Abstract
Ulcerative colitis is a chronic, idiopathic, inflammatory bowel disease characterized by a relapsing and remitting course. A substantial proportion of patients fail conventional therapies despite therapy with immunosuppressives and tumor necrosis factor antagonists. Accordingly, newer therapeutic agents that target disease-specific inflammation and minimize adverse events are required. Central to the pathogenesis of ulcerative colitis is an aberrant host response to commensal microorganisms with a resultant dysregulation of gut immune homeostasis and lymphocyte trafficking. Recently, a newer biologic, vedolizumab, which blocks lymphocyte trafficking, has been developed for use in moderate to severe ulcerative colitis. The efficacy of this agent has been demonstrated to be similar to other currently available biologics, and the selectivity of this agent in blocking lymphocyte migration to the gut has substantially reduced treatment-related adverse events. The drug has now been approved for use in the United States and Europe, and, although the exact positioning of this biologic in clinical practice is yet to be defined, it represents an important new chapter in our armamentarium of treatment options for this population. In this review, we will highlight key considerations to be made by providers when using this agent in clinical practice.
Keywords: Ulcerative colitis, inflammatory bowel disease, IBD, α4β7, MAdCAM-1, vedolizumab
Ulcerative colitis (UC) is a chronic, idiopathic, inflammatory bowel disease (IBD) that results from a pathological interaction between the innate gut flora and the systemic immune system. Aberrant host responses to commensal microorganisms, secondary to mucosal barrier dysfunction, culminate in dysregulated gut immune homeostasis. The ensuing alterations in vascular permeability, leukocyte trafficking, and T-cell migration lead to an uncontrolled immune reaction, which is primarily localized to the mucosal layer of colonic epithelium. The net result of this process is recurrent mucosal ulcerations, bloody diarrhea, and abdominal pain with associated systemic inflammatory symptoms such as fever and weight loss.1, 2
Traditional management algorithms for UC have featured nonspecific inhibition of the inflammatory cascade through broadly acting antiinflammatory agents such as corticosteroids and immunosuppressives. The first-line therapy for mildly to moderately active UC includes the use of topical therapy such as 5-aminosalicylic acid or rectal steroids that target the colonic mucosa.3 The favorable safety profile of these drugs results from the lack of significant systemic absorption4, 5; however, the effectiveness of these therapies for the maintenance of remission in severe disease is modest.6 Patients with refractory disease are often treated with thiopurine immunomosuppressives; however, these agents are associated with serious adverse events, including severe infection, skin cancer, and lymphoma.7–9
Recently, the development of the tumor necrosis factor (TNF) antagonists has shifted the focus toward disease-specific targeting of immunosuppression. Since the initial approval of infliximab in 2006, several TNF antagonists have been approved for use in moderately to severely active UC.10 Despite the robust initial response to these agents, long-term, corticosteroid-free remission rates are approximately 20%, and their use is associated with serious infections.9–11 Accordingly, a need exists for safer and more effective therapy.
Disease Mechanisms in Ulcerative Colitis: Targeting Leukocyte Adhesion Molecules
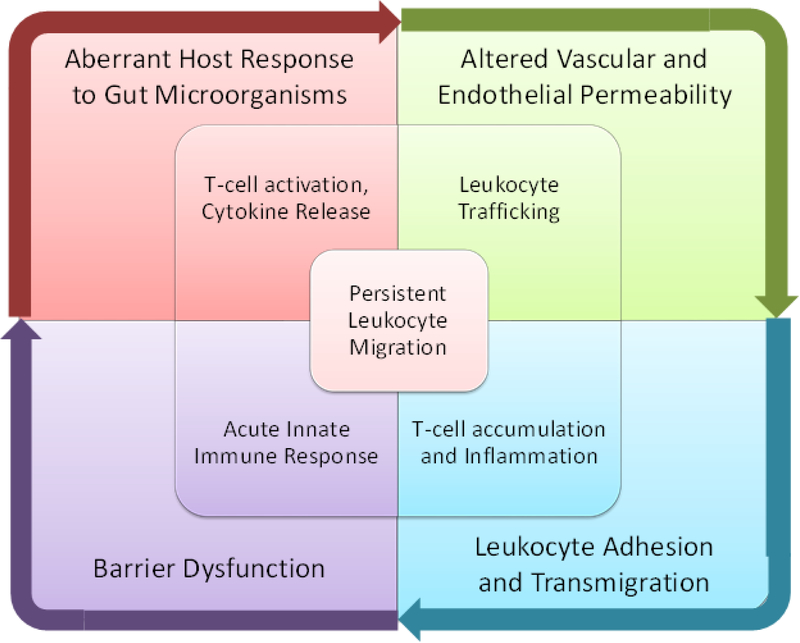
In health, a state of immune homeostasis exists between proinflammatory and antiinflammatory mediators. Although the exact pathophysiology of UC has not been fully defined, several factors play an integral role in the initiation and perpetuation of disease-related inflammation (Figure 1).12 One hypothesis is that mucosal barrier dysfunction occurs in response to a bacterial trigger, which facilitates the presentation of luminal antigens to the innate immune system. If the immune system is unable to clear these antigens, an intricate cascade occurs in which antigens are presented to T cells which, under specific environmental circumstances, become activated. These activated T cells undergo proliferation and clonal expansion in regional lymph nodes, eventually returning to the gut as mature antigen-differentiated lymphocytes. This cascade results in the release of proinflammatory chemokines and cytokines, such as TNF-α, which, in turn, increase endothelial and vascular permeability, and leukocyte trafficking to the gut.
Figure 1:
Factors considered to play a role in the pathogenesis of ulcerative colitis.
Leukocyte homing to areas of inflammation requires dynamic interactions between leukocyte surface ligands and endothelial cell surface adhesion molecules that allow leukocytes to roll across cell surfaces and, ultimately, to adhere to the endothelium. This adhesion is critical for diapedesis, the process of leukocyte migration into target tissues, and is dependent on the strength of attachment. To facilitate this process, proinflammatory cytokines upregulate and activate secondary adhesion molecules on leukocyte surfaces known as integrins. These integrins consist of two transmembrane glycoprotein subunits, α and β, that have the capability of binding specific cell adhesion molecules according to their subtype (Table 1).13 Since leukocyte adhesion molecules play a central role in the accentuation and perpetuation of the inflammatory cascade, they have become targets for drug development in several immune-mediated conditions, including IBD.
Table 1:
Integrins Involved in T-Cell Migration and Retention
| Integrin | Expression | Adhesion Molecule |
|---|---|---|
| LFA-1 or α2β2 | Neutrophils | ICAM-1 |
| α4β1 | Leukocytes (not neutrophils) |
VCAM-1 |
| α4β7 | Lymphocytes | MAdCAM-1 |
| αEβ7 | Mucosal intraepithelial T-lymphocytes |
E-Cadherin |
LFA = lymphocyte function–associated; ICAM-1 = intercellular adhesion molecule-1; VCAM-1 = vascular cell adhesion molecule-1; MAdCAM-1 = mucosal addressin cell adhesion molecule-1.
Previous Experience with Integrin Inhibitors: Natalizumab
Natalizumab, a humanized immunoglobulin G4 monoclonal antibody directed against the α4 integrin subunit, was the first integrin antagonist tested for the treatment of IBD.14 Based on initial placebo-controlled trials that demonstrated a modest degree of efficacy, two large-scale, randomized, placebo-controlled trials (Efficacy of Natalizumab as Active Crohn’s Therapy (ENACT-1) and the Evaluation of Natalizumab as Continuous Therapy (ENACT-2)) were conducted to further evaluate natalizumab for induction and maintenance of remission in patients with active Crohn’s disease (CD).15 The initial rates of response (56% vs 49%, p=0.05) and remission (37% vs 30%, p=0.12) at 10 weeks were similar for treatment compared with placebo groups. However, the overall long-term benefit in sustained response (61% vs 28%, p<0.001), remission (44% vs 26%, p=0.003) and corticosteroid-free remission (58% vs 28%, p<0.001), were significantly higher in patients assigned to natalizumab. Unfortunately, important safety concerns arose following approval of this drug for the treatment of multiple sclerosis. Specifically, cases of progressive multifocal leukoencephalopathy (PML), a severe and usually fatal neurologic disease, developed after long-term natalizumab exposure. PML is an opportunistic infection of the central nervous system (CNS) caused by the John Cunningham (JC) virus, which is latent in approximately 60% of the general population.16 Inhibition of the α4β1 integrin both permits uncontrolled viral replication in patients with latent JC virus and impairs CNS immune surveillance of viral infection, placing patients at risk for PML. Following recognition of this problem, natalizumab was temporarily removed from the market and the planned development program for UC was terminated. Although natalizumab was subsequently reinstated for the treatment of both CD and multiple sclerosis, the risk of PML has restricted use of the drug.
Vedolizumab
Targeting gut-specific integrins has potential to minimize off-target adverse effects during the treatment of IBD given that the risk of PML with natalizumab was related to its interaction with integrins in the CNS. After promising results in animal models, human studies were initiated with a murine-derived α4β7 monoclonal antibody (MLN02). In a small proof-of-concept study, 29 patients with moderately to severely active UC (Mayo clinical score ≥ 5, ≥ 3 bowel movements, and endoscopically active UC) were enrolled and randomized to varying doses of MLN02 (0.15 mg/kg subcutaneously or 0.15, 0.5, 2.0 mg/kg intravenously) or placebo.17 MLN02 was well tolerated, and after a single infusion of 0.5 mg/kg, α4β7 on peripheral blood T cells was saturated for up to 30 days. Furthermore, saturation of the target correlated with treatment efficacy. Subsequently, a phase II, dose-finding study randomized 181 patients with moderately to severely active UC (UC clinical score [UCCS] of 5–7 and a Modified Baron Endoscopic Score [MBS] ≤ 2) to either MLN02 0.5 mg/kg, MLN02 2.0 mg/kg, or placebo.18 The primary outcome, clinical remission (UCCS of 0 or 2, with an MBS of 0 or 1 and no rectal bleeding), occurred in a higher proportion of patients treated with MLN02 compared to placebo (33%, 32%, and 14%, respectively, p=0.03). Although statistically and clinically significant improvements in clinical, endoscopic, and histologic disease activity were observed, a high rate of antidrug antibody (44%) formation was also seen, and their presence was associated with decreased treatment efficacy. The latter finding prompted the development of a new cell line to produce the drug, resulting in the product that is hereafter referred to as vedolizumab (Entyvio; Millennium Pharmaceuticals, Cambridge, MA).
Treatment Efficacy
In a phase II, dose-finding study, vedolizumab therapy was evaluated in 46 patients with active UC (partial Mayo clinical score ≥ 1).19 Patients were randomized in a 4:4:4:3 ratio to receive intravenous vedolizumab 2, 6, or 10 mg/kg, or placebo at weeks 0, 2, 4, and 12. At week 36, vedolizumab-treated patients had higher rates of clinical response compared to patients assigned to placebo (50% vs 33%). Vedolizumab demonstrated dose-proportional pharmacokinetics, maximally saturating α4β7 receptors on peripheral lymphocytes at all measurable serum concentrations. These results confirmed that this new product had similar pharmacokinetic and pharmacodynamic properties as the previous product (MLN02). In addition, the rate of antidrug antibody formation was substantially lower (11%) than for the previous product. These data led to the conduct of an open-label study in which 53 patients with UC continued vedolizumab maintenance therapy for up to 18 months.20 Ten patients discontinued therapy due to adverse events (3 patients), lack of efficacy (5 patients), or withdrawal of consent (2 patients). Of the remaining 43 patients who completed the study, 38 (88%) were in clinical remission at the end of the study.
Subsequently, in a phase III, blinded, placebo-controlled trial (GEMINI-1), 374 patients with moderately to severely active UC (Mayo clinical score ≥ 6, with an endoscopic subscore ≥ 2) (induction cohort 1) were randomized to receive intravenous vedolizumab 300 mg (n=225) or placebo (n=149) at weeks 0 and 2.21 A second induction cohort of 521 patients received open-label vedolizumab at weeks 0 and 2, with disease evaluation performed for both cohorts at week 6 (Table 2). In the maintenance phase, patients in either induction cohort who had a response to vedolizumab at week 6 were randomly assigned to continue therapy every 8 (n=122) or 4 weeks (n=125), or to switch to placebo (n=126) for up to 52 weeks. The primary outcome, clinical response, was defined as a decrease in the Mayo clinical score of least 3 points and a decrease of at least 30% from baseline, with an accompanying decrease in the rectal bleeding subscore of at least 1 point or an absolute rectal bleeding subscore of 0 or 1.
Table 2:
Key Baseline Characteristics of Patients Included in the GEMINI 1 study21
| Characteristic | Induction Phase | Maintenance Phase | |
|---|---|---|---|
| Vedolizumab every 8 weeks |
Vedolizumab every 4 weeks |
||
| No. of patients | 746 | 122 | 125 |
| Age, yrs (mean ± SD) | 40 ± 13 | 41 ± 13 | 39 ± 14 |
| Male sex | 58% | 57% | 54% |
| Disease duration (yrs) | 6.8 ± 6.2 | 6.2 ± 5 | 7.6 ± 7 |
| Mayo clinical score (mean ± SD) | 8.6 ± 1.8 | 8.4 ± 1.8 | 8.3 ± 1.7 |
| Received concomitant glucocorticoids or immunosuppressive agentsa | 72% | 75% | 74% |
| Received prior anti-TNF therapy | 48% | 41% | 42% |
anti-TNF = anti–tumor necrosis factor.
Immunosuppressive agents were azathioprine or 6-mercaptopurine.
At week 6, the vedolizumab-treated patients in the randomized cohort had significantly higher rates of clinical response (47% vs 26%, p<0.001), clinical remission (17% vs 5%, p=0.001), and mucosal healing (41% vs 25%, p=0.001) than placebo-treated patients. Similar rates of response (44%), remission (19%), and mucosal healing (37%) were seen in the open-label vedolizumab cohort (Figure 2). At week 52, patients who continued with vedolizumab maintenance therapy were more likely to achieve and/or maintain clinical response, remission, and mucosal healing than patients who received placebo (Figure 3). On further analysis, no predictors of treatment response were identified; however, there appeared to be a trend toward higher response rates with increasing drug concentrations (Table 3). Based on these data, vedolizumab was approved in May 2014 by the United States Food and Drug Administration (FDA) for use in patients with moderately to severely active UC that is refractory to one or more standard therapies (glucocorticoids, immunosuppressants, TNF antagonists). Vedolizumab has similarly been approved by the European Commission and is pending review and approval in several other countries. The approved vedolizumab dosing regimen is 300 mg infused intravenously over approximately 30 minutes at 0, 2, and 6 weeks for induction therapy, followed by maintenance therapy with 300 mg every 8 weeks thereafter. The drug can be administered through infusion centers, and although the reported rates of infusion-related reactions are low, patients should be monitored until infusions are complete.
Figure 2:
Rates of response, remission, and mucosal healing in the induction phase of the GEMINI 1 trial.21 **Statistically significant difference for vedolizumab (Vedo) vs placebo (p≤0.001); p value not reported for open-label Vedo vs placebo.
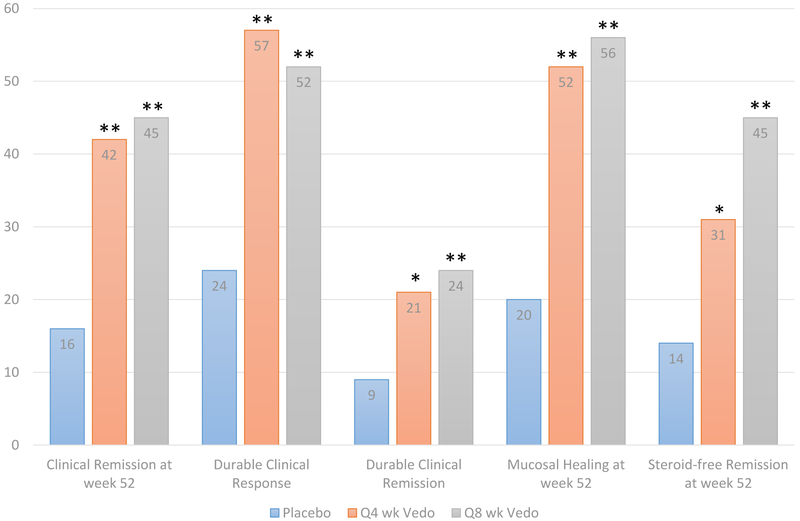
Figure 3:
Rates of response, remission, and mucosal healing in the maintanence phase of the GEMINI 1 trial.21 *Statistically significant difference for vedolizumab (Vedo) vs placebo (p≤0.01); **Statistically significant difference for Vedo vs placebo (p≤ 0.001). Durable clinical response was defined as response at both weeks 6 and 52; durable clinical remission was defined as remission at both weeks 6 and 52; steroid-free remission was assessed in patients receiving steroids at baseline.
Table 3:
Treatment Response and Remission Rates According to Drug Concentrations21
| 1st quartile | 2nd quartile | 3rd quartile | 4th quartile | |
|---|---|---|---|---|
| Induction Phase | ||||
| Clinical response rate | 30% | 47% | 46% | 71% |
| Clinical remission rate | 6% | 11% | 16% | 37% |
| Maintenance Phase | ||||
| Clinical response rate | 42% | 79% | 63% | 80% |
| Clinical remission rate | 45% | 50% | 100% | 67% |
Vedolizumab trough concentrations were as follows: 1st quartile, 0–16.8 μg/ml; 2nd quartile, 16.7–24.7 μg/ml; 3rd quartile, 24.8 – 33.2 μg/ml; and 4th quartile, 33.3 – 65.6 μg/ml.
Treatment-Related Adverse Events
Vedolizumab is approved for use in both UC and CD, and an extensive amount of safety data have accumulated from phase III and long-term safety extension studies.21–23 As of June 2013, the safety of vedolizumab has been analyzed in 3,326 patients, with the majority of patients (61%) receiving ≥ 6 months of exposure and only a minority with ≥ 2 years (27%) and ≥ 3 years (12%) of exposure.
Progressive Multifocal Leukoencephalopathy
Given the safety concerns with natalizumab, one of the major issues that arose during the vedolizumab clinical development program was the risk of PML. Blockade of α4β1 integrin vascular cell adhesion molecule interactions affects T-cell trafficking to all vascular beds, including the CNS.24 In distinction, blockade of the shared epitope of α4β7, which binds specifically to mucosal addressin cell adhesion molecule (MadCAM), a gut-specific adhesion molecule, should not affect the former processes. Despite clear evidence from both animal and human studies that vedolizumab has no impact on leukocyte trafficking to the CNS and should therefore carry no risk for PML,25 extensive clinical experience was needed before this concern could be allayed. Consequently, a comprehensive safety monitoring program (Risk Assessment and Minimization for PML [RAMP]) directed by internationally recognized experts in PML, was a critical component of the vedolizumab development program. Potential cases of PML were sought through a detailed screening and monitoring process. Based on prior experiences with Natalizumab, it can be calculated that if vedolizumab was associated with an increased risk of PML similar to that of natalizumab, the incidence of PML in vedolizumab-treated patients would be approximately 2.1 cases per 1,000 patients.26 No cases were observed in the 2,884 patients with IBD who were screened by the RAMP program, indicating that an association between vedolizumab and PML is highly unlikely.
Systemic and Gastrointestinal Infections
During the vedolizumab development program, a higher rate of infections was observed with vedolizumab therapy compared with placebo (43% vs 35%), and a higher rate of nonserious upper respiratory tract infections (24% vs 17%) was primarily responsible for this difference. A plausible biological mechanism, namely expression of MadCAM in the vasculature of the oropharynx, is likely responsible for this observation. The overall rate of serious infections in the placebo-controlled induction/maintenance trials, however, was similar for vedolizumab versus placebo in patients with UC (2% vs 3%; relative risk 0.56, 95% confidence interval [CI] 0.22–1.44). The majority of serious infections were abscess formation (33%) or respiratory infections (17%). Based on a long-term, open-label safety extension study in both patients with UC or CD, time-adjusted risks of therapy were determined.22 In this analysis, the rates of infection with vedolizumab were compared to those observed in the general IBD population and in patients with IBD who received TNF antagonist therapy. After adjusting for length of observation, the rates of extraintestinal or opportunistic infections were not significantly different between groups (Table 4).
Table 4:
Rates of Adverse Events of Interest with Vedolizumab Compared with Those in the General Population with IBD and in Patients with IBD Undergoing Anti-TNF Therapy22
| General IBD populationa | Vedolizumab Clinical Program | |||
|---|---|---|---|---|
| All patients | Patients Undergoing Anti-TNF Therapy | Patients Receiving Vedolizumab | Patients Receiving Placebo | |
| Serious Infections | ||||
| Anal Abscess | 6.18 (4.70 – 7.99) | 20.0 | 18.19 | 28.22 |
| Bacterial Pneumonia | 2.81 (1.85 – 4.09) | 4.39 | 11.16 | 18.75 |
| Sepsis | 6.20 (4.72 – 8.00) | 7.93 | 2.50 | 9.35 |
| Tuberculosis | 0.52 (0.17 – 1.21) | 1.46 | 0.83 | 0.00 |
| Clostridium difficile | 3.14 (2.12 – 4.49) | 4.10 | 7.11 | 0.00 |
| Salmonella gastroenteritis | 0.10 (0.00 – 0.58) | 0.29 | 1.25 | 4.67 |
| Campylobacter gastroenteritis | 0.00 (0.00 – 0.38) | 0.00 | 2.71 | 0.00 |
| CMV colitis | 0.52 (0.17 – 1.21) | 0.29 | 1.87 | 0.00 |
| Malignancy | ||||
| Solid Tumors | 6.89 (5.32 – 8.78) | 8.21 | 5.21 | 4.69 |
| Colon Cancer | 2.07 (1.26 – 3.20) | 1.45 | 0.62 | 0.00 |
| Lymphoma | 0.41 (0.11 – 1.06) | 0.29 | 0.21 | 0.00 |
| Melanoma | 0.41 (0.11 – 1.06) | 0.29 | 0.42 | 0.00 |
Rates are expressed as no. of events/1000 patient-years with or without 95% confidence intervals.
IBD = inflammatory bowel disease; anti-TNF: anti–tumor necrosis factor; CMV = cytomegalovirus.
Incidence rates for the general IBD population were derived from a retrospective claims-based cohort using the HealthCore Integrated Research Database (HIRDSM; HealthCore Inc., Wilmington, DE).
Despite the mechanism of action, substantial differences in the overall rates of gastrointestinal or abdominal infections with vedolizumab compared with placebo (6% vs 4%) were not observed. It is worth noting, however, that the incidence of two very important gastrointestinal infections, Clostridium difficile and cytomegalovirus (CMV) colitis, were increased with vedolizumab therapy. The incidence of C. difficile and CMV colitis has been steadily rising in patients with UC, and these infections are a major source of morbidity (hospitalizations, colectomies) and mortality in this population.22, 27 Based on the available data, it appears that the use of vedolizumab may increase the rate of C. difficile and CMV colitis beyond that seen in the general IBD population or in patients with IBD exposed to TNF antagonists (Table 4).28
Clostridium difficile infection is a toxin-mediated, luminal infection of the gastrointestinal tract. Given the mechanism of infection (toxin-mediated), it is not expected that vedolizumab, a drug that blocks lymphocyte trafficking in response to a processed antigen, would impact the rates of primary C. difficile infection. It is noteworthy, however, that all of the C. difficile infections occurred among vedolizumab-treated patients, and there were no reported cases of C. difficile in the placebo group.22 Although this raises concern for a potential drug-related risk, this may be partially attributable to key study design and patient factors. All patients required a negative C. difficile stool test prior to inclusion, and the placebo group had a much shorter mean duration of follow-up, as patients were allowed to switch to open-label vedolizumab after induction. Therefore, placebo-treated patients may have rolled over to vedolizumab before rates of C. difficile regressed to the expected rates in this population. This is supported by the fact that no cases of C. difficile occurred during the induction phase of the study for either group (placebo or vedolizumab), and all reported C. difficile cases occurred during the maintenance phase. Similarly, although CMV colitis cases were only seen in vedolizumab-treated patients, the vast majority of these occurred during the maintenance phase, and most of the reported cases with vedolizumab were not serious and did not lead to study discontinuation.
Providers should therefore feel somewhat reassured that although the overall rates of C. difficile and CMV infections were higher with vedolizumab, the majority of infections were readily manageable, and key patient factors may have influenced the observed rates. Given the clinical importance of these infections, however, this will still require careful monitoring as the drug is increasingly used in clinical practice, and further phase IV postmarketing registries will be needed to address this issue.
Malignancy and Death
There were 18 malignancies were observed in patients exposed to vedolizumab during the phase III and open-label extension studies.22, 29 Overall, the incidence of cancer in vedolizumab-treated patients appears to not be increased with vedolizumab compared with the general population, patients with IBD who are not exposed to biologic therapy, and patients with IBD who are exposed other biologic agents (Table 4).22, 29 Across all vedolizumab studies, 14 deaths occurred (5 patients with UC, 9 patients with CD), with 4 of these deaths attributed to vedolizumab therapy.22 The majority of these events were related to infections (sepsis in 2 patients with CD, viral encephalitis in 1 patient with UC), with a single death occurring secondary to hepatocellular carcinoma in a patient with a family history of hepatic neoplasm. The mortality rate with vedolizumab (0.001%) is similar to that seen in patients with IBD using anti-TNF (0.2%) or immunomodulator (0.3%) therapy, with sepsis-related deaths again contributing to the majority of deaths with these therapies.30
Clinical Considerations
Traditional treatment algorithms for UC are based on use of use of nonselective immunosuppressives or antiinflammatory agents such as aminosalicylates, corticosteroids, and thiopurines. In the past decade, the standard approach to the patient who has failed conventional therapy has been TNF antagonist therapy, alone or in combination with an immunosuppressive. With the recent approval of vedolizumab for patients with moderately to severely active refractory UC, several questions have emerged regarding the integration of this new drug into clinical practice.31–34,35–37
Should Vedolizumab Be Used as a First- or Second-Line Agent in Patients Who Are Anti-TNF Therapy Naïve or Have Failed Anti-TNF Therapy?
Although comparisons across trials is of questionable validity, the rates of clinical response and remission observed in the vedolizumab development program are generally similar to those in clinical trials of TNF antagonists (infliximab, adalimumab, golimumab) (Table 5),21, 34,35–37 despite the fact that many of the patients who participated in the vedolizumab studies had failed TNF antagonist therapy. These data would suggest that any of these agents may be suitable as first-line therapy, notwithstanding all the caveats of cross-trial comparisons. A recent network meta-analysis concluded that for biologically naïve patients, effect sizes for clinical response and clinical remission with infliximab (odds ratio [OR] 4.13, 95% CI 2.39–7.16, and OR 5.33, 95% CI 2.28–13.63, respectively) and vedolizumab (OR 3.23, 95% CI 1.42–7.42, and OR 4.51, 95% CI 1.13–20.76, respectively) were similar, whereas treatment effect sizes were lower with golimumab (OR 2.11, 95% CI 1.18–3.28, and OR 2.90, 95% CI 1.19–6.54, respectively) and adalimumab (OR 1.76, 95% CI 1.19–2.56, and OR 1.91, 95% CI 0.98–3.72, respectively).38 Collectively, these data indicate that vedolizumab can be an appropriate first-line biologic agent.
Table 5:
Treatment Efficacy of Biologics for Induction Therapy in Moderately to Severely Active Ulcerative Colitis21, 34,35–37
| Biologic | Clinical Response Rate | Clinical Remission Rate |
|---|---|---|
| Infliximab | 69% | 39% |
| Adalimumab | 50% | 17% |
| Golimumab | 55% | 18% |
| Vedolizumab | 47% | 17% |
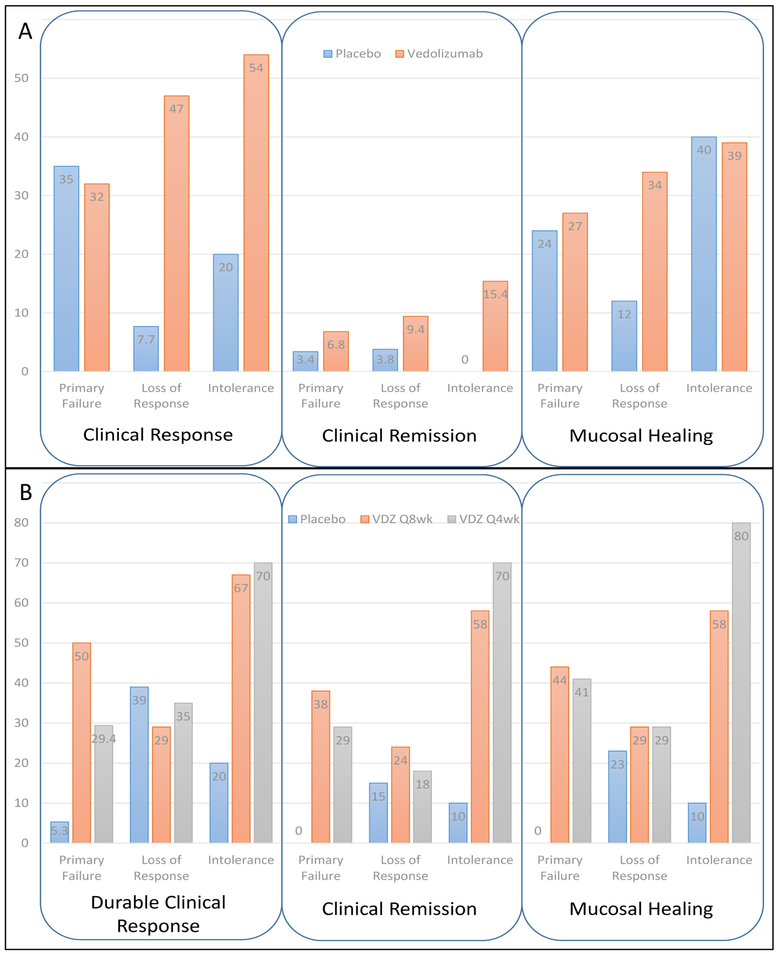
Vedolizumab is also an effective agent for patients who have failed therapy with one or more TNF antagonists. Given that the randomization was stratified for prior TNF exposure, the relative efficacy of the drug can be examined in TNF-exposed and TNF-naïve patients (Takeda Pharmaceuticals, unpublished data, 2014 (Figure 4). When evaluating vedolizumab induction therapy, patients with primary failure of TNF antagonists appeared to have the lowest treatment effect compared with those with secondary loss of response or intolerance (Figure 4A). This may have been due to key differences in disease characteristics, such as degree of inflammation and severity, known to impact TNF antagonist treatment response, as opposed to an actual difference in efficacy among groups. It could also be that factors such as serum albumin concentration, C-reactive protein concentration, and body surface area, which affect clearance of anti-TNF biologics, may also affect the clearance of vedolizumab, rendering the patient relatively resistant to therapy with all monoclonal antibodies. In the maintenance phase of the study, patients with the lowest treatment effect were those who had secondary loss of response to TNF antagonists (Figure 4B). The most common reason for secondary loss of response to TNF antagonists is immunogenicity, and immunogenicity to one TNF antagonist affects the outcome to a second TNF antagonist.39 Therefore, it is plausible to consider that immunogenicity to TNF antagonists may result in enhanced antidrug antibody formation and/or cross-reactivity of antidrug antibodies to vedolizumab, which may account for this difference.
Figure 4:
Clinical outcomes stratified by reason for tumor necrosis factor antagonist failure. Rates of clinical response, clinical remission, and mucosal healing at week 6 are shown in panel A; durable clinical response, durable clinical remission, and mucosal healing at week 52 are shown in panel B. VDZ = vedolizumab.
When considering the ideal positioning of vedolizumab within our current treatment algorithm, providers must take into consideration treatment-related risks. Anti-TNF therapy has been associated with several noninfectious adverse events, including congestive heart failure, demyelinating CNS disease, autoimmune vasculitis or systemic lupus erythematosus, worsening of psoriasis, and even interstitial lung disease.40 Furthermore, anti-TNF therapy has a black-box warning associated with it, stating that the use of these agents may result in an increased risk of serious infections leading to hospitalization or death, including tuberculosis, bacterial sepsis, invasive fungal infections (e.g., histoplasmosis), and infections due to other opportunistic pathogens.41 No such warning exists for vedolizumab, and to date, no other extraintestinal adverse events have been reported with its use.
Collectively, these data suggest that given the proposed safety and gut selectivity of vedolizumab, and similar treatment effect size when compared with infliximab, vedolizumab may emerge as a widely used therapy for moderately to severely active UC, particularly in patients at high risk for treatment-related adverse events or treatment failure with TNF antagonists. It is unknown if TNF antagonists are effective in patients who fail vedolizumab therapy, and further studies are needed to address this question.
Treatment Optimization: Role of Concomitant Immunosuppression and/or Dose Intensification
Following successful induction therapy, the subsequent goal is to maintain long-term remission. The rate of secondary nonresponders (i.e., those who initially responded to therapy but ultimately lost response during maintenance therapy) to TNF antagonists ranges from 25–40% in the reported literature.42 Therefore, this is an important clinical problem for which solutions are required. The use of concomitant immunosuppressants (azathioprine, methotrexate, 6-mercaptopurine) or dose intensification has been shown to reduce the rate of secondary nonresponse by both reducing the risk of immunogenicity and increasing drug concentrations.39, 43 The role of concomitant immunosuppressive therapy with vedolizumab is uncertain. Within the phase III studies of vedolizumab in patients with UC, the rate of secondary loss of response was approximately 25%, and although concomitant immunosuppressive therapy was associated with a reduction in immunogenicity, it had no clear impact on vedolizumab efficacy or maintenance of efficacy.21 When evaluating TNF antagonists, subanalyses of randomized controlled trials stratified by immunosuppressive use similarly demonstrated a reduction in immunogenicity, with no clear benefit for combination therapy; however, further studies powered to adequately assess this question demonstrated a clear benefit for combination therapy over TNF antagonist monotherapy.39, 44 Therefore, although this exploratory subanalysis failed to demonstrate a clinical benefit for immunosuppressive combination therapy with vedolizumab, combination therapy did protect against immunogenicity. Further prospective randomized studies of vedolizumab monotherapy versus combination therapy are needed.
Likewise, very little information is available regarding the role of dose intensification in patients with a secondary loss of response to vedolizumab therapy. Although the every-4-week schedule achieved higher mean trough concentrations compared to the every-8-week schedule (38.3 ± 24.4 μg/ml compared to 11.2 ± 7.2 μg/ml), and higher trough concentrations were associated with higher response rates (Table 3), there was no significant difference between the every-4-week and every-8-week groups with regard to loss of response during maintenance therapy (26% and 25%, respectively).21 It should be noted, however, that these studies were not designed to specifically address the issue of dose intensification, and based on the observed association between exposure and efficacy, it is likely that some patients will benefit from greater dug exposure. However, dedicated trials are needed to evaluate this question.
Use of Vedolizumab in High-Risk Populations
Although the efficacy and safety of vedolizumab has been established in general populations of patients with moderate to severe UC, providers should consider certain specific patient demographics when using this drug in clinical practice. The average age of patients included in these studies was 40 years, and therefore the safety of vedolizumab in the elderly population is yet to be established. Given the increased risk for serious infection and lymphoma with TNF antagonists and immunosuppressants in elderly (> 65 years) patients with IBD,29, 39 vedolizumab may actually be preferred in this population due to its gut selectivity and limited systemic immunosuppression. Similarly, the role of vedolizumab in pregnant patients has yet to be evaluated, as these patients were excluded from clinical trials due to uncertainties regarding placental transfer and fetal safety. Vedolizumab is classified by the FDA as a category B drug, and initial, limited experience in pregnant patients and neonates (exposed in utero) has not raised unique safety concerns45; however, transplacental transfer of vedolizumab during pregnancy is likely, and given that the half-life of vedolizumab is nearly twice that of TNF antagonists, infants born to mothers treated with the drug during the third trimester of pregnancy would be expected to have detectable serum drug concentrations for 6 months or more.19, 46 This exposure could increase the risk of gastrointestinal infections, and these infants should not receive the rotovirus immunization, which is usually administered at 3 months of age, since this is a live viral vaccine.47
Conclusion
Vedolizumab, a monoclonal antibody that selectively inhibits the α4β7 integrin and blocks T-lymphocyte trafficking to the gut, is a new agent for the treatment of UC. Although clinical studies have established efficacy and safety in patients with moderately to severely active UC, the exact role of vedolizumab in clinical practice has yet to be defined. Ultimately, the relative safety of this gut-selective approach may be an important treatment advance over existing agents.
Acknowledgements:
Dr. Dulai is supported by Grant number 5T32DK007202–39 from the National Institute of Diabetes and Digestive and Kidney Diseases.
Footnotes
Potential Conflicts and Disclosures: PSD and MM have no conflicts of interest. RK has attended Ad Boards for Janssen, AbbVie, and Takeda. BGL has served as a consultant for Takeda, Abbvie, and Nestle health sciences. BGF has received research support from, participated on scientific advisory boards, and served as a consultant for Abbott/Abbvie, Amgen, Astra Zeneca, Bristol-Myers Squibb, Janssen, Pfizer, Tillotts, and UCB Pharma; has participated on scientific advisory boards and served as a consultant for Avaxia Biologics Inc., Celgene, Biogen, Ferring, Merck, Novonordisk, Prometheus, Protagonist, Salix, Takeda, TiGenix, and Teva; has received research support and served as a consultant for Roche/Genentech, Millennium, and Receptos; has received research support from Santarus and Sanofi; has served on scientific advisory boards for Novartis, has served as a consultant forActogenix, Albireo Pharma, Avir Pharma, Axcan, Baxter Healthcare Corp., Boehringer-Ingelheim, Calypso Biotech, EnGene,, GiCare Pharma, Gilead, Given Imaging Inc., GSK, Ironwood Pharma, Kyowa Kakko Kirin Co Ltd., Lexicon, Lilly, Nektar, Serono, Shire, Sigmoid Pharma, Synergy Pharma Inc., Vertex Pharma, VHsquared Ltd., Warner-Chilcott, Wyeth, Zealand, Zyngenia; has participated in speakers bureaus for Abbott/AbbVie, JnJ/Janssen, Takeda, Warner-Chilcott, UCB Pharma; and is on the board of directors for Robarts Clinical Trials. WJS has received research support from Janssen, Abbvie, and Western University London Ontario (owner of Robarts Clinical Trials); and has served as a consultant for Janssen, Abbvie, UCB Pharma, Shire, Salix, and Takeda
REFERENCES
- 1.Abraham C, Cho JH. Inflammatory bowel disease. N Engl J Med 2009;361:2066–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ordas I, Eckmann L, Talamini M, et al. Ulcerative colitis. Lancet 2012;380:1606–19. [DOI] [PubMed] [Google Scholar]
- 3.Danese S, Siegel CA, Peyrin-Biroulet L. Review article: integrating budesonide-MMX into treatment algorithms for mild-to-moderate ulcerative colitis. Aliment Pharmacol Ther 2014;39:1095–103. [DOI] [PubMed] [Google Scholar]
- 4.Feagan BG, Chande N, MacDonald JK. Are there any differences in the efficacy and safety of different formulations of Oral 5-ASA used for induction and maintenance of remission in ulcerative colitis? evidence from cochrane reviews. Inflamm Bowel Dis 2013;19:2031–40. [DOI] [PubMed] [Google Scholar]
- 5.Sherlock ME, Seow CH, Steinhart AH, et al. Oral budesonide for induction of remission in ulcerative colitis. Cochrane Database Syst Rev 2010:Cd007698. [DOI] [PubMed] [Google Scholar]
- 6.Jani N, Regueiro MD. Medical therapy for ulcerative colitis. Gastroenterol Clin North Am 2002;31:147–66. [DOI] [PubMed] [Google Scholar]
- 7.Khan KJ, Dubinsky MC, Ford AC, et al. Efficacy of immunosuppressive therapy for inflammatory bowel disease: a systematic review and meta-analysis. Am J Gastroenterol 2011;106:630–42. [DOI] [PubMed] [Google Scholar]
- 8.Khan N, Abbas AM, Lichtenstein GR, et al. Risk of lymphoma in patients with ulcerative colitis treated with thiopurines: a nationwide retrospective cohort study. Gastroenterology 2013;145:1007–1015.e3. [DOI] [PubMed] [Google Scholar]
- 9.Dulai PS, Thompson KD, Blunt HB, et al. Risks of Serious Infection or Lymphoma With Anti-Tumor Necrosis Factor Therapy for Pediatric Inflammatory Bowel Disease: A Systematic Review. Clin Gastroenterol Hepatol 2014. [DOI] [PubMed] [Google Scholar]
- 10.Danese S, Colombel JF, Peyrin-Biroulet L, et al. Review article: the role of anti-TNF in the management of ulcerative colitis -- past, present and future. Aliment Pharmacol Ther 2013;37:855–66. [DOI] [PubMed] [Google Scholar]
- 11.Colombel JF, Loftus EV Jr., Tremaine WJ, et al. The safety profile of infliximab in patients with Crohn’s disease: the Mayo clinic experience in 500 patients. Gastroenterology 2004;126:19–31. [DOI] [PubMed] [Google Scholar]
- 12.Mosli MH, Rivera-Nieves J, Feagan BG. T-cell trafficking and anti-adhesion strategies in inflammatory bowel disease: current and future prospects. Drugs 2014;74:297–311. [DOI] [PubMed] [Google Scholar]
- 13.Lobaton T, Vermeire S, Van Assche G, et al. Review article: anti-adhesion therapies for inflammatory bowel disease. Aliment Pharmacol Ther 2014;39:579–94. [DOI] [PubMed] [Google Scholar]
- 14.Keeley KA, Rivey MP, Allington DR. Natalizumab for the treatment of multiple sclerosis and Crohn’s disease. Ann Pharmacother 2005;39:1833–43. [DOI] [PubMed] [Google Scholar]
- 15.Sandborn WJ, Colombel JF, Enns R, et al. Natalizumab induction and maintenance therapy for Crohn’s disease. N Engl J Med 2005;353:1912–25. [DOI] [PubMed] [Google Scholar]
- 16.Van Assche G, Van Ranst M, Sciot R, et al. Progressive multifocal leukoencephalopathy after natalizumab therapy for Crohn’s disease. N Engl J Med 2005;353:362–8. [DOI] [PubMed] [Google Scholar]
- 17.Brian G Feagan JM, Gordon Greenberg, Gary Wild, Pierre Pare, Richard Fedorak. Steven B Landau, Lee R Brettman. An Ascending Dose Trial of Humanized A4B7 Antibody in Ulcerative Colitis. Gastroenterology. Abstract ed, 2000, 118 (4 suppl 2): A874. [Google Scholar]
- 18.Feagan BG, Greenberg GR, Wild G, et al. Treatment of ulcerative colitis with a humanized antibody to the alpha4beta7 integrin. N Engl J Med 2005;352:2499–507 [DOI] [PubMed] [Google Scholar]; ** The first study to examine the effects of inhibiting alpha4beta7 as a therapeutic target for inflammatory bowel disease.
- 19.Parikh A, Leach T, Wyant T, et al. Vedolizumab for the treatment of active ulcerative colitis: A randomized controlled phase 2 dose-ranging study. Inflamm Bowel Dis 2011;18:1470–9 [DOI] [PubMed] [Google Scholar]; ** An important phase-IIb study that provided significant insight into the pharmacological profile of vedolizumab.
- 20.Parikh A, Fox I, Leach T, et al. Long-term Clinical Experience with Vedolizumab in Patients with Inflammatory Bowel Disease. Inflamm Bowel Dis 2013. [DOI] [PubMed] [Google Scholar]
- 21.Feagan BG, Rutgeerts P, Sands BE, et al. Vedolizumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med 2013;369:699–710. [DOI] [PubMed] [Google Scholar]
- 22.Takeda. Briefing Document Vedolizumab for Ulcerative Colitis and Crohn’s Disease. FDA; 2014. [Google Scholar]
- 23.Sandborn WJ, Feagan BG, Rutgeerts P, et al. Vedolizumab as induction and maintenance therapy for Crohn’s disease. N Engl J Med 2013;369:711–21. [DOI] [PubMed] [Google Scholar]
- 24.Major EO. Progressive multifocal leukoencephalopathy in patients on immunomodulatory therapies. Annu Rev Med 2010;61:35–47. [DOI] [PubMed] [Google Scholar]
- 25.Parikh A, Fedyk E, Soler D, et al. Gastrointestinal selectivity of vedolizumab (MLN0002), a humanized monoclonal antibody to the alpha4beta7 integrin. Inflamm Bowel Dis 2008;14:S18–S18.18816687 [Google Scholar]
- 26.Bloomgren G, Richman S, Hotermans C, et al. Risk of natalizumab-associated progressive multifocal leukoencephalopathy. N Engl J Med 2012;366:1870–80. [DOI] [PubMed] [Google Scholar]
- 27.Berg AM, Kelly CP, Farraye FA. Clostridium difficile infection in the inflammatory bowel disease patient. Inflamm Bowel Dis 2013;19:194–204. [DOI] [PubMed] [Google Scholar]
- 28.Maconi G, Lombardini M, Furfaro F, et al. Long-term outcome of inflammatory bowel diseases with cytomegalovirus colitis: effect of antiviral treatment. Eur J Gastroenterol Hepatol 2014. [DOI] [PubMed] [Google Scholar]
- 29.Dulai PS, Siegel CA. The Risk of Malignancy Associated with the Use of Biological Agents in Patients with Inflammatory Bowel Disease. Gastroenterology clinics of North America 2014. [DOI] [PubMed] [Google Scholar]
- 30.Lichtenstein GR, Rutgeerts P, Sandborn WJ, et al. A pooled analysis of infections, malignancy, and mortality in infliximab- and immunomodulator-treated adult patients with inflammatory bowel disease. Am J Gastroenterol 2012;107:1051–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Colombel JF, Sandborn WJ, Reinisch W, et al. Infliximab, azathioprine, or combination therapy for Crohn’s disease. N Engl J Med 2010;362:1383–95. [DOI] [PubMed] [Google Scholar]
- 32.D’Haens G, Baert F, van Assche G, et al. Early combined immunosuppression or conventional management in patients with newly diagnosed Crohn’s disease: an open randomised trial. Lancet 2008;371:660–7. [DOI] [PubMed] [Google Scholar]
- 33.Panaccione R, Ghosh S, Middleton S, et al. Combination therapy with infliximab and azathioprine is superior to monotherapy with either agent in ulcerative colitis. Gastroenterology 2014;146:392–400.e3. [DOI] [PubMed] [Google Scholar]
- 34.Rutgeerts P, Sandborn WJ, Feagan BG, et al. Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med 2005;353:2462–76. [DOI] [PubMed] [Google Scholar]
- 35.Reinisch W, Sandborn WJ, Hommes DW, et al. Adalimumab for induction of clinical remission in moderately to severely active ulcerative colitis: results of a randomised controlled trial. Gut 2011;60:780–7. [DOI] [PubMed] [Google Scholar]
- 36.Sandborn WJ, van Assche G, Reinisch W, et al. Adalimumab induces and maintains clinical remission in patients with moderate-to-severe ulcerative colitis. Gastroenterology 2012;142:257–65 e1-3. [DOI] [PubMed] [Google Scholar]
- 37.Sandborn WJ, Feagan BG, Marano C, et al. Subcutaneous golimumab induces clinical response and remission in patients with moderate-to-severe ulcerative colitis. Gastroenterology 2014;146:85–95; quiz e14–5. [DOI] [PubMed] [Google Scholar]
- 38.Danese S, Fiorino G, Peyrin-Biroulet L, et al. Biological agents for moderately to severely active ulcerative colitis: a systematic review and network meta-analysis. Ann Intern Med 2014;160:704–11. [DOI] [PubMed] [Google Scholar]
- 39.Dulai PS, Siegel CA, Colombel JF, et al. Systematic review: monotherapy with antitumour necrosis factor alpha agents versus combination therapy with an immunosuppressive for IBD. Gut 2014. [DOI] [PubMed] [Google Scholar]
- 40.Feuerstein JD, Cheifetz AS. Miscellaneous adverse events with biologic agents (excludes infection and malignancy). Gastroenterol Clin North Am 2014;43:543–63. [DOI] [PubMed] [Google Scholar]
- 41.Kopylov U, Afif W. Risk of infections with biological agents. Gastroenterol Clin North Am 2014;43:509–24. [DOI] [PubMed] [Google Scholar]
- 42.Danese S, Fiorino G, Reinisch W. Review article: Causative factors and the clinical management of patients with Crohn’s disease who lose response to anti-TNF-alpha therapy. Aliment Pharmacol Ther 2011;34:1–10. [DOI] [PubMed] [Google Scholar]
- 43.Ben-Horin S Loss of response to anti-tumor necrosis factors: what is the next step? Dig Dis 2014;32:384–8. [DOI] [PubMed] [Google Scholar]
- 44.Dulai PS, Siegel CA, Peyrin-Biroulet L. Anti-tumor necrosis factor-alpha monotherapy versus combination therapy with an immunomodulator in IBD. Gastroenterol Clin North Am 2014;43:441–56. [DOI] [PubMed] [Google Scholar]
- 45.Gisbert JP, Chaparro M. Safety of anti-TNF agents during pregnancy and breastfeeding in women with inflammatory bowel disease. Am J Gastroenterol 2013;108:1426–38. [DOI] [PubMed] [Google Scholar]
- 46.Mahadevan U, Wolf DC, Dubinsky M, et al. Placental transfer of anti-tumor necrosis factor agents in pregnant patients with inflammatory bowel disease. Clin Gastroenterol Hepatol 2013;11:286–92; quiz e24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wyant T, Leach T, Sankoh S, et al. Vedolizumab affects antibody responses to immunisation selectively in the gastrointestinal tract: randomised controlled trial results. Gut 2014. [DOI] [PubMed] [Google Scholar]