Abstract
Background:
Continuous-flow left ventricular assist devices (LVADs) cause blood trauma – von Willebrand factor degradation, platelet activation, and subclinical hemolysis. Blood trauma contributes to bleeding, thrombosis, and stroke, which cause significant morbidity and mortality. The TORVAD is a first-of-its kind, toroidal-flow LVAD designed to minimize blood trauma. We tested the hypothesis that the TORVAD causes less blood trauma than the HeartMate II LVAD.
Methods:
Whole human blood was circulated for 6 hours in ex vivo circulatory loops with a HeartMate II (n=8, 10,000 RPM, 70±6 mmHg, 4.0±0.1 L/min) or TORVAD (n=6, 144 RPM, 72±0.0 mmHg, 4.3±0.0 L/min). Von Willebrand factor degradation was quantified with electrophoresis and immunoblotting. Platelet activation was quantified by CD 41/61 ELISA. Hemolysis was quantified by plasma free hemoglobin ELISA.
Results:
The TORVAD caused significantly less degradation of high-molecular-weight von Willebrand factor multimers (−10±1 vs. −21±1%, p<0.0001), accumulation of low-molecular-weight von Willebrand factor multimers (22±2 vs. 45±2%, p<0.0001), and accumulation of von Willebrand factor degradation fragments (7±1 vs. 25±6%, p<0.05) than the HeartMate II. The TORVAD did not activate platelets whereas the HeartMate II caused significant platelet activation (645±20 vs. 1,581±150 ng/ml, p<0.001; normal human CD 41/61=593 ng/ml, range 400–800 ng/ml). Similarly, the TORVAD caused minimal hemolysis whereas the HeartMate II caused significant hemolysis (11±2 vs. 109±10 mg/dl, p<0.0001; normal human plasma free hemoglobin<4mg/dl).
Conclusions:
The TORVAD design, with markedly lower shear stress and pulsatile flow, caused significantly less blood trauma that the HeartMate II. LVADs with reduced blood trauma are likely to improve clinical outcomes and expand LVAD therapy into patients with less advanced heart failure.
Classifications: bioengineering; blood, coagulation/anticoagulation; circulatory support devices
Keywords: heart failure, left ventricular assist device (LVAD), mechanical circulatory support, blood trauma, von Willebrand factor degradation, platelet activation, hemolysis
Current-generation continuous-flow left ventricular assist devices (LVADs) generate peak shear stress that is two orders of magnitude greater than physiologic values1,2. Supraphysiologic shear stress causes blood trauma. The foremost types of LVAD-associated blood trauma that have known clinical consequences include von Willebrand factor (vWF) degradation3 (which contributes to bleeding), platelet activation4 (which contributes to thrombosis), and subclinical hemolysis5 (which contributes to thrombosis). As a result, LVAD hemocompatibility, a term used to characterize the clinical impact of biophysical interactions and blood trauma at the device-blood interface, is gaining attention as a major area for improvement of future-generation LVADs.
Strong evidence suggests that limited hemocompatibility with current-generation LVADs contributes to LVAD-associated bleeding, thrombosis, and stroke5–8. These complications cause significant morbidity and mortality in LVAD patients and have limited the role of mechanical circulatory support in patients with less advanced stages of heart failure9. As such, it is becoming increasingly clear that next-generation LVADs with less blood trauma and better hemocompatibility are urgently needed to reduce adverse events, improve outcomes, and expand the role of circulatory support devices in the treatment of heart failure.
With this goal in mind, Windmill Cardiovascular Systems, Incorporated (Austin, TX) has developed the TORVAD10–12, a novel, positive-displacement, toroidal-flow LVAD. The TORVAD is specifically designed with low shear stress and pulsatile blood flow to reduce blood trauma in order to improve hemocompatibility and reduce adverse events. We tested the hypothesis that the TORVAD causes less blood trauma than the HeartMate II.
Material and Methods
This study was conducted with approval from the Hospital of the University of Pennsylvania Institutional Review Board (#818944). Volunteer blood donors provided informed consent.
Blood Sample Collection
Fresh whole blood was obtained from human donors (n=14) via standard venipuncture. Blood was anticoagulated in citrate phosphate dextrose adenine-1 blood collection bags (Terumo Corporation, Tokyo, Japan).
LVAD Mock Circulatory Loops
Two mock circulatory loops were constructed with 3/8” Tygon tubing (Medtronic, Minneapolis, MN) and a 1 L reservoir with a blood sampling port as previously reported13–15. A HeartMate II (Thoratec Corporation, Pleasanton, CA) or TORVAD (Figure 1, Windmill Cardiovascular Systems, Austin, TX) was placed in series with each loop. Loops were primed with approximately 450 to 500 ml of whole human blood. Blood was circulated for 6 hours in a loop with either a HeartMate II (n=8) or a TORVAD (n=6).
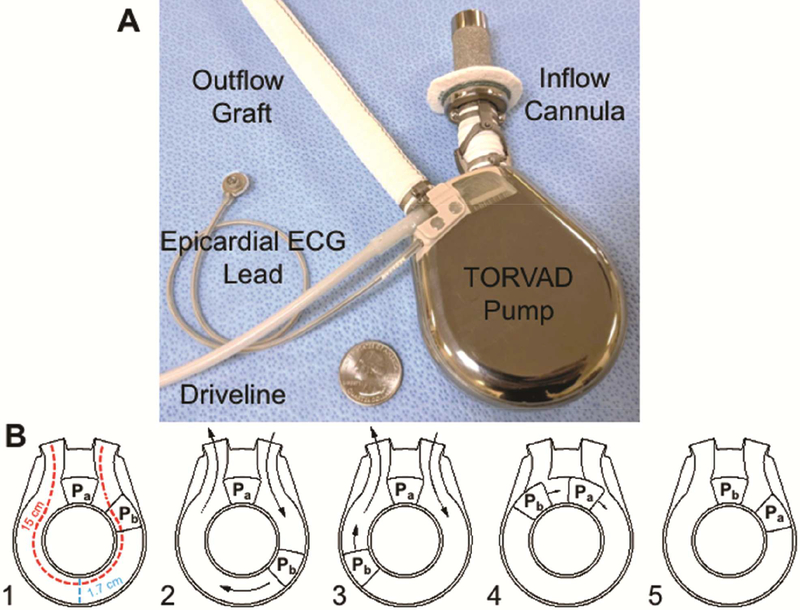
Figure 1.
A: The TORVAD is a novel, toroidal-flow left ventricular assist device (LVAD) specifically designed to reduce blood trauma to improve hemocompatibility and minimize adverse events. The core component is a 30-ml displacement torus chamber. The pumping chamber is connected to the left ventricular apex with a sintered titanium inflow cannula and systemic circulation with a 14 mm ePTFE graft. An epicardial ECG sensing lead triggers TORVAD filling and ejection either synchronously (pulsatile or counterpusatile modes) or asynchronously. B: Panels 1–5 demonstrate the novel, toroidal pumping mechanism of the TORVAD. To fill and eject, the TORVAD maintains one of two pistons (Pa) in a stationary position. The other piston (Pb) is actuated around the torus chamber to displace a stroke volume of 30 ml to generate flow (panels 2 and 3). As piston b completes a cycle around the torus and becomes stationary (panel 4), piston a begins a new cycle (panel 5). The result is unidirectional, pulsatile blood flow. Importantly, the TORVAD generates peak shear stress of approximately 10 Pa, which is near physiologic values (2–8 Pa). The dimensions of the wide flow path are show in red and blue.
The HeartMate II was operated at 10,000 RPM and the TORVAD was operated at 144 RPM to allow each device to maintain a flow of ≥4 L/min and a mean pressure of >65 mmHg. A c-clamp resistor (Fisher Scientific, Pittsburgh, PA) was placed distal to the LVAD to control pump-head pressure and total flow. An ultrasonic flow probe (Transonic, Ithaca, NY) and fluid-filled pressure transducer (BD, Franklin Lakes, NJ) measured volumetric flow and pump outlet pressures, respectively. Importantly, the HeartMate II generates peak shear stress of greater than 1,500 Pa1. In contrast, the TORVAD generates a peak shear stress of approximately 10 Pa11. For reference, normal physiologic intravascular shear stress is approximately 2 to 8 Pa16.
Blood samples were collected at baseline (prior to initiation of LVAD support) and after each 6-hour experiment.
Gel Electrophoresis for vWF Multimers and Fragments
Plasma vWF multimers were resolved with vertical agarose gel electrophoresis13–15. Plasma samples were diluted 1:40 in NuPAGE lithium dodecyl sulfate (LDS) sample buffer (Life Technologies, Carlsbad, CA). Samples were heated at 70°C for 10 minutes and loaded into 1.0% vertical agarose-sodium dodecyl sulfate (SDS) gels (0.1% SDS, 0.375 M Tris base). Electrophoresis was performed at 60 volts for 2.5 hours in tris-acetate SDS running buffer (Life Technologies) in an XCell SureLock Mini-Cell Electrophoresis System (Life Technologies).
Plasma vWF degradation fragments were resolved by polyacrylamide gel electrophoresis13–15. Plasma samples diluted in LDS sample buffer were loaded into NuPAGE 3–8% tris-acetate polyacrylamide gels (Life Technologies). Electrophoresis was performed at 150 volts for 1 hour and 25 minutes in tris-acetate SDS running buffer (Life Technologies).
vWF Immunoblotting
Protein was transferred to polyvinylidene difluoride membranes using the iBlot dry transfer system (Life Technologies). Membranes were blocked and incubated with polyclonal rabbit anti-human vWF primary antibody (1:500, Dako, Glostrup, Denmark) in 5% Non-fat Dry Milk Omniblot (AmericanBIO Inc., Natick, MA) in phosphate buffered saline (PBS) for one hour. Membranes were washed and incubated in monoclonal goat anti-rabbit IgG horseradish peroxidase (HRP)-conjugated secondary antibody (1:3,000, Cell Signaling, Danvers, MA) in 5% milk-PBS for one hour, developed with Luminata Forte Western Blot HRP Substrate (Millipore, Billerica, MA), and imaged with an ImageQuant LAS 4000 (GE Healthcare, Piscataway, NJ).
To confirm equal protein loading, blots were probed for human plasma albumin with a polyclonal goat anti-human albumin HRP-conjugated antibody (1:10,000, Abcam, Cambridge, MA) in 5% milk-PBS. Blots were washed, developed, and imaged as described above.
Quantification of vWF Multimers And Fragments
Loss of high-molecular-weight vWF multimers was quantified as percent decrease in total length of the profile of multimers during LVAD support versus baseline on each agarose immunoblot17. Accumulation of low-molecular-weight vWF was quantified as percent change in area under the curve for the lowest-molecular-weight band on each agarose immunoblot. VWF fragments were quantified as the percent change in area under the curve for all degradation fragments on each polyacrylamide immunoblot. ImageQuantTL (GE Healthcare) and ImageJ (National Institutes of Health, Bethesda, MD) were used to generate and analyze densitometric plots, respectively.
Measurement of CD 41/61
Plasma CD 41/61, a marker of platelet activation, was measured with an ELISA (Assaypro LLC, St. Charles, MO). Plasma samples were incubated in wells coated with an anti-human CD 41/61 polyclonal antibody. After washing, wells were incubated with biotinylated polyclonal anti-human CD 41/61 antibody followed by streptavidin-peroxidase conjugate. A peroxidase enzyme substrate was added to elicit a colorimetric reaction that was quantified with spectrophotometry. Plasma CD 41/61 values were interpolated from a standard curve.
Measurement of Plasma Free Hemoglobin
Plasma free hemoglobin (pfHgb), a marker of hemolysis, was measured with an ELISA (Abcam). Briefly, plasma samples were incubated in wells coated with a polyclonal anti-human hemoglobin antibody. After washing, wells were incubated with a HRP-conjugated anti-human hemoglobin antibody. Tetramethylbenzidine elicited a colorimetric reaction that was quantified with spectrophotometry. PfHgb values were interpolated from a standard curve.
Statistics
Prism, version 6.00 (GraphPad Software, Inc., La Jolla, CA) was used to perform statistical analyses and plot data. Unpaired Student’s t-tests were used for comparisons. Kolmogorov-Smirnov normality test was used to confirm normality of data sets. A p≤0.05 was considered statistically significant. All data were presented as mean±standard error.
Results
Hemodynamics
At 10,000 RPM, the HeartMate II generated flow of 4.0±0.1 L/min and a mean pressure of 70±6 mmHg. At 144 RPM, the TORVAD generated flow of 4.3±0.0 L/min and a mean pressure of 72±0.0 mmHg.
Degradation of vWF
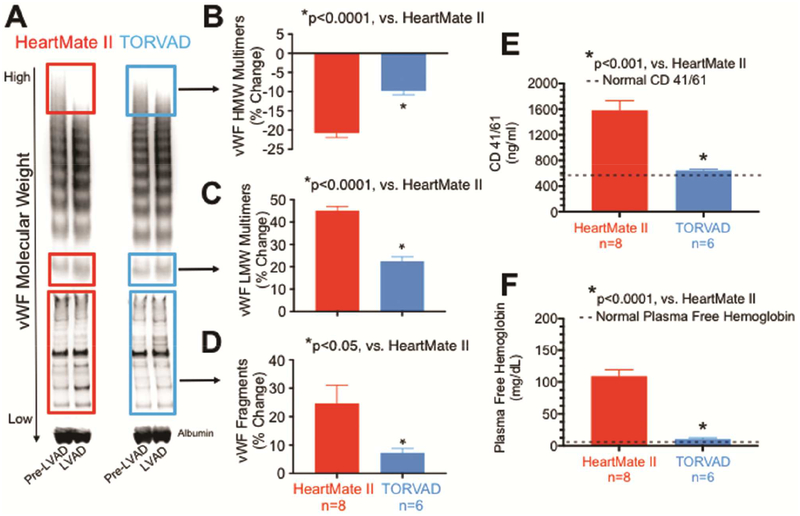
The TORVAD caused significantly less degradation of high-molecular-weight vWF multimers (−10±1 vs. −21±1%, p<0.0001), and accumulation of low-molecular-weight vWF multimers (22±2 vs. 45±2%, p<0.0001) and vWF fragments (7±1 vs. 25±6%, p<0.05) than the HeartMate II (Figure 2 A–D).
Figure 2.
A-D: Von Willebrand factor (vWF) degradation was examined in mock circulatory loops with the HeartMate II and the TORVAD. The TORVAD caused significantly less degradation of high-molecular-weight (HMW) vWF and accumulation of low-molecular-weight (LMW) vWF multimers and vWF fragments than the HeartMate II. E: The TORVAD did not cause platelet activation, whereas the HeartMate II caused significant platelet activation. F: Similarly, the TORVAD caused minimal hemolysis, whereas the HeartMate II caused significant hemolysis.
Platelet Activation
The TORVAD did not cause platelet activation (Figure 2 E). In contrast, the HeartMate II caused significant platelet activation (645±20 vs. 1,581±150 ng/ml, p<0.001). Normal human plasma CD 41/61 is 593 ng/ml (n=20, range 400–800 ng/ml)18.
Hemolysis
The TORVAD caused minimal hemolysis (Figure 2 F). In contrast, the HeartMate II caused significant hemolysis (11±2 vs. 109±10 mg/dl, p<0.0001). Normal human pfHgb is less than 4mg/dl.
Comment
Current-generation continuous-flow LVADs cause blood trauma characterized by vWF degradation, platelet activation, and subclinical hemolysis. Blood trauma contributes to multiple adverse events that include bleeding, thrombosis, and stroke. Next-generation LVADs with less blood trauma that are more hemocompatible are needed to improve outcomes. With this goal in mind, the TORVAD was specifically designed with low shear stress to minimize blood trauma and adverse events10–12.
In ex vivo circulatory loops with whole human blood, the TORVAD caused significantly less blood trauma than the HeartMate II (Figure 2). The toroidal-flow design caused less vWF degradation than the HeartMate II, minimal hemolysis, and did not cause platelet activation. These findings reaffirm that LVAD design modifications have pronounced effects on blood trauma. Improved hemocompatibility may translate into reduced adverse events, improve patient outcomes, and expand the use of mechanical circulatory support into patients with less advanced heart failure.
Current Generation LVADs Generate Supraphysiologic Shear Stress, Blood Trauma, And Adverse Events
Bleeding, thrombosis, and stroke are major obstacles to improving outcomes, reducing costs, and expanding the public health impact of LVAD support8,19–21. Intrinsic design constraints of continuous-flow LVADs result in very high shear stress in the blood flow path. Strong evidence now demonstrates that high shear stress causes significant blood trauma, which in turn contributes to adverse events. Specifically, current-generation continuous-flow LVADs generate peak shear stresses greater than 1,500 to 3,000 Pa1,2, which is more than two orders of magnitude greater than physiologic values of 2 to 8 Pa16.
Shear stress is a potent activator of multiple hematologic pathways22,23. VWF degradation occurs above 9 Pa, platelet activation occurs above 50 Pa, and hemolysis occurs above 150 Pa24. High shear stress with LVADs has multiple consequences. LVAD shear stress accelerates vWF degradation3, which causes an acquired vWF deficiency and bleeding diathesis in 30 to 75% of LVAD patients6. Similarly, continuous-flow LVADs activate platelets, the coagulation cascade, leukocytes, and the complement system, which contribute to a hypercoagulable state and device thromboembolism21. In parallel, subclinical hemolysis, which occurs to some degree in all continuous-flow LVAD patients, exacerbates microthrombosis and may trigger a dangerous cascade in which worsening hemolysis begets macrothrombosis and vice versa5. As a result, LVAD thrombosis occurs in up to 18% of LVAD patients21. These findings demonstrate that important mechanistic relationships exist between LVAD shear stress, blood trauma, and adverse events and underscore the clinical impact of LVAD hemocompatibility.
Device Design Influences Blood Trauma, Hemocompatibility, and Adverse Events
Different LVADs cause different amounts of blood trauma. Each device has a unique hematologic footprint and adverse event profile. Specific device design features influence blood trauma, hemocompatibility, and clinical outcomes25,26. For example, the EVAHEART LVAS, a centrifugal-flow LVAD designed with relatively low shear stress causes minimal vWF degradation27. As a result, the gastrointestinal bleeding rate in the first 96 patients studied was 0%28. In contrast, the HeartMate II causes significant vWF degradation3,29. Clinically, bleeding occurs in up to 70% of HeartMate II patients6. These data suggest that greater vWF degradation leads to more bleeding events.
Similarly, the HeartMate 3 LVAD was designed with large flow gaps and lower RPM to reduce shear stress and blood trauma and thereby improve hemocompatibility. As a result, the incidence of LVAD thrombosis was zero with the HeartMate 3, whereas LVAD thrombosis occurred in 10% of patients with a HeartMate II30. These recent reports demonstrate that device design has direct consequences for blood trauma and clinical outcomes. Indeed, specific device design features such as flow mechanism (pulsatile, axial, centrifugal, toroidal), internal components (impeller, stator, rotor, piston, contact-bearing, hydrodynamic levitation, magnetic levitation), device geometry (blood-film gaps, blood transient times), flow algorithm, and blood-contacting material each affect shear stress, blood trauma, hemocompatibility, and ultimately patient outcomes.
The Toroidal-Flow TORVAD Design Minimizes Blood Trauma Versus The Axial-Flow HeartMate II Design
The HeartMate II is an axial-flow rotary blood pump. The impeller spins on hydrodynamic ruby bearings at 6,000 to 15,000 RPM. The blood flow path within the HeartMate II (100% by volume) courses through 50 μm flow gaps. Tight flow gaps produce high shear stress. Indeed, at a flow rate of 5 L/min and mean pressure head of 70 mmHg, the HeartMate II generates peak shear stress of greater than 1,500 Pa1. For reference, normal physiologic intravascular shear stress is approximately 2 to 8 Pa16.
In contrast, the TORVAD is a novel toroidal-flow LVAD specifically designed with low shear stress to minimize blood trauma10–12 (Figure 1). The TORVAD has a 30-ml stroke volume and primarily operates in synchronous counterpulsation mode but can also be run asynchronously at flow rates from 1 to 8 L/min (40 to 260 RPM). Synchronous and asynchronous modes provide greater device versatility than current-generation LVADs to optimize ventricular unloading and facilitate device weaning in the setting of myocardial recovery.
The pumping mechanism of the TORVAD is distinctly different than first generation sac-type pulsatile LVADs. To simultaneously fill and eject, the TORVAD cyclically actuates one of two pistons around a torus chamber to generate unidirectional, pulsatile flow (Figure 1 B). Each piston includes a rare-earth (strong permanent) magnet hermetically-sealed within a ceramic shell that is independently actuated by a position-controlled motor that rotates a magnetic coupling outside of the pumping chamber. With each cycle, pistons exchange functional roles. While one is temporarily fixed as a “virtual valve”, the other spins around the torus and produces flow. The novel pumping mechanism allows the TORVAD to be significantly smaller than first-generation pulsatile LVADs (TORVAD = 270 g and 90 cc displacement volume versus Thoratec XVE = 1,150 g and 400 cc displacement volume) and comparable to current continuous-flow LVADs (HeartMate II = 290 g and 63 cc displacement volume, HeartMate 3 = 200 g and 80 cc displacement volume, HVAD = 160 g and 50 cc displacement volume).
The majority of blood in the TORVAD (99.7% by volume) flows through a single 15 cm long, 1.7 cm wide path in the pumping chamber, which has flow and shear stress comparable to a large artery. Remaining blood (0.3% by volume) flows through the annular gaps between the torus and the pistons. Low shear stress is achieved by the combination of low pump speeds (40 to 260 RPM) and a thin (75 μm) fixed gap between the piston and torus walls. The fixed gap is achieved with small ceramic hydrodynamic bearings. Each piston has two ceramic bearings that interface with a ceramic track on the top and bottom of the torus. The hydrodynamic bearing surface areas are small (approximately 0.010” wide) with gaps of approximately 50 nm. Each track is specifically angled to provide hydrodynamic forces in both the axial and radial directions. During device flow, the effective viscosity of the plasma in this extremely narrow gap increases and generates a plasma gel layer that provides a thin, protective fluid/protein buffer between each rotating piston and ceramic track. This phenomenon persists during brief pauses in piston motion, thus maintaining separation of the piston and track to effectively eliminate long-term device wear. By controlling gap size, shear is minimized and is orders of magnitude lower than shear stress caused by continuous-flow LVAD impellers1. At a flow rate of 5 L/min and pressure head of 70 mmHg, the TORVAD generates shear stress in the gap of approximately 10 Pa11, which is near physiologic values.
We observed that blood trauma was significantly lower with the TORVAD than the HeartMate II. The TORVAD caused significantly less vWF degradation than the HeartMate II, minimal hemolysis, and no platelet activation (Figure 2). These data are consistent with findings from chronic bovine studies. In these pre-clinical experiments, calves (n=3) were implanted with a TORVAD for 60 days. Importantly, the TORVAD caused minimal hemolysis and a near normal profile of vWF multimers for the duration of the study31. Animals did not receive anticoagulation or antiplatelet therapy during support. In the first two animals, small thrombus accumulated on an O-ring in the inner pump-mating seam. This defect was addressed by laser welding the seam, which corrected the issue in the third animal, in whom no thrombus deposition was observed. Otherwise, all other pump components (pistons, rails, and titanium walls) in all three animals were thrombus free at the time of explant, which suggested thromboresistance of the dual-piston toroidal pumping chamber. Together with our current data, findings of the chronic bovine studies suggested that toroidal-flow is a promising new LVAD design with significantly less blood trauma.
Limitations
It was not possible to compare the TORVAD to the HeartMate 3. However, there were major benefits to studying the HeartMate II, which is the current gold standard LVAD with more than 25,000 human implants worldwide to date. Our findings with the HeartMate II demonstrated the limitations of current-generation continuous-flow LVADs that were not designed with shear stress and blood trauma in mind. These findings may be extrapolated to other current-generation LVADs with high shear stress such as the HVAD and continuous-flow total artificial hearts under development that are expected to cause similar (or worse) blood trauma to current-generation LVADs. This study also demonstrated for clinicians, investigators, and especially LVAD engineers that it is possible to design a low-profile, rotary LVAD that generates full-support, pulsatile blood flow, which has low shear stress and minimal blood trauma.
“Hemocompatibility” is more complex than vWF degradation, platelet activation, and hemolysis. Other hematologic changes that occur during artificial circulation include activation of leukocytes, the coagulation cascade, and the complement system, as well as generation of microparticles. We did not measure these variables, which may have also occurred, and for which the clinical consequences are unknown.
This study was performed ex vivo with human blood. Although the TORVAD caused significantly less blood trauma than the HeartMate II, it remains to be determined clinically whether less blood trauma and improved hemocompatibility with the TORVAD (or any LVAD) will translate into fewer adverse events and improve clinical outcomes. Nonetheless, a major goal of this article is to raise awareness within the heart failure community of relationships that exist between LVAD shear stress, hemocompatibility, and adverse events. We hope that these data trigger further research and development of next-generation LVADs specifically designed to minimize blood trauma and reduce adverse events.
Conclusions
LVAD-induced blood trauma is associated with major adverse events such as bleeding, thrombosis, and stroke. The TORVAD, a novel toroidal-flow LVAD designed with low shear stress, caused significantly less vWF degradation, platelet activation, and hemolysis than the HeartMate II. Toroidal-flow is a promising new LVAD design that minimizes blood trauma. These findings should inform the design of next-generation LVADs. Reduced blood trauma may improve LVAD hemocompatibility, reduce adverse events, and improve clinical outcomes.
Abbreviations
- LVAD
left ventricular assist device
- vWF
von Willebrand factor
- CD
cluster of differentiation
- ELISA
enzyme-linked immunosorbent assay
- RPM
revolutions per minute
- LDS
lithium dodecyl sulfate
- SDS
sodium dodecyl sulfate
- PBS
phosphate buffered saline
- HRP
horse-radish peroxidase
- pfHgb
plasma free hemoglobin
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Thamsen B, et al. A two-stage rotary blood pump design with potentially lower blood trauma: a computational study. Int J Artif Organs 2016; 39(4): 178–83. [DOI] [PubMed] [Google Scholar]
- 2.Fraser KH, et al. A quantitative comparison of mechanical blood damage parameters in rotary ventricular assist devices: shear stress, exposure time and hemolysis index. J Biomech Eng 2012; 134(8): 081002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bartoli CR, et al. Pathologic von Willebrand factor degradation with a left ventricular assist device occurs via two distinct mechanisms: mechanical demolition and enzymatic cleavage. J Thorac Cardiovasc Surg 2015; 149(1): 281–9. [DOI] [PubMed] [Google Scholar]
- 4.Chen Z, et al. High shear induces platelet dysfunction leading to enhanced thrombotic propensity and diminished hemostatic capacity. Platelets 2017: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bartoli CR, et al. Clinical and In Vitro Evidence That Subclinical Hemolysis Contributes to LVAD Thrombosis. Ann Thorac Surg 2018; 105(3): 807–14. [DOI] [PubMed] [Google Scholar]
- 6.Proudfoot AG, et al. von Willebrand factor disruption and continuous-flow circulatory devices. J Heart Lung Transplant 2017; 36(11): 1155–63. [DOI] [PubMed] [Google Scholar]
- 7.Tran PL, et al. Hemolysate-mediated platelet aggregation: an additional risk mechanism contributing to thrombosis of continuous flow ventricular assist devices. Perfusion 2016; 31(5): 401–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Uriel N, et al. Hemocompatibility-Related Outcomes in the MOMENTUM 3 Trial at 6 Months: A Randomized Controlled Study of a Fully Magnetically Levitated Pump in Advanced Heart Failure. Circulation 2017; 135(21): 2003–12. [DOI] [PubMed] [Google Scholar]
- 9.Pagani FD, et al. The NHLBI REVIVE-IT study: Understanding its discontinuation in the context of current left ventricular assist device therapy. J Heart Lung Transplant 2016; 35(11): 1277–83. [DOI] [PubMed] [Google Scholar]
- 10.Letsou GV, et al. Improved left ventricular unloading and circulatory support with synchronized pulsatile left ventricular assistance compared with continuous-flow left ventricular assistance in an acute porcine left ventricular failure model. J Thorac Cardiovasc Surg 2010; 140(5): 1181–8. [DOI] [PubMed] [Google Scholar]
- 11.Gohean JR, et al. Scaling the Low-Shear Pulsatile TORVAD for Pediatric Heart Failure. ASAIO J 2017; 63(2): 198–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gohean JR, et al. Preservation of native aortic valve flow and full hemodynamic support with the TORVAD using a computational model of the cardiovascular system. ASAIO J 2015; 61(3): 259–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Restle DJ, et al. Preclinical Models for Translational Investigations of Left Ventricular Assist Device-Associated von Willebrand Factor Degradation. Artif Organs 2015; 39(7): 569–75. [DOI] [PubMed] [Google Scholar]
- 14.Dassanayaka S, et al. Mechanistic pathway(s) of acquired von willebrand syndrome with a continuous-flow ventricular assist device: in vitro findings. ASAIO J 2013; 59(2): 123–9. [DOI] [PubMed] [Google Scholar]
- 15.Kang J, et al. Reduced continuous-flow left ventricular assist device speed does not decrease von Willebrand factor degradation. J Thorac Cardiovasc Surg 2016; 151(6): 1747–54 e1. [DOI] [PubMed] [Google Scholar]
- 16.Resnick N, et al. Fluid shear stress and the vascular endothelium: for better and for worse. Prog Biophys Mol Biol 2003; 81(3): 177–99. [DOI] [PubMed] [Google Scholar]
- 17.Hennessy-Strahs S, et al. Establishing a standard practice for the evaluation of von Willebrand factor multimer degradation in rotary blood pumps. Proceedings of the International Society of Mechanical Circulatory Support 2017; (Tucson, AZ). [Google Scholar]
- 18.AssayPro. AssayMax Human GPIIb/IIIa ELISA Kit. Charles, MO: 2017. [Google Scholar]
- 19.Holley CT, et al. Gastrointestinal Bleeding during Continuous-Flow Left Ventricular Assist Device Support is Associated with Lower Rates of Cardiac Transplantation. ASAIO J 2015; 61(6): 635–9. [DOI] [PubMed] [Google Scholar]
- 20.Akhter SA, et al. Hospital Readmissions After Continuous-Flow Left Ventricular Assist Device Implantation: Incidence, Causes, and Cost Analysis. Ann Thorac Surg 2015; 100(3): 884–9. [DOI] [PubMed] [Google Scholar]
- 21.Bartoli CR, et al. Diagnosis, nonsurgical management, and prevention of LVAD thrombosis. J Card Surg 2014; 29(1): 83–94. [DOI] [PubMed] [Google Scholar]
- 22.Nesbitt WS, et al. A shear gradient-dependent platelet aggregation mechanism drives thrombus formation. Nat Med 2009; 15(6): 665–73. [DOI] [PubMed] [Google Scholar]
- 23.Zhang X, et al. Mechanoenzymatic cleavage of the ultralarge vascular protein von Willebrand factor. Science 2009; 324(5932): 1330–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thamsen B, et al. Numerical Analysis of Blood Damage Potential of the HeartMate II and HeartWare HVAD Rotary Blood Pumps. Artif Organs 2015; 39(8): 651–9. [DOI] [PubMed] [Google Scholar]
- 25.Meyer AL, et al. Acquired von Willebrand syndrome in patients with a centrifugal or axial continuous flow left ventricular assist device. JACC Heart Fail 2014; 2(2): 141–5. [DOI] [PubMed] [Google Scholar]
- 26.Birschmann I, et al. Ambient hemolysis and activation of coagulation is different between HeartMate II and HeartWare left ventricular assist devices. J Heart Lung Transplant 2014; 33(1): 80–7. [DOI] [PubMed] [Google Scholar]
- 27.Bartoli CR, et al. Left Ventricular Assist Device Design Reduces von Willebrand Factor Degradation: A Comparative Study Between the HeartMate II and the EVAHEART Left Ventricular Assist System. Ann Thorac Surg 2017; 103(4): 1239–44. [DOI] [PubMed] [Google Scholar]
- 28.Saito S, et al. Post-approval study of a highly pulsed, low-shear-rate, continuous-flow, left ventricular assist device, EVAHEART: a Japanese multicenter study using J-MACS. J Heart Lung Transplant 2014; 33(6): 599–608. [DOI] [PubMed] [Google Scholar]
- 29.Kang J, et al. Continuous-Flow LVAD Support Causes a Distinct Form of Intestinal Angiodysplasia. Circ Res 2017; 121(8): 963–9. [DOI] [PubMed] [Google Scholar]
- 30.Mehra MR, et al. A Fully Magnetically Levitated Circulatory Pump for Advanced Heart Failure. N Engl J Med 2017; 376(5): 440–50. [DOI] [PubMed] [Google Scholar]
- 31.Gohean JR, et al. Low Shear and Thromboresistance in the Synchronous Pulsatile Adult and Pediatric TORVAD Proceedings of the 63rd Annual ASAIO Conference 2017; (June 21–24; Chicago, IL): #244. [Google Scholar]