Abstract
This paper describes and compares methods and analyzers used to measure hemoglobin (Hb) in clinical laboratories and field settings. We conducted a literature review for methods used to measure Hb in clinical laboratories and field settings. We described methods to measure Hb and factors influencing results. Automated hematology analyzer (AHA) was reference for all Hb comparisons using evaluation criteria of ±7% set by College of American Pathologists (CAP) and Clinical Laboratory Improvement Amendments (CLIA). Capillary fingerprick blood usually produces higher Hb concentrations compared with venous blood. Individual drops produced lower concentrations than pooled capillary blood. Compared with the AHA: (1) overall cyanmethemoglobin (1.0–8.0 g/L), WHO Colour Scale (0.5–10.0 g/L), paper-based devices (5.0–7.0 g/L), HemoCue® Hb-201 (1.0–16.0 g/L) and Hb-301 (0.5–6.0 g/L), and Masimo Pronto® (0.3–14.0 g/L) overestimated concentrations; (2) Masimo Radical® −7 both under- and overestimated concentrations (0.3–104.0 g/L); and (3) other methods underestimated concentrations (2.0–16.0 g/L). Most mean concentration comparisons varied less than ±7% of the reference. Hb measurements are influenced by several analytical factors. With few exceptions, mean concentration bias was within ±7%, suggesting acceptable performance. Appropriate, high-quality methods in all settings are necessary to ensure the accuracy of Hb measurements.This paper describes and compares methods and analyzers used to measure hemoglobin (Hb) in clinical laboratories and field settings. With few exceptions, mean concentration bias was within ±7%, suggesting acceptable performance. Appropriate, high-quality methods in all settings are necessary to ensure the accuracy of Hb measurements.
Keywords: hemoglobin, anemia, blood collection, clinical, field, variability
Introduction
Reducing anemia by 50% in women of reproductive age (WRA) is a 2025 World Health Assembly Global Nutrition Target (WHO, 2014), and accurate assessment of hemoglobin (Hb) is a global priority. The use of Hb measurement in the blood banking environment as the screening method for donor eligibility is a necessary requirement. Anemia is a condition that develops due to a low level of circulating red blood cells (RBCs), which reduces their capacity to carry oxygen in the body.1,2 Hb is a protein in RBCs that carries the oxygen to the tissues. Anemia is defined as Hb concentration below a specific threshold (70–130 g/L depending on age, sex, and pregnancy status and/or severity level).3 WHO estimates that approximately 1.93 billion people, 27% of the world’s population, suffer from anemia and it is a major public health problem with the highest prevalence among preschool children and WRA in low- and middle-income countries.4 Iron deficiency is considered to be a leading cause of anemia, but multiple factors may contribute to the etiology of anemia including other micronutrient deficiencies (e.g., zinc and vitamins A and B12); helminth infection and malaria; other sources of blood loss, inflammation, and other chronic diseases; and blood disorders (e.g., sickle cell and thalassemia).1,5–7
Appropriate, high-quality methods for Hb measurement in clinical laboratories and field settings are necessary to ensure the accuracy of Hb measurements.8–11 Clinical laboratories are controlled environments. Field settings include areas in the natural environment outside of a controlled environment.12 Several factors to assess when considering methods and analyzers used for assessing Hb include the source of the blood sample, cost of the analysis, and reproducibility of the results.13–16 Factors to consider when choosing to measure Hb in a clinical laboratory or field setting include quality control (QC) needs, extreme environmental conditions, low-resource environments, poor infrastructure, and standardized training. Attention to these factors will potentially reduce the risk of any negative impact on Hb measurements.11,17–19 In all cases, postanalytical factors, including adjusting Hb concentrations for altitude and smoking status, and use of appropriate WHO recommended cutoffs for defining anemias (based on age, sex, and pregnancy status), must be included in the analysis.1
Early qualitative methods for assessing Hb in clinical settings include the copper sulfate technique (CST).20 Quantitative methods were later developed including the cyanmethemoglobin method (CM) for assessing Hb concentrations.21 The CM is the internationally recognized reference method for calibrating clinical and field equipment used to measure Hb and determination of Hb concentration in blood.22 Counting and sizing particles using automated hematology analyzers (AHAs) in clinical laboratories is a quantitative method that was developed due to the need to assess Hb in low-resource settings.1,22 Less expensive, field-friendly quantitative methods were later developed including the WHO Colour Scale and other paper- and color-based analytical devices and portable point-of-care (POC) analyzers.10,11,17,23,24
There are five objectives of this paper, including:
Describe the different methods and analyzers used to measure Hb in clinical laboratories and field settings.
Describe the preanalytical factors including blood source of collection, postural effect, and environmental factors.
Describe analytical and postanalytical factors and training requirements, which can potentially influence Hb concentrations.
Compare the performance of different methods and analyzers of Hb measurement to the AHA as reference.
Describe the feasibility and cost of assessing the etiology of anemia in public health population-based surveys.
In addition, we compared the results of portable invasive photometric POC analyzers with other portable POC analyzer methods. These are reported as Supplementary Text S3 (online only) and Figure S2A and S2B (online only) and not the main body of the paper, because although they may be models currently used in clinical settings or the field by population-based surveys to assess anemia, they were compared with other methods and analyzers other than the AHA or they are models no longer supported by the manufacturer (e.g., HemoCue® B-Hb).11
Methods
We conducted a systematic literature review to describe the different methods used to measure Hb concentrations, sources of blood, training, and other factors that might influence Hb concentrations, as well as to identify studies examining the performance of different methods compared with the AHA (reference) and the HemoCue. CM is the internationally recognized reference method for calibrating clinical and field equipment used to measure Hb and determination of Hb concentration in blood, but few studies examined methods and analyzers compared with this method.22 Information on the feasibility and cost of assessing multiple factors that may contribute to anemia in population-based surveys is from the recent experience of authors providing technical assistance in the design and implementation of such surveys and surveillance systems.1,5–7
We searched PubMed, PubMed Central, MEDLINE, and EMBASE databases for all studies ever written in English related to methods for Hb measurement in blood banks, clinical laboratories, and field settings. We used keywords alone and in combination for the search. Keywords included: anaemia; haemoglobin; automatic hematology analyzer; point-of-care analyzer; photometric; HemoCue; Hb-Quick; noninvasive; Masimo Radical-7®; Masimo Pronto-7®; ToucHb; copper sulfate technique, cyanmethaemoglobin method; WHO Colour Scale; paper-based analytical tests; earlobe puncture; venous blood; capillary blood; arterial blood; finger stick; heel stick; venipuncture; source of sample; accuracy; variability; postural effect; disease; illness; quality control; validation; hemolysis; training; preana-lytical factors; analytical factors; postanalytical factors; method comparison; and cost. One author screened all titles and two authors extracted data independently.
We compared studies of qualitative (CST) and quantitative methods (CM, WHO Colour Scale, and other paper-based analytical tests and portable POC analyzers) against AHAs as the reference as few studies identified were compared with the CM method. Only the study arms compared with AHAs were included. We also compared portable invasive photometric POC analyzers, specifically the HemoCue Hb-201+ and Hb-301 (currently available and supported by HemoCue), with other portable POC analyzers as these methods are feasible in field settings and population-based surveys. For these comparisons, only the study arms compared with the portable invasive photometric POC analyzers were included. There are two threshold evaluation criteria for Hb set by the College of American Pathologists (CAP) and the Westgard Clinical Laboratory Improvement Amendments (CLIA).170,171 These criteria target a threshold of ±7% of the reference as the acceptable difference between methods. We applied a bias threshold of ±7% for each method and analyzer compared with the reference to examine whether the variation in mean Hb concentration between the two methods was within this ±7% threshold.
Results
The literature search generated 2232 matches from all the search combinations. After excluding duplicates and studies that did not meet the objectives, we chose 257 articles for review. Of those articles reviewed, we included 113 as part of this review due to meeting the method comparison criteria, including preanalytical factors (i.e., blood source and sampling technique), analytical factors (i.e., QC and method accuracy), and postanalytical factors (i.e., adjusting Hb concentrations). We excluded 28 studies from the review that included comparisons of different methods, but did not meet the method criteria for the comparisons with the AHAs or the portable invasive photometric POC analyzers (Table S1, online only; see this Table for Refs. 105, 150–169).
Description of methods and analyzers to assess Hb concentrations
Table 1 provides a summary of various method characteristics used to measure Hb in clinical laboratories and field settings, including analytical considerations for each method for the analyzer. Qualitative and quantitative methods and analyzers are available to measure Hb in both settings. Qualitative methods for Hb measurement include the CST,20,25 which was developed in the late 1880s and was commonly used to identify healthy blood donors in the mid- to late-1900s. It is a qualitative method for measuring Hb based on the estimation of specific gravity from a blood sample. With this method, the specific gravity value of 1.053 corresponds to an Hb concentration of 125 g/L.26 Altitude effects the specific gravity of the liquid solution used by the CST, thus the Hb concentration must be adjusted based to sea level to ensure the final Hb concentration is accurately calculated.27,28 The CST is still used today by many laboratories, including the National Health Service (NHS) Blood and Transplant, the United Kingdom, although some publications mention the method is no longer used.20,29,30
Table 1.
Summary of various method and analyzer characteristics used for hemoglobin measurement
| Method/analyzer | Setting | Blood source: arterial (A), capillary (C), or venous (V) | Blood volume (μL) | Time of test | Calibration (quality control) | Method principle | Approximate equipment* costs, USD (2017) | Cost/test, USD (2018) |
|---|---|---|---|---|---|---|---|---|
| Copper sulfate technique (CST)a | Clinical laboratory | A, C, and V | 10 | 20 s | Limited/not well established; mustusefresh anticoagulated blood samples | Hb concentration based on the estimation of specific gravity from a blood sample where the specific gravity value of 1.053 corresponds to an Hb concentration of 125 g/L. | $200–500 | <$1.00 |
| Cyanmethemog-lobin method (CM)b | Clinical laboratory | A, C, and V | 10 | 5–10 min prep time and 60 s for the test | Drabkin’s reagent | Hb is converted into methemoglobin, which is then converted into cyanmethemoglobin. This is done by adding both potassium cyanide and ferricyanide whose absorbance is then measured at 540 nm using a photoelectric colorimeter against a standard quality control solution. The Hb concentration is then determined by the result produced by the photoelectric colorimeter. | $200–500 | <$1.00 |
| Automated hematology analyzers (AHA)c | Clinical laboratory | A, C, and V | 200 | 30 s | Quality control material specific to the analyzer | The automated hematology analyzer operates bypulling particles through an orifice with the use of an electric current in order to produce a change in resistance that is proportional to the volume of the particle traversing through the orifice. The automated hematology analyzer functions by counting cells of various sizes that are composed within whole blood. | $2000–15,000 equipment (depending on model) | <$10.00 |
| WHO Colour Scaled | Clinical laboratory and field settings | A, C,and V | 30 | 60 s | Drabkin’s reagent or fresh anticoagulated blood sample | Contains six shades of red (i.e., lighter to darker corresponding to an Hb concentration of 40, 60, 80, 100,120, and 140 g/L) that are mounted onto strips. A drop of blood is placed onto a moveable piece of filter and compared to the shades of red on the color scale. | ~$100/box 200 | <$0.50 |
| Invasive photometric point-of-care analyzers (e.g., HemoCue, Hemo-Control, Hb-Quick, DiaSpect, URIT, and TrueHb)e | Clinical laboratory and field settings | A, C, and V | 10 | 10s | Not required for Hb-201+, Hb-301, Hemo-Control, DiaSpect, TrueHb, and URIT, but HemoTrol and EuroTrol have liquid controls available for use | For the Hb-201+, Hemo-Control, and Hb-Quick, Hb is converted to methemoglobin by sodium nitrate from the ferrous to ferric state to form azidemethemoglobin,where the Hb concentration is then detected at 570 and 880 nm and read using a photoelectric colorimeter. For the Hb-301, the Hb concentration is simply determined by the photoelectric colorimeter. | $600–900 equipment |
$1.00–2.00 |
| Paper- or color-based analytical devices (e.g., μPADs and color-based filter testf | Clinical laboratory | A, C, and V | 20 | 45–60 min | Drabkin’s reagent | Based on microfluidic technology using chromatography paper with a wax finish that is heated at 150 °C for 3 min prior to use. Blood samples are diluted with Drabkin’s reagent and then incubated for 10 min. A 20 μL sample of blood is then placed onto the paper-based analytical device, and the Hb concentration is then read using a portable flatbed scanner after the sample dries. | $200–500 equipment | <$1.00 |
| Noninvasive point-of-care analyzers (e.g., Masimo Radical-7)h | Clinical laboratory and field settings | NA | NA | 30 s | NA | Noninvasive analyzers operate using a device called the CO-oximeter, which measures the oxygen saturation (SpO2), pulse rate, perfusion index (Pi), and total Hb by detecting the levels of oxygen and carbon monoxide (CO) bound to Hb in the individual. This is done simply by placing a monitor on the finger of the individual (i.e., appropriate to the size and age of the individual), requiring them to sit completely still, and measuring the total Hb. | $1800–2000 | <$2.00 |
| Color-based analytical devicesg | Clinical laboratory and field setting | A, C, and V | 5 | 60 s | Drabkin’s reagent | Uses small round tube with a cap that holds the solution, which mixes with the blood sample that enters the device via capillary action. The sample is then compared to a color chart with a range of colors. | $200–500 | <$1.00 |
Yang et al.25
Supplies are $5 per person for small survey assessing anemia and malaria versus $75 per person for large survey assessing anemia, malaria, serum ferritin, serum soluble transferrin receptor, inflammation, serum vitamin A, serum vitamin B12, folate, serum zinc, and urinary iodine (i.e., costs include supplies and analytical costs) (see Refs. 148 and 149).
The CM is a quantitative method that is the internationally recognized reference standard method for the determination of Hb concentration in blood.22 CM is used to calibrate clinical and field equipment prior to use as well as used as a regular method for the determination of Hb concentration. The principle of the CM, developed for clinical laboratories in the mid-1900s, is to convert Hb into methemoglobin (MetHb) and then metHb into cyanMetHb. The conversion occurs by adding a solution containing both potassium cyanide and ferricyanide to 10 μL arterial, venous, or capillary blood, mixing, waiting approximately 5–10 min, and measuring Hb using a photoelectric colorimeter with an absorbance of 540 nm, which takes approximately 60 seconds.26 The CM is still a commonly used method in many clinical laboratories even though newer methods are available.31
In 1953, Coulter developed a principle of rapid and accurate counting and sizing particles in a clinical laboratory setting. Coulter’s principle led to the development of quantitative automated cell counting, with advancements in technology during the 1960–1970s leading to the development of higher quality AHAs.17 Due to the size and lack of portability of the analyzers (i.e., requires electricity), AHAs are typically used by blood banks and clinical laboratories and are generally not considered feasible for field settings. AHAs are the most commonly used analyzers for clinical laboratories because they can also measure other blood indicators including hematocrit.15,32,33
Recent quantitative methods to measure Hb include the WHO Colour Scale.23,34–36 and other paper- and color-based analytical devices25 and portable POC analyzers (i.e., both noninvasive and photometric invasive).10,11,17 The WHO Colour Scale is a quantitative method developed by WHO in the late 1990s and available for purchase in 2001 as a replacement to the CST30 to be used by blood banks, clinical laboratories, and field settings to measure Hb. It uses six shades of red (i.e., lighter to darker corresponding to Hb concentrations of 40, 60,80,100,120, and 140 g/L) that are mounted onto strips. A drop of blood is placed onto a moveable piece of filter paper and compared with the shades of red on the color scale.34,36
In the late 1990s, development of portable invasive photometric POC analyzers started as a means to measure Hb quickly in all settings using small amounts of blood. Invasive photometric POC analyzers, such as the Hb-Quick® and HemoCue models, provide Hb measurements within 10 seconds using approximately 10 μL of fresh arterial, venous, or capillary blood.11,37–39 Two widely used invasive photometric POC analyzers are the handheld HemoCue models Hb-201+ and Hb-301. The Hb-201+ cuvettes contain sodium deoxycholate reagent that creates hemolysis of the blood when it enters the cuvette; however, this makes them sensitive to high temperatures and humidity. The Hb-301 cuvettes do not contain sodium deoxycholate reagent and are stable at a greater range of temperature and humidity levels, if stored correctly in the designated, closed containers; however, if the blood sample in the cuvette is open to air or on a piece of parafilm, it is constantly being exposed to oxygen and may result in an artificially higher Hb value (1.3% increase per minute), so it is essential to read the Hb concentrations within 20–30 s of filling the cuvette. These characteristics potentially make HemoCue model Hb-201+ better suited to more controlled settings in clinical laboratories, and HemoCue model 301 for less controlled field settings.
Due to the need for a portable, low-cost test for low- and middle-income settings, a simple paper-based analytical device (μPADs) based on microfluidic technology was developed in the early 2000s to quantitatively measure Hb.24 This paper-based analytical device uses chromatography paper with a wax finish that is heated at 150 °C for 3 min prior to use. Twenty microliters of the diluted blood sample is required and applied to the paper-based analytical device, which must dry for 25 min prior to reading the Hb concentration using a flatbed scanner. The scanner senses the colors red, green, and blue to measure intensity, which correlates with the Hb concentration of the blood sample. Clinical laboratories are currently the primary setting for this method due to having to complete the measurement of Hb in a clinical laboratory setting after sample preparation.24,25,34
Due to a need for a noninvasive device to assess Hb, noninvasive POC analyzers were developed for use in more controlled settings, with the potential for use in field settings.40–44 In 2006, Masimo was one of the first to develop noninvasive POC analyzers (Radical-7) followed by other model analyzers (and Pronto-7 pulse CO-oximeters) and companies, including Biosense (ToucHb) in 2008. These noninvasive POC analyzers operate using a device called the CO-oximeter, which measures the oxygen saturation (SpO2), pulse rate, perfusion index (Pi), and total Hb by detecting the levels of oxygen and carbon monoxide (CO) bound to Hb in the individual. This is done simply by placing a monitor on the finger of the individual (i.e., appropriate to the size and age of the individual), requiring them to sit completely still, and measuring the total Hb within 30 seconds.40–42,44–46
In 2013, a color-based POC analyzer was developed to measure Hb aiming to provide a rapid, simple to use, and disposable method that did not require electricity.47 The device consists of a small round tube with a cap that holds the solution, which mixes with the capillary blood sample that enters the device via capillary action. After 60 s, the Hb sample is compared with a color chart. Use of the test is applicable to all settings.47
Preanalytical factors influencing Hb measurements including blood source of collection, postural effect, and environmental factors
Capillary, arterial, and venous sources of blood collection.
There are three primary sources of blood collection: artery, vein, and capillary. Collection of cord blood among women who just gave birth is also used as a surrogate to venous blood (reference) to measure Hb concentrations. Earlobe puncture to collect capillary blood (~75 μL) is a historical form of blood collection.9,48–50 Arterial blood is collected from the radial artery in the forearm, a less common and more complex form of collection. Arterial blood can be useful when arterial blood gas measurements are required, and Hb can be measured using arterial blood. Arterial blood is oxygenated (i.e., flowing from the heart) and has higher amounts of oxygen-bound Hb compared with blood sources not flowing from the heart. Due to the complexity in collecting arterial blood, only well-trained personnel should collect it in clinical settings. It is not a field-friendly method and is not an appropriate source for blood collection in public health population-based surveys in field settings.51,52
Capillary blood collected from the finger or heel can be collected as individual drops or pooled blood. It is collected in all settings and is used for obtaining approximately ~50–500 μL of blood. Just below the dermal layer of skin, the finger and heel contain capillary loops, which are a collection of small blood vessels that contain a combination of arterial and venous blood, as well as interstitial and intracellular fluids. The highly oxygenated arterial blood flows into the capillaries via small arteries called arterioles and then leaves the capillaries deoxygenated into the small veins called venules. Since oxygen is bound to Hb when it enters capillaries, when collected with appropriate techniques, the measured level of Hb should be higher in capillaries compared with venous blood because venous blood is deoxygenated, that is, contains less oxygen.10,34,53–55 It is particularly important to not squeeze the finger or heel too hard when collecting capillary blood because this can cause interstitial fluid to mix with the blood diluting the sample, thus leading to an incorrect (lower) Hb concentration. Warming the hands is also important. Cold fingers can also lead to incorrect Hb results due to poor circulation in the fingers.
For multiple reasons, venous blood is the reference for blood collection. Venous blood is easier to collect compared with arterial blood. Venous blood is also the most common form of blood collection in clinical settings and blood banks; blood sample from the cubital vein provides a larger volume of blood (~2–5 mL blood) allowing for the assessment of multiple biological indicators compared with capillary blood, and larger blood volumes might be necessary when performing multiple biological tests. Blood banks also use capillary fingerprick blood samples as a screening method for donation eligibility. Field settings are also a prime location for venous blood collection when the assessment of multiple biological indicators requiring larger blood volumes is required.
Venous blood and capillary blood from the finger or heel (usually heel is among the youngest children aged less than 6 or 12 months, depending on country requirements) are currently the commonly used forms of blood collection for the estimation of Hb concentration in both clinical laboratories and field settings.10,11,53–55 For both sources, blood collection may be more challenging among younger children compared with older children and adults because their fingers and veins are smaller.1
Figure 1A and B include a summary of 25 comparisons from 18 studies comparing the sources of blood collection against venous or cord blood (reference) (Fig. 1A), and single drops of blood compared with pooled blood (reference) (Fig. 1B), as well as right compared with left-hand choice (reference) (Fig. 1B). The sample sizes and population groups comparing the source of blood varied, including both male and female adults and from infancy to 73 years (Fig. 1A). Twelve studies found higher mean Hb concentrations (1.0–7.0 g/L) by capillary blood compared with venous blood,11,19,51,52,56–63,140 and three studies foundlower mean Hb concentrations (0.8–8.1 g/L) bycapillaryblood compared with venous blood.65,87 Two studies measuring arterial blood found opposite findings with one having a higher mean Hb concentration (0.1 g/L) and one having a lower mean Hb concentration (2.0 g/L) compared with the venous blood.51,52 Eslami et al. also compared mean Hb concentrations among female and male infants by capillary blood compared with cord blood and found a higher mean Hb concentration (4.6 g/L) by capillary blood (Fig. 1A).62 When applying the mean difference threshold of ±7% for the comparison of sources of blood collection (venous or cord blood as the reference), all studies were within the ±7% variation threshold.11,19,51,52,54,56–65,76,87,140
Figure 1.
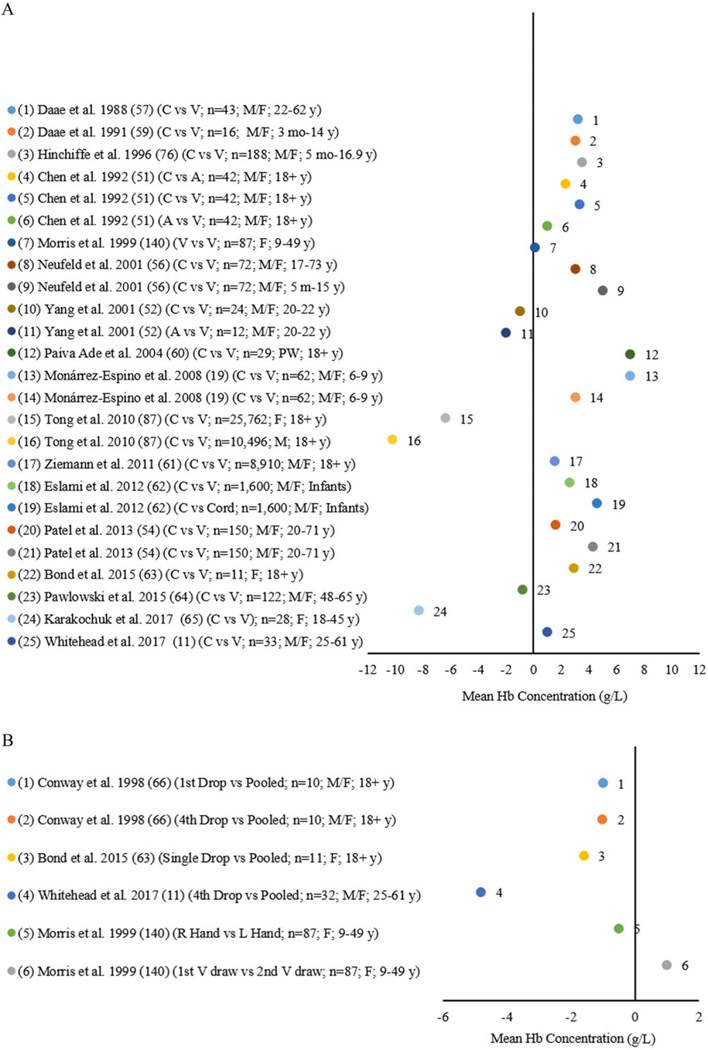
(A) Differences in mean Hb by arterial (A) and capillary (C) blood source compared with venous (V) or cord blood source as the reference* among infants, children, and adult males (M), females (F), and pregnant women (PW). (B) Differences in mean Hb by single drops of capillary (C) blood source compared with pooled capillary blood source as the reference*, right (R) hand compared with left (L) hand as the standard*, and first venous draw compared with second venous draw as the standard* among children and adult males (M) and females (F).
Figure 1B shows four comparisons examining drop to drop variability compared with pooled capillary blood as the reference among adults aged 18 years and older.11,63,66 All studies found a lower meanHb concentration (1.0,1.0,1.6, and 4.8 g/L) by single drops (first or fourth drop,66 single drops,63 fourth drop,11 respectively) compared with pooled capillary blood.11,63,66 Morris et al. studied mean Hb concentration differences in capillary blood collected in the right hand versus the left hand (reference) among 87 females aged 9–49 years (Fig. 1B) and found a lower mean Hb concentration (0.5 g/L) by the right hand compared with the left hand.140 Morris et al. also reported a higher mean Hb concentration (0.1 g/L) from the first venous blood draw (2 mL) compared with the second venous blood draw (2 mL) among 141 female infants 4 months of age from poor families who had been exclusively breastfed.140 When applying the ±7% threshold for mean difference between methods comparing drops of capillary blood to pooled capillary blood (reference), all studies were within the ±7% variation threshold.11,63,66,140
Postural effect.
Postural effect (sitting versus standing) during blood collection may influence Hb values and individuals should be seated to ensure accurate measurement of Hb.9,67 Standing during blood collection can cause Hb concentrations to become diluted in the lower extremities of the body due to pooling of fluids in these areas, which leads to lower Hb concentrations (up to 3.5 g/L lower).9 Lima-Oliveira et al. studied postural effects on Hb concentrations among 19 healthy adults (7 males and 12 females) with a mean age 33–55 years.67 The study included testing with the individuals laying down, sitting, and standing. Lower mean Hb concentrations (3.0 and 7.0 g/L) were found when a lying position was compared with a sitting position and when a sitting position was compared with standing, respectively.39 For all blood sources, it is important for participants to be seated (not standing) during blood collection to minimize any postural effects on the blood specimen9,67
Environmental factors.
Unfavorable environmental conditions (i.e., increased temperature and humidity); poor infrastructure (i.e., lack of electricity, clinical laboratory space, cold storage, and back-up generator); poor cold chain management; and inadequate training of laboratory personnel can affect Hb measurements.11,68 Following proper technique and protocols is essential to minimize the risk of hemolysis during collection of the blood sample; ensure proper storage of the blood sample after collection; and ensure proper processing of the blood sample in the clinical laboratory or field setting to prevent any delayed effects that may occur when processing the samples. Adequate temperature is necessary for the proper operation of instrumentation used by all methods in both clinical laboratories and field settings. Hb measurements can also be negatively affected by improper storage of supplies used in the assessment of Hb concentrations in the clinical laboratory and field setting, as well as the use of expired supplies.19,67,69–72
Field settings are of particular concern because of the less controlled and possibly harsh climatic settings. It is important to ensure appropriate methods and take into consideration the optimal operating temperatures for storage and use of supplies and equipment. Studies have shown that elevated temperatures and humidity can potentially be an issue for invasive photometric POC analyzers and their supplies, including cuvettes and liquid QCs where Hb concentrations are known to significantly increase (1.3 g/L) after 3 weeks of exposure to poor conditions.11,68,73
Analytical and postanalytical factors and training requirements
Table 1 describes analytical factors to consider when deciding which method to use, including the volume of the sample needed, time per test, and QC requirements. Postanalytical factors must also be considered in order to accurately use Hb concentration data to properly assess anemia in individuals and populations in both clinical laboratories and field settings. WHO provides recommendations on properly diagnosing anemia using age, sex, and pregnancy status specific cutoffs, as well as guidance on properly adjusting Hb values for altitude and smoking status.3 In population-based surveys, thresholds for referral to the public health facility for low Hb concentrations (determined by the Ministry of Health) maybe adjusted for altitude or smoking depending on the context. The practice of universal precautions with blood collection and standardized training of laboratory personnel are required regardless of the method selected for measuring Hb (Supplementary Text S1, online only) 11,20,34,68,69,74,75
Comparability of the automated hematology analyzer (reference) with other methods and analyzers in clinical laboratories and field settings
Eighty-three studies compared AHA (reference) to other methods and analyzers in clinical laboratories and field settings among infants, children, and adult males, females, and pregnant women (PW) aged newborn to >90 years.
Comparability of the automated hematology analyzer with CST and CM.
Figure 2A shows four studies where venous blood analyzed by the reference was compared with the CST among infants and children aged 6 months to 6 years, adult males, females, and PW aged 18 years and older.77–80 All studies reported lower mean Hb concentrations (2.0–16.0 g/L) in venous blood analyzed by the CST compared with the reference (Fig. 2A). When applying the ±7% threshold for mean difference for the studies comparing the CST with the reference, two studies were within the threshold range78,79 and two studies exceeded the ±7% bias77,80 (Fig. 2A). Figure 2B shows two studies where venous blood analyzed by the reference was compared with the CM among infants and children aged 5 months to 5 years and adult males and females aged 21–54.84,99 Sawant et al. compared the reference with the CM in venous and capillary blood and reported higher mean concentrations (8.0 g/L) for both blood sources compared with the reference.84 Nkrumah et al. compared the reference with the CM in capillary blood and reported a higher mean concentration (1.0 g/L) in capillary blood (Fig. 2B).99 When applying the ±7% mean difference threshold comparing the CM with the reference, all studies were within the ±7% variation threshold.84,99
Figure 2.
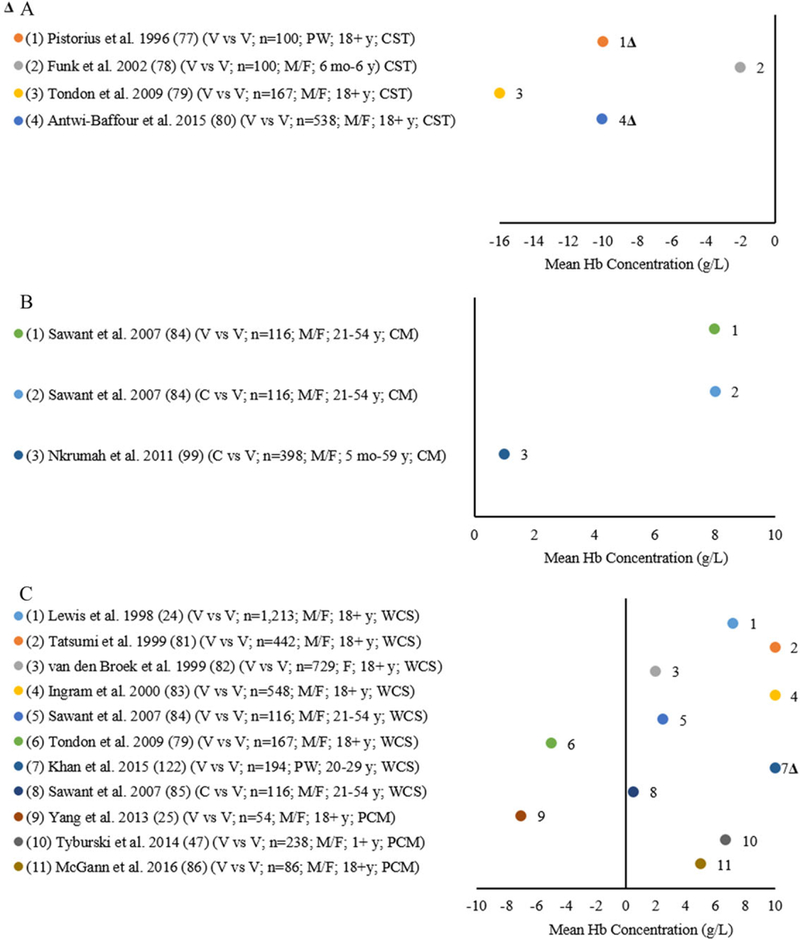
(A) Differences in mean Hb of the copper sulfate technique (CST) compared with an automated hematology analyzer (AHA) (reference ) by venous (V) source among infants and children and adult males (M), females (F), and pregnant women (PW). (2B) Differences in mean Hb of the cyanmetHb method (CM) compared with an AHA (reference ) by venous (V) source among infants and children and adult males (M) and females (F). (2C) Differences in mean Hb of the WHO Colour Scale and paper- and color-based methods (PCM) compared with an AHA (reference*) by venous (V) source among infants and children and adult males (M), females (F), and pregnant women (PW).
Comparability of the automated hematology analyzer with WHO Colour Scale and paper- and color-based methods.
In Figure 2C, seven studies used venous blood to compare the reference to the WHO Colour Scale among infants, children, and adult males, females, and PW aged 18 years and older.24,79,81,84,122 Six studies24,81–85,122 reported higher mean Hb concentrations (0.5–10.0 g/L) compared with the reference, while Tondon et al.79 reported a lower mean Hb concentration (5.0 g/L). Sawant et al. also collected capillary blood and reported higher mean Hb concentrations (0.5 g/L) for the WHO Colour Scale compared with the reference.84 When applying the mean difference variation threshold to the seven studies82,84,85,122 comparing the WHO Colour Scale with the reference, only one study122 exceeded the ±7% bias (Fig. 2C). Three studies using venous blood compared a novel device, including either a microfluidic paper-based analytic device (μPAD) or a color-based assay24,47,86 with the reference (Fig. 2C). McGann et al.86 and Tyburski et al.47 reported higher mean Hb concentrations (5.0 and 6.7 g/L, respectively) with a color-based assay compared with the reference. Yang et al. reported lower mean Hb concentrations (7.0 g/dL) comparing the paper-based analytic device to the reference.25 When applying the ±7% threshold for mean HB concentration difference for the studies comparing the microfluidic paper-based analytic device (μPAD) or a color-based assay with the reference, all three studies were within the threshold variation of ±7%24,47,86 (Fig. 2C).
Comparability of the automated hematology analyzer with HemoCue Hb-201+.
Twenty-eight comparisons from 23 studies compared the reference and a HemoCue Hb-201+ using either arterial, venous, or capillary blood in both the clinical laboratory and field setting among infants and children aged 6 months to 17 years and adult males, females, and PW aged 18 years and older (Fig. 3A). Using arterial blood, Seguin et al.115 reported a lower mean Hb concentration and Giraud et al.107 a higher mean concentration by Hb-201+ compared with the reference. Eleven studies analyzed venous blood with eight comparisons finding higher mean Hb concentrations (2.0–16.0 g/L) by the Hb-201+ compared with the reference8,19,54,103,104,106,107,139 and four comparisons found lower mean Hb concentrations (0.2–15.0 g/L).64,115,147 Fourteen comparisons from 13 studies compared capillary blood with venous blood8,54,64,65,103,108–114,133 with 9 comparisons finding higher mean Hb concentrations (2.0–9.0 g/L).8,54,103,108,110–113 Five found a lower mean Hb concentration (2.6–13.0 g/L for all findings).64,65,109,114,115 When applying the mean concentration variation threshold of ±7% for the studies comparing the Hb-201+ with the reference, 27 of the 28 comparisons8,19,54,64,65,104,106–114,133,139,147 met the allowable degree of variation with one comparison from one study exceeding the ±7% bias115 (Fig. 3A). Figure S1 and Supplementary Text S2 (both online only; see Fig. S1 for 89–94, 96–98, 100, 101, 141–145) describe 30 studies with 37 comparisons examining the HemoCue B-Hb invasive photometric POC analyzer to the reference.
Figure 3.
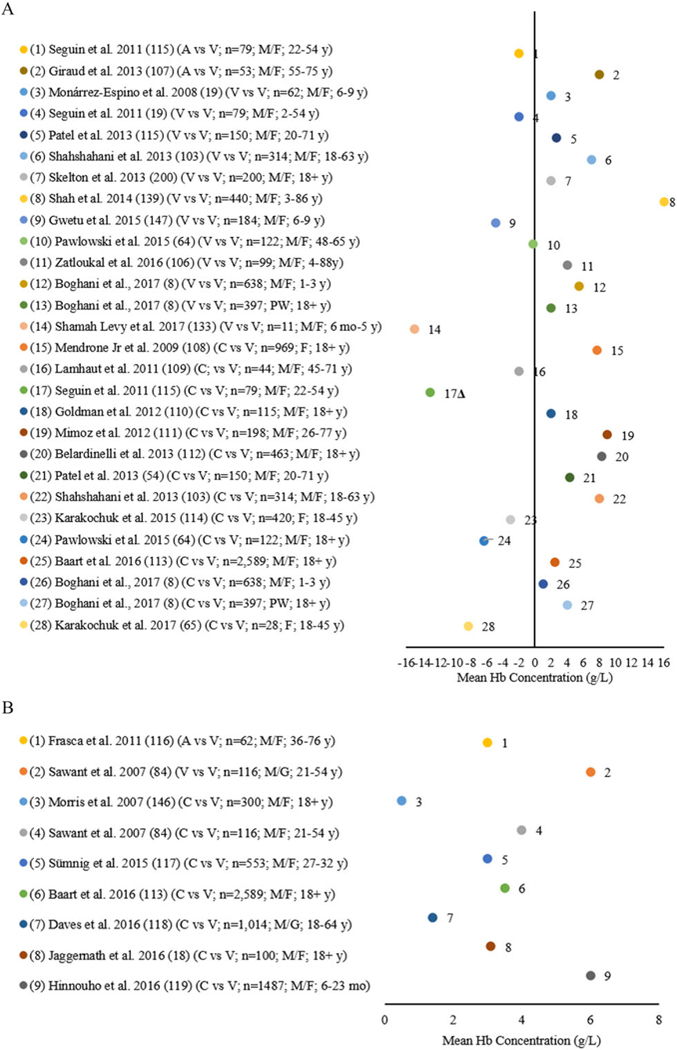
(A) Differences in mean Hb of the HemoCue Hb-201+ compared with an automated hematology analyzer (AHA) (reference*) by arterial (A), venous (V), cord, and capillary (C) blood source among infants and children and adult males (M), females (F), and pregnant women (PW). (B) Differences in mean Hb of the HemoCue Hb-301 compared with an AHA (reference ) by arterial (A), venous (V), and capillary (C) blood source among adult males (M) and females (F).
Comparability of the automated hematology analyzer with HemoCue Hb-301.
Eight studies conducted nine comparisons between the reference and a HemoCue Hb-301 using blood collected in both the clinical laboratory and field settings (Fig. 3B) and from arterial, venous, and capillary blood among adult males and females aged 18 years and older.18,84,95,113,116–119,146 All comparisons reported higher Hb concentrations compared with the AHA (0.5–6.1 g/L), but were within the ±7% threshold for mean difference in Hb concentrations.18,84,95,113,116–119,146
Comparability of the automated hematology analyzer with other portable photometric invasive POC analyzers or clinical blood gas analyzers.
Eleven studies included 18 comparisons between the reference and other portable photometric invasive POC analyzers or clinical blood gas analyzers using blood collected in both the clinical laboratory and field setting from venous and capillary blood sources among children 6 months to 17 years and adult males and females 18 years and older (Fig. 4). Eight of these studies reported 13 comparisons of venous or capillary blood for other portable photometric invasive POC analyzers that were compared with the reference.18,42,43,102,110,113,120,121 Six studies with nine comparisons analyzing venous blood found higher mean Hb concentrations (0.5–20.0 g/L) by the portable photometric invasive POC nanalyzer compared with the reference,18,113,120–122 including McNulty et al.,120 Singh et al.,121 and Jaggernath et al.18 who each compared two different analyzers to the reference in their studies. Goldman et al.,110 Rudolf-Oliveira et al.,102 and Ardin et al.42 analyzed capillary blood by a portable photometric invasive POC analyzer compared with the reference, with Ardin et al.42 comparing two different analyzers to the reference. Rudolf-Oliveira et al. found no difference in Hb concentration by the POC analyzer compared with the reference.102 Goldman et al. found higher mean Hb concentrations (1.0 g/L) by the portable POC analyzer compared with the reference.110 Ardin et al.42 reported a higher mean Hb concentration (1.2 g/L) using one invasive POC analyzer (IPOC-2)42,102,110 and Ardin et al.42 reported a different analyzer (IPOC-1) having a lower mean Hb concentration (4.9 g/L). Spielmann et al.,138 Broderick et al.,43 and Zatloukal et al.106 analyzed venous blood by clinical laboratory blood gas analyzers compared with the reference in five comparisons. Broderick et al.43 found a lower mean Hb concentration (1.5 g/L) and Zatloukal et al.106 found a higher mean Hb concentration (1.0 g/L). Spielmann et al. found two different clinical blood gas analyzers (CBGA-1 and CBGA-2) to have a higher mean Hb concentration (5.8–8.0 g/L) and one analyzer (CBGA-3) to have a lower mean Hb concentration (0.5 g/L) compared with the reference.138 When applying the mean concentration difference threshold of ±7% for the studies comparing the other portable photometric invasive POC analyzers or clinical laboratory blood gas analyzers with the reference, 11 studies including 17 comparisons18,31,43,102,106,110,113,120,121,138 met the allowable degree of variation with one study exceeding the ±7% bias42 (Fig. 4).
Figure 4.
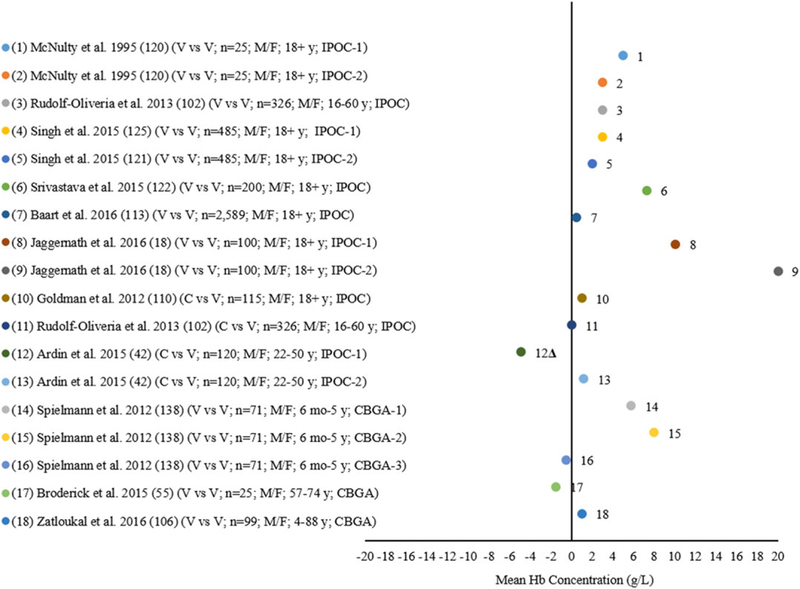
Differences in mean Hb of the other invasive point-of-care (IPOC) analyzers and clinical blood gas analyzer (CBGA) compared with an automated hematology analyzer (reference*) by venous (V) and capillary (C) blood source among children and adult males (M) and females (F).
Comparability of the automated hematology analyzer with noninvasive POC analyzers.
Twenty-five studies included 32 comparisons between the reference and other noninvasive POC analyzers in clinical laboratories (Fig. 5A and C). Eleven studies included 13 comparisons between the reference and the Masimo Radical-7 with the reference among children aged 3 years and 12–17 years and adult males and females aged 18 years and older41,43,107,116,123–128 (Fig. 5A). Frasca et al. analyzed arterial blood finding the same mean Hb concentration by the Masimo Radical-7 compared with the reference.116 Giraud et al. analyzed arterial blood finding a lower mean Hb concentration (10 g/L) for arterial blood.107 Ten studies with 11 comparisons analyzed venous blood with six comparisons finding a lower mean Hb concentration (1.4–10.0 g/L) and five comparisons having a higher mean Hb concentration (1.2–7.0 g/L) by Radical-7 compared with the reference.43,53,104,123,124,126–128 Von Schweinitz et al. also analyzed venous blood by the Masimo Radical-57 compared with the reference finding a higher mean Hb concentration (12.0 g/L) by the Masimo Radical-57 (data not shown).125 When applying the variation in mean difference threshold of ±7% to the studies comparing the Masimo Radical-7 with the reference, nine studies41,43,53,104,116,124,125,127,128 were within the allowable range of variation and four studies exceeded the ±7% bias range43,107,123,126 (Fig. 5A).
Figure 5.
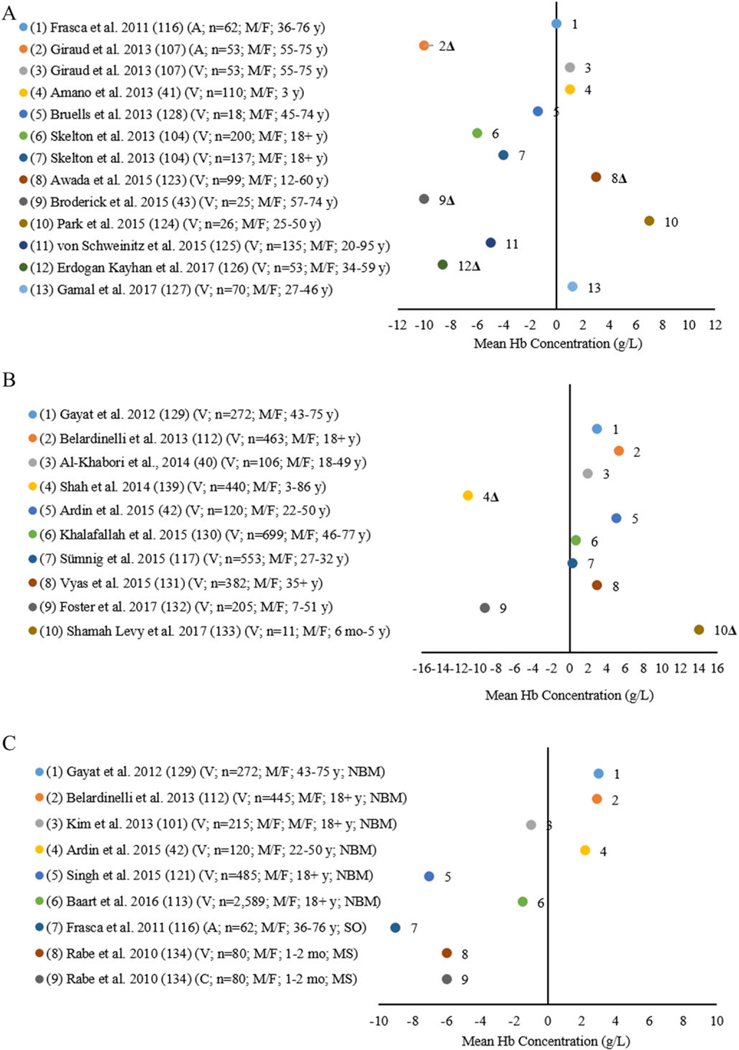
(A) Differences in mean Hb of the noninvasive Masimo Radical-7 analyzer compared with an automated hematology analyzer (AHA) (reference ) by arterial (A) and venous (V) blood source among children and adult males (M) and females (F). (B) Differences in mean Hb of the noninvasive Masimo Pronto-7 analyzer compared with an AHA (reference ) venous (V) blood source children and adult males (M) and females (F). (C) Differences in mean Hb of noninvasive OrSense NBM-200 analyzers (NBM) and other noninvasive point-of-care analyzers (Siemens CO-Oximeter (SO) and Mediscan (MS)) compared with an AHA (reference ) by arterial (A), venous (V), and capillary (C) blood source among infants and adult males (M) and females (F).
Ten studies analyzed venous blood by the Masimo Pronto-7 compared with the reference among children aged 6 months to 17 years and adult males and females aged 18 years and older40,42,112‘117‘129–133‘139 (Fig. 5B). Eight studies40,42,112,117,129–131,133 found higher mean Hb concentrations (0.3–14.0 g/L) and two132,139 found lower mean concentrations (9.2–11.0 g/L) by the Pronto-7 compared with the reference. Eight studies40,42,112,117,129–132 were within the ±7% threshold for mean concentration variation for comparisons of the Masimo Pronto-7 with the reference, while two studies exceeded the ±7% bias133,139 (Fig. 5B). Eight studies including nine comparisons compared the OrSense NBM-200 noninvasive analyzer, the Mediscan 2000, and the Siemens CO-Oximeter with the reference among infants aged 1 −2 months and adult males and females aged 18 years and older31,42,102,112,113,116,121,129 (Fig. 5C). Six studies analyzed venous blood by the OrSense NBM-200 noninvasive analyzer to compare with the reference42,102,112,113,121,129 and three reported lower mean Hb concentrations (1.0–7.0 g/L)31,102,121 and three found higher mean Hb concentrations (2.2–3.0 g/L).42,112,129 Rabe et al. compared Mediscan 2000 with the reference analyzing both venous and capillary blood and found lower mean Hb concentrations (6.0 and 6.0 g/L, respectively) for each blood source (Fig. 5C).134 Frasca et al. analyzed arterial blood and found a lower mean Hb concentration (9.0 g/L) by the Siemens CO-Oximeter compared with the reference.116 For the studies comparing the OrSense NBM-200 noninvasive analyzer, the Mediscan 2000, and the Siemens CO-Oximeter with the reference, all studies were within the ±7% mean concentration threshold for the degree of variation.31,42,102,112,113,116,121,129,134
Comparability of HemoCue models with portable invasive and noninvasive photometric POC analyzers
Figure S2A and S2B (online only) include 16 studies with 28 comparisons of different models of portable HemoCue invasive photometric POC analyzers to other invasive and noninvasive POC analyzers used in clinical laboratories and field settings comparing both venous and capillary samples among children aged 6–9 and 16–17 years and adult males and females aged 18 years and older.11,18,19,102,113 These results are presented in Supplementary Text S3 (online only; see this file for Ref. 173).
Review of indicators collected to assess the etiology of anemia, feasibility, and cost
Multiple factors may contribute to the condition of anemia, including micronutrient deficiencies (e.g., iron, zinc, vitamins A and B12); helminth infection and malaria; other sources of blood loss and inflammation (other morbidities and chronic disease); and blood disorders (e.g., sickle cell disease and thalassemia).1,5–7 Pasricha describes approximately 17 different factors related to the etiology of anemia based on a review using data from the Global Burden of Diseases, Injuries, and Risk Factors 2010 (GDB 2010) study.135 Table 2 describes biological indicators included in recent surveys assessing the etiology of anemia, along with matrix and the volume of the specimen, testing methods, and cost per test for these analytical tests. Critical factors to consider when designing surveys can be found in Supplementary Text S4 (online only; see this file for Refs. 136 and 137).
Table 2.
Potential biological indicators included in public health population-based surveys to assess the etiology of anemia
| Method implications |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Health status assessment | Indicator | Collection source: A, V, C, S, U, H, and W | Matrix: whole blood, serum, plasma, stool, and urine | Blood volume for analysis (μL) | Optimal sample processing time: 1. 1–2 h 2. Same day 3. Within 3–4 days (stored cold | Analysis setting (CL or FS) | Method | Needs electricity (Y/N) | Storage of blood matrix until analyzed: (R or F) | Optimal operating/storage conditions and equipment* (°C) | Equipment cost (USD) | Analysis cost/test (USD) |
| Anemia | Hemoglobin | A, V, and C | Whole blood | 200 | 2 | CL | Automated hematology analyzer* | Y | R | RT | $ $ $ $ $ | $ $ |
| 10 | 1 and 2 | CL/FS | Portable invasive POC analyzers | N | R | 15–30 (Hb-201+); 10–40 (Hb-301) | $ $ $ | $ | ||||
| NA | 1 and 2 | CL/FS | Portable noninvasive POC analyzers | N | R | RT | $ $ $ | $ | ||||
| 30 | 1 and 2 | CL/FS | WHO Colour Scale | N | R | RT | $ $ $ | $ | ||||
| 10 | 1 and 2 | CL | Cyanmethemoglobin method | Y | R | RT | $ $ $ | $ | ||||
| 20 | 1 and 2 | CL | Paper-based analytical device | Y | R | RT | $ $ $ | $ | ||||
| 5 | 1 and 2 | CL/FS | Color-based method | N | R | RT | $ $ $ | $ | ||||
| Malaria | Plasmodium species | A, V, and C | Whole blood | 5 | 1 and 2 | CL/FS | RTK | N | R | 10–40 | $ | $ |
| 50 | 1 and 2 | CL/FS | Microscopy* | N | R | RT | $ $ $ | $ | ||||
| Serum | 10 | 1 and 2 | CL | Indirect fluorescent antibody | Y | R | RT | $ $ $ $ | $ | |||
| Serum | 10 | 1 and 2 | CL | ELISA | Y | RT | $ $ $ $ | $ | ||||
| Gastric wellness | Helicobacter pylori | A, V, and C | Whole blood | 5 | 1 and 2 | CL/FS | RTK | N | R | 10–40 | $ | $ |
| S | Stool | 1 g | 1 and 2 | CL | Enzyme immunoassay | Y | R | R | $ $ $ $ | $ | ||
| Vitamin A status | Retinol | A, V, and C | Serum, plasma | 25–250 | 1 and 2 | CL | HPLC* | Y | F | RT | $ $ $ $ $ | $ $ |
| Retinol-binding protein (RBP) | 50 | 1 and 2 | CL | ELISA | Y | F | RT | $ $ $ $ | $ | |||
| Modified relative dose response (MRDR) | 250 | 1 and 2 | CL | HPLC* | Y | F | RT | $ $ $ $ $ | $ $ | |||
| Iron status | Ferritin | A, V, and C | Serum, plasma | 250 | 1 and 2 | CL | Clinical analyzer* | Y | F | RT | $ $ $ $ $ | $ $ |
| 50 | ELISA | Y | F | RT | $ $ $ $ | $ | ||||||
| Soluble transferrin receptor | A, V, and C | Serum, plasma | 250 | 1 and 2 | CL | Clinical analyzer* | Y | F | RT | $ $ $ $ $ | $ $ | |
| 50 | ELISA | Y | F | RT | $ $ $ $ | $ | ||||||
| Iodine status | Urinary iodine | U | Urine | 500 | 1, 2, and 3 | CL | Ammonium persulfate* | Y | F | RT | $ $ $ $ | $ |
| Zinc status | Zinc | A and V | Serum | 500 | 1 and 2 | CL | Atomic absorption | Y | F | RT | $ $ $ $ | $ $ |
| ICP-MS | RT | $ $ $ $ $ | $ $ | |||||||||
| Inflammation status | C-reactive protein | A, V, and C | Serum, plasma | 250 | 1 and 2 | CL | Clinical analyzer* | Y | F | RT | $ $ $ $ $ | $ $ |
| 50 | ELISA | Y | F | RT | $ $ $ $ | $ | ||||||
| Alpha-1-acid glycoprotein | A, V, and C | Serum, plasma | 250 | 1 and 2 | CL | Clinical analyzer* | Y | F | RT | $ $ $ $ $ | $ $ | |
| 50 | ELISA | Y | F | RT | $ $ $ $ | $ | ||||||
| Folate status | Red blood cell folate | A, V, and C | Whole blood | 100 | 1 and 2 | CL | Folate microbiological assay* | Y | F | RT | $ $ $ | $ $ |
| Serum folate | A, V, and C | Serum | 100 | 1 and 2 | CL | Folate microbiological assay* | Y | F | RT | $ $ $ | $ $ | |
| Vitamin B12 status | Vitamin B12 | A and V | Serum, plasma | 150 | 1 and 2 | CL | Immunoassay* | Y | F | RT | $ $ $ $ $ | $ $ |
| Soil transmitted helminths (STHs) infections | Round worm | S | S | 2g | 1 | CL and FS | Kato-Katz via microscopy* | N | R | 10–40 | $ $ $ | $ |
| Ascaris lumbricoides | S | S | 2g | 1 | CL and FS | Kato-Katz via microscopy* | N | R | 10–40 | $ $ $ | $ | |
| Trichuris trichiura (whipworm) | S | S | 2g | 1 | CL and FS | Kato-Katz via microscopy* | N | R | 10–40 | $ $ $ | $ | |
| Overweight/obesity | Body mass index | H | NA | NA | NA | CL and FS | Adult/infant/child expandable measuring length/height board | NA | NA | RT | $ $ $ | NA |
| W | NA | NA | NA | CL and FS | Mother/child tarred scale | NA | NA | RT | $ $ $ | NA | ||
= >$2000;
= $1000–1999;
= $100–999;
= $10–99;
= <$10.
A, arterial blood; C, capillary blood; CL, clinical laboratory; ELISA, enzyme-linked immunosorbent assay; FS, field setting; F, frozen; H, height; HPLC, high-performance liquid chromatography; ICP-MS, inductively coupled plasma mass spectrometry; NA, not applicable; POC, point-of-care; RTK, rapid test kit; R, refrigerated; RT, room temperature (23–28 °C); S, stool; U, urine; V, venous blood; W, weight.
Supplies are $5 per person for small survey assessing anemia and malaria versus $75 per person for large survey assessing anemia, malaria, serum ferritin, serum soluble transferrin receptor, inflammation, serum vitamin A, serum vitamin B12, folate, serum zinc, and urinary iodine (i.e., costs include supplies and analytical costs) (see Refs. 148 and 149).
Discussion
For all settings, appropriate and high-quality methods are necessary to ensure the accuracy of Hb assessment as measurement and interpretation can vary significantly by preanalytical, analytical, and postanalytical factors. CAP and CLIA have both set evaluation criteria of ±7% to be used as requirements for analytical quality for Hb.170,171 For this analysis, the vast majority of all studies compared with the AHA as the reference were within the ±7% mean concentration bias threshold with a few exceptions. Limited studies compared CM (international reference) to the AHA, but the three comparisons that did so were within the mean variation threshold of ±7%. All comparisons examining mean Hb concentration difference by blood source were also within the ±7% mean bias threshold. Overall, these comparisons suggest that different blood sources and most methods and analyzers had acceptable performance based on a ±7% bias threshold and maybe useful for Hb assessment depending on the purpose. It is relevant to consider that the ±7% threshold may not necessarily have clinical relevance.
Few studies in our review examined the sensitivity or specificity to identify anemia, which is a primary purpose of collecting Hb in all settings. In a study among low-income young children and PW in the United States that had all comparisons falling within the ±7% mean concentration bias threshold, Boghani et al. reported sensitivity and specificity for the different data collection sites and included various analyses examining either capillary or venous blood analyzed on HemoCue models compared with venous blood analyzed on a Coulter Counter as the reference.8 The sensitivities reported for all comparisons for young children ranged from 32.8% to 60.4% and the specificities from 85.6% to 97.7%. For PW, sensitivities were 66.7% and 92.6%; and specificities were 98.1% and 96.7%, respectively. Another study among adults in South Africa examining various POC Hb meters and blood sources in both central laboratory and community clinic settings reported sensitivities ranging from 72% to 100% and specificities of 50–100%.18
Ideally, both sensitivity and specificity will be high to avoid misdiagnosis of anemia and unnecessary treatment, but high sensitivity and specificity have not been consistently demonstrated in the limited available literature comparing populations, blood sources, methods, and analyzers.8,18,170,171 Criteria for determining acceptable levels of sensitivity and specificity may vary depending on factors related to purpose, as well as cost and feasibility of follow-up testing, particularly in settings where anemia is used as a proxy for diagnosing iron deficiency anemia and iron supplementation may be automatically prescribed upon anemia diagnosis. In a context of screening for anemia in a setting where additional testing will occur among those who screen positive before prescribing treatment, attention to higher sensitivity for anemia may be useful to avoid missing those who need treatment. Higher specificity will limit mistakenly treating people who do not need treatment. However, if follow-up assessment after positive screening is not possible, then a higher balance of sensitivity and specificity may be more important to avoid unnecessary treatment, especially in settings where malaria or other infections may be risks of giving iron to those who are iron replete. Notably, low sensitivity and specificity to identify anemia may result in erroneous population-based prevalence estimates of anemia, which may have significant consequences for national policies and—particularly in low-income and middle-income countries as anemia prevalence is often the basis of national nutrition policy prioritization, evaluation, and accountability. Further, discordant anemia prevalence results in countries with two nationally representative surveys collected close in time and causes confusion and uncertainty for policymakers, donors, and other users of these data. Recent discordant national anemia prevalences resulted when different blood sources (single drops, pooled samples, and venous) and analyzer models varied in household surveys, among other differences.172 Additional research in real-world (less controlled) population-based household survey settings on Hb variability and the impact on prevalence estimates when collecting drops, pooled capillary samples, and venous samples could help improve the accuracy of field estimates of anemia.
Our review found, regardless of setting or pur-pose, that the degree of attention to preanalytical, analytical, and postanalytical factors has important implications for the quality of the measurements and interpretation. In general, it is easier to control all potential factors influencing the quality of the measurement and interpretation in more controlled settings than in less controlled field settings, but high-quality data are possible in all settings. Innovation to develop new quantitative methods and analyzers is important, and for the methods to be practical in the field, both their performance, as well as their ability to be portable and field-friendly will need to be considered.10 New quantitative methods must be able to meet the same standards and QC measures as the reference while still meeting preanalytical, analytical, and postanalytical factors often presented in field settings and population-based surveys.170,171
Last, venous and capillary blood remain the most readily collected sources for blood for clinical and field settings, and venous blood is considered the reference. AHAs are optimal analyzers for measuring Hb for clinical laboratories while still calibrating with the CM method.25 Most studies compared the invasive photometric POC analyzers developed by HemoCue to the reference, as these are frequently used to measure Hb in public health population-based surveys in field settings and other settings requiring low technological solutions that provide immediate Hb results. There were a few studies of the HemoCue Hb-301 analyzer and most studies were limited to adults and did not include PW or children less than 18 years, which is a key research gap. Noninvasive and invasive POC analyzers were considered feasible for field settings because of their portability, cost, weight, and comparability to the reference. New technology, including noninvasive POC analyzers and paper- and color-based analytical devices, provides promise for the future and research that tests these methods in field settings, among young children less than 5 years of age and among PW, would fill a gap in the field.
Supplementary Material
Table S1. Summary of excluded* studies for comparison of methods for Hb measurement
Figure S1. Differences in mean Hb of theHemoCue B-Hb compared with an automated hematology analyzer (reference*) by arterial (A), venous (V), cord, and capillary (C) blood source among infants and children and adult males (M), females (F), and pregnant women (PW).
Figure S2A. Differences in mean Hb of the other invasive point-of-care analyzers compared with HemoCue point-of-care analyzers as the reference* by venous (V) and capillary (C) blood source among children and adult males (M) and females (F).
Figure S2B. Differences in mean Hb of the noninvasive point-of-care analyzers compared with HemoCue point-of-care analyzers as a reference* by arterial (A), venous (V), and capillary (C) blood source among children and adult males (M) and females (F).
Supplementary Text S1. Universal precautions and laboratory personnel training
Supplementary Text S2. HemoCue® B-Hb comparability with the automated hematology analyzer
Supplementary Text S3. HemoCue® comparability with other point-of-care analyzers
Supplementary Text S4. Critical factors to consider when designing surveys
Acknowledgments
Disclaimer
The mention of company, brand, or product names in this document is for descriptive or comparative purposes only and does not constitute an official endorsement by the Centers for Disease Control and Prevention (CDC). The findings and conclusions in this manuscript are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Statement
This manuscript was presented at the World Health Organization (WHO) technical consultation “Use and Interpretation of Haemoglobin Concentrations for Assessing Anaemia Status in Individuals and Populations,” held in Geneva, Switzerland on November 29–30 and December 1, 2017. This paper is being published individually but will be consolidated with other manuscripts as a special issue of Annals of the New York Academy of Sciences, the coordinators of which were Drs. Maria Nieves Garcia-Casal and Sant-Rayn Pasricha. The special issue is the responsibility of the editorial staff of Annals of the New York Academy of Sciences, who delegated to the coordinators preliminary supervision of both technical conformity to the publishing requirements of Annals of the New York Academy of Sciences and general oversight of the scientific merit of each article. The workshop was supported by WHO, the Centers for Disease Control and Prevention (CDC), the United States Agency for International Development (USAID), and the Bill & Melinda Gates Foundation. The authors alone are responsible for the views expressed in this paper; they do not necessarily represent the views, decisions, or policies of the WHO. The opinions expressed in this publication are those of the authors and are not attributable to the sponsors, publisher, or editorial staff of Annals of the New York Academy of Sciences.
Footnotes
Supporting information
Additional supporting information maybe found in the online version of this article.
Competing interests
As part of their routine work duties, the authors provide technical assistance to countries in the design, training, implementation, data management, analysis, and dissemination of public health population-based surveys, including collection of Hb and anemia.
References
- 1.World Health Organization (WHO). 2008. Worldwide prevalence of anemia, 1993–2005 In WHO Global Database on Anemia. de Benoist B, McLean E, Egli I & Cogswell M, Eds. Geneva: World Health Organization; Accessed October 25, 2018 http://whqlibdoc.who.int/publications/2008/9789241596657_eng.pdf?ua=1. [Google Scholar]
- 2.McLean E, Cogswell M, Egli I, et al. 2009. Worldwide prevalence of anaemia, WHO Vitamin and Mineral Nutrition System, 1993–2005. Public Health Nutr. 12: 444–454. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization (WHO). Haemoglobin concentrations for the diagnosis of anaemia and assessment of severity. World Health Organization; 2011. Accessed October 25, 2018 http://www.who.int/vmnis/indicators/haemoglobin.pdf?ua=1. [Google Scholar]
- 4.Kassebaum NJ 2016. GBD 2013 Anemia Collaborators. The global burden of anemia. Hematol. Oncol. Clin. North Am. 30: 247–308. [DOI] [PubMed] [Google Scholar]
- 5.Balarajan Y, Ramakrishnan U, Ozaltin E, et al. 2011. Anaemia in low-income and middle-income countries. Lancet 378: 2123–2135. [DOI] [PubMed] [Google Scholar]
- 6.Bougouma EC, Tiono AB, Ouedraogo A, et al. 2012. Haemoglobin variants and Plasmodium falciparum malaria in children under five years of age living in a high and seasonal malaria transmission area of Burkina Faso. Malaria J. 11: 154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dugdale M 2001. Anemia. Obstet. Gynecol. Clin. North Am. 28: 363–381. [DOI] [PubMed] [Google Scholar]
- 8.Boghani S, Mei Z, Perry GS, et al. 2017. Accuracy of capillary hemoglobin measurements for the detection of anemia in the U.S. low-income toddlers and pregnant women. Nutrients 9: E253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chaudhary R, Dubey A & Sonker A 2017. Techniques used for blood screening of hemoglobin levels in blood donors: current insights and future directions. J. Blood Med. 8: 75–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sanchis-Gomar F, Cortell-Ballester J, Pareja-Galeano H, et al. 2013. Hemoglobin point-of-care testing: the HemoCue system. J. Lab. Autom. 18: 198–205. [DOI] [PubMed] [Google Scholar]
- 11.Whitehead RD Jr., Zhang M, Sternberg MR, et al. 2017. Effects of preanalytical factors on hemoglobin measurement: a comparison of two HemoCue® point-of-care analyzers. Clin. Biochem. 50: 513–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aziz HA 2017. Comparison between field research and controlled laboratory research. Arch. Clin. Biomed. Res. 1: 101–104. [Google Scholar]
- 13.Hanneman SK 2008. Design, analysis and interpretation of method-comparison studies. AACN Adv. Crit. Care 19: 223–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lacher DA, Barletta J & Hughes JP 2012. Biological variation of hematology tests based on the 1999–2002 National Health and Nutrition Examination Survey. Natl. Health Stat. Rep. 54: 1–10. [PubMed] [Google Scholar]
- 15.Lara AM, Kandulu J, Chisuwo L, et al. 2007. Laboratory costs of a hospital-based blood transfusion service in Malawi. J. Clin. Pathol. 60: 1117–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin LIK 1989. A concordance correlation coefficient to evaluate reproducibility. Biometrics 45: 255–268. [PubMed] [Google Scholar]
- 17.Green R & Wachsmann-Hogiu S 2015. Development, history, and future of automated cell counters. Clin. Lab. Med. 35: 1–10. [DOI] [PubMed] [Google Scholar]
- 18.Jaggernath M, Naicker R, Madurai S, et al. 2016. Diagnostic accuracy of the HemoCue Hb 301, STAT-Site MHgb and URIT-12 Point-of-Care hemoglobin meters in a central laboratory and a community based clinic in Durban, South Africa. PLoS One 11: e0152184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Monárrez-Espino J & Roos N 2008. Comparison of the analytic performance between the B-HB and HB-201+ HemoCue® hemoglobinometers for venous and capillary blood under field work conditions. Ecol. Food Nutr. 47: 159–169. [Google Scholar]
- 20.Boynton MH 1946. The use of the copper sulfate method of hemoglobin estimation for screening blood donors. TransL Res. 31: 40–44. [PubMed] [Google Scholar]
- 21.Politzer WM, Myburgh WM & Van der Merwe JF 1988. Haemoglobin estimate—reliability of the copper sulphate specific gravity v. cyanmethaemoglobin colorimetric method. S. Afr. Med. J. 73: 111–112. [PubMed] [Google Scholar]
- 22.Prakash N & Banerji HN 1972. Evaluation of cyan-methaemoglobin method for haemoglobin estimation. Indian J. Chest Dis. 14: 102–105. [PubMed] [Google Scholar]
- 23.National Committee for Clinical Laboratory Standards. 2000. H15-A3: reference and selected procedures for the quantitative determination of hemoglobin in blood. 3rd ed. [Google Scholar]
- 24.Lewis SM, Stott GJ & Wynn KJ 1998. An inexpensive and reliable new haemoglobin colour scale for assessing anaemia. J. Clin. Pathol. 51: 21–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang X, Piety N, Vignes SM, et al. 2013. Simple paper-based test for measuring blood hemoglobin concentration in resource-limited settings. Clin. Chem. 59: 1506–1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Elwood PC & Jacobs A 1966. Haemoglobin estimation: a comparison of different techniques. Br. Med. J. 1: 20–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Al-Hashem FH 2006. Pattern of haemoglobin among high and low altitude children of southwestern Saudi Arabia. J. Family Community Med. 13: 35–40. [PMC free article] [PubMed] [Google Scholar]
- 28.World Health Organization (WHO). Global nutrition targets 2025: policy briefseries. Geneva: World Health Organization; 2014. Accessed October 25, 2018 http://apps.who.int/iris/bitstream/10665/149018/1/WHO_NMH_NHD_14.2_eng.pdftua=1. [Google Scholar]
- 29.Phillips RA, Van Slyke DD, Hamilton PB, et al. 1950. Measurement of specific gravities of whole blood and plasma by standard copper sulfate solutions. J. Biol. Chem. 183: 305–330. [Google Scholar]
- 30.Lewis SM 2002. Laboratory practice at the periphery in developing countries. Int. J. Hematol. 76(Suppl. 1): 294–298. [DOI] [PubMed] [Google Scholar]
- 31.Srivastava T, Negandhi H, Neogi SB, et al. 2014. Methods for hemoglobin estimation: a review of “what works.” J. Hematol. Transfus. 2: 1028. [Google Scholar]
- 32.Gulati GL & Hyun BH 1986. Quality control in hematology. Clin. Lab. Med. 6: 675–688. [PubMed] [Google Scholar]
- 33.Mohandas N, Kim Y, Tycko D, et al. 1986. Accurate and independent measurement of volume and hemoglobin concentration of individual red cells by laser light scattering. Blood 68: 506–513. [PubMed] [Google Scholar]
- 34.World Health Organization (WHO). Review of the haemoglobin colour scale. World Health Organization; 2004. Accessed October 25, 2018 http://apps.who.int/iris/bitstream/10665/68734/2/WHO_EHT_04.12.pdf. [Google Scholar]
- 35.Darshana LG & Uluwaduge DI 2014. Validation of the WHO Hemoglobin Color Scale method. Anemia 2014. 10.1155/2014/531670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marn H & Critchley JA 2016. Accuracy of the WHO Haemoglobin Colour Scale for the diagnosis of anaemia in primary health care settings in low-income countries: a systematic review and meta-analysis. Lancet Glob. Health 4: e251–e265. [DOI] [PubMed] [Google Scholar]
- 37.Gong AK & Backenstose B 1999. Evaluation of the HB-Quick: a portable hemoglobinometer. J. Clin. Monit. Com-put. 15: 171–177. [DOI] [PubMed] [Google Scholar]
- 38.HemoCue America. Hb-201+ Product Sheet. Accessed October 25, 2018 http://www.hemocue.us/~/media/hemocue-images/hemocue_us-images/pdf/hemoglobin-lit1056-hb-201product-sheet.pdf?la=en-US.
- 39.HemoCue America. Hb-301 Product Sheet. Accessed October 25, 2018 http://www.hemocue.us/~/media/hemocue-images/hemocue_us-images/pdf/hemoglobin-lit6052-hb-301-productprofile.pdf?la=en-US
- 40.Al-Khabori M, Al-Riyami AZ, Al-Farsi K, et al. 2014. Validation of a non-invasive pulse CO-oximetry based hemoglobin estimation in normal blood donors. Transfus. Apher. Sci. 50: 95–98. [DOI] [PubMed] [Google Scholar]
- 41.Amano I & Murakami A 2013. Use of non-invasive total hemoglobin measurement as a screening tool for anemia in children. Pediatr. Int. 55: 803–835. [DOI] [PubMed] [Google Scholar]
- 42.Ardin S, Störmer M, Radojska S, et al. 2015. Comparison of three noninvasive methods for hemoglobin screening of blood donors. Transfusion 55: 379–387. [DOI] [PubMed] [Google Scholar]
- 43.Broderick AJ, Desmond F, Leen G & Shorten G 2015. Clinical evaluation of a novel technology for non-invasive and continuous measurement of plasma haemoglobin concentration. Anaesthesia 70: 1165–1170. [DOI] [PubMed] [Google Scholar]
- 44.Neogi SB, Negandhi H, Kar R, et al. 2016. Diagnostic accuracy of haemoglobin colour strip (HCS-HLL), a digital haemoglobinometer (TrueHb) and a non-invasive device (TouchHb) for screening patients with anaemia. J. Clin. Pathol. 69: 164–170. [DOI] [PubMed] [Google Scholar]
- 45.Biosense. ToucHb. Accessed October 25, 2018 http://www.biosense.in/touchb.html.
- 46.Masimo. About Masimo. Accessed October 25, 2018 http://www.masimo.com/About-Masimo/about-masimo/.
- 47.Tyburski EA, Gillespie SE, Stoy WA, et al. 2014. Disposable platform provides visual and color-based point-of-care anemia self-testing. J. Clin. Invest. 124: 4387–4394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Avoy DR, Canuel ML, Otton BM & Mileski EB 1977. Hemoglobin screening in prospective blood donors: a comparison of methods. Transfusion 17: 261–264. [DOI] [PubMed] [Google Scholar]
- 49.Henderson AS 1953. The ear lobe as a source of blood in haemoglobin estimation. J. Physiol. 121: 43P–44P. [PubMed] [Google Scholar]
- 50.Lucy HC 1950. Fortuitous factors affecting the leucocyte count in blood from the ear. J. Clin. Pathol. 3: 146–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen PP, Short TG, Leung DHY & Oh TE 1992. A clinical evaluation of the HemoCue haemoglobinometer using capillary, venous, and arterial samples. Anaesth. Intensive Care 20: 497–503. [DOI] [PubMed] [Google Scholar]
- 52.Yang ZW, Yang SH, Chen L, et al. 2001. Comparison of blood counts in venous, fingertip and arterial blood and their measurement variation. Clin. Lab. Haematol. 23:155–159. [DOI] [PubMed] [Google Scholar]
- 53.Gutierrez G 1986. The rate of oxygen release and its effect on capillary O2 tension: a mathematical analysis. Respir. Physiol. 63: 79–96. [DOI] [PubMed] [Google Scholar]
- 54.Patel AJ, Wesley R, Leitman SF & Bryant BJ 2013. Capillary versus venous haemoglobin determination in the assessment of healthy blood donors. Vox Sang. 104: 317–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zavorsky GS, Cao J, Mayo NE, et al. 2007. Arterial versus capillary blood gases: a meta-analysis. Respir. Physiol. Neurobiol. 155: 268–279. [DOI] [PubMed] [Google Scholar]
- 56.Neufeld L, García-Guerra A, Sánchez-Francia D, et al. 2002. Hemoglobin measured by Hemocue and a reference method in venous and capillary blood: a validation study. Salud Publica Mex. 44: 219–227. [DOI] [PubMed] [Google Scholar]
- 57.Daae LNW, Halvorsen S, Mathisen PM & Mironska K 1988. A comparison between haematological parameters in ‘capillary’ and venous blood from healthy adults. Scand. J. Clin. Lab. Invest. 48: 723–726. [DOI] [PubMed] [Google Scholar]
- 58.Chambers LA & McGuff JM 1989. Evaluation of methods and protocols for hemoglobin screening of prospective whole blood donors. Am. J. Clin. Pathol. 91: 309–312. [DOI] [PubMed] [Google Scholar]
- 59.Daae LNW, Hallerud M & Halvorsen S 1991. A comparison between haematological parameters in ‘capillary’ and venous blood from hospitalized children aged 3 months to 14 years. Scand. J. Clin. Lab. Invest. 51: 651–654. [DOI] [PubMed] [Google Scholar]
- 60.Paiva Ade A, Rondo PH, Silva SS & Latorre Mdo R 2004. Comparison between the HemoCue and an automated counter for measuring hemoglobin. Rev. Saude Publica 38: 585–587. [DOI] [PubMed] [Google Scholar]
- 61.Ziemann M, Lizardo B, Geusendam G & Schlenke P 2011. Reliability of capillary hemoglobin screening under routine conditions. Transfusion 51: 2714–2719. [DOI] [PubMed] [Google Scholar]
- 62.Eslami Z, Ghilian R & Abbasi F 2012. Evaluation of hemoglobin concentration of cord, capillary and venous sampling in neonates. Iran J. Ped. Hematol. Oncol. 2: 159–163. [PMC free article] [PubMed] [Google Scholar]
- 63.Bond MM &Richards-Kortum RR 2015. Drop-to-drop variation in the cellular components of fingerprick blood. Am. J. Clin. Pathol. 144: 885–894. [DOI] [PubMed] [Google Scholar]
- 64.Pawlowski M, Latute F, Bardou-Jacquet E, et al. 2015. Portable hemoglobinometer is a reliable technology for the follow-up of venesections tolerance in hemochromatosis. Clin. Res. Hepatol. Gassroenterol. 39: 570–575. [DOI] [PubMed] [Google Scholar]
- 65.Karakochuk CD, Rappaport AI, Barr SI & Green TJ 2017. Mean hemoglobin concentrations in fasting venous and non-fasting capillary blood of Cambodian women using a hemoglobinometer and an automated hematology analyzer. Clin. Chem. Lab. Med. 95: e247–e250. [DOI] [PubMed] [Google Scholar]
- 66.Conway AM, Hinchiffe RF, Earland J & Anderson LM 1998. Measurement of haemoglobin using single drops of skin puncture blood: is precision acceptable? J. Clin. Pathol. 51: 248–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lima-Oliveira G, Guidi GC, Salvagno GL, et al. 2017. Patient posture for blood collection by venipuncture: recall for standardization after 28 years. Rev. Bras. Hematol. Hemoter. 39: 127–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Drammeh BS, Schleicher RL, Pfeiffer CM, et al. 2008. Effects of delayed sample processing and freezing on serum concentrations of selected nutritional indicators. Clin. Chem. 54: 1883–1891. [DOI] [PubMed] [Google Scholar]
- 69.Burger S & Pierre-Louis J 2002. A Procedure to Estimate the Accuracy and Reliability of HemoCue® Measurements of Survey Workers. New York, NY: Helen Keller International. [Google Scholar]
- 70.Morris LD, Pont A & Lewis SM 2001. Use of a new HemoCue system for measuring haemoglobin at low concentrations. Clin. Lab. Haematol. 23: 91–96. [DOI] [PubMed] [Google Scholar]
- 71.Nguyen HT 2002. High humidity affects HemoCue cuvette function and HemoCue haemoglobin estimation in tropical Australia. J. Paediatr. Child Health 38: 427–428. [DOI] [PubMed] [Google Scholar]
- 72.Nicholls PD 1990. An evaluation of the HemoCue for correcting the haemoglobin value of lipaemic samples. Med. Lab. Sci. 47: 226–229. [PubMed] [Google Scholar]
- 73.Henderson MA & Irwin MG 1995. High humidity affects HemoCue microcuvette function. Anaesth. Intensive Care 23: 407. [PubMed] [Google Scholar]
- 74.Lewis SM & Emmanuel J 2001. Validity of the haemoglobin colour scale in blood donor screening. Vox Sang. 80: 28–33. [DOI] [PubMed] [Google Scholar]
- 75.Centers for Disease Control and Prevention (CDC). 2016. Bloodborne infectious diseases: HIV/AIDS, hepatitis B, hepatitis C. Accessed October 25, 2018 https://www.cdc.gov/niosh/topics/bbp/universal.html.
- 76.Hinchliffe RF & Anderson LM 1996. Haemoglobin values in venous and skin puncture blood. Arch. Dis. Child. 75: 170–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pistorius LR, Funk M, Pattinson RC & Howarth GR 1996. Screening for anemia in pregnancy with copper sul¬fate densitometry. Int. J. Gynaecol. Obstet. 52: 33–36. [DOI] [PubMed] [Google Scholar]
- 78.Funk M, Hambrock T, van Niekerk GC & Witten¬berg DF 2002. Screening for childhood anaemia using copper sulphate densitometry. S. Afr. Med. J. 92: 978–982. [PubMed] [Google Scholar]
- 79.Tondon R, Verma A, Pandey P & Chaudhary R 2009. Quality evaluation of four hemoglobin screening methods in a blood donor setting along with their comparative cost analysis in an Indian scenario. Asian J. Transfus. Sci. 3: 66–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Antwi-Baffour S, Annor DK, Adjei JK, et al. 2015. Anemia in prospective blood donors deferred by the copper sulphate technique of hemoglobin estimation. BMC Hema-tol. 15: 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tatsumi N, Bunyaratvej A, Timan IS, et al. 1990. Field evaluation of who hemoglobin color scale in West Java. Southeast Asian J. Trop. Med. Public Health 30(Suppl. 3): 177–181. [PubMed] [Google Scholar]
- 82.van den Broek NR, Ntonya C, Mhango E & White SA 1999. Diagnosing anaemia in pregnancy in rural clinics: assessing the potential of the Haemoglobin Colour Scale. Bull. World Health Organ. 77: 15–21. [PMC free article] [PubMed] [Google Scholar]
- 83.Ingram CF & Lewis SM 2000. Clinical use of WHO haemoglobin colour scale: validation and critique. J. Clin. Pathol. 53: 933–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sawant RB, Bharucha ZS & Rajadhyaksha SB 2007. Evaluation of hemoglobin of blood donors deferred by the copper sulphate method for hemoglobin estimation. Transfus. Apher. Sci. 36: 143–148. [DOI] [PubMed] [Google Scholar]
- 85.Khan AA, Fatmi Z & Kadir MM 2015. Accuracy and use of WHO Hemoglobin Color Scale for diagnosis of anemia among pregnant women by health care providers in periurban settings in Karachi, Pakistan. Asia Pac. J. Public Health 27: 610–619. [DOI] [PubMed] [Google Scholar]
- 86.McGann PT, Tyburski EA, de Oliveira V, et al. 2015. An accurate and inexpensive color-based assay for detecting severe anemia in a limited-resource setting. Am. J. Hematol. 90: 1122–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tong E, Murphy WG, Kinsella A, et al. 2010. Capillary and venous haemoglobin levels in blood donors: a 42-month study of 36,258 paired samples. Vox Sang. 98: 547–553. [DOI] [PubMed] [Google Scholar]
- 88.Cohen AR & Seidl-Friedman J 1988. HemoCue system for hemoglobin measurement. Evaluation in anemic and nonanemic children. Am. J. Clin. Pathol. 90: 302–325. [DOI] [PubMed] [Google Scholar]
- 89.Mills AF & Meadows N 1989. Screening for anaemia: evaluation of a haemoglobinometer. Arch. Dis. Child. 64: 1468–1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Boulton FE, Nightingale MJ & Reynolds W 1994. Improved strategy for screening prospective blood donors for anaemia. Transfus. Med. 4: 221–225. [DOI] [PubMed] [Google Scholar]
- 91.Hudson-Thomas M, Bingham KC & Simmons WK 1994. An evaluation of the HemoCue for measuring haemoglobin in field studies in Jamaica. Bull. World Health Organ. 72: 423–436. [PMC free article] [PubMed] [Google Scholar]
- 92.Jaeger M, Ashbury T, Adams M & Duncan P 1996. Perioperative on-site haemoglobin determination: as accurate as laboratory values? Can. J. Anaesth. 43: 795–798. [DOI] [PubMed] [Google Scholar]
- 93.Krenzischek DA & Tanseco FV 1996. Comparative study of bedside and laboratory measurements of hemoglobin. Am. J. Crit. Care 5: 427–432. [PubMed] [Google Scholar]
- 94.Rechner IJ, Twigg A, Davies AF & Imong S 2002. Evaluation of the HemoCue compared with the Coulter STKS for measurement of neonatal haemoglobin. Arch. Dis. Child. Fetal Neonatal Ed. 86: F188–F189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Muñoz M, Romero A, Gómez JF, et al. 2005. Utility of point-of-care haemoglobin measurement in the HemoCue-B haemoglobin for the initial diagnosis of anaemia. Clin. Lab. Haematol. 27: 99–104. [DOI] [PubMed] [Google Scholar]
- 96.Van de Louw A, Lasserre N, Drouhin F, et al. 2007. Reliability of HemoCue in patients with gastrointestinal bleeding. Intensive Care Med. 33: 355–358. [DOI] [PubMed] [Google Scholar]
- 97.Zhou X, Yan H, Xing Y, et al. 2009. Evaluation of a portable hemoglobin photometer in pregnant women in a high altitude area: a pilot study. BMC Public Health 9: 228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mariño Z, Carrión JA, Bedini JL, et al. 2011. Evaluation of a portable hemoglobinometer (HemoCue) to control anemia in hepatitis C liver transplant recipients undergoing antiviral therapy. Eur. J. Gastroenterol. Hepatol. 23: 942–927. [DOI] [PubMed] [Google Scholar]
- 99.Nkrumah B, Nguah SB, Sarpong N, et al. 2011. Hemoglobin estimation by the HemoCue® portable hemoglobin photometer in a resource poor setting. BMC Clin. Pathol. 11: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Adam I, Ahmed S, Mahmoud MH & Yassin MI 2012. Comparison of HemoCue® hemoglobin-meter and automated hematology analyzer in measurement of hemoglobin levels in pregnant women at Khartoum hospital, Sudan. Diagn. Pathol. 7: 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kim MJ, Park Q, Kim MH, et al. 2013. Comparison of the accuracy of noninvasive hemoglobin sensor (NBM-200) and portable hemoglobinometer (HemoCue) with an automated hematology analyzer (LH500) in blood donor screening. Ann. Lab. Med. 33: 261–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Rudolf-Oliveira RC, Gonsalves KT, Martignago ML, et al. 2013. Comparison between two portable hemoglobin-nometers and a reference method to verify the reliability of screening in blood donors. Transfus. Apher. Sci. 49: 578–582. [DOI] [PubMed] [Google Scholar]
- 103.Shahshahani HJ, Meraat N & Mansouri F 2013. Evaluation of the validity of a rapid method for measuring high and low haemoglobin levels in whole blood donors. Blood Transfus. 11: 385–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Skelton VA, Wijayasinghe N & Sharafudeen S 2013. Evaluation of point-of-care haemoglobin measuring devices: a comparison of Radical-7TM pulse co-oximetry, HemoCue(®) and laboratory haemoglobin measurements in obstetric patients. Anaesthesia 68: 40–45. [DOI] [PubMed] [Google Scholar]
- 105.Shah PP, Desai SA, Modi DK & Shah SP 2014. Assessing diagnostic accuracy of Haemoglobin Colour Scale in real-life setting. J. Health Popul. Nutr. 32: 51–57. [PMC free article] [PubMed] [Google Scholar]
- 106.Zatloukal J, Pouska J, Kletecka J, et al. 2016. Comparison of the accuracy of hemoglobin point of care testing using HemoCue and GEM Premier 3000 with automated hematology analyzer in emergency room. J. Clin. Monit. Comput. 30:949–956. [DOI] [PubMed] [Google Scholar]
- 107.Giraud B, Frasca D, Debaene B & Mimoz O 2013;. Comparison of haemoglobin measurement methods in the operating theatre. Br. J. Anaesth. 111: 946–954. [DOI] [PubMed] [Google Scholar]
- 108.Mendrone A Jr., Sabino EC, Sampaio L, et al. 2009. Anemia screening in potential female blood donors: comparison of two different quantitative methods. Transfusion 49: 662–668. [DOI] [PubMed] [Google Scholar]
- 109.Lamhaut L, Apriotesei R, Combes X, et al. 2011. Comparison of the accuracy of noninvasive hemoglobin monitoring by spectrophotometry (SpHb) and HemoCue’® with automated laboratory hemoglobin measurement. Anesthesiology 115: 548–554. [DOI] [PubMed] [Google Scholar]
- 110.Goldman M, Uzicanin S, Yi QL, et al. 2012. Validation and implementation of a new hemoglobinometer for donor screening at Canadian Blood Services. Transfusion 52: 1607–1613. [DOI] [PubMed] [Google Scholar]
- 111.Mimoz O, Frasca D, Médard A, et al. 2011. Reliability of the HemoCue® hemoglobinometer in critically ill patients: a prospective observational study. Minerva Anestesiol. 77: 979–985. [PubMed] [Google Scholar]
- 112.Belardinelli A, Benni M, Tazzari PL & Pagliaro P 2011. Noninvasive methods for haemoglobin screening in prospective blood donors. Vox Sang. 105: 116–120. [DOI] [PubMed] [Google Scholar]
- 113.Baart AM, de Kort WL, van den Hurk K & Pasker-de Jong PC 2016. Hemoglobin assessment: precision and practicability evaluated in the Netherlands—the HAPPEN study. Transfusion 56: 1984–1993. [DOI] [PubMed] [Google Scholar]
- 114.Karakochuk CD, Janmohamed A, Whitfield KC, et al. 2015. Evaluation of two methods to measure hemoglobin concentration among women with genetic hemoglobin disorders in Cambodia: a method-comparison study. Clin. Chim. Acta 441: 148–155. [DOI] [PubMed] [Google Scholar]
- 115.Seguin P, Kleiber A, Chanavaz C, et al. 2011. Determination of capillary hemoglobin levels using the HemoCue system in intensive care patients. J. Crit. Care 26: 423–427. [DOI] [PubMed] [Google Scholar]
- 116.Frasca D, Dahyot-Fizelier C, Catherine K, et al. 2011. Accuracy of a continuous noninvasive hemoglobin monitor in intensive care unit patients. Crit. CareMed. 39:2277–2282. [DOI] [PubMed] [Google Scholar]
- 117.Sümnig A, Hron G, Westphal A, et al. 2015. The impact of noninvasive, capillary, and venous hemoglobin screening on donor deferrals and the hemoglobin content of red blood cells concentrates: a prospective study. Transfusion 55:2847–2854. [DOI] [PubMed] [Google Scholar]
- 118.Daves M, Cemin R, Zagler EM, et al. 2016. Evaluation of capillary haemoglobin determination for anaemia screening in blood donation settings. Blood Transfus. 14: 387–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hinnoucho GM,Barffour MA, Wessells KR, et al. 2018. Comparison of haemoglobin assessments by HemoCue and two automated hematology analyzers in young Laotian children. J. Clin. Pathol. 71: 532–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.McNulty SE, Torjman M, Grodecki W, et al. 1995. A comparison of four bedside methods of hemoglobin assessment during cardiac surgery. Anesth. Analg. 81: 1197–1202. [DOI] [PubMed] [Google Scholar]
- 121.Singh A, Dubey A, Sonker A & Chaudhary R 2015. Evaluation of various methods of point-of-care testing of haemoglobin concentration in blood donors. Blood Transfus. 13: 233–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Srivastava A, Koul V, Dwivedi SN, et al. 2015. Per-formance analysis of newly developed point-of-care hemoglobinometer (TrueHb) against an automated hematology analyzer (Sysmex XT 1800i) in terms of precision in hemoglobin measurement. Int. J. Lab. Hematol. 37: 483–485. [DOI] [PubMed] [Google Scholar]
- 123.Awada WN, Mohmoued MF, Radwan TM, et al. 2015. Continuous and noninvasive hemoglobin monitoring reduces red blood cell transfusion during neurosurgery: a prospective cohort study. J. Clin. Monit. Comput. 29: 733–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Park SG, Lee OH, Park YH, et al. 2015. The changes of non-invasive hemoglobin and perfusion index of Pulse CO-Oximetry during induction of general anesthesia. Korean J. Anesthesiol. 68: 352–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.von Schweinitz BA, De Lorenzo RA, Cuenca PJ, et al. 2015. Does a non-invasive hemoglobin monitor correlate with a venous blood sample in the acutely ill? Intern. Emerg. Med. 10: 55–61. [DOI] [PubMed] [Google Scholar]
- 126.Erdogan Kayhan G, Colak YZ, Sanli M, et al. 2017. Accuracy of non-invasive hemoglobin monitoring by pulse CO-oximeter during liver transplantation. Minerva Anestesiol. 83: 485–492. [DOI] [PubMed] [Google Scholar]
- 127.Gamal M, Abdelhamid B, Zakaria D, et al. 2018. Evaluation of non-invasive hemoglobin monitoring in trauma patients with low hemoglobin levels. Shock 49: 150–153. [DOI] [PubMed] [Google Scholar]
- 128.Bruells CS, Menon AK, Rossaint R, et al. 2013. Accuracy of the Masimo Pronto-7® system in patients with left ventricular assist device. J. Cardiothorac. Surg. 8: 159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Gayat E, Aulagnier J, Matthieu E, et al. 2012. Noninvasive measurement of hemoglobin: assessment of two different point-of-care technologies. PLoS One 7: e30065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Khalafallah AA, Chilvers CR, Thomas M, et al. 2015. Usefulness of non-invasive spectrophotometric haemoglobin estimation for detecting low haemoglobin levels when compared with a standard laboratory assay for preoperative assessment. Br. J. Anaesth. 144: 669–676. [DOI] [PubMed] [Google Scholar]
- 131.Vyas KJ, Danz D, Gilman RH, et al. 2015. Noninvasive assessment of excessive erythrocytosis as a screening method for chronic mountain sickness at high altitude. High Alt. Med. Biol. 16: 162–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Foster KE, Sahay RD, Zhang N & Hardie WD 2017. Results of a prospective study evaluating a noninvasive method of hemoglobin adjustment for determining the diffusing capacity of the lung. Ann. Am. Thorac. Soc. 14: 41–48. [DOI] [PubMed] [Google Scholar]
- 133.Shamah Levy T, Méndez-Gómez-Humarán I, Morales Ruán MD, et al. 2017. Validation of Masimo Pronto 7 and HemoCue 201 for hemoglobin determination in children from 1to5 years of age. PLoS One 12: e0170990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Rabe H, Alvarez RF, Whitfield T, et al. 2010. Spectroscopic noninvasive measurement of hemoglobin compared with capillary and venous values in neonates. Neonatology 98: 1–5. [DOI] [PubMed] [Google Scholar]
- 135.Pasricha SR 2014. Anaemia: a comprehensive global estimate. Blood 123: 611–612. [DOI] [PubMed] [Google Scholar]
- 136.Suchdev PS, Namaste SM, Aaron GJ, et al. 2016. Overview of the Biomarkers Reflecting Inflammation and Nutritional Determinants of Anaemia (BRINDA) Project. Adv. Nutr. 7: 349–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Erhardt JG, Estes JE, Pfeiffer CM, et al. 2004. Combined measurement of ferritin, soluble transferrin receptor, retinol binding protein, and C-reactive protein by an inexpensive, sensitive, and simple sandwich enzyme-linked immunosorbent assay technique. J. Nutr. 134:3127–3132. [DOI] [PubMed] [Google Scholar]
- 138.Spielmann N, Mauch J, Madjdpour C, et al. 2012. Accuracy and precision of hemoglobin point-of-care testing during major pediatric surgery. Int. J. Lab. Hematol. 34: 86–90. [DOI] [PubMed] [Google Scholar]
- 139.Shah N, Osea EA & Martinez GJ 2014. Accuracy of noninvasive hemoglobin and invasive point-of-care hemoglobin testing compared with a laboratory analyzer. Int. J. Lab. Hematol. 36: 56–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Morris SS, Ruel MT, Cohen RJ, et al. 1999. Precision, accuracy, and reliabilityofhemoglobin assessmentwith use of capillaryblood. Am. J. Clin. Nutr. 69: 1243–1248. [DOI] [PubMed] [Google Scholar]
- 141.Laifer SA,Kuller JA& Hill LM 1990Rapidassessment of fetal hemoglobin concentration with the HemoCue system. Obstet. Gynecol. 76: 723–724. [PubMed] [Google Scholar]
- 142.Berry SM, Dombrowski MP, Blessed WB, et al. 1993. Fetal hemoglobin quantitations using the Hemocue system are rapid and accurate. Obstet. Gynecol. 81: 417–420. [PubMed] [Google Scholar]
- 143.Herzog B & Felton B 1994. Hemoglobin screening for normal newborns. J. Perinatol. 14: 285–259. [PubMed] [Google Scholar]
- 144.Lardi AM, Hirst C, Mortimer AJ & McCollum CN 1998. Evaluation of the HemoCue for measuring intraoperative haemoglobin concentrations: a comparison with the Coulter Max-M. Anaesthesia 53: 349–352. [DOI] [PubMed] [Google Scholar]
- 145.Prakash S, Kapil U, Singh G, et al. 1999. Utility of HemoCue in estimation of hemoglobin against standard blood cell counter method. J. Assoc. Physicians India 47: 995–997. [PubMed] [Google Scholar]
- 146.Morris LD, Osei-Bimpong A, McKeown D, et al. 2007. Evaluation of the utility of the HemoCue 301 haemoglobi-nometer for blood donor screening. Vox Sang. 93: 64–69. [DOI] [PubMed] [Google Scholar]
- 147.Gwetu TP & Chhagan MK 2015. Evaluation of the diagnostic accuracy of the HemoCue device for detecting anaemia in healthy school-aged children in KwaZulu-Natal, South Africa. S. Afr. Med. J. 105: 596–599. [DOI] [PubMed] [Google Scholar]
- 148.Centers for Disease Control and Prevention (CDC). Micronutrient survey manual. 2017. Accessed October 25, 2018 http://surveytoolkit.micronutrient.org/.
- 149.Centers for Disease Control and Prevention (CDC). Micronutrient survey toolkit. 2016. Accessed October 25, 2018 http://surveytoolkit.micronutrient.org/.
- 150.von Schenck H, Falkensson M & Lundberg B 1986. Evaluation of “HemoCue,” a new device for determining hemoglobin. Clin. Chem. 32: 526–529. [PubMed] [Google Scholar]
- 151.Kwant G, Oeseburg B, Zwart A & Zijlstra WG 1987. Calibration of a practical haemoglobinometer. Clin. Lab. Haematol. 9: 387–393. [DOI] [PubMed] [Google Scholar]
- 152.Be WK, Kerkkamp HE & Booij LH 1991. Hemocue-a new haemoglobinometer in the clinic. Eur. J. Anaesthesiol. 8: 55–58. [PubMed] [Google Scholar]
- 153.Russell BL & Martin SM 1997. Blood donor rejection rate: hemoCue hemoglobin analyzer vs. microhematocrit. Clin. Lab. Sci. 10: 321–324. [PubMed] [Google Scholar]
- 154.Montresor A, Albonico M, Khalfan N, et al. 2000. Field trial of a haemoglobin colour scale: an effective tool to detect anaemia in preschool children. Trop. Med. Int. Health 5: 129–133. [DOI] [PubMed] [Google Scholar]
- 155.Cardigan R & Smith K 2002. Evaluation of the HemoCue plasma haemoglobin analyser for assessing haemolysis in red cell concentrates during storage. Vox Sang. 82: 76–79. [DOI] [PubMed] [Google Scholar]
- 156.Chowdhury ME, Chongsuvivatwong V, Geater AF, et al. 2002. Taking a medical history and using a colour scale during clinical examination of pallor improves detection of anaemia. Trop. Med. Int. Health 7: 133–139. [DOI] [PubMed] [Google Scholar]
- 157.Kapoor SK, Kapil U, Dwivedi SN, et al. 2002. Comparison of HemoCue method with cyanmethemoglobin method for estimation of hemoglobin. Indian Pediatr. 39: 743–746. [PubMed] [Google Scholar]
- 158.Paddle JJ 2002. Evaluation of the Haemoglobin Colour Scale and comparison with the HemoCue haemoglobin assay. Bull. World Health Organ. 80: 813–816. [PMC free article] [PubMed] [Google Scholar]
- 159.Bhaskaram P, Balakrishna N, Radhakrishna KV & Krishnaswamy K 2003. Validation of hemoglobin estimation using Hemocue. Indian J. Pediatr. 70: 25–28. [DOI] [PubMed] [Google Scholar]
- 160.Montresor A, Ramsan M, Khalfan N, et al. 2003. Performance of the Haemoglobin Colour Scale in diagnosing severe and very severe anaemia. Trop. Med. Int. Health 8: 619–624. [DOI] [PubMed] [Google Scholar]
- 161.Abrahams M, Ram D, Das S & Britt RP 2005. Use of the WHO hemoglobin color scale in family welfare clinics in India. Southeast Asian J. Trop. Med. Public Health 36: 976–978. [PubMed] [Google Scholar]
- 162.Lindblade KA, Mwololo K, van Eijk AM, et al. 2006. Evaluation of the WHO Haemoglobin Colour Scale for diagnosis of anaemia in children and pregnant women as used by primary health care nurses and community health workers in western Kenya. Trop. Med. Int. Health 11: 1679–1687. [DOI] [PubMed] [Google Scholar]
- 163.Akhtar K, Sherwani RK, Rahman K, et al. 2008. HemoCue photometer: a better alternative of hemoglobin estimation in blood donors? Indian J. Hematol. Blood Transfus. 24: 170–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Sinha N, Deshmukh PR & Garg BS 2008. Evaluation of WHO haemoglobin colour scale & palmar pallor for screening of anaemia among children (6–35 months) in rural Wardha, India. Indian J. Med. Res. 128: 278–281. [PubMed] [Google Scholar]
- 165.Aldridge C, Foster HM, Albonico M, et al. 2012. Evaluation of the diagnostic accuracy of the Haemoglobin Colour Scale to detect anaemia in young children attending primary healthcare clinics in Zanzibar. Trop. Med. Int. Health 17:423–429. [DOI] [PubMed] [Google Scholar]
- 166.M’baya B,Mbingwani I, Mgawi L, et al. 2014. Validation of the haemoglobin colour scale for screening blood donors in Malawi. Malawi Med. J. 26: 30–33. [PMC free article] [PubMed] [Google Scholar]
- 167.Bansal PG, Toteja GS, Bhatia N, et al. 2016. Comparison of haemoglobin estimates using direct & indirect cyan-methaemoglobin methods. Indian J. Med. Res. 144: 566–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Baulig W, Seifert B, Spahn DR & Theusinger OM 2017. Accuracy of non-invasive continuous total hemoglobin measurement by Pulse CO-Oximetry in severe traumatized and surgical bleeding patients. J. Clin. Monit. Comput. 31: 177–185. [DOI] [PubMed] [Google Scholar]
- 169.Sari M, de Pee S, Martini E, et al. 2001. Estimating the prevalence of anaemia: a comparison of three methods. Bull World Health Organ. 79: 506–511. [PMC free article] [PubMed] [Google Scholar]
- 170.College of American Pathologists. Accessed October 25, 2017 https://www.cap.org.
- 171.Westgard. CLIA requirements for analytical quality. West-gard. Accessed October 25, 2018 https://www.westgard.com/clia.htm.
- 172.SPRING. 2018. Anemia assessment in micronutrient and demographic and health surveys: comparisons in Malawi and Guatemala. Arlington, VA: Strengthening Partnerships, Results, and Innovations in Nutrition Globally (SPRING) Project. [Google Scholar]
- 173.Rippmann CE, Nett PC, Popovic D, et al. 1997. Hemocue, an accurate bedside method of hemoglobin measurement? J. Clin. Monit. 13: 373–377. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Summary of excluded* studies for comparison of methods for Hb measurement
Figure S1. Differences in mean Hb of theHemoCue B-Hb compared with an automated hematology analyzer (reference*) by arterial (A), venous (V), cord, and capillary (C) blood source among infants and children and adult males (M), females (F), and pregnant women (PW).
Figure S2A. Differences in mean Hb of the other invasive point-of-care analyzers compared with HemoCue point-of-care analyzers as the reference* by venous (V) and capillary (C) blood source among children and adult males (M) and females (F).
Figure S2B. Differences in mean Hb of the noninvasive point-of-care analyzers compared with HemoCue point-of-care analyzers as a reference* by arterial (A), venous (V), and capillary (C) blood source among children and adult males (M) and females (F).
Supplementary Text S1. Universal precautions and laboratory personnel training
Supplementary Text S2. HemoCue® B-Hb comparability with the automated hematology analyzer
Supplementary Text S3. HemoCue® comparability with other point-of-care analyzers
Supplementary Text S4. Critical factors to consider when designing surveys


