Abstract
Background
A combination of olanzapine and samidorphan (OLZ/SAM) is in development to provide the established antipsychotic efficacy of olanzapine while mitigating olanzapine-induced weight gain.
Methods
Two multicenter, open-label, parallel-cohort studies were performed to evaluate the effect of moderate hepatic impairment (Child-Pugh score 7–9 [class B]; study 1) and severe renal impairment (estimated glomerular filtration rate: 15–29 mL/min/1.73 m2; study 2) on the pharmacokinetics, safety, and tolerability of a single dose of OLZ/SAM 5/10 mg.
Results
There was a 1.67-fold increase in area under the plasma concentration-time curve from time 0 to infinity (AUC0-∞) and a 2.17-fold increase in maximum plasma concentration (Cmax) of olanzapine, and a 1.52-fold increase in AUC0-∞ and a 1.63-fold increase in Cmax of samidorphan, in subjects with moderate hepatic impairment compared with healthy control subjects. Compared with healthy control subjects, subjects with severe renal impairment had a 33% and 56% reduction in clearance, a 1.51- and 2.31-fold increase in AUC0-∞, and a 1.32- and 1.37-fold increase in Cmax of olanzapine and samidorphan, respectively.
Conclusion
OLZ/SAM 5/10 mg was generally well tolerated under the conditions of the studies, with a safety profile consistent with that observed in other clinical studies of OLZ/SAM.
Keywords: olanzapine, samidorphan, renal impairment, hepatic impairment, pharmacokinetics
Introduction
Schizophrenia is one of the leading causes of disability worldwide. While a number of effective medications for schizophrenia are currently available, limitations in the tolerability of these medications1 support continuous efforts in developing additional treatment options. Although clozapine is the most effective treatment available today for treatment-resistant symptoms, safety concerns (eg, agranulocytosis) preclude its use as a first-line antipsychotic.2 Olanzapine is considered to be a highly effective antipsychotic,3 but its use has also been curtailed by safety concerns related to long-term metabolic consequences, including weight gain.1
Preclinical studies have identified the key role of the opioid system in governing food-reward, feeding behavior, and metabolism. In μ-, κ-, and δ-opioid receptor knockout mice, a decrease in weight gain was found compared with non-knockout mice, despite no differences in caloric intake in μ- and κ-opioid receptor knockout mice.4–6 It is hypothesized that the addition of an opioid antagonist to central nervous system-active medications may mitigate metabolic dysregulation. Samidorphan is a new chemical entity. In vitro, samidorphan binds with high affinity to human μ-, κ-, and δ-opioid receptors and acts as an antagonist at μ-opioid receptors and partial agonist at κ- and δ-opioid receptors.7,8 In vivo, samidorphan has been demonstrated to function as an opioid antagonist.9 A combination bilayer tablet composed of a flexible dose of olanzapine (5, 10, 15, or 20 mg) and a fixed dose of 10 mg samidorphan (OLZ/SAM)10 is under development and designed to provide the established antipsychotic efficacy of olanzapine while mitigating olanzapine-induced weight gain.11 In phase 1 and phase 2 clinical studies, co-administration of samidorphan with olanzapine mitigated olanzapine-induced weight gain.11,12
Olanzapine is predominantly eliminated via hepatic metabolism, with less than 10% of the administered dose excreted renally as unchanged olanzapine.13 The disposition of samidorphan was evaluated in a clinical study of 10 healthy male volunteers. Following a single oral dose of 2 mg samidorphan with approximately 50 μCi [14C]-labeled samidorphan, approximately 20% of the dose was renally excreted as unchanged samidorphan, with the remainder eliminated via hepatic metabolism (data on file, Alkermes, Inc., Waltham, MA, USA). Impaired hepatic clearance may impact the pharmacokinetics of olanzapine and samidorphan, as both are extensively hepatically metabolized and eliminated. Although neither olanzapine nor samidorphan is predominantly renally eliminated, it was of interest to investigate the effect of renal impairment on the pharmacokinetics of olanzapine and samidorphan, as kidney disease is a comorbidity in some patients requiring treatment with antipsychotic medications. In addition, chronic renal impairment may affect the pharmacokinetics of hepatically cleared drugs.14 The effects of hepatic and renal impairment on the pharmacokinetics of olanzapine and samidorphan have been previously evaluated utilizing physiologically based pharmacokinetic (PBPK) modeling (unpublished data). PBPK modeling predicted that mild hepatic or mild renal impairment would have minimal impact on systemic exposure to olanzapine and samidorphan (<1.5-fold increase in total exposure), whereas moderate to severe hepatic impairment would result in >1.5-fold increase in both olanzapine and samidorphan exposure, and severe renal impairment would result in >1.5-fold increase in samidorphan exposure. Given that the concept of using PBPK modeling to prospectively predict drug pharmacokinetics in subjects with hepatic or renal impairment has not been systematically established,15 2 clinical studies were conducted to assess the effects of moderate hepatic impairment and severe renal impairment on the pharmacokinetics, safety, and tolerability of OLZ/SAM. Data from these clinical studies were used to confirm and refine the PBPK modeling predictions, and in conjunction with PBPK modeling, will be used to inform future dose recommendations of OLZ/SAM in subjects with varying degrees of hepatic and renal impairment.
Methods
Two separate studies were each conducted at 2 sites in the United States (hepatic impairment study: Clinical Pharmacology of Miami, Inc., Miami, FL and DaVita Clinical Research, Minneapolis, MN; renal impairment study: Orlando Clinical Research Center, Orlando, FL and DaVita Clinical Research, Minneapolis, MN). Approval was obtained from each site’s Institutional Review Board prior to subject enrollment (IntegReview IRB, Austin, TX for all study sites). Both studies were performed in accordance with the Declaration of Helsinki and the International Conference on Harmonization’s Good Clinical Practice Guideline. Subjects provided written informed consent.
Study design
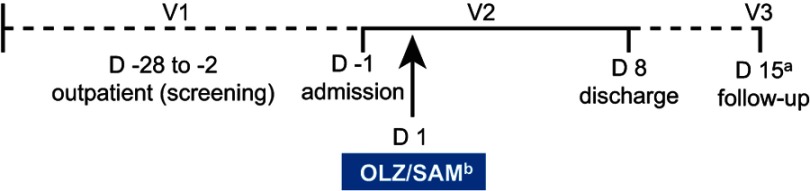
Both studies were phase 1, multicenter, open-label, single-dose, parallel-cohort studies. Each study was approximately 6 weeks in duration, including a screening period of up to 27 days, an 8-day in-clinic period, and a follow-up visit, approximately 7 days after discharge (Figure 1). On day 1, all subjects received a single oral dose of 5 mg olanzapine in combination with 10 mg samidorphan formulated in a bilayer tablet (OLZ/SAM), taken with 240 mL of water. Subjects were required to fast for at least 10 hrs predose and until 4 hrs postdose. Subjects also fasted 10 hrs prior to the morning assessments taken at days 2 through 8, any screening visit where blood was drawn, and the end-of-study follow-up visit.
Figure 1.
Study design schematic.
Notes: a±3-day window; b5 mg olanzapine/10 mg samidorphan.
Abbreviations: D, day; V, visit.
Study populations
Each study included 2 cohorts (hepatic impairment study: subjects with moderate hepatic impairment and healthy control subjects; renal impairment study: subjects with severe renal impairment and healthy control subjects). Subjects were aged ≥18 to ≤70 years in the hepatic impairment study and ≥18 to ≤79 years in the renal impairment study. For both studies, subjects had a body mass index of ≥18.0 and ≤40.0 kg/m2 and a total body weight >50 kg at time of screening.
In the hepatic impairment study, subjects with moderate hepatic impairment were defined as those having a Child-Pugh score of 7–9 (class B) at the time of screening. Further, these subjects had a history of chronic hepatic dysfunction (minimum of 6 months) due to primary hepatocellular disease, with their diagnosis documented by medical history, liver biopsy, hepatic ultrasound, computed tomography scan, or other standard procedure. They also fulfilled the following inclusion criteria preceding or at the time of screening: stable hepatic function for 2 months before screening, creatinine clearance (CrCl) >60 mL/min (Cockcroft-Gault formula), hemoglobin ≥9 g/dL, platelet count >30×109/L, and international normalized ratio ≤2.0. Healthy control subjects were required to have no clinically significant observed abnormality or psychiatric or medical condition.
In the renal impairment study, subjects with severe renal impairment met the following criteria preceding or at the time of screening: stable renal function for at least 60 days prior to screening; stage 4 renal disease (estimated glomerular filtration rate of 15–29 mL/min/1.73 m2 based on Modification of Diet in Renal Disease), with no existing or anticipated need for dialysis; hemoglobin ≥9 g/dL; alanine transaminase ≤1.5× upper limit of normal (ULN); aspartate transaminase ≤1.5× ULN; and total bilirubin within normal limits. Healthy control subjects were defined as those with CrCl >90 mL/min.
In both studies, healthy control subjects were enrolled after the cohort of subjects with hepatic or renal impairment. The demographics of the healthy control cohort were matched to the cohort of subjects with hepatic or renal impairment based on median age (±10 years), body mass index (±15%), and gender.
Key exclusion criteria in both studies were as follows: current evidence or history of any other clinically significant medical or psychiatric condition; observed abnormality anticipated to potentially compromise subject safety or affect pharmacokinetic evaluation; history of gastrointestinal surgery affecting drug absorption or biliary elimination, excluding appendectomy or cholecystectomy; consumption of alcohol within 48 hrs of visit 2 or had a positive urine toxicological screen for alcohol at visit 2; inability to abstain from recreational drug use, opioid medication, and any product containing nicotine from screening until study completion.
In the hepatic impairment study, subjects with history of a liver transplant were excluded. In the renal impairment study, subjects with a history of inadequately controlled diabetes were excluded.
Pharmacokinetic sample collection and bioanalytical methods
In both studies, blood samples for plasma pharmacokinetic assessments were collected within 1 hr before dosing (pre-dose) and at 0.5, 1, 2, 3, 4, 5, 6, 7, 8, 12, 16, 24, 36, 48, 72, 96, 120, and 168 hrs post-dose. In the renal impairment study, urine pharmacokinetic samples were also collected in intervals from −2 to 0, 0 to 4, 4 to 8, 8 to 12, 12 to 16, 16 to 24, 24 to 36, 36 to 48, 48 to 60, 60 to 72, 72 to 84, 84 to 96, 96 to 108, 108 to 120, 120 to 132, 132 to 144, 144 to 156, and 156 to 168 hrs post-dose. In both studies, blood samples were collected pre-dose and at 5 hrs post-dose for the determination of plasma protein binding of olanzapine.
Plasma concentrations of olanzapine and samidorphan were analyzed using a validated liquid chromatography system coupled with detection by tandem mass spectrometry (LC-MS/MS) method.16 The linear range of the plasma assay was 0.250 ng/mL to 100 ng/mL, and the lower limit of quantitation (LLOQ) was 0.250 ng/mL for both olanzapine and samidorphan. Similarly, concentrations of olanzapine and samidorphan in urine were analyzed using a validated LC-MS/MS method. The linear range of the urine assay was 2.50 ng/mL to 2500 ng/mL, and the LLOQ was 2.50 ng/mL for both olanzapine and samidorphan. Both plasma and urine samples were analyzed by an independent bioanalytic laboratory (Covance/Tandem, Salt Lake City, UT) in accordance with Good Laboratory Practice guidelines.
Outcome measures
Pharmacokinetic assessments of olanzapine and samidorphan included the following parameters: maximum observed plasma drug concentration (Cmax), area under the plasma drug concentration versus time curve (AUC) from time 0 to last quantifiable concentration (AUClast) and to infinity (AUC0-∞), time to Cmax (tmax), terminal elimination half-life (t1⁄2), total body clearance (CL/F), and volume of distribution (Vz/F). In addition, the extent of plasma protein binding of olanzapine was determined based on samples collected at 5 hrs postdose. In the renal impairment study, the following parameters were also determined: renal clearance (CLR), amount of drug excreted in urine (Ae) over each collection interval, and total amount of drug excreted in urine from 0 to 168 hrs after the dose (Ae0-168).
Safety assessments included monitoring for adverse events (AEs); vital sign changes; abnormal laboratory values; and electrocardiogram (ECG) changes.
Pharmacokinetic and statistical analyses
Pharmacokinetic parameters were calculated by a standard noncompartmental analysis using elapsed time from dosing to estimate individual plasma and urine pharmacokinetic parameters, and are summarized descriptively. An analysis of covariance model was used to analyze natural log-transformed systemic exposure parameters (ie, Cmax, AUClast, and AUC0-∞) for olanzapine and samidorphan. Geometric mean ratios of each of the exposure parameters between subjects with hepatic or renal impairment and healthy control subjects and their 2-sided 90% confidence intervals were calculated.
Results
Subject disposition
In the hepatic impairment study, 10 subjects with moderate hepatic impairment and 11 healthy control subjects were enrolled. All 21 subjects received a single dose of OLZ/SAM and were included in the safety population analysis. The pharmacokinetic population included all subjects in the safety population, except for 1 healthy control subject who had a serious AE (syncope) within hours after dosing. This resulted in insufficient samples collected for estimation of pharmacokinetic parameters for this subject.
In the renal impairment study, 10 subjects with severe renal impairment and 10 healthy control subjects were enrolled. All 20 subjects received a single dose of OLZ/SAM and were included in the safety and pharmacokinetic populations for analysis.
Subject demographics and baseline characteristics
Subject demographics and baseline characteristics are summarized in Table 1, and were balanced between cohorts. The mean (SD) Child-Pugh total score for subjects with moderate hepatic impairment was 7.5 (0.7), and the mean (SD) estimated glomerular filtration rate for subjects with severe renal impairment was 22.5 (3.8) mL/min/1.73 m2.
Table 1.
Demographics and baseline characteristics
| Variable category/statistics | Hepatic impairment study | Renal impairment study | ||
|---|---|---|---|---|
| Moderate hepatic impairment (N=10) | Healthy control (N=11) | Severe renal impairment (N=10) | Healthy control (N=10) | |
| Age (years) | ||||
| Mean (SD) | 62.0 (6.07) | 60.4 (4.37) | 63.3 (11.08) | 65.5 (4.53) |
| Sex, n (%) | ||||
| Male | 8 (80.0) | 8 (72.7) | 7 (70.0) | 7 (70.0) |
| Ethnicity, n (%) | ||||
| Not Hispanic or Latino | 6 (60.0) | 5 (45.5) | 9 (90.0) | 9 (90.0) |
| Hispanic or Latino | 4 (40.0) | 6 (54.5) | 1 (10.0) | 1 (10.0) |
| Race, n (%) | ||||
| White | 9 (90.0) | 8 (72.7) | 8 (80.0) | 9 (90.0) |
| Black or African American | 0 | 3 (27.3) | 2 (20.0) | 1 (10.0) |
| Other | 1 (10.0) | 0 | 0 | 0 |
| Height (cm) | ||||
| Mean (SD) | 169.9 (9.1) | 169.2 (11.4) | 168.2 (9.4) | 173.7 (6.7) |
| Weight (kg) | ||||
| Mean (SD) | 89.7 (15.6) | 87.7 (14.3) | 82.8 (11.8) | 86.4 (10.4) |
| Body mass index (kg/m2) | ||||
| Mean (SD) | 31.1 (4.3) | 30.4 (2.5) | 29.3 (3.9) | 28.6 (2.1) |
| Child-Pugh total score | ||||
| Mean (SD) | 7.5 (0.7) | — | — | — |
| eGFR (mL/min/1.73 m2) | ||||
| Mean (SD) | — | — | 22.5 (3.8) | — |
| CrCl (mL/min) | ||||
| Mean (SD) | — | — | — | 113.6 (13.4) |
Abbreviations: CrCl, creatinine clearance; eGFR, estimated glomerular filtration rate; SD, standard deviation.
Pharmacokinetics
Hepatic impairment study
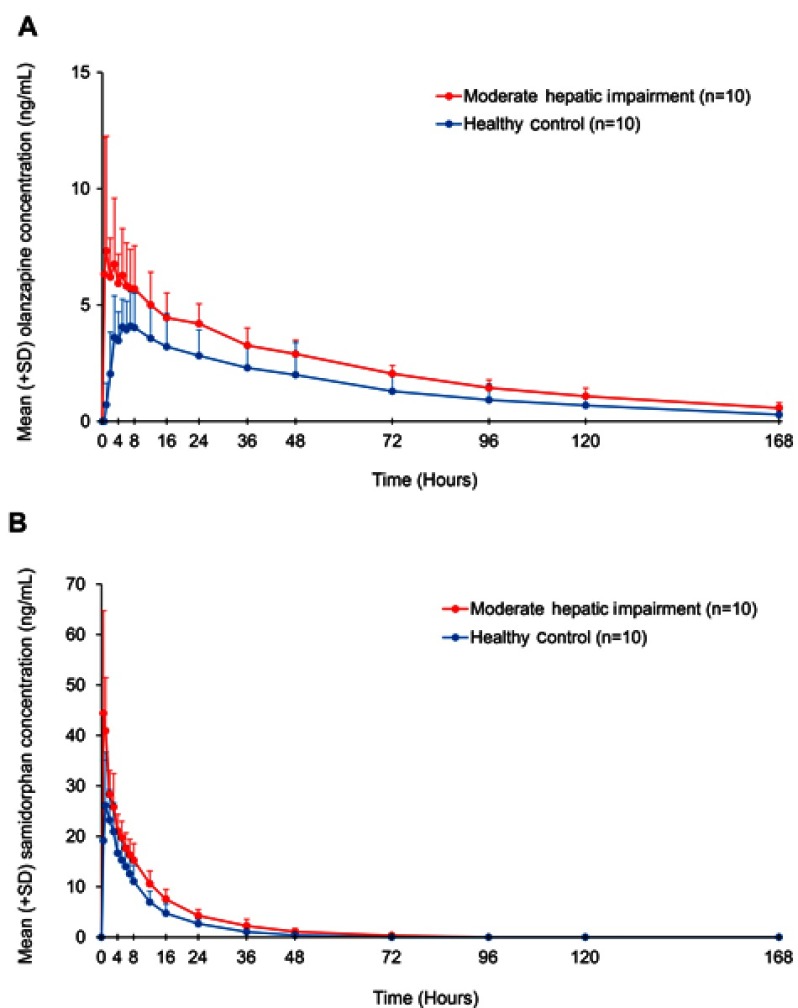
Pharmacokinetic parameters for olanzapine and samidorphan in subjects with moderate hepatic impairment and healthy control subjects are summarized in Table 2. Mean plasma concentrations of olanzapine (Figure 2A) and samidorphan (Figure 2B) were higher in subjects with moderate hepatic impairment compared with healthy control subjects after a single oral dose of OLZ/SAM (Table S1). Absorption of olanzapine was faster in subjects with moderate hepatic impairment compared with healthy control subjects, with median tmax values of 1.5 hrs and 7 hrs, respectively. Absorption of samidorphan was fast, with median tmax observed within 1 hr in both cohorts. The t1/2 of both olanzapine and samidorphan in subjects with moderate hepatic impairment was similar to that in healthy control subjects.
Table 2.
Summary of pharmacokinetic parameters for olanzapine and samidorphan in subjects with moderate hepatic impairment and healthy control subjects after a single oral dose of OLZ/SAM (5 mg olanzapine/10 mg samidorphan)
| Parameter, arithmetic mean (SD) | Olanzapine | Samidorphan | ||
|---|---|---|---|---|
| Moderate hepatic impairment (N=10) | Healthy control (N=10) | Moderate hepatic impairment (N=10) | Healthy control (N=10) | |
| Cmax (ng/mL) | 10.65 (4.21) | 4.91 (1.51) | 50.71 (13.6) | 30.77 (8.5) |
| tmax (hr)a | 1.5 (0.5–4.0) | 7.0 (2.0–12.0) | 0.5 (0.5–1.0) | 1.0 (0.5–3.0) |
| t1/2 (hr) | 53.3 (16.1) | 51.9 (18.0) | 11.9 (2.3) | 9.0 (0.8) |
| AUClast (hr×ng/mL) | 376 (71) | 237 (129) | 397 (84) | 256 (64) |
| AUC0-∞ (hr×ng/mL) | 424 (84) | 275 (149) | 408 (87) | 263 (65) |
| CL/F (L/hr) | 12.2 (2.4) | 22.2 (9.0) | 25.5 (5.1) | 39.8 (8.2) |
| Vz/F (L) | 920 (249) | 1555 (564) | 429 (86) | 514 (105) |
Note: aMedian (min, max) presented for tmax.
Abbreviations: AUClast, area under the plasma concentration-time curve from time 0 to last observed concentration above the lower limit of quantification; AUC0-∞, area under the plasma concentration-time curve from time 0 to infinity; CL/F, apparent clearance; Cmax, maximum observed concentration; OLZ/SAM, olanzapine/samidorphan; SD, standard deviation; tmax, time to maximum observed concentration; t½, terminal elimination half-life; Vz/F, apparent volume of distribution.
Figure 2.
Plasma concentrations (mean + SD) of olanzapine (A) and samidorphan (B) over time in subjects with moderate hepatic impairment and healthy control subjects after a single oral dose of OLZ/SAM (5 mg olanzapine/10 mg samidorphan).
Abbreviations: OLZ/SAM, olanzapine/samidorphan; SD, standard deviation.
Table S1.
Mean plasma levels of olanzapine and samidorphan in subjects with moderate hepatic impairment and healthy control subjects after a single oral dose of OLZ/SAM (5 mg olanzapine/10 mg samidorphan)
| Arithmetic mean (SD) postdose plasma concentration (ng/mL) | ||||
|---|---|---|---|---|
| Olanzapine | Samidorphan | |||
| Moderate hepatic impairment (N=10) | Healthy control (N=10) | Moderate hepatic impairment (N=10) | Healthy control (N=10) | |
| Time postdose, hr | ||||
| 0 (predose) | <LLOQ | <LLOQ | <LLOQ | <LLOQ |
| 0.5 | 6.32 (5.87) | <LLOQ | 44.37 (20.36) | 19.14 (15.94) |
| 1 | 7.32 (4.94) | 0.72 (0.93) | 40.90 (10.55) | 26.08 (10.60) |
| 2 | 6.20 (1.68) | 2.04 (1.80) | 28.31 (4.75) | 23.18 (6.04) |
| 3 | 6.75 (2.84) | 3.61 (1.79) | 25.81 (6.63) | 20.92 (5.73) |
| 4 | 5.93 (1.25) | 3.47 (1.24) | 21.03 (3.32) | 16.65 (4.21) |
| 5 | 6.27 (2.01) | 4.05 (1.20) | 19.74 (3.23) | 15.28 (4.30) |
| 6 | 5.82 (1.85) | 3.94 (1.23) | 17.63 (3.05) | 14.00 (4.19) |
| 7 | 5.71 (1.69) | 4.09 (1.48) | 16.34 (3.06) | 12.55 (4.13) |
| 8 | 5.69 (1.86) | 4.03 (1.48) | 15.23 (3.32) | 11.05 (3.15) |
| 12 | 5.00 (1.41) | 3.57 (1.38) | 10.60 (2.52) | 6.95 (2.19) |
| 16 | 4.45 (1.07) | 3.21 (1.43) | 7.52 (1.97) | 4.73 (1.88) |
| 24 | 4.20 (0.85) | 2.82 (1.11) | 4.26 (1.23) | 2.67 (0.94) |
| 36 | 3.27 (0.75) | 2.30 (1.03) | 2.24 (1.33) | 1.05 (0.41) |
| 48 | 2.90 (0.60) | 2.00 (1.39) | 1.08 (0.65) | 0.40 (0.26) |
| 72 | 2.05 (0.35) | 1.29 (0.80) | 0.31 (0.44) | <LLOQ |
| 96 | 1.44 (0.34) | 0.92 (0.68) | <LLOQ | <LLOQ |
| 120 | 1.08 (0.35) | 0.69 (0.46) | <LLOQ | <LLOQ |
| 168 | 0.58 (0.23) | 0.29 (0.35) | <LLOQ | <LLOQ |
Note: Values below the LLOQ (0.250 ng/mL) were set to 0 in the calculation of the summary statistics.
Abbreviation: LLOQ, lower limit of quantification.
Compared with healthy control subjects, subjects with moderate hepatic impairment had a 45% and 36% reduction in CL/F of olanzapine and samidorphan, respectively, and a 41% and 17% reduction in Vz/F, respectively. AUC0-∞ values of olanzapine and samidorphan were 1.67- and 1.52-fold higher, and Cmax values of olanzapine and samidorphan were 2.17- and 1.63-fold higher, respectively, in subjects with moderate hepatic impairment compared with healthy control subjects (Table 3). Olanzapine was approximately 92% bound to plasma protein in both cohorts.
Table 3.
Comparison of systemic exposure of olanzapine and samidorphan in subjects with moderate hepatic impairment and healthy control subjects after a single oral dose of OLZ/SAM (5 mg olanzapine/10 mg samidorphan)
| Parameter statistic | Olanzapine | Samidorphan | ||
|---|---|---|---|---|
| Moderate hepatic impairment (N=10) | Healthy control (N=10) | Moderate hepatic impairment (N=10) | Healthy control (N=10) | |
| Cmax (ng/mL) | ||||
| Geometric mean | 11.21 | 5.17 | 48.13 | 29.58 |
| Geometric mean ratio | 2.17 | 1.63 | ||
| 90% CI | (1.69, 2.79) | (1.32, 2.01) | ||
| AUClast (hr×ng/mL) | ||||
| Geometric mean | 411 | 237 | 411 | 271 |
| Geometric mean ratio | 1.73 | 1.52 | ||
| 90% CI | (1.38, 2.18) | (1.31, 1.75) | ||
| AUC0-∞ (hr×ng/mL) | ||||
| Geometric mean | 462 | 276 | 422 | 278 |
| Geometric mean ratio | 1.67 | 1.52 | ||
| 90% CI | (1.32, 2.13) | (1.31, 1.75) | ||
Abbreviations: AUClast, area under the plasma concentration-time curve from time 0 to last observed concentration above the lower limit of quantification; AUC0-∞, area under the plasma concentration-time curve from time 0 to infinity; CI, confidence interval; Cmax, maximum observed concentration; OLZ/SAM, olanzapine/samidorphan.
Renal impairment study
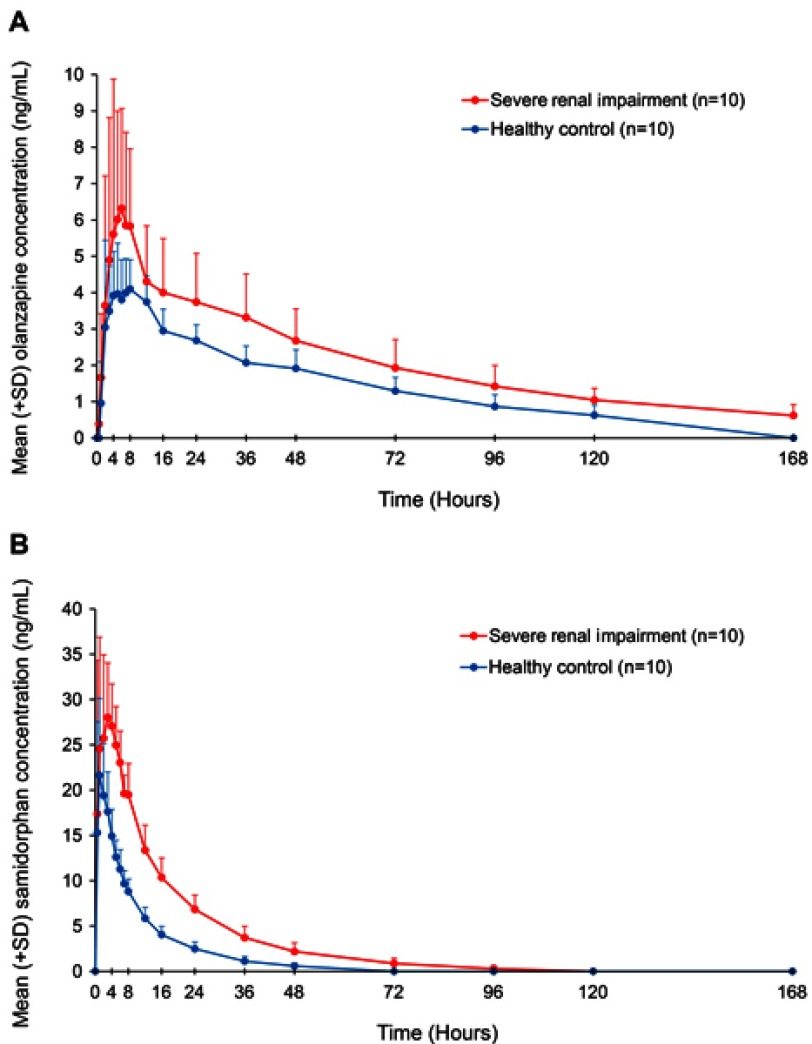
Pharmacokinetic parameters for olanzapine and samidorphan in subjects with severe renal impairment and healthy control subjects are summarized in Table 4. Mean plasma concentrations of olanzapine (Figure 3A) and samidorphan (Figure 3B) were higher in subjects with severe renal impairment compared with healthy control subjects after a single oral dose of OLZ/SAM (see Table S2, which also summarizes mean urine concentrations of olanzapine and samidorphan). The tmax values of olanzapine and samidorphan were similar in subjects with severe renal impairment and healthy control subjects (Table 4). The t1/2 of olanzapine was longer in subjects with severe renal impairment compared with healthy control subjects (58 hrs vs 46 hrs). Similarly, t1/2 of samidorphan was 17 and 11 hrs, respectively.
Table 4.
Summary of pharmacokinetic parameters for olanzapine and samidorphan in subjects with severe renal impairment and healthy control subjects after a single oral dose of OLZ/SAM (5 mg olanzapine/10 mg samidorphan)
| Parameter, arithmetic mean (SD) | Olanzapine | Samidorphan | ||
|---|---|---|---|---|
| Severe renal impairment (N=10) | Healthy control (N=10) | Severe renal impairment (N=10) | Healthy control (N=10) | |
| Cmax (ng/mL) | 7.36 (3.62) | 5.25 (1.59) | 34.58 (9.43) | 26.09 (6.45) |
| tmax (hr)a | 5.1 (3.0–7.0) | 4.5 (2.0–12.0) | 1.5 (0.5–4.0) | 1.0 (0.5–4.0) |
| t1/2 (hr) | 57.8 (15.0) | 45.6 (11.9) | 17.1 (4.7) | 11.4 (2.8) |
| AUClast (hr×ng/mL) | 347 (127) | 228 (58) | 519 (110) | 224 (42) |
| AUC0-∞ (hr×ng/mL) | 404 (138) | 256 (68) | 530 (109) | 233 (43) |
| CL/F (L/hr) | 13.9 (5.9) | 20.7 (4.9) | 19.7 (4.4) | 44.6 (10.2) |
| Vz/F (L) | 1122 (405) | 1306 (258) | 468 (104) | 706 (101) |
| CLR(L/hr) | 0.964 (0.812) | 1.93 (1.43) | 1.92 (0.69) | 9.87 (2.62) |
Note: aMedian (min, max) presented for tmax.
Abbreviations: Ae0-168, total amount of drug excreted from 0 to 168 hrs after dosing; AUClast, area under the plasma concentration-time curve from time 0 to last observed concentration above the lower limit of quantification; AUC0-∞, area under the plasma concentration-time curve from time 0 to infinity; CL/F, apparent clearance; CLR, renal clearance; Cmax, maximum observed concentration; OLZ/SAM, olanzapine/samidorphan; SD, standard deviation; tmax, time to maximum observed concentration; t½, terminal elimination half-life; Vz/F, apparent volume of distribution.
Figure 3.
Plasma concentrations (mean + SD) of olanzapine (A) and samidorphan (B) in subjects with severe renal impairment and healthy control subjects after a single oral dose of OLZ/SAM (5 mg olanzapine/10 mg samidorphan).
Abbreviations: OLZ/SAM, olanzapine/samidorphan; SD, standard deviation.
Table S2.
Mean plasma and urine levels of olanzapine and samidorphan in subjects with severe renal impairment and healthy control subjects after a single oral dose of OLZ/SAM (5 mg olanzapine/10 mg samidorphan)
| Arithmetic mean (SD) postdose plasma concentration (ng/mL) | ||||
|---|---|---|---|---|
| Olanzapine | Samidorphan | |||
| Severe renal impairment (N=10) | Healthy control (N=10) | Severe renal impairment (N=10) | Healthy control (N=10) | |
| Time postdose, hr | ||||
| 0 (predose) | <LLOQ | <LLOQ | <LLOQ | <LLOQ |
| 0.5 | 0.38 (0.56) | <LLOQ | 17.38 (16.93) | 15.30 (12.21) |
| 1 | 1.66 (1.76) | 0.96 (1.14) | 24.55 (12.33) | 21.64 (8.48) |
| 2 | 3.64 (3.57) | 3.04 (2.39) | 25.72 (9.21) | 19.4 (5.73) |
| 3 | 4.89 (3.92) | 3.49 (1.24) | 28.02 (6.01) | 17.62 (4.39) |
| 4 | 5.61 (4.27) | 3.92 (1.21) | 27.07 (4.64) | 14.92 (2.96) |
| 5 | 6.01 (2.98) | 3.97 (1.40) | 24.95 (4.26) | 12.60 (1.86) |
| 6 | 6.31 (2.76) | 3.80 (1.10) | 23.03 (3.48) | 11.28 (2.15) |
| 7 | 5.85 (2.56) | 3.99 (0.94) | 19.61 (2.02) | 9.68 (1.40) |
| 8 | 5.83 (2.13) | 4.10 (0.81) | 19.48 (3.45) | 8.82 (1.38) |
| 12 | 4.31 (1.53) | 3.74 (0.73) | 13.38 (2.76) | 5.85 (1.21) |
| 16 | 4.00 (1.49) | 2.95 (0.60) | 10.36 (2.16) | 4.05 (0.92) |
| 24 | 3.74 (1.34) | 2.68 (0.43) | 6.84 (1.59) | 2.49 (0.76) |
| 36 | 3.32 (1.20) | 2.07 (0.46) | 3.72 (1.26) | 1.13 (0.52) |
| 48 | 2.68 (0.88) | 1.91 (0.51) | 2.20 (0.95) | 0.59 (0.36) |
| 72 | 1.93 (0.78) | 1.30 (0.37) | 0.87 (0.57) | <LLOQ |
| 96 | 1.42 (0.57) | 0.87 (0.33) | 0.30 (0.33) | <LLOQ |
| 120 | 1.05 (0.32) | 0.63 (0.28) | <LLOQ | <LLOQ |
| 168 | 0.62 (0.30) | <LLOQ | <LLOQ | <LLOQ |
| Arithmetic mean (SD) postdose urine concentration (ng/mL) | ||||
| Time postdose, hr | ||||
| 0 (predose) | <LLOQ | <LLOQ | <LLOQ | <LLOQ |
| 0‒4 | 23.10 (19.81) | 9.07 (9.58) | 432.45 (213.66) | 461.10 (224.40) |
| 4‒8 | 84.81 (69.96) | 33.21 (36.45) | 573.67 (398.33) | 593.80 (197.93) |
| 8‒12 | 60.21 (30.98) | 58.91 (29.86) | 409.40 (110.92) | 720.00 (255.28) |
| 12‒16 | 60.39 (26.00) | 106.21 (73.04) | 340.43 (107.58) | 699.33 (433.96) |
| 16‒24 | 47.65 (18.60) | 85.11 (50.09) | 273.67 (98.91) | 490.90 (389.37) |
| 24‒36 | 45.08 (22.70) | 55.95 (30.92) | 145.72 (92.07) | 199.36 (66.83) |
| 36‒48 | 38.19 (21.65) | 57.13 (43.77) | 85.57 (38.76) | 119.11 (96.76) |
| 48‒60 | 36.22 (18.30) | 29.04 (18.53) | 56.02 (39.05) | 54.85 (43.34) |
| 60‒72 | 31.11 (21.37) | 21.87 (20.97) | 34.84 (22.85) | 30.44 (34.11) |
| 72‒84 | 28.57 (14.73) | 19.21 (14.88) | 26.10 (21.94) | 12.52 (8.37) |
| 84‒96 | 20.09 (13.57) | 12.95 (7.83) | 15.33 (13.81) | 11.61 (15.74) |
| 96‒108 | 15.60 (9.67) | 10.49 (7.70) | 7.96 (7.24) | 6.39 (7.20) |
| 108‒120 | 13.62 (11.22) | 10.66 (6.73) | 5.82 (5.45) | 4.33 (6.43) |
| 120‒132 | 12.67 (6.10) | 11.57 (5.71) | 4.30 (3.65) | 4.02 (8.12) |
| 132‒144 | 10.70 (9.51) | 9.84 (5.94) | 2.88 (3.47) | 3.23 (6.25) |
| 144‒156 | 8.91 (4.87) | 5.53 (3.73) | <LLOQ | <LLOQ |
| 156‒168 | 7.12 (3.58) | 7.99 (4.55) | <LLOQ | 2.95 (7.33) |
Notes: Values below the LLOQ (0.250 ng/mL for plasma and 2.50 ng/mL for urine) were set to 0 in the calculation of the summary statistics.
Abbreviation: LLOQ, lower limit of quantification.
Compared with healthy control subjects, subjects with severe renal impairment had a 33% and 56% reduction in CL/F of olanzapine and samidorphan, respectively, and a 50% and 81% reduction in CLR, respectively. AUC0-∞ values of olanzapine and samidorphan were 1.51- and 2.31-fold higher, and Cmax values of olanzapine and samidorphan were 1.32- and 1.37-fold higher, respectively, in subjects with severe renal impairment compared with healthy control subjects (Table 5). Olanzapine was approximately 90–91% bound to plasma protein in both cohorts.
Table 5.
Comparison of systemic exposure of olanzapine and samidorphan in subjects with severe renal impairment and healthy control subjects after a single oral dose of OLZ/SAM (5 mg olanzapine/10 mg samidorphan)
| Parameter statistic | Olanzapine | Samidorphan | ||
|---|---|---|---|---|
| Severe renal impairment (N=10) | Healthy control (N=10) | Severe renal impairment (N=10) | Healthy control (N=10) | |
| Cmax (ng/mL) | ||||
| Geometric mean | 6.71 | 5.07 | 34.51 | 25.21 |
| Geometric mean ratio | 1.32 | 1.37 | ||
| 90% CI | (0.954, 1.84) | (1.10, 1.71) | ||
| AUClast (hr×ng/mL) | ||||
| Geometric mean | 341 | 238 | 529 | 226 |
| Geometric mean ratio | 1.43 | 2.34 | ||
| 90% CI | (1.12, 1.83) | (1.98, 2.77) | ||
| AUC0-∞ (hr×ng/mL) | ||||
| Geometric mean | 403 | 268 | 540 | 234 |
| Geometric mean ratio | 1.51 | 2.31 | ||
| 90% CI | (1.19, 1.91) | (1.96, 2.72) | ||
Abbreviations: AUClast, area under the plasma concentration-time curve from time 0 to last observed concentration above the lower limit of quantification; AUC0-∞, area under the plasma concentration-time curve from time 0 to infinity; CI, confidence interval; Cmax, maximum observed concentration; OLZ/SAM, olanzapine/samidorphan.
Safety
Hepatic impairment study
In total, 18 (86%) subjects experienced at least 1 AE: 10 in the moderate hepatic impairment cohort and 8 in the control cohort (Table 6). All AEs were considered by the investigators to be related to the study drug. Overall, the most common AEs (reported in ≥2 subjects) included somnolence (81%), dizziness (29%), and hypotension (10%). These AEs had an earlier onset in subjects with moderate hepatic impairment; however, they were mild in severity. Two healthy control subjects experienced severe AEs (hypotension, n=1; syncope, n=1). The AE of syncope was considered a serious AE, occurred approximately 6 hrs after dosing, and resulted in an overnight hospitalization; the patient recovered the next day and continued in the study. No deaths or study discontinuations were reported.
Table 6.
Summary of adverse events in the hepatic impairment study
| Category, n (%) | Moderate hepatic impairment (N=10) | Healthy control (N=11) | All subjects (N=21) |
|---|---|---|---|
| Any AE | 10 (100.0) | 8 (72.7) | 18 (85.7) |
| Serious AE | 0 | 1 (9.1) | 1 (4.8) |
| AE leading to discontinuation | 0 | 0 | 0 |
| AE by highest severity | |||
| Mild | 8 (80.0) | 5 (45.5) | 13 (61.9) |
| Moderate | 2 (20.0) | 1 (9.1) | 3 (14.3) |
| Severe | 0 | 2 (18.2) | 2 (9.5) |
| AEs in ≥2 subjects in any cohort | |||
| Somnolence | 10 (100.0) | 7 (63.6) | 17 (81.0) |
| Dizziness | 3 (30.0) | 3 (27.3) | 6 (28.6) |
| Hypotension | 1 (10.0) | 1 (9.1) | 2 (9.5) |
Abbreviation: AE, adverse event.
Renal impairment study
In total, 12 (60%) subjects experienced at least 1 AE: 7 in the severe renal impairment cohort and 5 in the healthy control cohort (Table 7). All AEs were considered by the investigators to be related to the study drug. Overall, the most common AEs (reported in ≥2 subjects) were somnolence (25%), dizziness (15%), nausea (15%), abdominal pain (15%), and lethargy (10%). Except for somnolence, these AEs were reported more frequently in subjects with severe renal impairment. The majority of AEs were mild in severity. Two healthy control subjects experienced AEs that were severe in intensity (syncope and orthostatic hypotension, n=1; hypotension, n=1); neither of these met the criteria for a serious AE. No deaths or study discontinuations were reported.
Table 7.
Summary of adverse events in the renal impairment study
| Category, n (%) | Severe renal impairment (N=10) | Healthy control (N=10) | All subjects (N=20) |
|---|---|---|---|
| Any AE | 7 (70.0) | 5 (50.0) | 12 (60.0) |
| Serious AE | 0 | 0 | 0 |
| AE leading to discontinuation | 0 | 0 | 0 |
| AE by highest severity | |||
| Mild | 5 (50.0) | 2 (20.0) | 7 (35.0) |
| Moderate | 2 (20.0) | 1 (10.0) | 3 (15.0) |
| Severe | 0 | 2 (20.0) | 2 (10.0) |
| AEs in ≥2 subjects in any cohort | |||
| Somnolence | 2 (20.0) | 3 (30.0) | 5 (25.0) |
| Abdominal pain | 2 (20.0) | 1 (10.0) | 3 (15.0) |
| Dizziness | 2 (20.0) | 1 (10.0) | 3 (15.0) |
| Nausea | 2 (20.0) | 1 (10.0) | 3 (15.0) |
| Lethargy | 2 (20.0) | 0 | 2 (10.0) |
Abbreviation: AE, adverse event.
Discussion
In the hepatic impairment study, subjects with moderate hepatic impairment had faster absorption and higher systemic exposure of olanzapine compared with healthy control subjects. The effect of moderate hepatic impairment on the pharmacokinetics of olanzapine was more pronounced than previously reported, where there was no statistically significant differences in olanzapine pharmacokinetic parameters between subjects with mild/moderate hepatic impairment and healthy subjects.17 However, the results of the previous study are limited by 2 key considerations: 1) subjects were neither age- nor gender-matched and 2) 50% of subjects in the hepatic impairment cohort were smokers as compared to only 25% of subjects in the control cohort.17 Smoking is known to induce CYP1A2, the primary CYP enzyme responsible for metabolizing olanzapine.17 As olanzapine clearance is higher in smokers and in men,17 the previous study may have underestimated the effect of hepatic impairment on the pharmacokinetics of olanzapine due to imbalance of these confounding factors. Consistent with the increases in olanzapine and samidorphan exposure in subjects with moderate hepatic impairment, clearance of both drugs was decreased. However, as the volume of distribution of both drugs was also decreased, the t1/2 of both olanzapine and samidorphan was similar in subjects with moderate hepatic impairment and healthy control subjects, indicating that the elimination rate of either drug was not affected.
The increase in olanzapine exposure in subjects with severe renal impairment was small (geometric mean ratio of 1.32 and 1.51 for Cmax and AUC0-∞, respectively; Table 5). This is consistent with renal excretion being a minor clearance pathway for olanzapine and findings from the previous study by Callaghan et al, in which no significant changes in olanzapine pharmacokinetics were observed in subjects with severe renal impairment.17 Subjects with severe renal impairment had a higher systemic exposure of samidorphan compared with healthy control subjects, consistent with a 56% reduction in total body clearance. However, tmax was similar between the cohorts, whereas t1/2 was slightly longer in subjects with severe renal impairment. These results suggest that severe renal impairment largely affects elimination, but not absorption, of samidorphan. The lower reduction in urine excretion and renal clearance of olanzapine compared with samidorphan in subjects with severe renal impairment is likely because renal excretion plays a smaller role in the clearance of olanzapine than that of samidorphan (7%18 vs 20% [data on file, Alkermes, Inc., Waltham, MA, USA], respectively).
Consistent with published data,18 the fraction of olanzapine bound to plasma protein was 90–92% in both studies. Although hepatic impairment can alter protein binding and potentially lead to increased systemic concentrations of free active drug,19 in both studies, the fraction of olanzapine bound to plasma protein was similar in subjects with moderate hepatic impairment or severe renal impairment and in healthy control subjects. In the present study, a 5-mg olanzapine dose was chosen due to tolerability concerns in healthy volunteers, should exposures be elevated in subjects with hepatic and renal impairment. Given that the pharmacokinetics of olanzapine is linear over the commercial dose range of 2.5 mg to 20 mg,17 and there are no pharmacokinetic drug-drug interactions between olanzapine and samidorphan when the two drugs were administered in combination as a bilayer tablet,10,16 it is expected that the findings in the present studies are applicable to the entire intended commercial dose range of OLZ/SAM.
The sample size chosen for the studies reported here is in accordance with the regulatory guidelines on pharmacokinetic studies in patients with hepatic or renal impairment20–23 and not based on statistical considerations. Due to enrollment difficulties, it has been traditionally accepted that a sample size of approximately 8 subjects per group should be sufficient in providing a fit-for-purpose estimation of the effect of hepatic and renal impairment on pharmacokinetics.20,24 A sample size of 10 evaluable subjects per cohort utilized in the studies reported here was regarded as adequate to attain reliable results and to fulfill the objectives and requirements of the studies.
A higher intersubject variability in olanzapine and samidorphan pharmacokinetic profiles was noted in hepatically and renally impaired subjects as compared with the healthy control subjects (Figures 2 and 3). This result was likely caused by alterations in drug bioavailability and clearance due to chronic hepatic or renal disorder and/or potential pharmacokinetic interactions with concomitant medication received by subjects with hepatic or renal impairment.
Overall, OLZ/SAM was generally well tolerated. AEs were typically consistent with those observed in other studies of olanzapine alone or OLZ/SAM.10,18,25 The early onset of the most commonly reported AEs (somnolence, dizziness, and hypotension) in subjects with moderate hepatic impairment was consistent with the earlier tmax of olanzapine observed in this cohort; however, the AEs were mild in severity.
Conclusion
There was a 1.67- and 2.17-fold increase in AUC0-∞ and Cmax of olanzapine, respectively, and a 1.52- and 1.63-fold increase in AUC0-∞ and Cmax of samidorphan, respectively, in subjects with moderate hepatic impairment compared with healthy control subjects. Compared with healthy control subjects, subjects with severe renal impairment had a 33% and 56% reduction in total body clearance, a 1.51- and 2.31-fold increase in AUC0-∞, and a 1.32- and 1.37-fold increase in Cmax of olanzapine and samidorphan, respectively. OLZ/SAM was generally well tolerated under the conditions of the studies, with a safety profile consistent with that observed in other completed clinical studies to date.
Supplementary materials
Acknowledgments
This study was sponsored by Alkermes, Inc. The authors would also like to thank Mark S. Todtenkopf, PhD, who assisted in the preparation and proofreading of the manuscript. Medical writing and editorial support was provided by Peloton Advantage, LLC (Parsippany, NJ), and funded by Alkermes, Inc. Alkermes, Inc. is a pharmaceutical company developing OLZ/SAM, a combination product of olanzapine and samidorphan for the treatment of schizophrenia, and has funded this study.
Author contributions
All authors contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
Lei Sun, Sergey Yagoda, Yangchun Du, and Lisa von Moltke are employees and shareholders of Alkermes, Inc. The authors report no other conflicts of interest in this work.
References
- 1.Lehman AF, Lieberman JA, Dixon LB, et al. Practice Guideline for the Treatment of Patients with Schizophrenia. 2nded; 2010. Available from: https://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/schizophrenia.pdf. Accessed January28, 2019. [Google Scholar]
- 2.Agid O, Foussias G, Singh S, Remington G. Where to position clozapine: re-examining the evidence. Can J Psychiatry. 2010;55(10):677–684. doi: 10.1177/070674371005501007 [DOI] [PubMed] [Google Scholar]
- 3.Leucht S, Cipriani A, Spineli L, et al. Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: a multiple-treatments meta-analysis. Lancet. 2013;382(9896):951–962. doi: 10.1016/S0140-6736(13)60733-3 [DOI] [PubMed] [Google Scholar]
- 4.Tabarin A, Diz-Chaves Y, Carmona Mdel C, et al. Resistance to diet-induced obesity in mu-opioid receptor-deficient mice: evidence for a “thrifty gene”. Diabetes. 2005;54(12):3510–3516. doi: 10.2337/diabetes.54.12.3510 [DOI] [PubMed] [Google Scholar]
- 5.Czyzyk TA, Romero-Pico A, Pintar J, et al. Mice lacking delta-opioid receptors resist the development of diet-induced obesity. Faseb J. 2012;26(8):3483–3492. doi: 10.1096/fj.12-208041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Czyzyk TA, Nogueiras R, Lockwood JF, et al. kappa-Opioid receptors control the metabolic response to a high-energy diet in mice. Faseb J. 2010;24(4):1151–1159. doi: 10.1096/fj.09-143610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wentland MP, Lou R, Lu Q, et al. Syntheses of novel high affinity ligands for opioid receptors. Bioorg Med Chem Lett. 2009;19(8):2289–2294. doi: 10.1016/j.bmcl.2009.02.078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bidlack JM, Knapp BI, Deaver DR, et al. In vitro pharmacological characterization of buprenorphine, samidorphan, and combinations being developed as an adjunctive treatment of major depressive disorder. J Pharmacol Exp Ther. 2018;367(2):267–281. doi: 10.1124/jpet.118.249839 [DOI] [PubMed] [Google Scholar]
- 9.Shram MJ, Silverman B, Ehrich E, Sellers EM, Turncliff R. Use of remifentanil in a novel clinical paradigm to characterize onset and duration of opioid blockade by samidorphan, a potent mu-receptor antagonist. J Clin Psychopharmacol. 2015;35(3):242–249. doi: 10.1097/JCP.0000000000000320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sun L, McDonnell D, Liu J, von Moltke L. Bioequivalence of olanzapine given in combination with samidorphan as a bilayer tablet (ALKS 3831) compared with olanzapine-alone tablets: results from a randomized, crossover relative bioavailability study. Clin Pharmacol Drug Dev. 2019;8(4):459–466. doi: 10.1002/cpdd.601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Silverman BL, Martin W, Memisoglu A, DiPetrillo L, Correll CU, Kane JM. A randomized, double-blind, placebo-controlled proof of concept study to evaluate samidorphan in the prevention of olanzapine-induced weight gain in healthy volunteers. Schizophr Res. 2018;195:245–251. doi: 10.1016/j.schres.2017.10.014 [DOI] [PubMed] [Google Scholar]
- 12.Martin WF, Correll CU, Weiden PJ, et al. Mitigation of olanzapine-induced weight gain with samidorphan, an opioid antagonist: a randomized double-blind phase 2 study in patients with schizophrenia. Am J Psychiatry. 2019;176(6):457–467. doi: 10.1176/appi.ajp.2018.18030280 [DOI] [PubMed] [Google Scholar]
- 13.Kassahun K, Mattiuz E, Nyhart E Jr, et al. Disposition and biotransformation of the antipsychotic agent olanzapine in humans. Drug Metab Dispos. 1997;25(1):81–93. [PubMed] [Google Scholar]
- 14.Yuan R, Venitz J. Effect of chronic renal failure on the disposition of highly hepatically metabolized drugs. Int J Clin Pharmacol Ther. 2000;38(5):245–253. doi: 10.5414/cpp38245 [DOI] [PubMed] [Google Scholar]
- 15.Wagner C, Zhao P, Pan Y, et al. Application of physiologically based pharmacokinetic (PBPK) modeling to support dose selection: report of an FDA public workshop on PBPK. CPT Pharmacometrics Syst Pharmacol. 2015;4(4):226–230. doi: 10.1002/psp4.33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sun L, McDonnell D, von Moltke L. Pharmacokinetics and short-term safety of ALKS 3831, a fixed-dose combination of olanzapine and samidorphan, in adult subjects with schizophrenia. Clin Ther. 2018;40(11):1845–1854. doi: 10.1016/j.clinthera.2018.09.002 [DOI] [PubMed] [Google Scholar]
- 17.Callaghan JT, Bergstrom RF, Ptak LR, Beasley CM. Olanzapine. Pharmacokinetic and pharmacodynamic profile. Clin Pharmacokinet. 1999;37(3):177–193. doi: 10.2165/00003088-199937030-00001 [DOI] [PubMed] [Google Scholar]
- 18.Zyprexa (olanzapine) new drug approval package; 1996. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/nda/96/020592_Original_Approval_Pkg%20.pdf. Accessed September26, 2018.
- 19.Telles-Correia D, Barbosa A, Cortez-Pinto H, Campos C, Rocha NB, Machado S. Psychotropic drugs and liver disease: a critical review of pharmacokinetics and liver toxicity. World J Gastrointest Pharmacol Ther. 2017;8(1):26–38. doi: 10.4292/wjgpt.v8.i1.26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.US Food and Drug Administration. Guidance for industry pharmacokinetics in patients with impaired hepatic function: study design, data analysis, and impact on dosing and labeling; 2003. Available from: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/pharmacokinetics-patients-impaired-hepatic-function-study-design-data-analysis-and-impact-dosing-and. Accessed July8, 2019.
- 21.US Food and Drug Administration. Guidance for industry: pharmacokinetics in patients with impaired renal function—study design, data analysis, and impact on dosing and labeling. Revision 1; 2010. Available from: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/pharmacokinetics-patients-impaired-renal-function-study-design-data-analysis-and-impact-dosing-and. Accessed July8, 2019.
- 22.European Medicines Agency. Guideline on the evaluation of the pharmacokinetics of medicinal products in patients with impaired hepatic function; 2005. Available from: https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-evaluation-pharmacokinetics-medicinal-products-patients-impaired-hepatic-function_en.pdf. Accessed July8, 2019.
- 23.European Medicines Agency. Guideline on the evaluation of the pharmacokinetics of medicinal products in patients with decreased renal function; 2015. Available from: https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-evaluation-pharmacokinetics-medicinal-products-patients-decreased-renal-function_en.pdf. Accessed: July8, 2019.
- 24.Ibrahim S, Honig P, Huang SM, Gillespie W, Lesko LJ, Williams RL. Clinical pharmacology studies in patients with renal impairment: past experience and regulatory perspectives. J Clin Pharmacol. 2000;40(1):31–38. [DOI] [PubMed] [Google Scholar]
- 25.Markowitz JS, DeVane CL, Boulton DW, Liston HL, Risch SC. Hypotension and bradycardia in a healthy volunteer following a single 5 mg dose of olanzapine. J Clin Pharmacol. 2002;42(1):104–106. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Zyprexa (olanzapine) new drug approval package; 1996. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/nda/96/020592_Original_Approval_Pkg%20.pdf. Accessed September26, 2018.