Abstract
The purpose of this project was to assess the feasibility and acceptability of a hatha yoga program designed to target chronic pain in people receiving opioid agonist therapy for opioid use disorder. We conducted a pilot randomized trial in which people with chronic pain who were receiving either methadone maintenance therapy (n=20) or buprenorphine (n=20) were randomly assigned to weekly hatha yoga or health education (HE) classes for 3 months. We demonstrated feasibility in many domains, including recruitment of participants (58% female, mean age 43), retention for follow-up assessments, and ability of teachers to provide interventions with high fidelity to the manuals. Fifty percent of participants in yoga (95% CI: 0.28 – 0.72) and 65% of participants in HE (95% CI: 0.44 −0.87) attended at least 6 of 12 possible classes (p = 0.62). Sixty-one percent in the yoga group reported practicing yoga at home, with a mean number of times practicing per week of 2.67 (SD = 2.37). Participant mood improved pre-class to post-class, with greater decreases in anxiety and pain for those in the yoga group (p< .05). In conclusion, yoga can be delivered on-site at opioid agonist treatment programs with home practice taken up by the majority of participants. Future research may explore ways of increasing the yoga “dosage” received. This may involve testing strategies for increasing either class attendance or the amount of home practice or both.
Keywords: yoga, chronic pain, methadone, buprenorphine
1. Introduction
Chronic pain is a significant problem for people with opioid use disorder who are receiving opioid agonist therapy (OAT), including methadone maintenance therapy or buprenorphine. A large percentage of these patients report that pain preceded any use of addictive substances (Rosenblum et al., 2003; Rosenblum et al., 2007; Tsui et al., 2010), and that their primary reason for initiating opioid use was to reduce pain (Brands, Blake, Sproule, Gourlay, & Busto, 2004; Tsui et al., 2010). Thus, it is unfortunate, but not surprising, that approximately 40-50% of methadone patients suffer from chronic pain (Dunn, Finan, Tompkins, Fingerhood, & Strain, 2015; Voon et al., 2015; Weiss et al., 2014)—a prevalence far higher than what is reported in the general population (Kennedy, Roll, Schraudner, Murphy, & McPherson, 2014). There is a similar rate of chronic pain among buprenorphine patients (Dunn et al., 2015; Stein, Herman, et al., 2015).
Despite high rates of pain, there are many challenges to treating pain in OAT patients (Volkow & McLellan, 2016). Several issues may complicate pharmacologic pain treatment, including the possibility of opioid-induced hyperalgesia (Athanasos, Ling, Bochner, White, & Somogyi, 2019); increased tolerance of opioids; illicit drug use; use of benzodiazepines, alcohol, and marijuana to mitigate pain; and patients’ own fears about medications and addiction (Eyler, 2013). Further, providers may have insufficient education regarding pain management in OAT patients (Berg, Arnsten, Sacajiu, & Karasz, 2009) and have difficulty resolving the seeming inconsistencies between a pain management approach and an addiction management approach (Eyler, 2013). There are few studies of non-pharmacologic treatment approaches to pain in OAT patients. Cognitive-behavioral therapy (CBT) may be efficacious for treating pain and improving quality of life across a range of chronic pain conditions (Chou et al., 2017; Williams, Eccleston, & Morley, 2012), but studies of CBT for pain relief amongst substance users are rare and inconclusive (Barrett & Chang, 2016; Haibach, Beehler, Dollar, & Finnell, 2014; Hruschak, Cochran, & Wasan, 2018).
Hatha yoga may be a useful approach to treating chronic pain, decreasing pain-related disability, and preventing opioid misuse during OAT. Yoga is an ancient Indian system of philosophy and practice (Desikachar, 1999). Most people who practice yoga in the West practice hatha yoga, which includes a focus on physical postures (āsanas), breath control (prāṇāyāma), and meditative practices. There are several reasons hatha yoga may be useful in this population. First, a meta-analysis of yoga across chronic pain conditions (including back pain, headache, and rheumatoid arthritis; n = 16 studies) reported that yoga had a moderate-large effect on pain, with a standardized mean difference (SMD) of −0.74. Yoga also had a moderate-large impact on pain-related disability (SMD = −0.79) and a moderate impact on depressed mood (SMD = −0.65; Bussing, Ostermann, Ludtke, & Michalsen, 2012). More recent meta-analyses have also found that yoga has a small to medium-sized impact on chronic pain patients’ function compared to a non-exercise control condition (Wieland et al., 2017), although there is a need for more high-quality research in order to have confidence in these results. Second, there are plausible mechanisms by which yoga may reduce chronic pain, decrease pain-related disability, and reduce opioid misuse. Possible cognitive or affective mechanisms include increased ability to use mindfulness in everyday life, including the ability to notice the sensory component of pain with less punitive self-judgment and negative affect (Bishop et al., 2004; Park et al., 2018); decreased pain catastrophizing (Curtis, Osadchuk, & Katz, 2011; Vlaeyen & Linton, 2000) that may occur with experience in gently moving the body and mindful attention to pain sensations; and decreased depression and anxiety (Zou et al., 2018). Yoga’s impact on chronic pain may also be meditated by increased muscle strength, flexibility, and endurance from engaging in yoga āsanas (Beazley, Patel, Davis, Vinson, & Bolgla, 2017; Ikai et al., 2017). Consistent with this, a meta-regression analysis suggested that exercise that includes stretching and strengthening results in decreased pain and improved functioning for people with chronic low back pain (Hayden, van Tulder, & Tomlinson, 2005).
A third reason that yoga may be a promising approach to chronic pain for persons receiving OAT is that yoga can be adjunctive to – and complement-- other types of pain and substance use treatment. Because yoga is a non-pharmacologic approach to pain, it avoids many of the problems described above that surround additional pharmacologic treatment of chronic pain in OAT patients. Finally, OAT patients may like the positive and self-reliant approach to health inherent in yoga: they may find it empowering to take active steps to improve their own health and well-being.
Although yoga has been recommended as a complementary treatment for addiction (Khanna & Greeson, 2013), there is a relatively small amount of data available to support the use of yoga for people with addictions (Kuppili, Parmar, Gupta, & Balhara, 2018). There is preliminary evidence that a hatha yoga program is acceptable to people with alcohol use disorders (Hallgren, Romberg, Bakshi, & Andreasson, 2014) and people living with HIV/AIDS who use crack cocaine (Agarwal, Kumar, & Lewis, 2015). Many of the previous studies on yoga in the context of addiction have focused on tobacco use (Sarkar & Varshney, 2017). Indeed, a large study has shown that hatha yoga, relative to a wellness class, is an effective adjunctive treatment to help women quit smoking (Bock et al., 2018),
There has been little research on yoga in persons with opioid use disorder, with a literature review showing only four studies (Dhawan, Chopra, Jain, Yadav, & Vedamurthachar, 2015; Mallik, Bowen, Yang, Perkins, & Sandoz, 2019; Shaffer, LaSalvia, & Stein, 1997; Zhuang, An, & Zhao, 2013). Three studies found superior outcomes for opioid-dependent individuals randomized to a yoga group vs. a control group on outcomes such as abstinence (Mallik et al., 2019), quality of life (Dhawan et al., 2015; Zhuang et al., 2013), and mood (Zhuang et al., 2013). However, definitive conclusions are limited by lack of randomization (Mallik et al., 2019) and use of intensive program formats that are not feasible in many settings or for many people, such as a 3-day, 12 hour program (Dhawan et al., 2015), or 1-hour classes for 5 days per week for 6 months (Zhuang et al., 2013). Further, two studies did not employ hatha yoga; rather, one used Raja yoga meditation, which includes meditation and prāṇāyāma, but no āsana practice (Mallik et al., 2019), and another used Sudaráan Kriyā Yoga (SKY), which consists only of breath-based yoga practices (Dhawan et al., 2015). The one study that failed to find differences between yoga and a control group had a robust control group –i.e., psychotherapy (Shaffer et al., 1997). No studies looked specifically at pain as an outcome. Thus, there is little information available on acceptable and efficacious yoga programs for people with chronic pain who are taking OAT.
In this treatment development study, we developed an instructors’ manual for a yoga program for people with chronic pain who were enrolled in OAT. We then conducted a pilot randomized trial of 12 weeks of a weekly hatha yoga class (yoga) vs. a weekly health education class (HE). The primary treatment target was life interference due to pain. The purpose of this study was to assess feasibility of research procedures, and acceptability and feasibility of yoga and HE classes in this group of patients. We also assessed immediate changes in mood and pain from pre- to immediately post-class for each treatment arm, with the hypothesis that classes may result in small, immediate improvements in both conditions, albeit with larger changes for the yoga group. We planned this last set of analyses because it is possible that the immediate impact of yoga on pain and mood, whether it be following a class or home practices, serves to mediate the longer term and more generalized impact of yoga on pain-related interference and other pain outcomes.
2. Material and Methods
2.1. Participants and Setting
We recruited participants from a methadone maintenance clinic and from a primary care clinic where providers prescribed buprenorphine. Clinics were located at two separate sites of a federally-qualified health center in Fall River, MA. Please see Figure 1 for the CONSORT flow chart. Inclusion criteria were: 1) enrolled in methadone maintenance or buprenorphine treatment for 3 or more months; 2) plan to continue this treatment for next 6 months; 3) chronic pain, defined as pain duration for at least three months (Dworkin et al., 2012), a mean score of 4 or higher on the Brief Pain Inventory (BPI) Pain Interference Scale (Cleeland & Ryan, 1994), and pain severity of 4 or higher on a Visual Analog Scale (0-10) indicating “worst pain in the last week” (Breivik et al., 2008; Jensen, Chen, & Brugger, 2003); 4) aged 18 or older; 5) proficiency in English sufficient to engage in informed consent in English and understand classes taught in English; and 6) medically cleared by their primary care physician for mild-to-moderate physical activity. Exclusion criteria were: 1) medical conditions that would make participation in the study difficult or potentially dangerous, per Principal Investigator discretion; 2) joint surgery in the previous 6 months; 3) use of assistive ambulatory devices other than a cane; 4) pregnancy; 5) planned surgery in next 6 months; 6) current psychosis; 7) current home yoga practice or yoga class attendance; and 8) current meditation practice.
Figure 1.
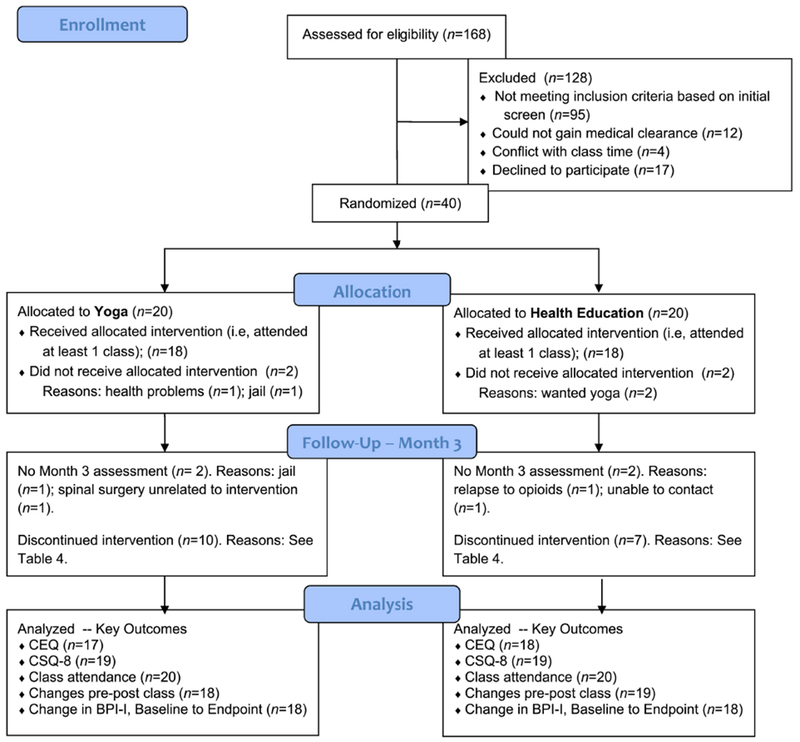
Flow Diagram of Study Participants
2.2. Procedures
The protocol was approved by the Butler Hospital Institutional Review Board. Because the methadone clinic and buprenorphine clinic were not at the same physical location, we first recruited 20 participants in the methadone clinic between March and December of 2017, followed by 20 in the buprenorphine clinic between January and August of 2018. Potential participants were recruited via provider referral or self-referral. In order to present the study interventions with equipoise, in our advertising materials we described the project as a study of “new ways of coping with chronic pain” that could include either health education or yoga. At their first in-person contact with potential participants, research staff conducted an initial eligibility screen and obtained consent to seek medical clearance from the participant’s physician. Once clearance was gained, participants provided informed consent and attended a baseline interview. They were then enrolled and randomized. Participants were stratified based on site (methadone clinic or buprenorphine clinic) and, for the buprenorphine group only, gender. (We added gender after noting that methadone participants were not evenly distributed by gender.) Randomization was also blocked to ensure approximately equal numbers in each study arm. Study staff had no access to the randomization table; when a participant was ready to be enrolled, a study staff member simply selected “randomize” in our data collection system (REDCap).
Participants were invited to attend once weekly classes in their respective study arm (yoga or HE), at their clinic site. Although we initially offered class twice per week, each class was so small that we switched to once per week in order to increase class size even if it limited people we could enroll to only those who could make that one class time. Further, although the first 10 participants began as a single cohort (i.e., all were randomized in the same 2.5-week period), we subsequently switched to rolling enrollment in the interventions for feasibility reasons. Participants were invited to attend class for 12 weeks. All classes were audiorecorded.
Formal assessments occurred after 1, 2, and 3 months (intervention endpoint). Further, each time a participant attended a class, they completed a brief self-report assessment before and after the class (i.e., pre-class and post-class assessments).
2.3. Assessment Instruments
2.3.1. Participant safety
Each week that participants attended class, they completed a questionnaire about whether they experienced any injuries as a result of yoga. At the 3-month timepoint, we administered the Systematic Assessment of Treatment-Emergent Events – General Inquiry (SAFTEE; Levine & Schooler, 1986) to elicit any additional adverse events that occurred over the previous 3 months. Finally, any report of a new medical problem made to study staff at any time was also recorded as an adverse event.
2.3.2. Teacher fidelity to the manual
We created a measure of yoga teacher fidelity with two components: an assessment of whether the teacher taught specific prescribed practices (content fidelity), and whether the teacher taught the class in the recommended style (style fidelity). To rate content fidelity, we created a checklist, and for each item, the rater chose a score ranging from 0-2, with 0 being “no-not done;” 1 being “partially done;” and 2 being “yes – done.” For the final content fidelity scale, total possible number of points was 36; in this report, we present scores as a percent of the total. For style fidelity, the rater rated specific items such as “encouraged acceptance of one’s own physical abilities” or “acknowledged pain experience [of participants]” on a 0-2 scale, with 0 being “poor” and 2 being “good.” There were descriptive anchors provided for each item. Total possible points were 24; in this report, we present scores as a percent of the total. Similarly, HE teacher fidelity ratings had two components: a content fidelity scale which was different for each class, with total possible points ranging from 10-15; and a style fidelity scale which included items such as “stayed on topic” and “did not conduct psychotherapy.” In the style fidelity scale, there were four items and a total of 7 possible points. In this report, we present scores for both scales as percent of the maximum possible.
2.3.3. Acceptability
We measured intervention credibility and patient expectations for intervention success at baseline with the Credibility Expectancy Questionnaire (CEQ; Devilly & Borkovec, 2000); this was administered after the initial class attended. We administered the Client Satisfaction Questionnaire (CSQ-8; Nguyen, Attkisson, & Stegner, 1983) at post-treatment (3-month assessment) to assess satisfaction with treatment. Internal consistency was good for both the CEQ and CSQ-8 in this sample. See Table 3 for values of Cronbach’s alpha. We used qualitative responses to a detailed post-treatment interview to gain participants’ feedback about intervention components and research procedures.
Table 3.
Acceptability and Feasibility Metrics
| Cronbach’s Alpha | Possible Range | Mean (± SD) |
|||
|---|---|---|---|---|---|
| Yoga | HE | t(p = ) | |||
| Credibility (CEQ), after first class | 0.90 | 0-1 | 0.79 (± 0.21) | 0.67 (± 0.20) | 1.82 (0.08) |
| Expectancy (CEQ), after first class | 0.91 | 0-1 | 0.59 (±0.19) | 0.48 (± 0.19) | 1.57 (0.13) |
| Program satisfaction, (CSQ-8) Endpoint | 0.83 | 8-32 | 27.5 (±3.4) | 27.4 (±3.9) | 0.09 (0.93) |
Note. For credibility and expectancy, n=17 and 18 in the yoga arm and HE arm, respectively. For program satisfaction, n=19 in each arm. This includes two people who did not complete the CSQ-8 at M3 assessment but did complete it 3 months later.
2.3.4. Intervention adherence
At formal assessment points (Months 1, 2, and 3), we administered a version of the International Physical Activity Questionnaire (IPAQ; IPAQ Research Committee, 2005) that we modified to include assessment of home yoga practice in the previous week. We administered this version of the IPAQ to participants in both arms.
2.3.5. Mood and pain pre- and post-class
Pre- and post-class, we asked participants to “rate how you feel right now” on 5 scales which assessed sadness, anxiety, irritability, fatigue, and pain. Participants used a 0-10 Numerical Rating Scale. As an example, for sadness, 0 represented “no sadness,” 5 represented “moderate sadness,” and 10 represented “extreme sadness.”
2.3.6. Pain
At baseline, month 1, month 2, and month 3, we used the Brief Pain Inventory - Pain Interference Scale (BPI-I) to measure our primary treatment target: pain-related disability. The pain interference subscale includes items assessing psychosocial/ affective functioning (relations with others, enjoyment of life, mood) and physical functioning (walking, general activity, work; Cleeland & Ryan, 1994). In this study, Cronbach’s alpha, representing internal consistency, was 0.90.
2.4. Interventions
2.4.1. Yoga program
The final version of the hatha yoga program was a manualized 1-hour class that included the following elements, in this order: greeting and discussion of home practice; centering body and breath awareness followed by a sounding tone (typically “Om”); prāṇāyāma practices of prayer breath, slow Bhastrikā, and Nāḏī Śoḏhana warm-up movements including cat-cow, a side stretch, and shoulder rolls and neck lengthening with neck release; a sequence of āsanas that included forward fold (uttānāsana), Warrior I, down dog or modified down dog with a chair, some additional optional āsanas, and a twist; and then final seated meditation and discussion of home practice. All participants started seated in a chair; they also each had a mat and blocks. Teachers customized practice to each participant’s physical abilities; thus, some participants continued to use the chair for balance throughout the class whereas others did not. The teachers’ manual also included guidance regarding style of teaching. Teachers were asked to: acknowledge the validity of the participants’ pain and gently challenge them to try a new way of coping with it; discuss and model acceptance of one’s own physical abilities; frequently demonstrate and encourage mindful attention to the present moment; emphasize breathing in every part of the class; and teach the class at a slow pace. All yoga teachers were Registered Yoga Teachers (RYTs) with the Yoga Alliance. Prior to teaching, the lead yoga instructors and study Primary Investigator provided instructors with approximately 5 hours of training in manual procedures and protection of human subjects. Throughout the study, teachers met monthly for supervision and provision of feedback on the manualized procedures.
Because this was a treatment development study, we did make some changes to the yoga manual over time. We developed the original manual based on: a) other yoga manuals for chronic pain; b) feedback from patients and staff at the clinic; c) our own previous experience with yoga research; and d) previous experience of yoga teachers. We mention the most substantive changes over time here. We do not believe that any of these changes substantially altered the substance of the program. In our first version of the manual, we had included a brief walking meditation; however, we dropped this practice due in part to the available clinic space feeling too small to teachers and participants. Teachers originally had more flexibility in choice of particular breathing practices or āsanas; however, as we learned what worked over time, we decreased the amount of choice the teachers had.
2.4.2. Health education (HE)
These classes occurred at the same time as the yoga classes, and lasted one hour. There were 12 classes on different topics so that participants could join at any class, thus accommodating rolling admission to the study. Class topics were: grains, fruits, and vegetables; meat, dairy, and oils; sodium, fats, and sugars; fad diets; exercise with chronic pain; sleeping with chronic pain; activities of daily living; smoking cessation; biology of pain; germs, colds, and the flu; protecting your heart; and being prepared as a patient. Classes were designed to be informative and engaging but not personally tailored. The teacher used slides, audio and visual clips, and questions to engage the class in discussion. Teachers were bachelor’s level research staff with substantive experience working with this population. Similar to the yoga teachers, all HE teachers were trained in the manual and topic areas before teaching. The major changes to the HE classes over time included provision of more information about how to eat in a healthy way while on a limited income, and increased information about specific apps to use to manage one’s health information.
2.5. Data Analysis
We calculated descriptive statistics to summarize the characteristics of the sample. We used t-tests for differences in means and χ2 tests for differences in counts to compare intervention arms with respect to demographic characteristics and baseline pain interference.
To compare pre- to post-class levels of sadness, anxiety, irritability, fatigue, and pain, we used a repeated measures (time = pre or post) multiple-level design. Depending on the number of classes attended, participants were observed on a varying number of pre-and post-class assessments. Therefore, to adjust for non-independence, we used fixed-effects linear regression (Allison, 2009). This method effectively controlled for all time-invariant unobserved between-subject heterogeneity. Score on the mood or pain rating was the dependent variable. Models estimated three parameters: a regression constant, a main effect for time that gave the mean within subject change in the arm coded 0, and the time by treatment interaction that represented the between group differences in mean change scores. We present 95% confidence interval estimates for all unstandardized differences in mean change scores.
We defined “high attenders” as people who attended 6 or more classes in either arm; “low attenders” were those who attended 5 or fewer classes. In order to look at changes in BPI-I from baseline to interim follow-up assessments (Month 1, Month 2) and end-of-treatment (Month 3), we examined changes in: a) the intent-to-treat sample (all available data) and b) in the sample of people whom were high attenders. We conducted a t-test comparing the change scores for the two groups, and then calculated Cohen’s d as a measure of effect size.
3. Results
3.1. Participant Demographics
Please see Table 1 for information about enrolled participants.
Table 1.
Background Characteristics and Baseline Pain Interference by Intervention Arm.
| Mean (± SD) or n (%) |
|||
|---|---|---|---|
| Yoga (n = 20) | HE (n = 20) | t or χ2 (p = ) | |
| Age (Years) | 43 (± 10.7) | 44 (± 10.8) | −0.29 (0.77) |
| Gender (Female) | 9 (45%) | 14 (70%) | 2.56 (0.11) |
| Race | |||
| Black/African American | 2 (10%) | 0 (0%) | 2.23 (0.29) |
| White | 16 (80%) | 17 (85%) | |
| Other or More than One | 2 (10%) | 3 (15%) | |
| Hispanic or Latino (Yes) | 3 (15%) | 1 (5%) | 1.11 (0.29) |
| Married/Lives w Partner (Yes) | 9 (45%) | 9 (50%) | 0.01 (0.76) |
| Education (Some College or More) | 15 (83%) | 11 (55%) | 3.52 (0.06) |
| Lives in Own Home (Yes) | 11 (61%) | 14 (70%) | 0.33 (0.56) |
| Brief Pain Inventory -- Interference Scale | 6.11 (±2.08) | 6.12 (±2.09) | 0.01 (0.99) |
3.2. Recruitment and retention
Our a priori recruitment target was 3 participants per month, and our retention target was that 80% of participants would complete the Month 3 (endpoint assessment). Regarding enrollment, we came close to achieving our goal, randomizing 2.4 people per month of recruitment in the methadone clinic, and 2.9 people per month in the buprenorphine clinic. Retention for endpoint assessments was excellent: 90% of participants completed their month 3 assessments (i.e., 18 of 20 participants in each group completed these assessments). Please see Figure 1 for the CONSORT flow sheet.
3.3. Teacher fidelity to the manuals
We evaluated yoga teacher and HE teacher fidelity to respective manuals. Because the yoga class manual changed somewhat over the course of the project, we evaluated 6 yoga classes that were taught in the last 6 months of the project. Across 3 teachers and 6 classes rated, the average content fidelity score was 90%, with the lowest score being 86%, and the average style fidelity score was 97% with the lowest score being 92%. When we evaluated 9 HE classes, across 3 teachers, taught over the course of the project, the average content fidelity score was 93%, with the lowest score being 90%, and the average style fidelity score was 94% with the lowest score being 86%. Thus, teachers showed very good fidelity to the manual in both arms.
3.4. Adverse events
We did not observe any serious adverse events that were possibly, probably, or definitely related to study participation. There was one adverse event, with severity graded as moderate, which was probably related to the study intervention (yoga). Please see Table 2 for details regarding adverse events that occurred while participants were in a study intervention. There were no significant differences in distribution of the severity of adverse events by study arm (X2(df=3) = 2.17, p = 0.54).
Table 2.
Adverse Events during 3 months of active intervention
| Yoga | HE | |||
|---|---|---|---|---|
| Grade | n | Description of events | n | Description of events |
| Minimal | 0 | 1 | Ear infection | |
| Mild | 3 | Increased low back pain HPV and ovarian cyst muscle spasm in calf |
4 | Skin rash Non-displaced fracture Increased ADHD symptoms Fall |
| Moderate | 10 | Increased back pain* Migraine Constipation (2 pts) Tooth pain and oral surgery Torn ligaments in knee Lapse in MAT Broken hand Bruised ribs Panic attack |
6 | Anemia Kidney pain Neuropathic pain Lump in breast Fluid in lungs/ COPD Knee pain |
| Severe | 2 | Diabetic ketoacidosis Septic left shoulder |
1 | Diabetic seizure |
| Total | 15 | 12 | ||
Note: For number of adverse events by study arm, X2 = 2.17, df = 3, p = 0.54
Eighteen participants reported at least one adverse event. Four participants had more than one adverse event.
There was only one adverse event probably related to the intervention. We graded it as moderate. A person with a substantive history of back pain and back spasms attended yoga class, where she had a painful back spasm while doing cat/cow and a seated side stretch with arms raised. Teacher had her rest and the spasm appeared to subside. She later reported that the severe level of pain (8/10) continued the rest of the day and into the next morning, only fully subsiding after about 4 days. Despite reassurance from her primary care provider, she chose not to return to classes.
3.5. Credibility, expectancy, and satisfaction
We assessed intervention credibility and expectancy for intervention success after participants attended their first class. For both measures, we set as a feasibility target that the average across participants would be greater than the score on the midpoint of the possible score range (i.e., at least 0.50, with the possible score range being 0-1) for both study arms. We achieved this with one exception: the average for expectancy was just below the midpoint (0.48) for HE. See Table 3. We assessed program satisfaction at the intervention endpoint (month 3). As shown in Table 3, we found that program satisfaction was high (mean >24) for both arms.
3.6. Class attendance and adherence to home practice
Other measures of intervention acceptability were class attendance and adherence to home practice. Class attendance fell below our expectations: we found that only 10/20 participants in yoga (i.e., 50%, 95% confidence interval = 0.28 – 0.72) and 13/20 participants in HE (65%, 95% confidence interval = 0.44 −0.87) could be considered “high attenders” (i.e., attended at least 6/12 classes; X2 (df=1) = 0.25, p = 0.62). See Table 4 for reasons for low attendance (i.e., attending 5 or fewer classes). Approximately two-thirds of the reasons for low attendance were considered unrelated to the study intervention. Including all participants (even those who attended 0 sessions), average number of classes attended was 5.2 (SD = 4.01) in the yoga arm and 6.55 (SD = 3.87) in the HE arm (t (df = 38) = 1.08, p = 0.29). We also assessed amount of home practice. When assessed on a monthly basis (averaged across the three months), eleven of 18 (61%) in the yoga arm reported practicing yoga at home, averaging two or more times per week (range= 0-6.33 times). This was close to our goal that 70% of yoga participants engage in yoga at least twice per week at home. Only one person (of 20 assessed) in the HE arm reported practicing yoga at home, thus confirming that intervention contamination was minimal. Across the 3-month intervention period, the mean number of times practicing yoga at home per week was 2.67 (SD = 2.37) for the yoga group, and 0.13 (SD = 0.60) for the HE group (t = −4.63 (df = 36), p = 0.000). The mean number of minutes per week practicing yoga at home was 58.91 (SD = 71.60) in the yoga group, and 3.33 (14.91) in the HE group (t = −3.40, df=36, p = 0.002).
Table 4.
Potential Reasons for Low Attendance (defined as attending 5 or fewer classes out of 12 possible classes)
| Yoga | HE | Examples | |||||
|---|---|---|---|---|---|---|---|
| Total | MMT | BUP | Total | MMT | BUP | ||
|
Related to specific aspects of the intervention | |||||||
| Wanted other arm | 1 | 1 | 2 | 1 | 1 | •Two attended 0 classes (Both in HE arm) •One person attended 1 class (Yoga arm) |
|
| Did not like class | 2 | 2 | 0 | •Attended 2 classes; stopped attending due to discomfort over “vibe” of class. Also was embarrassed by his physical limitations. Reported that he liked stretching and breathing and did these practices at home. (Yoga arm) •Attended 2 classes; felt anxious and self-conscious during class because other pts could get off the mat onto the floor while she had to remain in chair. (Yoga arm) |
|||
| Health problems, possibly related | 1 | 1 | 0 | •Attended 1 class; history of chronic back pain and back spasms; experienced a back spasm during first class while doing cat/cow; declined to return despite reassurance from PCP (Yoga arm) | |||
|
Unrelated to specific aspects of the intervention | |||||||
| Substance use disorder relapse | 1 | 1 | 2 | 1 | 1 | •Attended 5 classes; then stopped attending due to a respiratory infection and relapse to cocaine use (HE arm) •Attended 2 classes; thereafter missed several Bup clinic appointments; was arrested; was suspected of drug diversion (HE arm) •Attended 2 classes. Was switched from Bunavail to Suboxone; experienced increased craving and relapsed to heroin and cocaine. Thereafter, reported using a specific asana (child pose) at home to stretch. (Yoga arm) |
|
| Class time no longer acceptable | 0 | 1 | 1 | • Attended 2 classes, then started a new job that conflicted with class time (HE arm) | |||
| Health problems, unrelated | 4 | 2 | 2 | 0 | •Attended 3 classes, then started having health problems including the flu and ongoing GI problems. Reported using breath practices from class at home even after no longer attending class (Yoga, arm) •Attended 0 classes; experienced worsening migraines and TMJ (Yoga arm) •Attended 3 classes, then reported health problems including abdominal pain, increased low back pain (4 weeks from last yoga class attended), torn ligaments in knee (8 weeks from last yoga class attended), ongoing tooth pain and oral surgery (Yoga arm) •Attended 1 class; broke hand 4 weeks later. Also began experiencing worsening neck & back pain and received post-M.3 neck surgery (Yoga arm) |
||
| Homelessness | 1 | 1 | • Attended 2 classes; mom became ill then passed away; participant was subsequently homeless and searching for housing (HE arm) | ||||
| Jail | 1 | 1 | • Attended 0 classes; Arrested and jailed before attending any classes (Yoga arm) | ||||
| Other | 1 | 1 | •Attended 2 classes; reported stopped attending due to increased court obligations (HE arm) | ||||
| Totals | 10 | 5 | 5 | 7 | 3 | 4 | |
Note. MMT = Methadone maintenance treatment; BUP = buprenorphine treatment.
3.7. Mood and pain pre-to post-class
As seen in Table 5, in both arms we found significant changes from pre-class to post-class on all 5 variables: sadness, anxiety, irritability, fatigue, and pain. Directionally, between-group comparisons indicated that participants randomized to yoga had larger within-subject reductions on all 5 outcomes. Differences between groups were significantly different for anxiety and for pain. For example, on average, the yoga had a 1.79 point decrease in anxiety from pre- to post-class (on a 0-10 scale), whereas the HE group had only a 1.07 point decrease in anxiety from pre- to post-class. Similarly, the yoga group had a 2.08 decrease in pain severity from pre- to post-class, whereas the HE group had a 0.97 decrease in pain severity.
Table 5.
Change in Mood and Pain from Pre- to Post-Classa.
| Sadness | Anxiety | Irritability | Fatigue | Pain | |
|---|---|---|---|---|---|
| Within Arm | Δ (95%CI) |
Δ (95%CI) |
Δ (95%CI) |
Δ (95%CI) |
Δ (95%CI) |
| Yogab | −1.06*** (−1.52; −0.61) |
79*** (−2.22; −1.36) |
−1.55*** (−1.92; −1.18) |
−1.27*** (−1.62; −0.93) |
−2.08*** (−2.52; −1.64) |
| HEb | −0.75*** (−1.13; −0.38) |
−1.07*** (−1.44;−0.71) |
−1.36*** (−1.76; −0.96) |
−0.92*** (−1.31; −0.53) |
−0.97*** (−1.44; −0.49) |
|
Between Arm Difference |
|||||
| Yoga Δ – HE Δc | −0.31 (−0.90; 0.27) |
−0.71* (−1.27; −0.15) |
−0.19 (−0.75; 0.36) |
−0.35 (−0.89; 0.18) |
−1.11*** (−1.76; −0.47) |
p < .05
p < .01
p < .001
We used fixed-effects linear regression to estimate mean within-subject change. The fixed-effects estimator was used because participants were assessed on a varying number of occasions. For sadness, anxiety, irritability, and fatigue/energy, 18 participants randomized to yoga were observed on a total of 217 occasions, and 19 participants randomized to HE were observed on a total of 266 occasions. For weekly pain, 13 yoga participants were observed on 132 occasions and 10 participants in the HE arm were observed on a total of 138 occasions.
To provide values interpretable to the reader, we estimated coefficients for within-subject change in Yoga and HE in separate models with dummy coding reversed. Both models included the time (pre-post class) by intervention arm interaction. Thus, values provided are unstandardized regression coefficients that represent mean within-subject change in each arm.
Values presented are between-group differences in unstandardized pre-post change scores. These were estimated as the time (prepost class) by intervention arm interaction with Yoga and post-class coded as 1. These coefficients give the change in the Yoga arm, relative to change in the HE arm.
3.8. Change in primary outcome over time
Finally, we assessed change in our primary target (interference due to pain, as assessed by the BPI-I) over time. We present mean change scores in Table 6. Using an intent-to-treat analysis, we did not observe differences between groups, with a mean difference in change scores from baseline to Month 3 of −0.08, p = 0.94, Cohen’s d = 0.03 (95% CI = −0.62, 0.69). When we conducted an analysis comparing baseline and Month 3 using only high attenders in both arms, we observed an unstandardized mean difference in change scores of −0.84, p =0.38, Cohen’s d = −0.37 (95% CI = −0.46, 1.20), with greater reductions in interference due to pain observed in the yoga group. This represents a small-to-medium effect size.
Table 6.
Mean Change in Brief Pain Index-Interference (BPI-I) Scores from Baseline to Months 1, 2, and 3, by Intervention Arm.
| INTENT TO TREAT | HIGH ATTENDERS ONLY | |||||
|---|---|---|---|---|---|---|
| Month 1 | Month 2 | Month 3 | Month 1 | Month 2 | Month 3 | |
| Mean Change from Baseline (HE arm)a | −1.53 | −1.37 | −0.81 | −2.07 | −1.52 | −0.96 |
| Mean Change from Baseline (Yoga arm)a | −1.76 | −1.35 | −0.89 | −2.49 | −1.77 | −1.80 |
| Raw Differenceb | −0.23 | 0.02 | −0.08 | −0.42 | −0.25 | −0.84 |
| Cohen’s d | −0.10 | 0.01 | −0.03 | −0.23 | −0.14 | −0.36 |
| p-valuec | 0.769 | 0.978 | 0.920 | 0.592 | 0.736 | 0.384 |
BPI-I at follow-up timepoint – baseline BPI-I. Negative values indicate a reduction in BPI-I scores.
Mean change in the Yoga arm + mean change in the HE arm. Negative scores indicate larger reductions in BPI-I scores in the Yoga arm.
p-value for a t-test comparing mean change scores by intervention arm.
4. Discussion
The purpose of this study was to examine the feasibility and acceptability of a clinical trial of yoga vs. HE for people with chronic pain who were enrolled in methadone maintenance or buprenorphine treatment. We offered one yoga class per week and brought yoga teachers to the treatment site for the convenience of participants. We were able to recruit participants at a reasonable rate and retain them for follow-up assessments. Participants rated both the yoga classes and the HE classes as credible interventions for pain, and, on average, reported acceptable levels of satisfaction with the program. Teachers showed very good fidelity to the manuals in both arms. There were no serious adverse events related to the study intervention. In summary, we demonstrated feasibility and acceptability on multiple important indices.
Although this is an underpowered pilot study, we did find clinical effects that pointed in the expected direction. Persons who attended yoga sessions, on average, showed improved pain and anxiety immediately following the class. In addition, persons in the yoga condition had moderate improvement in pain interference over 3 months if they attended at least half of the 12 intervention sessions. However, because this is based on an analysis of high-attenders only, and consists of only a little over half the sample, the question of efficacy remains unanswered until tested in future, adequately powered clinical trials using intent-to-treat analyses.
Our treatment attendance rate was not as high as is typically desired in clinical trials (Cramer, Haller, Dobos, & Lauche, 2016). This is perhaps not surprising, as persons with opioid use disorder may have health and life concerns, at times chaotic and unpredictable, that disrupt clinical study participation (Stein, Anderson, Thurmond, & Bailey, 2015). This population is notable for their numerous medical comorbidities (Edelman, Tetrault, & Fiellin, 2014; Jiao et al., 2016; Samet, O’Connor, & Stein, 2018), and high rate of psychosocial stressors, including low income, homelessness, transportation problems, accidents, physical assault or fights, and incarceration (Hayaki, Stein, Lassor, Herman, & Anderson, 2005).
Indeed, our treatment attendance rate appears to be in line with other trials of behavioral interventions for substance misuse, and, specifically, opioid use disorder. A systematic review and meta-analysis of mindfulness-based treatments for substance misuse reported that “treatment completion” rates were 43% to 100%, with generally high attrition rates (Li, Howard, Garland, McGovern, & Lazar, 2017). In the studies reviewed, treatment attendance rates were not different between the mindfulness-based intervention and alternative treatment comparison groups (e.g., CBT, social support group; Li et al., 2017). As an example, in a pilot study of Mindfulness-Based Relapse Prevention for people in methadone maintenance treatment, investigators enrolled 15 participants, of whom only 10 attended at least one session. Overall, participants attended only 57% of the six scheduled 2-hour group sessions (Bowen, Somohano, Rutkie, Manuel, & Rehder, 2017). Similarly, in a trial of Mindfulness-Oriented Recovery Enhancement (MORE) for people recruited from chronic pain clinics who used daily or near-daily prescription opioid analgesics, Garland et al. (2014) randomized 115 participants. Of the 57 randomized to MORE, 15 (26%) did not attend any sessions, and an additional 5 (9%) discontinued the intervention early. Only 54% allocated to MORE were assessed postintervention. Attendance during CBT interventions in this population has also been low. For example, Moore et al. (2016) looked at the effects of CBT in persons enrolled in primary care-based buprenorphine treatment and found session attendance to be approximately 60% on average. Thus, class attendance in our pilot study was not out of the range reported in other behavioral interventions in opioid populations. Further, given the small sample size, the 95% confidence intervals surrounding the percent of people who we considered high attenders was quite large. Given that attendance in other studies of behavioral interventions is similar, research on methods that may improve intervention attendance in this population is certainly warranted.
One of the ways to increase treatment “dosage” is to encourage home practice. In this study, we found that yoga-assigned participants were willing to practice at home, and there may be ways to increase support for home practice that can be examined in future studies. Other yoga programs designed to target health problems such as chronic low back pain (Saper et al., 2014), depression (Uebelacker et al., 2017), or smoking cessation (Bock et al., 2018) also included yoga home practice, and there are some data indicating that amount of yoga home practice may improve outcomes such as stress reduction (Greenberg et al., 2018).
We believe that the next steps in this line of research should include investigation of ways of increasing the intervention dosage received, whether by increasing class attendance or increasing simple, safe, home yoga practice. As an example, it is possible that an initial 1:1 meeting with the yoga instructor could be used to increase comfort with participating in the yoga class as well as increase self-efficacy for home practice. A second possibility would be to return to offering classes more than once per week so that if a person was not feeling well on a certain day, or had some other scheduling conflict, they might be able to attend another day. Of course, offering yoga classes offsite (e.g., at a YMCA) might increase the number of classes available each week; however, we believe that the familiarity of the OAT clinic may be important for helping participants feel comfortable attending class. A third option might be to increase and extend resources for practice at home – such as through tailored videos made available to participants.
Limitations are inherent to a pilot study. First, given the small sample, it was not surprising that the groups were unbalanced on at least one demographic variable: the people assigned to the yoga arm had, as a group, fewer years of education than those assigned to the HE arm. Second, we conducted this study at two sites within one healthcare system with a small set of yoga teachers, and thus do not know how generalizable our results are to other sites. Third, reflecting the populations at these clinics, there were few non-white or Latino participants enrolled in the study. Fourth, we changed some of our procedures during the course of the study. For example, we originally offered two classes times per week, but then moved to only offering one class per week. We also changed from cohort enrollment to rolling enrollment. These changes, however, are consistent with the goal of pilot research to refine research procedures in preparation for the next step in a line of research.
In sum, the population of people engaged in OAT are clearly in need of more options for coping with chronic pain, as the potential costs of uncontrolled chronic pain include not only suffering and functional limitations but also higher risk for drug relapse. Hatha yoga shows promise, and future research might examine ways to maximize the “dosage,” as well as defining what a minimally important dose might be. Ultimately, a clinical efficacy trial may be warranted, with examination of the impact of yoga on outcomes such as pain interference, pain severity, anxiety and depression, cravings, illicit substance use, retention in OAT, and overall quality of life.
Highlights:
We assessed feasibility of a yoga program for pain in people with opioid use disorder.
Recruitment, retention, and instructor fidelity to the manual were good.
Participants practiced yoga at home, but class attendance was lower than desired.
Yoga participants showed decreases in anxiety and pain from pre- to post- class.
Future research should focus on increasing attendance and home practice.
Acknowledgements
This study was funded by a grant from the National Center for Complementary and Integrative Health (NCCIH) at the National Institutes of Health [R34AT009432], Its contents are solely the responsibility of the authors and do not necessarily represent the official views of NCCIH.
Authors would like to thank Jon Leaver, Tom Gillette, Katherine Conte, and Micah Conti for their excellent work on this project. We would also like to thank our Safety Monitoring Committee for their time and oversight– Beth Bock, Geetanjali Chander, and Erik Groessl.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Registered at clinicaltrials.gov:
References
- Agarwal RP, Kumar A, & Lewis JE (2015). A pilot feasibility and acceptability study of yoga/meditation on the quality of life and markers of stress in persons living with HIV who also use crack cocaine. Journal of Alternative and Complementary Medicine, 21(3), 152–158. doi: 10.1089/acm.2014.0112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allison PD (2009). Fixed effects regression models Quantitative applications in the social sciences. Number 160 Sage: Thousand Oaks, CA. [Google Scholar]
- Athanasos P, Ling W, Bochner F, White JM, & Somogyi AA (2019). Buprenorphine maintenance subjects are hyperalgesic and have no antinociceptive response to a very high morphine dose. Pain Medicine, 20(1), 119–128. doi: 10.1093/pm/pny025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett K, & Chang YP (2016). Behavioral interventions targeting chronic pain, depression, and substance use disorder in primary care. Journal of Nursing Scholarship, 48(4), 345–353. doi: 10.1111/jnu.12213 [DOI] [PubMed] [Google Scholar]
- Beazley D, Patel S, Davis B, Vinson S, & Bolgla L (2017). Trunk and hip muscle activation during yoga poses: Implications for physical therapy practice. Complementary Therapies in Clinical Practice, 29, 130–135. doi: 10.1016/j.ctcp.2017.09.009 [DOI] [PubMed] [Google Scholar]
- Berg KM, Arnsten JH, Sacajiu G, & Karasz A (2009). Providers’ experiences treating chronic pain among opioid-dependent drug users. J Gen Intern Med, 24(4), 482–488. doi: 10.1007/s11606-009-0908-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop SR, Lau M, Shapiro S, Carlson L, Anderson ND, Carmody J, et al. (2004). Mindfulness: A proposed operational definition. Clinical Psychology Science and Practice, 11, 230–241. [Google Scholar]
- Bock BC, Dunsiger SI, Rosen RK, Thind H, Jennings E, Fava JL, et al. (2018). Yoga as a complementary therapy for smoking cessation: Results from BreathEasy ,a randomized clinical trial. Nicotine and Tobacco Research. doi: 10.1093/ntr/nty212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowen S, Somohano VC, Rutkie RE, Manuel JA, & Rehder KL (2017). Mindfulness-based relapse prevention for methadone maintenance: a feasibility trial. Journal of Alternative and Complementary Medicine, 23(7), 541–544. doi: 10.1089/acm.2016.0417 [DOI] [PubMed] [Google Scholar]
- Brands B, Blake J, Sproule B, Gourlay D, & Busto U (2004). Prescription opioid abuse in patients presenting for methadone maintenance treatment. Drug and Alcohol Dependence, 73(2), 199–207. [DOI] [PubMed] [Google Scholar]
- Breivik H, Borchgrevink PC, Allen SM, Rosseland LA, Romundstad L, Hals EK, et al. (2008). Assessment of pain. British Journal of Anaesthesiology, 101(1), 17–24. doi: 10.1093/bja/aen103 [DOI] [PubMed] [Google Scholar]
- Bussing A, Ostermann T, Ludtke R, & Michalsen A (2012). Effects of yoga interventions on pain and pain-associated disability: a meta-analysis. Journal of Pain, 13(1), 1–9. doi: 10.1016/j.jpain.2011.10.001 [DOI] [PubMed] [Google Scholar]
- Chou R, Deyo R, Friedly J, Skelly A, Hashimoto R, Weimer M, et al. (2017). Nonpharmacologic therapies for low back pain: a systematic review for an American College of Physicians Clinical Practice Guideline. Annals of Internal Medicine, 166(7), 493–505. doi: 10.7326/M16-2459 [DOI] [PubMed] [Google Scholar]
- Cleeland CS, & Ryan KM (1994). Pain assessment: global use of the Brief Pain Inventory. Annals, Academy of Medicine, Singapore, 23(2), 129–138. [PubMed] [Google Scholar]
- Cramer H, Haller H, Dobos G, & Lauche R (2016). A systematic review and meta-analysis estimating the expected dropout rates in randomized controlled trials on yoga interventions. Evidence-Based Complementary and Alternative Medicine, 2016, 5859729. doi: 10.1155/2016/5859729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis K, Osadchuk A, & Katz J (2011). An eight-week yoga intervention is associated with improvements in pain, psychological functioning and mindfulness, and changes in cortisol levels in women with fibromyalgia. Journal of Pain Research, 4, 189–201. doi: 10.2147/JPR.S22761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desikachar TKV (1999). The Heart of Yoga: Developing a Personal Practice, Revised Edition. Rochester, Vermont: Inner Traditions International. [Google Scholar]
- Devilly GJ, & Borkovec TD (2000). Psychometric properties of the credibility/ expectancy questionnaire. Journal of Behavior Therapy and Experimental Psychiatry, 31, 73–86. [DOI] [PubMed] [Google Scholar]
- Dhawan A, Chopra A, Jain R, Yadav D, & Vedamurthacha r. (2015). Effectiveness of yogic breathing intervention on quality of life of opioid dependent users. International Journal of Yoga, 8(2), 144–147. doi: 10.4103/0973-6131.154075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn KE, Finan PH, Tompkins DA, Fingerhood M, & Strain EC (2015). Characterizing pain and associated coping strategies in methadone and buprenorphine-maintained patients. Drug and Alcohol Dependence, 157, 143–149. doi: 10.1016/j.drugalcdep.2015.10.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dworkin RH, Turk DC, Peirce-Sandner S, Burke LB, Farrar JT, Gilron I, et al. (2012). Considerations for improving assay sensitivity in chronic pain clinical trials: IMMPACT recommendations. Pain, 153(6), 1148–1158. doi: 10.1016/j.pain.2012.03.003 [DOI] [PubMed] [Google Scholar]
- Edelman EJ, Tetrault JM, & Fiellin DA (2014). Substance use in older HIV-infected patients. Current Opinion in HIV and AIDS, 9(4), 317–324. doi: 10.1097/COH.0000000000000069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyler EC (2013). Chronic and acute pain and pain management for patients in methadone maintenance treatment. American Journal of Addiction, 22(1), 75–83. doi: 10.1111/j.1521-0391.2013.00308.x [DOI] [PubMed] [Google Scholar]
- Garland EL, Manusov EG, Froeliger B, Kelly A, Williams JM, & Howard MO (2014). Mindfulness-oriented recovery enhancement for chronic pain and prescription opioid misuse: results from an early-stage randomized controlled trial. Journal of Consulting and Clinical Psychology, 82(3), 448–459. doi: 10.1037/a0035798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg J, Braun TD, Schneider ML, Finkelstein-Fox L, Conboy LA, Schifano ED, et al. (2018). Is less more? A randomized comparison of home practice time in a mind-body program. Behavior Research and Therapy, 111, 52–56. doi: 10.1016/j.brat.2018.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haibach JP, Beehler GP, Dollar KM, & Finnell DS (2014). Moving toward integrated behavioral intervention for treating multimorbidity among chronic pain, depression, and substance-use disorders in primary care. Medical Care, 52(4), 322–327. doi: 10.1097/MLR.0000000000000098 [DOI] [PubMed] [Google Scholar]
- Hallgren M, Romberg K, Bakshi AS, & Andreasson S (2014). Yoga as an adjunct treatment for alcohol dependence: a pilot study. Complementary Therapies in Medicine, 22(3), 441–445. doi: 10.1016/j.ctim.2014.03.003 [DOI] [PubMed] [Google Scholar]
- Hayaki J, Stein MD, Lassor JA, Herman DS, & Anderson BJ (2005). Adversity among drug users: relationship to impulsivity. Drug and Alcohol Dependence, 78(1), 65–71. doi: 10.1016/j.drugalcdep.2004.09.002 [DOI] [PubMed] [Google Scholar]
- Hayden JA, van Tulder MW, & Tomlinson G (2005). Systematic review: strategies for using exercise therapy to improve outcomes in chronic low back pain. Annals of Internal Medicine, 142(9), 776–785. [DOI] [PubMed] [Google Scholar]
- Hruschak V, Cochran G, & Wasan AD (2018). Psychosocial interventions for chronic pain and comorbid prescription opioid use disorders: A narrative review of the literature. Journal of Opioid Management, 77(5), 345–358. doi: 10.5055/jom.2018.0467 [DOI] [PubMed] [Google Scholar]
- Ikai S, Uchida H, Mizuno Y, Tani H, Nagaoka M, Tsunoda K, et al. (2017). Effects of chair yoga therapy on physical fitness in patients with psychiatric disorders: A 12-week single-blind randomized controlled trial. Journal of Psychiatric Research, 94, 194–201. doi: 10.1016/j.jpsychires.2017.07.015 [DOI] [PubMed] [Google Scholar]
- IPAQ Research Committee. (2005). Guidelines for data processing and analysis of the International Physical Activity Questionnaire (IPAQ)—Short and Long Forms.
- Jensen MP, Chen C, & Brugger AM (2003). Interpretation of visual analog scale ratings and change scores: a reanalysis of two clinical trials of postoperative pain. Journal of Pain, 7(7), 407–414. [DOI] [PubMed] [Google Scholar]
- Jiao JM, So E, Jebakumar J, George MC, Simpson DM, & Robinson-Papp J (2016). Chronic pain disorders in HIV primary care: clinical characteristics and association with healthcare utilization. Pain, 157(4), 931–937. doi: 10.1097/j.pain.0000000000000462 [DOI] [PubMed] [Google Scholar]
- Kennedy J, Roll JM, Schraudner T, Murphy S, & McPherson S (2014). Prevalence of persistent pain in the U.S. adult population: new data from the 2010 national health interview survey. Journal of Pain, 75(10), 979–984. doi: 10.1016/j.jpain.2014.05.009 [DOI] [PubMed] [Google Scholar]
- Khanna S, & Greeson JM (2013). A narrative review of yoga and mindfulness as complementary therapies for addiction. Complementary Therapies in Medicine, 27(3), 244–252. doi: 10.1016/j.ctim.2013.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuppili PP, Parmar A, Gupta A, & Balhara YPS (2018). Role of yoga in management of substance-use disorders: a narrative review. Journal of Neuroscience in Rural Practice, 9(1), 117–122. doi: 10.4103/jnrp.jnrp_243_17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine JL, & Schooler NR (1986). SAFTEE: a technique for the systematic assessment of side effects in clinical trials. Psychopharmacology Bulletin, 22, 343–381. [PubMed] [Google Scholar]
- Li W, Howard MO, Garland EL, McGovern P, & Lazar M (2017). Mindfulness treatment for substance misuse: A systematic review and meta-analysis. Journal of Substance Abuse Treatment, 75, 62–96. doi: 10.1016/j.jsat.2017.01.008 [DOI] [PubMed] [Google Scholar]
- Mallik D, Bowen S, Yang Y, Perkins R, & Sandoz EK (2019). Raja yoga meditation and medication-assisted treatment for relapse prevention: A pilot study. Journal of Substance Abuse Treatment, 96, 58–64. doi: 10.1016/j.jsat.2018.10.012 [DOI] [PubMed] [Google Scholar]
- Moore BA, Fiellin DA, Cutter CJ, Buono FD, Barry DT, Fiellin LE, et al. (2016). Cognitive Behavioral Therapy improves treatment outcomes for prescription opioid users in primary care buprenorphine treatment. Journal of Substance Abuse Treatment, 71, 54–57. doi: 10.1016/j.jsat.2016.08.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen TD, Attkisson CC, & Stegner BL (1983). Assessment of patient satisfaction: development and refinement of a service evaluation questionnaire. Evaluation and Program Planning, 6(299–313). [DOI] [PubMed] [Google Scholar]
- Park CL, Elwy AR, Maiya M, Sarkin AJ, Riley KE, Eisen SV, et al. (2018). The Essential Properties of Yoga Questionnaire (EPYQ): psychometric properties. International Journal of Yoga Therapy, doi: 10.17761/2018-00016R2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenblum A, Joseph H, Fong C, Kipnis S, Cleland C, & Portenoy RK (2003). Prevalence and characteristics of chronic pain among chemically dependent patients in methadone maintenance and residential treatment facilities. Journal of the American Medical Association, 289(18), 2370–2378. doi: 10.1001/jama.289.18.2370 [DOI] [PubMed] [Google Scholar]
- Rosenblum A, Parrino M, Schnoll SH, Fong C, Maxwell C, Cleland CM, et al. (2007). Prescription opioid abuse among enrollees into methadone maintenance treatment. Drug and Alcohol Dependence, 90(1), 64–71. doi: 10.1016/j.drugalcdep.2007.02.012 [DOI] [PubMed] [Google Scholar]
- Samet JH, O’Connor PG, & Stein MD (2018). Substance Use and Addiction Medicine, An issue of Medical Clinics of North America. Medical Clinics of North America, 102(2). [DOI] [PubMed] [Google Scholar]
- Saper RB, Sherman KJ, Delitto A, Herman PM, Stevans J, Paris R, et al. (2014). Yoga vs. physical therapy vs. education for chronic low back pain in predominantly minority populations: study protocol for a randomized controlled trial. Trials, 15, 67. doi: 10.1186/1745-6215-15-67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar S, & Varshney M (2017). Yoga and substance use disorders: a narrative review. Asian Journal of Psychiatry, 25, 191–196. doi: 10.1016/j.ajp.2016.10.021 [DOI] [PubMed] [Google Scholar]
- Shaffer HJ, LaSalvia TA, & Stein JP (1997). Comparing hatha yoga with dynamic group psychotherapy for enhancing methadone maintenance treatment: a randomized clinical trial. Alternative Therapies in Health and Medicne, 3(4), 57–66. [PubMed] [Google Scholar]
- Stein MD, Anderson BJ, Thurmond P, & Bailey GL (2015). Comparing the life concerns of prescription opioid and heroin users. Journal of Substance Abuse Treatment, 48(1), 43–48. doi: 10.1016/j.jsat.2014.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein MD, Herman DS, Bailey GL, Straus J, Anderson BJ, Uebelacker LA, & Weisberg RB (2015). Chronic pain and depression among primary care patients treated with buprenorphine. Journal of General Internal Medicine, 30(7), 935–941. doi: 10.1007/s11606-015-3212-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsui JI, Herman DS, Kettavong M, Alford D, Anderson BJ, & Stein MD (2010). Physician introduction to opioids for pain among patients with opioid dependence and depressive symptoms. Journal of Substance Abuse Treatment, 39(4), 378–383. doi: 10.1016/j.jsat.2010.06.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uebelacker LA, Tremont G, Gillette LT, Epstein-Lubow G, Strong DR, Abrantes AM, et al. (2017). Adjunctive yoga v. health education for persistent major depression: a randomized controlled trial. Psychological Medicine, 47, 2130–2142. doi: 10.1017/S0033291717000575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlaeyen JW, & Linton SJ (2000). Fear-avoidance and its consequences in chronic musculoskeletal pain: a state of the art. Pain, 85(3), 317–332. [DOI] [PubMed] [Google Scholar]
- Volkow ND, & McLellan AT (2016). Opioid abuse in chronic pain--misconceptions and mitigation strategies. New England Journal of Medicine, 374(13), 1253–1263. doi: 10.1056/NEJMra1507771 [DOI] [PubMed] [Google Scholar]
- Voon P, Hayashi K, Milloy MJ, Nguyen P, Wood E, Montaner J, & Kerr T (2015). Pain among high-risk patients on methadone maintenance treatment. Journal of Pain, 16(9), 887–894. doi: 10.1016/j.jpain.2015.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss RD, Potter JS, Griffin ML, McHugh RK, Haller D, Jacobs P, et al. D. (2014). Reasons for opioid use among patients with dependence on prescription opioids: the role of chronic pain. Journal of Substance Abuse Treatment, 47(2), 140–145. doi: 10.1016/j.jsat.2014.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieland LS, Skoetz N, Pilkington K, Vempati R, D’Adamo CR, & Berman BM (2017). Yoga treatment for chronic non-specific low back pain. Cochrane Database Systematic Review, 1, CD010671. doi: 10.1002/14651858.CD010671.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams AC, Eccleston C, & Morley S (2012). Psychological therapies for the management of chronic pain (excluding headache) in adults. Cochrane Database Systematic Review, 11, CD007407. doi: 10.1002/14651858.CD007407.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang SM, An SH, & Zhao Y (2013). Yoga effects on mood and quality of life in Chinese women undergoing heroin detoxification: a randomized controlled trial. Nursing Research, 62(4), 260–268. doi: 10.1097/NNR.0b013e318292379b [DOI] [PubMed] [Google Scholar]
- Zou L, Yeung A, Li C, Wei GX, Chen KW, Kinser PA, et al. (2018). Effects of meditative movements on major depressive disorder: a systematic review and meta-analysis of randomized controlled trials. Journal of Clinical Medicine, 7(8). doi: 10.3390/jcm7080195 [DOI] [PMC free article] [PubMed] [Google Scholar]


