Abstract
Context
Loss of consciousness (LOC) and impact seizures associated with concussion represent different clinical presentations of concussion; however, they are often investigated and treated similarly. The biomechanical parameters differentiating these 2 distinct signs of injury are poorly described.
Objective
To differentiate between cases of concussions with LOC and those with impact seizures by comparing the impact velocity, peak linear and peak rotational acceleration, as well as brain tissue deformation in the cerebral cortex, white matter, brainstem, cerebellum, thalamus, and corpus callosum.
Design
Descriptive laboratory study.
Patients or Other Participants
Elite American football players who sustained an LOC (n = 20) or impact seizures (n = 21).
Main Outcome Measure(s)
Impact velocity, peak linear and peak rotational acceleration, maximum principal strain, cumulative strain damage measure at 10%, and strain rate (SR).
Results
The SR in the cerebral white matter was greater in the LOC group than in the impact-seizure group. Similar trends were observed for SRs in the cerebral cortex, brainstem, and corpus callosum. No differences were present between groups for the other variables in this study.
Conclusions
A lower SR in certain brain regions helps to explain why motor function is preserved and can be observed in patients with impact seizures versus LOC from concussive injuries.
Keywords: loss of consciousness, impact seizure, strain rate, American football
Key Points
In general, loss of consciousness and impact seizures represent similar severities of brain trauma as defined by brain tissue deformation.
Only strain rate in the white matter was greater in the patients with loss of consciousness than in those with impact seizures.
On rare occasions, impact seizures are an abnormal motor response observed after concussive impacts. The clinical signs include flexor behavior, extensor behavior, or a combination of both in the upper, lower, or both extremities.1–3 The few researchers who have investigated impact seizures after sport-related concussions have used different terms to describe them.
McCrory et al1,4 retrospectively evaluated videos of Australian football and rugby league players after concussive impacts and described 22 cases of abnormal motor response as concussive convulsions. They characterized 6 players as having an initial tonic posture and 20 as having myoclonic jerks. In 17 patients, the abnormal responses were bilateral, whereas 7 players demonstrated some lateralizing features. All athletes had normal imaging, and all returned to play within 2 weeks of the injury.
McCrory and Berkovic5 then prospectively investigated acute motor and convulsive manifestations during sport-related events. They observed 102 cases of concussions that had convulsive or motor (or both) features and classified them as having loss of consciousness (LOC; n = 75), tonic posturing (n = 25), clonic movements (n = 6), unsteady gait (n = 42), and righting movement (n = 40). Again, none of the athletes displayed abnormalities on neuroimaging, and all returned to play without any sequelae.
Hosseini and Lifshitz2 screened videos for the presence of LOC and reported that 66% of patients with concussions who demonstrated LOC had a form of abnormal motor response that they defined as the fencing response. The authors then attempted to replicate similar responses in rats using fluid-percussion injury. They found that the fencing response could only be elicited using an impact consistent with a moderately severe injury but not with a mildly severe or sham impact. The investigators postulated that head impacts with a fencing response represented more severe injuries than those without. Studies using rodents and the fluid-percussion model may not translate well to sport concussions in humans. However, the work of Hosseini and Lifshitz is unique, as they were the only researchers who attempted to characterize the impact magnitudes that led to impact seizures in concussive injuries. To our knowledge, no authors have evaluated impact magnitudes in humans.
The current literature on impact seizures suggests they are closely related to LOC, and the National Football League recently announced that impact seizures would be treated the same as LOC in their concussion protocol (https://www.playsmartplaysafe.com/newsroom/videos/nfl-head-neck-spine-committees-concussion-protocol-overview/). Loss of consciousness and impact seizures seem to be closely related, as they often occur concomitantly; however, they represent different clinical manifestations of concussive injuries. An LOC is characterized by complete loss of motor tone, whereas in impact seizures, a motor response is still present. This difference could be explained by the biomechanics of the concussive events and the resulting brain tissue deformation.
Adams and Victor6 suggested that a sign or symptom of a brain injury is an expression of both the brain regions that are dysfunctional and those that are normally activated or overactivated. The authors expected that patients presenting with impact seizures would experience less brain tissue deformation in specific brain regions, allowing some motor function to be preserved.
Researchers3,7–11 have proposed that both LOC and impact seizures associated with head injuries are caused by dysfunction in the deep structures of the brain, such as the thalamus, brainstem, and cerebellum. The depth of brain tissue deformation is related to impact energy and impact velocity.12,13
Physical reconstruction and finite element (FE) modeling of head impact are tools for estimating brain tissue deformations associated with these impacts and understanding the biomechanics of the impacts that led to them. Past investigators14–16 have used physical reconstruction of head impacts in sports to differentiate between previous concussion and no-injury impacts. Previous authors16–19 have examined the relationship between maximum principal strain (MPS), cumulative strain damage at 10% (CSDM10), and strain rate (SR) and concussions and have reported the metrics associated with these types of injuries. Thresholds for a 50% risk of concussions ranged from 0.19 to 0.27 for MPS, from 0.35 to 0.47 for CSDM10, and from 48.5 to 57.4 s−1 for SR.16–19 These values varied depending on the type of impacts being reconstructed, the model used, and the region of the brain measured. Scientists15,16 have also used peak linear and peak rotational accelerations from laboratory reconstructions to differentiate between concussions and no-injury head impacts. Zhang et al16 reported a 50% risk of concussion in American football players with 82g and 5900 rad/s2 for peak linear and rotational acceleration, respectively. Viano et al15 reported average peak linear acceleration of 94g and average peak rotational acceleration of 6432 rad/s2.
Among American football players, LOC occurs in approximately 8% of reported concussions.20 The incidence of impact seizures in American football athletes has not been studied, nor have the biomechanical differences between these 2 clinical signs of concussion been defined. A better understanding of the biomechanics associated with LOC and impact seizures could provide information about the severity of the impacts that cause them. The purpose of our study was to differentiate between concussions with LOC versus impact seizures by comparing the impact velocity, peak linear and peak rotational acceleration, and brain tissue deformation (ie, MPS, CSDM10, and SR).
METHODS
Patient Selection
We reviewed 5 years of video footage (1280 games) and press releases from elite American football games to identify cases of concussions with abnormal motor responses, concussions with LOC, and concussions with impact seizures. Only players for whom a press release was issued to confirm the concussion diagnosis were included in this study.
We divided the cases of concussion into LOC and impact-seizure groups using video evidence of the impact and the period immediately following (0–2 seconds). The LOC group consisted of athletes who demonstrated a complete loss of motor tone and the inability to protect themselves after the impact. The impact-seizure group consisted of athletes who displayed flexion or extension behavior of the upper or lower extremity (or both) in any combination. Players whose injury status could not be confirmed were not included in this study.
In total, we studied 20 cases of LOC and 21 cases of impact seizures (Table 1). We performed a power analysis on pilot data and determined that 16 cases would be sufficient to observe differences in impact velocity, peak linear and rotational accelerations, and MPS in the brain regions studied. However, for CSDM10 and SR, the suggested sample size varied between 24 and 523 in each group, depending on the brain region. The low incidence of impact seizures in American football players and the strictness of our inclusion and exclusion criteria did not allow us to examine such a large number of cases. However, these data were reported and the trends interpreted in consideration of the statistical power.
Table 1.
Cases of Loss of Consciousness and Impact Seizures Caused by Falls, Helmet-to-Helmet Collisions, and Shoulder-to-Helmet Collisions, No.
| Injury Status |
Falls |
Helmet-to-Helmet Collisions |
Shoulder-to-Helmet Collisions |
Total |
| Loss of consciousness | 3 | 11 | 6 | 20 |
| Impact seizures | 2 | 10 | 9 | 21 |
Video Analysis for Injury Reconstructions
We performed a video analysis to obtain the impact velocity, impact location and direction, and type of event leading to the injury (helmet-to-helmet collision, shoulder-to-helmet collision, or fall). We calculated the impact velocity using Kinovea (version 0.8.20; Bordeaux, France) by determining the distance between the injured player's head and the impacting surface using a calibration grid placed on the field at a set time before the impact (0.04–0.20 seconds). We set the time before impact when we determined that no changes in the player's velocity before impact were observable (ie, not being slowed down by a tackle elsewhere on the body or not being slowed down in a fall by protecting himself with his arms). To measure velocity using this method, we had to be able to observe the head of the injured player at the time of the impact and at the required timeframe for distance measurement. We also had to observe known markers in the frame and on the field to apply a calibration grid and measure the distance before the impact. A calibration grid had to be placed on the playing surface in proximity to and in the general orientation of the impact. These methods yielded measurement errors of less than 10% in ice hockey.21 Given that the error is vastly influenced by the size and proximity of the calibration grid, it is likely to be similar or smaller in American football since there are more markings on the field to establish an optimal calibration grid.
Impact Reconstructions
We collected the 3-dimensional peak linear and rotational acceleration using a Hybrid III headform (Humanetics Innovative Solutions, Farmington Hills, MI) with a 3-2-2-2 array of accelerometers22 mounted on an unbiased neck that allowed similar movement in all planes of motion.23 Several anthropomorphic test devices exist for head-impact testing and have both benefits and disadvantages. We chose to perform the reconstruction using the Hybrid III because it is the most widely used human impact-test surrogate. Also, the unbiased neck was designed to mimic the Hybrid III headform without the directional bias.
We reconstructed injuries caused by falls using a monorail drop rig consisting of a drop carriage attached to a 4.7-m-long rail. A pneumatic piston releases the carriage once it has reached the height necessary to obtain the desired impact velocity. A photoelectric time gate placed within 0.02 m of the impact measures impact velocity. The ground was represented by a sample of artificial turf (ACT Global sports turf, Austin, TX) that included the layers and materials that are common in artificial American football fields.
We performed reconstructions of helmet-to-helmet and shoulder-to-helmet collisions using a pneumatic linear impactor consisting of an impacting arm, sliding table, and frame. For helmet-to-helmet collisions, the impacting arm mass was 15.9 kg and equipped with a Hybrid III headform wearing an American football helmet, for a total striking mass of 24.0 kg. We used an impacting arm of 13.1-kg mass with a 0.142-m-thick VN 602 foam (Rubatex International, Bedford, VA) and an American football shoulder pad24 to reconstruct the shoulder-to-helmet impacts. A photoelectric time gate placed within 0.02 m of the impact measured impact velocity. Figure 1 displays the reconstruction setups for each type of event.
Figure 1.
Reconstruction setups for each type of event. A, Fall. B, Helmet-to-helmet collision. C, Shoulder-to-helmet collision.
We used video analysis to determine the helmets the injured players were wearing and used helmets with the same type of liners for our reconstructions. We did not impact helmets at the same location for more than 1 case (3 trials) for the purpose of consistency.
Finite Element Modeling
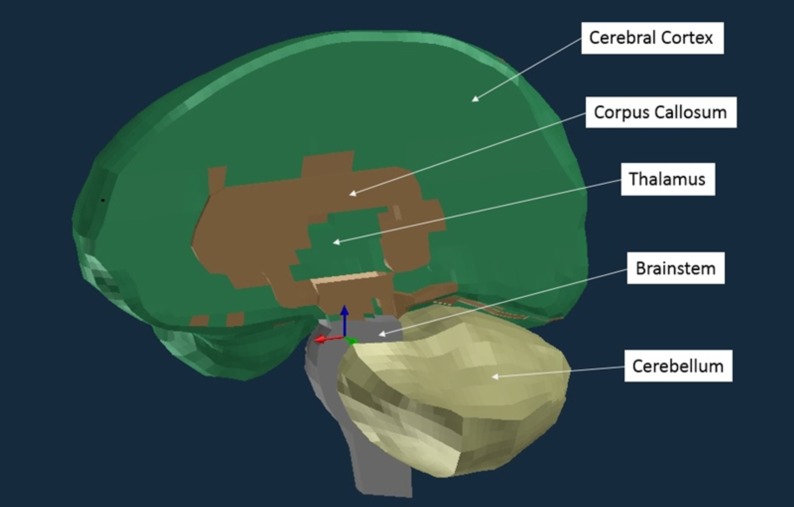
We used the Wayne State University Brain Injury Model (WSUBIM) to obtain the MPS, CSDM10, and SR for the cerebral cortex, cerebral white matter, brainstem, cerebellum, thalamus, and corpus callosum (Figure 2). We used PAM-CRASH software (ESI, Farmington Hills, MI) to run the simulations. The material properties and model validation can be found in Zhang et al.25 We chose the WSUBIM because, of the models available to us, it possessed the most stable brainstem, even though this region has not been validated in any FE brain model.
Figure 2.
Wayne State University Brain Injury Model of the cerebral cortex, cerebral white matter, brainstem, cerebellum, thalamus, and corpus callosum. The remainder of the cerebral white matter (located deep to the cerebral cortex) is not visible in this image.
Statistical Analysis
We performed independent-samples t tests to investigate the differences between cases of LOC and cases of LOC with impact seizures for peak linear acceleration, peak rotational acceleration, and impact velocity as well as MPS, CSDM10, and SR in the cerebral cortex, the remainder of the cerebral white matter, the brainstem, the cerebellum, the thalamus, and the corpus callosum. The remainder of the cerebral white matter was defined as the white matter identified by the WSUBIM without the portion corresponding to the corpus callosum. The α level was set to .05. Effect sizes were calculated using the Cohen d.
RESULTS
The kinematic data are reported in Table 2. Average brain tissue deformations (MPS, CSDM10, and SR) are reported in Tables 3 through 5. We did not find statistical differences between the LOC group and the impact-seizures group for any variables except SR in the white matter, which was higher for the LOC group.
Table 2.
Impact Velocity, Peak Linear Acceleration, and Peak Rotational Acceleration for Groups With Loss of Consciousness and Impact Seizures
| Kinematic Variables |
Mean ± SD |
Effect Size (Cohen d) |
P Value |
Power |
|
| Loss of Consciousness |
Impact Seizures |
||||
| Impact velocity, m/s | 8.7 ± 1.6 | 8.7 ± 1.5 | 0.02 | .975 | 0.979 |
| Peak linear acceleration, g | 80.9 ± 39.8 | 64.2 ± 32.3 | 0.46 | .150 | 0.835 |
| Peak rotational acceleration (rad/s2) | 5595 ± 2472 | 4972 ± 2379 | 0.25 | .416 | 0.829 |
Table 3.
Maximum Principal Strain in the Cerebral Cortex, Cerebral White Matter, Brainstem, Cerebellum, Thalamus, and Corpus Callosum Associated With Loss of Consciousness and Impact Seizures
| Brain Region |
Mean ± SD |
Effect Size (Cohen d) |
P Value |
Power |
|
| Loss of Consciousness |
Impact Seizures |
||||
| Cerebral cortex | 0.59 ± 0.27 | 0.48 ± 0.15 | 0.46 | .149 | 0.898 |
| Cerebral white matter | 0.47 ± 0.18 | 0.39 ± 0.10 | 0.57 | .077 | 0.951 |
| Brainstem | 0.38 ± 0.16 | 0.32 ± 0.10 | 0.34 | .286 | 0.914 |
| Cerebellum | 0.22 ± 0.08 | 0.20 ± 0.06 | 0.19 | .542 | 0.924 |
| Thalamus | 0.33 ± 0.15 | 0.28 ± 0.09 | 0.40 | .223 | 0.877 |
| Corpus callosum | 0.38 ± 0.18 | 0.32 ± 0.10 | 0.42 | .237 | 0.862 |
Table 5.
Strain Rates (s−1) in the Cerebral Cortex, Cerebral White Matter, Brainstem, Cerebellum, Thalamus, and Corpus Callosum Associated With Loss of Consciousness and Impact Seizuresa
| Brain Region |
Mean ± SD |
Effect Size (Cohen d) |
P Value |
Power |
|
| Loss of Consciousness |
Impact Seizures |
||||
| Cerebral cortex | 98.5 ± 58.9 | 70.5 ± 30.5 | 0.59 | .068 | 0.764 |
| Cerebral white matter | 83.7 ± 47.7 | 57.8 ± 23.4 | 0.69 | .037 | 0.842 |
| Brainstem | 60.8 ± 32.7 | 49.7 ± 21.5 | 0.40 | .205 | 0.769 |
| Cerebellum | 46.7 ± 22.8 | 42.4 ± 17.8 | 0.21 | .503 | 0.770 |
| Thalamus | 55.4 ± 30.4 | 53.8 ± 18.0 | 0.46 | .144 | 0.647 |
| Corpus callosum | 67.9 ± 40.3 | 51.9 ± 21.3 | 0.49 | .118 | 0.729 |
Significant differences are demonstrated in bold.
Table 4.
Cumulative Strain Damage at 10% in the Cerebral Cortex, Cerebral White Matter, Brainstem, Cerebellum, Thalamus, and Corpus Callosum Associated With Loss of Consciousness and Impact Seizures
| Brain Region |
Mean ± SD |
Effect Size (Cohen d) |
P Value |
Power |
|
| Loss of Consciousness |
Impact Seizures |
||||
| Cerebral cortex | 0.49 ± 0.19 | 0.47 ± 0.19 | 0.11 | .733 | 0.857 |
| Cerebral white matter | 0.45 ± 0.26 | 0.42 ± 0.23 | 0.11 | .720 | 0.569 |
| Brainstem | 0.21 ± 0.21 | 0.17 ± 0.20 | 0.15 | .628 | 0.050 |
| Cerebellum | 0.26 ± 0.23 | 0.19 ± 0.20 | 0.32 | .308 | 0.126 |
| Thalamus | 0.48 ± 0.33 | 0.43 ± 0.27 | 0.16 | .621 | 0.386 |
| Corpus callosum | 0.55 ± 0.30 | 0.55 ± 0.24 | 0.06 | .985 | 0.605 |
| Full brain | 0.45 ± 0.22 | 0.42 ± 0.18 | 0.15 | .641 | 0.746 |
DISCUSSION
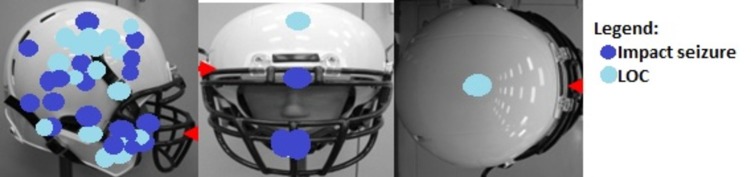
We hypothesized that the impact-seizures group would have lower magnitudes of brain tissue deformation. This hypothesis was true for the SR and significant in the white matter (effect size = 0.69). The cerebral cortex, brainstem, and corpus callosum showed similar trends in that the impact-seizures group had lower SRs, but the differences did not reach statistical significance. However, the observed statistical power was insufficient (<0.80) to dismiss the possibility that the SR in these regions might have been greater in the LOC group if we could have included a larger number of cases. Several researchers26–29 have described SR as being an important measure of neuronal dysfunction and neuronal cell death. This supports the notion that impact seizures may be the manifestation of function being preserved in certain brain regions or white matter tracts that allow certain muscle groups to be activated. Impact seizures were associated with a lower rotational acceleration around the x axis (P = .039), which explains the lower SRs. This difference is likely to be the result of differences in impact location between the groups. Our sample size did not allow for statistical analysis to determine differences among impact locations, but the LOC group seemed to be associated with 2 clusters of impact sites: 1 on the side and centric in nature and the other noncentric. In contrast, impact locations associated with impact seizures were sparser (Figure 3).
Figure 3.
Impact locations. Abbreviation: LOC, loss of consciousness.
For MPS and CSDM10, no statistical differences were present between the groups for any brain regions. Despite the low power associated with CSDM10, the differences between the groups were minimal and may not be clinically relevant. The power was sufficient to conclude that there were no differences in MPS between the LOC and impact-seizures groups for any brain regions. The LOC and impact-seizures group were homogeneous in terms of impact velocity and the types of events that led to the injury. It is possible that we did not detect differences in magnitudes and volume of strain between the groups because the MPS and CSDM10 are less sensitive to the interactions of the factors that characterize the impacts (impact velocity, impact location and direction, compliance, and striking mass) when the 2 groups are so similar. The magnitudes of strain in our injury groups were greater than those described by researchers using similar methods. This suggests that LOC or impact seizures (or both) are more severe than concussions without these signs in terms of the magnitude of the strain. Kleiven17 observed that an MPS of 0.21 indicated a 50% risk of concussion, whereas Patton et al18 suggested MPS values of 0.15, 0.15, and 0.27 indicated a 50% concussion risk in the midbrain, corpus callosum, and gray matter, respectively. As expected, both research teams17,18 reported mean values below those we obtained in all brain regions. Because these researchers used FE brain models with different brain material properties, comparisons across FE models should be cautious. Using the WSUBIM, Zhang et al16 found that an MPS of 0.24 defined an 80% risk of concussive injury in American football players. All the MPS values we demonstrated in this study were above the 80% risk value suggested by Zhang et al16 except the cerebellum. Magnitudes of brain tissue strain are linked to injury severity: ie, higher strains are associated with greater dysfunction and perhaps tissue death.27–29 Although this research was not designed to provide information on return-to-play protocols after LOC and impact seizures associated with concussive impacts, their association with severity of brain trauma suggests that further work is needed to understand the importance of delaying the return to contact sports when an athlete displays these signs.
We did not detect any differences in impact velocity, peak linear acceleration, or peak rotational acceleration between the 2 groups. Our groups consisted of cases involving the 3 most common types of concussive events among American football players: falls, helmet-to-helmet collisions, and shoulder-to-helmet collisions.30 These events resulted in different shapes of acceleration curves characterized by different peak values and durations of acceleration. The values of peak linear and peak rotational acceleration reported in this study were lower than those previously noted for concussions in American football athletes,15,16 despite resulting in greater brain tissue deformation. Thus, an interaction was present between event types and peak acceleration values; for meaningful interpretations, peak acceleration thresholds should be specific to the event types.
Limitations
Certain limitations are inherent to the methods used in this study. We assigned athletes with confirmed concussions to different groups based on video analysis and not onsite evaluations due to an inability to access this information. To ensure accuracy in group designation, we were careful to eliminate cases for which the response was ambiguous, but we could have misinterpreted an athlete's responses during the video analysis. Ambiguous cases of LOC consisted of videos in which it was unclear whether the athletes were simply resting on the ground for a period of time or were truly unconscious. Cases of impact seizures in which athletes displayed a brief motor response (<1 second) were discarded to avoid including those in which the motor response was either voluntary or the result of inertia of the limbs after the impacts. Impact seizures could have been misinterpreted as LOC if the motor response occurred during the fall that ensued from the collision or if the athlete was positioned in such a way that the motor response could not be seen.
Physical reconstructions and FE modeling of head impacts do not take into consideration individual anatomical and physiological differences that may influence an individual's tolerance to head impacts and associated responses. The biofidelity of headforms is sometimes compromised to improve reproducibility of testing. Comparisons of responses between headforms can be difficult. Furthermore, FE brain models only provide an approximation of the brain tissue deformation sustained during the head impact and have specific limitations. For example, the material properties are established based on in vivo and in vitro animal models of tissue deformation, and the methods used in these studies influenced the results. Zhang et al25 validated the WSUBIM for pressure and brain motion by studying cadavers, which may not fully represent what happens in a live athlete. In addition, these validations did not include all the brain regions we analyzed, such as the brainstem and cerebellum.25 Another limitation of the FE model is that it is a gross representation of the brain. Improving the details and axonal arrangement of certain brain regions could result in clearer distinctions among various outcomes of clinical presentation. Despite the limitations associated with FE models of the brain, the models were useful for comparisons between the groups.
CONCLUSIONS
We found that the SR in the white matter differentiated between the LOC and impact-seizures groups. Other regions, such as the cerebral cortex, brainstem, and corpus callosum, also presented with lower SRs in the impact-seizures group compared with the LOC group. In addition, we showed that athletes with LOC and impact seizures sustained similar magnitudes and volumes of strain. Overall, concussions with impact seizures were similar to concussions with LOC but both types were more severe than concussions without these signs.
REFERENCES
- 1.McCrory PR, Berkovic SF. Concussive convulsions. Incidence in sport and treatment recommendations. Sports Med. 1998;25(2):131–136. doi: 10.2165/00007256-199825020-00005. [DOI] [PubMed] [Google Scholar]
- 2.Hosseini AH, Lifshitz J. Brain injury forces of moderate magnitude elicit the fencing response. Med Sci Sports Exerc. 2009;41(9):1687–1697. doi: 10.1249/MSS.0b013e31819fcd1b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bricolo A, Turazzi S, Alexandre A, Rizzuto N. Decerebrate rigidity in acute head injury. J Neurosurg. 1997;47(5):680–689. doi: 10.3171/jns.1977.47.5.0680. [DOI] [PubMed] [Google Scholar]
- 4.McCrory PR, Bladin PF, Berkovic SF. Retrospective study of concussive convulsions in elite Australian Rules and rugby league footballers: phenomenology, aetiology, and outcome. BMJ. 1997;314(7075):171–174. doi: 10.1136/bmj.314.7075.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McCrory PR, Berkovic SF. Video analysis of acute motor and convulsive manifestations in sport-related concussion. Neurology. 2000;54(7):1488–1491. doi: 10.1212/wnl.54.7.1488. [DOI] [PubMed] [Google Scholar]
- 6.Adams R, Victor M. Principles of Neurology 3rd ed. New York, NY: McGraw-Hill Medical;; 1985. [Google Scholar]
- 7.Thiele FH. On the efferent relationship of the optic thalamus and Deiter's nucleus to the spinal cord, with special reference to the cerebellar influx of Dr Hughlings Jackson and the genesis of the decerebrate rigidity of Ord and Sherrington. J Physiol. 1905;32(5–6):358–384. doi: 10.1113/jphysiol.1905.sp001089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davis RA, Davis L. Decerebrate rigidity in humans. Neurosurgery. 1982;10(5):635–642. doi: 10.1227/00006123-198205000-00017. [DOI] [PubMed] [Google Scholar]
- 9.Gentry LR, Godersky JC, Thompson B. MR imaging of head trauma: review of the distribution and radiopathologic features of traumatic lesions. AJR Am J Roentgenol. 1988;150(3):663–672. doi: 10.2214/ajr.150.3.663. [DOI] [PubMed] [Google Scholar]
- 10.Levin HS, Mendelsohn D, Lilly MA, et al. Magnetic resonance imaging in relation to functional outcome of pediatric closed head injury: a test of the Ommaya-Gennarelli model. Neurosurgery. 1997;40(3):432–440. doi: 10.1097/00006123-199703000-00002. [DOI] [PubMed] [Google Scholar]
- 11.Levin HS, Williams D, Crofford MJ, et al. Relationship of depth of brain lesions to consciousness and outcome after closed head injury. J Neurosurg. 1988;69(6):861–866. doi: 10.3171/jns.1988.69.6.0861. [DOI] [PubMed] [Google Scholar]
- 12.Lighthall JW. Controlled cortical impact: a new experimental brain injury model. J Neurotrauma. 1988;5(1):1–15. doi: 10.1089/neu.1988.5.1. [DOI] [PubMed] [Google Scholar]
- 13.Goodman JC, Cherian L, Bryan RM, Jr, Robertson CS. Lateral cortical impact injury in rats: pathologic effects of varying cortical compression and impact velocity. J Neurotrauma. 1994;11(5):587–597. doi: 10.1089/neu.1994.11.587. [DOI] [PubMed] [Google Scholar]
- 14.Pellman EJ, Viano DC, Tucker AM, Casson IR, Waeckerle JF. Concussion in professional football: reconstruction of game impacts and injuries. Neurosurgery. 2003;53(4):799–814. doi: 10.1093/neurosurgery/53.3.799. [DOI] [PubMed] [Google Scholar]
- 15.Viano DC, Casson IR, Pellman EJ, et al. Concussion in professional football: brain responses by finite element analysis: part 9. Neurosurgery. 2005;57(5):891–916. doi: 10.1227/01.neu.0000186950.54075.3b. [DOI] [PubMed] [Google Scholar]
- 16.Zhang L, Yang KH, King AI. A proposed injury threshold for mild traumatic brain injury. J Biomech Eng. 2004;126(2):226–236. doi: 10.1115/1.1691446. [DOI] [PubMed] [Google Scholar]
- 17.Kleiven S. Predictors for traumatic brain injuries evaluated through accident reconstructions. Stapp Car Crash J. 2007;51:81–114. doi: 10.4271/2007-22-0003. [DOI] [PubMed] [Google Scholar]
- 18.Patton DA, McIntosh AS, Kleiven S. The biomechanical determinants of concussions: finite element simulations to investigate brain tissue deformations during sporting impacts to the unprotected head. J Appl Biomech. 2013;29(6):721–730. doi: 10.1123/jab.29.6.721. [DOI] [PubMed] [Google Scholar]
- 19.Viano DC, Casson IR, Pellman EJ. Concussion in professional football: biomechanics of the struck player–part 14. Neurosurgery. 2007;61(2):313–328. doi: 10.1227/01.NEU.0000279969.02685.D0. [DOI] [PubMed] [Google Scholar]
- 20.Casson IR, Viano DC, Powell JW, Pellman EJ. Twelve years of National Football League concussion data. Sports Health. 2010;2(6):471–483. doi: 10.1177/1941738110383963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Post A, Koncan D, Kendall M, et al. Analysis of speed accuracy using video analysis software. Sports Eng. 2018;21(3):235–241. [Google Scholar]
- 22.Padgaonkar AJ, Krieger KW, King AI. Measurement of angular acceleration of a rigid body using linear accelerometers. J Appl Mech. 1975;42(3):552–556. [Google Scholar]
- 23.Walsh ES, Kendall M, Post A, Meehan A, Hoshizaki TB. Comparative analysis of Hybrid III neckform and an unbiased neckform. Sports Eng. 2018;21(4):479–485. [Google Scholar]
- 24.Rousseau P, Hoshizaki TB. Defining the effective impact mass of elbow and shoulder strikes in ice hockey. Sports Biomech. 2015;14(1):57–67. doi: 10.1080/14763141.2015.1025236. [DOI] [PubMed] [Google Scholar]
- 25.Zhang L, Yang KH, Dwarampudi R, et al. Recent advances in brain injury research: a new human head model development and validation. Stapp Car Crash J. 2001;45:369–394. doi: 10.4271/2001-22-0017. [DOI] [PubMed] [Google Scholar]
- 26.Cullen DK, Simon CM, LaPlaca MC. Strain rate-dependent induction of reactive astrogliosis and cell death in three-dimensional neuronal-astrocytic co-cultures. Brain Res. 2007;1158:103–115. doi: 10.1016/j.brainres.2007.04.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morrison B, III, Cater HL, Wang CC, et al. A tissue tolerance criterion for living brain developed with an in vitro model of traumatic mechanical loading. Stapp Car Crash J. 2003;47:93–105. doi: 10.4271/2003-22-0006. [DOI] [PubMed] [Google Scholar]
- 28.Elkin BS, Morrison B., III Region-specific tolerance criteria for the living brain. Stapp Car Crash J. 2007;51:127–138. doi: 10.4271/2007-22-0005. [DOI] [PubMed] [Google Scholar]
- 29.Geddes DM, Cargill RS, II, LaPlaca MC. Mechanical stretch to neurons results in a strain rate and magnitude-dependent increase in plasma membrane permeability. J Neurotrauma. 2003;20(10):1039–1049. doi: 10.1089/089771503770195885. [DOI] [PubMed] [Google Scholar]
- 30.Pellman EJ, Powell J, Viano DC, et al. Concussion in professional football: epidemiological features of game injuries and review of the literature–part 3. Neurosurgery. 2004;54(1):81–96. doi: 10.1227/01.neu.0000097267.54786.54. [DOI] [PubMed] [Google Scholar]