Abstract
Oxytocin (OT) is widely known for promoting social interactions but there is growing appreciation that it can sometimes induce avoidance of social contexts. The social salience hypothesis posed an innovative solution to these apparently opposing actions, by proposing that OT enhances the salience of both positive and negative social interactions. The mesolimbic dopamine system was put forth as a likely system to evaluate social salience, due to its well described role in motivation. Evidence from several sources supports the premise that OT acting within the nucleus accumbens and ventral tegmental area facilitate social reward and approach behavior. However, in aversive social contexts, additional pathways play critical roles in mediating the effects of OT. Recent data indicate that OT acts in the bed nucleus of the stria terminalis to induce avoidance of potentially dangerous contexts. Here, we review evidence for neural circuits mediating the effects of OT in appetitive and aversive social contexts. Specifically, we propose that distinct but potentially overlapping neural circuits mediate OT-dependent social approach or social avoidance. We conclude that a broader and more inclusive consideration of neural circuits of social approach and avoidance is needed as the field continues to evaluate the potential of OT-based therapeutics.
Keywords: Nucleus Accumbens, bed nucleus of the stria terminalis, stress, anxiety, ventral tegmental area, intranasal oxytocin
Introduction
Social interactions are a central aspect of life for many species. In humans, social anxiety or reduced social motivation can be symptomatic of a wide range of clinical conditions. Avoidance of social contexts is a key symptom of social anxiety disorder (1) and autism spectrum disorder (2), while social isolation is an important risk factor for depression (3). Although existing therapeutics can increase social function in some individuals, many show no improvement. The neuropeptide oxytocin (OT) has been proposed as a novel therapeutic due to its important effects in preclinical studies on affiliative (4–6) and other social (7–9) behaviors. In humans, intranasal OT administration has been reported to enhance behaviors related to trust (10) and empathy (11). These findings have contributed to a widespread view that OT functions as a prosocial neuropeptide. However, other studies report that OT can increase social anxiety (12, 13) as well as other antisocial behaviors in humans and non-human animals (14–16). Findings such as these have prompted calls to move away from the idea that the primary function of OT is to promote prosocial behavior (17). An alternative hypothesis poses that OT increases the salience of social contexts (18, 19). The term social salience is used in a manner similar to “incentive salience” (20, 21), which refers to the attention grabbing and motivational features of rewards and their learned cues (22), or conversely to these cues in aversive contexts (23). The social salience hypothesis is valence neutral, and can account for findings that OT promotes both social approach and avoidance. Shamay-Tsoory & Abu-Akel proposed that the mesolimbic dopamine system was a key system within which OT signals social salience (18). This innovative assertion was supported by the presence of OT fibers and oxytocin receptors (OTR) in the mesolimbic dopamine system including the ventral tegmental area (VTA), nucleus accumbens (NAc), and prefrontal cortex (PFC). These nuclei are interconnected with circuits modulating social behavior (24). Previous work showed that OT interacts with dopaminergic circuits to enhance the salience of positive social contexts, while more recent work identified underlying molecular mechanisms. In contrast, interactions between OT and the mesolimbic dopamine system in aversive social contexts were more speculative. Adverse social experiences reduce OTR expression in the mesolimbic dopamine system, but this does not explain how OT can induce social anxiety. In this review, we propose that that the bed nucleus of the stria terminalis (BST) is an important site of OT action increasing social salience in aversive social contexts (13, 25, 26). The BST is heterogeneous, with subnuclei known to modulate social behavior and anxiety. Recent evidence suggests that OT acting in the BST induces a state of social anxiety, in which individuals monitor yet avoid unfamiliar social contexts. In this review, we hypothesize that distinct, but potentially overlapping, circuits modulate the effects of OT in positive and aversive social contexts.
Mesolimbic dopamine system facilitates motivation in appetitive and aversive contexts
The idea that OT-induced social salience is mediated by the mesolimbic dopamine system (18) was informed by preclinical studies showing that this system controls motivation in positive and aversive contexts (27, 28). Dopamine neurons are important for learning cues that predict rewards (29, 30) and for signaling internal motivation (31). The ability of the mesolimbic dopamine system to enhance salience, together with the strong connections with the social behavior network (32), provided a strong rationale for proposals that OT modulates social salience through the dopamine system (18). Recent studies strengthen this assertion. In female mice, neurotensin neurons in the medial preoptic area (MPOA) promoted sexual behavior and reacted to male odors during proestrus (33). Optical activation of these neurons resulted in dopamine release in to the NAc. In non-reproductive social contexts, optogenetic induction of burst firing in VTA dopamine neurons projecting to the NAc increased social interaction in female (34) and male (35) mice. Consistent with these preclinical studies, imaging studies in humans showed increased blood oxygen level-dependent (BOLD) signals in ventral striatum and ventral midbrain following positive social feedback (36, 37). Interestingly, negative social feedback also increased BOLD signals in ventral striatum, although this response seems to be stronger in adolescents (38). These observations suggest that the NAc can signal the salience of negative contexts as well. In rodents, aversive experiences such as electric shock (39) and social defeat stress (40) increased the activity of VTA dopamine neurons and increased dopamine release in the NAc (41). Inhibition of dopamine receptors in the NAc during social defeat blocked subsequent expression of submissive behaviors in male hamsters (42). In addition to these short-term responses, social defeat induced spontaneous burst firing in VTA dopamine neurons that persisted for weeks after social defeat concludes (43, 44). Burst firing of VTA dopamine neurons (45) and activation of D1 receptors in NAc shell (46) facilitated social avoidance, providing a strong rationale that OT modulation of dopamine signaling could affect social salience in aversive contexts.
Oxytocin in VTA and NAc facilitates social approach
Oxytocin neurons in the paraventricular nucleus of the hypothalamus (PVN) project directly to the NAc (47) and VTA (48) (Fig. 1). Previous reviews highlighted the importance of OT-dopamine interactions within the PVN-NAc circuit in modulating pair bonding behavior in prairie voles (49, 50). These data point to synchronous action of D2 receptor and OTR systems to signal the salience of a familiar partner. In men, intranasal OT induced stronger BOLD signals in the ventral striatum and midbrain in response to a female partner compared to other familiar women (51). The extent to which OT penetrates the brain via intranasal administration is controversial (52). At least some behavioral effects are likely mediated by OT action in the peripheral nervous system. Still, even if effects of intranasal OT on ventral striatum are indirect, these results are consistent with the hypothesis that NAc enhances the salience of familiar social cues. In rodents, activation of OTR in NAc is important for same-sex interactions. Prepubertal male mice formed place preferences for cage bedding associated with interactions with former cagemates over a 24 hr period, and this effect was abolished by selective deletion of presynaptic but not postsynaptic OTR in the NAc (53). This effect appears to be mediated by serotonergic neurons, as selective ablation of OTR from dorsal raphe (DRN) neurons projecting to the NAc abolished social place preferences (53). Currently it is unknown whether this mechanism generalizes to females or adult males. In adult male rats, serotonin release in the NAc is decreased while engaging in aggression (54). Oxytocin typically reduces male aggressive behavior (55), so OTR-induced serotonin release in the NAc might assign a positive valence to social interactions. In contrast, the absence of OTR driven serotonin release might signal a negative social context. Oxytocin also modulates dopaminergic neurotransmission by acting in the VTA.
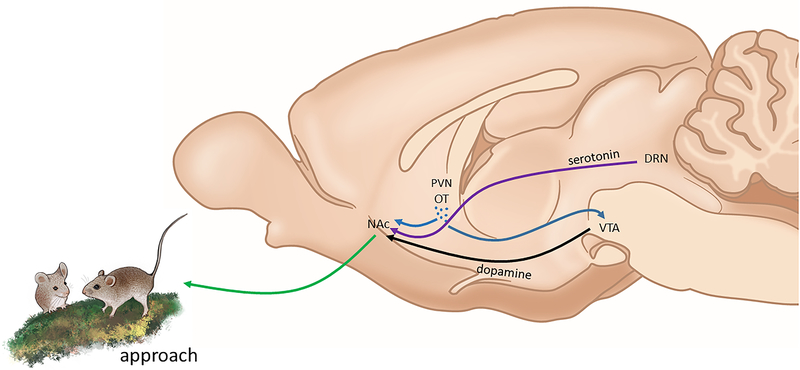
Figure 1:
Oxytocin acting in the mesolimbic dopamine system promotes social approach (green line) in positive social contexts. Oxytocin fibers (blue lines) originating from cell bodies (blue dots) in the paraventricular nucleus (PVN) are present in the nucleus accumbens (NAc) and ventral tegmental area (VTA). Activation of oxytocin receptors in NAc and VTA promote the formation of place preferences for positive social contexts. Activation of oxytocin receptor in the VTA induces release of dopamine (black line) in to the NAc. Effects of oxytocin receptor in NAc on social place preferences in juvenile male mice have been found to require the coactivation of 5HT1B on serotonergic fibers originating from the dorsal raphe (DRN, purple line). Mouse drawing by N. Duque-Wilckens.
Oxytocin acting in the VTA may signal social salience by increasing the activity of VTA dopamine neurons (56). In adult male Syrian hamsters, OTR antagonist infusions into the VTA during social place-preference conditioning (but not during preference testing) eliminated preferences for social contexts over non-social contexts (57). Similar results were seen in juvenile male mice, as optical activation of fibers from PVN OT neurons projecting to the VTA enhanced social place preferences (58). Ablation of OTR from dopamine neurons (but not GABA neurons) prevented the induction of social place preferences. Consistent with these data, OT increased excitatory post-synaptic potentials of dopamine neurons projecting to the NAc. Together, these results suggest that OT produced in the PVN and acting within the NAc and VTA increase the salience of appetitive social contexts to facilitate social approach. How OT modulates these nuclei in aversive contexts is less clear.
Aversive social experiences such as social defeat reduce both OT and OTR in the mesolimbic dopamine system. Social defeat reduced OT fiber density in NAc shell of female mandarin voles (59) and OTR binding within the NAc shell and core of male and female California mice (13). Furthermore, intracranial infusion of OT reduced defeat-induced social avoidance in female mandarin voles (59), and male mice and rats (60). These data suggest that exogenous OT can overcome deficits in OTR expression. However, female California mice previously exposed to social defeat had more OT/c-fos cells in PVN after a social interaction test (61) and intranasal OT failed to increase social interaction in these mice (61). Instead, systemic OTR antagonist treatment reduced stress-induced social avoidance in females(13). These results suggest that OTR activation can induce social avoidance. Since OTR antagonist infusion into the NAc did not decrease social avoidance in stressed females (13), OTR-dependent social avoidance may depend on an alternate circuit.
Studies examining how defeat affects OT action in the mesolimbic dopamine system have primarily analyzed behavior days or weeks after the last episode of defeat. However, PVN OT neurons are activated during episodes of defeat in both sexes (25, 61). Defeat-induced OT release has been observed with microdialysis (62) while intranasal OT increased the subjective perception of social stress in men (12). Currently, it is unknown whether OT released during social stressors encodes the salience of these experiences by acting in the mesolimbic dopamine system. An additional complexity is that OT is a promiscuous ligand, and can activate vasopressin V1a receptor (V1aR, (63)), which is attracting attention as an alternative therapeutic target for modulating social behavior (64, 65). Evidence for OT-V1aR interactions comes from studies using infusions of α-melanocyte stimulating hormone (α-MSH), which stimulates endogenous OT but not vasopressin (AVP) release (66). ICV infusion of α-MSH increased territorial behavior in male hamsters but not if a V1aR antagonist was also infused (67). These results are exciting because induced release of OT can require V1aR activity to affect behavior. One possible site of OT-V1aR cross-talk is the lateral septum (LS). The dorsal LS has V1aR expression and abundant AVP and OT fibers (68), and V1aR modulate social play behavior by inducing dopamine release (69). Ventral LS has strong OTR expression as well as AVP and OT fibers (68). Ventral LS enhances social salience in a variety of contexts. Social defeat before a fear conditioning session enhanced context-dependent fear memories in male mice in an OTR-dependent fashion (70). In contrast, OTR activation inhibits fear memory formation when paired with non-stressful social encounters (71). The LS is also important in an interesting model in which neutral social contexts are paired with aversive foot shocks (72). Infusion of OT into LS enhanced the extinction of social avoidance following social fear conditioning in both male (73) and female (74) mice. Social fear conditioning also increased OTR binding in LS (73). A last consideration is that neuropeptide fibers and receptors need not be adjacent for cross-talk to occur, as both AVP and OT can diffuse outside of synapses. For a full discussion of OT/AVP cross-talk with V1aR/OTR see (75).
Bed nucleus of the stria terminalis modulates anxiety and social behavior
The BST is considered to be a component of the “extended amygdala” due to evolutionarily conserved cell types and neuroanatomical connections with the amygdala (76, 77). The BST is heterogeneous, with at least 16 subregions identified (78, 79). Here, we use a broad classification scheme proposed by Dong and colleagues (78) based primarily on neuroanatomical connections and embryological development (Table 1). The posterior division of the BST modulates sexual (80) and aggressive (81) behaviors, and also anxiety-like behavior (82). The anterior division of BST modulates fear and anxiety (83, 84). A lateral group of nuclei has extensive connections with the CEA and is referred to as the central extended amygdala (77). The medial extended amygdala has stronger connections to the medial amygdala. This pattern of connectivity is evolutionarily conserved across rodents, Rhesus monkeys, and humans (83). The BST was thought to have stronger effects on sustained fear responses while the CEA was more important for acute fear responses (85), but subsequent work demonstrated more functional overlap. Optical inhibition of corticotropin releasing hormone (CRH) neurons in the CEA projecting to the anterolateral BST (BSTal) reduced sustained fear responses (86). Imaging studies in humans also showed that BST and CEA exhibit similar responses to acute and sustained threatening contexts (87). Adjacent pathways may independently modulate responses to acute versus diffuse threats. Optical activation of the oval nucleus of the BST (BSTov) had anxiogenic effects in an elevated plus maze while optical stimulation of anteromedial BST (BSTam) had anxiolytic effects (88). It would be interesting to compare how these circuits respond to acute versus diffuse threats. Overall there is strong evidence that the central extended amygdala modulates anxiety-like behavior (84). Studies of social behavior focused more on nuclei within the medial extended amygdala.
Table 1.
Nomenclature of Subnuclei of the BST Based on Rat [Dong et al. (78)] and Mouse [Paxinos and Franklin (79)]
| Dong & Swanson 2006 | Paxinos & Watson 2012 | ||
|---|---|---|---|
| Anterior division | Medial extended amygdala | ||
| BSTam | BSTMA | ||
| BSTav | BSTMV | ||
| Central extended amygdala | BSTal | BSTLP | |
| BSTju | BSTLJ | ||
| BSTov | BSTLD | ||
| BSTfu | Fu | ||
| BSTrh | BSTLP | ||
| Posterior division | BSTtr | BSTMP | |
| BTSif | BSTMP | ||
| BSTpr | BSTMP | ||
BST, bed nucleus of the stria terminalis; BSTal, anterolateral BST; BSTam, anteromedial BST; BSTav, anteroventral BST; BSTfu, fusiform nucleus BST; BSTif, interfasicular nucleus BST; BSTju, juxtacapsular BST; BSTLD, lateral division dorsal part BST; BSTLJ, lateral division juxtacapsular BST; BSTLP, lateral division posterior BST; BSTMA, anteromedial BST; BSTMP, medial division posterior part BST; BSTMV, medioventral BNST; BSTov, oval nucleus BST; BSTpr, principal nucleus BST; BSTrh, rhomboid nucleus BST; BSTtr, transverse nucleus BST; Fu, fusiform nucleus BST.
As with anxiety, circuits modulating social approach and avoidance are in close proximity. Activation of V1aR in anteroventral BST (BSTav) was necessary for social approach in male and female California mice (89). In contrast activation of the adjacent BSTam was necessary for the expression of submissive and defensive responses in stressed male hamsters (90). Furthermore, social defeat increased immediate early gene expression in BSTam of male hamsters (91) and rats (92), while chronic mild stress increased average dendritic length of BSTam neurons (93). These responses may lead to long-term changes. Two weeks after social defeat, female California mice had increased brain derived neurotrophic factor (BDNF) protein in BSTam and BSTav (94). Social defeat induced social avoidance in females, but this effect was blocked by an infusion of a TrkB antagonist into BSTam. In contrast, no effects of defeat on BDNF in BST were observed in male California mice (94) or Syrian hamsters (95). The CRH signaling pathways may be more responsive to stress in males. Restraint stress increased CRH immunoreactivity in BSTov in male but not female rats (96), while defeat-induced social anxiety in male hamsters was blocked with BST infusions of type II CRH receptor antagonist (97, 98). Whether CRH receptors in BST modulate social anxiety in females is unknown. Overall, circuits within the anterior divisions of BST can modulate both social approach and avoidance. Social avoidance may be mediated through projections to the anterior hypothalamus and periaqueductal gray (99), which modulate defensive behaviors (100). Recently it was proposed that the BST assesses the valence of social contexts, and modulates an individual’s decision to approach or avoid (101). Oxytocin acting within the BST may signal the salience of aversive social contexts.
OTR acting in the BST induces social anxiety response
Several lines of evidence indicate that OT acting within the BST can increase social anxiety (12, 13, 70)(Fig. 2). Recent data suggest that BSTav and BSTam mediate anxiogenic effects of OT. Hypothalamic nuclei (e.g. PVN) are usually considered to be the main source of OT, but OT cell bodies are also present in BSTav (25, 61, 68). Social defeat has a sex-specific, biphasic, effect on these neurons. In male C57Bl6/J (25) and California mice (61), one hour after exposure to social defeat, increased OT/c-fos colocalizations were observed in the BSTav. In C57Bl6/J, colocalizations were positively correlated with submissive postures. In contrast, there were no increases in OT/c-fos colocalizations one hour after defeat in female California mice. However, 10 weeks after the last episode of social defeat, increased OT/c-fos colocalizations were observed following social interaction testing in female California mice but not males (61). Furthermore, stressed females had more OT neurons in BSTav as well as more Oxt mRNA in BST punch samples. These results suggest that defeat induces enduring changes in the activity and transcription of OT in BSTav neurons. To date, no published study has directly tested the function of BSTav OT neurons, which are evolutionarily conserved across rodents (25, 68) and primates (102). However, pharmacology studies indicate that OTR in the BSTam modulates social anxiety.
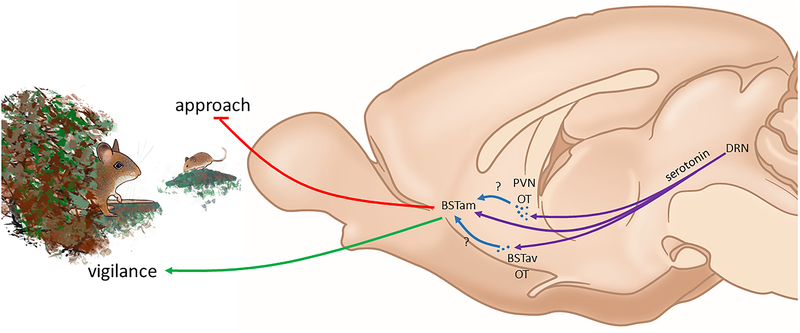
Figure 2:
Oxytocin acting in the bed nucleus of the stria terminalis promotes social avoidance and vigilance (green line) and inhibits social approach (red line). Social stress induces a long term increases in activity of OT neurons (blue dots) in anteroventral BST (BSTav) and PVN. Infusion of OTR antagonist into anteromedial BST (BSTam) blocks stress-induced social avoidance and vigilance. Currently it is unknown whether the source of OT within the BSTam originates from BSTav or PVN OT neurons. Optical activation of dorsal raphe nucleus (DRN) fibers (red lines) in BST is anxiogenic. This suggests that if OTR facilitates serotonin release in BNSTam as in NAc, increased serotonergic transmission would induce anxiogenic responses. Mouse drawing by N. Duque-Wilckens.
The BSTam became a site of interest based on analyses of the immediate early gene EGR-1. In female but not male California mice, intranasal administration of OT induced social avoidance (61) and increased EGR-1 immunoreactivity in BSTam and NAc core but not other nuclei that modulate social behavior (13). Site-specific infusions of OTR antagonist into BSTam but not NAc core increased social interaction behavior in stressed females. As described above, the BSTam is highly sensitive to aversive social contexts. Although the mechanism of OTR-dependent social avoidance is still uncertain, serotonergic signaling is a possible target. Non-social stressors induced serotonin release in the anterior division of BST to increase anxiety-like responses (103), and optical stimulation of DRN fibers in BST induced anxiety-like behavior through activation of 5-HT2C receptors (104). If OTR facilitates serotonin release in the BSTam as in NAc (53), the behavioral effect would likely be anxiogenic. Interestingly, inhibition of OTR within the BSTam also affected another behavioral response that suggested that this pathway modulates social anxiety. Stressed female California mice oriented towards a cage containing an unfamiliar target mouse without approaching it (13, 105), a response that did not occur in the absence of the target mouse. Moreover, when stressed females were treated with systemic or intraBSTam OTR antagonist, this orientation response was abolished. Similarly, female C57Bl6/J that witnessed social defeat of another male showed social avoidance (106) and vigilance (S. Iñiguez personal communication) and female OTR knockout mice showed fewer stretch-attend responses (considered a vigilance response) to intruders in a resident-intruder test (107). We propose that the combination of avoidance and vigilance represents a state of social anxiety.
In humans, social anxiety is characterized by an aroused physiological profile in which individuals attend to novel social cues while simultaneously avoiding them (108). The combination of increased vigilance and social avoidance is characteristic of behavioral inhibition (109), a temperamental trait associated with increased risk for social anxiety disorders (110). Social stress exposure may induce individuals to interpret an unfamiliar social context as a threatening one. Indeed, intranasal OT increased anxiety responses in men and women exposed to unpredictable shocks but had no effect when shocks were predictable (111). Evidence from rodents implicating BSTam are consistent with human imaging studies showing increased responsiveness of the BST and amygdala to social threat (87, 112). Independent quantification of social approach and vigilance in preclinical studies may lead to the identification of distinct circuits modulating social behavior. For example, infusion of OTR antagonist into NAc core of male California mice reduced social approach without increasing vigilance (A.V. Williams & Trainor unpublished). Decreased social approach in the absence of vigilance could represent a state of social anhedonia. Interestingly, reduced interest in social activity is a key symptom of depression and is linked to weaker BOLD responses in ventral striatum in positive social contexts (113). The delineation of neural circuits modulating social anxiety versus social anhedonia could have important implications for the use of OT-based therapeutics.
A relevant question is whether OTR-modulation of social vigilance is sex-specific. Dumais and Veenema reviewed the extent of sex differences in OT and OTR expression (114). When sex differences in OT expression are observed, females typically have more OT positive cells (e.g. in PVN and SON) than males. When sex differences in OTR expression are observed, males typically show increased binding (e.g. in BNST and NAc). However, many studies report no sex differences in OTR binding. The extent to which OTR modulates a behavior could be very different in males and females even in the absence of sex differences in OT or OTR expression. For example, social isolation induces stronger increases in OT/c-fos colocalizations in the PVN of female prairie voles than males (115). Thus, sex differences in the activity of OT neurons (in the absence of differences in OT cell number or OTR expression) could result in important sex differences in the relative activation of OTR. Sex differences in the effects of OTR on behavior have also been reported. While inhibition of OTR in VTA reduced social place preferences in male and female hamsters, infusion of OTR agonists reduced place preferences in females but enhanced them in males (116). In humans, functional connectivity analysis was used to determine the effect of intranasal OT on a social behavior network of hypothalamic and limbic nuclei (117). In men intranasal OT increased functional connectivity in a positive social context while in women intranasal OT reduced functional connectivity in a negative social context. One possible mechanism for this effect is that there could be sex differences in the cell types which express OTR. Difficulties in visualizing OTR have limited knowledge of which cell types express OTR. Single-cell RNAseq methods (118) could provide an ideal approach for high-throughput identification cells types expressing OTR males and females.
Translational Considerations and Future Directions
There is intense interest in using OT-based pharmaceuticals to treat illnesses such as depression (119), anxiety (120), schizophrenia (121), and autism (122). However, early clinical studies using intranasal OT have produced mixed results ((123–125), but see (126)). The impact of patient selection, pharmacokinetics, and statistical power has been reviewed elsewhere (127–129). Here we focus on how preclinical findings could inform future clinical work. First, future studies could take a more balanced approach in evaluating the effects of OT-based therapeutics on social approach and anxiety. Many clinical trials focus on variables related to social cognition, such as emotion recognition. A major premise of this approach is that OT increases salience by engaging the mesolimbic dopamine system. However, OT can act in the extended amygdala to enhance social anxiety. Evaluating effects of OT-based therapeutics on measures such as behavioral inhibition (130), could provide a more balanced assessment. Second, future clinical studies could consider how the social context during OT administration affects behavioral outcomes. Positive or negative contexts are likely to engage different neural circuits that could be modulated by OT (Fig. 3). A region-of-interest based meta-analysis found that intranasal OT increased amygdala activity in positive social contexts and decreased activity in negative contexts (131). However, this pattern was not detected in whole brain analyses (132). Social contexts used in many imaging studies may not be strong enough to generate robust neural responses across studies. Here the steroid literature could provide a useful example. Relationships between testosterone (T) and survey-based measures of behavior are weak (133), but more robust relationships are observed if T is measured while a participant is engaged in a competitive task (134). Tasks such as cyberball (135) or chat room (136) protocols that are more socially engaging might produce more robust results in OT studies. Integration of anticipation of positive and negative outcomes could also be informative. Stronger BOLD responses in the BST were observed during electric shock anticipation while increased BOLD responses in amygdala occurred during shock exposure (137). This could be important because activation of OTR was anxiogenic in BST (13) but anxiolytic in CEA (138, 139). These results suggest that the neural circuits modulated by OT could vary over a timescale of seconds or minutes. Finally, it will be important to have more comparisons between clinical populations and healthy controls. As discussed previously, preclinical studies demonstrate that OT-based manipulations have different behavioral effects in stressed versus unstressed individuals. Thus, there is a strong premise to expect that OT may have different effects in clinical populations. The development of new tools such as brain accessible OTR ligands as well as tracers would be extremely useful for evaluating the extent to which OTR can modulate social approach and anxiety in clinical contexts.
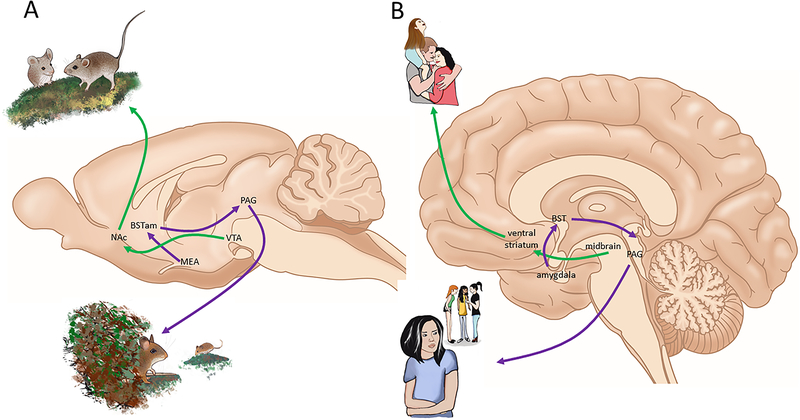
Figure 3:
Hypothesized neural circuits of social approach (green lines) and avoidance (purple lines) in rodents (A) and humans (B). Several lines of evidence indicate that dopamine neurons in the ventral tegmental area (VTA) projecting to the nucleus accumbens (NAc) facilitate social approach. Imaging studies in humans do not have the same level of resolution, but suggest that similar circuits (midbrain and ventral striatum) are active in positive social contexts. A circuit including the medial amygdala (MEA) and anterior bed nucleus of the stria terminalis (BNSTam) facilitate social avoidance in rodents. Imaging studies in humans frequently report increased BOLD responses in amygdala and BST in aversive contexts. Drawings by N. Duque-Wilckens.
Here we focused on OT action within the mesolimbic dopamine system and extended amygdala, networks that have strong functional connections (77, 140). However, it is unclear how these OT-sensitive systems interact with each other across different social contexts. Furthermore, OT can act within other cortical systems to modulate social salience (141–144). A major challenge for developing any novel therapeutic is that while mechanisms of action may be circuit-specific, in most cases systemic administration is the only realistic treatment option. Oxytocin is no exception. However, applying OT-based ligands in specific social contexts could amplify (or dampen) the affected neural circuits. Successful implementation of this type of approach will require a broader understanding of how OT modulates neural circuits in positive and negative contexts. Eventually these data could contribute to the development novel therapeutic strategies for individuals that are resistant to existing therapies.
Acknowledgements
BCT and NDW were supported by NIH R01 MH103322 and MQS was supported by T32-AA007456. The authors thank D. S. Stolzenberg for helpful conversations, A. S. Fox and several anonymous reviewers for helpful comments on the manuscript.
Footnotes
Disclosures
The authors have nothing to disclose.
References
- 1.Taylor JH, Lebowitz ER, Silverman WK (2018): Anxiety disorders In: Martin A, Bloch MH, Volkmar FR, editors. Lewis’s child Adolesc psychiatry a Compr Textb, 5th ed Philadelphia, PA: Wolters Kluwer, pp 509–518. [Google Scholar]
- 2.Simonoff E, Pickles A, Charman T, Chandler S, Loucas T, Baird G (2008): Psychiatric Disorders in Children With Autism Spectrum Disorders: Prevalence, Comorbidity, and Associated Factors in a Population-Derived Sample. J Am Acad Child Adolesc Psychiatry.. doi: 10.1097/CHI.0b013e318179964f. [DOI] [PubMed] [Google Scholar]
- 3.Cacioppo JT, Hawkley LC, Thisted RA (2010): Perceived social isolation makes me sad: 5-year cross-lagged analyses of loneliness and depressive symptomatology in the Chicago Health, Aging, and Social Relations Study. Psychol Aging. 25: 453–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ross HE, Young LJ (2009): Oxytocin and the neural mechanisms regulating social cognition and affiliative behavior. Front Neuroendocr. 30: 534–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Neumann ID (2008): Brain oxytocin: a key regulator of emotional and social behaviours in both females and males. J Neuroendocr. 20: 858–865. [DOI] [PubMed] [Google Scholar]
- 6.Carter CS, Grippo AJ, Pournajafi-Nazarloo H, Ruscio MG, Porges SW (2008): Oxytocin, vasopressin, and sociality. Prog Brain Res. 170: 312–322. [DOI] [PubMed] [Google Scholar]
- 7.Goodson JL, Thompson RR (2010): Nonapeptide mechanisms of social cognition, behavior and species-specific social systems. Curr Opin Neurobiol. 20: 784–794. [DOI] [PubMed] [Google Scholar]
- 8.Meyer-Lindenberg A, Buckholtz JW, Kolachana B, Hariri AR, Pezawas L, Blasi G, et al. (2006): Neural mechanisms of genetic risk for impulsivity and violence in humans. Proc Natl Acad Sci USA. 103: 6269–6274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johnson ZV, Young LJ (2017): Oxytocin and vasopressin neural networks: implications for social behavioral diversity and translational neuroscience. Neurosci Biobehav Rev. 76: 87–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kosfeld M, Heinrichs M, Zak PJ, Fischbacher U, Fehr E (2005): Oxytocin increases trust in humans. Nature. 435: 673–676. [DOI] [PubMed] [Google Scholar]
- 11.Domes G, Heinrichs M, Glascher J, Buchel C, Braus DF, Herpertz SC (2007): Oxytocin attentuates amygdala responses to emotional faces regardless of valence. Biol Psychiatry. 62: 1187–1190. [DOI] [PubMed] [Google Scholar]
- 12.Eckstein M, Scheele D, Weber KS, Stoffel-Wagner B, Maier W, Hurlemann R (2014): Oxytocin facilitates the sensation of social stress. Hum Brain Mapp. 35: 4741–4750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Duque-Wilckens N, Steinman MQ, Busnelli M, Chini B, Yokoyama S, Pham M, et al. (2018): Oxytocin Receptors in the Anteromedial Bed Nucleus of the Stria Terminalis Promote Stress-Induced Social Avoidance in Female California Mice. Biol Psychiatry. 83: 203–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Dreu CK, Greer LL, Handgraaf MJ, Shalvi S, Van Kleef GA, Baas M, et al. (2010): The neuropeptide oxytocin regulates parochial altruism in intergroup conflict among humans. Science (80-). 328: 1408–1411. [DOI] [PubMed] [Google Scholar]
- 15.De Dreu CKW, Greer LL, Van Kleef GA, Shalvi S, Handgraaf MJJ (2010): Oxytocin promotes human ethnocentrism. Proc Natl Acad Sci U S A. 108: 1262–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beery AK (2015): Antisocial oxytocin: complex effects on social behavior. Curr Opin Behav Sci. 6: 174–182. [Google Scholar]
- 17.van Anders SM, Goodson JL, Kingsbury MA (2013): Beyond “oxytocin=good”: neural complexities and the flipside of social bonds. Arch Sex Behav. 42: 1115–1118. [DOI] [PubMed] [Google Scholar]
- 18.Shamay-Tsoory S, Abu-Akel A (2016): The social salience hypothesis of oxytocin. Biol Psychiatry. 79: 197–202. [DOI] [PubMed] [Google Scholar]
- 19.Bartz J, Zaki J, Bolger N, Ochsner KN (2011): Social effects of oxytocin in humans: context and person matter. Trends Cog Sci. 15: 301–309. [DOI] [PubMed] [Google Scholar]
- 20.Berridge KC (2007): The debate over dopamine’s role in reward: the case for incentive salience. Psychopharmacology (Berl). 191: 391–431. [DOI] [PubMed] [Google Scholar]
- 21.Olney JJ, Warlow SM, Naffziger EE, Berridge KC (2018): Current perspectives on incentive salience and applications to clinical disorders. Curr Opin Neurobiol. 22: 59–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bindra D (1978): How adaptive behavior is produced: a perceptual-motivational alternative to response reinforcements. Behav Brain Sci. 1: 41–91. [Google Scholar]
- 23.Berridge KC, Kringelbach ML (2013): Neuroscience of affect: Brain mechanisms of pleasure and displeasure. Curr Opin Neurobiol.. doi: 10.1016/j.conb.2013.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Skuse DH, Gallagher L (2008): Dopaminergic-neuropeptide interactions in the social brain. Trends Cogn Sci. 13: 27–35. [DOI] [PubMed] [Google Scholar]
- 25.Nasanbuyan N, Yoshida M, Takayanagi Y, Inutsuka A, Nishimori K, Yamanaka A, Onaka T (2018): Oxytocin-Oxytocin Receptor Systems Facilitate Social Defeat Posture in Male Mice. Endocrinology. 159: 763–775. [DOI] [PubMed] [Google Scholar]
- 26.Steinman MQ, Trainor BC (2016): Sex differences in the effects of social defeat on brain and behavior in the California mouse: Insights from a monogamous rodent. Semin Cell Dev Biol.. doi: 10.1016/j.semcdb.2016.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Laman-Maharg A, Trainor BC (2017): Stress, sex, and motivated behaviors. J Neurosci Res. 95. doi: 10.1002/jnr.23815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Salamone JD, Correa M (2012): The mysterious motivational functions of mesolimbic dopamine. Neuron. 76: 470–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schultz W (2016): Dopamine reward prediction error coding. Dialogues Clin Neurosci. 18: 23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Keiflin R, Janak PH (2017): Error-driven learning: dopamine signals more than value-based errors. Curr Biol. 27: R1321–R1323. [DOI] [PubMed] [Google Scholar]
- 31.Berridge KC (2012): From prediction error to incentive salience: mesolimbic computation of reward motivation. Eur J Neurosci. 35: 1124–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.O’Connell LA, Hofmann HA (2011): The vertebrate mesolimbic reward system and social behavior network: a comparative synthesis. J Comp Neurol. 3599–3639. [DOI] [PubMed] [Google Scholar]
- 33.McHenry JA, Otis JM, Rossi MA, Robinson JE, Kosyk O, Miller NW, et al. (2017): Hormonal gain control of a medial preoptic area social reward circuit. Nat Neurosci. 20: 449–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gunaydin LA, Grosenick L, Finkelstein JC, Kauvar IV, Fenno LE, Adhikari A, et al. (2014): Natural neural projection dynamics underlying social behavior. Cell. 157: 1535–1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bariselli S, Tzanoulinou S, Glangetas C, Prévost-Solié C, Pucci L, Viguié J, et al. (2016): SHANK3 controls maturation of socal reward circuits in the VTA. Nat Neurosci. 19: 926–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Izuma K, Saito DN, Sadato N (2008): Processing of social and monetary rewards in the human striatum. Neuron. 58: 284–294. [DOI] [PubMed] [Google Scholar]
- 37.Davey CG, Allen NB, Harrison BJ, Dwyer DB, Yucel M (2010): Being liked activates primary reward and midline self-related brain regions. Hum Brain Mapp. 31: 660–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Masten CL, Eisenberger NI, Borofsky LA, Pfeifer JH, McNealy K, Mazziotta JC, Mirella D (2009): Neural correlates of social exclusion during adolescence: understanding the distress of peer rejection. Soc Cogn Affect Neurosci. 4: 143–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brischoux F, Chakraborty S, Brierley DI, Ungless MA (2009): Phasic excitation of dopamine neurons in ventral VTA by noxious stimuli. Proc Natl Acad Sci U S A. 106: 4894–4899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Greenberg GD, Steinman MQ, Doig IE, Hao R, Trainor BC (2015): Effects of social defeat on dopamine neurons in the ventral tegmental area in male and female California mice. Eur J Neurosci. 42. doi: 10.1111/ejn.13099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tidey JW, Miczek KA (1996): Social defeat stress selectively alters mesocorticolimibic dopamine release: an in vivo microdialysis study. Brain Res. 721: 140–149. [DOI] [PubMed] [Google Scholar]
- 42.Gray CL, Norvelle A, Larkin K, Huhman KL (2015): Dopamine in the nucleus accumbens modulates the memory of social defeat in Syrian hamsters (Mesocricetus auratus). Behav Brain Res. 286: 22–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Krishnan V, Han M-H, Graham DL, Berton O, Renthal W, Russo SJ, et al. (2007): Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell. 131: 391–404. [DOI] [PubMed] [Google Scholar]
- 44.Anstrom KK, Miczek KA, Budygin EA (2009): Increased phasic dopamine signaling in the mesolimbic pathway during social defeat in rats. Neuorscience. 161: 3–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chaudhury D, Walsh JJ, Friedman AK, Juarez B, Ku SM, Koo JW, et al. (2013): Rapid regulation of depression-related behaviours by control of midbrain dopamine neurons. Nature. 493: 532–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Campi KL, Greenberg GD, Kapoor A, Ziegler TE, Trainor BC (2014): Sex differences in effects of dopamine D1 receptors on social withdrawal. Neuropharmacology. 77. doi: 10.1016/j.neuropharm.2013.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bosch OJ, Dabrowska J, Modi ME, Johnson ZV, Keebaugh AC, Barrett CE, et al. (2016): Oxytocin in the nucleus accumbens shell reverses CRFR2-evoked passive stress-coping after partner loss in monoagmous male prairie voles. Psychoneuroendocrinology. 64: 66–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shahrokh DK, Zhang T-Y, Diorio J, Gratton A, Meaney MJ (2010): Oxytocin-dopamine interactions mediate variations in maternal behavior in the rat. Endocrinology. 151: 2276–2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Young LJ, Wang Z (2004): The neurobiology of pair bonding. Nat Neurosci. 7: 1048–1054. [DOI] [PubMed] [Google Scholar]
- 50.Wang Z, Aragona BJ (2004): Neurochemical regulation of pair bonding in male prairie voles. Physiol Behav. 83: 319–328. [DOI] [PubMed] [Google Scholar]
- 51.Scheele D, Wille A, Kendrick KM, Stoffel-Wagner B, Becker B, Gunturkun O, et al. (2013): Oxytocin enhances brain reward system responses in men viewing the face of their female partner. Proc Natl Acad Sci U S A. 110: 20308–20313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Leng G, Ludwig M (2016): Intranasal oxytocin:myths and delusions. Biol Psychiatry. 79: 243–250. [DOI] [PubMed] [Google Scholar]
- 53.Dolen G, Darvishzadeh A, Huang KW, Malenka RC (2013): Social reward requires coordinated activity of nucleus accumbens oxytocin and serotonin. Nature. 501: 179–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ferrari PF, Van erp AMM, Tornatzky W, Miczek KA (2003): Accumbal dopamine and serotonin in anticipation of the next aggressive episode in rats. Eur J Neurosc. 17: 371–378. [DOI] [PubMed] [Google Scholar]
- 55.Calcagnoli F, Meyer N, De Boer SF, Althaus M, Koolhaas J (2014): Chronic enhancement of brain oxytocin levels causes enduring anti-aggressive and pro-social explorative behavioral effects in male rats. Horm Behav. 65: 427–433. [DOI] [PubMed] [Google Scholar]
- 56.Xiao L, Priest MF, Nasenbeny J, Lu T, Kozorovitskiy Y (2017): Biased Oxytocinergic Modulation of Midbrain Dopamine Systems. Neuron. 95: 368–384 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Song Z, Borland JM, Larkin TE, O’Malley M, Albers HE (2016): Activation of oxytocin receptors, but not arginine-vasopressin V1a receptors, in the ventral tegmental area of male Syrian hamsters is essential for the reward-like properties of social interactions. Psychoneuroendocrinology. 74: 164–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hung LW, Neuner S, Polepalli JS, Beier KT, Wright M, Walsh JJ, et al. (2017): Gating of social reward by oxytocin in the ventral tegmental area. Science (80-). 357: 1406–1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang L, Hou W, He Z, Yuan W, Yang J, Yang Y, et al. (2018): Effects of chronic social defeat on social behaviors in adult female mandarin voles (Microtus mandarinus): Involvement of the oxytocin system in the nucleus accumbens. Prog Neuropsychopharmacol Biol Psychiatry. 82: 278–288. [DOI] [PubMed] [Google Scholar]
- 60.Lukas M, Toth I, Reber SO, Slattery DA, Veenema AH, Neumann ID (2011): The neuropeptide oxytocin facilitates pro-social behavior and prevents social avoidance in rats and mice. Neuropsychopharmacology. 36: 2159–2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Steinman MQ, Duque-Wilckens N, Greenberg GD, Hao R, Campi KL, Laredo SA, et al. (2016): Sex-Specific Effects of Stress on Oxytocin Neurons Correspond With Responses to Intranasal Oxytocin. Biol Psychiatry. 80. doi: 10.1016/j.biopsych.2015.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Engelmann M, Ebner K, Landgraf R, Holsboer F, Wotjak CT (1999): Emotional stress triggers intrahypothalamic but not periperal release of oxytocin in male rats. J Neuroendocr. 11: 867–872. [DOI] [PubMed] [Google Scholar]
- 63.Busnelli M, Sauliere A, Manning M, Bouvier M, Gales C, Chini B (2012): Functional selective oxytocin-derived agonists discriminate between individual G protein family subtypes. J Biol Chem. 287: 3617–3629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Umbricht D, Del Valle Rubido M, Hollander E, McCracken JT, Shic F, Scahill L, et al. (2017): A single dose, randomized, controlled proof-of-mechanism study of a novel vasopressin 1a receptor antagonist (rg7713) in high-functioning adults with autism spectrum disorder. Neuropsychopharmacology.. doi: 10.1038/npp.2016.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Parker KJ, Garner JP, Oztan O, Tarara ER, Li J, Sclafani V, et al. (2018): Arginine vasopressin in cerebrospinal fluid is a marker of sociality in nonhuman primates. Sci Transl Med.. doi: 10.1126/scitranslmed.aam9100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sabatier N, Caquineau C, Dayanithi G, Bull P, Douglas AJ, Guan XMM, et al. (2003): α-Melanocyte-Stimulating Hormone Stimulates Oxytocin Release from the Dendrites of Hypothalamic Neurons While Inhibiting Oxytocin Release from Their Terminals in the Neurohypophysis. J Neurosci. 23: 10351–10358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Song Z, McCann KE, McNeill JK 4th, Larkin TE 2nd, Huhman KL, Albers HE (2014): Oxytocin induces social communication by activating arginine-vasopressin V1a receptors and not oxytocin receptors. Psychoneuroendocrinology. 50: 14–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.DiBenedictis BT, Nussbaum ER, Cheung HK, Veenema AH (2017): Quantitative mapping reveals age and sex differences in vasopressin, but not oxytocin, immunoreactivity in the rat social behavior neural network. J Comp Neurol. 525: 2549–2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bredewold R, Nascimento NF, Ro GS, Cieslewski SE, Reppucci CJ, Veenema AH (2018): Involvement of dopamine, but not norepinephrine, in the sex-specific regulation of juvenile socially rewarding behavior by vasopressin. Neuropsychopharmacology.. doi: 10.1038/s41386-018-0100-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Guzman YF, Tronson NC, Jovasevic V, Sato K, Guedea AL, Mizukami H, et al. (2013): Fear-enhancing effects of septal oxytocin receptors. Nat Neurosci. 16: 1185–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Guzman YF, Tronson NC, Sato K, Mesic I, Guedea AL, Nishimori K, Radulovic J (2014): Role of oxytocin receptors in modulation of fear by social memory. Psychopharmacol. 231: 2097–2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Toth I, Neumann ID, Slattery DA (2012): Social fear conditioning: a novel and specific animal model to study social anxiety disorder. Neuropsychopharmacology.. doi: 10.1038/npp.2011.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zoicas I, Slattery DA, Neumann ID (2014): Brain oxytocin in social fear conditioning and its extinction: involvement of the lateral septum. Neuropsychopharmacology. 39: 3027–3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Menon R, Grund T, Zoicas I, Althammer F, Fiedler JL, Biermeier V, et al. (2018): Oxytocin signaling in the lateral septum prevents social fear during lactation. Curr Biol. 28: 1066–1078. [DOI] [PubMed] [Google Scholar]
- 75.Song Z, Albers HE (2017): Cross-talk among oxytocin and arginine-vasopressin receptors: Relevance for basic and clinical studies of the brain and periphery. Front Neuroendocrinol.. doi: 10.1016/j.yfrne.2017.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ju G, Swanson LW, Simerly RB (1989): Studies on the cellular architecture of the bed nucleus of the stria terminalis in the rat: II. chemoarchitecture. J Comp Neurol. 280: 603–621. [DOI] [PubMed] [Google Scholar]
- 77.Alheid GF, Heimer L (1988): New perspectives in basal forebrain organization of special relevance for neropsychiatric disorders: the striatopallidal amygdaloid and corticopetal components of the substantia innominata. Neuroscience. 27: 1–39. [DOI] [PubMed] [Google Scholar]
- 78.Dong H-W, Petrovich GD, Swanson LW (2001): Topography of projections from amygdala to bed nuclei of the stria terminalis. Brain Res Rev. 38: 192–246. [DOI] [PubMed] [Google Scholar]
- 79.Paxinos G, Franklin KBJ (2003): The mouse brain in stereotaxic coordinates, 2nd ed New York: Academic Press. [Google Scholar]
- 80.Dominguez J, Riolo JV, Xu Z, Hull EM (2001): Regulation by the medial amygdala of copulation and medial preoptic dopamien release. J Neurosci. 21: 349–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hong W, Kim D, Anderson DJ (2014): Antagonistic control of social versus repetitive self-grooming behaviors by separable amygdala neuronal subsets. Cell. 158: 1348–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Henckens MJAG, Printz Y, Shamgar U, Dine J, Lebow M, Drori Y, et al. (2017): CRF receptor type 2 neurons in the posterior bed nucleus of the stria terminalis critically contribute to stress recovery. Mol Psychiatry. 22: 1691–1700. [DOI] [PubMed] [Google Scholar]
- 83.Fox AS, Oler JA, Tromp DPM, Fudge JL, Kalin NH (2015): Extending the amygdala in theories of threat processing. Trends Neurosci. 38: 319–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Daniel SE, Rainnie DG (2016): Stress modulation of opposing circuits in the bed nucleus of the stria terminalis. Neuropsychopharmacology. 41: 103–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Walker DL, Toufexis DJ, Davis M (2003): Role of the bed nucleus of the stria terminalis versus the amygdala in fear, stress, and anxiety. Eur J Pharmacol. 463: 199–216. [DOI] [PubMed] [Google Scholar]
- 86.Asok A, Draper A, Hoffman AF, Schulkin J, Lupica CR, Rosen JB (2018): Optogenetic silencing of a corticotropin-releasing factor pathway from the central amygdala to the bed nucleus of the stria terminalis disrupts sustained fear. Mol Psychiatry. 23: 914–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Shackman AJ, Fox AS (2016): Contributions of the central extended amygdala to fear and anxiety. J Neurosci. 36: 8050–8063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kim S-Y, Adhikari A, Lee SY, Marshel JH, Kim CK, Mallory CS, et al. (2013): Diverging neural pathways assemble a behavioural state from separable features in anxiety. Nature. 496: 219–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Duque-Wilckens N, Steinman MQ, Laredo SA, Hao R, Perkeybile AM, Bales KL, Trainor BC (2016): Inhibition of vasopressin V1a receptors in the medioventral bed nucleus of the stria terminalis has sex- and context-specific anxiogenic effects. Neuropharmacology. 110. doi: 10.1016/j.neuropharm.2016.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Markham CM, Norvelle A, Huhman KL (2009): Role of the bed nucleus of the stria terminalis in the acquisition and expression of conditioned defeat in Syrian hamsters. Behav Brain Res. 198: 69–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kollack-Walker S, Watson SJ, Akil H (1997): Social stress in hamsters: defeat activates specific neurocircuits within the brain. J Neurosci. 17: 8842–8855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Martinez M, Phillips PJ, Herbert J (1998): Adaptation in patterns of c-fos expression in the brain associated with exposure to either single or repeated social stress in male rats. Eur J Neurosci. 10: 20–33. [DOI] [PubMed] [Google Scholar]
- 93.Pego JM, Morgado P, Pinto LG, Cerqueira JJ, Almeida OFX, Souss (2008): Dissociation of the morphological correlates of stress-induced anxiety and fear. Eur J Neurosci. 27: 1503–1516. [DOI] [PubMed] [Google Scholar]
- 94.Greenberg GD, Laman-Maharg A, Campi KL, Voigt H, Orr VN, Schaal L, Trainor BC (2014): Sex differences in stress-induced social withdrawal: Role of brain derived neurotrophic factor in the bed nucleus of the stria terminalis. Front Behav Neurosci. 7. doi: 10.3389/fnbeh.2013.00223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Taylor SL, Stanek LM, Ressler KJ, Huhman KL (2011): Differential BDNF expression in limbic brain regions following social defeat or territorial aggression. Behav Neurosci. 125: 911–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sterrenburg L, Gaszner B, Boerrigter J, Stantbergen L, Bramini M, Roubos EW, et al. (2012): Sex-dependent and differential responses to acute restraint stress of corticotropin-releasing factor-producing neurons in the rat paraventricular nucleus, central amygdala, and bed nucleus of the stria terminalis. J Neurosci Res. 90: 179–192. [DOI] [PubMed] [Google Scholar]
- 97.Jasnow AM, Davis M, Huhman KL (2004): Involvement of central amygdalar and bed nucleus of the stria terminalis corticotropin-releasing factor in behavioral responses to social defeat. Behav Neurosci. 118: 1051–1061. [DOI] [PubMed] [Google Scholar]
- 98.Cooper MA, Huhman KL (2005): Corticotropin-releasing factor type II (CRF-sub-2) receptors in the bed nucleus of the stria terminalis modulate conditioned defeat in Syrian hamsters (Mesocricetus auratus). Behav Neurosci. 119: 1042–1051. [DOI] [PubMed] [Google Scholar]
- 99.Dong HW, Swanson LW (2006): Projections from bed nuclei of the stria terminalis, anteromedial area: Cerebral hemisphere integration of neuroendocrine, autonomic, and behavioral aspects of energy balance. J Comp Neurol. 494: 142–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gross CT, Canteras NS (2012): The many paths to fear. Nat Rev Neurosci. 13: 651–658. [DOI] [PubMed] [Google Scholar]
- 101.Lebow MA, Chen A (2016): Overshadowed by the amygdala: the bed nucleus of the stria terminalis emerges as key to psychiatric disorders. Mol Psychiatry. 21: 450–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wang Z, Moody K, Newman JD, Insel TR (1997): Vasopressin and oxytocin immunoreactive neurons and fibers in the forebrain of male and female common marmosets (Callithrix jacchus). Synapse. 27: 14–25. [DOI] [PubMed] [Google Scholar]
- 103.Hammack SE, Guo J-D, Hazra R, Dabrowska J, Myers KM, Rainnie DG (2009): The response of neurons in the bed nucleus of the stria terminalis to serotonin: implications for anxiety. Prog Neuropsychopharmacol Biol Psychiatry. 33: 1309–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Marcinkiewcz CA, Mazzone CM, D’Agostino G, Halladay LR, Hardaway JA, DiBerto JF, et al. (2016): Serotonin engages an anxiety and fear-promoting circuit in the extended amygdala. Nature. 537: 97–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Williams AV, Laman-Maharg A, Armstrong CV, Ramos-Maciel S, Minie VA, Trainor BC (2018): Acute inhibition of kappa opioid receptors before but not after social defeat blocks anhedonia and social avoidance in female California mice. Prog Neuropsychopharmacol Biol Psychiatry. 86: 166–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Iñiguez SD, Flores-Ramirez FJ, Riggs LM, Alipio JB, Garcia-Carachure I, Hernandez MA, et al. (2018): Vicarious social defeat strress induces depression-related outcomes in female mice. Biol Psychiatry. 83: 9–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Choleris E, Gustaffson J-A, Korach KS, Muglia LJ, Pfaff DW, Ogawa S (2003): An estrogen-dependent four-gene micronet regulating social recognition: a study with oxytocin and estrogen receptor-alpha and -beta knockout mice. Proc Natl Acad Sci USA. 100: 6192–6197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Fox NA, Henderson HA, Marshall PJ, Nichols KE, Ghera MM (2005): Behavioral inhibition: linking biology and behavior within a developmental framework. Annu Rev Psychol. 56: 235–262. [DOI] [PubMed] [Google Scholar]
- 109.Kagan J, Reznick JS, Snidman N (1987): The physiology and psychology of behavioral inhibition in children. Child Dev. 58: 1459–1473. [PubMed] [Google Scholar]
- 110.Clauss JA, Blackford JU (2012): Behavioral inhibition and risk for developing social anxiety disorder: a meta-analytic study. J Am Acad Child Adolesc Psychiatry. 51: 1066–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Grillon C, Krimsky M, Charney DR, Vytal K, Ernst M, Cornwell B (2013): Oxytocin increases anxiety to unpredictable threat. Mol Psychiatry. 18: 958–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Silk JS, Siegle GJ, Lee KH, Nelson EE, Stroud LR, Dahl RE (2014): Increased neural response to peer rejection associated with adolescent depression and pubertal development. Soc Cogn Affect Neurosci. 9: 1798–1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Germine LT, Garrido L, Bruce L, Hooker C (2011): Social anhedonia is associated with neural abnormalities during face emotion processing. Neuroimage. 58: 935–945. [DOI] [PubMed] [Google Scholar]
- 114.Dumais KM, Veenema AH (2016): Vasopressin and oxytocin receptor systems in the brain: sex differences and sex-specific regulation of social behavior. Front Neuroendocr. 40: 1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Grippo AJ, Gerena D, Huang J, Kumar N, Shah M, Ughreja R, Sue Carter C (2007): Social isolation induces behavioral and neuroendocrine disturbances relevant to depression in female and male prairie voles. Psychoneuroendocrinology. 32: 966–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Borland JM, Rilling JK, Frantz KJ, Albers HE (2018): Sex-dependent regulation of social reward by oxytocin: an inverted U hypothesis. Neuropsychopharmacology.. doi: 10.1038/s41386-018-0129-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Rilling JK, Chen X, Chen X, Haroon E (2018): Intranasal oxytocin modulates neural functional connectivity during human social interaction. Am J Primatol.. doi: 10.1002/ajp.22740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Macosko EZ, Basu A, Satija R, Nemesh J, Shekhar K, Goldman M, et al. (2015): Highly parallel genome-wide expression profiling of individual cells using nanoliter droplets. Cell.. doi: 10.1016/j.cell.2015.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.McQuaid RJ, McInnis OA, Abizaid A, Anisman H (2014): Making room for oxytocin in understanding depression. Neurosci Biobehav Rev. 45: 305–322. [DOI] [PubMed] [Google Scholar]
- 120.Neumann ID, Slattery DA (2016): Oxytocin in general anxiety and social fear: a translation approach. Biol Psychiatry. 79: 213–221. [DOI] [PubMed] [Google Scholar]
- 121.Fulford D, Treadway M, Woolley J (2018): Social motivation in schizophrenia: the impact of oxytocin on vigor in the context of social and nosnocial reinforcement. J Abnorm Psych. 127: 116–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Andari E, Hurlemann R, Young LJ, Hurlemann R, Grinevich V (2018): A precision medicine approach to oxytocin trials. Curr Top Behav Neurosci. (Vol. 35). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Leppanen J, Ng KW, Kim Y-R, Tchanturia K, Treasure J (2018): Meta-analytic review of the effects of a single dose of intranasal oxytocin on threat processing in humans. J Affect Disord. 225: 167–179. [DOI] [PubMed] [Google Scholar]
- 124.MacDonald K, Feifel D (2014): Oxytocin’s role in anxiety: a critical appraisal. Brain Res. 1580: 22–56. [DOI] [PubMed] [Google Scholar]
- 125.Hofmann SG, Fang A, Brager DN (2015): Effect of intranasal oxytocin administration on psychiatric symptoms: a meta-analysis of placebo-controlled studies. Psychiatry Res. 228: 708–714. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 126.Quintana DS (2018): Revisiting non-significant effects of intranasal oxytocin using equivalence testing. Psychoneuroendocrinology.. doi: 10.1016/j.psyneuen.2017.10.010. [DOI] [PubMed] [Google Scholar]
- 127.Quintana DS, Guastella AJ, Westlye LT, Andreassen OA (2016): The promise and pitfalls of intranasally administering psychopharmacological agents for the treatment of psychiatric disorders. Mol Psychiatry. 21: 29–38. [DOI] [PubMed] [Google Scholar]
- 128.Guastella AJ, Hickie IB (2016): Oxytocin treatment, circuitry, and autism: a critical review of the literature placing oxytocin into the autism context. Biol Psychiatry. 79: 234–242. [DOI] [PubMed] [Google Scholar]
- 129.Walum H, Waldman ID, Young LJ (2016): Statistical and Methodological Considerations for the Interpretation of Intranasal Oxytocin Studies. Biol Psychiatry.. doi: 10.1016/j.biopsych.2015.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Biederman J, Rosenbaum JF, Hirshfeld-Becker DR, Faraone SV, Bolduc EA, Gersten M, et al. (1990): Psychiatric correlates of behavioral inhibition in young children of parents with and without psychiatric disorders. Arch Gen Psychiatry. 47: 21–26. [DOI] [PubMed] [Google Scholar]
- 131.Wang D, Yan X, Li M, Ma Y (2017): Neural substrates underlying the effects of oxytocin: A quantitative meta-analysis of pharmaco-imaging studies. Soc Cogn Affect Neurosci. . doi: 10.1093/scan/nsx085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Grace SA, Rossell SL, Heinrichs M, Kordsachia C, Labuschagne I (2018): Oxytocin and brain activity in humans: A systematic review and coordinate-based meta-analysis of functional MRI studies. Psychoneuroendocrinology.. doi: 10.1016/j.psyneuen.2018.05.031. [DOI] [PubMed] [Google Scholar]
- 133.Fuxjager MJ, Trainor BC, Marler CA (2017): What can animal research tell us about the link between androgens and social competition in humans? Horm Behav. 92. [DOI] [PubMed] [Google Scholar]
- 134.Goetz SMM, Tang L, Thomason ME, Diamond MP, Hariri AH, Carré JM (2014): Testosterone rapidly increases neural reactivity to threat in healthy men: a novel two-step pharmacological challenge paradigm. Biol Psychiatry. 76: 324–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Eisenberger NI, Lieberman MD, Williams KD (2003): Does rejection hurt? An fMRI study of social exclusion. Science (80-).. doi: 10.1126/science.1089134. [DOI] [PubMed] [Google Scholar]
- 136.Guyer AE, Choate VR, Detloff A, Benson B, Nelson EE, Perez-Edgar K, et al. (2012): Striatal functional alteration during incentive anticipation in pediatric anxiety disorders. Am J Psychiatry. 169: 205–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Klumpers F, Kroes MCW, Baas JMP, Fernandez G (2017): How human amygdala and bed nucleus of the stria terminalis may drive distinct defensive responses. J Neurosci. 37: 9645–9656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Knobloch HS, C A, Hoffmann LC, Eliava M, Khrulev S, Cetin AH, et al. (2012): Evoked axonal oxytocin release in the central amygdala attenuates fear response. Neuron. 73: 553–566. [DOI] [PubMed] [Google Scholar]
- 139.Viviani D, Charlet A, van den Burg E, Robinet C, Hurni N, Abatis M, et al. (2011): Oxytocin selectively gates fear responses through distinct outputs from the central amygdala. Science (80-). 333: 104–107. [DOI] [PubMed] [Google Scholar]
- 140.Jennings JH, Sparta DR, Stamatakis AM, Ung RL, Pleil KE, Kash TL, Stuber GD (2013): Distinct extended amygdala circuits for divergent motivational states. Nature. 496: 224–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Rogers-Carter MM, Varela JA, Gribbons KB, Pierce RC, McGoey MT, Ritchey M, Christianson JP (2018): Insular cortex mediates approach and avoidance responses to social affective stimuli. Nat Neurosci. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Choe HK, Reed MD, Benavidez N, Montgomery D, Soares N, Yim YS, Choi GB (2015): Oxytocin mediates entrainment of sensory stimuli to social cues of opposing valence. Neuron. 87: 152–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Sabihi S, Durosko NE, Dong SM, Leuner B (2014): Oxytocin in the prelimbic medial prefrontal cortex redcues anxiety-like behavior in female and male rats. Psychoneuroendocrinology. 45: 31–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Marlin BJ, Mitre M, D’amour JA, Chao MV, Froemke RC (2015): Oxytocin enables maternal behaviour by balancing cortical inhibition. Nature. 520: 499–504. [DOI] [PMC free article] [PubMed] [Google Scholar]