Summary
Background
Delirium during critical illness results from numerous insults, which might be interconnected and yet individually contribute to long-term cognitive impairment. We sought to describe the prevalence and duration of clinical phenotypes of delirium (ie, phenotypes defined by clinical risk factors) and to understand associations between these clinical phenotypes and severity of subsequent long-term cognitive impairment.
Methods
In this multicentre, prospective cohort study, we included adult (≥18 years) medical or surgical ICU patients with respiratory failure, shock, or both as part of two parallel studies: the Bringing to Light the Risk Factors and Incidence of Neuropsychological Dysfunction in ICU Survivors (BRAIN-ICU) study, and the Delirium and Dementia in Veterans Surviving ICU Care (MIND-ICU) study. We assessed patients at least once a day for delirium using the Confusion Assessment Method-ICU and identified a priori-defined, non-mutually exclusive phenotypes of delirium per the presence of hypoxia, sepsis, sedative exposure, or metabolic (eg, renal or hepatic) dysfunction. We considered delirium in the absence of hypoxia, sepsis, sedation, and metabolic dysfunction to be unclassified. 3 and 12 months after discharge, we assessed cognition with the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS). We used multiple linear regression to separately analyse associations between the duration of each phenotype of delirium and RBANS global cognition scores at 3-month and 12-month follow-up, adjusting for potential confounders.
Findings
Between March 14, 2007, and May 27, 2010, 1048 participants were enrolled, eight of whom could not be analysed. Of 1040 participants, 708 survived to 3 months of follow-up and 628 to 12 months. Delirium was common, affecting 740 (71%) of 1040 participants at some point during the study and occurring on 4187 (31%) of all 13 434 participant-days. A single delirium phenotype was present on only 1355 (32%) of all 4187 participant-delirium days, whereas two or more phenotypes were present during 2832 (68%) delirium days. Sedative-associated delirium was most common (present during 2634 [63%] delirium days), and a longer duration of sedative-associated delirium predicted a worse RBANS global cognition score 12 months later, after adjusting for covariates (difference in score comparing 3 days vs 0 days: –4·03, 95% CI –7·80 to –0·26). Similarly, longer durations of hypoxic delirium (–3·76, 95% CI –7·16 to –0·37), septic delirium (–3·67, –7·13 to –0·22), and unclassified delirium (–4·70, –7·16 to –2·25) also predicted worse cognitive function at 12 months, whereas duration of metabolic delirium did not (1·14, –0·12 to 3·01).
Interpretation
Our findings suggest that clinicians should consider sedative-associated, hypoxic, and septic delirium, which often co-occur, as distinct indicators of acute brain injury and seek to identify all potential risk factors that may impact on long-term cognitive impairment, especially those that are iatrogenic and potentially modifiable such as sedation.
Introduction
Delirium, a major complication of critical illness that occurs in response to numerous insults,1 is associated with short-term and long-term adverse outcomes.2–7 Though animal models have facilitated the study of specific forms of cognitive impairment (eg, septic),8,9 most clinical investigations of delirium have analysed delirium as a homogeneous syndrome. Indeed, diagnostic assessments10,11 and interventions12–14 directed at delirium in the intensive care unit (ICU) rarely distinguish between clinical phenotypes that can result from diverse underlying mechanisms and might be differentially related to poor outcomes. Thus, it is unclear whether clinicians should distinguish between various phenotypes of delirium as they seek to mitigate delirium and its associated adverse outcomes. For example, no published data exist to suggest whether a clinician caring for a septic patient with delirium should focus solely on treating sepsis or additionally seek to reduce the patient’s exposure to other delirium risk factors.
Sedative-associated delirium is of particular interest in the ICU because clinicians control patients’ exposure to sedatives yet almost no evidence is available regarding the long-term effects of sedative-associated delirium. Although duration of delirium during critical illness is known to predict risk of a long-term cognitive impairment that is similar in severity to dementia for many patients,5,6 it is unclear whether sedative-associated delirium and other delirium phenotypes are associated with differential effects on long-term cognitive impairment. Clinicians and researchers alike might assume that hypoxia and sepsis are important contributors to delirium and subsequent long-term cognitive impairment but view sedative-associated delirium as less innocuous without evidence to suggest otherwise.
To address widely acknowledged gaps in the current understanding of delirium during critical illness, we aimed to assess the prevalence and duration of various clinical phenotypes of delirium (ie, phenotypes defined by clinical risk factors) during critical illness and to understand associations between these clinical phenotypes of delirium and long-term cognitive outcomes (appendix p 4). In keeping with common clinical perspectives, we hypothesised that delirium in the setting of hypoxia and sepsis would be associated with worse long-term cognitive impairment. Alternatively, we hypothesised that delirium in the setting of sedation and metabolic derangements would not be associated with long-term cognitive impairment.
Methods
Study Design and Population
We did this multicentre, prospective cohort study as part of two parallel studies: the Bringing to Light the Risk Factors and Incidence of Neuropsychological Dysfunction in ICU Survivors (BRAIN-ICU) study,6 which enrolled participants at Vanderbilt University Medical Center and Saint Thomas Hospital (both in Nashville, TN, USA), and the Delirium and Dementia in Veterans Surviving ICU Care (MIND-ICU) study,15 which enrolled participants at the Department of Veterans Affairs (VA) Tennessee Valley Healthcare System (Nashville campus, TN, USA), the George E Wahlen Department of Veterans Affairs Medical Center (Salt Lake City, UT, USA), and the VA Puget Sound Health Care System (Seattle, WA, USA). We prespecified the hypotheses, covariates, and statistical analyses described herein based on previous research findings and biological plausibility.16
We included adults (≥18 years of age) managed in a medical or surgical ICU with respiratory failure, septic or cardiogenic shock, or both (appendix p 2) unless they met one or more exclusion criteria. These included a life expectancy of less than 24 h, blindness, deafness, or inability to understand English (because these characteristics could impede assessments for delirium and cognitive function at follow-up); residence more than 200 miles from the enrolling centre, homelessness, active substance abuse, or psychotic disorder (any of which might prevent long-term follow-up); and chronic neurodegenerative disease or severe dementia, identified initially via medical record review and validated surrogate-based questionnaires (because we sought to identify newly acquired cognitive impairment). Specifically, we excluded patients who scored higher than 2·0 on the Clinical Dementia Rating (CDR) scale.17 We also excluded patients with recent significant critical illness (defined as >72 h with organ dysfunction from the time of ICU admission to the time of screening for enrolment in the current study, longer than 5 days in an ICU during the 30 days before screening for enrolment in the current study, or an ICU admission requiring mechanical ventilation during the 2 months before screening), cardiac surgery during the 3 months before screening, or anoxic brain injury. Finally, we excluded patients for whom informed consent was not obtained.
At the time of enrolment, we obtained informed consent from authorised surrogates because most participants did not have capacity to consent; when participants regained decision-making capacity, they could choose to consent to further participation or to withdraw. The institutional review boards at all study sites approved the study protocol.
Procedures
From enrolment until hospital discharge, death, or for a maximum of 30 days, trained research personnel assessed participants twice a day in the ICU and once a day outside the ICU for delirium and coma using the validated Confusion Assessment Method for the ICU (CAM-ICU)10 and the Richmond Agitation-Sedation Scale (RASS).18,19 In analyses, we considered any day during which at least one CAM-ICU assessment was positive to be a day of delirium. If neither CAM-ICU assessment was positive and one or both RASS assessments were –4 (unresponsive to voice but responsive to physical stimulation) or –5 (unresponsive to voice and physical stimulation), we considered the day to be a day of coma.
Every day, we collected data from electronic medical records (eg, medication administration records, laboratory results, and progress notes) and bedside physiological monitors (eg, blood pressure and pulse oximetry monitors) to identify four clinical delirium phenotypes, which we defined a priori based on clinical judgment and research regarding the common risk factors for delirium during critical illness as well as hypothesised mechanisms of long-term cognitive impairment after critical illness:20 hypoxic (ie, tissue hypoxia due to either hypoxaemia or shock),21 septic,22 sedative-associated,23 and metabolic delirium.24 The panel provides definitions used to classify the presence of each of these phenotypes. Additional details regarding our approach to identifying sepsis are available in the appendix (p 2). The most common clinical phenotypes of delirium often coexist so we did not consider the phenotypes mutually exclusive—ie, a participant could be classified as having more than one delirium phenotype on a given day, in which case the sum of the durations of each of their delirium phenotypes would be greater than the number of days they were delirious (appendix p 6). If the participant was CAM-ICU positive on a day when none of the delirium phenotype definitions were met, we considered that a day of unclassified delirium.
Panel:
Delirium phenotypes
| Hypoxic delirium |
| • Hypoxemia* or |
| • Shock† |
| Septic delirium |
| • Known or suspected infection and |
| • 2+ systemic inflammatory response syndrome criteria‡ |
| Sedative-associated delirium |
| • Receipt of benzodiazepine or |
| • Propofol or |
| • Opioid or |
| • Dexmedetomidine |
| Metabolic delirium |
| • Blood urea nitrogen > 17·85 mmol/L or |
| • Glucose < 2·5 mmol/L or |
| • INR > 2·5 and [aspartate transaminase or alanine transaminase] >200 U/L or |
| • Sodium < 120 mmol/L or |
| • Sodium > 160 mmol/L |
| Unclassified delirium |
| • None of the above |
Two or more 15-minute intervals during which lowest blood oxygen saturation was <90%.
Lactate > 4·4 mmol/L or two or more 15-minute intervals during which lowest mean arterial pressure was <65 mmHg.
Temperature > 38°C or < 36°C, heart rate > 90 beats per minute, respiratory rate > 20 breaths per minute or PaCO2 < 32 mmHg, or leukocyte count > 12,000/mm3 or < 4,000/mm3.
The data collected to identify delirium phenotypes (panel) were also used as covariate data. Additionally, we collected data from the electronic medical record to establish the following covariates: age, Charlson Comorbidity Index,24 Short Informant Questionnaire on Cognitive Decline in the Elderly (Short IQCODE),25 Framingham stroke risk profile,26 and mean modified Sequential Organ Failure Assessment (SOFA)27 during the ICU stay. The appendix (p 2) provides additional details regarding covariates.
Outcomes
3 and 12 months after hospital discharge, trained psychology professionals assessed participants’ cognitive function. We used the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS)29 and Mini-Mental State Examination (MMSE) to assess global cognition and the Trail Making Test, Part B (Trails B),30 to assess executive function. Results of the RBANS were age-adjusted according to published normative data, with the age-adjusted population mean for the RBANS global cognition score—the primary outcome of interest—being 100 (SD 15). Results of Trails B were adjusted for age, sex, and education according to published normative data, with the adjusted population mean being 50 (SD 10).
Statistical Analysis
To establish whether longer durations of the phenotypes of delirium were associated with worse long-term cognitive impairment, we used separate multiple linear regression models to analyse the associations between the duration of each delirium phenotype (which we analysed as continuous exposure variables) and 3 month or 12 month RBANS global cognition scores (as well as secondary outcomes, including individual cognitive domains assessed with RBANS, Trails B, and MMSE scores), adjusting for age, Charlson Comorbidity Index, Short IQCODE, years of education, Framingham stroke risk profile, duration of coma, duration of severe sepsis, number of 15 min intervals of hypoxaemia, mean modified SOFA during the ICU stay, and mean 24 h doses of sedating medications received in the ICU (including benzodiazepines, opiates, propofol, dexmedetomidine, and haloperidol). We selected all covariates a priori based on biological plausibility and previous research suggesting they might confound the association between a delirium phenotype and long-term cognitive outcomes. Because delirium phenotypes were allowed to have a non-linear association with RBANS global cognition, the magnitude of association depends on the comparison chosen. For all delirium phenotypes, we generated the point estimates reported in the main text by comparing 3 versus 0 days of the exposure variable, chosen because these values represent the 75th to the 25th percentile values of the most common delirium phenotypes.
In addition to the a priori-specified analyses, we also did several post-hoc analyses: we used different thresholds to identify hypoxic and metabolic delirium, considered different sedatives as separate exposures, and assessed whether delirium phenotypes were associated with long-term mortality. These analyses are described in the appendix along with additional details regarding imputation, non-linearity, and multicollinearity. We used R (version 3.3) for all statistical analyses and considered a two-sided alpha of 0·05 to indicate significance.
Role of the Funding Source
The funder had no role in study design, data collection, data analysis, data interpretation, or in the writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
From March 14, 2007, to May 27, 2010, we enrolled 1048 participants, eight of whom could not be analysed because they withdrew and requested their data be destroyed. Of 1040 participants analysed, 201 died in the hospital and another 131 died after hospital discharge but before 3-month follow-up, leaving 708 participants who survived to 3-month follow-up. We assessed 564 (80%) of these 708 participants, and we assessed 471 (75%) of the 628 participants who survived to 12-month follow-up (80 participants died between 3-month and 12-month follow-up). Participant characteristics of the follow-up cohort were similar to those of the enrolment cohort with the exception that severity of illness in the ICU was slightly lower for participants included in the follow-up cohort (table 1). The appendix (p 5) shows the full details of participants screened, excluded, enrolled, and assessed during the 1-year follow-up. The 1040 participants analysed had a wide range of ages (IQR 53–72) and admission diagnoses, but nearly all had a high severity of illness (table 1). Only 66 (6%) had a Short IQCODE score higher than 3·6, indicating pre-existing cognitive impairment.
Table 1:
Participant characteristics
| Enrollment cohort (n=1,040) | Follow-up cohort (n=586) | |
|---|---|---|
| Age (years) | 62 [53, 72] | 61 [52, 70] |
| Race | ||
| White | 946 (91%) | 524 (89%) |
| African American | 87 (8%) | 59 (10%) |
| Sex | ||
| Women | 413 (40%) | 239 (41%) |
| Men | 627 (60%) | 347 (59%) |
| Education (years) | 12 [12, 14] | 12 [12, 14] |
| Short IQCODE | 3·00 [3·00, 3·12] | 3·00 [3·00, 3·12] |
| Preexisting cognitive impairment* | 66 (6%) | 35 (6%) |
| Charlson Comorbidity Index | 2 [1, 4] | 2 [1, 4] |
| Admission diagnosis | ||
| Sepsis/ARDS | 373 (36%) | 202 (34%) |
| Surgery | 168 (16%) | 100 (16%) |
| COPD, asthma, or other pulmonary disease† | 134 (13%) | 72 (12%) |
| Congestive heart failure, myocardial infarction, or cardiogenic shock | 161 (15%) | 92 (16%) |
| Airway protection | 109 (10%) | 62 (11%) |
| Other | 95 (9%) | 52 (9%) |
| APACHE II at ICU admission | 24 [18, 30] | 23 [17, 29] |
| SOFA at enrollment | 9 [7, 11] | 8 [7, 11] |
| Mean daily SOFA in the ICU | 7·2 [5·7, 9·6] | 6·6 [5·3, 8·5] |
| Mechanically ventilated | ||
| Ever | 923 (89%) | 516 (88%) |
| Days after enrollment‡ | 3·1 [1·0, 8·6] | 2·2 [1·0, 6·0] |
| Benzodiazepine exposure | ||
| Ever | 686 (66%) | 364 (62%) |
| Mean 24 h dose in ICU (mg)‡§ | 5·8 [1·2, 19·0] | 5·7 [1·5, 18·3] |
| Opioid exposure | ||
| Ever | 834 (80%) | 458 (78%) |
| Mean 24 h dose in ICU (mcg)ঠ| 550 [148, 1,442] | 586 [153, 1,307] |
| Propofol exposure | ||
| Ever | 521 (50%) | 299 (51%) |
| Mean 24 h dose in ICU (mg)‡ | 655 [185, 1,648] | 589 [185, 1,578] |
| Dexmedetomidine exposure | ||
| Ever | 128 (12%) | 79 (13%) |
| Mean 24 h dose in ICU (mcg)‡ | 147 [46, 386] | 139 [48, 369] |
| Length of stay (days) | ||
| ICU | 5·0 [2·8, 11·1] | 4·9 [2·7, 10·0] |
| Hospital | 10·0 [5·9, 17·2] | 10·0 [6·1, 18·0] |
Data are median (IQR) or n/total (%). ARDS=acute respiratory distress syndrome. COPD=chronic obstructive pulmonary disease. ICU=intensive care unit. IQCODE=Informant Questionnaire on Cognitive Decline in the Elderly. SOFA=Sequential Organ Failure Assessment. APACHE=Acute Physiologic Assessment and Chronic Health Evaluation.
Short IQCODE>3·6.
Including pulmonary embolus and pulmonary fibrosis.
Among those exposed.
In midazolam equivalents.
In fentanyl equivalents.
Delirium was common, with 740 (71%) of all participants being delirious at some point during the study. Delirium occurred during 4187 (31%) of all 13 434 participant-days during which mental status could be assessed during the study (ie, study days when participants were alive and were still in the hospital). Of the 4187 days of delirium studied, one delirium phenotype was present during 1355 (32%), two were present during 1213 (29%), three were present during 1231 (29%), and four were present during 388 (9%)—ie, during most delirium days, multiple phenotypes were identified. More than half of participants who experienced delirium had hypoxic, septic, or sedative-associated delirium at some point during the study (table 2), whereas metabolic and unclassified delirium (ie, delirium in the absence of hypoxia, sedation, sepsis, and metabolic dysfunction) occurred less often.
Table 2:
Prevalence and duration of delirium phenotypes
| Prevalence among participants | Frequency among delirium days | Duration among participants affected | |
|---|---|---|---|
| Any delirium | 740 (71%) | 4,187 (100%) | 4 [2, 7] |
| Hypoxic | 579 (56%) | 2,247 (54%) | 3 [1, 5] |
| Septic | 534 (51%) | 2,405 (57%) | 3 [2, 6] |
| Sedative-associated | 663 (64%) | 2,634 (63%) | 3 [1, 5] |
| Metabolic | 260 (25%) | 1,149 (27%) | 3 [1, 6] |
| Unclassified | 224 (22%) | 591 (14%) | 2 [1, 3] |
Data are n (%) or median [IQR].
Figure 1 shows the prevalence of the delirium phenotypes according to study day, and the median duration of each phenotype is shown in table 2. Early in hospital stay, sedative-associated, hypoxic, and septic delirium were common and were often one of multiple delirium phenotypes present on the same day. Metabolic delirium occurred less often, and unclassified delirium was the least common phenotype. The percentage of participants with septic and metabolic delirium remained relatively stable over time, whereas the percentage with hypoxic delirium and especially sedative-associated delirium fell over time.
Figure 1: Prevalence of delirium phenotypes according to study day.
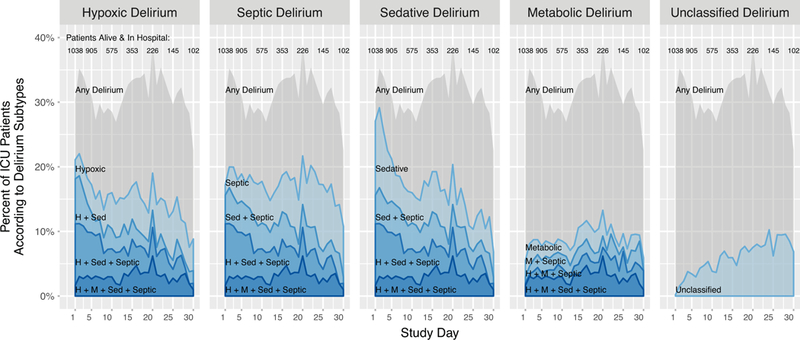
Each area plot shows (on the y-axis) the percentage of study participants in the hospital who had any delirium, a single delirium phenotype, or a combination of multiple delirium phenotypes according to study day (shown on the x-axis). The grey shading indicates the overall percentage of participants with delirium on each study day. The red lines and shaded areas represent the number of phenotypes of delirium present, with darker reds representing the presence of more phenotypes of delirium. The lightest red regions, therefore, show the percentage of participants with a given single phenotype. H=hypoxic. M=metabolic. Sed=sedative. Sep=septic.
The associations between delirium phenotypes and long-term cognitive outcomes are displayed in figure 2, and point estimates and confidence intervals for these associations are provided in table 3 and the appendix (pp 8, 9). After adjusting for age, Charlson Comorbidity Index, Short IQCODE, education, Framingham stroke risk profile, coma, sepsis, hypoxaemia, SOFA, and doses of sedating medications, longer durations of hypoxic delirium predicted worse RBANS global cognition scores at both 3-month and 12-month follow-up (table 3). Similarly, longer durations of sedative-associated and unclassified delirium also predicted worse cognitive function at 3 and 12 months. Although duration of septic delirium was not associated with 3-month cognitive outcomes, longer durations of septic delirium (like hypoxic, sedative-associated, and unclassified delirium) did predict worse 12-month cognitive outcomes (table 3). In contrast to the other delirium phenotypes studied, duration of metabolic delirium had no association with cognitive outcomes at 3-month or 12-month follow-up. In general, the delirium phenotypes that were associated with worse long-term RBANS global cognition scores were also associated with worse MMSE scores and worse scores in multiple cognitive domains assessed (appendix p 10). That is, none of the delirium phenotypes had an association with long-term cognitive impairment that was driven by an association with deficits in only one cognitive domain.
Figure 2: Associations between duration of delirium phenotypes and global cognition scores at 3- and 12-month follow-up.

Each line graph shows the association between the duration of a delirium phenotype (on the x-axis) and global cognitive performance on the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS) at 3 months (red lines, with the 95% CI represented by red shading) and 12 months (blue lines, with the 95% CI represented by blue shading) after hospital discharge.
Table 3:
Point estimates of the associations between delirium phenotypes and long-term cognition
| Comparison | RBANS global cognition at 3 months | RBANS global cognition at 12 months | |
|---|---|---|---|
| Hypoxic | 3 vs 0 days | −3·85 (−7·07 to −0·64) | −3·76 (−7·16 to −0·37) |
| Septic | 3 vs 0 days | −2·65 (−6·05 to 0·75) | −3·67 (−7·13 to −0·22) |
| Sedative-associated | 3 vs 0 days | −6·52 (−9·66 to −3·37) | −4·03 (−7·80 to −0·26) |
| Metabolic | 3 vs 0 days | 0·15 (−1·52 to 1·81) | 1·44 (−0·12 to 3·01) |
| Unclassified | 3 vs 0 days | −4·72 (−6·93 to −2·51) | −4·70 (−7·16 to −2·25) |
Data are estimate (95% CI). The point estimates indicate the change in RBANS global cognition score associated with the specified increase in the number of days of the delirium phenotype according to a multiple linear regression model that adjusted for potential confounders; because delirium phenotypes were allowed to have a non-linear association with RBANS global cognition, the magnitude of association depends on the comparison chosen. For all delirium phenotypes, we generated point estimates by comparing 3 vs 0 days of the exposure variable, chosen because these values represent the 75th to the 25th percentile values of the most common delirium phenotypes.
RBANS=Repeatable Battery for the Assessment of Neuropsychological Status
In post-hoc analyses, we found that using more severe thresholds to identify hypoxic or metabolic delirium did not qualitatively change associations with 3-month outcomes, but associations with 12-month outcomes were diminished (appendix p 12). Additionally, analyses that considered benzodiazepine-associated, propofol-associated, and opioid-associated delirium as separate exposures showed that no specific subtype of sedative-associated delirium stood out as the main driver to explain the results of the strong association between sedative-associated delirium (as a broader phenotype) and severity of long-term cognitive impairment (appendix p 13). However, most sedative-associated delirium days involved exposure to multiple sedating drugs (appendix p 14). Finally, none of the delirium phenotypes examined were associated with long-term mortality (appendix p 15).
Discussion
In this large, multicentre, prospective cohort, more than half of the critically ill patients studied with acute respiratory failure, shock, or both developed multiple clinical phenotypes of delirium, with sedative-associated, hypoxic, and septic being the most common types. Importantly, longer durations of the most common delirium phenotypes, including sedative-associated delirium, predicted more profound long-term cognitive impairment 12 months after hospital discharge even after adjusting for age, Charlson Comorbidity Index, Short IQCODE, education, Framingham stroke risk profile, coma, and the risk factors for the various phenotypes: SOFA (accounting for renal, liver, cardiovascular, and other organ dysfunctions), sepsis, hypoxaemia, and doses of sedating medications. Only delirium in the setting of metabolic disturbances did not predict worse long-term cognition. Although clinicians and researchers have speculated that certain delirium phenotypes are less relevant to long-term outcomes, our findings show that multiple delirium phenotypes, including sedative-associated delirium (which is both common and modifiable), have far-reaching clinical relevance, as defined by their association with the patient-centred outcome of long-term cognitive impairment.
Our finding that prolonged sedative-associated delirium predicts worse long-term cognitive impairment refutes our original hypothesis that posited sedative-associated delirium is not associated with long-term harm. Although sedative doses received during critical illness do not consistently predict long-term cognitive outcomes,6 we found that an adverse neurological response to sedation (ie, sedative-associated delirium) predicts severity of cognitive impairment 3 and 12 months later, a finding that suggests there are long-term adverse effects of sedatives when used to manage critically ill patients for several days. Clinicians must be mindful that, although many sedatives have short half-lives in healthy people, the clearances of these drugs are altered in the presence of critical illness and can be dramatically delayed after prolonged use—ie, over days rather than hours.31 Although significant controversy exists about the short-term and long-term adverse effects of sedatives and whether delirium should be assessed during sedative administration,32,33 our approach is in keeping with the recommendations of international scientific organisations that state that diagnostic criteria for delirium “should include all states of altered arousal (except coma) in the spectrum of delirium on scientific, practical and clinical safety grounds”.34 When taking this approach, a clinician assesses a patient for the presence or absence of delirium in the setting of all diseases and treatments and then, if delirium is present, considers associated risk factors (including exposure to deliriogenic sedatives) to work out the clinical phenotypes of delirium.
Sedative-associated delirium is the most modifiable clinical delirium phenotype to occur commonly during critical illness, and these data suggest that its management could have implications for long-term cognitive function and disability.5,6,35 So far, only one other study has attempted to assess the clinical importance of sedative-associated delirium during critical illness. Patel and colleagues36 assessed 97 mechanically ventilated ICU patients for delirium and considered patients who were delirious before but not immediately after sedative interruption to have rapidly reversible sedative-related delirium. Of 97 patients, the 12 (12%) who only had rapidly reversible sedative-related delirium had mortality and length of stay outcomes similar to the ten (10%) patients who never developed delirium, whereas the 75 (77%) patients with persistent delirium (all of whom had received sedation) had significantly greater mortality and longer lengths of stay. This important study by Patel and colleagues collected valuable data regarding the timing of delirium in the setting of sedation, but it probably lacked the power needed to establish whether rapidly reversible sedative-related delirium was associated with mortality or other important clinical outcomes, including long-term cognitive function. In view of the relatively low frequency of rapidly reversible sedative-related delirium, a much larger prospective study is now needed before we can confidently consider this phenotype a benign problem. In view of our finding that the duration of sedative-associated delirium—both rapidly reversible and persistent together because we did not distinguish between these subtypes—has a clear predictive association with severity of long-term cognitive impairment, the safest interpretation of the currently available evidence is that delirium diagnosed in the setting of sedation is more often than not indicative of brain injury that places the patient at higher risk for long-term cognitive impairment.
We hypothesised that both hypoxic delirium and septic delirium would be associated with long-term cognitive impairment because hypoxia and sepsis have well established mechanisms of cellular injury that can affect the brain. Whereas intermittent hypoxia is known to cause brain injury,37,38 the effects of sepsis on the brain have only recently been elucidated in animal models. These models show that systemic inflammation during sepsis leads to monocyte and neutrophil infiltration in the brain, where pro-inflammatory cytokines and chemokines are expressed and microglia are activated.9 These processes eventually result in chronic neuroinflammation and neurodegeneration, including cortical and subcortical neuronal loss, leading to clinically significant cognitive impairment.39 By including hypoxemia and sepsis as separate covariates in our regression models, we sought to establish whether the brain’s acute response (in the form of delirium) to these potentially injurious factors provides additional information about risk of long-term cognitive impairment. The fact that the durations of hypoxic delirium and of septic delirium predicted long-term cognitive outcomes after adjusting for the durations of hypoxaemia and sepsis suggests that delirium in these settings might be a useful biomarker of acute brain injury that affects a high percentage of critically ill patients.
Delirium during acute kidney injury, liver failure, or other metabolic disturbances was the only phenotype studied that had no association with long-term cognitive outcomes, a finding that has multiple potential explanations, including confounding of delirium assessments due to coma (known to be more likely during acute kidney injury24 or liver failure),40 type II error due to fewer patients having metabolic delirium, and differences in the underlying mechanisms of delirium, which might be more amenable to treatment in certain cases (eg, with lactulose or rifaximin, in the setting of liver failure). As with other forms of delirium, the pathophysiology of delirium during acute kidney injury or hepatic failure remains poorly understood and could differ greatly from that of delirium resulting from hypoxia, sepsis, or sedative exposure. Our results suggest that future investigations of the mechanisms of delirium should distinguish between various delirium phenotypes rather than assume that all delirium has a common mechanism.
Notably, one of the strongest predictors of worse long-term cognitive impairment in our study was a prolonged period of delirium that did not meet the criteria for any of the delirium phenotypes (ie, unclassified delirium). The reason for this finding is unknown, but the association might reflect that delirium lasting beyond the period of the ICU stay is a strong indicator of persistent brain injury given that our study design did not allow us to identify certain delirium phenotypes after an ICU discharge. For example, hypoxic delirium could not be identified because continuous pulse oximetry data were not consistently recorded outside the ICU, so some days of unclassified delirium could have been prolonged hypoxic delirium. Alternatively, unclassified delirium might represent one or more distinct delirium phenotypes that warrant additional study.
Our study has limitations that warrant comment. First, in patients with multiple delirium phenotypes, we were not able to identify the primary cause of delirium. When analysing each phenotype, we adjusted for other causes of delirium (eg, sepsis and hypoxaemia), but we were not able to specifically quantify the additive effect of each phenotype because participants had different combinations of phenotypes. Future studies, including trials of interventions that target specific delirium phenotypes, are needed to establish which phenotypes carry the greatest risk and which are amenable to intervention. Including patients with multiple phenotypes enhanced our study’s generalisability because the large cohort reflects the clinical reality that most critically ill patients experience multiple, related delirium risk factors. We were also unable to establish whether or not delirium at a specific timepoint was a response to risk factors experienced at that time or earlier, a challenge that mirrors that faced by clinicians at the bedside. Nevertheless, by adjusting for a large number of well defined covariates, we sought to isolate the association between each phenotype of delirium and long-term cognition. Some misclassification of delirium phenotypes may have occurred—eg, we did not alter sedation orders (ie, drug, dose, route, and timing ordered by physician) and were unable to systematically distinguish rapidly reversible sedative-associated delirium from persistent sedative-associated delirium. As in clinical practice, we could not disentangle the effects of different sedatives as only a small percentage of participants were exposed to just one sedative. Our findings therefore speak directly to the management of most mechanically ventilated ICU patients, who typically receive multiple sedatives either concurrently or during the course of their illness. We also analysed delirium and coma durations by tallying the calendar days on which these mental states were detected, but both delirium and coma can begin or end mid-day, leading to imprecision in these calculations.
Furthermore, we did not directly measure exposures before or after the in-hospital study period that might have altered cognitive outcomes. For example, the unplanned nature of critical illness prevented us from directly assessing premorbid cognitive function. However, we employed a rigorous multistep process using validated surrogate-based questionnaires to identify pre-existing cognitive impairment. Another characteristic of critical illness, a high mortality rate, could have resulted in survivor bias, although this would probably bias results towards the null given that the sickest patients were most likely to die and therefore not be included in our analyses. The results of this observational study cannot prove a causative association between the phenotypes of delirium and long-term cognitive impairment. Finally, in keeping with authoritative recommendations and to avoid type II errors, we chose not to adjust for multiple comparisons when testing our a priori hypotheses, an approach that can increase the risk of type I errors.
Despite these limitations, our investigation had multiple strengths. As well as including a diverse study population that mirrors those treated every day by critical care clinicians, this multicentre study included more than 1000 participants from the medical and surgical ICUs of five academic, community, and Veterans Administration hospitals across the USA, making it the largest study of delirium phenotypes in the ICU and enhancing the generalisability of our findings. Second, the large sample size provided statistical power to adjust for numerous covariates with low risk of overfitting. Third, highly trained research personnel assessed patients for delirium using a well validated method that is both sensitive and specific. Fourth, we collected granular physiological, pharma-cological, and clinical data so that delirium phenotypes were carefully identified. And finally, personnel who were masked to all events of the critical illness hospitalisation, including all delirium data, performed detailed assessments 3 and 12 months after hospital discharge to assess long-term cognitive function, a patient-centred outcome.
In conclusion, in this large multicentre cohort of patients with medical and surgical critical illness due to acute respiratory failure, shock, or both, sedative-associated, hypoxic, and septic delirium were common and often occurred concurrently. Longer periods of each of these delirium phenotypes predicted worse long-term cognitive impairment up to 1 year after discharge from the hospital even after adjusting for multiple relevant covariates. These findings suggest that specific phenotypes of delirium during critical illness—including sedative-associated delirium, which is the most modifiable phenotype affecting ICU patients—have important clinical implications regarding long-term outcomes and should therefore be an important focus of future research and clinical efforts to improve patient safety. Clinicians should consider delirium in the settings of sedation, hypoxia, and sepsis to be a red flag indicating increased risk for long-term cognitive impairment and should seek to minimise the duration of exposure to associated risk factors.
Supplementary Material
Research in context.
Evidence before this study
We searched PubMed for relevant original research articles assessing prevalence and duration of clinical phenotypes of delirium during critical illness (ie, phenotypes defined by clinical risk factors) and seeking to understand associations between these delirium phenotypes and long-term cognitive impairment. Limiting the search to studies published in English before June 1, 2017, we used various combinations of the following search terms: “delirium”, “phenotype”, “hypoxia”, “sepsis”, “sedative”, “acute kidney injury”, “liver failure”, “intensive care”, and “cognitive impairment”. We found 17 studies that identified motoric phenotypes of delirium (defined by level of arousal) during critical illness, whereas only one study (Patel et al, 2014), a single-centre prospective cohort study analysing 97 patients, identified delirium phenotypes according to associated risk factors, specifically focusing on sedative-associated delirium. We found no published studies describing the prevalence of other clinical phenotypes of delirium during critical illness. Additionally, although duration of delirium during critical illness is known to predict long-term cognitive outcomes, it is not known whether various clinical phenotypes of delirium are associated with differential effects on long-term cognition.
Added value of this study
This large, multicentre, prospective cohort study is the first, to our knowledge, to show that more than half of the critically ill patients with acute respiratory failure, shock, or both develop multiple clinical phenotypes of delirium, with sedative-associated, hypoxic, and septic delirium being most common. Importantly, longer durations of hypoxic, septic, sedative-associated, and unclassified delirium predict worse long-term cognitive impairment up to 12 months after hospital discharge.
Implications of all the available evidence
Our findings suggest that sedative-associated, hypoxic, and septic delirium, which are common and frequently co-occur during critical illness, have important implications regarding long-term cognitive impairment. As potentially modifiable aspects of clinical care related to patients’ long-term cognition, attention to these delirium phenotypes is an aspect of patient safety that should be the focus of improvements in patient care and future research.
Acknowledgments
This study and TDG, JLT, PPP, NEB, JCJ, MBP, RC, RSD, and EWE received support from the National Institutes of Health (AG034257, AG031322, AG027472, AG035117, GM120484, HL111111, and HL135144). In addition, TDG, MRE, RBG, RSD, and EWE received support from the Department of Veterans Affairs (Merit Review Grant, Geriatric Research, Education and Clinical Center [GRECC]).
Funding National Institutes of Health (AG034257, AG031322, AG027472, AG035117, GM120484, HL111111, HL135144) and the Veterans Affairs Tennessee Valley Geriatric Research, Education and Clinical Center (GRECC).
Footnotes
Declaration on Interests
PPP received a research grant from Hospira in collaboration with the NIH. EWE received honoraria from Orion and Hospira for continuing medical education activities. NEB served on an advisory board for ArjoHuntleigh. All other authors declare no competing interests.
References
- 1.Girard TD, Pandharipande PP, Ely EW. Delirium in the intensive care unit. Crit Care 2008; 12 Suppl 3: S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ely EW, Shintani A, Truman B, et al. Delirium as a predictor of mortality in mechanically ventilated patients in the intensive care unit. JAMA 2004; 291(14): 1753–62. [DOI] [PubMed] [Google Scholar]
- 3.Pisani MA, Kong SY, Kasl SV, Murphy TE, Araujo KL, Van Ness PH. Days of delirium are associated with 1-year mortality in an older intensive care unit population. Am J Respir Crit Care Med 2009; 180(11): 1092–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shehabi Y, Riker RR, Bokesch PM, Wisemandle W, Shintani A, Ely EW. Delirium duration and mortality in lightly sedated, mechanically ventilated intensive care patients. Crit Care Med 2010; 38(12): 2311–8. [DOI] [PubMed] [Google Scholar]
- 5.Girard TD, Jackson JC, Pandharipande PP, et al. Delirium as a predictor of long-term cognitive impairment in survivors of critical illness. Crit Care Med 2010; 38(7): 1513–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pandharipande PP, Girard TD, Jackson JC, et al. Long-term cognitive impairment after critical illness. N Engl J Med 2013; 369(14): 1306–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saczynski JS, Marcantonio ER, Quach L, Fong TG, Gross A, Inouye SK, Jones RN. Cognitive trajectories after postoperative delirium. N Engl J Med 2012; 367: 30–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bozza FA, Garteiser P, Oliveira MF, et al. Sepsis-associated encephalopathy: a magnetic resonance imaging and spectroscopy study. J Cereb Blood Flow Metab 2010; 30(2): 440–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Singer BH, Newstead MW, Zeng X, et al. Cecal ligation and puncture results in long-term central nervous system myeloid inflammation. PLoS One 2016; 11(2): e0149136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ely EW, Inouye SK, Bernard GR, et al. Delirium in mechanically ventilated patients: validity and reliability of the confusion assessment method for the intensive care unit (CAM-ICU). JAMA 2001; 286(21): 2703–10. [DOI] [PubMed] [Google Scholar]
- 11.Bergeron N, Dubois MJ, Dumont M, Dial S, Skrobik Y. Intensive Care Delirium Screening Checklist: evaluation of a new screening tool. Intensive Care Med 2001; 27(5): 859–64. [DOI] [PubMed] [Google Scholar]
- 12.Girard TD, Pandharipande PP, Carson SS, et al. Feasibility, efficacy, and safety of antipsychotics for intensive care unit delirium: the MIND randomized, placebo-controlled trial. Crit Care Med 2010; 38(2): 428–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Devlin JW, Roberts RJ, Fong JJ, et al. Efficacy and safety of quetiapine in critically ill patients with delirium: a prospective, multicenter, randomized, double-blind, placebo-controlled pilot study. Crit Care Med 2010; 38(2): 419–27. [DOI] [PubMed] [Google Scholar]
- 14.Page VJ, Ely EW, Gates S, et al. Effect of intravenous haloperidol on the duration of delirium and coma in critically ill patients (Hope-ICU): a randomised, double-blind, placebo-controlled trial. Lancet Respir Med 2013; 1(7): 515–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Patel MB, Jackson JC, Morandi A, et al. Incidence and Risk Factors for Intensive Care Unit-related Post-traumatic Stress Disorder in Veterans and Civilians. Am J Respir Crit Care Med 2016; 193(12): 1373–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.National Institutes of Health. 5K23AG034257–05: Predictors of cognitive impairment in survivors of critical illness. https://projectreporter.nih.gov/project_info_description.cfm?aid=8523720 (accessed April 4, 2017).
- 17.Berg L Clinical Dementia Rating (CDR). Psychopharmacology bulletin 1988; 24(4): 637–9. [PubMed] [Google Scholar]
- 18.Sessler CN, Gosnell MS, Grap MJ, et al. The Richmond Agitation-Sedation Scale: validity and reliability in adult intensive care unit patients. Am J Respir Crit Care Med 2002; 166(10): 1338–44. [DOI] [PubMed] [Google Scholar]
- 19.Ely EW, Truman B, Shintani A, et al. Monitoring sedation status over time in ICU patients: reliability and validity of the Richmond Agitation-Sedation Scale (RASS). JAMA 2003; 289(22): 2983–91. [DOI] [PubMed] [Google Scholar]
- 20.Girard TD, Dittus RS, Ely EW. Critical illness brain injury. Annu Rev Med 2016; 67: 497–513. [DOI] [PubMed] [Google Scholar]
- 21.Hsieh SJ, Soto GJ, Hope AA, Ponea A, Gong MN. The association between acute respiratory distress syndrome, delirium, and in-hospital mortality in intensive care unit patients. Am J Respir Crit Care Med 2015; 191(1): 71–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ebersoldt M, Sharshar T, Annane D. Sepsis-associated delirium. Intensive Care Med 2007; 33(6): 941–50. [DOI] [PubMed] [Google Scholar]
- 23.Pandharipande P, Shintani A, Peterson J, et al. Lorazepam is an independent risk factor for transitioning to delirium in intensive care unit patients. Anesthesiology 2006; 104(1): 21–6. [DOI] [PubMed] [Google Scholar]
- 24.Siew ED, Fissell WH, Tripp CM, et al. Acute kidney injury as a risk factor for delirium and coma during critical illness. Am J Respir Crit Care Med 2017; 195(12): 1597–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987; 40(5): 373–83. [DOI] [PubMed] [Google Scholar]
- 26.Jorm AF. A short form of the Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE): development and cross-validation. Psychol Med 1994; 24: 145–53. [DOI] [PubMed] [Google Scholar]
- 27.Wolf PA, D’Agostino RB, Belanger AJ, Kannel WB. Probability of stroke: a risk profile from the Framingham Study. Stroke 1991; 22(3): 312–8. [DOI] [PubMed] [Google Scholar]
- 28.Vincent JL, Mendonca Ad, Cantraine F, et al. Use of the SOFA score to assess the incidence of organ dysfunction/failure in intensive care units: Results of a multicenter, prospective study. Crit Care Med 1998; 26: 1793–800. [DOI] [PubMed] [Google Scholar]
- 29.Randolph C, Tierney MC, Mohr E, Chase TN. The Repeatable Battery for the Assessment of Neuropsychological Status (RBANS): preliminary clinical validity. Journal of clinical and experimental neuropsychology 1998; 20(3): 310–9. [DOI] [PubMed] [Google Scholar]
- 30.Reitan RM, Wolfson D. The Halstead Reitan Neuropsychological Test Battery. Tuscon, AZ: Neuropsychology Press; 1985. [Google Scholar]
- 31.Tse AH, Ling L, Joynt GM, Lee A. Prolonged infusion of sedatives and analgesics in adult intensive care patients: a systematic review of pharmacokinetic data reporting and quality of evidence. Pharmacol Res 2017; 117: 156–65. [DOI] [PubMed] [Google Scholar]
- 32.Can delirium Assessments Be Accurately Labelled (CABAL) Investigators group, Devlin JW, Fraser GL, Joffe AM, Riker RR, Skrobik Y. The accurate recognition of delirium in the ICU: the emperor’s new clothes? Intensive Care Med 2013; 39(12): 2196–9. [DOI] [PubMed] [Google Scholar]
- 33.Haenggi M, Blum S, Brechbuehl R, Brunello A, Jakob SM, Takala J. Effect of sedation level on the prevalence of delirium when assessed with CAM-ICU and ICDSC. Intensive Care Med 2013; 39(12): 2171–9. [DOI] [PubMed] [Google Scholar]
- 34.European Delirium Association, American Delirium Society. The DSM-5 criteria, level of arousal and delirium diagnosis: inclusiveness is safer. BMC Med 2014; 12: 141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brummel NE, Jackson JC, Pandharipande PP, et al. Delirium in the ICU and subsequent long-term disability among survivors of mechanical ventilation*. Crit Care Med 2014; 42(2): 369–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Patel SB, Poston JT, Pohlman A, Hall JB, Kress JP. Rapidly reversible, sedation-related delirium versus persistent delirium in the intensive care unit. Am J Respir Crit Care Med 2014; 189(6): 658–65. [DOI] [PubMed] [Google Scholar]
- 37.Zhang SX, Wang Y, Gozal D. Pathological consequences of intermittent hypoxia in the central nervous system. Compr Physiol 2012; 2: 1767–77. [DOI] [PubMed] [Google Scholar]
- 38.McMorris T, Hale BJ, Barwood M, Costello J, Corbett J. Effect of acute hypoxia on cognition: a systematic review and meta-regression analysis. Neurosci Biobehav Rev 2017; 74: 225–32. [DOI] [PubMed] [Google Scholar]
- 39.Semmler A, Hermann S, Mormann F, et al. Sepsis causes neuroinflammation and concomitant decrease of cerebral metabolism. J Neuroinflammation 2008; 5: 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ferenci P, Lockwood A, Mullen K, Tarter R, Weissenborn K, Blei AT. Hepatic encephalopathy--definition, nomenclature, diagnosis, and quantification: final report of the working party at the 11th World Congresses of Gastroenterology, Vienna, 1998. Hepatology 2002; 35(3): 716–21. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


