Abstract
Protecting the health of the public—particularly the most vulnerable groups, such as children—requires rethinking current approaches to reducing environmental risks. We review the evolving understanding of the relationship between exposure to chemicals in the environment and disease, as well as the current state of managing those chemicals. We present recommendations to improve current approaches, including a renewed focus on health and acknowledging the contribution of the environment, flipping the burden of proof so that chemicals are not presumed safe in the absence of scientific data, and modernizing approaches to assessing health risks.
It is widely recognized that the environment contributes to many cancers, other chronic diseases, and disabilities.(1) Key early evidence for this fact came from observations that the children or grandchildren of immigrants from countries with lower (or higher) rates of cancer than the new home country had cancer rates typical of their new home.(2,3) Natural and man-made substances in the environment are believed to be important contributors to most human cancers.(3) The President’s Cancer Panel—an advisory committee of physicians and scientists appointed in 2006 by President George W. Bush—“was particularly concerned to find that the true burden of environmentally induced cancer has been grossly underestimated.”(4)
Observed increases over the past twenty to thirty years in the incidence of many chronic conditions—including diabetes, childhood asthma, and childhood and adult obesity(5,6)—raise concerns about potentially underappreciated environmental risk factors.(7) Chronic diseases such as these are now the leading cause of disability and death in the US population,(8) and genetic predisposition and increased screening and diagnostic sensitivity cannot fully explain the increases.(9,10)
Many factors can increase the risk of chronic diseases, a risk that increases further with the likely interaction among factors.(1) For example, genetic predisposition, age, lifestyle, nutrition, and exposure to chemicals in the environment have all been identified as risk factors for children’s chronic conditions such as asthma, obesity, and behavioral and learning problems.(5) The role of environmental chemicals is receiving increasing attention as a preventable risk factor for many chronic diseases.(7)
In this paper, we review the current state of environmental decision making and our evolving understanding of the relationship between disease and exposure to chemicals in the environment. We then recommend changes in the assessment and management of that exposure to improve the management of public health risks.
The Current Situation
The Magnitude Of The Environmental Chemical Problem
In the past seventy years, the manufacture and use of industrial chemicals has increased more than twentyfold,(11) an outgrowth of innovation and discovery. People are now exposed to multiple chemicals in the air, food, water, and a variety of consumer products, such as phthalates, polybrominated flame retardants, and perfluorinated chemicals. Many of these chemicals appear in measurable quantities in Americans’ bodies(12) and are associated with adverse health effects.(13–15) Approximately 84,000 chemical substances are listed by the Environmental Protection Agency (EPA) as manufactured or processed in the United States, or imported into the country, but this is probably an overestimate of the number of chemicals currently in commercial use. The EPA believes that not all of these chemicals are being produced or imported at any given time, and it is currently reassessing the total. Approximately 700 new industrial chemicals are introduced each year.(16). About 3,000–4,000 chemicals are identified as high volume chemicals, meaning that more than a million pounds of each of them are manufactured or imported annually.(17) These may pose special risks by virtue of their volume.
The US government has made important progress in reducing exposures to some chemicals that adversely affect health, including specific air pollutants identified in the Clean Air Act of 1963, such as particulate matter and lead; hazardous air pollutants named in the Clean Air Act Amendments of 1990, including benzene and dioxin; asbestos; persistent chlorinated insecticides such as DDT; and dibromochloropropane and other pesticides that are highly toxic and mutagenic, or damaging to DNA. As shown in Exhibit 1, national ambient air concentrations of critical air pollutants—sulfur dioxide, nitrogen dioxide, carbon monoxide, particulate matter, ozone, and lead—have declined as a result of this legislation, although some are still above the current national air quality standard. Similarly, Exhibit 2 shows the changes in annual average air concentrations of benzene, whose health effects include increasing the risk of leukemia.
Exhibit 1 (figure). National Ambient Air Concentrations Of Pollutants Compared To National Air Quality Standard, 1990–2008.
NOTES: PM2.5 is particulate matter particles 2.5 micrometers or smaller. PM10 is particulate matter particles 10 micrometers or smaller. NO2 is nitrogen dioxide. CO is carbon monoxide. SO2 is sulfur dioxide.
SOURCE Environmental Protection Agency. Our nation’s air—status and trends through 2008 [Internet]. Washington (DC): EPA; 2010 Feb [cited 2011 Apr 11]. Available from: http://www.epa.gov/airtrends/2010/
Exhibit 2 (figure). Annual Air Concentrations Of Benzene, 1994–2009.
NOTE The average line represents average annual concentrations from twenty-two monitoring sites nationwide. The shaded area displays the concentration range where 80 percent of measured values occurred for each year.
SOURCE Environmental Protection Agency. Our nation’s air—status and trends through 2008 [Internet]. Washington (DC): EPA; 2010 Feb [cited 2011 Apr 11]. Available from: http://www.epa.gov/airtrends/2010/
Some of these improvements can be measured by reduced concentrations of the chemicals in human blood or fat, detected through biomonitoring studies—which measure the presence of chemicals in human biological tissues such as blood or urine.(18,19) The observed declines in these chemicals can be directly linked to government regulatory activity.(20) However, although exposures to some chemicals have dropped, exposures to others have not,(21) and the introduction of new chemicals leads to new exposures.
Managing Exposures to Chemicals
The variations in successfully managing exposures to chemicals is partly driven by the availability of data related to health. Management decisions require both data about the extent of exposures to chemicals in the environment and toxicity data, to understand the extent of potential health risks in the general population and which groups are particularly sensitive to these exposures.
Differences In Legal Requirements
Multiple laws determine regulatory agencies’ ability to obtain data from manufacturers and legal authority to manage exposures to chemicals. For example, there are generally no toxicity data requirements prior to release of chemicals into different environmental media—such as air and water—although many statutes require risk assessments for certain pollutants after their release. These include emissions of chemicals into the air, under the Clean Air Act Amendments of 1990; into water under the Safe Drinking Water Act of 1972; and into hazardous waste, under the Comprehensive Environmental Response, Compensation, and Liability Act of 1980 and the Resource Conservation and Recovery Act of 1976. On the other hand, pre-market toxicity testing is generally required for pesticides under the Federal Insecticide, Fungicide, and Rodenticide Act of 1976 and the Food Quality Protection Act of 1996. Such testing can provide data about potential health risks or other hazards resulting from release of the pesticide into the environment.
The Toxic Substances Control Act
One of the largest identified policy gaps is in the Toxic Substances Control Act of 1976, which gives the EPA only limited authority to require data on the potential health risks from exposures to a large number of nonpesticidal chemicals, both those that have long been in use and those that have more recently appeared on the market.(22) Toxicity and exposure data for thousands of chemicals currently in use never been requested or assessed.(16) In addition, if a substances is discovered to be hazardous and its use is strictly regulated, it is often replaced by an untested substance—which may be just as bad, or worse.(23)
Under the Toxic Substances Control Act, chemicals are essentially assumed to be safe until shown to be harmful, with few requirements for manufacturers to supply data on potential exposures or risks.(24,25). Not surprisingly, manufacturers have little incentive to generate data that might show their products are unsafe, and as a result it is difficult for the EPA to assess and regulate chemicals in a timely fashion.(25) In contrast, manufacturers of pharmaceuticals and pesticides must test them for toxicity and receive approval for their use from the Food and Drug Administration or the EPA, respectively, before they enter the market.(24).
For pesticides containing active ingredient, , the Food Quality Protection Act and the Federal Insecticide, Fungicide, and Rodenticide Act do require premarket testing. This has led to certain safeguards.(26) For example, the use of mutagenic and carcinogenic pesticides is strongly curtailed. The system of premarket testing and registration is not perfect. For example, a variety of registered pesticide replacements were found to pose significant environmental or health risks and either had their registrations for particular uses revoked or were withdrawn from the market. Nonetheless, the act provides more protection from pesticides than from other chemicals.
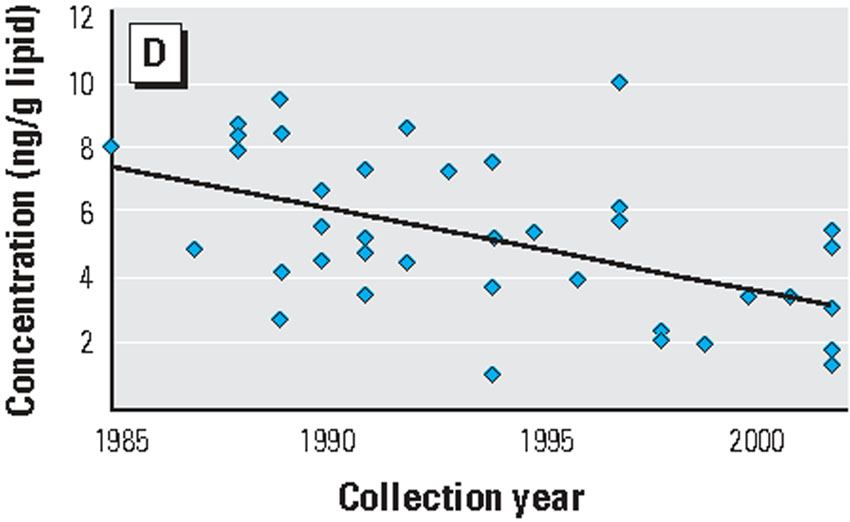
The case of two flame retardants provide an illustration of the problem with the act. In 1974 Firemaster flame retardants containing polybrominated biphenyls (PBBs) were pulled off the market due to toxicity concerns after an inadvertent and widespread poisoning of animal feed in Michigan.(27) However, use of similar flame retardant chemicals was not discontinued. These include polybrominated diphenyl ethers (PBDEs), compounds whose effects on human health have still not been adequately tested. Concentrations of polybrominated biphenyls in human fat samples decreased between 1985 and 2000 (Exhibit 3), and concentrations of the similar, but unregulated, polybrominated biphenyl ethers increased (Exhibit 4).
Exhibit 3 (figure). Levels Of Polybrominated Biphenyls (PBBs) In Human Body Fat, 1985–2002.
SOURCE Note 18 in text.
NOTES The use of PBBs in the United States was discontinued in the 1970s. Polybrominated diphenyl ethers were then introduced as replacement fire retardants.
Exhibit 4 (figure) NEED PLOTTING POINTS. Levels Of Polybrominated Diphenyl EthersI In Human Body Fat, 1985–2002.
SOURCE: Note 18 in text.
In the early 2000s growing recognition of the increasing concentrations of polybrominated diphenyl ethers in people and wildlife,(18) and increasing concern about the chemicals’ effects on thyroid hormones, led to a voluntary phasing out in the United States of two of the three commercial formulations of PBDEs (pentaPBDE and octaPBDE). However, people continue to be exposed to the chemicals because they were widely used in furniture and other indoor consumer products and they persist in the environment.
Furthermore, recent scientific studies have found that polybrominated biphenyl ethers do have effects on thyroid hormones and children’s development.(13, 28) The federal National Toxicology Program plans to perform long-term carcinogenesis studies on PBDE 153,(29) one of the 209 polybrominated diphenyl ethers on the market, which is important because it increasingly is appearing in the food chain and in people.(21,30)By the time the test results are published, use of the chemical will have long ceased but people will continue to be exposed to it
Similarly, a study of the carcinogenic effects of polybrominated biphenyls flame retardant chemicals whose structure is very similar to polybrominated diphenyl ethers, was published a decade after the use of these chemicals was discontinued. Their long half life means that people may still have the chemicals in their bodies, which could pose a low, but not inconsequential, risk.(31)
The Dangers Of Substitutes
There are numerous other halogenated flame retardants—flame retardants containing fluorine, chlorine, bromine, iodine, or another chemical with one or more halogen atoms—in use that substitute for polybrominated diphenyl ethers and polybrominated biphenyls. Many have been found in either wildlife or people.(32,33) Because many of them are similar to chemicals that are known to be toxic, it is not surprising that some give indications of being toxic or carcinogenic.(34) Regulatory agencies have yet to formally consider the risks that these substitutes pose.
Other substitutes abound, including those for diethylhexylphthalate (DEHP), a chemical often used in plastic products such as in children’s toys and medical devices such as blood bags, which has toxic effects on the developing male reproductive system. Substitutes for this chemical have been shown to disrupt thyroid hormones in experiments involving animals.(35) The toxicity of most of these substitutes has not been adequately studied.(36)
Risk-Assessment
Sometimes a state or federal agency can take action to protect the environment or reduce risk to human health without a detailed risk assessment. For example, the Clean Air Act mandates that the EPA assess and require “maximum achievable control technologies” for certain sources of the contaminants defined in the act as hazardous air pollutants. The act also includes provisions for adding other substances to the list of hazardous air pollutants, provisions that could be used to expand this regulatory approach to other air pollutants. Research has identified potential candidates for inclusion based on their known toxicities.(37,38).
Furthermore, the approach of requiring the use of “maximum achievable control technologies” to reduce emissions of toxic substances could be expanded to other media. The advantage of this approach is that the best technology for reducing emissions has already been identified and can be applied to all industries without requiring specific risk assessments for each emitted pollutant, , which can save a good deal of time.(39,40) As of 2000, the EPA estimates that full implementation of the “maximum achievable control technologies”—as mandated in the Clean Air Act Amendments of 1990—has led to an almost fifteenfold improvement in the reduction of air pollutants (40)
However, in many cases, an agency cannot proceed without assessing the level of risk posed to the population. The primary approach is risk assessment. Using biostatistics and biological analysis, risk assessment summarizes the scientific toxicity information, for example describing the probability that an exposure will result in harm.
Typically, a risk assessment consists of four parts. These are assessing the type of health effects associated with exposure to a chemical, typically called hazard assessment; assessing the relationship between the exposure and the response, which is known as dose-response; assessing the amount of exposure and how it varies across uses and individuals; and risk characterization, which combines exposures with the dose-response to estimate risks to the populations of interest.
In addition, risk assessment for cancer is different than risk assessment for noncancer effects. The assumption is that there is no safe exposure level for a carcinogen, but when cancer is not an effect, the assumption is that a no-risk or safe exposure level does exist.
Newer science, reviewed by the National Academy of Sciences,(41) has shown that approaches to risk assessment need to be modernized to reflect the current scientific understanding of the relationship between exposure and adverse health effects, as we will explain below.
Susceptibility And Vulnerability
Intrinsic And Extrinsic Factors
Susceptibility to the harmful effects of exposure can vary dramatically from person to person and can be influenced by both intrinsic and extrinsic factors. The relevant intrinsic factors are age, genetics, and preexisting conditions.
During specific stages of development—including the fetal stage, infancy, early childhood, and puberty—young people can be substantially more susceptible to chemicals than adults.(42) Behavior during early childhood, such as crawling and putting a hand in the mouth, can also increase exposures to certain environmental chemicals. Biomonitoring studies find larger levels of certain chemicals in children compared to their parents or other adults.(43,44) The elderly can also be more susceptible to exposure to certain chemicals in the environment, such as those involved in air pollution.(45)
Preexisting conditions—such as those that compromise the function of the immune system,(46) asthma, coronary heart disease, heart arrhythmias, and other less serious disorders(47)—can leave individuals more susceptible to additional environmental stressors. And individuals’ varying genetic makeup is clearly related to varying risks from environmental chemicals.(48,49)
The relevant extrinsic factors are socioeconomic status, race or ethnicity (that is, the external manifestations of these factors and their effect on the treatment a person receives in society), and exposures to other chemicals. There is a growing amount of evidence, mainly from air pollution epidemiology, that social factors affect a person’s vulnerability to chemicals in the environment.(50–52)
People are exposed to a wide array of environmental chemicals, such as those produced by fuel combustion, industry, drinking water disinfection, and the use of consumer products. Biomonitoring studies of the US population show measurable levels of numerous chemicals in every person studied (12, 21). Studies show that exposure to multiple chemicals that have the same adverse impact on health can have a cumulative effect, increasing the risk still further.(53) For example, the National Academy of Sciences notes that phthalates and other chemicals—such as those found in certain pesticides—that affect testosterone levels can cumulatively act together to adversely impact male reproductive development.(53) Exhibit 5 illustrates how these factors may affect individual and population response to an environmental exposure.
Exhibit 5 (figure). The Effect Of Multiple Exposures On Susceptibility To Exposure To Chemicals In The Environment.
This is Figure 2 in the publication “Meeting report: moving upstream—evaluating adverse upstream end points for improved risk assessment and decision-making” in Environmental Health Perspectives
SOURCE Woodruff TJ, Zeise L, Axelrad DA, Guyton KZ, Janssen S, Miller M, et al. Meeting report: moving upstream—evaluating adverse upstream end points for improved risk assessment and decision-making. Environ Health Perspect. 2008;116(11):1568–75.
Translating Animal Data Into Human Risk
Much of human health risk assessment is based on animal data rather than observational or experimental human studies.(16) Most animal experiments test the effects of individual chemicals using homogeneous adult animal strains—that is, the studies are done on animals that are all genetically similar. Although this can clarify relationships between exposure and effect for any individual chemical, it does not fully account for other factors that can influence risk of disease. As noted above, those other factors include age, genetics, preexisting conditions, and exposure to other chemicals.(41) Given those limitations, the results of animal experimentation probably underestimate human risk.(50)
To translate the findings from animals to humans, researchers use a quantitative factor that takes into account differences in the way small bodies—such as those of rodents—and the large bodies of humans handle chemicals, and differences in pharmacodynamics, or the ways chemicals may influence the process of a disease in a particular species. Sometimes a model is used instead of the quantitative factor—such models are called pharmacokinetic models.
Interindividual variability, or differing susceptibility and vulnerability among humans, is taken into account only when the chemical is not carcinogenic. The numerical factor used to account for interindividual variability for health effects other than cancer, which generally is no larger than 10, may not adequately describe the full range of vulnerability and susceptibility in a population. The National Academy of Sciences has advocated the use of better models of difference among humans.(41)
For cancer, the EPA quantitatively incorporates the increased susceptibility from childhood exposures to mutagenic chemicals. However, the EPA does not make a similar adjustment for prenatal exposures(41) or for childhood exposures to nonmutagenic chemicals, even though some of those have been shown to be significantly more potent when exposure occurs early in life. For carcinogens, the standard default approach assumed in predicting human risk from animal data is that equal exposure leads to the same risk of cancer for each person in the population, but epidemiological and other findings show that in fact the risk varies.(54,55).
Other Needs In Risk Assessments
Sometimes another factor is used to account for the lack of specific data, such as when no studies were conducted to evaluate chronic effects, or to assess particular adverse health effects—for example, neurodevelopmental toxicity. However, where no data at all exist, the risk assessment typically presumes that the chemical poses no risk. The National Academy of Science characterizes this as a missing default, meaning that it amounts to having a default assumption of no effect. The academy advocates the development of new approaches to address such cases.(41)
Finally, risk assessments generally do not account for “background” exposures to the multitude of other chemicals outside (12,21) and inside the body that may affect responses to any given chemical in the environment. There may be some accounting for a small subset of chemicals, such as those measured at a hazardous waste site, through a “hazard index” methodology for effects that do not lead to cancer, a methodology that cumulates chemicals producing the same general type of toxicity. But the wide range of background exposures and physiological processes that may affect health outcomes and potentially result in risks at low doses are not addressed.(41) The National Academy of Science has identified the failure to account for background exposure in risk assessments not involving carcinogens as a major deficiency in current risk assessment methodology.(41)
Some of these newer scientific understandings were recognized in changes to risk assessments for pesticides mandated by the Food Quality Protection Act. This act requires additional consideration of health risks to children, as well as consideration of aggregate exposure to pesticides from multiple sources and exposures to multiple pesticides that produce effects through the same mechanism of action.
The National Academy of Science has recently recommended extending the approaches in the Food Quality Protection Act to account for other susceptibilities and vulnerabilities, and to adequately cover background exposures to multiple chemicals in the environment. Although the Food Quality Protection Act adopts some of the concepts of this approach, , it still does not require that risk assessments consider exposures to non-pesticide chemicals that can contribute to health risks. And although the act considers together pesticides with the same mechanism of action, newer science has led the academy to recommend that chemicals that act on the same common adverse health outcome also be considered together.(53).
Furthermore, the extent of vulnerability and susceptibility and the fact that similar biological pathways can lead to both cancer and noncancer effects led the National Academy of Science to conclude that risk assessment for cancer and noncancer should be unified, and that an assessment should not assume a chemical’s effects are at a threshold or safe level unless the underlying science is reviewed and supports that assumption.(41)
Assessing Exposures
Two problems limit our ability to understand the effects of exposures to chemicals. First, we do not know all of the chemicals in the environment or their sources because no standard reporting is required and information about how chemicals are produced is proprietary in many cases. Second, after toxic chemicals are released into the environment, they may be transformed into by-products that are also be toxic.
For example, the pesticide dichlorodiphenyltrichloroethane (DDT) was banned in 1972. The levels of it found in the environment and humans are now lower than during the time it was used, but one of its by-products, dichlorodiphenyldichloroethylene, (DDE), is still found in over 90 percent of people measured,(12) The ideal risk assessment would consider the toxicity of such by-products, including those produced by combustion related to manufacturing, energy generation and transportation, and processes such as water disinfection.
Interpreting The Science
The complexity and uncertainty in the science lends itself to various interpretations. Different groups, often influenced by financial motives, promote their own interpretations and claim that alternative interpretations are biased or based on poor science.(56,57) On top of this is a continuing call by scientific peer reviewers for assessments that fully characterize the risk and quantitatively capture all major uncertainties and unknowns. Furthermore, policy makers’ requests for peer review to resolve scientific differences in a risk assessment lengthens the process. this sort of peer review is a very high bar, as the scientific understanding of these assessments is almost always incomplete. Finally, as discussed above, the current legal burden of some statutes to determine that chemicals pose harm to human health before regulatory action can be taken is an additional complication.
All of these problems contribute to paralysis in the decision-making process. Toxicity assessments for major chemicals can take ten years or longer. The assessments of some chemicals—such as dioxin and trichloroethylene—that the EPA started in the 1980s have yet to be completed.(41) Stakeholders can become disengaged and distrustful. And the public remains exposed to potentially harmful chemicals while scientific debate continues.
Conflicts Between Science And Policy
Much of that debate focuses on finding a safe level for each individual chemical, even though recent science shows that exposures to different chemicals can combine to produce effects at experienced in the general population (53) and that some low exposures can produce risks.(41) Although there is a growing recognition among scientists that absolute safe levels may not exist, policy makers and the public want to be assured that any exposure is only to an “acceptable” risk. Once a “safe” level of exposure is identified and enforced through policy, they consider the problem to have been solved.
Debates about the science are often motivated by underlying concerns about the costs of regulation. For example, the recent decision by the EPA to subject the research on the health effects of ozone to further peer review before revising the national ambient air quality standard may reflect concerns about the costs of the standard, as the statutorily mandated scientific review panel expressed unanimous support for the agency’s proposed standard.(58) Costs and other societal concerns besides health are important considerations in decision making. However, as the National Academy of Sciences has recognized since the 1980s, descriptions of environmental health risk, cost, and other societal concerns should be made explicit and trade-offs among them should be transparent.(59) Decisions typically involve accepting some risk, and putting off a decision for reasons of political expediency often increases the eventual health burden.
The Way Forward
Taken together, the rise in certain chronic diseases, the multitude of chemicals as potential risk factors, and the large gaps in data based on inadequate testing requirements make it imperative that we update the way we manage chemicals in the environment. This is critical for the prevention, and sometimes the treatment, of many chronic diseases.
We can use what we have learned over the past thirty years to adjust chemical policy for the next thirty years. The National Academy of Science’s Science and Decisions report(41) offers a framework for progress. The academy recommends first identifying the actions that could be taken to address the problem, then developing a way to characterize the risks, costs, and benefits to discriminate among each of the possible actions and then implementing the assessment plan; and presenting the results to the decision makers so that they can make an informed choice. The academy also recommends that stakeholders be engaged at each step of this process.
The goal should be a flexible analytic and deliberative approach to policy making that can be tailored to individual circumstances. In addition, we make the following recommendations.
The focus of environmental policy should always be on health. In addition, approaches to health policy should acknowledge the role of the environment as a determinant of health at the individual and population levels. The management of chemicals is critical for addressing chronic conditions and preventing lifelong disabilities.
It is essential to ask the right questions. The first step in identifying possible ways to address potential health risks from chemical exposure should be to identify the scale and complexity of the assessment, and the issues it should address. The assessment is designed to discriminate among different options and related trade-offs. One approach to managing chemicals in the absence of direct evidence from animal or human studies is to use structural chemical alerts and other related chemicals information. For example, the introduction of chemicals such as polybrominated diphenyls that exhibit persistent or bioaccumulative properties could automatically trigger a consideration of removing them from the marketplace or reducing their use. Such a process could be based on similar approaches already used for pesticides.
The burden of proof should be flipped, so it is on the manufacturer rather than the regulator. The current approach of assuming that chemicals are safe until proved harmful is an incentive for manufacturers to delay acquiring data necessary for assessing products’ safety. Manufacturers of all chemicals—not just pesticides with active ingredients and pharmaceuticals—should be required to produce data to support risk assessments of their products before they enter the marketplace.
To avoid responding to uncertainty with inaction, which often leads to delays and greater health risk, default approaches should be established as temporary measures. For example, in the absence of data about different populations’ response to an exposure, the default assumption could be that people with preexisting diseases are at higher risk than healthy people.(41)
Substantial health gains and cost savings are most likely to come from policies that concentrate on susceptible and vulnerable populations, groups that are also liable to have unequal access to health care and other essential services. For example, California takes into account children’s particular susceptibility to carcinogens in its management of all chemicals, not just those that damage DNA.(41,60)
Absolute safety or the complete absence of risk from exposure to a chemical in the environment is often an unrealistic and unscientific objective. Thus we need to modify current approaches to integrating science into decision making. The National Academy of Science has supported use of a unified approach to risks of cancer and other conditions that starts with an assumption that there is risk at low doses unless scientific data indicate otherwise,(41) an approach we endorse. Policy makers and the public need to realize that there will always be questions about trade-offs in risk management. Should decision makers focus on reducing a pollutant in the air, in drinking water, or both at once?—A transparent presentation and description of the risks will make those trade-offs easier to understand. -And political, economic, and business considerations should be separate from the health risk assessment. The cost of managing a health risk does not affect the magnitude of the risk, but it may affect the choice of risk management options.
Approaches to risk assessment and management should be modified to take advantage of new scientific knowledge as it emerges. For example, studying how a chemical can affect key biological events involved in toxicity will permit more rapid assessment of certain types of chemicals, although the uncertainties in those assessments will be large.(61) Although it may take many years of committed research to develop a full suite of advanced testing and assessment approaches(16), a narrow set of applications—such as prioritizing chemicals for testing—may be ready within five years.
At least one of these recommendations—requiring manufacturers to submit data on the potential health effects of their chemicals—would require new legislative authority to implement. Others—such as using new scientific knowledge—could be integrated into existing regulatory programs, although specific legislation or other signals from Congress for regulatory agencies to move in new directions makes it more likely that they will do so.
Conclusion
Evolving science has provided many new insights about exposures to chemicals in the environment and previously unrecognized biological effects. As the scientific community continues to study the links between exposure and disease, the public is confronted with a barrage of mixed messages about risks. By reexamining our current approaches and applying new science to make risk management decisions faster and more transparent, we can continue to reduce the roll of chronic diseases and disabilities.
Notes
- 1.Bookman EB, McAllister K, Gillanders E, Wanke K, Balshaw D, Rutter J, et al. Gene-environment interplay in common complex diseases: forging an integrative model—recommendations from an NIH workshop. Genet Epidemiol. 2011. February 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dunn JE. Cancer epidemiology in populations of the United States—with emphasis on Hawaii and California—and Japan. Cancer Res. 1975;35(11 Pt. 2):3240–5. [PubMed] [Google Scholar]
- 3.National Institutes of Health. Cancer and the environment: what you need to know, what you can do [Internet]. Washington (DC): Department of Health and Human Services; 2003. August [cited 2011 Apr 11]. Available from: http://www.niehs.nih.gov/health/scied/documents/CancerEnvironment.pdf [Google Scholar]
- 4.Reuben SH for the President’s Cancer Panel. Reducing environmental cancer risk: what we can do now [Internet]. Washington (DC): Department of Health and Human Services; 2010. April [cited 2011 Apr 11]. (2008–2009 Annual Report). Available from: http://deainfo.nci.nih.gov/advisory/pcp/annualReports/pcp08-09rpt/PCP_Report_08-09_508.pdf [Google Scholar]
- 5.Van Cleave J, Gortmaker SL, Perrin JM. Dynamics of obesity and chronic health conditions among children and youth. JAMA. 2010;303(7):623–30. [DOI] [PubMed] [Google Scholar]
- 6.National Center for Health Statistics. Health, United States, 2010: with special feature on death and dying [Internet]. Hyattsville (MD): The Center; 2011. [cited 2011 Apr 11]. Available from: http://www.cdc.gov/nchs/data/hus/hus10.pdf [PubMed] [Google Scholar]
- 7.Diamanti-Kandarakis E, Bourguignon JP, Giudice LC, Hauser R, Prins GS, Soto AM, et al. Endocrine-disrupting chemicals: an Endocrine Society scientific statement. Endocr Rev. 2009;30(4):293–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention. Chronic diseases and health promotion [Internet]. Atlanta (GA): CDC; last updated 2010. July 7; cited 2011 Mar 16]. Available from: http://www.cdc.gov/chronicdisease/overview/index.htm [Google Scholar]
- 9.Cramer JD, Fu P, Harth KC, Margevicius S, Wilhelm SM. Analysis of the rising incidence of thyroid cancer using the Surveillance, Epidemiology and End Results national cancer data registry. Surgery. 2010;148(6):1147–52; discussion 1152–3. [DOI] [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention. Prevalence of autism spectrum disorders—Autism and Developmental Disabilities Monitoring Network, United States, 2006. MMWR Surveill Summ. 2009;58(10):1–20. [PubMed] [Google Scholar]
- 11.Federal Reserve Board. G.17 - Industrial Production and Capacity Utilization, Industrial Production for Chemicals (NAICS=325). The Federal Reserve Board Data Download Program; 2008. [updated 2008 August 14, 2009; cited 2008 August 18, 2008]. Available from:http://www.federalreserve.gov/datadownload/Download.aspx?rel=G17&series=4374e8caec6beeaa874d28776fa68c1e&filetype=spreadsheetml&label=include&layout=seriescolumn&from=01/01/1919&to=12/31/2009 [Google Scholar]
- 12.Centers for Disease Control and Prevention. Fourth national report on human exposure to environmental chemicals [Internet]. Atlanta (GA): CDC; 2009. [cited 2011 Apr 11]. Available from: http://www.poison.org/current/cdc%20fourth%20report%20human%20exposure%20to%20env%20chemicals.pdf [Google Scholar]
- 13.Herbstman JB, Sjödin A, Kurzon M, Lederman SA, Jones RS, Rauh V, et al. Prenatal exposure to PBDEs and neurodevelopment. Environ Health Perspect. 2010;118(5):712–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lederman SA, Jones RL, Caldwell KL, Rauh V, Sheets SE, Tang D, et al. Relation between cord blood mercury levels and early child development in a World Trade Center cohort. Environ Health Perspect. 2008;116(8):1085–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Swan SH, Main KM, Liu F, Stewart SL, Kruse RL, Calafat AM, et al. Decrease in anogenital distance among male infants with prenatal phthalate exposure. Environ Health Perspect. 2005;113(8):1056–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.National Research Council. Toxicity testing in the 21st century: a vision and strategy. Washington (DC): National Academies Press; 2007. [Google Scholar]
- 17.Environmental Protection Agency. Chemical testing and data collection: master testing list—introduction [Internet]. Washington (DC): EPA; last updated 2010. April 29 [cited 2011 Apr 13]. Available from: http://epa.gov/opptintr/chemtest/pubs/mtlintro.html [Google Scholar]
- 18.Sjodin A, Jones RS, Focant JF, Lapeza C, Wang RY, McGahee EE 3rd, et al. Retrospective time-trend study of polybrominated diphenyl ether and polybrominated and polychlorinated biphenyl levels in human serum from the United States. Environ Health Perspect. 2004;112(6):654–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Solomon GM, Weiss PM. Chemical contaminants in breast milk: time trends and regional variability. Environ Health Perspect. 2002;110(6):A339–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Environmental Protection Agency. Measure D6: types of childhood cancer [Internet]. Washington (DC): last updated 2010. December 20 [cited 2011 Apr 11]. Available from: http://www.epa.gov/envirohealth/children/child_illness/d6-graph.html [Google Scholar]
- 21.Woodruff TJ, Zota AR, Schwartz JM. Environmental chemicals in pregnant women in the US: NHANES 2003–2004. Environ Health Perspect. 2011. January 14 (epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Government Accountability Office. High-Risk Series: an update [Internet]. Washington (DC): GAO; 2011. February [cited 2011 May 3]. Available from: http://www.gao.gov/new.items/d11278.pdf [Google Scholar]
- 23.University of California Centers for Occupational and Environmental Health. Green chemistry: cornerstone to a sustainable California. Berkeley (CA): The Centers; 2008. [Google Scholar]
- 24.Cranor CF. Legally poisoned: how the law puts us at risk from toxicants. Cambridge (MA): Harvard University Press; 2011. [Google Scholar]
- 25.Wagner WE. Commons ignorance: the failure of environmental law to produce needed information on health and the environment. Duke Law J. 2004;53(6):1619–745. [PubMed] [Google Scholar]
- 26.Cranor CF. Toxic torts: science, law, and the possibility of justice. New York (NY): Cambridge University Press; 2006. [Google Scholar]
- 27.National Toxicology Program. Toxicology and carcinogenicity studies of a polybrominated biphenyl mixture (Firemaster FF-1). Research Triangle Park (NC): The Program; 1983. [PubMed] [Google Scholar]
- 28.Chevrier J, Harley KG, Bradman A, Gharbi M, Sjödin A, Eskenazi B. Polybrominated diphenyl ether (PBDE) flame retardants and thyroid hormone during pregnancy. Environ Health Perspect. 2010;118(10):1444–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.National Toxicology Program. Testing status of agents at NTP [Internet] Research Triangle Park (NC): The Program; [cited 2011 Apr 15]. Available from: http://ntp.niehs.nih.gov/index.cfm?objectid=BD450D45-123F-7908-7BE898D621DE54E0 [Google Scholar]
- 30.Schecter A, Päpke O, Harris TR, Tung KC, Musumba A, Olson J, et al. Polybrominated diphenyl ether (PBDE) levels in an expanded market basket survey of U.S. food and estimated PBDE dietary intake by age and sex. Environ Health Perspect. 114(10):1515–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.National Toxicology Program. 11th report on carcinogens [Internet]. Washington (DC): Department of Health and Human Services; 2011. [cited 2011 Apr 11]. Available from: http://ntp.niehs.nih.gov/ntp/roc/toc11.html [Google Scholar]
- 32.California Biomonitoring Program. Brominated and chlorinated organic chemical compounds used as flame retardants: additional information on four flame retardants [Internet]. City (State): The Program; 2009. [cited 2010 Jan 19]. Available from: http://oehha.ca.gov/multimedia/biomon/pdf/FlameRetardants_FourMore.pdf [Google Scholar]
- 33.California Biomonitoring Program. Brominated and chlorinated organic chemical compounds used as flame retardants [Internet]. City (State): The Program; 2008. [cited 2010 Jan 20]. Available from: http://oehha.ca.gov/multimedia/biomon/pdf/120408flamedoc.pdf [Google Scholar]
- 34.National Research Council. Toxicological risks of selected flame-retardant chemicals. Washington (DC): National Academies Press; 2000. [PubMed] [Google Scholar]
- 35.COWI A/S. Data on manufacture, import, export, uses and release of dibutyl phthalate (DBP) as well as information on potential alternatives to its use. Kongens Lyngby; 2009. January 29, 2009 Contract No.: Document Number|. [Google Scholar]
- 36.Versar I Review of Exposure and Toxicity Data for Phthalate Substitutes. Prepared for US Consumer Product Safety Commission; 2010. [Google Scholar]
- 37.Lunder S, Woodruff TJ, Axelrad DA. An analysis of candidates for addition to the Clean Air Act list of hazardous air pollutants. J Air Waste Manag Assoc. 2004;54(2):157–71. [DOI] [PubMed] [Google Scholar]
- 38.National Research Council. Air quality management in the United States. Washington (DC): National Academies Press; 2004. [Google Scholar]
- 39.Sexton K Science and policy in regulatory decision-making: getting the facts right about hazardous air-pollutants. Environ Health Perspect. 1995;103:213–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Environmental Protection Agency. Taking toxics out of the air [Internet]. Washington (DC): EPA; 2007. [cited 2011 May 3].Available from: http://www.epa.gov/oar/toxicair/takingtoxics/ [Google Scholar]
- 41.National Academy of Science. Science and decisions: advancing risk assessment. Washington (DC): National Academies Press; 2009. [PubMed] [Google Scholar]
- 42.Woodruff TJ, Janssen SJ, Guillette LJ Jr., Giudice LC Environmental impacts on reproductive health and fertility. New York (NY): Cambridge University Press; 2010. [Google Scholar]
- 43.Lunder S, Hovander L, Athanassiadis I, Bergman A. Significantly higher polybrominated diphenyl ether levels in young U.S. children than in their mothers. Environ Sci Technol. 2010;44(13):5256–62. [DOI] [PubMed] [Google Scholar]
- 44.Calafat AM, Ye XY, Wong LY, Reidy JA, Needham LL. Exposure of the US population to bisphenol A and 4-tertiary-octylphenol: 2003–2004. Environ Health Perspect. 2008;116(1):39–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sandström T, Frew AJ, Svartengren M, Viegi G. The need for a focus on air pollution research in the elderly. Eur Respir J Suppl. 2003;40:92s–95s. [DOI] [PubMed] [Google Scholar]
- 46.Nadeau K, McDonald-Hyman C, Noth EM, Pratt B, Hammond SK, Balmes J, et al. Ambient air pollution impairs regulatory T-cell function in asthma. J Allergy Clin Immunol. 2010;126(4):845–52.e10. [DOI] [PubMed] [Google Scholar]
- 47.Annesi-Maesano I, Agabiti N, Pistelli R, Couilliot MF, Forastiere F. Subpopulations at increased risk of adverse health outcomes from air pollution. Eur Respir J Suppl. 2003;40:57s–63s. [DOI] [PubMed] [Google Scholar]
- 48.National Academy of Science. Copper in drinking water. Washington (DC): National Academies Press; 2000. [PubMed] [Google Scholar]
- 49.ICRP. Genetic Susceptibility to Cancer; 1998. Contract No.: Document Number|.
- 50.Krewski D, Burnett RT, Goldberg M, Hoover K, Siemiatycki J, Abrahamowicz M, et al. Reanalysis of the Harvard Six Cities Study, part II: sensitivity analysis. Inhal Toxicol. 2005;17(7–8):343–53. [DOI] [PubMed] [Google Scholar]
- 51.Clougherty JE, Levy JI, Kubzansky LD, Ryan PB, Suglia SF, Canner MJ, et al. Synergistic effects of traffic-related air pollution and exposure to violence on urban asthma etiology. Environ Health Perspect. 2007;115(8):1140–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shankardass K, McConnell RS, Milam J, Berhane K, Tatalovich Z, Wilson JP, et al. The association between contextual socioeconomic factors and prevalent asthma in a cohort of Southern California school children. Soc Sci Med. 2007;65(8):1792–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.National Research Council. Phthalates and cumulative risk assessment: the task ahead. Washington (DC): National Academies Press; 2008. [PubMed] [Google Scholar]
- 54.Ginsberg G, Guyton K, Johns D, Schimek J, Angle K, Sonawane B. Genetic polymorphism in metabolism and host defense enzymes: implications for human health risk assessment. Crit Rev Toxicol. 2010;40(7):575–619. [DOI] [PubMed] [Google Scholar]
- 55.Bois FY, Krowech G, Zeise L. Modeling human interindividual variability in metabolism and risk: the example of 4-aminobiphenyl. Risk Anal. 1995;15(2):205–13. [DOI] [PubMed] [Google Scholar]
- 56.Michaels D Doubt is their product: how industry’s assault on science threatens your health. New York (NY): Oxford University Press; 2008. [Google Scholar]
- 57.McDaniel PA, Solomon G, Malone RE. The tobacco industry and pesticide regulations: case studies from tobacco industry archives. Environ Health Perspect. 2005;113(12):1659–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Environmental Protection Agency. Summary minutes of the Clean Air Scientific Advisory Committee Ozone Review Panel for the reconsideration of the 2008 Ozone NAAQS: public teleconference [Internet]. Washington (DC): EPA; 2010. January 25 [cited 2011 Apr 14]. Available from: http://yosemite.epa.gov/sab/sabproduct.nsf/MeetingCalCASAC/3B22E6307E3ABE778525767F00612270/$File/CASAC+Ozone+Rev+Panel+1-25-10+Teleconference+Minutes.pdf [Google Scholar]
- 59.National Academy of Sciences. Risk assessment in the federal government: managing the process. Washington (DC): National Academies Press; 1983. [PubMed] [Google Scholar]
- 60.California Environmental Protection Agency, Office of Environmental Health Hazard Assessment. Technical support document for cancer potency factors: methodologies for derivation, listing of available values, and adjustments to allow for early life stage exposures [Internet]. City (State): The Agency; 2009. April [cited 2011 Apr 11]. Available from: http://oehha.ca.gov/air/hot_spots/pdf/TSDCPFApril_09.pdf [Google Scholar]
- 61.Crump KS, Chen C, Louis TA. The future use of in vitro data in risk assessment to set human exposure standards: challenging problems and familiar solutions. Environ Health Perspect. 2010;118(10):1350–4. [DOI] [PMC free article] [PubMed] [Google Scholar]