A coordinated electrical propagation throughout the heart is necessary for appropriate cardiac function. When such coordination in cardiac action potential (AP) fails, cardiac arrhythmias occur [1]. Several sub-cellular factors contribute to AP heterogeneity, mainly related to sarcolemmal ion channels; however, a growing attention is focused on the action of mitochondria on cell excitability. Indeed, albeit the mitochondrial role in energy production and apoptotic pathways has been determined, the contribution of this highly dynamic organelle in the regulation of excitation–contraction coupling is less clear.
Mitochondria form a functional network within the cardiomyocyte that represents 20–30% of myocardial volume and produces over 95% of cellular ATP. ATP production is made possible by mitochondrial membrane potential (ΔΨm) that generate a proton motive force liberating the energy necessary to phosphorylate ADP to ATP [2]. This mechanism is also one of the main sources of reactive oxygen species (ROS) in the cell. In physiological conditions ΔΨm is highly regulated, so that ATP production sufficiently responds to energy demand and ROS does not exceed cell-detoxifying capacity. In response to pathological stimuli, including ischemia and structural injury, alterations of ΔΨm cause reduction of ATP generation, and increase of ROS production that once exceeds the detoxifying capacity, leads to oxidative stress.
The involvement of mitochondria in arrhythmogenesis derives from their ability to produce both ATP and ROS; indeed, on one side, mitochondrial dysfunction can affect electrical function of the heart through reduced ATP production, altering sarcolemmal K+ fluxes via ATP-sensitive potassium channels. On the other side, excessive mitochondrial ROS production can introduce heterogeneity into cardiac action potential since during oxidative stress the oxidation of mitochondrial ion channels can alter their kinetic opening loading to current dispersion and eventually collapse of ΔΨm. The ATP- and ROS-based mechanisms are part of the same cellular response: during mitochondrial dysfunction ΔΨm reduction occurs, resulting in attenuated ATP production and increased ROS level; the latter, in turn, induces further mitochondrial dysfunction, with ΔΨm reduction and consequent reduced ATP generation. This vicious cycle, in which different responses act in synergic manner, causes electrophysiological alterations, thereby conferring a central role to mitochondria in arrhythmogenesis.
Functional Role of Mitochondria in Linking Metabolism and Cell Excitability
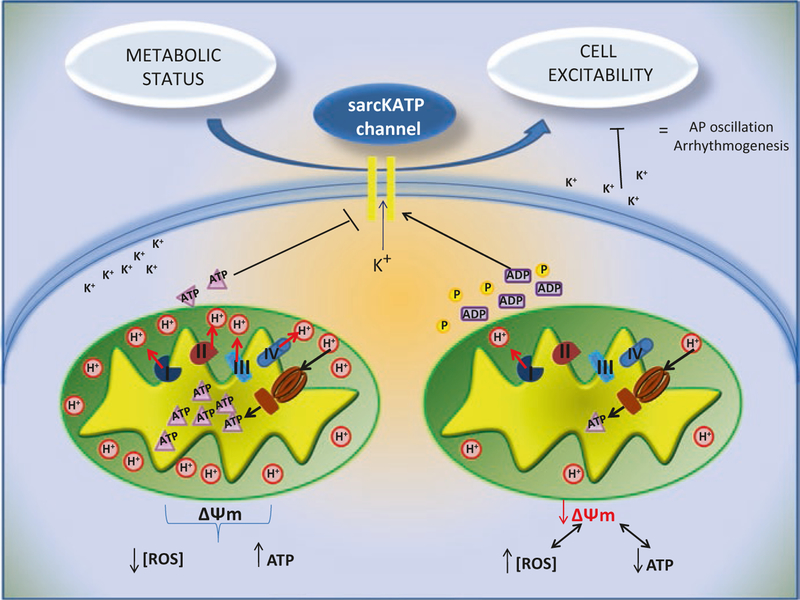
Several studies have shown the influence of mitochondrial energetic status on the sarcolemmal action potential and heart excitability. The first experimental evidence derives from simultaneous detection of ΔΨm and AP. ΔΨm oscillations, induced by photo-oxidation, coincide with AP oscillations: in particular, during ΔΨm collapse also AP collapses until a cell non-excitable state, while ΔΨm recovery is mirrored by AP recovery. The impact of mitochondria on cellular excitability is mainly mediated by a class of ion channels abundant in cardiac tissue: energy sensing, ATP sensitive K+ channels on sarcolemmal membrane (sarcKATP). These channels are heteromultimers inhibited by intracellular ATP and activated by ADP, Pi, Mg2+ and extremely sensitive to oxidation. Therefore, sarcKATP channels represent a crucial link between electrical function and metabolism, a dynamic relationship that has been confirmed by independent investigators [3, 4] (Fig. 10.1). During metabolic stress, oscillations of sarcKATP current occur in phase with variation of NADH concentration, with losses of ΔΨm, and that these oscillations are responsible of reduced length of AP [5]. Therefore, metabolic stress like nutrient deprivation can impair mitochondrial function, with reduction of ΔΨm and consequent diminished production of ATP, which in physiological conditions inhibits sarKATP channels. The result is the activation of these channels with K+ dispersion and reduction of cell excitability, allowing mitochondria to indirectly affect the AP [6]. Such effect has a citoprotective role for myocytes during ischemia, when mitochondria cannot provide adequate ATP to support cardiac energy demand; thus, the sarKATP-mediated reduction in excitability and calcium (Ca2+) transients attenuates cell death during metabolic stress [7–10].
Fig. 10.1.
Role of sarcKATP channels in linking cell metabolic status and cell excitability. Mitochondrial dysfunction lead to increase of ROS and reduction of mitochondrial potential with consequent decrease of ATP production. ATP decrease, ATP and Pi activate sarcKATP channel, with K dissipating current and reduction of AP that predispose to arrhythmia
Mounting evidence supports the mechanistic role of sarKATP in arrhythmogenesis as a sensor of metabolic condition of the cell. Importantly, a reduction in ventricular arrhythmia development after blocking sarcKATP channels using HMR1883 has been reported in studies in both animal and human studies [11–14]. However, other studies have shown that the blocking of these channels with glibenclamide is not enough to rescue an arrhythmic phenotype [15]. Because of these conflicting findings further studies are necessary to elucidate the molecular mechanisms that occur before and after activation of these channels, and to clarify the role of several mitochondrial ion channels that could modify their kinetics in response to metabolic stress. The correct dynamic activity of cellular ion channels is synergistically linked to mitochondria, not only for ATP demand but also for mitochondrial ROS generation.
Role of Mitochondrial ROS in the Pathogenesis of Cardiac Arrhythmias
If reduced ATP production as consequence of mitochondrial dysfunction can directly affect the activity of several ion channels, concomitant ROS generation can activate a complex response which in turn amplifies ΔΨm alterations that further inhibit ATP production and increase ROS generation. Sallott and colleagues demonstrated a ROS-dependent oscillation of ΔΨm, with collapse of ΔΨm prevented by ROS scavenger [16, 17]. As mentioned above, given that ΔΨm oscillations promote alterations of myocyte AP, the ability of ROS to affect mitochondrial membrane potential confers them a pro-arrhythmogenic role. In particular such arrhythmogenic effect of ROS depends on complex and multifactorial response of cardiac mitochondria, named ROS-induced ROS release (RIRR), an autocatalytic process by which high levels of ROS induce further ROS release from mitochondria [17]. Mitochondrial-dependent RIRR regulates electro-chemical equilibrium, through oxidation of several proteins that control current fluxes inside the myocyte; the main consequences of oxidative stress are: activation of sarcKATP channels (that are sensible to oxidation, too), with reduction of Na+ and K+ currents [18]; altered kinetic of L-Type Ca2+ channels; increase of intracellular Ca2+ leak from RyR on the sarcoplasmic reticulum [19]. The functional role of mitochondria-dependent RIRR in arrhythmogenesis has been demonstrated also in whole heart, in ex-vivo experiments; stimulation of the heart with H2O2 leads to a two-phase ROS production: the first pick of ROS is directly linked to H2O2 action, while the second pick corresponds to ROS of endogenous production derived by mitochondrial RIRR and is associated with arrhythmia [20]. The mechanism of RIRR represents a concerted response mediated by different mitochondrial channels, that are sensitive to ROS concentration, including internal Mitochondria Anion Channel (IMAC), Permeability Transition Pore (PTP), Translocator Protein (TSPO), Mitochondrial Ca2+ Uniporter (MCU), and mitochondrial ATP- sensitive K+ channel (mKATP channel). These channels change their activities in response to crescent levels of ROS and then activate RIRR. Despite the specific contribute of each of these channels to RIRR is not fully understood, the important evidence is that mitochondrial ion channels have a central role in the regulation of electrophysiology equilibrium; indeed through them cell excitability is affected by metabolic status of the cell; therefore, they could represent new and innovative therapeutic target for pathological conditions characterized by electrical alterations, typically arrhythmias, mostly in response to metabolic stress.
Mitochondrial Channels and Arrhythmogenesis
The metabolism-excitation axis is finely regulated by several mitochondrial channels including the inner membrane anion channel (IMAC), the mitochondrial permeability transition pore (mPTP) and the translocator protein (TSPO), and by their crosstalk.
IMAC is a channel deputed to anion efflux from mitochondria [21]. Although its chemical structure is not completely determined, it is known to be one of the channels proposed to participate in energy dissipation in mitochondria, determining ΔΨm collapse and consequent AP reduction [22–24]. In vitro, the blockade of IMAC during metabolic stress stabilizes ΔΨm and determines recovery of AP; on the other hand, activation of IMAC accelerates the shortening of AP through RIRR [10]. These results are confirmed in intact mammalian hearts [10, 25, 26]. Indeed, in guinea pig hearts IMAC inhibition reduces the ischemia-induced AP shortening and coincides with decreased tachycardia/fibrillation during reperfusion [10]. This evidence opens the possibility to target IMAC as a primary mitochondrial mediator of ROS amplification, ΔΨm reduction, and AP oscillations, for novel anti-arrhythmic therapeutic strategies.
The electrochemical equilibrium in mitochondria is finely orchestrated by mPTP, a large conductance channel located on the inner mitochondrial membrane that plays a crucial role in the regulation of cell death [27]. Although some studies demonstrate that the inhibition of mPTP is protective for cardiac cells during ischemia, there are controversial results about the role of this channel in determining arrhythmia. In isolated cells, the collapse of ΔΨm induced by laser flash is not inhibited by mPTP blocker, cyclosporine A [4, 16, 28]. A non-protective effect of cyclosporine on arrhythmia was confirmed in rat, pig, and rabbit [10, 25, 29]. However, mPTP is directly involved in RIRR; therefore this channel contributes to the effects of metabolism on the cell excitability, but its inhibition is not enough to prevent arrhythmia [30].
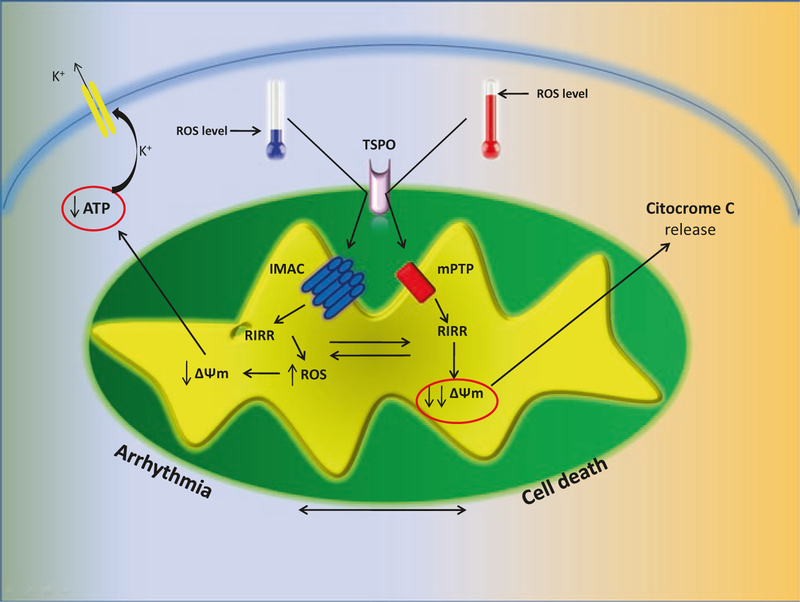
Interestingly, IMAC-mediated RIRR and mPTP-mediated RIRR are both regulated by a third protein, located on the outer mitochondrial membrane, TSPO. Altered expression and activity of this translocator protein have been reported in ischemia/reperfusion injury and myocardial infarction, both of which are considered mayor risk factors for arrhythmias. Growing evidence suggests that TSPO can affect arrhythmic response regulating RIRR, directly through IMAC and indirectly through mPTP. TSPO ligands abolish metabolic and electrophysiological oscillations induced by oxidative stress, and reduce ROS levels in cardiomyocytes [31]; moreover, TSPO inhibition abolishes the second pick of ROS production in response to H2O2 that represents the RIRR- derived ROS, suppressing ventricular fibrillation and the frequency of arrhythmogenic triggers [20]. This ability of TSPO to promote RIRR with consequent pro-arrhythmogenic effect, is mediated mainly by its action on IMAC. IMAC is tight regulated by several TSPO -acting -ligands, suggesting a direct interaction between pore- forming subunit of IMAC in the inner-mitochondria-membrane, and the regulatory protein TSPO in out mitochondrial membrane [32–34]. Several studies, demonstrate that TSPO is able to regulate mPTP too, indirectly through interaction with VDAC and ANT [35, 36]. Indeed, TSPO block results in inhibition of ΔΨm depolarization after ROS production, but at the same time also in increase of the cell survival in response to oxidative stress, with reduction of cytochrome C release, caspase-3 activation and DNA fragmentation [37]. This evidence suggests a hierarchal activation pattern of mitochondrial ion channels, with TSPO playing a key regulatory role. In response to moderate levels of ROS, TSPO could mediate activation of IMAC with initial energy dissipation that results in RIRR activation, ΔΨm partial depolarization and consequent AP reduction, ensuring a protective status for the cell characterized by reduced excitability to lower energy demand. When metabolic stress is persistent and ROS levels, derived also from RIRR, mediate extreme oxidative stress, TSPO oxidation leads to the activation of the large conductance mPTP and eventually to irreversible mitochondrial membrane potential depolarization, promoting cell dysfunction and death (Fig. 10.2). This hypothesis confers to mitochondria a central role in determining cell destiny: on one hand this organelle activates cardio-protection at the expense of cell excitability; on the other hand, mitochondrial dysfunction can cause cell death. In this complex response TSPO acts as a sensor of metabolic stress tolerability; not surprisingly, TSPO ligands are promising in preventing ischemia-induced ventricular fibrillation [38].
Fig. 10.2.
TSPO regulates mitochondrial dissipating energy channel. In presence of low levels of ROS, TSPO is able to activate IMAC with consequent RIRR response that can induce arrhythmia. In presence of high levels of ROS, TSPO becomes able to activate mPTP with terminal collapse of mitochondrial potential and cell death
mitoKATP Channels
A crucial role in arrhythmogenesis is played by a specific class of mitochondrial channels, initially identified in hepatic mitochondria and then found also in the heart: mitochondrial ATP-sensitive potassium channels (mitoKATP) [39]. Little is known about these channels and some studies yielded conflicting results, probably because of the low specificity of the compounds used to target mitoKATP [40–43]. Nevertheless, recent evidence supports a protective role of these channels against arrhythmia, since their opening before metabolic stress (ischemia) induces partial dissipation of ΔΨm, reducing the drive force for mitochondrial Ca2+ uptake and improving cellular respiration. The ATP sensibility of these channels allow them to behave as sensors of metabolic stress [40–42].
Mechanistic Role of Mitochondrial Ca2+ in Arrhythmogenesis
Ca2+ homeostasis in mitochondria is mainly ensured by Ca2+ influx through MCU (see Chap. 2), and Ca2+ efflux through mitochondrial Na+/Ca2+ exchanger [44]. The administration of MCU blockers reduces the incidence of ventricular fibrillation in anesthetized rats [45]. Most likely, the inhibition of MCU opening plays a protective role keeping mitochondrial Ca2+ concentration low. Indeed, mitochondrial Ca2+ overload can induce mitochondrial dysfunction with increase of open probability of mPTP [45].
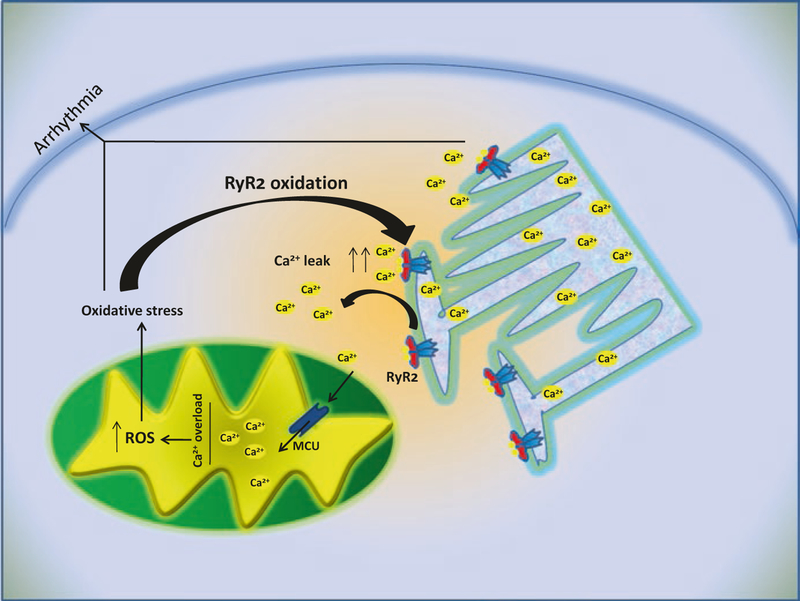
Mitochondrial Ca2+ overload might trigger opening of the mPTP, causing uncoupling of oxidative phosphorylation, swelling of the mitochondria, and rupture of the mitochondrial outer membrane. It is not easy to define precisely the role of MCU in arrhythmogenesis given the non-specific effects of the compounds used as blockers (ruthenium red and Ru360); however, mitochondrial dysfunction as result of Ca2+ overload suggests that these organelles can contribute also indirectly to arrhythmogenesis, through ROS production and ΔΨm collapse in response to high Ca2+ influx. In cardiac myocytes, mitochondria and SR are structurally and functionally related, co-localizing in the so-called mitochondrial microdomain; mitochondrial Ca2+ influx through MCU is tightly linked to SR Ca2+ release [46]. Alterations of RyR2 on SR, and consequent increase of Ca2+ leak in the cytosol, are among the causes of cardiac arrhythmias [47]. Mitochondria, while not representing the primary cause, can contribute strongly to the arrhythmic phenotype, being a key component of the following vicious cycle (Fig. 10.3):
Fig. 10.3.
Ca2+ dependent pathways and mitochondrial function in arrhythmogenesis. Alterations of Ca2+ release from sarcoplasmic reticulum can induce mitochondrial dysfunction as consequence of mitochondrial Ca2+ overload. Mitochondrial dysfunction in turn, through means of oxidative stress, can mediate further alterations with increase of Ca2+ leak from SR
RyR alterations → increased Ca2+ leak → mitochondrial Ca2+ overload → mitochondrial dysfunction → ROS production → RyR2 oxidation → further increase of Ca2+ leak.
We have shown the importance of this vicious cycle, indicating that mitochondrial Ca2+ overload plays a key role in atrial fibrillation [48], one of the most common arrhythmias [49, 50]. We used murine models characterized by RyR2 mutations causing SR Ca2+ leak, and these mice exhibited mitochondrial dysfunction, RyR2 oxidation, high ROS level and atrial fibrillation. The most important evidence is that not only RyR2 pharmacological block, but also inhibition of mitochondrial ROS production, can prevent arrhythmias in these models [48]. Therefore, Ca2+ mediated crosstalk between mitochondria and SR represents an interesting field of investigation to identify new and innovative therapeutic strategies for prevention and treatment of arrhythmia.
Mitochondria as Therapeutic Target
As mentioned above, the role of mitochondria in cardiac arrhythmia is the result of a complex network between mitochondrial environment and extramitochondrial environment (cytosol, SR, plasmatic membrane). In particular, these dynamic organelles can contribute to electrical alterations in different circumstances, both when excitability dysregulation is a consequence of metabolic alterations, and when electrical dysfunction is the result of altered Ca2+ homeostasis.
Approximately 80% of clinical arrhythmia are consequence of alterations of coronary circulation that induce ischemic events, resulting in mitochondrial metabolic dysfunction [51, 52]. During these events, the mechanisms of RIRR orchestrated by mitochondria are essential, eventually inducing opening of sarcKATP channels with inhomogeneous AP alterations and consequent arrhythmia. After ischemia, restoration of normal blood flow results in additional cardiac damage known as reperfusion injury, with high ROS production that promotes mechano- electrical dysfunction. In these circumstances, the possibility to modulate mitochondrial response could represent an efficient strategy of intervention to prevent or to treat arrhythmia; for instance, the block of IMAC to inhibit RIRR and prevent AP oscillations, or increasing the anti-oxidant capacity of mitochondria in order to neutralize the first ROS production induced by deprivation of oxygen and metabolite during ischemia, preventing RIRR.
Left ventricular hypertrophy (LVH) is another pathological condition where mitochondrial dysfunction is essential for arrhythmogenesis. During LVH, an increase in ΔΨm occurs, with reduction of apoptosis and bioenergetic alterations since high ΔΨm can interfere with the transport of substrates inside mitochondria with consequent metabolic shift from fatty acid to glucose utilization [53]. The modifications of ΔΨm during LVH, could be attributed to altered expression of mitochondrial uncoupling protein (UCP) that is observed in hypertrophy [54]. Indeed, UCP regulates ΔΨm and ROS production, and mice with low UCP3 develop arrhythmia [55]. Therefore, mitochondrial uncoupling via UCP alterations can induce ventricular fibrillation through AP heterogeneity as consequence of ΔΨm dependent sarcKATP activation [56]. Also in this case, mitochondria can be exploited as therapeutic targets, with possibility to modulate UCP activity to prevent hypertrophy dependent arrhythmia. Moreover, IMAC blockers show to produce beneficial effects in isoproterenol-dependent hypertrophy, most likely via inhibition of RIRR after ROS production, as result of UCP alterations [57].
All these findings indicate that mitochondria could offer several molecular targets for prevention and/or treatment of a broad spectrum of arrhythmic conditions, from ion channels involved in RIRR (IMAC, mPTP), regulatory proteins (TPSO), to channels directly involved in mitochondrial electrical homeostasis and energetic coupling (MCU, UCP). The main problems are represented by difficulties in realizing pharmacological compounds that are specific and efficient ligands of these mitochondrial proteins, since several are able to interact also with other substrates outside mitochondria.
Given the central role of mitochondrial ROS production and RIRR mechanism as primary or secondary cause of cardiac arrhythmias, many investigators are focused on the development of compounds with ROS- scavenger properties, or at least with the ability to increase the anti-oxidant capacity of mitochondria (see Chap. 32). For instance, treatment with superoxide dismutase mimics or mitochondrial-targeted anti-oxidant peptides has been successful in decreasing incidence of arrhythmia [58, 59]. Moreover, administration of N-acetylcysteine to humans after cardiac surgery significantly decreases the probability of arrhythmia development [60].
Further studies are necessary to expand our knowledge on the molecular composition and regulation of mitochondrial targets, in order to develop selective pharmacological compounds able to suppress cardiac arrhythmias.
Acknowledgements
Dr. Gaetano Santulli, MD, PhD is supported by the National Institutes of Health (NIH, Grant NIDDK107895).
Contributor Information
Jessica Gambardella, Columbia University Medical Center, New York, NY, USA; Department of Medicine, Surgery and Dentistry, University of Salerno, Baronissi, Italy.
Daniela Sorriento, Department of Advanced Biomedical Sciences, “Federico II” University, Naples, Italy.
Michele Ciccarelli, Department of Medicine, Surgery and Dentistry, University of Salerno, Baronissi, Italy.
Carmine Del Giudice, Department of Advanced Biomedical Sciences, “Federico II” University, Naples, Italy.
Antonella Fiordelisi, Department of Advanced Biomedical Sciences, “Federico II” University, Naples, Italy.
Luigi Napolitano, Department of Advanced Biomedical Sciences, “Federico II” University, Naples, Italy.
Bruno Trimarco, Department of Advanced Biomedical Sciences, “Federico II” University, Naples, Italy.
Guido Iaccarino, Department of Medicine, Surgery and Dentistry, University of Salerno, Baronissi, Italy.
Gaetano Santulli, Dept. of Biomedical Advanced Sciences, Federico II University, Naples, Italy.
References
- 1.Santulli G, Iaccarino G, De Luca N, Trimarco B, Condorelli G Atrial fibrillation and microRNAs. Front Physiol 2014;5:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mitchell P Coupling of phosphorylation to electron and hydrogen transfer by a chemi-osmotic type of mechanism. Nature. 1961;191:144–8. [DOI] [PubMed] [Google Scholar]
- 3.Ryu SY, Lee SH, Ho WK Generation of metabolic oscillations by mitoKATP and ATP synthase during simulated ischemia in ventricular myocytes. J Mol Cell Cardiol 2005;39: 874–81. [DOI] [PubMed] [Google Scholar]
- 4.Aon MA, Cortassa S, Marban E, O’Rourke B Synchronized whole cell oscillations in mitochondrial metabolism triggered by a local release of reactive oxygen species in cardiac myocytes. J Biol Chem 2003;278:44735–44. [DOI] [PubMed] [Google Scholar]
- 5.O’Rourke B, Ramza BM, Marban E Oscillations of membrane current and excitability driven by metabolic oscillations in heart cells. Science. 1994;265:962–6. [DOI] [PubMed] [Google Scholar]
- 6.Terzic A, Jahangir A, Kurachi Y Cardiac ATP-sensitive K+ channels: regulation by intracellular nucleotides and K+ channel-opening drugs. Am J Phys 1995;269:C525–45. [DOI] [PubMed] [Google Scholar]
- 7.Johnson MS, Moore RL, Brown DA Sex differences in myocardial infarct size are abolished by sarcolemmal KATP channel blockade in rat. Am J Physiol Heart Circ Physiol 2006;290: H2644–7. [DOI] [PubMed] [Google Scholar]
- 8.Chicco AJ, Johnson MS, Armstrong CJ, Lynch JM, Gardner RT, Fasen GS, Gillenwater CP, Moore RL Sex-specific and exercise-acquired cardioprotection is abolished by sarcolemmal KATP channel blockade in the rat heart. Am J Physiol Heart Circ Physiol 2007;292: H2432–7. [DOI] [PubMed] [Google Scholar]
- 9.Brown DA, Chicco AJ, Jew KN, Johnson MS, Lynch JM, Watson PA, Moore RL Cardioprotection afforded by chronic exercise is mediated by the sarcolemmal, and not the mitochondrial, isoform of the KATP channel in the rat. J Physiol 2005;569:913–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Akar FG, Aon MA, Tomaselli GF, O’Rourke B The mitochondrial origin of postischemic arrhythmias. J Clin Invest 2005;115:3527–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wirth KJ, Rosenstein B, Uhde J, Englert HC, Busch AE, Scholkens BA ATP-sensitive potassium channel blocker HMR 1883 reduces mortality and ischemia-associated electrocardiographic changes in pigs with coronary occlusion. J Pharmacol Exp Ther 1999;291:474–81. [PubMed] [Google Scholar]
- 12.Vajda S, Baczko I, Lepran I Selective cardiac plasma-membrane K(ATP) channel inhibition is defibrillatory and improves survival during acute myocardial ischemia and reperfusion. Eur J Pharmacol 2007;577:115–23. [DOI] [PubMed] [Google Scholar]
- 13.Fischbach PS, White A, Barrett TD, Lucchesi BR Risk of ventricular proarrhythmia with selective opening of the myocardial sarcolemmal versus mitochondrial ATP-gated potassium channel. J Pharmacol Exp Ther 2004;309:554–9. [DOI] [PubMed] [Google Scholar]
- 14.Aronson D, Mittleman MA, Burger AJ Effects of sulfonylurea hypoglycemic agents and adenosine triphosphate dependent potassium channel antagonists on ventricular arrhythmias in patients with decompensated heart failure. Pacing Clin Electrophysiol 2003;26:1254–61. [DOI] [PubMed] [Google Scholar]
- 15.del Valle HF, Lascano EC, Negroni JA, Crottogini AJ Glibenclamide effects on reperfusion- induced malignant arrhythmias and left ventricular mechanical recovery from stunning in conscious sheep. Cardiovasc Res 2001;50:474–85. [DOI] [PubMed] [Google Scholar]
- 16.Zorov DB, Filburn CR, Klotz LO, Zweier JL, Sollott SJ Reactive oxygen species (ROS)-induced ROS release: a new phenomenon accompanying induction of the mitochondrial permeability transition in cardiac myocytes. J Exp Med 2000;192:1001–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zorov DB, Juhaszova M, Sollott SJ Mitochondrial ROS-induced ROS release: an update and review. Biochim Biophys Acta 2006;1757:509–17. [DOI] [PubMed] [Google Scholar]
- 18.Wang J, Wang H, Zhang Y, Gao H, Nattel S, Wang Z Impairment of HERG K(+) channel function by tumor necrosis factor-alpha: role of reactive oxygen species as a mediator. J Biol Chem 2004;279:13289–92. [DOI] [PubMed] [Google Scholar]
- 19.Santulli G, Nakashima R, Yuan Q, Marks AR Intracellular calcium release channels: an update. J Physiol 2017. (in press). doi: 10.1113/JP272781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Biary N, Xie C, Kauffman J, Akar FG Biophysical properties and functional consequences of reactive oxygen species (ROS)-induced ROS release in intact myocardium. J Physiol 2011;589:5167–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garlid KD, Beavis AD, Ratkje SK On the nature of ion leaks in energy-transducing membranes. Biochim Biophys Acta 1989;976:109–20. [DOI] [PubMed] [Google Scholar]
- 22.Brierley GP Energy-linked alteration of the permeability of heart mitochondria to chloride and other anions. Biochemistry. 1970;9:697–707. [DOI] [PubMed] [Google Scholar]
- 23.Beavis AD Properties of the inner membrane anion channel in intact mitochondria. J Bioenerg Biomembr. 1992;24:77–90. [DOI] [PubMed] [Google Scholar]
- 24.Garlid KD, Beavis AD Evidence for the existence of an inner membrane anion channel in mitochondria. Biochim Biophys Acta 1986;853:187–204. [DOI] [PubMed] [Google Scholar]
- 25.Brown DA, Aon MA, Akar FG, Liu T, Sorarrain N, O’Rourke B Effects of 4′-chlorodiazepam on cellular excitation-contraction coupling and ischaemia-reperfusion injury in rabbit heart. Cardiovasc Res 2008;79:141–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brown DA, Aon MA, Frasier CR, Sloan RC, Maloney AH, Anderson EJ, O’Rourke B Cardiac arrhythmias induced by glutathione oxidation can be inhibited by preventing mitochondrial depolarization. J Mol Cell Cardiol 2010;48:673–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McCully JD, Wakiyama H, Hsieh YJ, Jones M, Levitsky S Differential contribution of necrosis and apoptosis in myocardial ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol 2004;286:H1923–35. [DOI] [PubMed] [Google Scholar]
- 28.Romashko DN, Marban E, O’Rourke B Subcellular metabolic transients and mitochondrial redox waves in heart cells. Proc Natl Acad Sci U S A 1998;95:1618–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sato T, Takizawa T, Saito T, Kobayashi S, Hara Y, Nakaya H Amiodarone inhibits sarcolemmal but not mitochondrial KATP channels in guinea pig ventricular cells. J Pharmacol Exp Ther 2003;307:955–60. [DOI] [PubMed] [Google Scholar]
- 30.Piot C, Croisille P, Staat P, Thibault H, Rioufol G, Mewton N, Elbelghiti R, Cung TT, Bonnefoy E, Angoulvant D, Macia C, Raczka F, Sportouch C, Gahide G, Finet G, Andre-Fouet X, Revel D, Kirkorian G, Monassier JP, Derumeaux G, Ovize M Effect of cyclosporine on reperfusion injury in acute myocardial infarction. N Engl J Med 2008;359:473–81. [DOI] [PubMed] [Google Scholar]
- 31.Aon MA, Cortassa S, O’Rourke B The fundamental organization of cardiac mitochondria as a network of coupled oscillators. Biophys J 2006;91:4317–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Akar FG Mitochondrial targets for arrhythmia suppression: is there a role for pharmacological intervention? J Interv Card Electrophysiol 2013;37:249–58. [DOI] [PubMed] [Google Scholar]
- 33.Beavis AD On the inhibition of the mitochondrial inner membrane anion uniporter by cationic amphiphiles and other drugs. J Biol Chem 1989;264:1508–15. [PubMed] [Google Scholar]
- 34.Kinnally KW, Zorov DB, Antonenko YN, Snyder SH, McEnery MW, Tedeschi H Mitochondrial benzodiazepine receptor linked to inner membrane ion channels by nanomolar actions of ligands. Proc Natl Acad Sci U S A 1993;90:1374–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sileikyte J, Petronilli V, Zulian A, Dabbeni-Sala F, Tognon G, Nikolov P, Bernardi P, Ricchelli F Regulation of the inner membrane mitochondrial permeability transition by the outer membrane translocator protein (peripheral benzodiazepine receptor). J Biol Chem 2011;286:1046–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McEnery MW, Snowman AM, Trifiletti RR, Snyder SH Isolation of the mitochondrial benzodiazepine receptor: association with the voltage-dependent anion channel and the adenine nucleotide carrier. Proc Natl Acad Sci U S A 1992;89:3170–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Leducq N, Bono F, Sulpice T, Vin V, Janiak P, Fur GL, O’Connor SE, Herbert JM Role of peripheral benzodiazepine receptors in mitochondrial, cellular, and cardiac damage induced by oxidative stress and ischemia-reperfusion. J Pharmacol Exp Ther 2003;306:828–37. [DOI] [PubMed] [Google Scholar]
- 38.Motloch LJ, Hu J, Akar FG The mitochondrial translocator protein and arrhythmogenesis in ischemic heart disease. Oxidative Med Cell Longev 2015;2015:234104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Paucek P, Mironova G, Mahdi F, Beavis AD, Woldegiorgis G, Garlid KD Reconstitution and partial purification of the glibenclamide-sensitive, ATP-dependent K+ channel from rat liver and beef heart mitochondria. J Biol Chem 1992;267:26062–9. [PubMed] [Google Scholar]
- 40.Rajesh KG, Sasaguri S, Suzuki R, Xing Y, Maeda H Ischemic preconditioning prevents reperfusion heart injury in cardiac hypertrophy by activation of mitochondrial KATP channels. Int J Cardiol 2004;96:41–9. [DOI] [PubMed] [Google Scholar]
- 41.Headrick JP, Willems L, Ashton KJ, Holmgren K, Peart J, Matherne GP Ischaemic tolerance in aged mouse myocardium: the role of adenosine and effects of A1 adenosine receptor over-expression. J Physiol 2003;549:823–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fryer RM, Hsu AK, Nagase H, Gross GJ Opioid-induced cardioprotection against myocardial infarction and arrhythmias: mitochondrial versus sarcolemmal ATP-sensitive potassium channels. J Pharmacol Exp Ther 2000;294:451–7. [PubMed] [Google Scholar]
- 43.Schwartz LM, Welch TS, Crago MS Cardioprotection by multiple preconditioning cycles does not require mitochondrial K(ATP) channels in pigs. Am J Physiol Heart Circ Physiol 2002;283:H1538–44. [DOI] [PubMed] [Google Scholar]
- 44.Belmonte S, Morad M ‘Pressure-flow’-triggered intracellular Ca2+ transients in rat cardiac myocytes: possible mechanisms and role of mitochondria. J Physiol 2008;586:1379–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Garcia-Rivas Gde J, Carvajal K, Correa F, Zazueta C Ru360, a specific mitochondrial calcium uptake inhibitor, improves cardiac post-ischaemic functional recovery in rats in vivo. Br J Pharmacol 2006;149:829–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Santulli G, Pagano G, Sardu C, Xie W, Reiken S, D’Ascia SL, Cannone M, Marziliano N, Trimarco B, Guise TA, Lacampagne A, Marks AR Calcium release channel RyR2 regulates insulin release and glucose homeostasis. J Clin Invest 2015;125:4316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xie W, Santulli G, Guo X, Gao M, Chen BX, Marks AR Imaging atrial arrhythmic intracellular calcium in intact heart. J Mol Cell Cardiol 2013;64:120–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xie W, Santulli G, Reiken SR, Yuan Q, Osborne BW, Chen BX, Marks AR Mitochondrial oxidative stress promotes atrial fibrillation. Sci Rep 2015;5:11427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Santulli G, D’Ascia SL, D’Ascia C Development of atrial fibrillation in recipients of cardiac resynchronization therapy: the role of atrial reverse remodelling. Can J Cardiol 2012;28:245 e17; author reply 245 e17–8. [DOI] [PubMed] [Google Scholar]
- 50.D’Ascia SL, D’Ascia C, Marino V, Lombardi A, Santulli R, Chiariello M, Santulli G Cardiac resynchronisation therapy response predicts occurrence of atrial fibrillation in non-ischaemic dilated cardiomyopathy. Int J Clin Pract 2011;65:1149–55. [DOI] [PubMed] [Google Scholar]
- 51.Halestrap AP Calcium, mitochondria and reperfusion injury: a pore way to die. Biochem Soc Trans 2006;34:232–7. [DOI] [PubMed] [Google Scholar]
- 52.Panth N, Paudel KR, Parajuli K Reactive oxygen species: a key hallmark of cardiovascular disease. Adv Med 2016;2016:9152732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Leong HS, Brownsey RW, Kulpa JE, Allard MF Glycolysis and pyruvate oxidation in cardiac hypertrophy – why so unbalanced? Comp Biochem Physiol A Mol Integr Physiol 2003;135: 499–513. [DOI] [PubMed] [Google Scholar]
- 54.Laskowski KR, Russell RR 3rd Uncoupling proteins in heart failure. Curr Heart Fail Rep 2008;5:75–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ozcan C, Palmeri M, Horvath TL, Russell KS, Russell 3rd RR. Role of uncoupling protein 3 in ischemia-reperfusion injury, arrhythmias, and preconditioning. Am J Physiol Heart Circ Physiol 2013;304:H1192–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Brennan JP, Southworth R, Medina RA, Davidson SM, Duchen MR, Shattock MJ Mitochondrial uncoupling, with low concentration FCCP, induces ROS-dependent cardioprotection independent of KATP channel activation. Cardiovasc Res 2006;72:313–21. [DOI] [PubMed] [Google Scholar]
- 57.Jin H, Nass RD, Joudrey PJ, Lyon AR, Chemaly ER, Rapti K, Akar FG Altered spatiotemporal dynamics of the mitochondrial membrane potential in the hypertrophied heart. Biophys J 2010;98:2063–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Konya L, Kekesi V, Juhasz-Nagy S, Feher J The effect of superoxide dismutase in the myocardium during reperfusion in the dog. Free Radic Biol Med 1992;13:527–32. [DOI] [PubMed] [Google Scholar]
- 59.Cho J, Won K, Wu D, Soong Y, Liu S, Szeto HH, Hong MK Potent mitochondria-targeted peptides reduce myocardial infarction in rats. Coron Artery Dis 2007;18:215–20. [DOI] [PubMed] [Google Scholar]
- 60.Erdogan O N-acetylcysteine for the prevention of postoperative atrial fibrillation: a prospective, randomized, placebo-controlled pilot study. Eur Heart J 2008;29:1591; author reply 1591. [DOI] [PubMed] [Google Scholar]