Abstract
Purpose
Chronic obstructive pulmonary disease (COPD) is a major pulmonary disease. However, few studies have investigated the relationship between COPD and prostate cancer (PCa). This study aimed to investigate the association between COPD severity and PCa risk.
Patients and methods
We conducted a nationwide population-based cohort study utilizing data from 2001 to 2013 from the National Health Insurance Research Database of Taiwan. Cox proportional hazards models with 1:1 propensity score-matched analysis were used to investigate the association between COPD and PCa risk. We further divided the COPD group according to severe complications (including acute respiratory failure, cardiopulmonary arrest, pneumonia, and acute exacerbation) to test for the relationship between COPD severity and PCa risk.
Results
This study included 47,634 patients (23,817 COPD patients and 23,817 matched non-COPD controls). Among them, 756 (1.59%) were diagnosed with PCa during a mean follow-up period of 7.05±4.13 years; 387 (1.62%) were from the COPD group and 369 (1.55%) were from the control group. Compared with the patients without COPD, the adjusted hazard ratio (HR) for PCa in the COPD patients was 1.10 (95% confidence interval [CI] 0.95–1.27), while that in the COPD patients with complications was 2.46 (95% CI 1.96–3.61).
Conclusions
An increased risk for PCa was found among the COPD patients with complications. COPD complications included acute respiratory failure, cardiopulmonary arrest, pneumonia, and acute exacerbation. These findings may help physicians in treating COPD with complications and in remaining alert to the potential development of PCa.
Keywords: National Health Insurance Research Database, prostate cancer, chronic obstructive pulmonary disease
Introduction
Prostate cancer (PCa) is the cancer most commonly diagnosed in men in the United States, with 161,360 new cases in 2017.1 Recently, the incidence of PCa has been increasing in Taiwan.2 At present, PCa is the fifth most common cancer among men in Taiwan, with 5,106 new cases in 2015.2 The etiological factors of PCa include age, family history, and race.3 Additionally, chronic inflammation has been identified as a precursor of prostate carcinogenesis,4 and proliferative inflammatory atrophy (PIA) has been reported to progress to PCa.5 Inflammation of the prostate may increase the production of inflammatory cytokines and reactive oxygen species (ROS), which increase cellular proliferation or carcinogenesis.4
Chronic obstructive pulmonary disease (COPD) is a major disease worldwide6 and has been predicted to be the third leading cause of death in 2020.7 A recent epidemiological survey found that the prevalence of COPD in Taiwan is 6.1%.8 Smoking, occupational pollution, and air pollution are major etiological factors for COPD.9 The condition is characterized by progressive persistent airflow limitation. Airflow limitation develops from chronic inflammation due to the presence of noxious particles or gases in the airways.10
The parthogeneses of both PCa and COPD include chronic inflammation. However, there have been only a limited number of studies discussing the association between PCa and COPD. We thus conducted a nationwide population-based cohort study in Taiwan using a nationwide database to investigate the association between COPD severity and the risk of PCa.
Materials and methods
Data source and collection
This was a retrospective population-based study that used data from Taiwan’s National Health Insurance Research Database (NHIRD), a huge database containing data from the National Health Insurance (NHI) program. The NHI program is a unique health and medical insurance system in Taiwan that was started in 1995. As of the end of 2013, the NHI program covered 99.9% of Taiwan’s 23 million residents.11 The NHIRD contains demographic data, medical records, and data on clinical procedures administered to all inpatients, outpatients, and emergency room patients. We used a sub-dataset of the NHIRD called the Longitudinal Health Insurance Database 2000 (LHID2000), a database that contains the data of one million people randomly selected from the larger NHIRD in 2000. The LHID2000 and NHIRD are similar in terms of demographic data and origin population.12 The clinical diagnoses of patients in the database were made according to the International Classification of Diseases, 9th revision, Clinical Modification (ICD-9-CM). This study was approved by the Institutional Review Board of the Tri-Service General Hospital (approval number: TSGHIRB NO B-104-21).
Study population
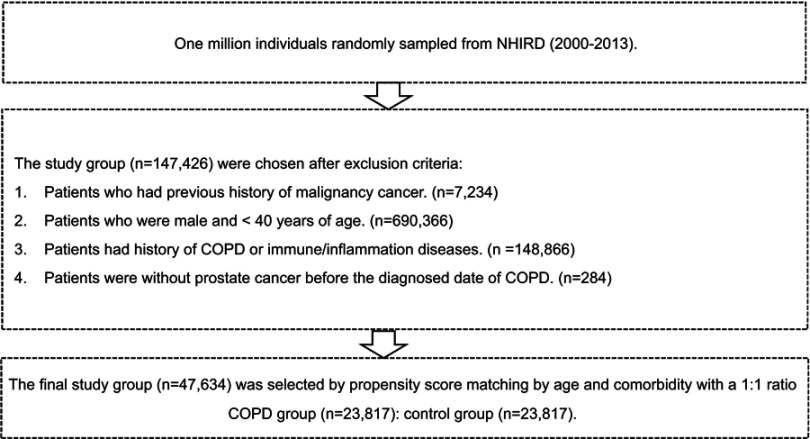
The study subjects were selected using LHID2000 data from January 2000 to December 2013. Patients who were newly diagnosed with COPD (ICD-9-CM: 491,492, and 496) between 2001 and 2013 were enrolled in this study (Figure 1). The COPD diagnoses were performed by licensed physicians. The exclusion criteria were as follows: (1) patients who were diagnosed with a malignancy before January 1, 2001 (n=7,234), (2) patients who were male and <40 years old (n=690,366), (3) patients with a history of COPD or any immune or inflammatory diseases (n=148,866), and (4) patients with incomplete medical records (n=284) (Figure 1). A total of 23,817 patients with COPD were ultimately enrolled in the study group.
Figure 1.
Flowchart of study design.
Abbreviation: NHIRD, National Health Insurance Research Database.
The control group was determined through a 1:1 matching with the study group considering age and income. Thus, 23,817 subjects were enrolled in the control group. For the study group, each patient’s date of COPD diagnosis was assigned as the index date and considered the starting point for the investigation of that patient, whereas for the control group, the year of the index date was a matched year in which the control subjects had utilized a medical service.
We also defined COPD severity according to any acute respiratory events subsequent to the COPD diagnosis. Subsequent acute respiratory events included acute exacerbation (ICD-9-CM: 491.21), pneumonia (ICD-9-CM: 480-486), acute respiratory failure (ICD-9-CM: 518.81), and cardiopulmonary arrest (ICD-9-CM: 799.1, 798, and 427.5).
Outcomes
There were 47,634 subjects in this study (23,817 COPD patients and 23,817 control group patients). Each subject was monitored until December 2013 to identify those who were newly diagnosed with PCa (ICD-9-CM: 185).13
All medical diagnoses of the patients and any procedures and drugs administered to them were recorded completely during the follow-up period. Each COPD diagnosis (ICD-9-CM: 491, 492, and 496) was made by chest physicians based on at least two outpatient department visits or at least one admission during the follow-up period. More specifically, each clinical diagnosis of COPD was made according to typical symptoms such as chronic cough, dyspnea, and sputum production; exposure to risk factors such as tobacco smoking, occupational pollution, and indoor air pollution; and spirometry test results. The acute respiratory events subsequent to COPD, including pneumonia, acute exacerbation, acute respiratory failure, and cardiopulmonary arrest, were also diagnosed by chest physicians, cardiologists, or emergency physicians based on emergency room and admission records during the follow-up period. We selected patients aged >40 years due to the extremely low prevalence of COPD in patients aged <40 years.14
The diagnosis of PCa was based on the ICD-9-CM coding system (ICD-9-CM: 185) and was included in the Registry for Catastrophic Illness Patient Database (RCIPD).15 Patients with PCa who were listed in the RCIPD were relieved of any medical payment obligations after receiving a catastrophic illness certification. After experts confirmed the accuracy of a PCa diagnosis by the NHI administration with pathological and cytological reports, patients with PCa were given catastrophic illness certifications.
Variables
Various comorbidities, including alcohol abuse, tobacco use disorder, obesity, diabetes mellitus, hypertension, hyperlipidemia, liver disease, cerebrovascular disease, coronary heart disease (CHD), and chronic kidney disease (CKD) (ICD-9-CM codes in Table S1), were regarded as variables.
Table S1.
ICD-9 diagnostic codes of variables
| Covariates | ICD-9-CM codes |
|---|---|
| Alcohol abuse | 303, 305.0, V11.3 |
| Tobacco use disorder | 305.1, 491.0, 491.2, 492.8, 496, 523.6, 649.0, 989.84, V15.82 |
| Obesity | 278.0 |
| Diabetes mellitus | 250 |
| Hypertension | 401, 402, 403, 404, 405 |
| Hyperlipidemia | 272 |
| Coronary heart disease | 410, 411, 412, 413, 414 |
| Chronic kidney disease | 585, 586, 588 |
| Liver disease | 456, 571, 572 |
| Cerebral vascular accident | 430, 431, 432, 433, 434, 435, 436, 437, 438 |
Abbreviation: ICD-9, International Classification of Diseases-9th revision.
The patients were classified into four age groups: 40–49, 50–59, 60–69, and ≥70 years. They were also categorized into four groups based on monthly income in New Taiwan Dollars (NTD): <NTD 16,500; NTD 16,500–19,199; NTD 19,200–33,299; and ≥NTD 33,300.
Statistical analysis
Categorical variables were expressed as frequencies and percentages, and numerical variables were expressed as means and standard deviations. The Chi-square test was performed to examine differences in the categorical variables among the study groups, and an independent t-test was performed to examine differences in the numerical variables among the study groups. Moreover, we used Cox proportional hazards regression models to estimate the effects of risk factors on the hazard ratios (HRs) and 95% confidence intervals (CIs). The proportion of the study group with PCa was analyzed using Kaplan-Meier curves and the log-rank test. All multivariable Cox regression models were adjusted for variables including age, COPD, and comorbidities. A two-sided p-value of <0.05 was considered statistically significant. All statistical methods were performed using SAS for Windows, version 9.4 (SAS Institute Inc., Cary, NC).
Results
There were 47,634 patients (23,817 COPD patients and 23,817 control group patients) with a mean age of 63.72±12.29 years enrolled in this study. The demographic characteristics of the subjects are listed in Table 1. The COPD patients mostly belonged to the older-age group, and hypertension, CHD, and liver disease were the three most common comorbidities among them. Compared with the non-COPD patients, the COPD patients had higher Charlson comorbidity index scores; higher rates of CHD, CKD, liver disease, and cerebrovascular accident; and lower rates of hypertension and hyperlipidemia. Regarding complications, 126 (0.26%) of the COPD patients had pneumonia, acute exacerbation, and acute respiratory failure. Among all the patients, 756 (1.59%) were diagnosed with PCa during a mean follow-up period of 7.05±4.13 years; 387 (1.62%) of them were from the COPD group and 369 (1.55%) were from the control group.
Table 1.
Characteristics of study population
| Male subjects | Total | Non-COPD | COPD | p-value | |||
|---|---|---|---|---|---|---|---|
| n=47,634 | n=23,817 | n=23,817 | |||||
| n | % | n | % | n | % | ||
| Age (mean ± SD, years) | 63.72±12.29 | 63.49±12.22 | 63.95±12.34 | 0.858 | |||
| 40–49 | 7,871 | 16.52 | 3,938 | 16.53 | 3,933 | 16.51 | |
| 50–59 | 9,227 | 19.37 | 4,620 | 19.40 | 4,607 | 19.34 | |
| 60–69 | 12,204 | 25.62 | 6,103 | 25.62 | 6,101 | 25.62 | |
| >70 | 18,332 | 38.49 | 9,156 | 38.44 | 9,176 | 38.53 | |
| Insurance income (monthly) | 0.571 | ||||||
| 0 | 6,281 | 13.19 | 3,294 | 13.84 | 3,364 | 13.54 | |
| 0–16,500 | 10,224 | 21.46 | 4,868 | 20.44 | 4,856 | 20.48 | |
| 16,500–19,200 | 3,558 | 7.47 | 1,775 | 7.45 | 1,783 | 7.58 | |
| 19,200–33,300 | 18,930 | 39.74 | 9,123 | 38.30 | 8,918 | 39.18 | |
| ≧33,300 | 8,641 | 18.14 | 4,757 | 19.97 | 4,896 | 19.22 | |
| Charlson comorbidity index (mean ± SD) | 1.38±1.60 | 1.23±1.55 | 1.53±1.64 | <0.0001** | |||
| 0 | 16,732 | 35.13 | 9,992 | 41.95 | 6,740 | 28.30 | |
| 1 | 14,077 | 29.55 | 6,341 | 26.62 | 7,736 | 32.48 | |
| 2 | 8,070 | 16.94 | 3,561 | 14.95 | 4,509 | 18.93 | |
| ≥3 | 8,755 | 18.38 | 3,923 | 16.47 | 4,832 | 20.29 | |
| Comorbidity | |||||||
| Alcohol abuse | 525 | 1.10 | 282 | 1.18 | 243 | 1.02 | 0.095 |
| Tobacco use disorder | 1,300 | 2.73 | 659 | 2.77 | 641 | 2.69 | 0.632 |
| Obesity | 156 | 0.33 | 72 | 0.30 | 84 | 0.35 | 0.377 |
| Diabetes | 10,434 | 21.9 | 5,220 | 21.92 | 5,214 | 21.89 | 0.955 |
| Hypertension | 24,341 | 51.10 | 12,326 | 51.75 | 12,015 | 50.45 | 0.004* |
| Hyperlipidemia | 10,294 | 21.61 | 5,250 | 22.04 | 5,044 | 21.18 | 0.022* |
| Liver disease | 8,289 | 17.4 | 3,013 | 12.65 | 5,276 | 22.15 | <0.0001** |
| Chronic kidney disease | 2,372 | 4.98 | 1,132 | 4.75 | 1,240 | 5.21 | 0.024* |
| Coronary heart disease | 12,908 | 27.1 | 6,347 | 26.65 | 6,561 | 27.55 | 0.028* |
| Cerebral vascular accident | 8,067 | 16.94 | 3,882 | 16.3 | 4,185 | 17.57 | 0.0002** |
| COPD’s severity | |||||||
| COPD only | 23,689 | 99.46 | |||||
| COPD with complications | 126 | 0.26 | |||||
| Acute exacerbation | 26 | 0.05 | |||||
| Pneumonia | 113 | 0.24 | |||||
| Acute respiratory failure | 9 | 0.02 | |||||
| Prostate Cancer | 0.5331 | ||||||
| No | 46,878 | 98.41 | 23,430 | 98.38 | 23,448 | 98.45 | |
| Yes | 756 | 1.59 | 387 | 1.62 | 369 | 1.55 | |
| Follow-up time (years) | <0.0001** | ||||||
| Mean ± SD | 7.05±4.13 | 7.46±4.02 | 6.64±4.19 | ||||
Notes: *p<0.05 ; **p<0.01.
The Cox regression analysis of risk factors of PCa is shown in Table 2. Compared to the control group, the adjusted HR for PCa in the COPD group was 1.10 (95% CI 0.95–1.27), while that in the COPD group with complications was 2.46 (95% CI 1.96–3.61). Elderly patients had a significantly higher risk of PCa (those aged ≥70 years had an adjusted HR [aHR] of 3.47 [95% CI 1.71–4.52] compared with those aged 40–49 years). Other significant risk factors of PCa were tobacco use disorder (aHR 2.30, 95% CI 1.61–3.27) and hypertension (aHR 1.19, 95% CI 1.02–1.39).
Table 2.
Independent predictors of risk factors of PCa by Cox regression analysis
| Crude HR | 95% CI | p-value | Adjusted HR | 95% CI | p-value | |||
|---|---|---|---|---|---|---|---|---|
| Lower | Upper | Lower | Upper | |||||
| Non-COPD | 1. | 1. | ||||||
| COPD | 1.082 | 0.938 | 1.248 | 0.279 | 1.103 | 0.955 | 1.273 | 0.1817 |
| COPD with complications | 3.37 | 3.243 | 4.653 | <0.0001** | 2.463 | 1.960 | 3.615 | <0.0001** |
| Age group | ||||||||
| 40–49 | 1 | 1. | ||||||
| 50–59 | 1.072 | 1.160 | 2.133 | <0.0001** | 1.761 | 1.042 | 1.980 | <0.0001** |
| 60–69 | 2.195 | 1.259 | 2.534 | <0.0001** | 2.006 | 1.285 | 3.271 | <0.0001** |
| >70 | 3.413 | 1.848 | 4.511 | <0.0001** | 3.473 | 1.717 | 4.522 | <0.0001** |
| Comorbidity | ||||||||
| Alcohol abuse | 0.161 | 0.023 | 1.141 | 0.068 | 0.268 | 0.038 | 1.911 | 0.1890 |
| Tobacco use disorder | 1.965 | 1.386 | 2.786 | <0.0001** | 2.300 | 1.613 | 3.278 | <0.0001** |
| Obesity | 1.307 | 0.421 | 4.061 | 0.643 | 2.022 | 0.648 | 6.310 | 0.2254 |
| Diabetes | 1.151 | 0.965 | 1.374 | 0.117 | 0.965 | 0.802 | 1.161 | 0.7068 |
| Hypertension | 1.540 | 1.334 | 1.779 | <0.0001** | 1.195 | 1.025 | 1.393 | 0.023* |
| Hyperlipidemia | 1.080 | 0.904 | 1.289 | 0.399 | 1.198 | 0.992 | 1.448 | 0.0609 |
| Liver disease | 1.037 | 0.855 | 1.259 | 0.711 | 1.047 | 0.854 | 1.284 | 0.6565 |
| Chronic kidney disease | 1.091 | 0.753 | 1.583 | 0.644 | 0.952 | 0.655 | 1.383 | 0.7950 |
| Coronary heart disease | 1.216 | 1.038 | 1.425 | 0.016* | 1.142 | 0.968 | 1.347 | 0.1153 |
| Cerebral vascular accident | 1.082 | 0.879 | 1.333 | 0.457 | 0.824 | 0.666 | 1.019 | 0.0734 |
Notes: *p<0.05 ; **p<0.01.
Abbreviation: PCa, prostate cancer.
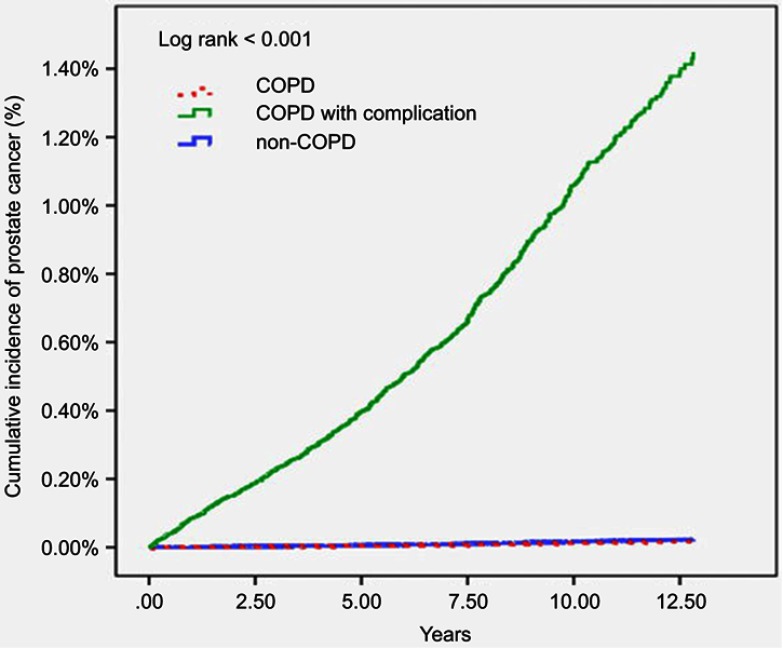
As shown in the Kaplan-Meier curves in Figure 2, patients with severe COPD with complications had a higher cumulative incidence of PCa (log-rank test, p<0.001) by the end of the follow-up period than the non-COPD and COPD groups.
Figure 2.
Kaplan-Meier analysis of developing prostate cancer in COPD, COPD with complication, and non-COPD groups.
Discussion
To our knowledge, no other cohort studies have investigated the association between COPD with complications and PCa in detail. In this large-scale cohort study, males with severe COPD complications including acute respiratory failure, cardiopulmonary arrest, pneumonia, and acute exacerbation were found to have a higher risk of PCa than males without COPD.
The association between COPD and PCa can be attributed to several plausible mechanisms. First, COPD is a chronic inflammatory disease in both airways and outside the respiratory system.16,17 Meanwhile, chronic inflammation of the prostate is associated with PCa, and PIA has been reported to progress to PCa.5 Furthermore, severe COPD may have an acute exacerbated inflammatory process, and there is evidence of oxidative stress in COPD, particularly during acute exacerbations.18 Reactive oxygen species (ROS) levels are elevated in severe COPD. ROS cause tissue damage, resulting in carbonyl stress that further causes nonenzymatic posttranslational modifications of proteins. These protein carbonylation reactions may induce the pathophysiological mechanisms of many chronic diseases.19 COPD severity is correlated with carbonyl stress levels.20 Carbonyl stress further causes DNA damage, inducing the autoimmune response and pro-inflammatory signaling (nuclear factor [NF]-κB pathway).21,22 The mechanisms of exacerbations in COPD also include increased airway inflammation and elevated levels of pro-inflammatory cytokines.23
The exposure of airway epithelial cells to acute oxidative stress triggers increased glutathione synthesis, but COPD patients fail to show this phenomenon.24 Moreover, severe COPD has also been associated with glutathione S-transferase (GST) activity.25 The GST family of enzymes plays an important role in environmental carcinogens. Thakur et al revealed that GSTT1 null genotypes increase the risks of both COPD and PCa in the Indian population.26
Pro-inflammatory cytokines also play an important role in chronic inflammation in both COPD and PCa. Increased levels of interleukin (IL)‐1β, IL‐6, IL‐8, IL-17, and tumor necrosis factor (TNF)‐α have been observed in sputum and bronchoalveolar lavage fluid from COPD patients.27,28 Moreover, several inflammatory cytokines including IL-1, IL-6, and IL-17 have been reported to be induced by PCa.4 IL-6 is an activator of STAT3 and NF-κB complexes,29,30 and NF-κB activation has been reported in PCa development.31 Furthermore, higher IL-6 and IL-8 levels have been associated with more severe COPD,23 and increased IL-6 levels in patients with severe COPD may further affect PCa development.
Studies have indicated that older men have an increased risk of COPD and PCa.32,33 Increasing age is a risk factor for severe COPD, especially in patients aged >80 years,34 and for PCa. Meanwhile, inhaled anticholinergic agents (ipratropium and tiotropium) are widely administered as maintenance treatment for COPD. However, such agents are linked to increased risk of acute urinary retention.35 Severe COPD is also treated with anticholinergic agents and systemic corticosteroids, and Foley insertion is performed to monitor daily urine output in ICU patients with severe COPD. In PCa, acute urinary retention and urine reflux are also well-known risk factors.36
This study has some limitations. First, all of the conditions were diagnosed using the ICD-9-CM coding system from the NHIRD, which is an administrative database; thus, detailed laboratory data or tumor marker results were not obtained. Although pulmonary function tests, FEV1/FVC ratios, prostate-specific antigen levels, computed tomography scans, and bone scans were not available, the diagnoses of PCa were ascertained by catastrophic illness certifications, which were reviewed by experts to confirm the given diagnosis. Second, instead of using the Global Initiative for Chronic Obstructive Lung Disease (GOLD) classification, we classified COPD by subsequent acute respiratory events, namely pneumonia, acute exacerbation, acute respiratory failure, and cardiopulmonary arrest. Third, some confounding factors such as cigarette smoking, obesity, and occupational exposure status might have been underestimated in the NHIRD database. Finally, this study has a retrospective nature; hence, more prospective studies are warranted to further investigate the association between COPD and PCa.
Conclusion
This study showed that COPD patients with complications have an increased risk of PCa. Thus, severe COPD may be a determining factor for PCa incidence. These findings may help physicians in treating COPD with complications and in remaining alert to the potential development of PCa. Relatedly, physicians may want to consider screening for PCa in those COPD patients with complications.
Supplementary material
Acknowledgments
This study was supported by the Tri-Service General Hospital Taiwan (TSGH-PH-108-10 and TSGH-C108-048) and Hualien Tzu Chi Hospital Taiwan (TCRID108-30). The implications and conclusions of this study do not represent the opinions of the Bureau of National Health Insurance, the Department of Health, or the National Health Research Institutes of Taiwan.
Author contributions
FWC and HYC wrote the manuscript. RJH and WLH wrote the proposal and designed the manuscript. FWC and HYC contributed to the conception of the study. FWC and HYC collected the data and conducted data analysis. RJH revised the manuscript and data analysis. All authors contributed toward data analysis, drafting and critically revising the paper, read and approved the final manuscript, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67(1):7–30. doi: 10.3322/caac.21387 [DOI] [PubMed] [Google Scholar]
- 2.Department of Health, the Executive Yuan, Republic of China. Cancer Registry Annual Report 2000–2015. Taiwan; 2017. Available from: http://www.hpa.gov.tw/Pages/List.aspx?nodeid=269. [Google Scholar]
- 3.Grönberg H. Prostate cancer epidemiology. Lancet. 2003;361(9360):859–864. doi: 10.1016/S0140-6736(03)12713-4 [DOI] [PubMed] [Google Scholar]
- 4.De Nunzio C, Kramer G, Marberger M, et al. The controversial relationship between benign prostatic hyperplasia and prostate cancer: the role of inflammation. Eur Urol. 2011;60(1):106–117. doi: 10.1016/j.eururo.2011.03.055 [DOI] [PubMed] [Google Scholar]
- 5.Nelson WG, De Marzo AM, Isaacs WB. Prostate cancer. N Engl J Med. 2003;349(4):366–381. doi: 10.1056/NEJMoa030969 [DOI] [PubMed] [Google Scholar]
- 6.Divo M, Cote C, de Torres JP, et al. Comorbidities and risk of mortality in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2012;186(2):155–161. doi: 10.1164/rccm.201201-0034OC [DOI] [PubMed] [Google Scholar]
- 7.Chen JC, Mannino DM. Worldwide epidemiology of chronic obstructive pulmonary disease. Curr Opin Pulm Med. 1999;5(2):93–99. doi: 10.1097/00063198-199903000-00003 [DOI] [PubMed] [Google Scholar]
- 8.Cheng SL, Chan MC, Wang CC, et al. COPD in Taiwan: a national epidemiology survey. Int J Chron Obstruct Pulmon Dis. 2015;10:2459–2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Salvi SS, Barnes PJ. Chronic obstructive pulmonary disease in non-smokers. Lancet. 2009;374(9691):733–743. doi: 10.1016/S0140-6736(09)61069-2 [DOI] [PubMed] [Google Scholar]
- 10.Global strategy for the diagnosis, management, and prevention of COPD - 2016. Global Initiative for Chronic Obstructive Lung Disease (GOLD); 2016. Available from: https://goldcopd.org/global-strategy-diagnosis-management-prevention-copd-2016/. Accessed August 13, 2019.
- 11.Cheng TM. Reflections on the 20th anniversary of Taiwan’s single-payer National Health Insurance System. Health Aff (Millwood). 2015;34(3):502–510. doi: 10.1377/hlthaff.2014.1332 [DOI] [PubMed] [Google Scholar]
- 12.National Health Research Institutes. National Health Insurance Research Database. Available from: http://nhird.nhri.org.tw/date_01.htm. Accessed October 1, 2018.
- 13.Liu JM, Yu CP, Chuang HC, et al. Androgen deprivation therapy for prostate cancer and the risk of autoimmune diseases. Prostate Cancer Prostatic Dis. Epub 2019. Jan 28. [DOI] [PubMed] [Google Scholar]
- 14.Menezes AMB, Perez-Padilla R, Jardim JB, et al. Chronic obstructive pulmonary disease in five Latin American cities (the PLATINO study): a prevalence study. Lancet. 2005;366(9500):1875–1881. doi: 10.1016/S0140-6736(05)67528-9 [DOI] [PubMed] [Google Scholar]
- 15.Wu CY, Chen YJ, Ho HJ, et al. Association between nucleoside analogues and risk of hepatitis B virus–related hepatocellular carcinoma recurrence following liver resection. JAMA. 2012;308(18):1906–1914. doi: 10.1001/2012.jama.11975 [DOI] [PubMed] [Google Scholar]
- 16.Agusti A, Soriano JB. COPD as a systemic disease. COPD. 2008;5(2):133–138. doi: 10.1080/15412550801941349 [DOI] [PubMed] [Google Scholar]
- 17.Fabbri LM, Rabe KF. From COPD to chronic systemic inflammatory syndrome? Lancet. 2007;370(9589):797–799. doi: 10.1016/S0140-6736(07)61383-X [DOI] [PubMed] [Google Scholar]
- 18.Kirkham PA, Barnes PJ. Oxidative stress in COPD. Chest. 2013;144(1):266–273. doi: 10.1378/chest.12-2664 [DOI] [PubMed] [Google Scholar]
- 19.Negre-Salvayre A, Coatrieux C, Ingueneau C, Salvayre R. Advanced lipid peroxidation end products in oxidative damage to proteins. Potential role in diseases and therapeutic prospects for the inhibitors. Br J Pharmacol. 2008;153(1):6–20. doi: 10.1038/bjp.2008.22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rahman I, van Schadewijk AA, Crowther AJ, et al. 4-hydroxy-2-nonenal, a specific lipid peroxidation product, is elevated in lungs of patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2002;166(4):490–495. doi: 10.1164/rccm.2110101 [DOI] [PubMed] [Google Scholar]
- 21.Di Stefano A, Caramori G, Oates T, et al. Increased expression of nuclear factor-kappaB in bronchial biopsies from smokers and patients with COPD. Eur Respir J. 2002;20(3):556–563. doi: 10.1183/09031936.02.00272002 [DOI] [PubMed] [Google Scholar]
- 22.Kurien BT, Scofield RH. Autoimmunity and oxidatively modified autoantigens. Autoimmun Rev. 2008;7(7):567–573. doi: 10.1016/j.autrev.2008.04.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bhowmik A, Seemungal TA, Sapsford RJ, Wedzicha JA. Relation of sputum inflammatory markers to symptoms and lung function changes in COPD exacerbations. Thorax. 2000;55(2):114–120. doi: 10.1136/thorax.55.8.685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harju T, Kaarteenaho-Wiik R, Soini Y, Sormunen R, Kinnula VL. Diminished immunoreactivity of gamma-glutamylcysteine synthetase in the airways of smokers’ lung. Am J Respir Crit Care Med. 2002;166(5):754–759. doi: 10.1164/rccm.2112014 [DOI] [PubMed] [Google Scholar]
- 25.Mohammed A, Gutta V, Ansari MS, Venkata RS, Jamil K. Altered antioxidant enzyme activity with severity and comorbidities of chronic obstructive pulmonary disease (COPD) in South Indian population. COPD Res Pract. 2017;3:4. doi: 10.1186/s40749-017-0023-z [DOI] [Google Scholar]
- 26.Thakur H, Gupta L, Sobti RC, Janmeja AK, Seth A, Singh SK. Association of GSTM1T1 genes with COPD and prostate cancer in north Indian population. Mol Biol Rep. 2011;38(3):1733–1739. doi: 10.1007/s11033-010-0287-8 [DOI] [PubMed] [Google Scholar]
- 27.Keatings VM, Collins PD, Scott DM, Barnes PJ. Differences in interleukin-8 and tumor necrosis factor-alpha in induced sputum from patients with chronic obstructive pulmonary disease or asthma. Am J Respir Crit Care Med. 1996;153(2):530–534. doi: 10.1164/ajrccm.153.6.8665036 [DOI] [PubMed] [Google Scholar]
- 28.Chen K, Pociask DA, McAleer JP, et al. IL-17RA is required for CCL2 expression, macrophage recruitment, and emphysema in response to cigarette smoke. PLoS One. 2011;6(5):e20333. doi: 10.1371/journal.pone.0020333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nadiminty N, Lou W, Lee SO, et al. Stat3 activation of NF-{kappa}B p100 processing involves CBP/p300-mediated acetylation. Proc Natl Acad Sci USA. 2006;103(19):7264–7269. doi: 10.1073/pnas.0509808103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lou W, Ni Z, Dyer K, Tweardy DJ, Gao AC. Interleukin-6 induces prostate cancer cell growth accompanied by activation of stat3 signaling pathway. Prostate. 2000;42(3):239–242. [DOI] [PubMed] [Google Scholar]
- 31.Huang S, Pettaway CA, Uehara H, Bucana CD, Fidler IJ. Blockade of NF-κB activity in human prostate cancer cells is associated with suppression of angiogenesis, invasion, and metastasis. Oncogene. 2001;20(31):4188–4197. doi: 10.1038/sj.onc.1204535 [DOI] [PubMed] [Google Scholar]
- 32.Lindberg A, Jonsson AC, Rönmark E, Lundgren R, Larsson LG, Lundbäck B. Ten-year cumulative incidence of COPD and risk factors for incident disease in a symptomatic cohort. Chest. 2005;127(5):1544–1552. doi: 10.1378/chest.127.2.630 [DOI] [PubMed] [Google Scholar]
- 33.Bostwick DG, Burke HB, Djakiew D, et al. Human prostate cancer risk factors. Cancer. 2004;101(10 Suppl):2371–2490. [DOI] [PubMed] [Google Scholar]
- 34.Stone RA, Lowe D, Potter JM, Buckingham RJ, Roberts CM, Pursey NJ. Managing patients with COPD exacerbation: does age matter? Age Ageing. 2012;41(4):461–468. doi: 10.1093/ageing/afs039 [DOI] [PubMed] [Google Scholar]
- 35.Afonso AS, Verhamme KM, Stricker BH, Sturkenboom MC, Brusselle GG. Inhaled anticholinergic drugs and risk of acute urinary retention. BJU Int. 2011;107(8):1265–1272. doi: 10.1111/j.1464-410X.2010.09648.x [DOI] [PubMed] [Google Scholar]
- 36.Moul JW, Davis R, Vaccaro JA, Sihelnik SA, Belville WD, McLeod DG. Acute urinary retention associated with prostatic carcinoma. J Urol. 1989;141(6):1375–1377. doi: 10.1016/S0022-5347(17)41312-7 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Global strategy for the diagnosis, management, and prevention of COPD - 2016. Global Initiative for Chronic Obstructive Lung Disease (GOLD); 2016. Available from: https://goldcopd.org/global-strategy-diagnosis-management-prevention-copd-2016/. Accessed August 13, 2019.