Abstract
Background
As physicians in a referral hospital, we observed the association between history of enteric fever and somatic disorders associated with low mood. At the Al-Hussein University Hospital, Cairo and the National Liver Institute Hospital, Menoufia, we receive patients from all over Egypt, including rural areas where enteric fever is endemic.
Aim
Here in, 60 Egyptian patients referred to us for evaluation of different somatic disorders are reported.
Methods
After extensive evaluations, the patients’ symptoms were function-related. Also, their typhoid carrier states were documented, and the severity of depression using Hamilton-D (HAM-D) questionnaire was evaluated and recorded. All patients were treated with ceftriaxone, 2 gm, IV, daily for 15 days. The clinical evaluation and Hamilton score were reassessed at the end of the treatment and 6 weeks thereafter. The patients did not receive any anti-depressant nor anti-anxiety treatment during their course. Typhoid carrier was defined by documenting the history of typhoid fever that was diagnosed by culturing the Salmonella species, and not by serology, isolated from stool culture along with febrile condition, plus the absence of fever in the past 3 weeks. The Widal test was not accepted as a criterion for enrollment.
Results
Patients showed clinically significant improvement in the somatic complaints, and their HAM-D score immediately post-treatment that was consolidated for 6 weeks post-treatment completion.
Conclusion
In this study, the typhoid carrier was associated with the psychosomatic depression that improved by antibiotic therapy.
Keywords: psychosomatic, depression, typhoid carriers, Egypt
Introduction
Typhoid fever is a major health problem, with about 21 million new cases infected by Salmonella typhi (S. typhi) each year.1 In southeast Asia and sub-Saharan Africa, the effect of typhoid fever resulting in mortality is comparable to that of prostate and breast cancers and leukemia in Western societies.2 In addition, the mortality from typhoid fever has increased by 39% between 1990 and 2010, with approximately 190,000 deaths annually.2 Approximately, 2–5% of patients with typhoid fever turn into chronic carriers,3 who are asymptomatic but continue to excrete the organism for an ill-defined, prolonged period and act as reservoirs for the organism.4 Low mood and depression are associated with considerable morbidity and mortality5 and will represent the second leading cause of disability worldwide by 2020, only after ischemic heart disease.6
Chronic inflammatory disorders are associated with depression.7–10 Abundant levels of inflammatory cytokines, such as interleukin-1 (IL-1), interleukin-6 (IL-6), and interferon gamma (IFN-γ), are associated with major depressive disorders.11 The elevated level of serum C-reactive protein (CRP) is associated with an increased risk of major depressive disorder.12 Strikingly, the association between inflammatory cytokines and depression is attributed to hepatitis C patients, treated with IFN-α, an inflammatory cytokine. It has been reported that up to 50% of patients treated with interferon-α developed clinically significant depression.13,14 This inflammatory model of depression provides a putative link between infection and depression. The association between typhoid fever and neuropsychiatric illness is documented in previous studies.15–17 Through increasing inflammatory cytokines, typhoid vaccine was associated with significant short-term depression together with increased activity within the anterior cingulate cortex.18,19 The current report is based mainly on our clinical observations as physicians in the referral hospital in the endemic country of Egypt.
Patients and methods
Study design and selection of patients
This was a multicenter prospective observational study on a cohort of 60 patients, aged 19–63 years, treated at Al-Hussein University Hospital, Cairo, Egypt and the National Liver Institute Hospital, Menoufia, Egypt from May 2015 to November 2017. The patients were referred to us for the evaluation of different somatic complaints. All patients were Egyptiansfrom rural areas who presented a combination of different somatic complaints. The study was conducted in accordance with the Good Clinical Practice guidelines and Declaration of Helsinki after obtaining approval of the local Ethics Committee and the Institutional Review Board National Liver Institute (IRB number 00003477), Menoufia University. An informed, written consent was obtained from all patients prior to enrollment. Baseline comorbidities such as the presence of diabetes mellitus, hypertension, chronic obstructive pulmonary disease, and history of smoking were recorded. Table 1 shows the frequency of somatic complaints at presentation and 6 weeks after the end of the treatment.
Table 1.
Basic, clinical, laboratory, radiological and microbiological data
| Characteristics. | Cases, number, (%). |
|---|---|
| Sex. | |
| Male. | 32, (53%) |
| Female. | 28, (47%) |
| Age, years, median (range) | 47 (19–63) |
| Rural destinations. | 60 (100%) |
| Chronic diseases. | |
| No | 40 (66%) |
| Diabetes Mellitus | 12 (20%) |
| HTN | 16 (26%) |
| Asthma | 4 (6%) |
| Smoking. | |
| Yes | 24 (40%) |
| No | 36 (60%) |
| Positive examination findings at presentations, at 6th week point | |
| Pallor | 48 (80%), 12 (20%) |
| White coated tongue | 48 (80%), 8 (13%) |
| Right iliac fossa tenderness by deep palpations | 52 (86%), 4 (6%) |
| Imaging finding at presentations, at 6th week point | |
| Splenomegaly | 32 (53%), 16 (26%) |
| 12–15 cm | 0 (0%), 0 (0%) |
| >15 cm | 24 (40%), 24 (40%) |
| Hepatomegaly | 48 (80%), 48 (80%) |
| Thick wall gall bladder | 12 (20%), 12 (20%) |
| Gall bladder stone | |
| Laboratory finding | |
| Leukocytes (×103/μL), mean, (SD) | 6.9 (3.6) |
| Hemoglobin (gm/dl), mean, (SD) | 12.4 (1) |
| Platelets (×103/μL), mean, (SD) | 329.1 (159) |
| ALT (IU/L), mean, (SD) | 46 (19.5) |
| AST (IU/L), mean, (SD) | 42.5 (14.1) |
| Creatinine (mg/dl), mean (SD) | 1.1 (0.2) |
| CRP (mg/L), mean (SD) | 4 (1.3) |
| ESR (ml/hr.), mean (SD) | 16.1 (8.6) |
| Widal test positivity cutoff 1/160 | 13 (86%) |
| Bacteria isolated from stool culture | |
| S. Typhi | 40 (66%) |
| S. para-typhi A | 20 (33%) |
| S. para-typhi B/C | 0 (0%) |
| Time since the diagnosis of typhoid fever | |
| Chronic >1 year. | 20 (33%) |
| Temporarily 3 months-1 year. | 32 (53%) |
| Convalescent 3 weeks-3 months. | 4 (6%) |
Patient data such as age, gender, residence, education, job position, past medical history (including any liver disease), current symptoms (including those related to typhoid carriers), and other risk factors were collected through a written questionnaire adapted from the “Exposure Prevention Information Network” (EPIN; http://www.healthsystem.virginia.edu/internet/epinet).
Exclusion criteria
Key exclusion criteria for the enrolled patients were family history of any psychiatric illness and drug history(patients taking any psychotropic medications, including steroids (except for inhaled steroid), anti-depressant, anxiolytics, or interferon therapy).Also, patients with hypothyroidism, hypopituitarism, Cushing syndrome or hypercortisolism, chronic kidney disease, liver cell failure, heart failure, and respiratory failure, presence of active infections, or malignancies were excluded. Pregnant women were also excluded from our study.
Clinical and investigational workup for the exclusion of organic diseases
A complete history of the patients was recorded. A thorough clinical examination, supplemented by the targeted laboratory, radiological, and endoscopic assessment was performed. The laboratory investigations included complete blood count using Sysmex XT-1800i automated hematology analyzer (Sysmex, Japan), evaluation of liver functions, renal functions, blood sugar, CRP using Cobas e501 Auto analyzer (Roche, Germany), and thyroid functions using Cobas e601 Autoanalyzer (Roche). In addition, thelevels of anti-nuclear antibodies (Algeria automated analyzer), calcium (Ca), and electrolytes were determined using AU480 chemistry analyzer(Beckman Coulter Diagnostics).Erythrocyte sedimentation rate (ESR), serum protein electrophoresis (Minilite fully automated electrophoresis analyzer, Italy), abdominal ultrasound, chest X-ray, echocardiography, resting and stress electrocardiography, computed tomography of abdomen and pelvis, and upper and lower gastrointestinal endoscopy were also carried out. Clinical evaluation was routinely carried out at the relevant specialized clinics at Al-Hussein University Hospital, Cairo, Egypt and the National Liver Institute Hospital, Menoufia, Egypt; the evaluating physicians were not aware of the ongoing study. Only after exclusion of the organic causes, the patients were enrolled. The medical records of the patients were reviewed, the basic laboratory and radiological data were extracted, and the positive clinical findings were recorded. Bacterial isolates from stool cultures were identified using the API 20E system (bioMérieux, Marcy l’Etoile, France).
Case definition for typhoid carrier
Typhoid carrier was defined as follows:
No history of fever in the past 3 weeks and body temperature was documented to be <37.5 °C for >48 h without antipyretics after inpatient admission.
The history of enteric fever, diagnosed by appropriate culturing from blood, urine, stool, or bone marrow, was documented by reviewing the patients’ medical records.
At least one occasion of isolation of S.typhi or Salmonella para-typhi A, B, or C from the stool of patients without fever.
Throughout this study, we did not differentiate between the carriers of S.typhi or Salmonella para-typhi A, B, or C and were referred to as typhoid carriers.
All patients fulfilled the above criteria.
Case identification of depression
The patients were evaluated using the standard clinical interview that was conducted by an expert physician under standard conditions of doctor-patient relationships. The Hamilton Rating Scale for Depression (HAM-D)20 is a well-validated questionnaire designed for assessing depression in adults. It is also used to assess the severity of depression, mood, guilty sensation, anxiety, weight loss, agitation, insomnia, somatic symptoms, and suicidal ideation. The assessment is based on 17 items, and each item is scored on 3 or 5 points and interpreted as follows:
0–7= Normal
8–13= Mild Depression
14–18= Moderate Depression
19–22= Severe Depression
>23= Very severe depression
The score was recorded three times for every patient: on the first assessment, at the end of antibiotics, and 6 weeks after completion of the antibiotics course.
Treatment and follow-up
After enrollment, all patients were treated empirically with intravenous ceftriaxone, 2 g every 24 h, for 14 days. No psychotropic drugs were administered, and only ceftriaxone was given. This protocol was adopted for all patients due to the high prevalence of ciprofloxacin-resistant strains in Egypt (based on our clinical experiences).
Documenting the recovery of carrier states
We documented clearance of the carrier state based on the culture-negative in 3 consecutive stool samples taken 1 month after the completion of the antibiotic course.
Statistical analyses
The data collected were tabulated and analyzed using the Statistical Package for the Social Sciences (SPSS) program, version 23. Numerical data are expressed as mean ± standard deviation, while categorical data are expressed as percentages. The Kolmogorov–Smirnov test was used to assess the normality of numerical data. Differences between two groups were compared using the χ2 test or Fisher’s exact test for categorical variables, and the Student’s t-test was utilized for continuous variables. Two paired t-test was applied to determine the mean differences and the significance of the HAM-D scale at different points of assessment. P-values <0.05 were considered to indicate significant difference, and P-value of <0.01 was considered as a highly significant difference.
Results
Baseline characteristics of the cases
Typhoid carrier status was diagnosed in 60 patients, consisting of 32 males (53%) and 28 females (47%). The median age of the cohort was 47, and the age range was 19–63 years. All patients were Egyptians and referred to us from the rural/village area. Of these, 40 patients (66%) did not complain of any chronic diseases, while 12 (20%) had diabetes mellitus, 16 (26%) had hypertension, and 4 (6%) were suffering from bronchial asthma; the conditions of these patients were well-controlled by therapy. Regarding smoking, 24 patients were smokers (40%), while 60% were nonsmokers.
Clinical examination, laboratory findings, and imaging findings
The mean leukocyte count was 6.9±3.6 (×103/μL), the mean hemoglobin concentration was 12.4±1 gm/dL, and the mean platelet count was 329.1±159 (×103/μL). The mean ALT was 46±19.5 (IU/mL), the mean AST was 42.5±14.1 (IU/mL), the mean serum creatinine was 1.1±0.2, the mean CRP level was 4±1.3 mg/L, and the mean ESR was 16.1±8.6 mL/h. Widal test was positive in 52 patients (86%). A cut-off value of 1/160 for either “O” or “H” antigen was positive Widal test.
Pallor and white-coated tongue (Figure 1) was detected in 48 patients (80%), right iliac fossa tenderness by deep palpation using more than average pressure by overlapping hands was present in 52 (86%), mild splenomegaly (12–15 cm) in 32 (53%), hepatomegaly in 24 (40%), thick wall gallbladder in 48 (80%), and calculary gallbladder in 12 (20%). Repeated assessment at 6th week post-antibiotic completion showed pallor in 12 patients (20%), white-coated tongue (Figure 1) in 8 (13%), right iliac fossa tenderness in 4 (6%), mild splenomegaly in 16 (26%), hepatomegaly in 24 (40%), thick wall gall bladder in 48 (80%), and calculary gall bladder in 12 (20%).
Figure 1.
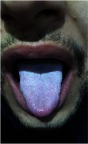
White coated tongue.
Bacterial isolates
This was the most time-consuming factor in the current study as the excretion of Salmonella in typhoid carriers is intermittent, and thus, the possibility of detecting it from one sample is considerably low. This phenomenon limits the number of patients enrolled and prolongs the study time to >2 years. However, in routine practice, we relied on the clinical assessment supplemented by Widal test to initiate the antibiotic treatment, even in stool culture-negative patients. S.a typhi was isolated from 10 patients (66%) and Salmonella para-typhi A from 5 patients (33%). No isolate for para-typhi B/C was found.
Duration of carriage
We did not classify our patients based on the carriage time (see discussion section); however, the current cohort comprised 20 patients (33%) with chronic carriage >1 year, 32 patients (53%) with chronic carriage, 3 months–1 year, and 1 patient (6%)with convalescence, 3 weeks–3 months. Table 1 shows the baseline, clinical, laboratory, radiological, and microbiological data.
Presenting somatic complaints
Table 2 shows the frequency of somatic complaints at presentation and 6th week post-antibiotic completion. The changes in the presenting somatic complaints were statistically significant (P<0.01). Furthermore, fatigue and myalgia were present in 60 (100%) patients, anorexia in 48 (80%), dyspepsia in 44 (73%), changes in bowel habits in 52 (86%), myalgia in 28 (46%), atypical chest pain in 16 (26%), palpitations in 36 (60%), dyspnea in 32 (53%),urination frequency in 24 (40%), erectile dysfunction in 18/32 males (50%), and menstrual disruption in 12/28 females (42%). At the 6th week, these figures and percentages were 4 (6%), 12 (20%), 16 (26%), 28 (46%), 24 (40%), 0 (0%), 0 (0%), 4 (6%), 8 (13%), 4 (6%), 12 (20%), 4 (6%), and un-applicable, respectively. The un-applicability indicates that we re-evaluated the patients after 6 weeks, which was an extremely short time point for assessing menstruation.
Table 2.
Frequency of somatic complaints at presentation and 6 weeks post-treatment
| Symptoms | Before | At 6th week point | P-value |
|---|---|---|---|
| Fatigue | 60 (100%) | 4 (6%) | <0.001* |
| Headache | 60 (100%) | 12 (20%) | <0.001* |
| Anorexia | 48 (80%) | 16 (26%) | <0.001* |
| Change in bowel habits | 52 (86%) | 28 (46%) | <0.001* |
| Dyspepsia | 44 (73%) | 24 (40%) | <0.001* |
| Myalgia | 28 (46%) | 0 (0%) | <0.001* |
| Atypical chest pain | 16 (26%) | 4 (6%) | <0.001* |
| Palpitations | 36 (60%) | 8 (13%) | <0.001* |
| Dyspnea | 32 (53%) | 4 (6%) | <0.001* |
| Urinary frequency/dysuria | 24(40%) | 12 (20%) | <0.001* |
| Erectile dysfunctions | 16 (50%) | 4 (6%) | <0.01* |
| Menstrual disturbances | 12 (20%) | un/applicable |
Notes: P-value <0.01* is considered as statistically highly significant.
HAM-D scale changes with antibiotic treatment
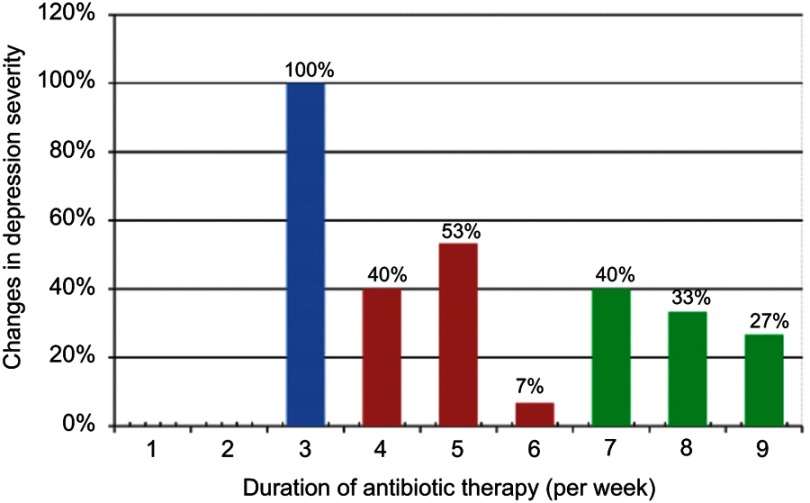
Figure 2 shows the classification of depression based on the HAM-D scale at presentation, 2 weeks, and 6 week time points. At presentation, all patients exhibited severe depression, while at the end of antibiotic treatment, only 4 patients (7%) continued to have severe depression, 32 patients (53%) had moderate depression, and 24 patients (40%) showed mild depression. By the 6th week post-treatment completion, none of the patients had severe depression, 20 patients (33%) showed moderate depression, 24 patients (40%) exhibited mild depression, and 16 patients (27%) were normal. Table 3 and Figure 3 showed the changes in the means of HAM-D scale with treatment which indicated statistically significant differences in the mean of HAM-D scale at the 3 points of evaluations; P<0.01 for each pair.
Figure 2.
Changes in depression severity during study time.
Notes: Blue colour: before treatment, Pink colour: end of treatment, Green colour: 6 weeks after treatment.
Table 3.
Changes in HAM-D scale with treatment
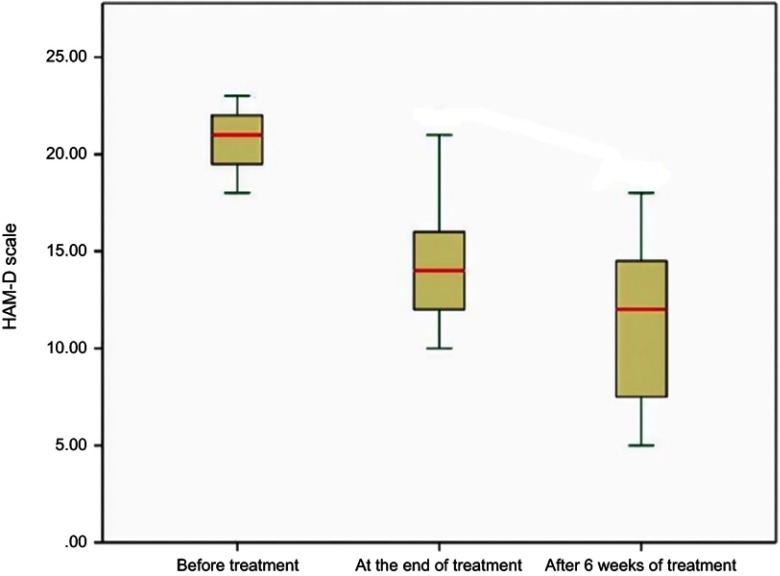
| Minimum | Maximum | Mean | Std. Deviation | Paired Samples t-test | P-value | ||
|---|---|---|---|---|---|---|---|
| t | df | ||||||
| HAM-D before treatment | 18.00 | 23.00 | 20.8000 | 1.61245 | 7.267 | 14 | <0.001* |
| HAM-D at end of treatment | 10.00 | 21.00 | 14.2667 | 3.08143 | 8.119 | 14 | <0.001** |
| HAM-D at 6th week post treatment completion | 5.00 | 18.00 | 11.2000 | 4.00357 | 4.258 | 14 | <0.001*** |
Notes: *For comparing HAM-D before treatment and at the end of treatment, **For comparing HAM-D before treatment and at the end of treatment, ***For comparing HAM-D at the end of treatment and at 6th week post treatment completion.
Figure 3.
Box plots demonstrating changes in HAM-D scale in patients during evaluation points.
Discussion
Typhoid fever is associated with depression as reported previously.15–17
To the best of our knowledge, this is the first report about the association between typhoid carriage and low mood. HAM-D has been proven to be useful in determining a patient’s level of depression before, during, and after treatment. It should be administered by a clinician experienced in working with psychiatric patients with 86.4% sensitivity and 92.2% specificity.21
The current study showed that all cases with typhoid carrier states presented severe depression. Based on the HAM-D scale, statistically significant improvement was observed in their depression scale, solely after antibiotic treatment. The improvement in the depression scale was evident immediately after the completion of antibiotic courses and consolidated at the 6th-week post-antibiotic completion. Also, the improvement in the presenting somatic complaints was statistically significant (Table 2).
These findings were inconsistent with most of the previous reports, which acknowledged that the majority of typhoid carriers in endemic areas are asymptomatic and up to 25% of them do not have any history of typhoid fever.22,23
Previous studies dealing with typhoid carrier have attempted to separate the convalescent, temporary, and chronic carriers. Convalescent carriers excrete the bacteria from the feces from 3 weeks to 3 months post-infection, temporary excretion between 3 and 12 months, and chronic >1 year.23 This separation is mainly for epidemiological interest; however, this distinction might not be of clinical interest in terms of depression as all the categories in the current study presented and responded similarly.
We did not include Widal test in our criteria for enrollment because of its unreliability and non-validity; however, it still represents a critical clinical tool in the diagnosis of acute typhoid fever in developing countries, such as Egypt.24 If this test was included, the number of cases reported in this study would be multiplied many-fold because it is widely used in the diagnosis of typhoid fever in our country. We found many patients with a clinical history of typhoid fever, yet their diagnosis was based only on the positivity of the Widal test without confirmation by bacterial culture. Also, many patients presented a clear history of typhoid fever that was diagnosed by culturing, yet we failed to find Salmonella in stool culture at the time of enrollment, which failed to prove their carrier states. These patients were excluded from reporting; however, we treated them in the same manner, and they responded similarly.
Many typhoid carriers are excretory of S. typhi;25 however, the bacteria isolated in our series was alsoS. typhi in 40 patients (66%) and para-typhi-A in 20 patients (33%). S. para-typhi was also isolated from the gall bladder of Nepali patients.26
Abnormal gallbladder with thick wall in imaging was prevalent in 48 (80%) and calculi gallbladder in 12 (20%) patients. This was consistent with previous reports.27–29
The highly specific characteristic for acute enteric fever was white-coated tongue as demonstrated by Haq et al.30 In the current study, this phenomenon was found in 80% of patients despite being a febrile.
Furthermore, right iliac fossa tenderness by deep palpation using more than average pressure by overlapping hands was found in 52 (86%) patients. The tenderness elicited was not associated with any peritoneal irritation signs. Thus, ileum could be incriminated as a site for the carriage, which results in local inflammation and tenderness. Similarly, mild splenomegaly (12–15 cm) was presented in 53% of the patients, best explained by the local chronic inflammation in the terminal ileum. Right iliac fossa tenderness and mesenteric adenitis or appendicitis are known to be a presenting feature of enteric fever.31–34
Depression is a heterogeneous disorder with multiple psychosocial, immunological, infectious, and biological risk factors. The role of inflammation in depression has been extensively studied over the past decades. Peripheral blood and cerebrospinal fluid of patients with major depressive illness was found to have an increased expression of inflammatory cytokines, their receptors, acute phase reactants, and soluble cell adhesion molecules.10,35,36 Accumulating evidence from a meta-analysis confirmed the association of peripheral blood level of IL-1β, IL-6, TNF, and CRP with depression.10 Polymorphisms in the genes of the inflammatory cytokines, IL-1β, TNF, and CRP was associated with depression and its response to treatment.37 Patients treated with the inflammatory cytokine, IFN-α, developed clinically significant depression.13,14 Similarly, patients with rheumatoid arthritis, psoriasis, and cancer, treated with anti-TNF, showed improvement in their depression symptoms.38–40 Patients responded to anti-TNF with improvement in the severity of depression with treatment-resistant depression along with high levels of inflammatory markers.41 Also, uncontrolled inflammation has been associated with poor response to anti-depressants.42–44
The strong selective pressure by microbial exposure promoted the selection of pro-inflammatory alleles, associated with the initiation of depressive behaviors. The model explained the association between inflammation and depression as the “pathogen host defense theory.”The negative depressive behaviors, the social avoidance, and anhedonia characteristic of depression are not considered as bystander effects, rather a protective effect is exerted through shunting energy sources towards combating infections.42,45
Many infectious agents have been suspected as risk factors for depression, including hepatitis C virus, enterovirus, herpes simplex 1, Epstein-Barr virus, human immunodeficiency virus, varicella-zoster virus, human T-cell lymphotropic virus, Borna disease virus, brucellosis, and Chlamydophila trachomatis.46–52 A recent study from Egypt reported that patients treated for chronic hepatitis C decompensated liver cirrhosis using new direct-acting antiviral drugs, which in turn, showed an improvement in liver tests and health-related quality of life. However, longer duration of follow-up for decompensation events are essential.53
We showed that all 60 patients with documented typhoid carrier states were suffering from low mood associated with somatic complaints, which improved after documenting the clearance of carrier states. Recently, the typhoid vaccine was used in experimental models for the induction of inflammatory states associated with low mood.18,19,54 This evolving association between a single 0.5 mL subcutaneous polysaccharide antigen typhoid vaccine only proposes strong criticisms in the asymptomatic model of Salmonella carriers when the subjects excrete Salmonella intermittently for a prolonged ill-defined period. Herein, we criticize this asymptomatic model.
One can look to our results as a support for the growing theory of approaching depression - “the leaky gut hypothesis.” This hypothesis assumes that the infection of intestinal epithelium by Gram-negative bacteria makes the intestinal epithelium leaky to inflammatory cytokines that translocate to the systemic circulation and are presumed to play a pathogenic role in depression. Supporting this finding, an elevated serum IgM and IgA was observed against lipopolysaccharides of the Gram-negative enterobacteria in depressed patients.55,56
Similarly, the leaky gut was implicated in the pathogenesis and clinical severity of schizophrenia, which might be mediated by inflammatory cytokines.57–59
Thus, we postulated that the intermittent excretion of Salmonellaby typhoid carrier patients result in local intestinal inflammatory reactions that damage the epithelium, allowing the inflammatory cytokines to translocate. Moreover, these inflammatory cytokines result in depression. However, we did not measure the inflammatory cytokines in the current study, and this was a limiting factor in our findings. Thus, we recommend additional studies measuring the inflammatory cytokines in typhoid carriers that correlate with low mood before and after clearance of the carrier states.
Instead of the traditional asymptomatic view for typhoid carrier, we present “the chronic infection model”; the patients are chronically infected, yet an a febrile and chronic infection is reflected as “psycho-somatic depression.”
Nevertheless, the present study has other limitations, such as the small number of patients and the short duration of follow-up. Thus, further studies using a large number of patients and prolonged follow-up durations are imperative.
The current results are inconsistent with the conventional view for typhoid carriers as asymptomatic populations. Although this theory might not alter dramatically based on 60 patients, we encourage physicians in endemic countries to report similar associations.
Finally, we found that diagnosis of Salmonella carriers is difficult due to the intermittent nature of excretions, especially in developing countries where the accessibility for accurate stool cultures is restrained, and the unreliability of Widal test. Therefore, the development of additional tests is encouraged for the detection of Salmonella carriers.
Acknowledgment
Forms of support received by each author for this study included a good selection of cases, instructive supervision, continuous guidance, valuable suggestions and good instructions.
No grant or other financial support was received for this study.
An informed written consent was obtained from all individual participants included in the study. The study was reviewed and approved by the ethical committee at the National Liver Institute. We did not receive any fund.
Data availability statement
All data are available upon request.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Crump JA, Luby SP, Mintz ED. The global burden of typhoid fever. Bull World Health Organ. 2004;82:346–353. [PMC free article] [PubMed] [Google Scholar]
- 2.Lozano R, Naghavi M, Foreman K, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2095–2128. doi: 10.1016/S0140-6736(12)61728-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Levine MM, Black RE, Lanata C. Precise estimation of the numbers of chronic carriers of Salmonella typhi in Santiago, Chile, an endemic area. J Infect Dis. 1982;146:724–726. doi: 10.1093/infdis/146.6.770 [DOI] [PubMed] [Google Scholar]
- 4.Gonzalez-Escobedo G, Marshall JM, Gunn JS. Chronic and acute infection of the gall bladder by Salmonella Typhi: understanding the carrier state. Nat Rev Microbiol. 2011;9:9–14. doi: 10.1038/nrmicro2490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ustun TB, Rehm J, Chatterji S, et al. Multiple-informant ranking of the disabling effects of different health conditions in 14 countries WHO/NIH Joint Project CAR Study Group. Lancet. 1999;354:111–115. doi: 10.1016/S0140-6736(99)00434-1 [DOI] [PubMed] [Google Scholar]
- 6.Murray CJ, Lopez AD. Evidence-based health policy–lessons from the Global Burden of Disease Study. Science. 1996;274:740–743. doi: 10.1126/science.274.5288.740 [DOI] [PubMed] [Google Scholar]
- 7.Dickens C, McGowan L, Clark-Carter D, Creed F. Depression in rheumatoid arthritis: a systematic review of the literature with meta-analysis. Psychosom Med. 2002;64:52–60. doi: 10.1097/00006842-200201000-00008 [DOI] [PubMed] [Google Scholar]
- 8.Felger JC, Lotrich FE. Inflammatory cytokines in depression: neurobiological mechanisms and therapeutic implications. Neuroscience. 2013;246:199–229. doi: 10.1016/j.neuroscience.2013.04.060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pillai V, Kalmbach DA, Ciesla JA. A meta-analysis of electroencephalographic sleep in depression: evidence for genetic biomarkers. Biol Psychiatry. 2011;70:912–919. doi: 10.1016/j.biopsych.2011.07.016 [DOI] [PubMed] [Google Scholar]
- 10.Miller AH, Maletic V, Raison CL. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol Psychiatry. 2009;65:732–741. doi: 10.1016/j.biopsych.2008.11.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dahl J, Ormstad H, Aass HCD, et al. The plasma levels of various cytokines are increased during ongoing depression and are reduced to normal levels after recovery. Psycho Neuroendocrinol. 2014;45:77–86. doi: 10.1016/j.psyneuen.2014.03.019 [DOI] [PubMed] [Google Scholar]
- 12.Pasco JA, Nicholson GC, Williams LJ, et al. Association of high sensitivity C-reactive protein with de novo major depression. Br J Psychiatry. 2010;197:372–377. doi: 10.1192/bjp.bp.109.076430 [DOI] [PubMed] [Google Scholar]
- 13.Taylor MJ, Godlewska B, Near J, et al. Effect of interferon-α on cortical glutamate in patients with hepatitis C: a proton magnetic resonance spectroscopy study. Psychol Med. 2014;44:789–795. doi: 10.1017/S0033291713001621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Udina M, Hidalgo D, Navinés R, et al. Prophylactic antidepressant treatment of interferon-induced depression in chronic hepatitis C: a systematic review and meta-analysis. J Clin Psychiatry. 2014;75:1113–1121. doi: 10.4088/JCP.13r08800 [DOI] [PubMed] [Google Scholar]
- 15.Osuntokun BO, Bademosi O, Ogunremi K, Wright SG. Neuropsychiatric manifestations of typhoid fever in 959 patients. Arch Neurol. 1972;27:7–13. doi: 10.1001/archneur.1972.00490130009002 [DOI] [PubMed] [Google Scholar]
- 16.Venkatesh S, Grell GA. Neuropsychiatric manifestations of typhoid fever. West Indian Med J. 1989;38:137–141. [PubMed] [Google Scholar]
- 17.Muhangi, JR. Psychiatric symptoms in typhoid fever. African Journal of Medical Sciences. 1972;3: 319. doi: 10.1136/bmj.2.5864.436 [DOI] [PubMed] [Google Scholar]
- 18.Harrison NA, Brydon L, Walker C, Gray MA, Steptoe A, Critchley HD. Inflammation causes mood changes through alterations in subgenual cingulate activity and mesolimbic connectivity. Biol Psychiatry. 2009;66:407–414. doi: 10.1016/j.biopsych.2009.03.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wright C, Strike PC, Brydon L, Steptoe A. Acute inflammation and negative mood: mediation by cytokine activation. Brain Behav Immun. 2005;19:345–352. doi: 10.1016/j.bbi.2004.05.002 [DOI] [PubMed] [Google Scholar]
- 20.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Strik JJ, Honig A, Lousberg R, Denollet J. Sensitivity and specificity of observer and self-report questionnaires in major and minor depression following myocardial infarction. Psychosomatics. 2001;42(5):423–428. doi: 10.1176/appi.psy.42.5.423 [DOI] [PubMed] [Google Scholar]
- 22.Mortimer PP. Mr N the milker, and Dr Koch’s concept of the healthy carrier. Lancet. 1999;353:1354–1356. doi: 10.1016/S0140-6736(98)09449-5 [DOI] [PubMed] [Google Scholar]
- 23.Parry CM, Hien TT, Dougan G, White NJ, Farrar JJ. Typhoid fever. N Engl J Med. 2002;347:1770–1782. doi: 10.1056/NEJMra020201 [DOI] [PubMed] [Google Scholar]
- 24.Olopoenia LA, King AL. Widal agglutination test– 100 years later: still plagued by controversy. Postgrad Med J. 2000;76:80–84. doi: 10.1136/pmj.76.892.80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roumagnac P, Weill F-X, Dolecek C, et al. Evolutionary history of Salmonella Typhi. Science. 2006;314:1301–1304. doi: 10.1126/science.1134933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dongol S, Thompson CN, Clare S, et al. The microbiological and clinical characteristics of invasive Salmonella in gallbladders from cholecystectomy patients in Kathmandu, Nepal. PLoS One. 2012;7:e47342. doi: 10.1371/journal.pone.0047342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schiøler H, Christiansen ED, Hoybye G, Rasmussen SN, Greibe J. Biliary calculi in chronic Salmonella carriers and healthy controls: a controlled study. Scand J Infect Dis. 1983;15:17–19. doi: 10.3109/inf.1983.15.issue-1.04 [DOI] [PubMed] [Google Scholar]
- 28.Karaki K, Matsubara Y. Surgical treatment of chronic biliary typhoid and paratyphoid carriers. Nippon Shokakibyo Gakkai Zasshi. 1984;81:2978–2985. in Japanese. [PubMed] [Google Scholar]
- 29.Lai CW, Chan RC, Cheng AF, Sung JY, Leung JW. Common bile duct stones: a cause of chronic salmonellosis. Am J Gastroenterol. 1992;87:1198–1199. [PubMed] [Google Scholar]
- 30.Haq SA, Alam MN, Hossain SM, Ahmed T, Tahir M. Value of clinical features in the diagnosis of enteric fever. Bangladesh Med Res Counc Bull. 1997;23(2):42–46. [PubMed] [Google Scholar]
- 31.Martin HC, Goon HK. Salmonella ileocaecal lymphadenitis masquerading as appendicitis. J Pediatr Surg. 1986;21:377–378. doi: 10.1016/s0022-3468(86)80020-3 [DOI] [PubMed] [Google Scholar]
- 32.Likitnukul S, Wongsawat J, Nunthapisud P. Appendicitis-like syndrome owing to mesenteric adenitis caused by Salmonella typhi. Ann Trop Paediatr. 2002;22:97. doi: 10.1179/027249302125000247 [DOI] [PubMed] [Google Scholar]
- 33.García-Corbeira P, Ramos JM, Aguado JM, Soriano F. Six cases in which mesenteric lymphadenitis due to non-typhi Salmonella caused an appendicitis-like syndrome. Clin Infect Dis. 1995;21:231–232. doi: 10.1093/clinids/21.1.231 [DOI] [PubMed] [Google Scholar]
- 34.Meng GR. Acute mesenteric lymphadenitis due to Salmonella enteritidis mimicking appendicitis: case report. Mil Med. 1974;139:277. doi: 10.1093/milmed/139.4.277 [DOI] [PubMed] [Google Scholar]
- 35.Andrew H, Raison CL. The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nat Rev Immunol. 2016;16(1):22–34. doi: 10.1038/nri.2015.5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yirmiya R, Pollak Y, Morag M, et al. Illness, cytokines, and depression. Ann NY Acad Sci. 2000;917:478–487. doi: 10.1111/j.1749-6632.2000.tb05412.x [DOI] [PubMed] [Google Scholar]
- 37.Bufalino C, Hepgul N, Aguglia E, Pariante CM. The role of immune genes in the association between depression and inflammation: a review of recent clinical studies. Brain Behav Immun. 2012;31:31–47. doi: 10.1016/j.bbi.2012.04.009 [DOI] [PubMed] [Google Scholar]
- 38.Tyring S, Gottlieb A, Papp K, et al. Etanercept and clinical outcomes, fatigue, and depression in psoriasis: double-blind placebo-controlled randomised phase III trial. Lancet. 2006;367:29–35. doi: 10.1016/S0140-6736(05)67763-X [DOI] [PubMed] [Google Scholar]
- 39.Abbott R, Whear R, Nikolaou V, et al. Tumour necrosis factor-α inhibitor therapy in chronic physical illness: a systematic review and meta-analysis of the effect on depression and anxiety. J Psychosom Res. 2015;79:175–184. doi: 10.1016/j.jpsychores.2015.04.008 [DOI] [PubMed] [Google Scholar]
- 40.Köhler O, Benros ME, Nordentoft M, et al. Effect of anti-inflammatory treatment on depression, depressive symptoms, and adverse effects: a systematic review and meta-analysis of randomized clinical trials. JAMA Psychiatry. 2014;71:1381–1391. doi: 10.1001/jamapsychiatry.2014.1611 [DOI] [PubMed] [Google Scholar]
- 41.Raison CL, Rutherford RE, Woolwine BJ, et al. A randomized controlled trial of the tumor necrosis factor antagonist infliximab for treatment-resistant depression: the role of baseline inflammatory biomarkers. JAMA Psychiatry. 2013;70:31–41. doi: 10.1001/2013.jamapsychiatry.4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Slavich GM, Irwin MR. From stress to inflammation and major depressive disorder: a social signal transduction theory of depression. Psychol Bull. 2014;140:774–815. doi: 10.1037/a0035302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Eurelings LS, Richard E, Eikelenboom P, van Gool WA, van Charante EP. Low-grade inflammation differentiates between symptoms of apathy and depression in community-dwelling older individuals. Int Psychogeriatr. 2015;27:639–647. doi: 10.1017/S1041610215001581 [DOI] [PubMed] [Google Scholar]
- 44.Cattaneo A, Gennarelli M, Uher R, et al. Candidate genes expression profile associated with antidepressants response in the GENDEP study: differentiating between baseline ‘predictors’ and longitudinal ‘targets’. Neuropsychopharmacology. 2013;38:377–385. doi: 10.1038/npp.2012.191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Raison CL, Miller AH. The evolutionary significance of depression in Pathogen Host Defense (PATHOS-D). Mol Psychiatry. 2013;18:15–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liao Y-T, Hsieh MH, Yang YH, et al. Association between depression and enterovirus infection. Medicine. 2017;96:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Elbaz T, Abdo M, Omar H, et al. Efficacy and safety of sofosbuvir and daclatasvir with or without ribavirin in eldery patients with chronic hepatitis C virus infection. J Med Virol . 2019. Feb;91(2):272–277. doi: 10.1002/jmv.25287 Epub 2018 Nov 8 [DOI] [PubMed] [Google Scholar]
- 48.Lucaciu LA, Dumitrascu DL. Depression and suicide ideation in chronic hepatitis C patients untreated and treated with interferon: prevalence, prevention, and treatment. Ann Gastroenterol. 2015;28:440–447. [PMC free article] [PubMed] [Google Scholar]
- 49.Chen MH, Wei HT, Su TP, et al. Risk of depressive disorder among patients with herpes zoster: a nationwide population-based prospective study. Psychosom Med. 2014;76:285–291. doi: 10.1097/PSY.0000000000000051 [DOI] [PubMed] [Google Scholar]
- 50.Stumpf BP, Carneiro-Proietti AB, Proietti FA, Rocha FL; INTERDISCIPLINARY HTLV RESEARCH GROUP (GIPH). Higher rate of major depression among blood donor candidates infected with human t-cell lymphotropic virus type 1. Int J Psychiatry Med. 2008;38:345–355. doi: 10.2190/PM.38.3.i [DOI] [PubMed] [Google Scholar]
- 51.Wang X, Zhang L, Lei Y, et al. Meta-analysis of infectious agents and depression. Sci Rep. 2014;4:4530. doi: 10.1038/srep04530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mandel GL, Bennett J Dolin R. Principles and Practice of Infectious Diseases. 7th ed. Philadelphia (PA): Churchil Livingtone, Elsivier; 2010:2921–2924. [Google Scholar]
- 53.Essa M, Sabry A, Abdelsameea E, Tharwa ES, Salama M. Impact of new direct-acting antiviral drugs on hepatitis C virus-related decompensated liver cirrhosis. Eur J Gastroenterol Hepatol. 2019;31(1):53–58. doi: 10.1097/MEG.0000000000001250 [DOI] [PubMed] [Google Scholar]
- 54.Brydon L, Harrison NA, Walker C, Steptoe A, Critchley HD. Peripheral inflammation is associated with altered substantia nigra activity and psychomotor slowing in humans. Biol Psychiatry. 2008;63:1022–1029. doi: 10.1016/j.biopsych.2007.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Maes M, Kubera M, Leunis J-C, Berk M. Increased IgA and IgM responses against gut commensals in chronic depression: further evidence for increased bacterial translocation or leaky gut. J Affect Disord. 2012;141:55–62. doi: 10.1016/j.jad.2012.02.023 [DOI] [PubMed] [Google Scholar]
- 56.Maes M, Kubera M, Leunis JC. The gut-brain barrier in major depression: intestinal mucosal dysfunction with an increased translocation of LPS from gram negative enterobacteria (leaky gut) plays a role in the inflammatory pathophysiology of depression. Neuro Endocrinol Letters. 2008;29:117–124. [PubMed] [Google Scholar]
- 57.Fan X, Goff DC, Henderson DC. Inflammation and schizophrenia. Expert Rev Neurother. 2007;7:789–796. doi: 10.1586/14737175.7.7.789 [DOI] [PubMed] [Google Scholar]
- 58.Fan X, Liu EY, Freudenreich O, et al. Higher white blood cell counts are associated with an increased risk for metabolic syndrome and more severe psychopathology in non-diabetic patients with schizophrenia. Schizophr Res. 2010;118:211–217. doi: 10.1016/j.schres.2010.02.1028 [DOI] [PubMed] [Google Scholar]
- 59.Hope S, Ueland T, Steen NE, et al. Interleukin 1 receptor antagonist and soluble tumor necrosis factor receptor 1 are associated with general severity and psychotic symptoms in schizophrenia and bipolar disorder. Schizophr Res. 2013;145:36–42. doi: 10.1016/j.schres.2012.12.023 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data are available upon request.