Abstract
Background
The addition of antibiotics reportedly augments the efficacy of gemcitabine (GEM) in tumor-bearing mice. However, whether this phenomenon is also observed in cancer patients remains unclear. In the present study, we aimed to assess whether antibiotics for treatment or prevention of infection augments treatment efficacies of GEM-containing regimens in patients with any type of cancer.
Methods
Medical records of patients diagnosed with cancer histopathologically and treated with GEM-containing regimens (n=169) were retrospectively reviewed. Patients were assigned into two groups: antibiotics-untreated group (patients who were treated with GEM-containing regimens but without antibiotics) and antibiotics-treated group (patients who were treated with GEM-containing regimens plus antibiotics). Response rates, progression-free survival (PFS) time, and overall survival (OS) time were analyzed for each group.
Results
The response rates of the antibiotics-untreated and antibiotics-treated groups with GEM-containing regimens were 15.1% and 27.6%, respectively. The median PFS times of the antibiotics-untreated and antibiotics-treated groups were 2.5 (95% CI: 1.86–3.73) and 4.9 (95% CI: 3.47–6.0) months, respectively. The median OS times of the antibiotics-untreated and antibiotics-treated groups were 7.53 (95% CI: 5.63–9.57) months and 13.83 (95% CI: 10.83–16.43) months, respectively.
Conclusion
The addition of antibiotics augments the treatment efficacies of GEM-containing regimens, and it may be a potential therapeutic option to improve treatment efficacies of GEM-containing regimens in patients with advanced cancer.
Keywords: antibiotics, bacteria, gemcitabine, multivariate analysis, univariate analysis
Background
Gemcitabine (GEM) is one of the anticancer drugs that is often used for patients with advanced cancer.1 GEM-containing regimens are used for patients with pancreatic cancer, biliary tract cancer, lung cancer, sarcoma, urothelial cancer, or breast cancer.2–7 Literature is limited on the responses of GEM-containing regimens in patients with advanced cancers5,8–10; this necessitates the improvement of treatment efficacies of GEM-containing regimens in patients with advanced cancers.
A previous study reported that GEM (2ʹ,2ʹ-difluorodeoxycytidine) is metabolized into an inactive metabolite 2,2ʹ-difluorodeoxyuridine by various microbes that express a long isoform of the bacterial enzyme cytidine deaminase (CDDL).11 In other previous reports, the treatment of tumor-bearing mice with antibiotics eradicates the bacteria from the tumor tissue and consequently increases the concentration of GEM in the tumor tissue.12 Increased concentration of GEM in the tumor tissue resulted in robust tumor regression, whereas the mouse not treated with GEM did not exhibit tumor regression.12 Moreover, various bacteria expressed CDDL in human pancreatic cancer tissue, and these bacteria potently conferred the resistance of GEM in the cancer cell line in vitro.12 Therefore, the bacteria that express CDDL in tumor tissue may be related to the low treatment efficacies of GEM in human and that the addition of antibiotics to a regimen-containing GEM would augment its efficacy. However, no previous report had examined whether the addition of antibiotics augments the treatment efficacy of GEM in patients with advanced cancer.
In this study, we tried to assess whether antibiotics given for treatment or prevention of infection augment the treatment efficacy of GEM-containing regimens in patients with various types of advanced cancers.
Methods
Patients
Medical records of patients who were diagnosed with cancer histopathologically and were treated with GEM-containing regimens (n=169) were retrospectively reviewed at the Department of Medical Oncology, Tohoku University Hospital from 2006 to 2018. Patients with advanced stage of pancreatic cancer, biliary tract cancer, duodenal cancer, cancer of unknown primary, neuroendocrine carcinoma, sarcoma, and urinary bladder cancer were included in this study. Patients with stage III or stage IV cancers were included in the antibiotics-untreated and antibiotics-treated group. Proportions of patients with stage III or IV cancer were similar between the two groups.
Inclusion criteria of this study included: 1) patients who had been histologically confirmed carcinoma or sarcoma; 2) patients who had unresectable cancer (or sarcoma) or metastatic lesion; 3) patients who had been treated with at least one course of GEM-containing regimen; 4) patients who had at least one measurable cancer (or sarcoma) lesion; 5) patient in whom the treatment efficacies of GEM-containing regimen in cancer (or sarcoma) had been assessed by computed tomography (CT) at least once. In all, there were 196 patients who met the inclusion criteria. Patients who did not meet inclusion criteria were all excluded from the analyses in this study.
Treatment methods
The doses and schedules of GEM treatment in this study were as follows. GEM alone (plus erlotinib): GEM 1000 mg/m2, days 1, 8 and 15 (erlotinib 100 mg/body, days 1–28) every 4 weeks; GEM plus nanoparticle albumin binding paclitaxel (nabPTX): GEM 1000 mg/m2, nabPTX 125 mg/m2, days 1, 8, 15, every 4 weeks; GEM plus cisplatin (plus S-1): GEM 1000 mg/m2, cisplatin 25 mg/m2, days 1, 8 (S-1 80 mg/m2, days 1–14, every 3 weeks; GEM plus docetaxel: GEM 900 mg/m2, day 1, 8, docetaxel 70 mg/m2 day 8, every 3 weeks.
Antibiotics were administered according to the drug attachment (e.g., levofloxacin hydrate: oral administration, 500 mg/body/day; cefdinir: oral administration, 300 mg/body/day; meropenem hydrate: intravenous administration, 0.5–1 g/body/day.) The administration period of antibiotics was determined by the chief physician of each patient.
Evaluation
Patients were assigned into two groups. The first was the antibiotics-treated group where patients had been treated with antibiotics from the start of the GEM-containing regimen to the first imaging evaluation of the efficacy of GEM-containing regimen using CT (antibiotics-treated group). The other group was the antibiotics-untreated group where patients had not been treated with antibiotics from the start of the GEM-containing regimen to the first CT evaluation of the efficacy of the GEM-containing regimen.
Responses were assessed using Response Criteria in Solid Tumor version 1.0.13 The rates of complete response (CR; all signs of cancer disappeared by treatment with GEM-containing regimen) and partial response (PR; defined as a ≥30% reduction in the diameter of measurable lesions on CT) were combined and defined as the response rate. CR, PR, and stable disease (defined as a <30% reduction and a <20% increase in the diameter of measurable lesions as shown on CT) rates were combined, and these rates were defined as the disease control rate. In this study, the relative dose intensity of GEM was defined as the ratio of the total actual dose of GEM delivered to patients to the planned dose of GEM. All toxicities were reviewed from medical records and were evaluated according to the Common Terminology Criteria for Adverse Events version 4.0.14
Statistical analysis
The median progression-free survival (PFS) time and median overall survival (OS) time were calculated using the Kaplan–Meier method. P-values of the response rate and disease control rate were based on Fisher’s exact test. All statistical analyses including univariate analysis, multivariate analysis, Pearson’s chi-squared test, and Wilcoxon Mann–Whitney test were performed using JMP® 11 (SAS Institute Inc., Cary, NC, USA). All differences were regarded as statistically significant when P<0.05.
Results
Patient characteristics
We identified 169 patients who were treated with GEM-containing regimen (antibiotics-untreated group=93; antibiotics-treated group=76). Patient characteristics are presented in Table 1. Approximately, 80% of the subjects had pancreatic or biliary tract cancer. Relative dose intensities of GEM in the antibiotics-untreated and antibiotics-treated groups were 81.1% and 78.9%, respectively. Proportions of sex, previous surgery, types of GEM-containing regimens were similar between the two groups.
Table 1.
Patient characteristics
| Antibiotics-untreated group | Antibiotics-treated group | P-value | |
|---|---|---|---|
| Number | 93 | 76 | |
| Sex (%) | 0.785 | ||
| Male | 56 (60.2) | 46 (60.5) | |
| Female | 37 (39.8) | 30 (39.5) | |
| Mean age (range) | 63.9 (29–80) | 63.0 (31–84) | |
| Cancer type (%) | 0.346 | ||
| Pancreatic cancer | 60 (64.5) | 45 (59.2) | |
| Biliary tract cancer | 16 (17.2) | 18 (23.7) | |
| Sarcoma | 9 (9.7) | 9 (11.8) | |
| CUP | 3 (3.2) | 2 (2.6) | |
| Duodenal cancer | 3 (3.2) | 1 (1.3) | |
| Neuroendocrine carcinoma | 1 (1.1) | 0 (0.0) | |
| Breast cancer | 1 (1.1) | 0 (0.0) | |
| Ulinary bladder cancer | 0 (0.0) | 1 (1.3) | |
| Tumor stage | 0.891 | ||
| III | 8 (8.6) | 7 (9.2) | |
| IV | 85 (91.4) | 69 (90.8) | |
| Operation history (%) | 0.755 | ||
| + | 23 (24.7) | 23 (30.3) | |
| − | 70 (75.3) | 53 (69.7) | |
| GEM including regimen (%) | 0.412 | ||
| GEM alone | 48 (52.1) | 34 (44.7) | |
| GEM plus nabPTX | 20 (22.9) | 20 (26.3) | |
| GEM plus cisplatin | 12 (12.5) | 12 (15.8) | |
| GEM plus docetaxel | 9 (9.4) | 9 (11.8) | |
| GEM plus cisplatin plus S-1 | 3 (2.1) | 0 (0.0) | |
| GEM plus elrotinib | 1 (1.0) | 1 (1.3) | |
| Relative dose intensity of GEM (%) | 81.1 | 78.9 | 0.788 |
| Treated antibiotics | |||
| New quinolone | 38 (50.0) | ||
| Second-generation cephem | 3 (3.9) | ||
| Third-generation cephem | 15 (19.7) | ||
| Fourth-generation cephem | 13 (17.1) | ||
| Carbapenem | 3 (3.9) | ||
| β-Lactamase inhibitor | 2 (2.6) | ||
| Penicillin | 1 (1.3) | ||
| Reason of antibiotics treatment | |||
| Because of infection | 16 (21.1) | ||
| To prevent infection | 60 (78.9) |
Note: P-values were calculated using chi-squared test or Wilcoxon or Mann–Whitney test.
Abbreviations: CUP, cancer of unknown primary; nabPTX, nanoparticle albumin binding paclitaxel; GEM, gemcitabine.
Efficacies of GEM-containing regimens
We calculated the response rate of patients to GEM-containing regimens. As shown in Table 2, the response rates in the antibiotics-untreated and antibiotics-treated groups by GEM-containing regimens were 15.1% and 27.6%, respectively. Disease control rates in the antibiotics-untreated and antibiotics-treated groups by GEM-containing regimens were 51.6% and 72.4%, respectively. The response and disease control rates were significantly higher in the antibiotics-treated group than in the antibiotics-untreated group.
Table 2.
Response rate of gemcitabine-containing regimens
| CR | PR | SD | PD | RR (%) | |
|---|---|---|---|---|---|
| Antibiotics-untreated group | 0 | 14 | 34 | 45 | 15.1 |
| Antibiotics-treated group | 0 | 21 | 34 | 21 | 27.6 |
Notes: P-value of response rate between two groups. Antibiotics-untreated group vs antibiotics-treated group P=0.0356. P-value of disease control rate between each group. Antibiotics-untreated group vs antibiotics-treated group P=0.0071.
Abbreviations: CR, complete response; DCR, disease control rate; PD, progression disease; PR, partial response; RR, response rate; SD, stable didease.
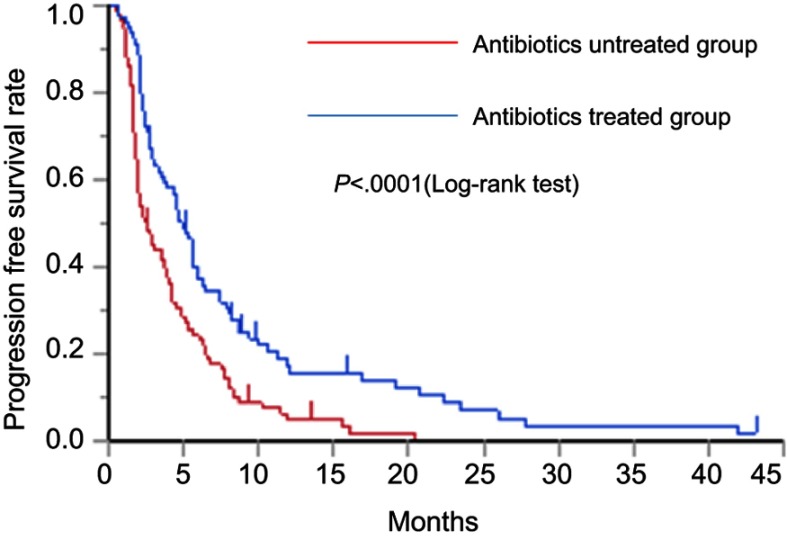
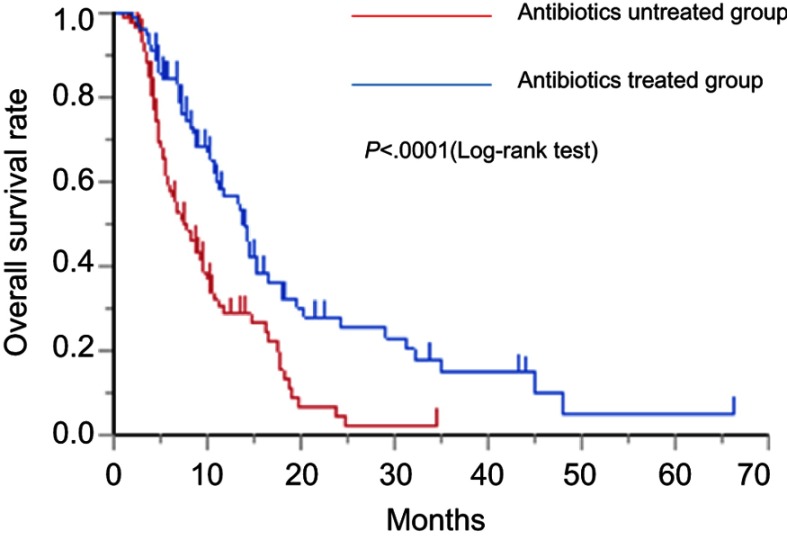
As shown in Figure 1, the median PFS times of the antibiotics-untreated and antibiotics-treated groups were 2.5 (95% CI: 1.86–3.73) days and 4.93 (95% CI: 3.47–6.0) months, respectively. The median PFS rate was significantly higher in the antibiotics-treated group than in the antibiotics-untreated group (P<0.0001, log-rank test). As shown in Figure 2, the median OS times of the antibiotics-untreated and antibiotics-treated groups were 7.53 (95% CI: 5.63–9.57) months and 13.83 (95% CI: 10.83–16.43) months, respectively. The median OS rate was significantly higher in the antibiotics-treated group than in the antibiotics-untreated group (P<0.0001, log-rank test). The median PFS and the median OS of the patients with each cancer type in antibiotics-treated group and antibiotics-untreated group were shown in Table S1. In all cancer types, both the median PFS and the median OS of antibiotics-treated group were longer than these of antibiotics-untreated group. Especially, in pancreatic cancer, both the median PFS and the median OS of the antibiotics-treated group were significantly longer than those of the antibiotics-untreated group. In sarcoma, the median OS of the antibiotics-treated group was significantly longer than that of antibiotics-untreated group. Original data of each patient were shown in Table S2.
Figure 1.
Kaplan–Meier curve of the PFS rate in the antibiotics-untreated group and antibiotics-treated group.
Abbreviation: PFS, progression-free survival.
Figure 2.
Kaplan–Meier curve of the OS rate with the antibiotics-untreated group and antibiotics-treated group.
Abbreviation: OS, overall survival
Table S1.
The median progression free survival time (PFS) or the median OS of the patients with biliary tract cancer, pancreatic cancer, sarcoma and other cancers in antibiotics-untreated group and antibiotics-treated group
| Primary site | Median PFS (months) | P-value | Median OS (months) | P-value | ||
|---|---|---|---|---|---|---|
| Antibiotics-untreated gourp | Antibiotics-treated group | Antibiotics-untreated gourp | Antibiotics-treated group | |||
| Biliary tract | 3.4 | 5.4 | 0.1580 | 10.6 | 14.3 | 0.4305 |
| Pancreas | 2.5 | 4.2 | 0.0035 | 6.6 | 13.8 | 0.0020 |
| Sarcoma | 1.9 | 5.1 | 0.2642 | 4.0 | 10.9 | 0.0400 |
| Other cancers | 4.0 | 7.9 | 0.1445 | 9.9 | 10.8 | 0.5997 |
Notes: Other cancer: CUP, NEC, duodenal cancer, breast cancer, urinary bladder cancer. P-value was calculated using log-rank test.
Table S2.
Patient’s original data in the present study
| Age | Sex | Primary site | Operation history | GEM-containing regimen | GEM containing regimen | Date of death | Antibiotics treatment | |
|---|---|---|---|---|---|---|---|---|
| Date of start | Date of discontinuation | |||||||
| 69 | Female | Pancreas | No | GC | 11-05-2017 | 31-08-2018 | 31-08-2018 | Carbapenem |
| 63 | Female | Biliary tract | No | GC | 14-11-2008 | 01-05-2009 | 29-07-2009 | Carbapenem |
| 67 | Female | Pancreas | No | GEM | 27-06-2008 | 24-09-2008 | 14-11-2008 | Carbapenem |
| 54 | Male | Biliary tract | No | GC | 05-07-2017 | 12-10-2017 | 31-08-2018 | Cephem |
| 67 | Male | Biliary tract | No | GC | 15-10-2015 | 17-12-2015 | 08-04-2017 | Cephem |
| 62 | Male | Biliary tract | No | GC | 17-10-2013 | 02-07-2015 | 20-08-2015 | Cephem |
| 69 | Male | Pancreas | No | GC | 25-07-2016 | 26-09-2016 | 23-05-2017 | Cephem |
| 75 | Female | Pancreas | No | GC | 16-06-2015 | 29-04-2016 | 24-08-2016 | Cephem |
| 25 | Male | Sarcoma | No | GD | 16-12-2013 | 25-05-2017 | 25-11-2017 | Cephem |
| 32 | Female | Sarcoma | No | GD | 30-10-2017 | 16-11-2017 | 12-01-2018 | Cephem |
| 68 | Male | Biliary tract | No | GEM | 24-11-2015 | 20-06-2017 | 31-08-2018 | Cephem |
| 57 | Male | CUP | No | GEM | 18-08-2017 | 10-11-2017 | 02-01-2018 | Cephem |
| 74 | Female | Pancreas | No | GEM | 05-04-2010 | 02-06-2010 | 04-06-2010 | Cephem |
| 76 | Female | Pancreas | No | GEM | 11-12-2006 | 10-10-2008 | 11-12-2008 | Cephem |
| 70 | Female | Pancreas | No | GEM | 24-08-2006 | 04-12-2008 | 12-01-2009 | Cephem |
| 49 | Male | Pancreas | No | GEM | 24-07-2014 | 05-12-2014 | 21-02-2015 | Cephem |
| 67 | Male | Pancreas | No | GEM | 06-02-2018 | 27-02-2018 | 29-04-2018 | Cephem |
| 66 | Male | Pancreas | No | GEM | 23-03-2017 | 24-05-2017 | 26-06-2018 | Cephem |
| 66 | Female | Pancreas | No | GEM | 11-12-2017 | 31-08-2018 | 31-08-2018 | Cephem |
| 68 | Female | Pancreas | No | GEM | 12-05-2017 | 19-07-2017 | 24-09-2017 | Cephem |
| 50 | Male | Pancreas | No | GEM | 30-09-2016 | 09-12-2016 | 23-04-2017 | Cephem |
| 72 | Male | Pancreas | No | GEM | 04-08-2016 | 15-09-2016 | 17-11-2016 | Cephem |
| 84 | Female | Biliary tract | No | GnP | 27-12-2017 | 31-08-2018 | 31-08-2018 | Cephem |
| 42 | Female | Biliary tract | No | GnP | 16-03-2016 | 12-09-2016 | 01-02-2017 | Cephem |
| 62 | Female | Biliary tract | No | GC | 31-05-2016 | 01-11-2016 | 01-11-2016 | New quinolone |
| 58 | Male | CUP | No | GC | 26-03-2010 | 18-11-2010 | 14-02-2011 | New quinolone |
| 70 | Male | Pancreas | No | GC | 31-10-2015 | 12-01-2016 | 25-03-2016 | New quinolone |
| 66 | Male | Pancreas | No | GC | 22-08-2014 | 29-01-2015 | 19-03-2015 | New quinolone |
| 68 | Female | Ulinary bladder | No | GC | 12-09-2011 | 05-12-2011 | 16-04-2012 | New quinolone |
| 36 | Male | Sarcoma | No | GD | 19-06-2015 | 07-04-2016 | 07-04-2016 | Ne w quinolone |
| 59 | Male | Biliary tract | No | GEM | 30-08-2016 | 11-04-2017 | 31-07-2017 | New quinolone |
| 61 | Male | Biliary tract | No | GEM | 04-06-2012 | 23-08-2012 | 25-10-2012 | New quinolone |
| 58 | Male | Biliary tract | No | GEM | 01-11-2012 | 31-01-2013 | 03-01-2014 | New quinolone |
| 80 | Female | Biliary tract | No | GEM | 28-02-2011 | 16-05-2011 | 21-10-2011 | New quinolone |
| 59 | Female | Biliary tract | No | GEM | 31-07-2009 | 12-11-2009 | 01-04-2011 | New quinolone |
| 78 | Male | Biliary tract | No | GEM | 18-09-2007 | 06-11-2009 | 10-05-2010 | New quinolone |
| 54 | Male | Pancreas | No | GEM | 26-03-2015 | 28-05-2015 | 16-07-2015 | New quinolone |
| 72 | Male | Pancreas | No | GEM | 13-02-2015 | 31-08-2018 | 31-08-2018 | New quinolone |
| 64 | Male | Pancreas | No | GEM | 21-02-2011 | 08-08-2011 | 29-03-2012 | New quinolone |
| 66 | Male | Pancreas | No | GEM | 24-09-2010 | 11-03-2011 | 28-05-2011 | New quinolone |
| 74 | Male | Pancreas | No | GEM | 26-05-2010 | 05-01-2011 | 03-08-2011 | New quinolone |
| 48 | Male | Pancreas | No | GEM | 06-04-2009 | 21-08-2009 | 08-05-2010 | New quinolone |
| 71 | Male | Pancreas | No | GEM | 06-09-2007 | 16-11-2007 | 18-01-2008 | New quinolone |
| 70 | Male | Pancreas | No | GEM | 26-05-2006 | 23-05-2007 | 05-02-2010 | New quinolone |
| 69 | Female | Pancreas | No | GEM | 05-10-2006 | 21-02-2007 | 23-11-2007 | New quinolone |
| 73 | Male | Biliary tract | No | GnP | 18-05-2015 | 16-07-2015 | 11-09-2015 | New quinolone |
| 77 | Male | Pancreas | No | GnP | 02-07-2015 | 26-10-2015 | 18-06-2016 | New quinolone |
| 58 | Male | Pancreas | No | GnP | 12-12-2011 | 20-01-2012 | 10-02-2012 | New quinolone |
| 87 | Female | Pancreas | No | GnP | 16-02-2018 | 23-04-2018 | 19-06-2018 | New quinolone |
| 70 | Male | Pancreas | No | GnP | 04-09-2017 | 24-05-2018 | 05-07-2018 | New quinolone |
| 80 | Male | Pancreas | No | GnP | 30-08-2017 | 18-12-2017 | 19-02-2018 | New quinolone |
| 63 | Male | Pancreas | No | GEM | 28-06-2017 | 21-08-2017 | 31-08-2018 | Penicilline |
| 66 | Male | Pancreas | No | GEM | 25-11-2014 | 01-09-2015 | 01-04-2016 | β-lactamase inhibitor |
| 61 | Female | Biliary tract | No | GC | 25-08-2014 | 18-09-2014 | 25-09-2014 | None |
| 70 | Male | Biliary tract | No | GC | 24-03-2014 | 30-05-2014 | 29-07-2014 | None |
| 78 | Female | Biliary tract | No | GC | 16-02-2016 | 13-06-2016 | 31-07-2016 | None |
| 71 | Male | Biliary tract | No | GC | 12-08-2016 | 07-10-2016 | 05-05-2017 | None |
| 68 | Female | Biliary tract | No | GC | 14-07-2014 | 07-10-2014 | 15-05-2015 | None |
| 75 | Female | Biliary tract | No | GC | 29-01-2013 | 12-09-2013 | 17-08-2014 | None |
| 58 | Male | Biliary tract | No | GC | 21-01-2013 | 21-09-2013 | 06-12-2013 | None |
| 69 | Female | Biliary tract | No | GC | 10-05-2010 | 29-06-2010 | 21-02-2011 | None |
| 79 | Female | CUP | No | GC | 31-05-2012 | 24-05-2013 | 30-09-2013 | None |
| 50 | Male | NEC | No | GC | 06-10-2014 | 20-04-2015 | 27-09-2015 | None |
| 64 | Female | Biliary tract | No | GCS | 09-10-2015 | 16-06-2016 | 06-04-2017 | None |
| 74 | Male | Biliary tract | No | GCS | 06-01-2016 | 15-07-2016 | 12-10-2016 | None |
| 32 | Male | Sarcoma | No | GD | 06-03-2017 | 21-03-2017 | 04-04-2017 | None |
| 52 | Female | Sarcoma | No | GD | 31-10-2016 | 26-12-2016 | 28-02-2017 | None |
| 70 | Male | Biliary tract | No | GEM | 10-02-2011 | 12-04-2011 | 28-06-2011 | None |
| 72 | Female | Biliary tract | No | GEM | 04-09-2008 | 28-10-2008 | 17-04-2009 | None |
| 78 | Male | CUP | No | GEM | 27-06-2017 | 25-07-2017 | 05-10-2017 | None |
| 70 | Male | CUP | No | GEM | 14-04-2008 | 18-08-2008 | 28-08-2008 | None |
| 46 | Female | Breast | No | GEM | 10-04-2008 | 22-05-2008 | 18-02-2009 | None |
| 76 | Male | Pancreas | No | GEM | 20-09-2016 | 05-01-2017 | 13-05-2017 | None |
| 29 | Male | Pancreas | No | GEM | 06-01-2014 | 28-02-2014 | 20-06-2014 | None |
| 80 | Female | Pancreas | No | GEM | 04-09-2014 | 30-10-2014 | 14-02-2015 | None |
| 42 | Male | Pancreas | No | GEM | 22-08-2013 | 15-12-2013 | 15-12-2013 | None |
| 61 | Female | Pancreas | No | GEM | 17-01-2014 | 17-09-2014 | 14-11-2014 | None |
| 65 | Male | Pancreas | No | GEM | 16-05-2013 | 08-10-2013 | 02-10-2013 | None |
| 45 | Female | Pancreas | No | GEM | 23-04-2013 | 06-06-2013 | 12-07-2013 | None |
| 61 | Male | Pancreas | No | GEM | 05-10-2012 | 06-11-2012 | 21-11-2012 | None |
| 55 | female | Pancreas | no | GEM | 19-04-2013 | 18-06-2013 | 23-09-2013 | none |
| 69 | male | Pancreas | no | GEM | 15-01-2013 | 25-06-2013 | 05-07-2014 | none |
| 63 | female | Pancreas | no | GEM | 26-04-2012 | 01-06-2012 | 01-09-2012 | none |
| 56 | male | Pancreas | no | GEM | 22-09-2011 | 31-10-2011 | 17-11-2011 | none |
| 52 | male | Pancreas | no | GEM | 13-12-2010 | 11-04-2011 | 11-04-2011 | none |
| 69 | male | Pancreas | no | GEM | 12-08-2010 | 14-10-2010 | 02-11-2010 | none |
| 62 | female | Pancreas | no | GEM | 14-01-2010 | 18-02-2010 | 15-03-2010 | none |
| 66 | female | Pancreas | no | GEM | 14-12-2009 | 08-02-2010 | 01-06-2010 | none |
| 68 | male | Pancreas | no | GEM | 06-10-2009 | 10-02-2010 | 30-11-2010 | none |
| 73 | male | Pancreas | no | GEM | 14-07-2009 | 13-08-2009 | 21-11-2009 | none |
| 69 | male | Pancreas | no | GEM | 07-07-2009 | 27-08-2009 | 17-10-2009 | none |
| 74 | female | Pancreas | no | GEM | 02-02-2009 | 23-03-2009 | 23-03-2009 | none |
| 64 | male | Pancreas | no | GEM | 27-11-2008 | 21-01-2009 | 24-02-2009 | none |
| 57 | male | Pancreas | no | GEM | 25-11-2008 | 08-06-2009 | 17-08-2009 | none |
| 63 | male | Pancreas | no | GEM | 26-09-2008 | 07-11-2008 | 11-04-2009 | none |
| 75 | female | Pancreas | no | GEM | 29-05-2008 | 17-07-2008 | 10-06-2010 | none |
| 77 | male | Pancreas | no | GEM | 21-05-2008 | 04-09-2008 | 02-06-2009 | none |
| 70 | male | Pancreas | no | GEM | 11-01-2008 | 04-03-2008 | 14-04-2008 | none |
| 44 | male | Pancreas | no | GEM | 10-10-2007 | 17-03-2008 | 23-05-2008 | none |
| 67 | male | Pancreas | no | GEM | 27-07-2007 | 14-09-2007 | 10-11-2007 | none |
| 72 | male | Pancreas | no | GEM | 02-04-2007 | 28-05-2007 | 06-08-2007 | none |
| 60 | male | Pancreas | no | GEM | 27-12-2006 | 17-08-2007 | 20-02-2008 | none |
| 57 | female | Pancreas | no | GEM | 22-11-2006 | 10-01-2007 | 24-04-2007 | none |
| 72 | female | Pancreas | no | GEM | 30-03-2006 | 13-06-2006 | 04-02-2007 | none |
| 69 | male | Pancreas | no | GEM | 10-08-2012 | 11-01-2013 | 13-04-2013 | none |
| 63 | female | Pancreas | no | GEM plus elrotinib | 16-09-2010 | 11-01-2012 | 29-02-2012 | none |
| 71 | male | Pancreas | no | GnP | 20-02-2018 | 21-08-2018 | 31-08-2018 | none |
| 70 | male | Pancreas | no | GnP | 28-11-2017 | 16-01-2018 | 27-03-2018 | none |
| 60 | male | Pancreas | no | GnP | 02-10-2017 | 26-02-2018 | 12-03-2018 | none |
| 72 | female | Pancreas | no | GnP | 08-09-2017 | 16-03-2018 | 27-05-2018 | none |
| 61 | male | Pancreas | no | GnP | 16-01-2018 | 21-02-2018 | 12-05-2018 | none |
| 68 | male | Pancreas | no | GnP | 06-09-2017 | 09-01-2018 | 26-01-2018 | none |
| 69 | female | Pancreas | no | GnP | 21-03-2017 | 27-04-2017 | 22-01-2018 | none |
| 66 | male | Pancreas | no | GnP | 22-11-2017 | 31-08-2018 | 31-08-2018 | none |
| 65 | female | Pancreas | no | GnP | 10-03-2017 | 01-05-2017 | 28-07-2017 | none |
| 65 | male | Pancreas | no | GnP | 31-01-2017 | 20-07-2017 | 08-10-2017 | none |
| 75 | male | Pancreas | no | GnP | 20-09-2016 | 31-01-2017 | 28-03-2017 | none |
| 75 | female | Pancreas | no | GnP | 26-09-2016 | 17-10-2016 | 15-11-2016 | none |
| 73 | female | Pancreas | no | GnP | 30-08-2016 | 20-12-2016 | 20-03-2017 | none |
| 81 | male | Pancreas | no | GnP | 28-07-2016 | 13-10-2016 | 18-01-2017 | none |
| 62 | male | Pancreas | no | GnP | 03-03-2017 | 06-04-2017 | 13-05-2017 | none |
| 67 | male | Pancreas | no | GnP | 22-06-2016 | 21-09-2016 | 16-12-2016 | none |
| 77 | female | Pancreas | no | GnP | 12-05-2015 | 04-08-2015 | 03-09-2015 | none |
| 74 | male | Sarcoma | yes | GD | 14-10-2015 | 29-02-2016 | 29-08-2018 | cephem |
| 38 | female | Sarcoma | yes | GD | 11-12-2014 | 17-06-2015 | 27-08-2015 | cephem |
| 51 | female | Sarcoma | yes | GD | 13-08-2015 | 22-02-2016 | 15-03-2016 | cephem |
| 58 | female | Pancreas | yes | GEM | 07-06-2017 | 04-09-2017 | 15-11-2017 | cephem |
| 75 | female | Biliary tract | yes | GnP | 27-09-2017 | 22-02-2018 | 04-08-2018 | cephem |
| 78 | male | Pancreas | yes | GnP | 30-10-2017 | 19-01-2018 | 26-09-2018 | cephem |
| 50 | male | Pancreas | yes | GnP | 03-10-2016 | 21-06-2017 | 27-03-2018 | cephem |
| 63 | female | Pancreas | yes | GnP | 23-07-2015 | 07-01-2016 | 30-06-2016 | cephem |
| 67 | female | Pancreas | yes | GnP | 18-07-2017 | 19-03-2018 | 12-04-2018 | cephem |
| 59 | female | Pancreas | yes | GnP | 29-06-2016 | 26-12-2016 | 22-01-2017 | cephem |
| 31 | male | Sarcoma | yes | GD | 19-12-2013 | 11-12-2014 | 22-06-2015 | new quinolone |
| 64 | male | Sarcoma | yes | GD | 16-04-2014 | 20-06-2014 | 25-11-2015 | new quinolone |
| 51 | male | Sarcoma | yes | GD | 10-11-2015 | 15-12-2015 | 04-10-2016 | new quinolone |
| 61 | female | Biliary tract | yes | GEM | 24-11-2016 | 27-04-2017 | 13-01-2018 | new quinolone |
| 78 | female | Pancreas | yes | GEM | 09-11-2009 | 26-04-2010 | 26-07-2010 | new quinolone |
| 66 | female | Pancreas | yes | GEM plus erlotinib | 20-06-2008 | 04-11-2008 | 09-01-2009 | new quinolone |
| 45 | Fe male | Biliary tract | Yes | GnP | 08-07-2015 | 10-06-2016 | 04-10-2016 | new Quinolone |
| 46 | Female | Pancreas | Yes | GnP | 22-03-2013 | 26-02-2015 | 31-08-2018 | New quinolone |
| 63 | Male | Pancreas | Yes | GnP | 22-08-2013 | 24-10-2013 | 06-02-2014 | New quinolone |
| 63 | Male | Pancreas | Yes | GnP | 30-01-2014 | 09-04-2014 | 21-08-2016 | New quinolone |
| 58 | Male | Pancreas | Yes | GnP | 10-08-2015 | 14-10-2015 | 14-03-2016 | New quinolone |
| 69 | Male | Pancreas | Yes | GnP | 07-06-2017 | 02-02-2018 | 31-08-2018 | New quinolone |
| 64 | FEMALE | Biliary tract | Yes | GC | 24-05-2016 | 08-02-2017 | 14-12-2017 | None |
| 62 | Male | Biliary tract | Yes | GC | 25-08-2015 | 12-04-2016 | 01-01-2017 | None |
| 36 | Male | Biliary tract | Yes | GCS | 21-07-2006 | 24-03-2008 | 21-05-2009 | None |
| 75 | Male | Sarcoma | Yes | GD | 24-08-2012 | 30-04-2013 | 03-02-2014 | None |
| 61 | Female | Sarcoma | Yes | GD | 21-07-2017 | 31-08-2018 | 31-08-2018 | None |
| 38 | Male | Sarcoma | Yes | GD | 30-01-2015 | 06-03-2015 | 27-04-2015 | None |
| 44 | Male | Sarcoma | Yes | GD | 18-08-2016 | 27-10-2016 | 16-12-2016 | None |
| 80 | Female | Sarcoma | yes | GD | 10-02-2014 | 08-04-2014 | 12-05-2014 | None |
| 56 | Female | Sarcoma | Yes | GD | 21-02-2017 | 10-04-2017 | 09-05-2017 | None |
| 59 | Male | Sarcoma | Yes | GD | 04-06-2008 | 05-08-2008 | 05-09-2008 | None |
| 70 | Male | Biliary tract | Yes | GEM | 26-03-2007 | 14-05-2007 | 06-08-2007 | None |
| 60 | Male | DK | Yes | GEM | 04-02-2010 | 27-05-2010 | 09-09-2010 | None |
| 66 | Male | DK | Yes | GEM | 26-02-2010 | 17-09-2010 | 11-02-2012 | None |
| 78 | Female | DK | Yes | GEM | 09-02-2006 | 28-03-2006 | 02-05-2006 | None |
| 60 | Male | Pancreas | Yes | GEM | 12-11-2013 | 28-01-2014 | 04-06-2014 | None |
| 67 | Female | Pancreas | Yes | GEM | 08-11-2011 | 20-12-2011 | 20-12-2011 | None |
| 63 | Male | Pancreas | Yes | GEM | 05-11-2009 | 14-10-2010 | 18-01-2011 | None |
| 57 | Female | Pancreas | Yes | GEM | 30-09-2009 | 19-11-2009 | 18-06-2010 | None |
| 62 | Female | Pancreas | Yes | GEM | 20-11-2006 | 26-01-2007 | 15-04-2007 | None |
| 41 | Female | Pancreas | Yes | GEM | 13-04-2012 | 17-08-2012 | 09-01-2013 | None |
| 74 | Male | Pancreas | Yes | GnP | 12-02-2015 | 16-12-2015 | 12-01-2016 | None |
| 62 | Male | Pancreas | Yes | GnP | 05-06-2015 | 14-09-2016 | 20-01-2017 | None |
| 54 | Male | Pancreas | Yes | GnP | 10-04-2015 | 06-07-2015 | 22-01-2016 | None |
| 81 | Male | DK | Yes | GEM | 16-01-2015 | 03-06-2016 | 31-08-2018 | β-lactamase inhibitor |
Abbreviations: CUP, Cancer of unknown primary; NEC, Neuroendocrine carcinoma; GEM, Gemcitabine; GnP, GEM+nabPTX; GC, GEM plus cisplatin; GD, GEM plus docetaxel; GCS, GEM plus cisplatin plus S-1.
Toxicities
Toxicities by GEM-containing regimens in the antibiotics-untreated and antibiotics-treated group are shown in Table 3. The proportions of patients with severe leukopenia and neutropenia by GEM-containing regimens in the antibiotics-treated group were higher than those in the antibiotics-untreated group. Patients with a febrile neutropenia were included only in the antibiotics-treated group. The incidence rates of anemia, thrombocytopenia, and elevated aspartate aminotransferase (AST) or alanine aminotransferase (ALT) level were similar between the two groups. No patients died from adverse events of GEM-containing regimens.
Table 3.
Severe (grade 3 or 4) toxicities by gemcitabine-containing regimens
| Antibiotics-untreated group (n=93) | Antibiotics-treated group (n=76) | |
|---|---|---|
| Leukopenia | 14 (15.1) | 36 (47.4) |
| Neutropenia | 27 (29.0) | 42 (55.3) |
| Anemia | 15 (16.1) | 12 (15.8) |
| Thrombocytopenia | 10 (10.7) | 7 (9.2) |
| Febrile neutropenia | 0 (0.0) | 2 (2.6) |
| Elevated AST/ALT | 7 (7.5) | 6 (7.9) |
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase.
Univariate and multivariate analyses
We performed univariate and multivariate analyses for the relationship between the responses to GEM-containing regimens and patient background or a severe neutropenia by GEM-containing regimens. Results of univariate and multivariate analyses are shown in Table 4. We found statistically significant correlations between the response by GEM-containing regimens and antibiotic treatment (univariate analysis: P=0.0305, multivariate analysis: P=0.0314). Seven factors (age, sex, severe neutropenia, operation history, tumor stage, cancer primary site, and type of GEM-containing regimens) analyzed did not significantly correlate with the response of GEM-containing regimens.
Table 4.
Univariate and multivariate analyses for the relationship between the response to the gemcitabine-containing regimens and patients’ background or toxicity by gemcitabine-containing regimens
| n (%) | Univariete analysis | Multivariate analysis | ||
|---|---|---|---|---|
| P-value | OR (95% CI) | P-value | ||
| Sex | ||||
| Male | 102 (60.3) | 0.5621 | 1.39 (0.632–3.058) | 0.4129 |
| Female | 67 (39.7) | |||
| Age | ||||
| ≧65 | 90 (53.3) | 0.4144 | 1.733 (0.765–3.926) | 0.1877 |
| <65 | 79 (46.7) | |||
| Antibiotics | ||||
| Untreated | 93 (55.0) | 0.0305 | 2.444 (1.083–5.519) | 0.0314 |
| Treated | 76 (45.0) | |||
| Severe (grade 3 or 4) neutropenia | ||||
| Negative | 110 (65.1) | 0.6975 | 0.696 (0.293–1.651) | 0.4103 |
| Positive | 59 (34.9) | |||
| Operaion history | ||||
| Negative | 123 (72.8) | 0.1148 | 0.364 (0.129–1.033) | 0.0577 |
| Positive | 46 (27.2) | |||
| Tumor stage | ||||
| III | 15 (8.9) | 0.4360 | 2.321 (0.473–11.392) | 0.2995 |
| IV | 154 (91.1) | |||
| Cancer type | ||||
| Pancreatic cancer | 105 (62.1) | 0.7799 | 0.919 (0.383–2.205) | 0.8500 |
| Other cancers | 64 (37.9) | |||
| Type of GEM-containing regimen | ||||
| GEM alone | 82 (48.5) | 0.0700 | 1.997 (0.842–4.738) | 0.1165 |
| Combination of GEM and other anticancer drug | 87 (51.5) | |||
Note: P-values were analyzed using Pearson’s chi-square test.
Abbreviation: GEM, gemcitabine.
Discussion
A previous study12 revealed that the antitumor efficacy of GEM was augmented by the addition of antibiotics in tumor-bearing mice compared to the antitumor efficacy of GEM alone. However, no previous report has demonstrated the augmentation of antitumor efficacy of GEM by addition of antibiotics in cancer patients. In this study, we observed that the treatment efficacy of GEM-containing regimens with antibiotics was augmented compared to that of GEM-containing regimens without antibiotics in patients with various types of advanced cancer. In all cancer type in this study, there had been tendency that both the median PFS and the median OS in the antibiotics-treated group were longer than these of antibiotics-untreated group.
A previous study12 demonstrated that antibiotics therapy (150 mg/kg of new quinolone) even for 2 days significantly removed bacteria from the tumor tissue in mice and consequently reduced the CDDL from bacteria. The reduction of CDDL resulted in the low metabolism of GEM by bacteria and the high concentration of GEM in the tumor tissue.12 The dosage of antibiotics in that study12 was similar to those usually used in patients in clinical practice. In the present study, all antibiotics were given in doses similar to those in clinical practice. In this study, as we did not investigate the amount of bacteria in the cancer tissue from patients, it is unclear whether bacteria were sufficiently removed from the tumor tissue by the antibiotics therapy. However, based on a previous study,12 the dosage of antibiotics used in the present study appeared to be sufficient to reduce the bacteria from the tumor tissue. Moreover, in the present study, the augmentation of the treatment efficacy of GEM-containing regimen by the addition of antibiotics might be attributable to the removal of bacteria from the cancer tissue, which consequently increased the concentration of GEM in cancer tissues.
In this study, the incidence rates of severe leukopenia and neutropenia by GEM-containing regimens were higher in the antibiotics-treated group than in the antibiotics-untreated group. Usually, patients who have grade 3 or 4 of leucopenia or neutropenia during chemotherapy are treated with antibiotics to prevent infections.15 Therefore, it is inevitable that the antibiotics-treated group includes patients with severe leukopenia or neutropenia. The proportions of anemia, thrombocytopenia, or elevated AST or ALT level were similar between two groups, suggesting that the addition of antibiotics do not increase the adverse effects by GEM-containing regimens.
Alteration of gut microbiota by antibiotics influenced the efficacies and toxicities of irinotecan as irinotecan metabolism was affected by bacteria in mice gut.16 Antibiotic treatment might change the gut microbiota in patients in the present study and might influence the metabolism of GEM by the bacteria in the gut similar to that in a previous report.16 These changes might elevate the blood concentration of GEM, resulting in higher toxicities with GEM-containing regimen in the antibiotics-treated group. However, the incidence rates of anemia, thrombocytopenia, and elevated AST or ALT level were similar between the two groups in the present study. Therefore, it is assumed that the general concentration of GEM is not elevated but elevated locally in the tumor tissue.
The univariate and multivariate analyses in the present study revealed that antibiotic treatment significantly correlated to the response of GEM-containing regimens. These results suggest that the addition of antibiotics was the cause of improvement of the treatment of efficacies of GEM-containing regimens.
This study has some limitations. First, this study has a retrospective design. Second, the number of patients is relatively small. Third, several previous studies have reported the influence of antibiotics on the activity of cytochrome P450 (CYP) or on the induction of CYP in humans.17–20 The change in CYP activity or in CYP induction by antibiotics influences the metabolisms of other anticancer drugs.21–24 The metabolism of GEM is possibly modified by CYP mediated by antibiotics. However, no study has reported about GEM metabolism by CYP. Therefore, it is still uncertain whether the blood concentration of GEM changes via CYP. Fourth, the timing and duration of antibiotic treatment during GEM-containing regimens varied with each patient. However, the background of the two groups was very similar, except that antibiotics were added to GEM-containing regimens only in the antibiotics-treated group. Thus, the improvement of treatment efficacy of GEM-containing regimens might be attributable to the addition of antibiotics to patients in the antibiotics-treated group. Forth, although there are several mechanisms modulating the sensitivities of GEM in cancer patients, we did not investigate the GEM resistant mechanisms in patients in this study. It has been reported that the dysregulation of proteins participating in GEM metabolism pathway or the high expression of GEM efflux pump is the mechanisms responsible for GEM resistance.25–27 Moreover, it was also reported that BRCA1 associated protein 1 gene (BAP1) mutation is responsible for the sensitivity of GEM in patients with malignant mesothelioma.28 To investigate whether these resistant mechanisms influence on efficacies of the antibiotics and GEM-containing regimen combination therapy or not is needed.
Conclusion
The addition of antibiotics to GEM-containing regimens might be a potential therapeutic option to improve treatment efficacies of GEM-containing regimens in patients with advanced cancer.
Supplementary materials
Ethics approval and consent to participate
This study protocol was approved by the ethics committee of Tohoku University Hospital. The ethics committee of Tohoku University Hospital has permitted to conduct retrospective studies without consent statements by patients (opt-out system). All data in the current study had no personal identifiers and were kept confidential.
Abbreviations
ALT, alanine aminotransferase; AST, aspartate aminotransferase; CDDL, long isoform of the bacterial enzyme cytidine deaminase; CR, complete response; CT, computed tomography; CYP, cytochrome P450; OS, overall survival; PFS, progression-free survival; PR, partial response.
Author contributions
Hiroo Imai designed the study and wrote the initial draft of the manuscript. Chikashi Ishioka is the corresponding author and contributed to analysis and interpretation of data and assisted in the preparation of the manuscript. All other authors have contributed to data collection and interpretation and critically reviewed the manuscript. All authors approved the final version of the manuscript and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Disclosure
Chikashi Ishioka received research funding from the Tokyo Cooperative Oncology Group. Chikashi Ishioka also received contributions from Chugai Pharmaceutical, Ono Pharmaceutical, MSD, Pfizer, AstraZeneca, Bristol-Myers Squibb, Janssen Pharmaceutical, Taiho Pharmaceutical, Daiichi Sankyo Company, Limited, and Takeda Pharmaceutical. Chikashi Ishioka is a representative of Tohoku Clinical Oncology Research and Education Society, a specified nonprofit corporation. Dr Masahiro Takahashi reports grants from Ono Pharmaceutical, outside the submitted work. The authors report no other conflicts of interest in this work.
References
- 1.Lund B, Kristjansen PE, Hansen HH. Clinical and preclinical activity of 2ʹ,2ʹ-difluorodeoxycytidine (gemcitabine). Cancer Treat Rev. 1993;19(1):45–55. [DOI] [PubMed] [Google Scholar]
- 2.Kamisawa T, Wood LD, Itoi T, Takaori K. Pancreatic cancer. Lancet (London, England). 2016;388(10039):73–85. doi: 10.1016/S0140-6736(16)00141-0 [DOI] [PubMed] [Google Scholar]
- 3.Gruenberger B, Schueller J, Heubrandtner U, et al. Cetuximab, gemcitabine, and oxaliplatin in patients with unresectable advanced or metastatic biliary tract cancer: a phase 2 study. Lancet Oncol. 2010;11(12):1142–1148. doi: 10.1016/S1470-2045(10)70247-3 [DOI] [PubMed] [Google Scholar]
- 4.Wu YL, Zhou C, Hu CP, et al. Afatinib versus cisplatin plus gemcitabine for first-line treatment of Asian patients with advanced non-small-cell lung cancer harbouring EGFR mutations (LUX-Lung 6): an open-label, randomised phase 3 trial. Lancet Oncol. 2014;15(2):213–222. doi: 10.1016/S1470-2045(13)70604-1 [DOI] [PubMed] [Google Scholar]
- 5.Seddon B, Strauss SJ, Whelan J, et al. Gemcitabine and docetaxel versus doxorubicin as first-line treatment in previously untreated advanced unresectable or metastatic soft-tissue sarcomas (GeDDiS): a randomised controlled phase 3 trial. Lancet Oncol. 2017;18(10):1397–1410. doi: 10.1016/S1470-2045(17)30622-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Naiki T, Iida K, Etani T, et al. Gemcitabine and docetaxel as second-line chemotherapy in elderly patients with metastatic urothelial carcinoma: a retrospective analysis. Cancer Manag Res. 2018;10:3669–3677. doi: 10.2147/CMAR.S172913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang J, Lin Y, Sun XJ, et al. Biomarker assessment of the CBCSG006 trial: a randomized phase III trial of cisplatin plus gemcitabine compared with paclitaxel plus gemcitabine as first-line therapy for patients with metastatic triple-negative breast cancer. Ann Oncol. 2018;29(8):1741–1747. doi: 10.1093/annonc/mdy209 [DOI] [PubMed] [Google Scholar]
- 8.Ramanathan RK, Goldstein D, Korn RL, et al. Positron emission tomography response evaluation from a randomized phase III trial of weekly nab-paclitaxel plus gemcitabine versus gemcitabine alone for patients with metastatic adenocarcinoma of the pancreas. Ann Oncol. 2016;27(4):648–653. doi: 10.1093/annonc/mdw020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee JK, Capanu M, O’Reilly EM, et al. A phase II study of gemcitabine and cisplatin plus sorafenib in patients with advanced biliary adenocarcinomas. Br J Cancer. 2013;109(4):915–919. doi: 10.1038/bjc.2013.432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Katakami N, Felip E, Spigel DR, et al. A randomized, open-label, multicenter, phase 3 study to compare the efficacy and safety of eribulin to treatment of physician’s choice in patients with advanced non-small cell lung cancer. Ann Oncol. 2017;28(9):2241–2247. doi: 10.1093/annonc/mdx284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baker JA, Wickremsinhe ER, Li CH, et al. Pharmacogenomics of gemcitabine metabolism: functional analysis of genetic variants in cytidine deaminase and deoxycytidine kinase. Drug Metab Dispos. 2013;41(3):541–545. doi: 10.1124/dmd.112.048769 [DOI] [PubMed] [Google Scholar]
- 12.Geller LT, Barzily-Rokni M, Danino T, et al. Potential role of intratumor bacteria in mediating tumor resistance to the chemotherapeutic drug gemcitabine. Science (New York, NY). 2017;357(6356):1156–1160. doi: 10.1126/science.aah5043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92(3):205–216. doi: 10.1093/jnci/92.3.205 [DOI] [PubMed] [Google Scholar]
- 14.Tobinai K, Kohno A, Shimada Y, et al. Toxicity grading criteria of the Japan Clinical Oncology Group. The clinical trial review committee of the Japan Clinical Oncology Group. Jpn J Clin Oncol. 1993;23(4):250–257. [PubMed] [Google Scholar]
- 15.Freifeld AG, Bow EJ, Sepkowitz KA, et al. Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the infectious diseases society of america. Clin Infect Dis. 2011;52(4):e56–e93. doi: 10.1093/cid/cir073 [DOI] [PubMed] [Google Scholar]
- 16.Wallace BD, Wang H, Lane KT, et al. Alleviating cancer drug toxicity by inhibiting a bacterial enzyme. Science (New York, NY). 2010;330(6005):831–835. doi: 10.1126/science.1191175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li A, Yeo K, Welty D, Rong H. Development of guanfacine extended-release dosing strategies in children and adolescents with ADHD using a physiologically based pharmacokinetic model to predict drug-drug interactions with moderate CYP3A4 inhibitors or inducers. Paediatr Drugs. 2018;20(2):181–194. doi: 10.1007/s40272-017-0270-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang L, Wei MJ, Zhao CY, Qi HM. Determination of the inhibitory potential of 6 fluoroquinolones on CYP1A2 and CYP2C9 in human liver microsomes. Acta Pharmacol Sin. 2008;29(12):1507–1514. doi: 10.1111/j.1745-7254.2008.00908.x [DOI] [PubMed] [Google Scholar]
- 19.Chattopadhyay N, Kanacher T, Casjens M, et al. CYP3A4-mediated effects of rifampicin on the pharmacokinetics of vilaprisan and its UGT1A1-mediated effects on bilirubin glucuronidation in humans. Br J Clin Pharmacol. 2018. doi: 10.1111/bcp.13750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pan X, Li Y, Qiu Y, et al. Efficacy and tolerability of first-line triple therapy with levofloxacin and amoxicillin plus esomeprazole or rabeprazole for the eradication of Helicobacter pylori infection and the effect of CYP2C19 genotype: a 1-week, randomized, open-label study in Chinese adults. Clin Ther. 2010;32(12):2003–2011. doi: 10.1016/j.clinthera.2010.11.005 [DOI] [PubMed] [Google Scholar]
- 21.Paolini M, Poul L, Berjaud C, et al. Nano-sized cytochrome P450 3A4 inhibitors to block hepatic metabolism of docetaxel. Int J Nanomed. 2017;12:5537–5556. doi: 10.2147/IJN.S141145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Parra-Guillen ZP, Berger PB, Haschke M, et al. Role of cytochrome P450 3A4 and 1A2 phenotyping in patients with advanced non-small-cell lung cancer receiving erlotinib treatment. Basic Clin Pharmacol Toxicol. 2017;121(4):309–315. doi: 10.1111/bcpt.12801 [DOI] [PubMed] [Google Scholar]
- 23.Chugh R, Wagner T, Griffith KA, et al. Assessment of ifosfamide pharmacokinetics, toxicity, and relation to CYP3A4 activity as measured by the erythromycin breath test in patients with sarcoma. Cancer. 2007;109(11):2315–2322. doi: 10.1002/cncr.22669 [DOI] [PubMed] [Google Scholar]
- 24.Niwa T, Shiraga T, Hashimoto T, Kagayama A. Effect of cefixime and cefdinir, oral cephalosporins, on cytochrome P450 activities in human hepatic microsomes. Biol Pharm Bull. 2004;27(1):97–99. doi: 10.1248/bpb.27.97 [DOI] [PubMed] [Google Scholar]
- 25.Zhou BS, Tsai P, Ker R, et al. Overexpression of transfected human ribonucleotide reductase M2 subunit in human cancer cells enhances their invasive potential. Clin Exp Metastasis. 1998;16(1):43–49. doi: 10.1023/A:1006559901771 [DOI] [PubMed] [Google Scholar]
- 26.Zhou J, Oliveira P, Li X, Chen Z, Bepler G. Modulation of the ribonucleotide reductase-antimetabolite drug interaction in cancer cell lines. J Nucleic Acids. 2010;2010:597098. doi: 10.4061/2010/597098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen M, Xue X, Wang F, et al. Expression and promoter methylation analysis of ATP-binding cassette genes in pancreatic cancer. Oncol Rep. 2012;27(1):265–269. doi: 10.3892/or.2011.1475 [DOI] [PubMed] [Google Scholar]
- 28.Guazzelli A, Meysami P, Bakker E, et al. BAP1 status determines the sensitivity of malignant mesothelioma cells to gemcitabine treatment. Int J Mol Sci. 2019;20(2). doi: 10.3390/ijms20020429 [DOI] [PMC free article] [PubMed] [Google Scholar]