Abstract
Background
Ghana started its national programme to eliminate lymphatic filariasis (LF) in 2000, with mass drug administration (MDA) with ivermectin and albendazole as main strategy. We review the progress towards elimination that was made by 2016 for all endemic districts of Ghana and analyze microfilaria (mf) prevalence from sentinel and spot-check sites in endemic districts.
Methods
We reviewed district level data on the history of MDA and outcomes of transmission assessment surveys (TAS). We further collated and analyzed mf prevalence data from sentinel and spot-check sites.
Results
MDA was initiated in 2001–2006 in all 98 endemic districts; by the end of 2016, 81 had stopped MDA after passing TAS and after an average of 11 rounds of treatment (range 8–14 rounds). The median reported coverage for the communities was 77–80%. Mf prevalence survey data were available for 430 communities from 78/98 endemic districts. Baseline mf prevalence data were available for 53 communities, with an average mf prevalence of 8.7% (0–45.7%). Repeated measurements were available for 78 communities, showing a steep decrease in mean mf prevalence in the first few years of MDA, followed by a gradual further decline. In the 2013 and 2014 surveys, 7 and 10 communities respectively were identified with mf prevalence still above 1% (maximum 5.6%). Fifteen of the communities above threshold are all within districts where MDA was still ongoing by 2016.
Conclusions
The MDA programme of the Ghana Health Services has reduced mf prevalence in sentinel sites below the 1% threshold in 81/98 endemic districts in Ghana, yet 15 communities within 13 districts (MDA ongoing by 2016) had higher prevalence than this threshold during the surveys in 2013 and 2014. These districts may need to intensify interventions to achieve the WHO 2020 target.
Author summary
Lymphatic filariasis (LF) control in Ghana has relied on ivermectin and albendazole since the year 2000 when the Ghana Filariasis Elimination Programme started. We analyzed trends in microfilaraemia prevalence during MDA, reported coverage, and transmission assessment survey using data obtained from the Ghana Health Services (GHS). The median reported treatment coverage varied between 77–80% over the years. Our results show that the treatment in Ghana made a significant impact in reducing infections <1% in majority of sentinel sites in endemic districts (81/98) by 2016. In the remaining 17 districts, extra efforts may be needed to achieve the same goal. Some of the challenges could be low coverage in some communities, high baseline endemicity, programme logistical challenges etc. The required average rounds of MDA needed to reach mf prevalence < 1% was 11, higher than that proposed by the Global Filariasis Elimination Programme. This article is relevant to LF control programmes in assessing the impact of MDA. It is important for programmes to monitor infections especially within communities where mf prevalence is still above the 1% threshold to ensure that the WHO 2020 target is achieved.
Introduction
Lymphatic filariasis (LF), commonly known as elephantiasis, is a debilitating and disfiguring tropical disease caused by lymphatic-dwelling filarial parasites Wuchereria bancrofti, Brugia malayi and Brugia timori. The disease is transmitted by different species of mosquitoes depending on the geographical location, including Culex, Anopheles and Aedes species. About 90% of the worldwide cases are caused by W. bancrofti and 10% caused by B. malayi and B. timori. Based on re-assessment of the global prevalence and distribution of LF [1], more than 120 million people were found to be infected and 40 million disfigured and incapacitated in the year 2000 [2]. In the same year, the Global Programme to Eliminate Lymphatic Filariasis (GPELF) was established, aiming to eliminate the disease as a public health problem by 2020 through annual mass drug administration (MDA) with albendazole in combination with diethylcarbamazine citrate (DEC) or ivermectin to all individuals at risk [3].
By the end of 2016, 20 out of 73 countries originally listed by the World Health Organization (WHO) as being endemic for LF have stopped interventions after passing the first transmission assessment survey and are conducting surveillance to validate elimination as a public health problem. Additional 30 countries have delivered MDA at least once in all endemic areas and are also on track to achieve the 2020 target [4]. While many have passed the TAS, there are also reports of failure [5] and of ongoing transmission in spite of passing the TAS [5–7].
A national survey carried out in Ghana in 1994 showed that the microfilaraemia prevalence varied from 0–20% between regions [8]. In the highly-endemic Kassena Nankana district (Upper East Region of Ghana), the prevalence of hydrocele was 30.8% and elephantiasis of the leg was 3.8% in the population aged 10 years and above [9,10]; 12% of extended families reported to have at least one family member with elephantiasis of the leg [10]. The extensive mapping of endemic communities [11] provided a database on areas in Ghana and neighboring countries that needed more efforts to eliminate the disease.
The LF elimination programme in Ghana started in 2000 and gradually scaled up over the years and by 2006 all endemic districts were covered. The implementation and outcomes by district were described in two recent papers [12,13]. By 2016, 81 of 98 initially endemic districts had reached an microfilaria (mf) prevalence <1%, had passed TAS survey and stopped MDA, while the remaining districts still had mf prevalence >1% [13] in spite of at least 10 years of MDA. The required duration of MDA turned out to be longer than the anticipated 5–6 years, which might be due to relatively high baseline mf prevalence levels. There were no major differences with other districts in reported coverage of MDA or long-lasting insecticide treated bednets [13].
Expected trends in infection during MDA will depend on multiple factors, including local baseline endemicity (depending on local transmission conditions) and the achieved coverage and compliance with MDA [7,14–16]. We aim to assess the impact of MDA on mf prevalence levels and to review progress towards LF elimination in Ghana from 2000–2016. For this purpose, we analyze community-level data from mf prevalence surveys and transmission assessment surveys (TAS) from sentinel and spot-check sites for all endemic districts in Ghana.
Methods
Ghana Filariasis Elimination Programme
The Ghana Filariasis Elimination Programme (GFEP) was established in June 2000 following the establishment of the Global Programme to Eliminate Lymphatic Filaraisis. Mapping of communities started in 2000 using the 50-km sample grid, rapid assessment procedure for antigenaemia in sample villages and spatial analysis to plot prevalence contours from 2000 to 2001 [11,17]. Forty nine districts out of 110 were initially identified as endemic and therefore selected for implementation of MDA. The GFEP implementation, programme outcomes, challenges and districts re-demarcation have been described in Biritwum et al. (2017a). Based on current demarcation, 98/216 districts (45%) are endemic with LF in Ghana.
The treatment implemented in Ghana was the combination of ivermectin (150 μg/kg) and albendazole (400 mg) given annually by the community-directed treatment approach [18] and implemented at the district level. MDA usually took place between March and June in all endemic communities across the country. Individuals eligible for treatment were those aged ≥5 years (excluding pregnant women, lactating mothers and the sick), and selection was solely based on height (≥90 cm) for those whose ages were not known. MDAs usually lasted for about 1–2 weeks per community. Individual treatment information (whether treated, absent, pregnant, sick, etc) was recorded in the community treatment book and summarized into treatment records by the Ghana Health Services (GHS). Community-level treatment coverage data (number treated out of total population at risk) across the country were reviewed and summarized by the GHS. For the purpose of this study, summary reports were reviewed.
There was no treatment offered in 2011 due to logistic and funding challenges; 2009 and 2012 treatment data were not available.
Monitoring and evaluation
Parasitological surveys
Parasitology data were collected by the GHS in programmatic yearly surveys (2000–2014) in selected endemic communities. In 2000, baseline mf surveys were carried out in 24 purposefully selected endemic communities (based on known high endemicity and population stability) [19,20] from the 8 districts where MDA was first initiated. From 2001–2004, baseline mf surveys were done in sentinel sites of remaining districts, as MDA was being extended into these districts. Subsequently, the previously selected sentinel sites per district were repeatedly surveyed to monitor progress to elimination (usually once every 2 or 3 years, but sometimes the interval was much longer due to financial constrains). Additional surveys were done in spot-check sites (same characteristics as sentinel) that were surveyed only once and often selected randomly from the same district where sentinel site is located to cross check the MDA performance in that district.
The mf surveys were usually done at the end of the year between November and December (before MDA treatment was done the following year between March-July). The target number of persons for sampling increased over time based on WHO guidelines; between 2000–2002 the target was 100 persons per community, between 2003–2009 it was 500 persons, and after 2009 it was 1000–1500 persons. The method of sampling for the community survey was convenient sampling, where participants who presented themselves were sampled from a central location until the minimum requirements for WHO was met. The surveys were usually preceded by a community gathering or announcement by the team informing members of the community to converge for the night blood collection (9pm − 2am). The eligible were verbally consenting individuals (parent consented for their children) aged ≥ 5years or with height ≥ 90cm height for those whose ages were not known, including pregnant women and lactating mothers (There was no effort to seek balance of age, sex or any other factor of the selected participants). Blood was sampled from these individuals by finger pricking (middle or forth finger) and a volume of 60μl taken for thick blood smear test (2000–2009) [21]. In later years (2010–2014), the volume of blood sampled was increased to 100μl and the microfilariae were counted using a regular microscope with a rafter counting chamber [22], by trained GHS laboratory technicians.
Transmission assessment surveys
The transmission assessment surveys (TAS) in Ghana followed the WHO guidelines, using antigenaemia prevalence in children aged 6–7 years as indicator of active transmission of LF [23]. TAS has a different sampling system and a different target population than a full mf survey. In Ghana, the elimination programme used a district as an implementation unit (IU) for MDA. The evaluation unit (EU) to assess progress of programme may also be a district or a cluster of districts with a population not more than 2 million. In some of the cities where the district population was more than 2 million, the district was divided into different EUs. An EU qualified to be assessed after achieving treatment coverage of ≥65% for 5 years and also recording mf prevalence of <1%. Those EUs who met the criteria were selected for the TAS. The TAS involved sampling of children 6–7 years in primary schools within the EU after written consent from their parents. The schools to be surveyed, the number of children to be tested and the critical cut-off point (maximum number of positives to fail TAS) were estimated using a survey sample builder software recommended by WHO [24]. A volume of 100 μl blood was taken from the children by finger pricking and test done using immunochromatographic card test (ICT) [25]. The EUs passed TAS if the number of positive children was less than or equal to the critical cut-off and MDA was stopped after passing TAS-1. Subsequently, two more TAS surveys (TAS-2 & TAS-3) are done after 2–3 years and 5 years respectively before elimination of LF as a public health problem is said to be achieved. No mf surveys continued in any community within EUs that passed TAS-1. During TAS-1, the EU with number of positive children above the critical cut-off point, failed the TAS and continued MDA for 2–3 years. In such EUs, a community survey was required to achieve an mf prevalence of <1% before TAS-1 was repeated [13].
Data collation and analysis
The longitudinal parasitological and treatment data from 2000–2014 were collated along with background information from the GHS and updates on TAS results until early 2016. Parasitological data comprised of number examined and number that were microfilarial positive in each community. Community mf prevalence was estimated as the number of microfilarial positives as a percentage of number examined in the community. Mf prevalence (for the districts or country) were calculated by estimating the percentage of total positive / total examined of communities included in the sampling for the district or country. Similarly, the treatment coverage (for the districts or country) were calculated by estimating the percentage of total treated / total population of communities included in the sampling for the district or country. We present data on community, district and country level.
Data limitations and special situations
We only consider mf prevalence data in our analysis of community-level trends in infection prevalence. No mf prevalence data were available for 2001 and data for 2002 were limited to few sites, as all community surveys in 2001 and part of the survey in 2002 only used ICT antigenaemia tests. For 3 districts in 2003 (with 2 communities surveyed per district) and all districts in 2005 (with 3–4 communities surveyed per district) mf prevalence was not reported for each community separately, and we only know the overall mf prevalence—aggregated over the surveyed communities. These aggregated data points were included in combined trends (averages) for all communities, but were not matched to community specific data for analysis for time trend analysis. There were no mf surveys carried out in 2006. Mf prevalence data in 2008 and 2010 were excluded from trends and analysis since communities sampled in these years were not randomly selected (mf data from individuals closely related to school children who were positive using ICT during school surveys).
Ethics statement
Ethical clearance was obtained from the Ghana Health Service Ethical Review Committee (ID NO: GHS-ERC-10/0/06) and the Liverpool School of Tropical Medicine’s Research Ethics Committee’s Research Protocol Approval (06.47). The study obtained oral informed consent from adult participants while parents and guardians orally consented for their children and wards to be part of this study. Due to the programmatic nature of the study with regular MDA and mf surveys done in many sites, participants in these communities were aware of the program. Given that the communities were mainly rural with study participants having minimal or no education and being suspicious of signing documents they did not well understand, oral consent was applied and noted as part of questionnaires during the surveys. Oral informed consent was approved by the Ghana Health Service and Liverpool School of Tropical Medicine’s ethical review committees.
Results
Overview of MDA implementation in Ghana
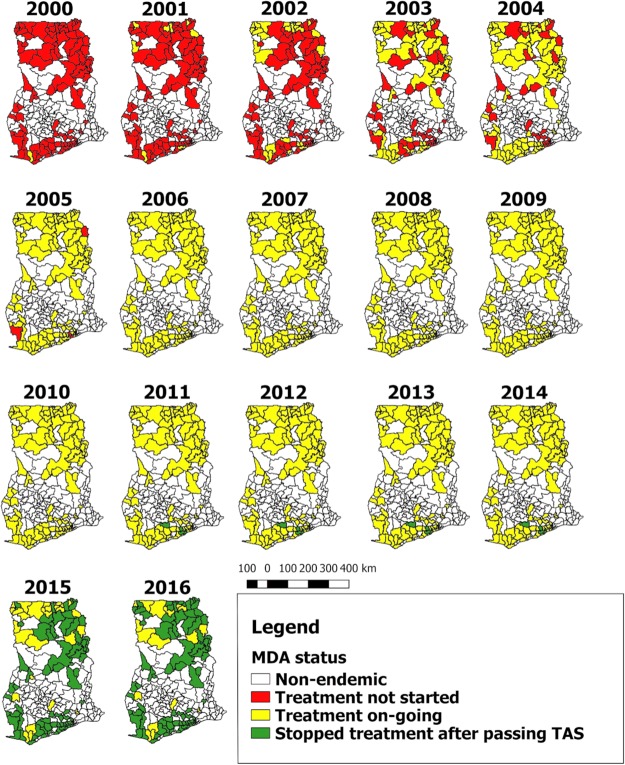
MDA started between 2000 and 2001 in 10/98 districts selected from the northern and coastal regions of Ghana. In 2002, 17 more districts were enrolled onto the MDA programme and in 2003, 2004, 2005 and 2006 a number of 27, 16, 25 and 3 more districts were enrolled onto the MDA programme respectively (Figs 1 and 2). All communities in each district were expected to be treated in the same year MDA started, thus geographical coverage within a district was expected to be 100%.
Fig 1. Progress of MDA implementation in Ghana by district.
NB: In the year 2011, there was no treatment due to some logistical challenges. The maps give an overview of the treatment progression to cover all the endemic districts in Ghana.
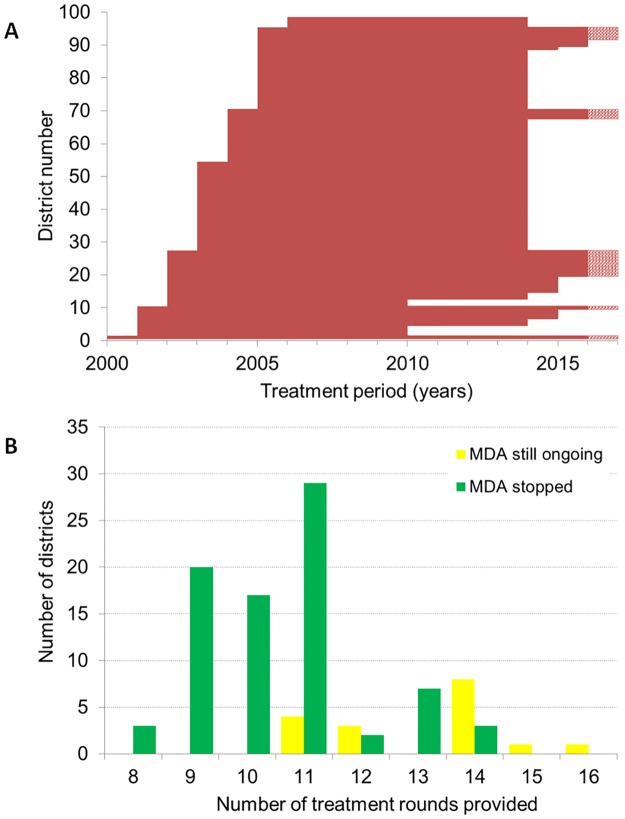
Fig 2. Duration of MDA by district in Ghana.
A) Period of MDA by each district in order of start year. Each horizontal line represents a district. Bars with a dashed section on the right-hand side represent districts where MDA is still ongoing after 2016 with unknown end year. See supplementary S2 Table for more details. B) Frequency distribution of the number of years of treatments provided by district through 2016, presented separately for districts that had stopped MDA by 2016 and those with still ongoing MDA.
Reported coverage of MDA by calendar year in Ghana
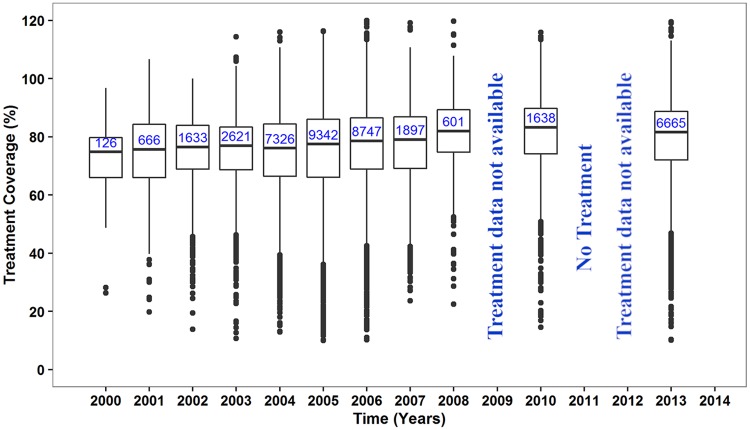
The median reported treatment coverage in treated districts of Ghana seemed to be constant over time, around 77–80% between 2000–2010, and the interquartile range and distribution of outliers were also similar over time (Fig 3). Although mean reported coverage per year seem to be high, there are large differences between communities. Community-level coverage estimates varied from 10 to 120%, with at least 7952/41265 (19.3%) surveys having a coverage under 65% and 198/41265 (0.5%) surveys over 100%, indicating wrong denominators.
Fig 3. Reported treatment coverage in treated communities in Ghana.
The box at each time point represents the interquartile range of coverage and the thick horizontal lines across each box represent the median coverage. The bullets outside each box (above or below) represent the outliers and are calculated as 1.5 times the interquartile range above or below the ends of the box (25th and 75th percentile). The vertical lines (whiskers) extend to the first value (coverage) before the outlier cut-off and where there are no outliers, they represent the minimum and maximum coverage at each time point. The numbers in the boxes are the total number of communities treated at each time point. There was no treatment offered in 2011 due to some challenges; 2009 and 2012 treatment data not available.
TAS was done in 5 districts in 2010, and all passed. Another 65, 9 and 2 districts had their first TAS in 2014, 2015 and early 2016, respectively and all passed. By the end of 2016, 81 out of the 98 endemic districts had passed the TAS in Ghana and had stopped MDA (Figs 1 and 2). 17 are left, of whom 4, 3, 8, 1 and 1 district have done 11, 12, 14, 15 and 16 rounds of MDA, respectively (see S2 Table, supplementary data for details). The average number of treatment rounds in districts that stopped MDA was 11 rounds, varying from 8–14. TAS-2 was performed in 69 districts in 2012 or 2015, and all districts passed. Details of the TAS surveillance in Ghana are given in S2 Table, supplementary data.
Trends in Mf prevalence
Mf prevalence data were available from 613 community mf surveys (datapoints), carried out between 2000 to 2014 in 430 communities (292 sentinel sites; 138 spot-check sites) in 78 out of the 98 endemic districts (within 8/10 regions of Ghana). Twenty districts were not represented in our compiled database, either because only antigenaemia data or TAS data were available or because no surveys had been done after re-demarcation of districts. 352 communities were measured only once and 78 measured multiple times (sampled between 2–6 times). Out of those measured multiple times, 35 communities also had data including baseline. Most of the single time point surveys were observed after 2007 (Fig 4A). Overall, the total number of individuals sampled per year ranged between 1,784–19,268.
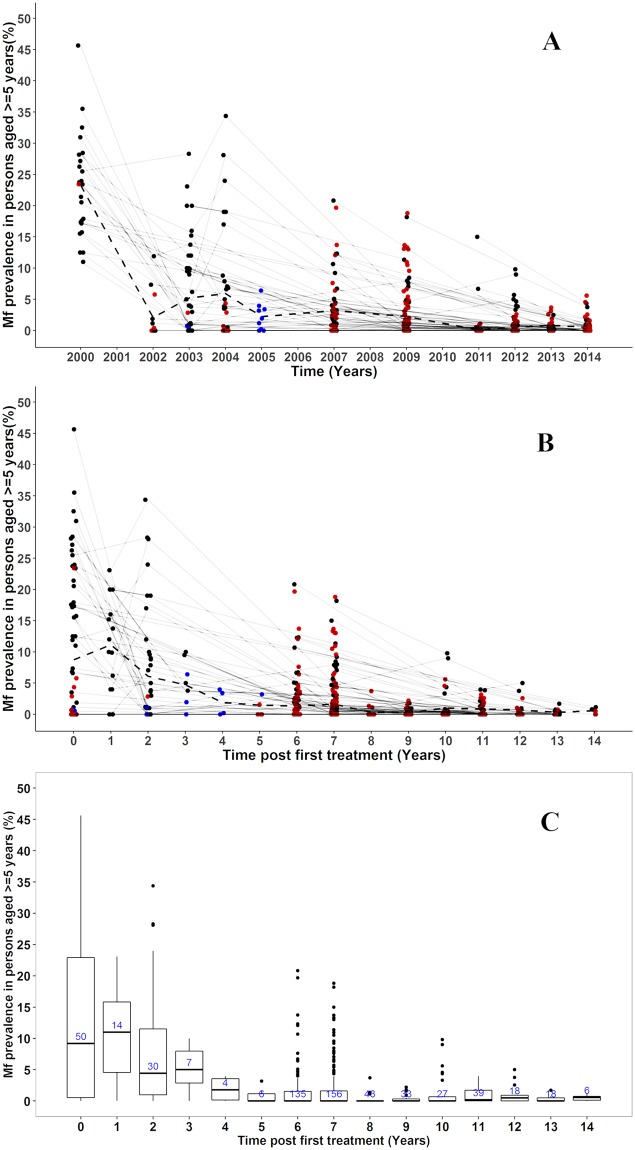
Fig 4. Observed lymphatic filariasis mf prevalence in sentinel and spot-check sites in Ghana, measured in the population aged 5 and above, for the period 2000–2014.
A) Data presented by calendar year. Multiple observations from the same community are connected through thin grey lines. Observations from communities surveyed only once are highlighted in brown. Observations presenting aggregated prevalence over multiple communities are displayed in blue (in 2003 and 2005). Dashed lines represent the average prevalence from all surveyed communities at each time point. Bullets at the same time point have been jittered to avoid overlapping of points at the same position; these do not represent time in months. B) As panel A but with time since first treatment on the horizontal axis. C) As B, but with data summarized in boxplots. The box at each time post treatment represents the interquartile range of mf prevalence in ≥5 years and the thick horizontal lines across each box represent the median mf prevalence. The bullets outside each box (above or below) represent the outliers and are defined as 1.5 times the interquartile range above or below the ends of the box (25th and 75th percentile). The vertical lines (whiskers) extend to the first value (mf prevalence) before the outlier cut-off and where there are no outliers, they represent the minimum and maximum mf prevalence at each time post treatment. The numbers in the boxes are the total number of communities examined at each time post treatment.
Baseline mf prevalence
Baseline parasitological surveys were carried out in the years 2000–2004, before the start of MDA, examining 7,882 individuals from 53/430 communities within 21/98 districts. The number of individuals examined per community at baseline ranged between 52–441 (mean 137, median 112). The average mf prevalence at baseline was 8.7% (range 0–45.7%) with the highest recorded in Gyahadze located in the central region of Ghana (See supplementary S1 Table).
Trends in mf prevalence over time (2000–2014)
Community-level mf prevalence data are presented by calendar year (Fig 4A). The impact of MDA on mf prevalence cannot clearly be seen from this figure, due to the differences between communities in start year of MDA. In Fig 4B, therefore, the same data are presented by time since first treatment, while Fig 4C presents these data in boxplots to better visualize the distribution of the observed community-level mf prevalence. The variation in baseline prevalence was large (ranged between 0–45.7% with interquartile range of 0.46–22.9%). The mean and median mf prevalence in surveyed communities declined strongly with increasing duration of MDA. Although 6–7 years after the onset of MDA the median prevalence had fallen below 1%, the variation was still large (range = 0–20.8%; interquartile range = 0–1.5%) between communities and many communities still had mf prevalence levels above 5% (Fig 4C). Yet, we still see a continued decline in the maximum observed prevalence levels with increasing duration of MDA. In most communities with multiple measurement the mf prevalence steadily decreased over time, but 12 out of 78 (15%) communities had at least once an increase between 2 time points (Fig 4A & 4B). In the 2013 and 2014 surveys, 7 and 10 communities respectively were identified with mf prevalence still above 1% (maximum 5.6%). Fifteen out of these 17 communities with threshold above 1% are within 13 districts (out of 17 districts) where MDA was still ongoing by 2016.
In 34 districts, one or more communities were surveyed at least twice during the period of MDA. Data for these districts are shown in supplementary file S1 Fig. When community data were aggregated at district level, there was a general decrease in average mf prevalence over time to approach zero in most districts (S1 Fig, red line). In 4 districts (Bongo, Jirapa, Lambussie-K and Lawra) there were slight increases in mf prevalence after baseline before decreasing steadily. Almost all the districts we assessed, apart from two (Lawra and Wa-West), showed mf prevalence less than 5% after 6 years of MDA (S1 Fig, supplementary data). In 31 out of these 34 districts (91%), mf prevalence eventually fell below <1% after 6–14 rounds of treatment; this was not the case in three districts (Bole, Jirapa and Wa-West) where the mf prevalence was still ≥1% in 2013 or 2014 and MDA still ongoing by 2016. 51 out of the 78 examined districts/IUs (65%) needed more than 6 rounds of MDA to reach mf prevalence of <1%. There was a moderately positive correlation (correlation coefficient = + 0.5) between baseline mf prevalence versus years of MDA required for each district (limited to districts that have stopped MDA) [S2 Fig, supplementary data].
Discussion
Ghana has made good progress towards elimination since the start of its elimination programme in 2000. The baseline mf prevalence in sentinel sites was 8.7% on average, ranging from 0 to 45.7%. The mf prevalence declined steeply during the first few years after starting MDA in communities, followed by a more gradual decline thereafter (Fig 4A). Surveys performed after 6–7 rounds of MDA showed high variation between communities in mf prevalence, with the mf prevalence often exceeding 1% or even 5% (Fig 4B & 4C). By the end of 2016, 81 out of 98 endemic districts had stopped mass drug administration (MDA) after an average of 11 rounds (range 8–14) and treatment ongoing in 17 districts.
We have created a unique longitudinal database on the long-term impact of MDA for lymphatic filariasis (LF) elimination in Ghana, containing data from 430 sentinel and spot-check sites. There are at least 12 countries that have reported longitudinal trend data on at least 3 microfilaria (mf) prevalence surveys of LF after at least 3 rounds of MDA, of whom 5 in Africa: Tanzania, Kenya, Nigeria, Egypt and Mali [26–31]. These African studies have reported the impact of 4–10 rounds of MDA on antigenaemia/mf prevalence within 4–20 sentinel/study sites where about 50–2000 participants were tested per year. Since we have more data (15 years MDA, 430 communities, 1,784–19,268 participants), this gives us more insight into the impact of MDA on mf prevalence, the dynamics involved over a period of time and the variability in outcomes between sites.
At country-level we observed a huge variation in baseline endemicity level and trends towards reaching the threshold below 1% (Fig 4A & 4B). The mean and median mf prevalence in surveyed communities declined strongly with increasing duration of MDA. The small increase in median prevalence observed 1 year after the onset of sampling is a selection effect and does not indicate a lack of impact, because surveys were only done in districts with a relatively high baseline mf prevalence. The number of districts and communities surveyed declines over time, because districts that have stopped MDA were no longer included in surveys. In addition, surveys were selectively performed in communities in districts with low reported coverage or relatively high prevalence in previous surveys. For these reasons, trends in mean and median mf prevalence of surveyed villages during later stages of the control are difficult to interpret. Patterns became clearer with less variation within districts when we plotted and analyzed data by districts (S1 Fig). For some districts only few observations were available, especially in districts with relatively low baseline prevalence, where mf prevalence was relatively rapidly reduced below 1%, obviating the need for further surveys.
When GPELF was initiated, it was expected that 5 or 6 rounds of MDA with good coverage–≥65% would be sufficient to eliminate LF as a public health problem [3,32]. Although few countries were indeed able to reach the goal within the 5–6 years of MDA [2], the required treatment duration in Ghana was always longer, often considerably longer. This experience can help other African countries with planning their interventions. Previous modelling studies already suggested that 5–6 rounds of MDA would not be enough in case of low coverage and/or high baseline endemicity [7,14–16], and the same factors may explain why the required treatment duration in some Ghanaian districts is much longer than in others [13]. In districts with relatively high baseline mf prevalence, sometimes many rounds of MDA were needed to ensure that mf prevalence reach below 1%. Our data shows a moderately positive correlation between baseline mf prevalence versus years of MDA required for each district (S2 Fig). However, the decision to stop MDA was at the district level (IU), and therefore also influenced by other communities. Additionally, the programme in Ghana did not evaluate yearly to assess whether treatment could be stopped. These factors could have influenced the timelines somehow.
Our data confirm the importance of baseline endemicity for the required treatment duration, but the role of coverage was more difficult to proof. Reported coverage data at community level were collated for all endemic districts of Ghana (Fig 3). Although the reported coverage was good for the majority of communities, coverage levels <50% are also frequently encountered. However, such data are notoriously unreliable, as also becomes clear from the frequent occurrence of reported coverage levels >100% and hence difficult to interpret [33]. Low coverage is problematic, particularly if it is sustained over multiple treatment rounds. We could not assess the importance of this phenomenon in our data, as it appeared difficult to match coverage data from subsequent years at community level and to match them to the mf prevalence data.
The high variation in mf prevalence after a given number of treatment rounds within districts, as observed in Ghana (this study) and elsewhere [5–7] complicates decision making. If communities with high residual mf prevalence are by chance not included in surveys, MDA may be stopped prematurely with danger of resurgence [5]. This could be prevented by targeting pre-TAS surveys to communities at high risk of residual transmission. High risk may occur due to programmatic or demographic factors [12], including migration during treatment period, treatment fatigue, high numbers of middle aged women (child bearing age; majority not taking drug due to pregnancy) etc.
Other local factors contributing to transmission may be high biting rate of the mosquitoes and behavior of residents that influence exposure to mosquito bites [16,34]. The vectorial capacities of different LF vector species influence the ability to interrupt transmission using MDA in different settings [35]. In Ghana, for example, high biting rates of the major vector An. gambiae and initial infection prevalence, coupled with high densities of other vector species such as An. melas and Mansonia have explained the ongoing LF transmission in some hotspot communities [36]. The impact of bednets on mf prevalence cannot be overruled. Bednet coverage in Ghana was <25% throughout the country until 2005. This varied between 25% − 50% in 2005–2010; and further increased after 2010 [37]. Bednet coverage leads to a gradual reduction in mf prevalence, due to the long lifespan of worms, whereas immediate strong declines results from MDA. Trends in mf prevalence are therefore mostly defined by MDA and not by bednet usage. Bednets however, may be crucial in the end stage to prevent resurgence of infection after stopping MDA.
Although TAS has been designed to cover a larger geographical area with the hope that pockets with residual transmission would be identified in TAS surveys, it is unclear whether TAS is sufficiently sensitive to pick up such pockets. This is because not all communities within the evaluation unit (district) are sampled, furthermore, some have reported transmission ongoing in spite of passing TAS [5,6]. Thus, the validity of TAS for longer-term post-MDA surveillance requires further investigation [38]. We could not assess this in the current study, as most districts are usually not resurveyed shortly after stopping MDA.
Our data had some limitations. Firstly, we only considered mf data, as antigenaemia prevalence data were not always collected. Survey sites (apart from spot-check sites) were not randomly chosen, but rather based on previous results and location, and most mf sites have been surveyed only once. The low number of persons sampled combined with less sensitive mf tests in early years makes the mf prevalence observed in early years less reliable (wide 95% CI, data for baseline shown in supplementary data, S1 Table). Also, the selection of participants for night blood collection in each community was also not random since some households were more likely to attend than others. This is particularly problematic if those not participating in surveys are also more likely not to participate in MDA, resulting in biased and possibly flattered mf prevalence estimates. The mf prevalence data did not provide details such as age and sex, therefore the depth of analysis was limited.
Conclusions and recommendations
The Ghana Filariasis Elimination programme has had large impact, reducing mf prevalence <1% in 81/98 endemic districts. The remaining 17 districts still need MDA but also seem to be approaching this target. There was variation in the required treatment rounds between and within districts. Stopping MDA must be done with caution, taking into account the risk that communities with residual transmission remain which could present a source for the resurgence of infection after stopping MDA. Monitoring at the community level is required to be maintained to sustain the gains that have already been made towards elimination of LF in Ghana.
Supporting information
(XLSX)
(TIF)
(TIF)
(DOCX)
(DOCX)
Acknowledgments
We acknowledge the support from the chiefs, opinion leaders and the community drug distributors in the various communities for mobilizing participants during treatment and mf surveys. We also acknowledge the contributions from the NTD Programme team of the Ghana Health Services (GHS) for planning, implementing and coordinating all field activities and collation of data made available for this article.
Data Availability
Data is available in the Ghana Health Service NTD Program’s in-country database and on the WHO ESPEN Portal (http://espen.afro.who.int/countries/ghana) at the African Regional level.
Funding Statement
The authors received no specific funding for this work. MDA within the endemic communities in Ghana over the years has received financial and technical support from the Government of Ghana, USAID’s NTDCP and End in Africa Projects, FHI360, Sightsavers (Ghana), Liverpool School of Tropical Medicine’s Centre for Neglected Tropical Diseases (LSTM/CNTD) and the Volta River Authority. SV, PK, SJdV and WS acknowledge funding by the Bill and Melinda Gates Foundation in partnership with the Task Force for Global Health through the NTD Modelling Consortium (OPP1053230). Data collation, organization, and analyses were also supported through the WHO-TDR fellowship awarded to KKF and the collaborative activities of the GHS, NMIMR and Erasmus MC institutions.
References
- 1.Michael E, Bundy DA, Grenfell BT (1996) Re-assessing the global prevalence and distribution of lymphatic filariasis. Parasitology 112 (Pt 4): 409–428. [DOI] [PubMed] [Google Scholar]
- 2.WHO (2015) Global programme to eliminate lymphatic filariasis: progress report, 2014. Wkly Epidemiol Rec 90: 489–504. [PubMed] [Google Scholar]
- 3.Ottesen EA (2000) The global programme to eliminate lymphatic filariasis. Trop Med Int Health 5: 591–594. [DOI] [PubMed] [Google Scholar]
- 4.WHO (2017) Summary of global update on preventive chemotherapy implementation in 2016: crossing the billion. Weekly epidemiological records 40: 589–608. [PubMed] [Google Scholar]
- 5.Rebollo MP, Mohammed KA, Thomas B, Ame S, Ali SM, et al. (2015) Cessation of mass drug administration for lymphatic filariasis in Zanzibar in 2006: was transmission interrupted? PLoS Negl Trop Dis 9: e0003669 10.1371/journal.pntd.0003669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rao RU, Samarasekera SD, Nagodavithana KC, Punchihewa MW, Dassanayaka TD, et al. (2016) Programmatic Use of Molecular Xenomonitoring at the Level of Evaluation Units to Assess Persistence of Lymphatic Filariasis in Sri Lanka. PLoS Negl Trop Dis 10: e0004722 10.1371/journal.pntd.0004722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Subramanian S, Perumal V, Adinarayanan S, Kaliannagounder K, Rengachari R, et al. (2012) Epidemiological assessment of eight rounds of mass drug administration for lymphatic filariasis in India: implications for monitoring and evaluation. PLoS Negl Trop Dis 6: e1926 10.1371/journal.pntd.0001926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gyapong JO, Adjei S, Sackey SO (1996) Descriptive epidemiology of lymphatic filariasis in Ghana. Trans R Soc Trop Med Hyg 90: 26–30. 10.1016/s0035-9203(96)90466-6 [DOI] [PubMed] [Google Scholar]
- 9.Gyapong JO, Badu JK, Adjei S, Binka F (1993) Bancroftian filariasis in the Kassena Nankana District of the upper east region of Ghana: a preliminary study. J Trop Med Hyg 96: 317–322. [PubMed] [Google Scholar]
- 10.Gyapong JO, Dollimore N, Binka FN, Ross DA (1995) Lay reporting of elephantiasis of the leg in northern Ghana. Trans R Soc Trop Med Hyg 89: 616–618. 10.1016/0035-9203(95)90410-7 [DOI] [PubMed] [Google Scholar]
- 11.Gyapong JO, Kyelem D, Kleinschmidt I, Agbo K, Ahouandogbo F, et al. (2002) The use of spatial analysis in mapping the distribution of bancroftian filariasis in four West African countries. Ann Trop Med Parasitol 96: 695–705. 10.1179/000349802125001735 [DOI] [PubMed] [Google Scholar]
- 12.Biritwum NK, de Souza DK, Marfo B, Odoom S, Alomatu B, et al. (2017a) Fifteen years of programme implementation for the elimination of Lymphatic Filariasis in Ghana: Impact of MDA on immunoparasitological indicators. PLoS Negl Trop Dis 11: e0005280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Biritwum N-K, Yikpotey P, Marfo BK, Odoom S, Mensah EO, et al. (2017b) Persistent ‘hotspots’of lymphatic filariasis microfilaraemia despite 14 years of mass drug administration in Ghana. Trans R Soc Trop Med Hyg 110: 690–695. [DOI] [PubMed] [Google Scholar]
- 14.Jambulingam P, Subramanian S, de Vlas SJ, Vinubala C, Stolk WA (2016) Mathematical modelling of lymphatic filariasis elimination programmes in India: required duration of mass drug administration and post-treatment level of infection indicators. Parasit Vectors 9: 501 10.1186/s13071-016-1768-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kastner RJ, Stone CM, Steinmann P, Tanner M, Tediosi F (2015) What Is Needed to Eradicate Lymphatic Filariasis? A Model-Based Assessment on the Impact of Scaling Up Mass Drug Administration Programs. PLoS Negl Trop Dis 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stolk WA, Walker M, Coffeng LE, Basanez MG, de Vlas SJ (2015) Required duration of mass ivermectin treatment for onchocerciasis elimination in Africa: a comparative modelling analysis. Parasit Vectors 8: 552 10.1186/s13071-015-1159-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gyapong JO, Remme JH (2001) The use of grid sampling methodology for rapid assessment of the distribution of bancroftian filariasis. Trans R Soc Trop Med Hyg 95: 681–686. 10.1016/s0035-9203(01)90115-4 [DOI] [PubMed] [Google Scholar]
- 18.Gyapong M, Gyapong JO, Owusu-Banahene G (2001) Community-directed treatment: the way forward to eliminating lymphatic filariasis as a public-health problem in Ghana. Ann Trop Med Parasitol 95: 77–86. 10.1080/00034980020035942 [DOI] [PubMed] [Google Scholar]
- 19.WHO (2000) Preparing and implementing a national plan to eliminate lymphatic filariasis (in countries where onchocerciasis is co-endemic). WHO/CDS/CPE/CEE/200016: 25–27.
- 20.WHO (2005) Monitoring and epidemiological assessment of the programme to eliminate lymphatic fi lariasis at implementation unit level. WHO/CDS/CPE/CEE/200550.
- 21.WHO (1997) Bench Aids for the diagnosis of filarial infections. http://appswhoint/iris/bitstream/10665/37156/1/9241544899_engpdf; Accessed on 21 June,2016.
- 22.McMahon JE, Marshall TF, Vaughan JP, Abaru DE (1979) Bancroftian filariasis: a comparison of microfilariae counting techniques using counting chamber, standard slide and membrane (nuclepore) filtration. Ann Trop Med Parasitol 73: 457–464. 10.1080/00034983.1979.11687285 [DOI] [PubMed] [Google Scholar]
- 23.WHO (2012b) Transmission assessment surveys in the Global Programme to Eliminate Lymphatic filariasis. http://appswhoint/iris/bitstream/10665/77690/1/WHO_HTM_NTD_PCT_2012_9_engpdf; Accessed on 13th May 2016. [PubMed]
- 24.WHO (2011) Monitoring and epidemiological assessment of mass drug administration in the GPELF; a manual for national elimination programmes. http://appswhoint/iris/bitstream/10665/44580/1/9789241501484_engpdf; Acessed on 21 June, 2016.
- 25.Weil GJ, Lammie PJ, Weiss N (1997) The ICT Filariasis Test: A rapid-format antigen test for diagnosis of bancroftian filariasis. Parasitol Today 13: 401–404. [DOI] [PubMed] [Google Scholar]
- 26.Mohammed KA, Molyneux DH, Albonico M, Rio F (2006) Progress towards eliminating lymphatic filariasis in Zanzibar: a model programme. Trends Parasitol 22: 340–344. 10.1016/j.pt.2006.05.010 [DOI] [PubMed] [Google Scholar]
- 27.Coulibaly YI, Dembele B, Diallo AA, Konate S, Dolo H, et al. (2015) The Impact of Six Annual Rounds of Mass Drug Administration on Wuchereria bancrofti Infections in Humans and in Mosquitoes in Mali. Am J Trop Med Hyg 93: 356–360. 10.4269/ajtmh.14-0516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Njenga SM, Mwandawiro CS, Wamae CN, Mukoko DA, Omar AA, et al. (2011) Sustained reduction in prevalence of lymphatic filariasis infection in spite of missed rounds of mass drug administration in an area under mosquito nets for malaria control. Parasit Vectors 4: 90 10.1186/1756-3305-4-90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ramzy RM, El Setouhy M, Helmy H, Ahmed ES, Abd Elaziz KM, et al. (2006) Effect of yearly mass drug administration with diethylcarbamazine and albendazole on bancroftian filariasis in Egypt: a comprehensive assessment. Lancet 367: 992–999. 10.1016/S0140-6736(06)68426-2 [DOI] [PubMed] [Google Scholar]
- 30.Richards FO, Eigege A, Miri ES, Kal A, Umaru J, et al. (2011) Epidemiological and entomological evaluations after six years or more of mass drug administration for lymphatic filariasis elimination in Nigeria. PLoS Negl Trop Dis 5: e1346 10.1371/journal.pntd.0001346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Simonsen PE, Derua YA, Magesa SM, Pedersen EM, Stensgaard AS, et al. (2014) Lymphatic filariasis control in Tanga Region, Tanzania: status after eight rounds of mass drug administration. Parasit Vectors 7: 507 10.1186/s13071-014-0507-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gyapong JO, Kumaraswami V, Biswas G, Ottesen EA (2005) Treatment strategies underpinning the global programme to eliminate lymphatic filariasis. Expert Opin Pharmacother 6: 179–200. 10.1517/14656566.6.2.179 [DOI] [PubMed] [Google Scholar]
- 33.de Souza DK, Yirenkyi E, Otchere J, Biritwum NK, Ameme DK, et al. (2016) Assessing Lymphatic Filariasis Data Quality in Endemic Communities in Ghana, Using the Neglected Tropical Diseases Data Quality Assessment Tool for Preventive Chemotherapy. PLoS Negl Trop Dis 10: e0004590 10.1371/journal.pntd.0004590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Irvine MA, Reimer LJ, Njenga SM, Gunawardena S, Kelly-Hope L, et al. (2015) Modelling strategies to break transmission of lymphatic filariasis—aggregation, adherence and vector competence greatly alter elimination. Parasit Vectors 8: 547 10.1186/s13071-015-1152-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.de Souza DK, Koudou B, Kelly-Hope LA, Wilson MD, Bockarie MJ, et al. (2012) Diversity and transmission competence in lymphatic filariasis vectors in West Africa, and the implications for accelerated elimination of Anopheles-transmitted filariasis. Parasit Vectors 5: 259 10.1186/1756-3305-5-259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pi-Bansa S, Osei JHN, Frempong KK, Elhassan E, Akuoko OK, et al. (2019) Potential factors influencing lymphatic filariasis transmission in "hotspot" and "control" areas in Ghana: the importance of vectors. Infect Dis Poverty 8: 9 10.1186/s40249-019-0520-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.The Malaria Atlas Project (2019) ITN Coverage. https://mapoxacuk/explorer/#/; Retreived on 27th April, 2019.
- 38.Chu BK, Deming M, Biritwum NK, Bougma WR, Dorkenoo AM, et al. (2013) Transmission assessment surveys (TAS) to define endpoints for lymphatic filariasis mass drug administration: a multicenter evaluation. PLoS Negl Trop Dis 7: e2584 10.1371/journal.pntd.0002584 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
(TIF)
(TIF)
(DOCX)
(DOCX)
Data Availability Statement
Data is available in the Ghana Health Service NTD Program’s in-country database and on the WHO ESPEN Portal (http://espen.afro.who.int/countries/ghana) at the African Regional level.