Abstract
Purpose
The incidence rate of thyroid cancer, the most common endocrine malignancy, has increased rapidly over the past 10 years. However, the fundamental molecular mechanisms underlying the malignant progression of thyroid cancer are unclear.
Materials and methods
Firstly, quantitative real-time PCR analysis and Western blot analysis were used to investigate the expression of Cbp/p300 interacting transactivator with Glu/Asp rich carboxy-terminal domain 1 (CITED1) in papillary thyroid carcinoma (PTC) cell lines. Then, we investigated the effects of CITED1 knockdown on cell proliferation, apoptosis, and invasion in in vitro and in vivo models of PTC.
Results
CITED1 was upregulated in PTC cell lines, and CITED1 knockdown significantly suppressed the proliferation, migration, and invasion of K1 cells resulting in a G0/G1 phase block. Furthermore, the silencing of CITED1 significantly promoted cell apoptosis. In the in vivo study, the growth speed and weight of the transplanted tumor were significantly suppressed in nude mice infected with short hairpin RNA targeting CITED1 (CITE1-shRNA) cells. Furthermore, we found that CITED1-shRNA activated Wnt/β-catenin signaling in PTC.
Conclusion
Taken together, our findings suggest that CITED1 knockdown facilitates apoptosis and inhibits proliferation and invasion in K1 cells via the Wnt/β-catenin signaling pathway.
Keywords: papillary thyroid carcinoma, CITED1, proliferation, invasion, Wnt/β-catenin signaling
Introduction
Thyroid cancer is the most common endocrine malignancy. It is estimated that by the end of 2018 there will be 53,990 new cases of thyroid cancer and that an estimated 2,060 people will die of this disease in the United States alone. Moreover, the incidence of thyroid cancer in women has become the fifth highest incidence of female tumors.1 Papillary thyroid carcinoma (PTC) and follicular thyroid carcinoma (FTC) are the most common subtypes of thyroid cancer accounting for 90–95% of all cases. PTC and FTC are differentiated thyroid cancers, which have a good prognosis with appropriate surgery and radioactive iodine therapy.2,3 However, 2–5% of these tumors will lose their differentiated status and become iodine-refractory and associated with a high mortality rate. In recent years, rapid advances in molecular biology and genetic engineering have established gene therapy as a viable treatment strategy in addition to the standard therapeutic approaches.4–7
Recent studies in our laboratory have characterized the significant overexpression of the transcriptional regulator CITED1 in PTC.8 CITED1 was originally identified in mouse melanoma cell lines.9 During vertebrate development, CITED1 is expressed in progenitors of the heart, limb, axial skeleton, kidney, and placenta. Moreover, CITED1 may function as a key coordinator during renal epithelial morphogenesis and is involved in mammary gland development.10 Several studies have shown that CITED1 is associated with the development and progression of PTC;11–14 however, the exact mediating mechanisms remain unclear.
We used a lentiviral vector to block the expression of CITED1 in K1 cells and constructed a PTC xenograft model to investigate the effects of CITED1 and determine whether the transcriptional regulator acts via the Wnt/β-catenin pathway.
Materials and methods
Cell culture
Normal human thyroid cells (Nthy-ori 3-1) and human thyroid papillary cancer cell lines (K1, BCPAP, and TPC-1) were purchased from the Cell Bank at Shanghai Institute of Cell Biology, Chinese Academy of Science (Shanghai, China). All cell lines were cultured in Dulbecco’s modified Eagle’s medium (DMEM; Gibco, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (FBS; HyClone, Los Angeles, CA, USA) and maintained a humidified atmosphere of 5% CO2 at 37°C.
Lentivirus and transfection
Short hairpin RNA (shRNA) targeting the human CITED1 gene and non-targeting shRNA were synthesized by Shanghai R&S Biotechnology Co., Ltd (Shanghai, China). The following RNA interference sequence was transfected into K1 cells to block the expression of CITED1: 5′-TGCTGTATTGGAGATCCCGAGGAACTGTTTTGGCCACTGACTGACAGTTCCTCGATCTCCAATA-3′. K1 cells were seeded in 6-well plates at a concentration of 5×104 cells/well. After K1 cells seeded in 6-well plates were grown to 30% confluence, they were infected with shRNA at multiplicity of infection (MOI) of 50. 5 µL titers of 1×108 TU/mL shRNA were added to each well. Green fluorescent protein (GFP) expression was observed using a fluorescence microscope (Eclipse Ti-S, Nikon, Japan). 72 h after transfection, and the infection efficiency was estimated according to the percentage of green fluorescent chromogenic cells. Followup experiments were conducted when the infection efficiency was above 80%. After that, the transfected cells were evaluated by fluorescence quantitative reverse transcriptase-polymerase chain reaction (qRT-PCR) and Western blotting analyses.
RNA extraction and qRT-PCR
Total RNA was extracted from the xenograft tumors or cells using TRIzol reagent (TaKaRa, Tokyo, Japan) following the manufacturer’s protocol, reverse transcribed into complementary DNA using a PrimeScript RT-PCR kit (TaKaRa Bio Inc., Tokyo, Japan) at 37°C for 25 min, and then incubated at 85°C for 5 s in 20 µL reaction volume. Real-time PCR was performed using 10 µL SYBR ®Premix Ex Taq™ II (TaKaRa Bio, Inc.) on a C-1000TM Thermal Cycler (Bio-Rad, Hercules, CA, USA). Actin was used as an internal control for CITED1. Expression fold changes were calculated using 2-ΔΔCt methods. The expression levels were relative to the fold change in the corresponding controls, which were defined as 1.0. The primers used in this study were as follows: Actin forward: 5′-CCACGAAACTACCTTCAACTCC-3′, and reverse: 5′-GTGATCTCCTTCTGCATCCTGT-3′; CITED1 forward: 5′-TCTGCCAAGGCTCTGAAATGAAATGC-3′, and reverse: 5′-AGACGGTTCCGAGACTTTACG-3′.
Western blotting analyses
The cell and xenograft tumors were lysed and liquid supernatant was collected. The protein concentration was determined using a bicinchoninic acid assay (Beyotime, Haimen, China). The sample proteins were electrophoresed on 12% and 8% sodium dodecyl sulfate-polyacrylamide gel (Beyotime) and transferred to a polyvinylidene fluoride membrane (Beyotime) for 60 min at 100 V. Next, the membranes were blocked with 5% skim milk at room temperature and then incubated and shaken overnight at 4°C with mouse anti-CITED1 (1:250, Abcam, Boston, MA, USA), anti-actin (1:5000, Proteintech, Wuhan, China), and rabbit anti-beta-catenin (1:5000, Abcam), anti-c-myc (1:1000, Abcam), anti-cyclinD1 (1:1000, Abcam). After washing, the membranes were treated with goat anti-mouse antibody (1:10000, MultiSciences, Hangzhou, China) and goat anti-rabbit antibody (1:4000, Proteintech) at room temperature for 1 h. Membranes were visualized by chemiluminescence (Thermo Fisher Scientific, New York, NY, USA) using the ECL-advance Western blotting Detection System (ChemiDocXRS+, Bio-Rad). Actin was used as the endogenous control.
Cell proliferation assay
Cells were seeded in 96-well plates at a concentration of 1×103 cells/well with four replicate wells and cultured for 24, 48, 72, 96, or 120 h. At each time interval, 10 µL cell proliferation assay reagent (Cell Counting Kit-8 [CCK-8]; Beijing Biodragon Immunotechnologies Co., Ltd, Beijing, China) was added to each well, taking care to avoid the formation of air bubbles to ensure accurate optical density readings, and incubated at 37°C for another 1 h. Then experimental cells were placed on a Perkin Elmer Enspire 2300 multifunction microplate reader (Perkin-Elmer, Waltham, MA, USA) to measure the optical density at 450 nm.
Cell cycle and cell apoptosis analyses
The cell cycle and cell apoptosis were assessed by flow cytometry (FACSVantage SE, Becton Dickinson, Mountain View, CA, USA). The harvested cells were fixed in 75% ethanol overnight at 4°C. After rinsing with phosphate-buffered saline (PBS), the cells were incubated with RNase at 37°C for 30 min. Then the cells were stained with propidium iodide (PI, Beyotime) for 30 min. Annexin V-FITC/PI double‑staining (Beyotime) was used to detect cell apoptosis. The cells were washed with ice-cold PBS and resuspended in the binding buffer at a density of 2×106 cells/mL. Then the cells were stained with 5 μL annexin V-FITC and 10 μL PI away from light for 15 min at room temperature. The apoptotic rate was calculated as the early apoptotic rate plus the late apoptotic rate.
Scratch wound healing assay
A monolayer wound-healing assay was used to assess cell migration ability. When cells grew to 100% confluence in the 6-well plate, straight scratches were made using a sterile 10 µL pipette tip. The remaining cells were washed three times to remove any cell debris and incubated at 37°C in serum-free DMEM. At 0, 12, and 24 h, three different cleared zones per well of migrating cells at the wound front were photographed and compared. The cell migration distance was determined by measuring the width of the wound.
Transwell migration and invasion experiments
We used 24-well Transwell plates with or without 8.0 µm pore Matrigel-coated membranes (Corning, NY, USA) for the migration and invasion experiments. Then the cells (1×106 cells) were suspended in 100 µL serum-free DMEM and added to the upper chamber, and 700 µL 20% FBS medium was placed in the lower chamber to serve as a chemoattractant stimulus. After incubation at 37°C for 24 h, the cells on the upper surface of the filters were removed using a cotton swab. The chamber was maintained at room temperature for 30 min and then immersed in 0.5% crystal violet containing 1% methanol for another 30 min. The crystal violet was washed with PBS three times. Cells on the lower chamber were counted under a microscope in four randomly selected fields.
Animal experiments
The animal experiment was approved by the Ethics Committee of the First Affiliated Hospital of Chongqing Medical University. Fifteen female BALB/C nude mice (aged 4–6 weeks) were purchased from Beijing Huafukang Bioscience Co., Inc. (Beijing, China) and raised in a specific-pathogen-free animal facility with constant humidity and controlled temperature. Nude mice were randomly divided into three groups and then CITED1–shRNA cells, NC–shRNA cells, or untreated cells were injected subcutaneously at a dose of 2×107 cells per animal for each group. Tumor volume was measured every 3 days using a caliper. After 27 days, the nude mice were sacrificed by cervical dislocation, and the xenograft tumors were harvested. Tumor volume was calculated as volume (mm3) = width2(mm2)*length (mm)/2.
Statistical analyses
The statistical tests were performed using the Statistical Package for the Social Sciences (SPSS) statistical software version 22 (IBM SPSS, Chicago, IL, USA). All experimental data are expressed as means ± standard deviations (SDs). The comparisons among groups were made using one-way analysis of variance, and least significant difference t-tests were used for multiple comparisons (a=0.05 as the level of significance). P-values <0.05 were considered to indicate statistically significant differences.
Ethical approval
All animals were maintained and used in accordance with the guidelines of the Institutional Animal Care and Use Committee of the Chongqing Medical University. All of the experimental procedures were approved by the Chongqing Medical University ethics commission.
Results
CITED1 overexpression in PTC cell lines and construction of stable knockout K1 cells
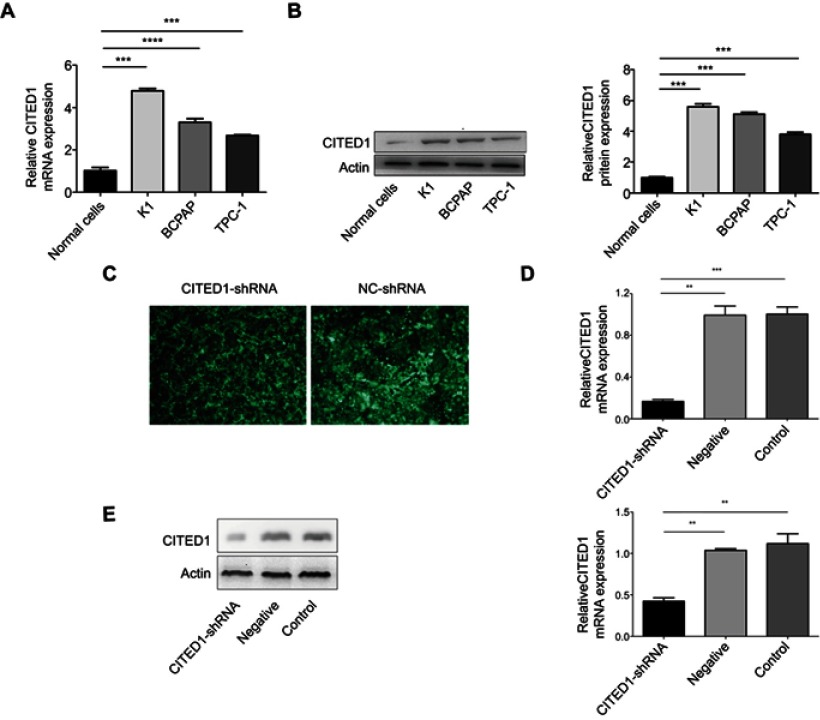
Quantitative real-time PCR and Western blotting analyses of CITED1 expression in human PTC cells and normal human thyroid cells revealed that CITED1 was overexpressed in PTC cells compared to normal thyroid cells (Figure 1A, B). Furthermore, CITED1 expression in the K1 cells was relatively high in the three PTC cell lines. Therefore, K1 cells were subjected to stable transfection with CITED1-shRNA. The expression of fluorescent protein observed by fluorescence microscopy was considered an indicator of transfection efficiency in K1 cells, which was more than 90% 3 days after transfection (Figure 1C). After CITED1-shRNA transfection, the expression of CITED1 in K1 cells was measured using qRT-PCR and Western blotting analyses, which revealed that CITED1 mRNA and protein expression decreased significantly in the CITED1-shRNA group compared to the Negative group (Figure 1D, E). Therefore, the corresponding stable transfectants of CITED1-shRNA were selected for further study.
Figure 1.
CITED1 was overexpressed in human PTC cells and showed stable silencing in K1 cells. CITED1 mRNA and protein levels in Nthy-ori 3–1, K1, BCPAP, and TPC-1 cells detected by (A) qRT-PCR and (B) Western blotting analyses. (C) The expression of green fluorescent protein in K1 cells after infection with CITED1-shRNA and NC-shRNA at 72 h observed by fluorescence microscopy (x40). CITED1 mRNA and protein expression in K1 cells after transfection with CITED1-shRNA and NC-shRNA detected by (D) real-time PCR and (E) Western blotting analyses, respectively. **P<0.01,***P<0.001 compared to the Negative and Control groups.
CITED1 contributes to progression of the malignant phenotype of PTC in vitro
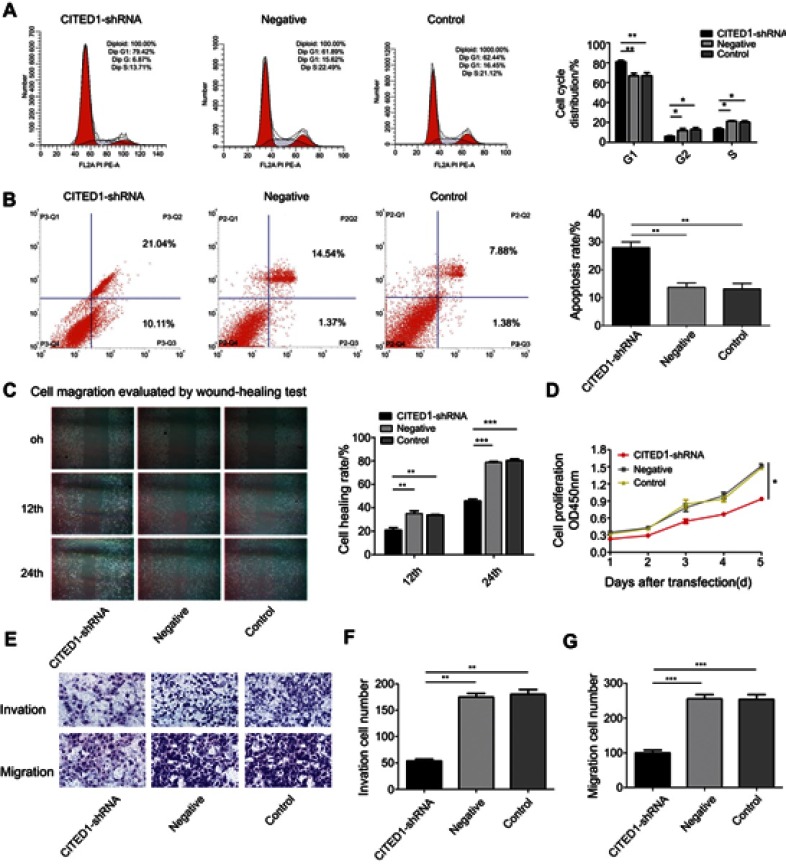
To investigate the role of CITED1 in PTC, we examined the effects of CITED1 knockdown on proliferation, apoptosis, migration, and invasion in the K1 cell line. The CCK-8 assay revealed that CITED1 downregulation significantly inhibited cell proliferation (Figure 2D). Moreover, flow cytometric analyses revealed that CITED1-shRNA cells were arrested in the G0/G1 phase and the apoptosis rate was markedly higher than those of the Negative and Control groups (Figure 2A and B). The transwell migration (Figure 2E, G) and invasion (Figure 2E, F) and wound healing (Figure 2C) assays revealed a significant decrease in the number of transmigrated CITED1-shRNA cells compared to the Negative and Control groups. Collectively, these findings suggest that CITED1 plays an important role in the malignant phenotype of K1 cells in vitro.
Figure 2.
CITED1 contributes to the progression of the malignant phenotype of PTC in vitro. The effects of CITED1 knockdown on (A) K1 cell cycle progression (flow cytometry). (B) K1 cell apoptosis (flow cytometry). (C) K1 cell migration (cell scratch wound healing assay, ×40). (D) K1 cell proliferation (CCK-8 assay). (E–G) K1 cell migration and invasion (transwell assay). *P<0.05, **P<0.01, ***P<0.001 compared to the negative and control groups.
CITED1 contributes to progression of the malignant phenotype of PTC in vivo
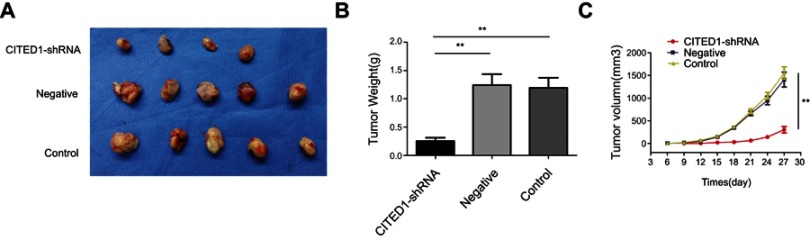
Subsequently, to further explore the effect of CITED1 on tumor growth in vivo, xenograft tumor models were established by injecting with K1 cells infected with CITED1-shRNA into nude mice. After 6 days, nude mice in the Control and Negative groups developed visible tumors, but only four mice in the CITED1-shRNA group developed visible tumors. The subcutaneous tumors were measured every 3 days. Downregulation of CITED1 had significant effect on tumor size (Figure 3A) and growth curve (Figure 3C). Compared with the Control and Negative groups, the CITED1-shRNA group had significantly smaller tumor volumes at day 27 (Figure 3C). Subsequently, tumors were removed and weighed, the tumor weighed significantly less in the CITED1-shRNA group (Figure 3B). Therefore, consist with our in vitro study, overexpressed CITED1 contributes to PTC tumorigenesis in vivo.
Figure 3.
CITED1 plays a suppressive role in tumor growth in a subcutaneous xenograft model (A) Xenograft tumors were harvested on day 27. (B) The tumors weighed significantly less in the CITED1-shRNA group than in the control and negative groups. (C) Graph showing the growth curve of the transplanted tumor in nude mice. **P<0.01 compared to the negative and control groups.
CITED1 suppresses activation of the Wnt/β‑catenin signaling pathway in vitro and in vivo
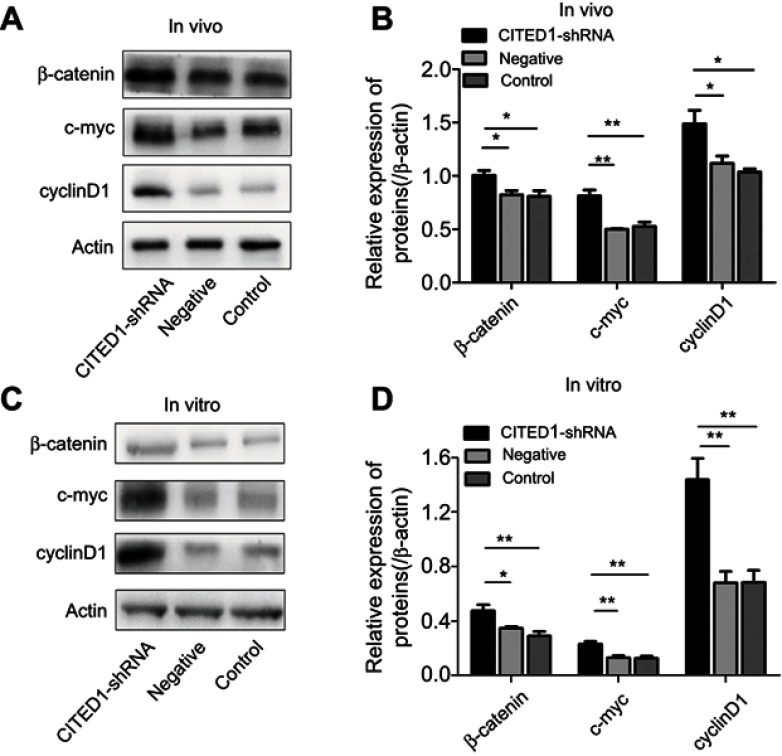
Previous studies have shown that CITED1 is closely associated with the Wnt/β-catenin pathway in various tumors.10,15,16 Therefore, we hypothesized that the action shown by CITED1 in PTCs may be partially mediated via Wnt/β-catenin signaling. We found that interference with CITED1 increased the expression of β-catenin, c-myc, and cyclinD1 in K1 cells and the xenograft tumors in the CITED1-shRNA group compared to the Control and Negative groups in vivo (Figure 4A, B) and in vitro (Figure 4C, D). These findings suggest that CITED1 suppresses activation of the Wnt/β‑catenin signaling pathway.
Figure 4.
The overexpression of CITED1 activates the Wnt/β-catenin signaling pathway in vitro and in vivo. (A, B) Western blotting analyses of β-catenin, c-myc, and cyclinD1 expression in the xenograft tumor tissue. (C, D) β-catenin, c-myc, and cyclinD1 expression in K1 cells on day 3 after CITED1-shRNA infection. *P<0.05, **P<0.01 compared to the negative and control groups.
Discussion
At present, the treatment options for patients with iodine-refractory thyroid cancer are limited. Therefore, the development of effective tumor markers and gene-targeting therapies signal important advances in research and clinical applications for thyroid cancer.7 With the development of molecular biology of thyroid cancer, many molecular targeted therapies have shown promising prospects. Molecular targeted drugs can inhibit malignant biological behavior of tumor cells at the molecular level by intervening or blocking specific gene or molecular changes of tumor cells. At the time of this writing, the FDA has approved four different drugs targeting the Mitogen-Activated Protein Kinase (MAPK) signaling pathway in the treatment of advanced thyroid cancers.17,18 These include Lenvatinib and Sorafenib for advanced, recurrent, and radioiodine-refractory differentiated thyroid cancer (RR-DTC); and Cabozantinib and Vandetanib for MTC. Significant improvement in progression-free survival (PFS) was achieved in patients with progressive RR-DTC using sorafenib or Lenvatinib, which were compared with placebo. Lenvatinib, an oral multikinase inhibitor, is the most recent drug approved by the FDA for the treatment of RR-DTC.19,20
CITED1 is the first member of the CITED family of cofactors to be found to be involved in regulating a wide variety of CBP/p300-dependent transcriptional responses.21 CITED1 interacts with beta-catenin at the protein level and thereby negatively regulates beta-catenin transcription.22 Furthermore, it is an oncogene in various cancers. Its overexpression has been observed in a variety of cancers including hepatoblastoma, Wilms’ tumor, thyroid cancer, melanoma, and intestinal cancer; moreover, high CITED1 levels are strongly correlated with a poor prognosis.10,15,16,23–25 Furthermore, its overexpression in cancer cells significantly inhibits the Wnt/β-catenin pathway.10,15,16 In previous studies, we found that CITED1 was overexpressed in PTC, and we recently showed that CITED1 upregulation is associated with lymph node metastasis and clinical stage;26 however, the underlying molecular mechanism remains unclear. To further clarify the role of CITED1 in PTC, we constructed a shRNA lentiviral vector to knock down the expression of CITED1 in K1 cells.
We investigated the role of CITED1 in PTC K1 cell lines. We hypothesized that abnormal expression of CITED1 was closely correlated with the pathological processes of PTC including proliferation, migration, invasion, and apoptosis. We found that the proliferation, migration, and invasion potential of K1 cells was significantly inhibited after silencing CITED1, whereas the percentage of apoptotic cells significantly increased. Furthermore, the cell cycle study revealed a G0/G1 phase block in K1 knockdown cells. The xenograft tumors from the CITED1 knockdown cells were consistently smaller and weighed less than those of the Control and Negative groups. Indeed, our in vitro and in vivo functional studies have shown that the knockdown of CITED1 significantly impaired cell growth and decreased the tumorigenic potential of K1 cells, suggesting that CITED1 plays an essential oncogenic role in the development of PTC.
CITED1 and the Wnt/β-catenin pathway are closely linked in the pathogenesis of cancer.10,15,16 For example, previous studies have implicated CITED1 as a modulator of Wnt signaling in hepatoblastoma and possibly liver development.15 Importantly, CITED1 modulation of Wnt signaling may reveal targets to induce wild-type cancer stem cell differentiation and to repress β-catenin-driven oncogenicity.16 The Wnt signaling pathway has been implicated in maintaining the critical, coordinated balance between differentiation and proliferation in multiple developmental contexts.27–31 Specifically, coordinated control of Wnt signaling has been linked to the transcriptional coactivators, CREB-binding protein (CBP) and P300, the principal proteins with which CITED1 interacts.32,33 Therefore, CITED1 may participate as a coregulator in the modulation and balance of CBP/β-catenin–mediated transcription, which is critical for stem/progenitor cell maintenance and P300/β-catenin–mediated transcription, which in turn is important for the initiation of cellular differentiation. Because CITED1 is a potential repressor of the Wnt pathway, we investigated the effects of CITED1 knockdown on Wnt pathway genes in in vitro and in vivo studies. We showed that CITED1 knockdown increased the expression of β-catenin, c-myc, and cyclinD1 in K1 cells and xenograft PTC tumors, indicating that CITED1 plays a tumorigenic role by suppressing the Wnt/β-catenin pathway. This hyperactivation of Wnt signaling appears to be mediated, at least in part, by increased levels of dephosphorylated β-catenin. Therefore, we showed that CITED1 knockdown increased the pool of active β-catenin, which is consistent with the enhanced Wnt pathway activation we observed.
In summary, our findings suggest that CITED1 may be a useful diagnostic tool and prognostic marker for PTC. As a novel oncogene, CITED1 plays an important role in the development and progression of PTC by promoting malignant cell proliferation via activation of the Wnt/β-catenin signaling pathway. Although we were unable to elucidate the CITED1 mechanism of action in PTC, our findings provide a useful theoretical basis and experimental data for further investigation of the precise function and molecular mechanisms underlying CITED1 involvement in PTC.
Acknowledgment
This work was supported by the Graduate Innovation Research Project of Chongqing (grant number CYS17152).
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7–30. doi: 10.3322/caac.21442 [DOI] [PubMed] [Google Scholar]
- 2.Ibrahim EY, Busaidy NL. Treatment and surveillance of advanced, metastatic iodine-resistant differentiated thyroid cancer. Curr Opin Oncol. 2017;29(2):151–158. doi: 10.1097/CCO.0000000000000349 [DOI] [PubMed] [Google Scholar]
- 3.Faugeras L, Pirson AS, Donckier J, et al. Refractory thyroid carcinoma: which systemic treatment to use? Ther Adv Med Oncol. 2018;10:1758834017752853. doi: 10.1177/1758834017752853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jin Y, Van Nostrand D, Cheng L, Liu M, Chen L. Radioiodine refractory differentiated thyroid cancer. Crit Rev Oncol Hematol. 2018;125:111–120. doi: 10.1016/j.critrevonc.2018.03.012 [DOI] [PubMed] [Google Scholar]
- 5.Cabanillas M, Terris DJ, Sabra M. Information for clinicians: approach to the patient with progressive radioactive iodine refractory thyroid cancer- when to use systemic therapy. Thyroid. 2017. doi: 10.1089/thy.2016.0578 [DOI] [PubMed] [Google Scholar]
- 6.Jaber T, Waguespack SG, Cabanillas ME, et al. Targeted therapy in advanced thyroid cancer to resensitize tumors to radioactive iodine. J Clin Endocrinol Metab. 2018. doi: 10.1210/jc.2018-00612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Naoum GE, Morkos M, Kim B, Arafat W. Novel targeted therapies and immunotherapy for advanced thyroid cancers. Mol Cancer. 2018;17(1):51. doi: 10.1186/s12943-018-0786-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li WB, Zhou J, Xu L, Su XL, Liu Q, Pang H. Identification of genes associated with Papillary Thyroid Carcinoma (PTC) for diagnosis by integrated analysis. Horm Metab Res. 2016;48(4):226–231. doi: 10.1055/s-0035-1569289 [DOI] [PubMed] [Google Scholar]
- 9.Howlin J, Cirenajwis H, Lettiero B, et al. Loss of CITED1, an MITF regulator, drives a phenotype switch in vitro and can predict clinical outcome in primary melanoma tumours. PeerJ. 2015;3:e788. doi: 10.7717/peerj.788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Meniel V, Song F, Phesse T, et al. Cited1 deficiency suppresses intestinal tumorigenesis. PLoS Genet. 2013;9(8):e1003638. doi: 10.1371/journal.pgen.1003638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sassa M, Hayashi Y, Watanabe R, et al. Aberrant promoter methylation in overexpression of CITED1 in papillary thyroid cancer. Thyroid. 2011;21(5):511–517. doi: 10.1089/thy.2010.0295 [DOI] [PubMed] [Google Scholar]
- 12.Schulten HJ, Hussein D, Al-Adwani F, et al. Microarray expression profiling identifies genes, including cytokines, and biofunctions, as diapedesis, associated with a brain metastasis from a papillary thyroid carcinoma. American Journal of Cancer Research. 2016;6(10):2140–2161. [PMC free article] [PubMed] [Google Scholar]
- 13.Huang Y, Prasad M, Lemon WJ, et al. Gene expression in papillary thyroid carcinoma reveals highly consistent profiles. Proc Natl Acad Sci U S A. 2001;98(26):15044–15049. doi: 10.1073/pnas.251547398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scognamiglio T, Hyjek E, Kao J, Chen Y-T. Diagnostic usefulness of HBME1, Galectin-3, CK19, and CITED1 and evaluation of their expression in encapsulated lesions with questionable features of papillary thyroid carcinoma. American Journal of Clinical Pathology. 2006;126(5):700–708. doi: 10.1309/044V-86JN-2W3C-N5YB [DOI] [PubMed] [Google Scholar]
- 15.Murphy AJ, de Caestecker C, Pierce J, et al. CITED1 expression in liver development and hepatoblastoma. Neoplasia. 2012;14(12):1153–IN1123. doi: 10.1593/neo.12958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Murphy AJ, Pierce J, de Caestecker C, et al. CITED1 confers stemness to Wilms tumor and enhances tumorigenic responses when enriched in the nucleus. Oncotarget. 2014;5(2):386–402. doi: 10.18632/oncotarget.1566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cooper DS, Doherty GM, Haugen BR, et al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009;19(11):1167–1214. doi: 10.1089/thy.2009.0110 [DOI] [PubMed] [Google Scholar]
- 18.Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid. 2016;26(1):1–133. doi: 10.1089/thy.2015.0373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schlumberger M, Tahara M, Wirth LJ, et al. Lenvatinib versus placebo in radioiodine-refractory thyroid cancer. N Engl J Med. 2015;372(7):621–630. doi: 10.1056/NEJMoa1406470 [DOI] [PubMed] [Google Scholar]
- 20.Brose MS, Nutting CM, Jarzab B, et al. Sorafenib in radioactive iodine-refractory, locally advanced or metastatic differentiated thyroid cancer: a randomised, double-blind, phase 3 trial. Lancet. 2014;384(9940):319–328. doi: 10.1016/S0140-6736(14)60421-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shi G, Boyle SC, Sparrow DB, Dunwoodie SL, Shioda T, de Caestecker MP. The transcriptional activity of CITED1 is regulated by phosphorylation in a cell cycle-dependent manner. J Biol Chem. 2006;281(37):27426–27435. doi: 10.1074/jbc.M602631200 [DOI] [PubMed] [Google Scholar]
- 22.Plisov S, Tsang M, Shi G, et al. Cited1 is a bifunctional transcriptional cofactor that regulates early nephronic patterning. J Am Soc Nephrol. 2005;16(6):1632–1644. doi: 10.1681/ASN.2004060476 [DOI] [PubMed] [Google Scholar]
- 23.Lovvorn HN, Westrup J, Opperman S, et al. CITED1 expression in Wilms’ tumor and embryonic kidney. Neoplasia. 2007;9(7):589–600. doi: 10.1593/neo.07358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao H, Wu G, Zhu J, et al. Melanocyte-specific gene 1 promotes melanoma progression by enhancing the expression of Bcl-2. Oncol Lett. 2018;15(2):2413–2418. doi: 10.3892/ol.2017.7592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cantelli G, Orgaz JL, Rodriguez-Hernandez I, et al. TGF-beta-induced transcription sustains amoeboid melanoma migration and dissemination. Curr Biol. 2015;25(22):2899–2914. doi: 10.1016/j.cub.2015.09.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xia E, Wang Y, Bhandari A, et al. CITED1 gene promotes proliferation, migration and invasion in papillary thyroid cancer. Oncol Lett. 2018;16(1):105–112. doi: 10.3892/ol.2018.8653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wen D, Liao T, Ma B, et al. Downregulation of CSN6 attenuates papillary thyroid carcinoma progression by reducing Wnt/beta-catenin signaling and sensitizes cancer cells to FH535 therapy. Cancer Med. 2018;7(2):285–296. doi: 10.1002/cam4.1272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li K, Xu X, He Y, et al. P21-activated kinase 7 (PAK7) interacts with and activates Wnt/beta-catenin signaling pathway in breast cancer. J Cancer. 2018;9(10):1821–1835. doi: 10.7150/jca.24934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhan T, Rindtorff N, Boutros M. Wnt signaling in cancer. Oncogene. 2017;36(11):1461–1473. doi: 10.1038/onc.2016.304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Anastas JN, Moon RT. WNT signalling pathways as therapeutic targets in cancer. Nat Rev Cancer. 2013;13(1):11–26. doi: 10.1038/nrc3419 [DOI] [PubMed] [Google Scholar]
- 31.Clevers H, Nusse R. Wnt/beta-catenin signaling and disease. Cell. 2012;149(6):1192–1205. doi: 10.1016/j.cell.2012.05.012 [DOI] [PubMed] [Google Scholar]
- 32.Sun Y, Kolligs FT, Hottiger MO, Mosavin R, Fearon ER, Nabel GJ. Regulation of beta -catenin transformation by the p300 transcriptional coactivator. Proc Natl Acad Sci U S A. 2000;97(23):12613–12618. doi: 10.1073/pnas.220158597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Takemaru KI, Moon RT. The transcriptional coactivator CBP interacts with beta-catenin to activate gene expression. The Journal of Cell Biology. 2000;149(2):249–254. doi: 10.1083/jcb.149.2.249 [DOI] [PMC free article] [PubMed] [Google Scholar]