Abstract
Background:
Standard treatment of squamous cell carcinoma (SCC) of the anterior nasal mucosa is surgical resection with or without postoperative radiation.
Methods:
Retrospective review of patients diagnosed with SCC of the nasal cavity between 1/2000 and 7/2018 who refused total rhinectomy and were treated with radiation with or without chemotherapy with curative intent.
Results:
Eleven patients were identified, 73% had stage III or IV disease. Four patients were treated with intensity-modulated radiotherapy (IMRT), and 7 with intensity-modulated proton radiotherapy (IMPT). Concurrent chemoradiotherapy was used in 9 patients (82%). With a median follow-up of 15 months (3–124 months) 2 patients experienced recurrence and one developed distant metastasis and died from disease. The 2-year rhinectomy-free survival rate was 88%. Two-year overall survival and recurrence-free survival were 100% and 75%, respectively.
Conclusion:
A radiation-based approach for SCC of the nasal cavity mucosa is a valid option for selected patients that refuse up-front surgery.
Keywords: nasal cavity, squamous cell carcinoma, radiotherapy, rhinectomy, outcomes
INTRODUCTION
Cancers of the nasal cavity and paranasal sinuses are uncommon. Approximately 2,000 new cases are diagnosed per year in the United States, representing 3% of all head and neck cancers1. Squamous cell carcinoma (SCC) is the most frequent histology, accounting for 50%–75% of cases, followed by adenocarcinomas and other rare histologies such as adenoid cystic carcinoma, melanoma, and lymphoma.2,3
There are no randomized studies to guide management, and current treatment guidelines are based on observational studies and consensus opinion.4,5 Further, interpretation of retrospective data is complex, as cancers of the nasal cavity are frequently analyzed with cancers of the paranasal sinuses, and most series include a variety of tumor histologies along with various disease stages. These series often span decades, include patients treated with a variety of surgical and radiotherapy techniques, and with inconsistency in the use of systemic therapy. Reported outcomes for cancers of the nasal cavity are typically focused on complete surgical resection, followed by post-operative radiotherapy.2,6 For SCCs, locoregional control of 80%–85% can be achieved following total resection and post-operative radiotherapy.7 Several retrospective series report that patients treated with surgery and radiation have better outcomes than patients treated with radiation alone, but such direct comparisons4,5 are hampered by selection bias, older radiotherapy techniques which utilized inadequate doses of radiotherapy, and the inconsistent use of chemotherapy to enhance the effects radiotherapy.
In contrast to early-stage cancers of the ethmoid or maxillary sinuses, resection of early-stage anterior nasal cavity cancers with partial or total rhinectomy can be disfiguring. The development of modern prostheses and reconstruction techniques has significantly improved cosmetic outcomes, but this remains a significant source of psychosocial distress for many patients.8,9
Few studies evaluate the feasibility of non-operative management for patients with initially operable cancers of the anterior nasal cavity, and the criteria for operability are institution-specific and continue to evolve. As patients are increasingly voicing their concern about appearance and quality of life, there are case reports on the outcomes of patients who refused NCCN recommended surgical treatment.10 Because these patients have resectable tumors, surgical salvage of local and regional failures from non-surgical treatment is still potentially possible and curative. However, the evidence supporting such an approach is scarce. This study aims to evaluate outcomes among patients with initially resectable mucosal SCCs of the anterior nasal cavity who declined partial or total rhinectomy and were treated with definitive radiotherapy with and without chemotherapy.
MATERIALS AND METHODS
Following approval by the institutional review board at Memorial Sloan Kettering Cancer Center (MSK), we reviewed the records of all patients diagnosed with mucosal SCC of the nasal cavity or nasal septum who refused rhinectomy and were treated with definitive radiation therapy with or without chemotherapy from January 2000 to July 2018 at MSK. Patients were excluded if they had cancers originating from the skin, nasal vestibule, or paranasal sinuses; received definitive treatment at an outside institution; or if they were treated with brachytherapy.
Data collection
We retrieved data from electronic medical records, including demographic data (age and sex), comorbidities, risk factors, extent of disease, and pathology. Histopathological information was obtained from diagnostic biopsies and reviewed at MSK. Tumors were staged according to the American Joint Committee on Cancer (AJCC) 8th edition for nasal cavity and ethmoid tumors.11
All patients were discussed in the multidisciplinary tumor board meeting to determine the best treatment strategy. Records were reviewed for details of patient treatment including radiation planning and systemic chemotherapy. Data regarding treatment toxicity was collected from the medical record and graded according to the toxicity criteria of the Radiation Therapy Oncology Group (RTOG). Disease status was obtained from the last disease management team visit.
Statistics
We evaluated overall survival, recurrence-free survival, and rhinectomy-free survival as endpoints. Overall survival was estimated from the diagnostic biopsy until the date of last follow-up or death, whichever occurred first. Recurrence-free survival was estimated from the diagnostic biopsy to the first disease recurrence. Rhinectomy-free survival was estimated from the diagnostic biopsy to the salvage rhinectomy date or death. Statistical analysis was carried out using SPSS Statistics software, version 25 (IBM).
RESULTS
Patients demographics
From January 2000 to July 2018, 11 patients with initially resectable SCC of the nasal cavity refused rhinectomy or partial rhinectomy were identified. Demographic and clinical characteristics of the patients are summarized in Table 1. The median age at diagnosis was 66 years (range 38–86 years), and 64% were male. The most common symptoms at presentation were epistaxis (46%), nasal mass (36%) and nasal ulcer (18%). Tobacco consumption was identified in 9 patients (82%). Clinical T classification were as follows: one T1, two T2, four T3, and four T4. In three cases the skin of the nose was involved. Eighteen percent of patients had clinical nodal disease diagnosed in a PET/CT at presentation. Seventy-three percent of patients had stage III or IVA disease. SCC was moderately-differentiated in 8 patients and in 4 cases immunohistochemistry for p16 status was positive.
Table 1:
| Characteristic | No. of patients (%) |
|---|---|
| Median age (range) | 66 (38–86) |
| Sex | |
| Male | Female |
| 7 (64%) | 4 (36%) |
| Smoking history | |
| Never | 2 (11%) |
| < 20 pack a year | 3 (33%) |
| ≥ 20 Pack a year | 6 (55%) |
| Symptoms at presentation | |
| Epistaxis | 5 (46%) |
| Nasal mass | 4 (36%) |
| Nasal ulcer | 2 (18%) |
| Site | |
| Nasal septum | 6(55%) |
| Nasal Vestibule | 2(18%) |
| Nasal Floor | 1(9%) |
| Nasal Vault | 2(18%) |
| Clinical T classification | |
| T1 | 1 (9%) |
| T2 | 2 (18%) |
| T3 | 4 (36%) |
| T4 | 4 (36%) |
| Clinical N classification | |
| N (−) | 9 (82%) |
| N (+) | 2 (18%) |
| Skin involvement | |
| No | 8(73%) |
| Yes | 3(27%) |
| Tumor differentiation | |
| Well-differentiated | 0 (0%) |
| Moderately-differentiated | 8 (73%) |
| Poorly-differentiated | 1 (9%) |
| Unknown | 2 (18%) |
| P 16 status | |
| Negative | 1(9%) |
| Positive | 4(36%) |
| Unknown | 6(55%) |
SCC, squamous cell carcinoma;
MSK, Memorial Sloan Kettering Cancer Center.
Treatment
All patients were offered surgery, in 3 cases a partial resection / near total resection and in 8 cases a total rhinectomy. Arguments for refusing surgery were related to the extent of the surgery and the cosmetic impact. Two patients had severe chronic pulmonary disease. Therefore, they were treated with definitive radiation therapy with or without chemotherapy. Treatment details are summarized in Table 2.
Table 2.
Summary of treatment of patients
| Patients | Stage | Induction chemotherapy |
Radiotherapy | Radiotherapy Target |
Concurrent Chemoradiotherapy |
Recurrence | Status | Follow up (Months) |
|---|---|---|---|---|---|---|---|---|
| 1 | T3N0 | No | IMRT* | Primary and neck | Cisplatin | No | Alive | 124 |
| 2 | T3N0 | No | IMRT | Primary and neck | Cisplatin | Yes (Regional) | Died of disease | 9.6 |
| 3 | T3N2C | Cisplatin, Docetaxel, Everolimus | IMRT | Primary and neck | Cisplatin | No | Alive | 96 |
| 4 | T3N0 | No | IMRT | Primary and neck | No | No | Alive | 23.9 |
| 5 | T4N2C | No | IMPT† | Primary and neck | Cisplatin | No | Alive | 14.5 |
| 6 | T1N0 | No | IMPT | Primary | Cisplatin | Yes (Local) | Alive rhinectomy | 33.4 |
| 7 | T4N0 | Carboplatin, Paclitaxel | IMPT | Primary and neck | Cisplatin | No | Alive | 7.4 |
| 8 | T2N0 | No | IMPT | Primary and neck | Carboplatin, Paclitaxel | No | Alive | 11.6 |
| 9 | T4N0 | Carboplatin, Paclitaxel | IMPT | Primary and neck | Cisplatin | No | Alive | 11.8 |
| 10 | T2N0 | No | IMPT | Primary and neck | Carboplatin, Paclitaxel | No | Alive | 3.3 |
| 11 | T4N0 | No | IMPT | Primary and neck | Carboplatin, Paclitaxel | No | Alive | 6.6 |
IMRT, intensity-modulated radiotherapy;
IMPT, intensity-modulated proton radiotherapy.
Four patients were treated with intensity-modulated radiotherapy (IMRT), and 7 patients were treated with intensity-modulated proton beam radiotherapy (IMPT). The median radiation dose was 70 Gy (69.96 – 70.2 Gy) for photon-based cases and 70 Cobalt Gray Equivalent (CGE) (46 – 70 CGE) for proton cases using a generic relative biological effectiveness (RBE) of 1.1. Patients were treated in 1.8 – 2 Gy / CGE fractions.
Radiotherapy Field Design
During the study period, the field design and technical details remained relatively consistent. Patients were immobilized with a 5-point thermoplastic mask and a bite block. Pre-treatment 18-FDG PET/CT and MRI were used for target delineation. The gross tumor volume (GTV), high-risk clinical target volume (HR-CTV), and low-risk clinical target volume (LR-CTV) were treated to 70, 60, and 50–54 Gy / CGE, respectively. The HR-CTV included the primary tumor and entirety of the nasal cavity, including any extension into the skull base or paranasal sinuses. At-risk nodal volumes included the bilateral facial and retropharyngeal nodes, as well as stations I-III. In patients with clinically involved cervical nodes, the elective nodal stations were extended to include level IV nodal stations.12,13
The primary site and regional nodes were treated in all but one patient. Treatment to the neck included bilateral IB to IV levels and retropharyngeal lymph nodes. One patient with a T1N0M0 AJCC stage I SCC of the nasal cavity was treated with protons to the primary site using a uniform scanning (not spot scanning) technique without coverage of the regional nodes.
Three patients received induction chemotherapy, all of whom had stage IVA disease. Two patients were treated with carboplatin, docetaxel, and fluorouracil, and a third received cisplatin, docetaxel, and everolimus on a clinical trial. Concurrent chemoradiotherapy was used in 9 patients (82%), of whom 6 received concurrent cisplatin and 3 received concurrent carboplatin/docetaxel. One patient was not able to complete treatment due to significant mucositis, and skin reactions and stopped further treatments at 46.05 CGE. Follow up imaging protocols were heterogeneous, but generally included a PET/CT and a magnetic resonance imaging (MRI) 3 months post radiation treatment and then as clinically indicated. In office endoscopic assessment generally took place at least every three months for the first year post treatment and every 6 months after the first year up to the fifth year post treatment.
Outcomes
With a median follow-up of 15 months (3–124 months), 2 patients experienced recurrence and one of the patients eventually developed distant metastasis and died from disease. The 2-year rhinectomy-free survival rate was 88%. Two-year overall survival and recurrence-free survival were 100% and 75%, respectively (Figure 1). At last follow-up, locoregional control was achieved in 9 patients, 8 of whom received concurrent chemoradiotherapy. One that was treated with IMPT without concurrent chemotherapy, developed locally recurrent disease after 10 months identified in a routine PET/CT and required a salvage total rhinectomy and neck dissection. Final pathology showed a T1N0 disease, and the patient was free of recurrence at last follow-up. One patient that was treated with IMRT to the primary site and neck with concurrent cisplatin developed recurrent disease in the neck 9 months after initial diagnosis; he required bilateral neck dissection, ultimately developed metastatic disease to the lung and mediastinum 16 months after diagnosis and died from disease 30 months from diagnosis.
Figure 1.
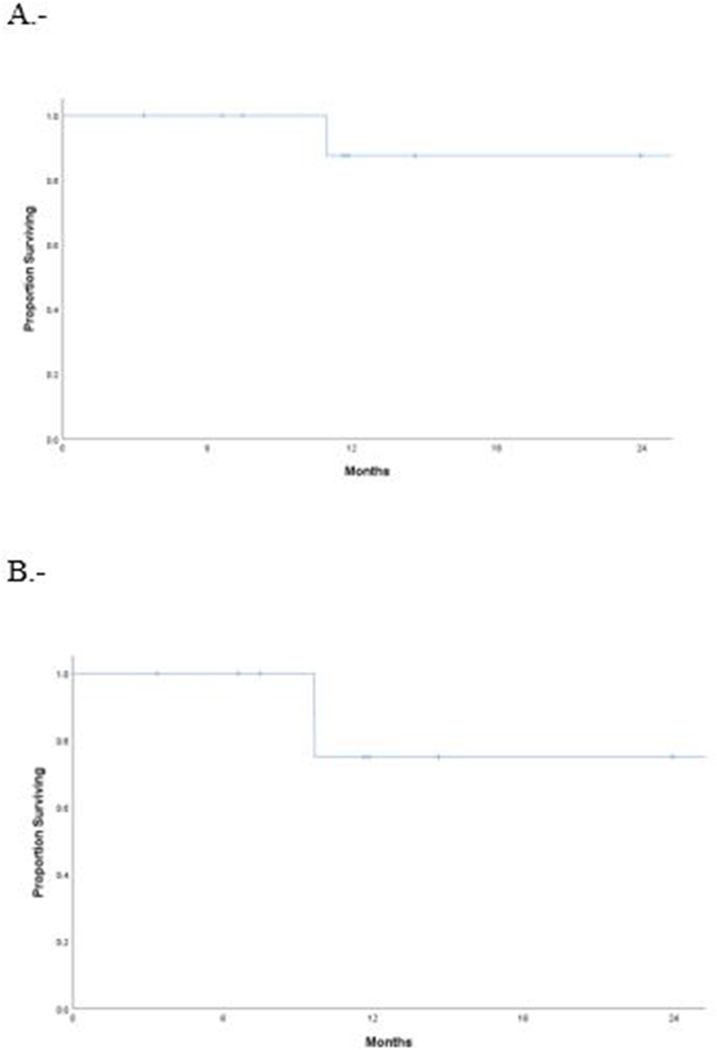
A, Rhinectomy-free survival. B, Recurrence-free survival
Toxicity
During treatment, the most common side effects were mucositis, dermatitis, dry mouth, and dysphagia; 72% were grade 1 or 2 according to RTOG. Of the 11 patients, 2 had a grade 4 toxicity and required hospitalization. Out of the 2 patients with grade 4 toxicity, one had a dermatitis complicated with a facial cellulitis that was treated with broad spectrum antibiotics and another developed mucositis, dermatitis, and dehydration and did not completed treatment.
During follow-up, 4 patients developed xerostomia, 2 developed septal perforation without functional impact, 4 had post treatment synechiae and partial stenosis. One patient went on to have endoscopic sinus surgery to divide the synechiae and 1 patient had a saddle nose deformity and underwent cosmetic surgery for aesthetic improvement.
DISCUSSION
The present study shows that non-operative management of anterior nasal cavity SCC is a feasible, well-tolerated approach. This study differs from prior studies of definitive radiotherapy and chemoradiotherapy for sinonasal malignancies in the following ways: (1) all patients were treatment-naïve and had resectable SCC of the nasal cavity; (2) high doses of radiation were uniformly employed; (3) concurrent platinum-based chemotherapy was given in almost 90% of the patients; and (4) 56% of patients received proton therapy in the form of IMPT.
This series compares to our historical cohort published in 2007 by Hoppe et al. 14 that includes 85 patients with tumors of the nasal cavity and paranasal sinuses treated with surgery and post-operative radiotherapy. In this study the most frequent histology was SCC and 24 patients had a tumor located in the nasal cavity, the reported 5-year OS and RFS was 67% and 55% respectively.
The locoregional control achieved in this series is comparable to historical outcomes using surgery and post-operative radiotherapy for nasal cavity and sinonasal malignancies2,6,15. Although historical patient series using radiotherapy alone have documented local control of 30%–50%6,16, inferior to what has been reported with surgery and adjuvant radiotherapy, these studies have been hampered by unresectable disease, limited use of concurrent chemotherapy and older radiotherapy techniques that did not allow adequate dose delivery to the nasal cavity due to the presence of multiple surrounding critical normal tissues such as the optic structures. Although it is difficult to compare outcomes from different series due to the heterogeneous nature of the disease in this location, some authors have reported more favorable outcomes. Investigators from MD Anderson Cancer Center reported a series of 68 patients with cancers of the nasal cavity, 32 (47%) of whom were treated with definitive radiotherapy, resulting in a 5-year locoregional control of 81.1%, similar to what was achieved with surgery followed by adjuvant radiotherapy (P = 0.10).15 More recently, Russo and colleagues at Massachusetts General Hospital reported a 5-year locoregional control of 80% in a series of 54 patients treated with surgery and post-operative radiation or definitive radiotherapy for sinonasal SCC.7. Although overall survival was worse for patients with less than a gross total resection, no difference in locoregional control was identified.
Proton radiotherapy has demonstrated clinical efficacy and the potential for toxicity reduction compared with photon therapy.17The nasal cavity and paranasal sinuses are challenging to treat with high-dose radiotherapy. Older series using 2D techniques report severe late toxicities, with 10%–30% of patients developing permanent vision loss. The risk of these severe toxicities has been dramatically reduced or eliminated with more modern photon-based techniques, such as IMRT.18 This, in turn, has increased the doses of radiation that can safely be delivered, potentially improving locoregional control. For instance, Askoxylakis and colleagues recently reported a 3-year locoregional control in a series of 76% for patients treated to ≥ 60 Gy, compared with 56% for patients treated to < 60 Gy.19
Although there is no level I evidence for concurrent chemoradiotherapy for sinonasal SCCs, current practice has been extrapolated from the management of SCCs from other sites in the head and neck.20 In a series using induction chemotherapy, approximately two-thirds of patients had some response, suggesting these agents are active for this disease type.21
In nasal cavity tumors, the treatment of the neck remains controversial. Generally, the literature supports the treatment of N0 neck when the probability of occult metastasis is greater than 20%.22 Disparities in regional failure have been reported in untreated necks, ranging from 0%–18%.6,23,24 In our series, 8 of 9 patients had neck radiotherapy, which is in concordance with our high rate of advanced disease.
In our cohort, all 4 cases with known immunohistochemistry for p16 status were p16-positive, and 2 cases with in-situ hybridization for high-risk human papillomavirus (HPV) RNA were HPV-positive. The sensitivity of p16-positive oropharyngeal cancers to chemoradiotherapy is well documented,25 but the prognostic value of p16 status for sinonasal cancers is less clear. Two relatively small series have reported improved oncologic outcomes associated with p16 positivity in SCCs. Treatment details were not reported in the series from Johns Hopkins Hospital, and in the series from Barcelona, patients were uniformly treated with surgery and radiotherapy.26,27
An RTOG analysis demonstrated that p16 positivity in non-oropharyngeal SCCs is associated with improved progression-free and overall survival after chemoradiotherapy, although the included trials excluded patients with sinonasal carcinomas.28
In this retrospective series, we show that non-operative management of anterior nasal cavity SCCs is feasible resulting in favorable outcomes with high-dose radiotherapy and concurrent chemoradiotherapy. There are several limitations to this study, including the small number of patients, the short follow up and its retrospective nature, which may have introduced selection bias. We also note that all 4 tumors tested for p16 with IHC were p16-positive, potentially resulting in a more treatment-sensitive cohort.
In our current practice patients with anterior SCC of the nasal cavity are offered standard treatment, surgery, post-operative radiation and reconstruction either with prosthesis or free flap reconstruction. When a carefully selected patient refuses upfront surgery, we offer a non-surgical approach. A chemoradiation approach is not free from side effects and careful consideration should be taken in target delineation and selection of the concurrent chemotherapy regimen.
CONCLUSION
In patients with SCC of the anterior nasal cavity that refuse standard treatment an organ preservation approach with definitive chemoradiation is feasible.
Acknowledgment:
This research was funded in part through the NIH/NCI Cancer Center Support Grant P30 CA008748.
References
- 1.Sanghvi S, Khan MN, Patel NR, Yeldandi S, Baredes S, Eloy JA. Epidemiology of sinonasal squamous cell carcinoma: a comprehensive analysis of 4994 patients. The Laryngoscope. 2014;124(1):76–83. [DOI] [PubMed] [Google Scholar]
- 2.Dulguerov P, Jacobsen MS, Allal AS, Lehmann W, Calcaterra T. Nasal and paranasal sinus carcinoma: are we making progress? A series of 220 patients and a systematic review. Cancer. 2001;92(12):3012–3029. [DOI] [PubMed] [Google Scholar]
- 3.Scurry WC Jr., Goldenberg D, Chee MY, Lengerich EJ, Liu Y, Fedok FG. Regional recurrence of squamous cell carcinoma of the nasal cavity: a systematic review and meta-analysis. Archives of otolaryngology--head & neck surgery. 2007;133(8):796–800. [DOI] [PubMed] [Google Scholar]
- 4.Siddiqui F, Smith RV, Yom SS, et al. ACR appropriateness criteria (R) nasal cavity and paranasal sinus cancers. Head Neck. 2017;39(3):407–418. [DOI] [PubMed] [Google Scholar]
- 5.Robbins KT, Ferlito A, Silver CE, et al. Contemporary management of sinonasal cancer. Head & neck. 2011;33(9):1352–1365. [DOI] [PubMed] [Google Scholar]
- 6.Mendenhall WM, Amdur RJ, Morris CG, et al. Carcinoma of the nasal cavity and paranasal sinuses. The Laryngoscope. 2009;119(5):899–906. [DOI] [PubMed] [Google Scholar]
- 7.Russo AL, Adams JA, Weyman EA, et al. Long-Term Outcomes After Proton Beam Therapy for Sinonasal Squamous Cell Carcinoma. International journal of radiation oncology, biology, physics. 2016;95(1):368–376. [DOI] [PubMed] [Google Scholar]
- 8.Becker C, Becker AM, Pfeiffer J. Health-related quality of life in patients with nasal prosthesis. Journal of cranio-maxillo-facial surgery : official publication of the European Association for Cranio-Maxillo-Facial Surgery. 2016;44(1):75–79. [DOI] [PubMed] [Google Scholar]
- 9.Subramaniam T, Lennon P, O’Neill JP, Kinsella J, Timon C. Total rhinectomy, a clinical review of nine cases. Irish journal of medical science. 2016;185(3):757–760. [DOI] [PubMed] [Google Scholar]
- 10.Ng SP, Ludmir EB, Oyervides MA, Wu RY, Frank S, Gunn GB. Combination Intensity Modulated Proton Therapy and Passive Scatter Boost for Rapidly Progressing Nasal Cavity Squamous Cell Carcinoma. Cureus. 2017;9(9):e1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Amin MB, Edge S, Greene F, et al. (eds). AJCC Cancer Staging Manual. 8 ed Philadelphia, PA: Springer International Publishing; 2017. [Google Scholar]
- 12.Dagan R, Bryant C. Sinonasal Cancers In: Lee NY, Leeman JE, Cahlon O, et al. , eds. Target Volume Delineation and Treatment Planning for Particle Therapy: A Practical Guide. Cham: Springer International Publishing; 2018:141–151. [Google Scholar]
- 13.Spratt DE, Lee NY. Carcinoma of the Paranasal Sinuses In: Lee NY, Riaz N, Lu JJ, eds. Target Volume Delineation for Conformal and Intensity-Modulated Radiation Therapy. Cham: Springer International Publishing; 2015:83–89. [Google Scholar]
- 14.Hoppe BS, Stegman LD, Zelefsky MJ, et al. Treatment of nasal cavity and paranasal sinus cancer with modern radiotherapy techniques in the postoperative setting--the MSKCC experience. International journal of radiation oncology, biology, physics. 2007;67(3):691–702. [DOI] [PubMed] [Google Scholar]
- 15.Allen MW, Schwartz DL, Rana V, et al. Long-term radiotherapy outcomes for nasal cavity and septal cancers. International journal of radiation oncology, biology, physics. 2008;71(2):401–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Katz TS, Mendenhall WM, Morris CG, Amdur RJ, Hinerman RW, Villaret DB. Malignant tumors of the nasal cavity and paranasal sinuses. Head & neck. 2002;24(9):821–829. [DOI] [PubMed] [Google Scholar]
- 17.Blanchard P, Gunn GB, Lin A, Foote RL, Lee NY, Frank SJ. Proton Therapy for Head and Neck Cancers. Semin Radiat Oncol. 2018;28(1):53–63. [DOI] [PubMed] [Google Scholar]
- 18.Al-Mamgani A, van Rooij P, Mehilal R, Tans L, Levendag PC. Combined-modality treatment improved outcome in sinonasal undifferentiated carcinoma: single-institutional experience of 21 patients and review of the literature. European archives of oto-rhino-laryngology : official journal of the European Federation of Oto-Rhino-Laryngological Societies (EUFOS) : affiliated with the German Society for Oto-Rhino-Laryngology-Head and Neck Surgery. 2013;270(1):293–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Askoxylakis V, Hegenbarth P, Timke C, et al. Intensity modulated radiation therapy (IMRT) for sinonasal tumors: a single center long-term clinical analysis. Radiation oncology (London, England). 2016;11:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pignon JP, le Maitre A, Maillard E, Bourhis J. Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): an update on 93 randomised trials and 17,346 patients. Radiother Oncol. 2009;92(1):4–14. [DOI] [PubMed] [Google Scholar]
- 21.Hanna EY, Cardenas AD, DeMonte F, et al. Induction chemotherapy for advanced squamous cell carcinoma of the paranasal sinuses. Archives of otolaryngology--head & neck surgery. 2011;137(1):78–81. [DOI] [PubMed] [Google Scholar]
- 22.Shah JP, Patel SG, Singh B, (eds). Jatin Shah’s Head and Neck Surgery and Oncology. 4th ed Philadelphia: Elsevier/Mosby; 2012. [Google Scholar]
- 23.Fornelli RA, Fedok FG, Wilson EP, Rodman SM. Squamous cell carcinoma of the anterior nasal cavity: a dual institution review. Otolaryngology--head and neck surgery : official journal of American Academy of Otolaryngology-Head and Neck Surgery. 2000;123(3):207–210. [DOI] [PubMed] [Google Scholar]
- 24.DiLeo MD, Miller RH, Rice JC, Butcher RB. Nasal septal squamous cell carcinoma: a chart review and meta-analysis. The Laryngoscope. 1996;106(10):1218–1222. [DOI] [PubMed] [Google Scholar]
- 25.Ang KK, Harris J, Wheeler R, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363(1):24–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alos L, Moyano S, Nadal A, et al. Human papillomaviruses are identified in a subgroup of sinonasal squamous cell carcinomas with favorable outcome. Cancer. 2009;115(12):2701–2709. [DOI] [PubMed] [Google Scholar]
- 27.Larque AB, Hakim S, Ordi J, et al. High-risk human papillomavirus is transcriptionally active in a subset of sinonasal squamous cell carcinomas. Mod Pathol. 2014;27(3):343–351. [DOI] [PubMed] [Google Scholar]
- 28.Chung CH, Zhang Q, Kong CS, et al. p16 protein expression and human papillomavirus status as prognostic biomarkers of nonoropharyngeal head and neck squamous cell carcinoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2014;32(35):3930–3938. [DOI] [PMC free article] [PubMed] [Google Scholar]


