® treatment on scar tissue following surgical treatment for breast cancer: a pilot study. Rehabil Oncol. 2010;28(3):3–12. [Google Scholar]
- 36.Lee JH, Lee DK, Oh JS. The effect of Graston technique on the pain and range of motion in patients with chronic low back pain. J Phys Ther Sci. 2016;28(6):1852–1855. doi: 10.1589/jpts.28.1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Baker RT, Nasypany A, Seegmiller JG, Baker JG. Instrument-assisted soft tissue mobilization treatment for tissue extensibility dysfunction. Int J Athl Ther Train. 2013;18(5):16–21. [Google Scholar]
- 38.Brantingham JW, Globe GA, Jensen ML, et al. A feasibility study comparing two chiropractic protocols in the treatment of patellofemoral pain syndrome. J Manipulative Physiol Ther. 2009;32(7):536–548. doi: 10.1016/j.jmpt.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 39.Gulick DT. Influence of instrument assisted soft tissue treatment techniques on myofascial trigger points. J Bodyw Mov Ther. 2014;18(4):602–607. doi: 10.1016/j.jbmt.2014.02.004. [DOI] [PubMed] [Google Scholar]
- 40.Englebert DJ, Hartwigsen NR, Krueger MC, Laporte KJ. The effects of myofascial release on medial tibial stress syndrome in collegiate track and field athletes. J Undergrad Kinesiol Res. 2012;7(2):63–72. [Google Scholar]
- 41.Blanchette MA, Normand MC. Augmented soft tissue mobilization vs natural history in the treatment of lateral epicondylitis: a pilot study. J Manipulative Physiol Ther. 2011;34(2):123–130. doi: 10.1016/j.jmpt.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 42.Burke J, Buchberger DJ, Carey-Loghmani MT, Dougherty PE, Greco DS, Dishman JD. A pilot study comparing two manual therapy interventions for carpal tunnel syndrome. J Manipulative Physiol Ther. 2007;30(1):50–61. doi: 10.1016/j.jmpt.2006.11.014. [DOI] [PubMed] [Google Scholar]
- 43.Crothers AL, French SD, Hebert JJ, Walker BF. Spinal manipulative therapy, Graston technique® and placebo for non-specific thoracic spine pain: a randomised controlled trial. Chiropr Man Therap. 2016;24:16. doi: 10.1186/s12998-016-0096-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Heinecke ML, Thuesen ST, Stow RC. Graston technique on shoulder motion in overhead athletes. J Undergrad Kinesiol Res. 2014;10(1):27–39. [Google Scholar]
- 45.Kivlan BR, Carcia CR, Clemente FR, Phelps AL, Martin RL. The effect of Astym® therapy on muscle strength: a blinded, randomized, clinically controlled trial. BMC Musculoskelet Disord. 2015;16:325. doi: 10.1186/s12891-015-0778-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Laudner K, Compton BD, McLoda TA, Walters CM. Acute effects of instrument assisted soft tissue mobilization for improving posterior shoulder range of motion in collegiate baseball players. Int J Sports Phys Ther. 2014;9(1):1–7. [PMC free article] [PubMed] [Google Scholar]
- 47.McCormack JR, Underwood FB, Slaven EJ, Cappaert TA. Eccentric exercise versus eccentric exercise and soft tissue treatment (Astym) in the management of insertional Achilles tendinopathy. Sports Health. 2016;8(3):230–237. doi: 10.1177/1941738116631498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schaefer JL, Sandrey MA. Effects of a 4-week dynamic-balance-training program supplemented with Graston instrument-assisted soft-tissue mobilization for chronic ankle instability. J Sport Rehabil. 2012;21(4):313–326. doi: 10.1123/jsr.21.4.313. [DOI] [PubMed] [Google Scholar]
- 49.Sevier TL, Stegink-Jansen CW. Astym treatment vs. eccentric exercise for lateral elbow tendinopathy: a randomized controlled clinical trial. PeerJ. 2015;3:e967. doi: 10.7717/peerj.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vardiman JP, Siedlik J, Herda T, et al. Instrument-assisted soft tissue mobilization: effects on the properties of human plantar flexors. Int J Sports Med. 2015;36(3):197–203. doi: 10.1055/s-0034-1384543. [DOI] [PubMed] [Google Scholar]
- 51.Wilson JK, Sevier TK, Helfts R, Honing EW, Thomann A. Comparison of rehabilitation methods in the treatment of patellar tendinitis. J Sport Rehabil. 2000;9(4):304–314. [Google Scholar]
- 52.PEDro statistics. Physiotherapy Evidence Database Web site. https://www.pedro.org.au/english/downloads/pedro-statistics Accessed October 23, 2018.
- 53.Tokunaga H. Estimating the mean of a population. In: Knight V, McDuffee Y, Bierach K, DeRosa K, Dickens G, editors. Fundamental Statistics for the Social and Behavioral Sciences. Thousand Oaks, CA: SAGE Publications;; 2016. pp. 271–311. [Google Scholar]
- 54.Prentice WE. Using therapeutic modalities to affect the healing process. In: Morita J, Boyle PJ, editors. Therapeutic Modalities in Rehabilitation 4th ed. New York, NY: McGraw-Hill;; 2011. pp. 19–36. [Google Scholar]
- 55.McKeon Medina JM, McKeon PO, King MA. Building a case for case studies. Int J Athl Ther Train. 2015;20(5):1–5. [Google Scholar]
- 56.Wilk KE, Macrina LC, Fleisig GS, et al. Correlation of glenohumeral internal rotation deficit and total rotational motion to shoulder injuries in professional baseball pitchers. Am J Sports Med. 2011;39(2):329–335. doi: 10.1177/0363546510384223. [DOI] [PubMed] [Google Scholar]
- 57.Shanley E, Kissenberth MJ, Thigpen CA, et al. Preseason shoulder range of motion screening as a predictor of injury among youth and adolescent baseball pitchers. J Shoulder Elbow Surg. 2015;24(7):1005–1013. doi: 10.1016/j.jse.2015.03.012. [DOI] [PubMed] [Google Scholar]
- 58.Shanley E, Rauh MJ, Michener LA, Ellenbecker TS, Garrison JC, Thigpen CA. Shoulder range of motion measures as risk factors for shoulders and elbow injuries in high school softball and baseball players. Am J Sports Med. 2011;39(9):1997–2006. doi: 10.1177/0363546511408876. [DOI] [PubMed] [Google Scholar]
- 59.Hindle KB, Whitcomb TJ, Briggs WO, Hong J. Proprioceptive neuromuscular facilitation (PNF): its mechanisms and effects on range of motion and muscular function. J Hum Kinet. 2012;31:105–113. doi: 10.2478/v10078-012-0011-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cheatham SW, Kolber MJ, Cain M, Lee M. The effects of self-myofascial release using a foam roller or roller massager on joint range of motion, muscle recovery, and performance: a systematic review. Int J Sports Phys Ther. 2015;10(6):827–838. [PMC free article] [PubMed] [Google Scholar]
- 61.Minor MA, Brown JD. Exercise maintenance of persons with arthritis after participation in a class experience. Health Educ Q. 1993;20(1):83–95. doi: 10.1177/109019819302000108. [DOI] [PubMed] [Google Scholar]
- 62.Dobkin PL, Abrahamowicz M, Fitzcharles MA, Dritsa M, da Costa D. Maintenance of exercise in women with fibromyalgia. Arthritis Rheum. 2005;53(5):724–731. doi: 10.1002/art.21470. [DOI] [PubMed] [Google Scholar]
- 63.Robinson ME, Bulcourf B, Atchison JW, et al. Compliance in pain rehabilitation: patient and provider perspectives. Pain Med. 2004;5(1):66–80. doi: 10.1111/j.1526-4637.2004.04002.x. [DOI] [PubMed] [Google Scholar]
- 64.Abbott JH, Patla CE, Jensen RH. The initial effects of an elbow mobilization with movement technique on grip strength in subjects with lateral epicondylalgia. Man Ther. 2001;6(3):163–169. doi: 10.1054/math.2001.0408. [DOI] [PubMed] [Google Scholar]
- 65.Häkkinen A, Salo P, Tarvainen U, Wiren K, Ylinen J. Effect of manual therapy and stretching on neck muscle strength and mobility in chronic neck pain. J Rehabil Med. 2007;39(7):575–579. doi: 10.2340/16501977-0094. [DOI] [PubMed] [Google Scholar]
- 66.El Miedany Y, El Gaarfary M, El Arousy N, Ahmed I, Youssef S, Palmer D. Arthritis education: the integration of patient-reported outcome measures and patient self-management. Clin Exp Rheumatol. 2012;30(6):899–904. [PubMed] [Google Scholar]
- 67.Wagner J, Olson K. A novel treatment tool to address soft tissue dysfunction. J Hand Ther. 2015;28(3):314–318. doi: 10.1016/j.jht.2014.10.005. [DOI] [PubMed] [Google Scholar]
- 68.Therapy, soft tissue. National Library of Medicine Web site. https://meshb.nlm.nih.gov/record/ui?name=Therapy,%20Soft%20Tissue Accessed October 23, 2018.