Abstract
Objective: Cell migration is an essential process in skin wound healing. Photodynamic therapy (PDT) enhances wound healing by photoactivating a photosensitizer with a specific wavelength of light. Cystic fibrosis transmembrane conductance regulator (CFTR) is an ion channel expressed in multiple layers of keratinocytes. Recent studies showed that the activation of CFTR-related downstream signaling affects skin wound healing. We examined whether indocyanine green (ICG)-mediated PDT-enhanced cell migration is related to CFTR activation.
Approach: The spatial and temporal expression levels of CFTR and proteins involved in focal adhesion, including focal adhesion kinase (FAK) and paxillin, were evaluated during cell migration in vitro and in vivo for wound healing.
Results: ICG-PDT-conditioned medium collected from cells exposed to 5 J/cm2 near-infrared light in the presence of 100 μg/mL ICG activated CFTR and enhanced HaCaT cell migration. The expression of phosphorylated FAK Tyr861 and phosphorylated paxillin in focal adhesions was spatially and temporally regulated in parallel by ICG-PDT-conditioned medium. Curcumin, a nonspecific activator of CFTR, further increased PDT-enhanced cell migration, whereas inhibition of CFTR and FAK delayed cell migration. The involvement of CFTR in ICG-PDT-enhanced skin wound healing was confirmed in a mouse back skin wound model.
Innovation: CFTR is a potential new therapeutic target in ICG-PDT to enhance wound healing.
Conclusion: ICG-PDT-enhanced cell migration may be related to activation of the CFTR and FAK pathway. Conditioned medium collected from ICG-PDT may be useful for treating patients with chronic skin ulcer by regulating CFTR expression in keratinocytes.
Keywords: cell migration, cystic fibrosis transmembrane conductance regulator, focal adhesion, photodynamic therapy, wound healing

Tak-Wah Wong, MD, PhD
Introduction
Every year in the United States, chronic wounds affect ∼6.5 million patients, and the government spends approximately $25 billion (U.S.) annually on medical treatments.1 It is estimated that 1–2% of people in developed countries will suffer a chronic wound during their lifetime.2 Human skin wound healing is a complex biological process that occurs in response to tissue injury.3 This process occurs in three overlapping phases, including inflammation, tissue formation, and tissue remodeling. Normal wound healing relies on the well-orchestrated combination of the sophisticated biologic and molecular events associated with cell migration, adequate circulation, nutrition, and immune responses. However, some chronic skin wounds caused by pressure, venous dysfunction, or diabetes mellitus exhibit healing difficulties.4 New treatments are urgently needed to improve wound healing.
Recently, systematic reviews have shown that photodynamic therapy (PDT) associated with a laser or LED light source improves human skin wound healing, possibly by inducing a localized acute inflammatory response.5 PDT is widely used to treat a variety of cancers, benign and malignant skin diseases, and wounds.5,6 PDT is a noninvasive procedure implemented by administering a photosensitizer, which is activated with light of defined wavelength in the presence of oxygen in the tissue. The photodynamic effects on cell targets are mainly attributed to the singlet oxygen/reactive oxygen species (ROS) generated during irradiation, which cause apoptosis and necrosis of the target cells.7 The photosensitizer tends to accumulate in organelles inside the cell and triggers selective cell death. Singlet oxygen/ROS are generated during irradiation through Type I and Type II mechanisms in mammalian cells and microbes.8,9 The treatment is commonly well tolerated by patients with minimal side effects. Most, if not all, photosensitizers currently in use are relatively costly10,11 and have an absorption peak in the visible light spectrum, limiting light penetration into the tissue and their applications in the clinic. Indocyanine green (ICG) has a high safety profile and has been approved by the United States Food and Drug Administration for use as a contrast agent in retinal and choroidal vascular imaging and liver function testing since 1956.12 ICG-mediated PDT has been applied in oncological research and antibacterial research since the early 2000s.13,14 ICG is a less costly photosensitizer with an absorption peak in the longer near-infrared (NIR) spectrum, indicating its treatment potential in PDT translational medicine.11 However, the mechanism underlying PDT-enhanced wound healing largely remains unknown, and the ICG-mediated PDT effect on wound healing has never been studied. Additionally, PDT requires a specific light source and photosensitizer, which are not available in all clinics. If key molecules can be identified in the PDT-conditioned medium, new drugs may be developed to treat chronic ulcers.
Cystic fibrosis (CF) is the most common genetically inherited disease among Caucasians. The disease is caused by mutations in the cystic fibrosis transmembrane conductance regulator (CFTR), which encodes a chloride-conducting transmembrane channel that regulates anion transport and mucociliary clearance in the airways. Functional failure of CFTR results in mucus retention and chronic infection, leading to local chronic airway inflammation that is harmful to the lungs.15 To date, more than 2,000 mutations in CFTR have been identified; of the known mutations, a deletion of phenylalanine at position 508 (Phe508del) is the most common in populations with northern European ancestry.15 Cell migration plays critical roles in multiple physiological and pathophysiological processes in tissue repair. During cell migration, both the composition and morphology of the focal adhesion exhibit dynamic changes.16 The number, size, distribution, and function of focal adhesions are regulated by site-specific phosphorylation of focal adhesion kinase (FAK), c-Src, paxillin, and p130Cas.17 In halide killifish, CFTR and phosphorylated FAK are colocalized in the apical membrane and subjacent membrane vesicles of mitochondria-rich salt-transporting cells.18
Most wound healing studies have focused on how CFTR affects airway epithelial repair. Although the skin sweat chloride test remains the gold standard for CF diagnosis,19 little is known about how CFTR affects skin physiology other than sweating. The expression of CFTR in the epidermis in both humans and mice was not reported until recently.20 Dong et al. found that delta F508cftr−/− mice with defective CFTR exhibited delayed wound healing compared with wild-type mice and CFTR did not appear to have ion channel function in keratinocytes.20 The physiological functions of CFTR in ICG-PDT-enhanced cell migration in vitro and skin wound healing have not been evaluated.
In this study, we investigated how CFTR is involved in ICG-mediated PDT-regulated cell migration in vitro and in skin wound healing.
Clinical Problem Addressed
Chronic skin ulcer remains a major challenge to the health care system globally. Despite advances in biotechnology, many chronic ulcers, such as venous ulcer and diabetic ulcer, show healing difficulties. PDT appears to be a new therapeutic modality for enhancing wound healing, and many clinical trials have shown promising results. However, the specific mechanism induced by PDT in wound healing is not completely understood. Searching for a new target for this treatment may help to improve skin wound healing. The role of CFTR in skin wound healing was not explored until recently. In this study, we found that ICG-PDT enhances in vitro cell migration and wound healing in mice, which may be related to activation of CFTR. ICG-PDT is safe and inexpensive and the conditioned medium is easy to collect. Therefore, CFTR might be a potential target in ICG-PDT-conditioned medium to improve wound healing in patients with chronic ulcer.
Materials and Methods
PDT system
The PDT system was modified from a previous report.11 It was composed of a NIR lamp on top of a metal box to reflect light. A plate or dish containing cells was placed at the center during irradiation. ICG has been reported to act as a photothermal agent when activated with NIR irradiation. It can produce heat up to 48.5°C to kill hepatocellular carcinoma in a mouse model.21 To avoid photothermal effects, the irradiation system was maintained at 37°C ± 1°C by an electric fan. The NIR lamp (PAR38E; Philips, Amsterdam, The Netherlands) emits infrared light at a wavelength ranging from 700 to 2,200 nm and peaking at 1,100 nm. The light power on the irradiated surface was set at 65.5 mW/cm2 at 780 nm by adjusting the distance of the lamp and confirmed by a power meter (TD300-3W-V1-SENSOR; Ophir, Jerusalem, Israel).
Chemicals and photosensitizer
ICG (Diagnogreen; Daiichi Sankyo, Taipei, Taiwan) was dissolved in sterile water following the manufacturer's instructions before use as a photosensitizer.11 Curcumin and CFTR inhibitor 172 were dissolved in dimethyl sulfoxide (DMSO) (final concentrations of DMSO was less than 0.1%) immediately before use. FAK inhibitor 14 was dissolved in Milli-Q water. All chemicals were purchased from Sigma (MO).
Cells and PDT-conditioned medium
Skin wound healing combines the production of extracellular matrix by fibroblasts in the dermis and cell migration of keratinocytes in the epidermis. To study how ICG-PDT-conditioned medium affects keratinocyte migration, an immortal HaCaT keratinocyte cell line purchased from American Type Culture Collection (ATCC) was used in the experiments. HaCaT cells (5 × 105) were seeded in a 2.5-cm dish and grown for 24 h in Dulbecco's modified Eagle's medium (DMEM) complete medium. The cells were incubated with 100 μg/mL ICG for 10 min. After washing with phosphate-buffered saline (PBS), the cell culture was replaced with 1 mL of fresh DMEM (without phenol red) containing 10% fetal bovine serum before exposure to different doses of NIR (0, 2.5, 5, and 15 J/cm2). After exposure to light, the dish was incubated for 6 h in an incubator. The supernatant (ICG-PDT-conditioned medium) was collected and stored at −20°C until used.
Cytotoxicity
A total of 5 × 104 cells/100 μL were seeded into a well of a 96-well plate and grown for 24 h at 37°C. The 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT; Sigma Aldrich, MO) assay was used to determine cell cytotoxicity after exposure to different chemicals or ICG-PDT treatments. After treatment, a final concentration of 0.5 mg/mL MTT was added to each well for 2–4 h and an absorbance at 590 nm was measured. ICG-PDT-conditioned medium-induced cell death was measured with ethidium homodimer-1 (EthD-1, E1169; ThermoFisher Scientific, OR), a membrane-impermeable fluorescent dye, which binds to DNA. The dye enters a disrupted cell membrane and emits red fluorescence after excitation at 528 nm.
In vitro cell migration assay
To study the effects of CFTR-related ICG-PDT-initiated cell migration in vitro, 5 × 105 HaCaT cells in 70 μL complete medium were seeded into silicon inserts (Culture-Insert 2 Well; Ibidi, Martinsried, Germany). The insert created a central gap in the cell sheet. Cells were rinsed once with PBS to remove debris after removal of the silicon insert after 16 h of culturing. The conditioned medium collected from different doses of ICG-PDT (0, 2.5, 5, and 15 J/cm2 NIR light exposure in the presence of 100 μg/mL ICG) was added to the cells with/without CFTR enhancer or inhibitor, and FAK inhibitor, respectively. The cells were cultured until total confluence. Cell migration was monitored and recorded with a digital camera every 3 h under a microscope (Olympus, Japan). The images were analyzed with ImageJ software (NIH).
Localization of CFTR and FAK in focal adhesion
After incubation with ICG-PDT-conditioned medium at different time points, the cells were fixed with 4% paraformaldehyde for 15 min and permeabilized with 0.1% Triton X-100 for 10 min. Nonspecific background was reduced with blocking solution (CAS-Block; Thermo Fisher, CA) for 1 h at room temperature. HaCaT cells were incubated with CFTR (No. ab2784; Abcam, Cambridge, United Kingdom) and phospho-FAK Y861 (No. 4084; Abcam) for 24 h at 4°C. The cells were washed with PBS every 5 min for 1 h and then incubated with Alexa Fluor-conjugated secondary antibody (Abcam) and Hoechst 33342 (Thermo Fisher) for 1 h at room temperature. Fluorescence images were captured under a confocal microscope (FV1000; Olympus). ImageJ was used to analyze the images.
Immunoblotting
Cell lysates were harvested in a cold radioimmune precipitation assay lysis buffer (50 mM Tris-Cl pH 7.4, 150 mM NaCl, 1% NP-40, 0.5% sodium deoxycholate, 1 mM PMSF, 1 mM NaF, and 1 mM Na3VO4) and stored at −80°C. Protein concentration was analyzed using the Lowry assay method, which used bovine serum albumin as a standard. Equal amounts of samples were electrophoresed on 6 − 10% SDS-PAGE gels and transferred to a nitrocellulose membrane (Amersham Biosciences, Aylesbury, United Kingdom). The membranes were blocked with 5% nonfat dry milk in washing buffer for 1 h at room temperature. After blocking, the membranes were then incubated with different primary antibodies, including CFTR (No. ACL-006; Alomone Labs), phospho-FAK Tyr861 (No. 4084; Abcam), total-FAK (No. 32658; Abcam), phospho-paxillin Tyr118 (No. AB3837; Chemicon, Thermo Fisher, CA), paxillin (No. AB3794; Chemicon, Thermo Fisher), and β-actin (No. 16039; Abcam) for 24 h at 4°C. Immunocomplexes were detected by incubation with 1:5,000 dilutions of horseradish peroxidase-conjugated IgG antibodies (Abcam) for 1 h at room temperature. The bands were visualized by using an Enhanced Chemiluminescence Detection Kit (Amersham Biosciences) and western blotting detection system (ImageQuant LAS 4000, Germany). ImageJ (NIH) was used for images analysis.
Animal wound healing model
An incisional wound model is more predictive than excisional wound model to detect altered healing and thus can reduce the number of animals used in the experiments.22 A mouse central back incisional wound model23 was used to examine whether CFTR is involved in ICG-PDT enhancing skin wound healing in vivo. Twenty 8–11-week-old female C57BL/6 mice were purchased from the Laboratory Animal Center at National Cheng Kung University. Animals were kept in a temperature-controlled room with a 12-h day/12-h night cycle with food and water ad libitum. All experimental procedures were performed under approval from the Laboratory Animal Center of the University. Mice were anesthetized by intraperitoneal injection of a fivefold diluted solution of Zoletil 50 (0.5 mL/100 g body weight; Virbac, Carros, France) and xylazine (80 mg/kg body weight; Virbac). A 2-cm full-thickness skin wound was created on the central dorsal aspect of the mouse back. The wound was treated with ICG-PDT. After a 10-min incubation of 100 μg/mL ICG (20 μL) followed by PBS washing, wounds were exposed to 0, 5, and 15 J/cm2 NIR. The wounds were healed by secondary intention and were recorded with a digital camera every 2–3 days. Digital images were analyzed by ImageJ (NIH).
Histology, immunofluorescence staining
Mice were sacrificed by CO2 inhalation. The wounds were collected and bisected along the horizontal axis of the wounded skin. The tissue was fixed in 4% formaldehyde and embedded in paraffin for H&E staining. For immunofluorescence staining, sections were blocked with a blocking solution (Dako, CA) for 1 h at room temperature after antigen retrieval. The sections were incubated with primary antibodies overnight at 4°C and incubated with fluorescent conjugated secondary antibodies and 4′,6-diamidino-2-phenylindole (DAPI).
Statistical analysis
One-way or two-way analysis of variance (ANOVA) with post hoc tests were used to analyze the data using GraphPad Prism (Version 7.0; GraphPad Software, CA). Results were expressed as the mean with standard deviations. At least three separate, independent experiments in duplicate for all in vitro experiments and six replicates in two separate animal experiments were done to reach significant results. A p-value of less than 0.05 was considered statistically significant.
Results
ICG-PDT-conditioned medium affects HaCaT cell migration
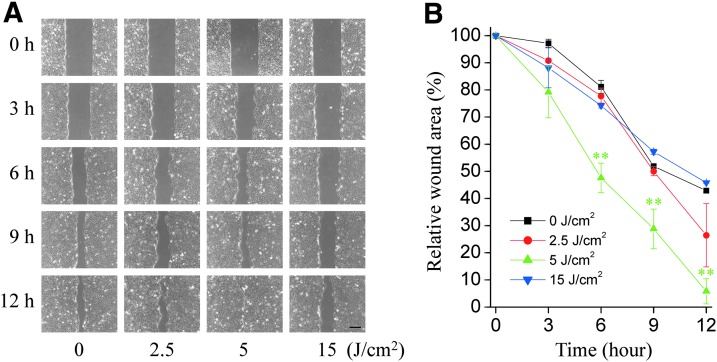
To investigate whether ICG-PDT promotes cell migration, the conditioned medium collected after exposure to different doses of PDT (NIR irradiation at 0, 2.5, 5, and 15 J/cm2 in the presence of 100 μg/mL ICG) was added to the HaCaT cell sheet with a gap in the center (Fig. 1A). The cell gaps became smaller at as early as 3 h and healed at 12 h as compared with those in the other groups (**p < 0.01 at 6, 9, and 12 h) after incubation with conditioned medium collected from the 5 J/cm2 PDT sample. A higher ICG-PDT dose of 15 J/cm2 did not enhance cell migration (Fig. 1B). These results suggest that there is an optimal level of ICG-PDT stress for enhancing cell migration. The conditioned medium from 5 J/cm2 PDT was used for further experiments. Supplementary Figure S1 shows the controls of ICG-PDT from Figure 1, including the absolute control (no ICG, no light), dark control (incubated cells with ICG without exposure to light), and light control (light exposure without ICG). Supplementary Figure S2 shows that a higher light dose (15 J/cm2) induced cell death, which may explain the inhibition of cell migration under this condition as shown in Figure 1.
Figure 1.
ICG-PDT-conditioned medium enhances cell migration. (A) A gap was created at the center of a HaCaT cell sheet. The cells were incubated with ICG-PDT-conditioned medium collected from different ICG-PDT conditions (0, 2.5, 5, and 15 J/cm2 with 100 μg/mL ICG). The gaps were monitored with a digital camera at different time points until one of the gaps was completely healed. (B) Data were pooled from three separate experiments (**p < 0.01 compared with the control, two-way ANOVA, Bonferroni comparison test). Scale bar = 100 μm. ANOVA, analysis of variance; ICG, indocyanine green; PDT, photodynamic therapy.
CFTR and FAK expression in focal adhesion
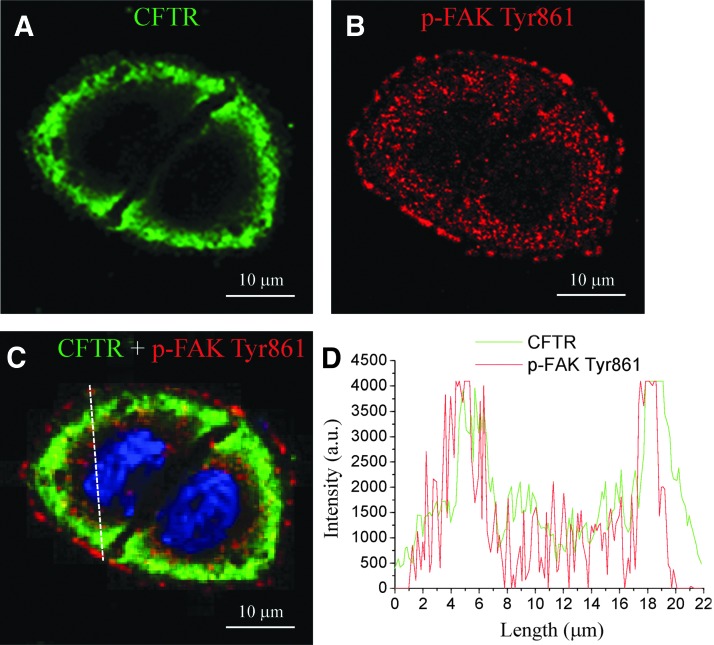
To investigative the spatial relationship of CFTR and FAK, both proteins were examined under a confocal microscope after 6 h of incubation in 5 J/cm2 ICG-PDT-treated conditioned medium. Figure 2 shows representative confocal images from HaCaT cells expressing CFTR (green spots in two connected cells; Fig. 2A), phospho-FAK Tyr861 (red; Fig. 2B), and a merge of both images (Fig. 2C). The merged fluorescent images and graph images highlight the colocalization of CFTR and phospho-FAK Tyr861 in the focal adhesion structure (Fig. 2D).
Figure 2.
Colocalization of CFTR and phosphorylated FAK Tyr861. (A) Representative confocal images from HaCaT cells expressing CFTR (green) in two connected cells from three separated experiments. (B) Phospho-FAK Tyr861 (p-FAK Tyr861; red) and (C) merge were examined 6 h after incubation with ICG-PDT-treated (5 J/cm2 and 100 μg/mL ICG) conditioned medium. (D) Line scan of fluorescence intensity (arbitrary unit, a.u.) showing high colocalization of CFTR and phospho-FAK Tyr861. Scale bar = 10 μm. CFTR, cystic fibrosis transmembrane conductance regulator; FAK, focal adhesion kinase.
Temporal and spatial expression of CFTR, phospho-FAK Tyr861, and paxillin
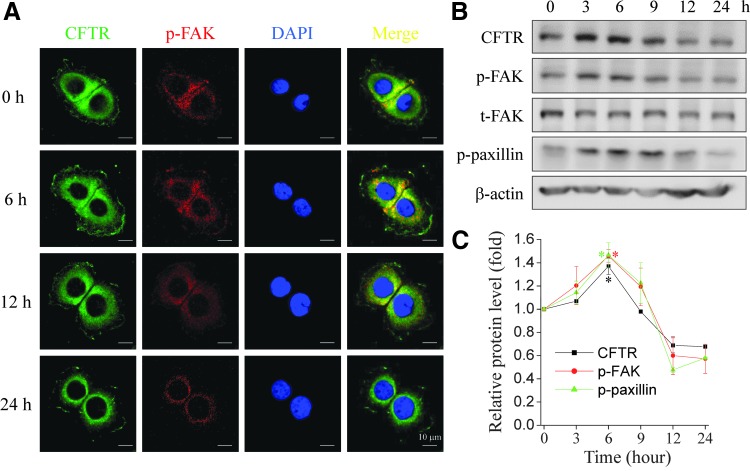
The temporal and spatial expression of CFTR, phospho-FAK, and phospho-paxillin in HaCaT cells after exposure to conditioned medium (collected from 5 J/cm2 NIR with 100 μg/mL ICG) was examined by fluorescence microscopy and immunoblotting. Immunofluorescence staining revealed that CFTR and phospho-FAK Tyr 861 were colocalized at 6 h after conditioned medium treatment (Fig. 3A). Western blotting showed that CFTR, phospho-FAK, and phospho-paxillin increased by as early as 3 h and peaked at 6 h. The proteins decreased thereafter and returned to the baseline level at 9 h after treatment with conditioned medium (Fig. 3B). The expression level of CFTR paralleled that of phospho-FAK and phospho-paxillin proteins in a temporal manner (Fig. 3C). These results suggest that ICG-PDT-promoted cell migration may be related to the activation of CFTR, which subsequently regulates the downstream FAK signaling pathway.
Figure 3.
Expression of CFTR, phospho-FAK Tyr861, and phospho-paxillin in HaCaT cells after treatment with ICG-PDT-conditioned medium. (A) Immunofluorescence images of HaCaT cells treated with ICG-PDT-conditioned medium (5 J/cm2 and 100 μg/mL ICG) at different time points. The cells were fixed and stained with the primary antibodies anti-CFTR (green) and phospho-FAK Tyr861 (p-FAK; red), and DAPI (blue). The figures show representative images from three separated experiments. (B) Representative immunoblots from three separate experiments revealed dynamic expressions of CFTR, phospho-FAK Tyr861 (p-FAK), total FAK (t-FAK), phospho-paxillin Tyr 118 (p-paxillin), and β-actin in HaCaT cells treated with the same conditioned medium. (C) The protein expression levels were quantified with ImageJ software (*p < 0.05, two-way ANOVA, Bonferroni comparison test). Scale bar = 10 μm. Figures show representative data from three separate experiments. DAPI, 4′,6-diamidino-2-phenylindole.
Activation or inhibition of CFTR affects cell migration
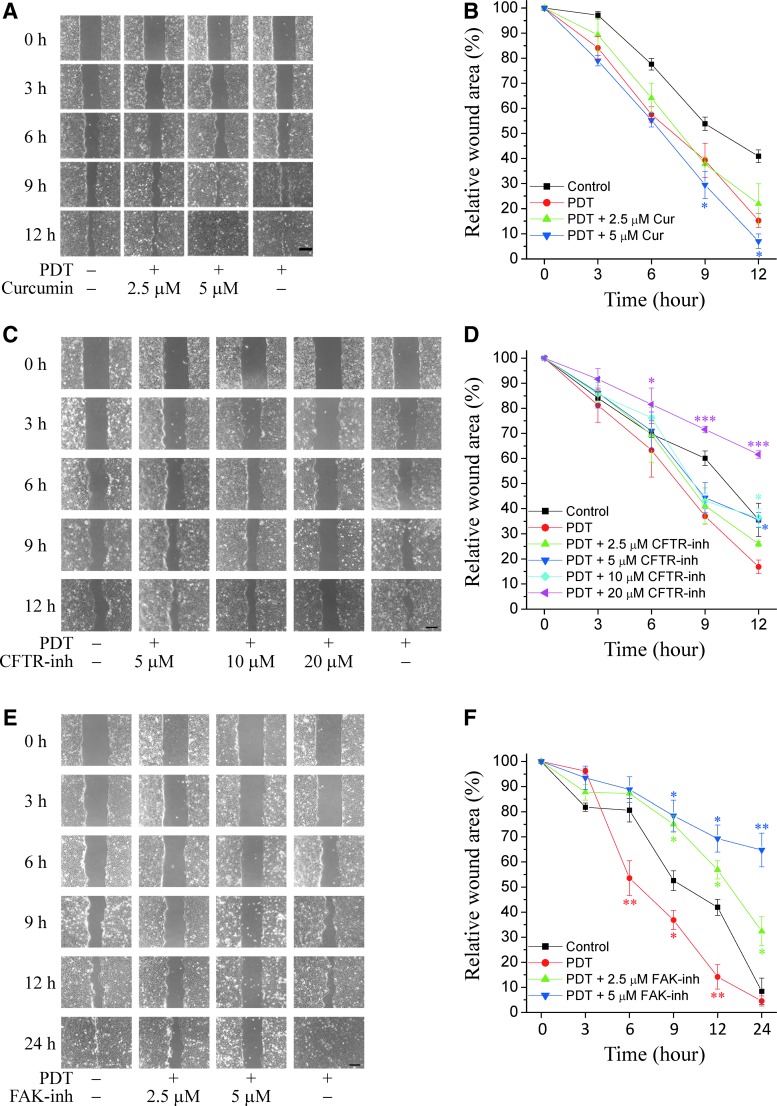
In addition to the increased CFTR expression by 1.5-fold at 6-h incubation following treatment with ICG-PDT-conditioned medium, as shown in Figure 3B, the role of CFTR in cell migration in response to ICG-PDT was further investigated by the activation or inhibition of the proteins using chemicals. Wild-type and mutant CFTR channels can be activated by curcumin.24 Curcumin, CFTR inhibitor 172,25 and FAK inhibitor 1426 were added to ICG-PDT-conditioned medium-treated HaCaT cells. The cytotoxicity of these chemicals was examined in an MTT assay with HaCaT cells in separate experiments (Supplementary Fig. S3). Curcumin concentrations lower than 10 μM (*p < 0.05, **p < 0.01 compared with the control; Supplementary Fig. S3A), CFTR inhibitor 172 concentrations less than 40 μM (Supplementary Fig. S3B), and FAK inhibitor 14 concentrations lower than 10 μM (Supplementary Fig. S3C) did not affect cell viability.
Incubation with 2.5 μM curcumin enhanced cell migration to a similar extent as 5 J/cm2 ICG-PDT-conditioned medium (Fig. 4A). Addition of 5 μM curcumin further enhanced cell migration compared with ICG-PDT-conditioned medium alone (9-h, *p < 0.05; Fig. 4B). Higher concentrations of curcumin (10 and 20 μM) inhibited cell migration (data not shown), which may be explained by the cytotoxic effects at these concentrations (Supplementary Fig. S3). Cell migration was delayed after incubation with 5 and 10 μM CFTR inhibitor 172 at 12 h (*p < 0.05, compared with ICG-PDT; Fig. 4C, D). The inhibition was more prominent at a higher concentration (20 μM, ***p < 0.001, compared with ICG-PDT, pink curve; Fig. 4D). Figure 4F shows the enhancement of cell migration (red curve, *p < 0.05, **p < 0.01, compared with the control) with ICG-PDT, whereas FAK-inhibitor 14 robustly inhibited cell migration at 2.5 and 5 μM (*p < 0.05, **p < 0.01, compared with ICG-PDT).
Figure 4.
Cell migration after CFTR activation and inhibition. (A) Cell migration in the presence of ICG-PDT-conditioned medium (5 J/cm2 and 100 μg/mL ICG) and curcumin (Cur). (B) Mean values of cell migration are expressed as % of wound areas. The addition of 5 μM curcumin to conditioned medium increased further cell migration at 12 h compared with PDT (time 0 as 100%, *p < 0.05). (C) Cell migration after addition of CFTR inhibitor 172 (CFTR-inh). (D) Mean values of cell migration in the presence of CFTR-inh and ICG-PDT-conditioned media. CFTR-inh at 5 and 10 μM inhibited cell migration at 12 h, whereas the robust inhibition at 20 μM may be attributed to cytotoxicity at this high concentration (time 0 as 100%, *p < 0.05, ***p < 0.001 compared with PDT, two-way ANOVA, Bonferroni comparison test). (E) Cell migration after addition of FAK inhibitor 14 (FAK-inh). (F) Mean values of cell migration in the presence of FAK-inh and ICG-PDT-conditioned media. PDT enhanced cell migration at 6-, 9-, 12-, 24-h compared with the control (time 0 as 100%, *p < 0.05, **p < 0.01, two-way ANOVA, Bonferroni comparison test). Addition of FAK-inh at the concentrations of 2.5 and 5 μM inhibited cell migration at 9-, 12-, and 24-h time points compared with the control. Results are expressed as % of wound areas (time 0 as 100%, *p < 0.05, **p < 0.01). Data are representative results from one of the three separate experiments.
ICG-PDT enhances wound healing in mice
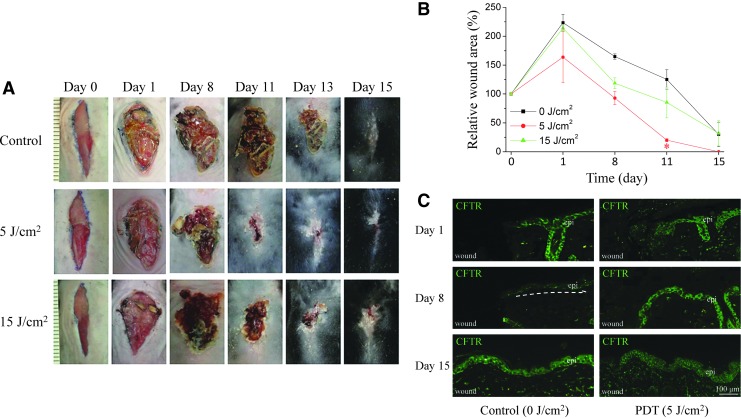
To determine the optimal ICG-PDT dosage for enhancing wound healing in vivo, irradiation of the wounds with 0, 5, and 15 J/cm2 PDT with different concentrations of ICG were evaluated on mouse skin. After exposure to 5 J/cm2 PDT and 100 μg/mL ICG, the wounds healed faster compared with those exposed to 15 J/cm2 PDT and the same concentration of ICG and the control (Fig. 5A). By day 11, ICG-PDT-treated wounds were reduced by 76%, compared with 35% in the controls (*p < 0.05; Fig. 5B). Figure 5C shows that CFTR increased on day 1 after PDT treatment and returned to normal levels on day 15 in the mouse epidermis.
Figure 5.
Wound closure in ICG-PDT-treated C57BL/6 mice. (A) A 2-cm wound on the central back of the mouse was treated with different light doses of PDT (0, 5, and 15 J/cm2) in the presence of 100 μg/mL ICG. ICG-PDT-treated wounds healed faster than wounds of the controls. The difference in wound closure was significant by day 11 postwounding. (B) Data were pooled from six mice in two independent experiments (*p < 0.05 compared with the control at day 11, two-way ANOVA, Bonferroni comparison test). (C) CFTR expression in the epidermis during wound healing in C57BL/6 mice. Representative immunofluorescence images showing the expression of CFTR of wound healing skin on different days after ICG-PDT treatment (0 and 5 J/cm2 with 100 μg/mL ICG).
Possible mechanism of CFTR regulates cell migration in ICG-PDT
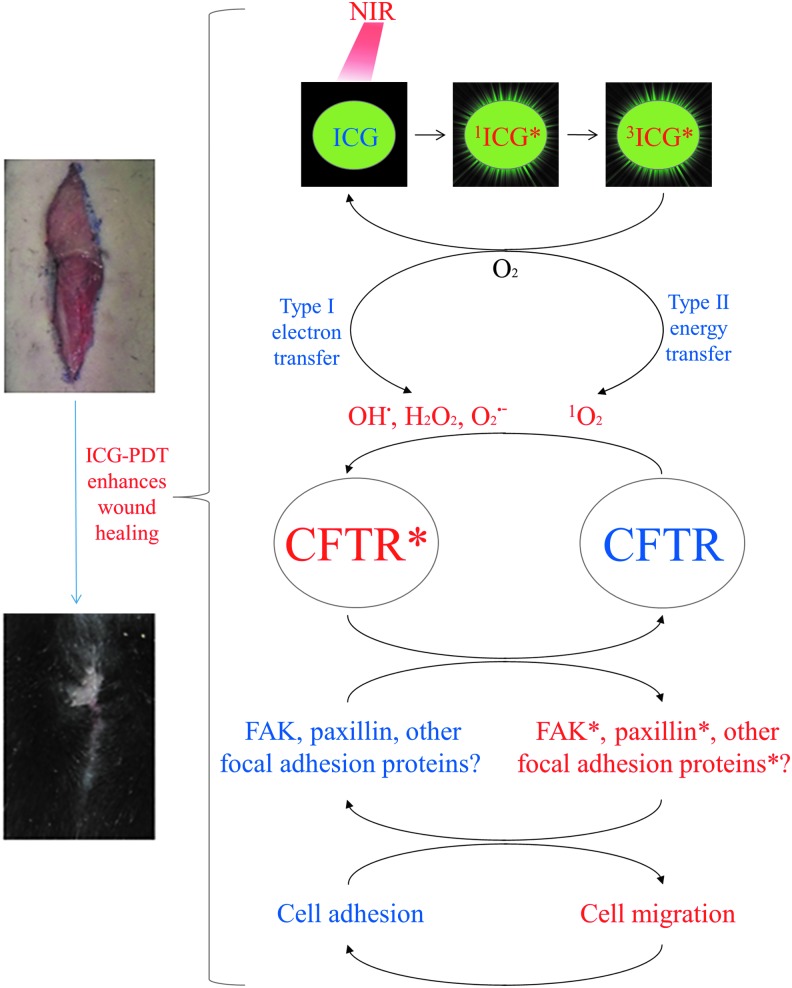
Figure 6 shows a schematic diagram summarizing the results of the present study, how ICG-PDT may affect CFTR, and downstream related signals involved in cell migration and wound healing. Oxidative stress induced by ICG-PDT through type I and type II reactions activates CFTR, which turns on downstream molecules in the focal adhesion and switches cell migration on/off.
Figure 6.
Schematic diagram showing the proposed mechanism by which ICG-PDT activates cell migration and wound healing by activating CFTR and the related downstream molecules in the focal adhesion.
Discussion
This study demonstrated that conditioned medium collected from ICG-mediated PDT with a low light dose (less than 15 J/cm2) enhanced cell migration in vitro and wound healing in mice. This enhancement may be related to the activation of CFTR, which affects the related downstream FAK signaling pathway. Our results agree with those of previous studies showing that a lower light dose is beneficial for cell migration and wound healing.5,27,28 Tedesco and Jesus used the photosensitizer silicon–naphthalocyanine in a liposomal formulation and irradiation with a laser at 670 nm with low light doses of 0.5, 1, 3, and 5 J/cm2 on an ex vivo human skin model.27 They found that low-dose (1 J/cm2) PDT prevented elastic network degradation. An even lower light dose (0.07 J/cm2) initiated the formation of new collagen, as reported by the same authors.28 In our model, 5 J/cm2 ICG-PDT improved cell migration, whereas 15 J/cm2 ICG-PDT inhibited cell migration in HaCaT cells. Similar effects were observed in mouse skin wound healing: a 15 J/cm2 light dose enhanced wound closure to a lesser extent than 5 J/cm2. We measured the 780-nm wavelength of the NIR lamp as a representative absorption peak of ICG. Notably, 810 nm may also activate ICG.29 The light power of the 810-nm irradiation was 64.8 ± 0.2 mW/cm2. Cells and mouse wounds may receive a nearly doubled light dose if this wavelength is accounted for in the effects. A low light-dose PDT is believed to produce better biostimulation,5 whereas a light dose greater than 30 J/cm2 is typically applied to kill cancer cells.6 Singlet oxygen/ROS is well known to have dual effects in cellular processes. Excessive singlet oxygen/ROS levels kill cells, whereas low levels of singlet oxygen/ROS affect cell signaling, particularly at the level of redox modulation.30 The low-dose ICG-PDT may produce a low level of singlet oxygen/ROS that ultimately promotes cell migration and skin wound healing through the activation of CFTR and modulation of different downstream cellular signaling events. The light dose for treating benign skin lesions, including acne, viral infection, and wound is usually around 10-times less than the light dose for treating a tumor in clinic.6 The therapeutic range in the present study on mouse wound is 5–15 J/cm2 with 100 μg/mL ICG. However, there could be a big difference between animal and human dose. There is no clinical report of topical ICG-PDT on wound healing in human and more studies are needed to clarify the optimal therapeutic range of ICG-PDT.
Huang et al. found that the CFTR/nuclear factor-κB-urokinase receptor signaling pathway plays a critical role in endometrial cell migration in patients with endometriosis.31 Chen et al. demonstrated that activation of epidermal CFTR leads to mitogen-activated protein kinase/nuclear factor-κB suppression, which alleviates inflammation, reduces proliferation, promotes the differentiation of human keratinocytes (HaCaT cells), and promotes cutaneous wound healing in CFTR mutant (DF508) mice.32 Our findings suggest that the activation of CFTR and subsequent regulated downstream FAK signaling can be attributed to ICG-PDT-enhanced cell migration. However, additional studies are needed to explore how CFTR affects inflammation, proliferation, and differentiation in wound healing following treatment with ICG-PDT.
CFTR is a well-studied ion channel target of curcumin. Both wild-type and mutant CFTR channels can be activated by curcumin. Exposure of different CFTR deletion constructs and airway epithelial cell lines to 30 μM curcumin for 30 min induced CFTR crosslinking.33 However, curcumin itself did not potentiate CFTR channels, whereas a cyclic derivative of curcumin with no crosslinking activity showed the opposite results.33 Curcumin not only allows ΔF508-CFTR to escape from the endoplasmic reticulum and to anchor in the plasma membrane, but also stimulates its channel activity once it reaches the plasma membrane34 and thus promotes cell migration. In contrast, another study showed that 10 μM curcumin retards cellular growth and migration by downregulating Src and FAK kinase activity.35 We found that 5 μM curcumin further increased cell migration in ICG-PDT-conditioned medium. Concentrations higher than and equal to 10 μM inhibited cell migration. A possible explanation is that CFTR activation was maximized by ICG-PDT and the addition of curcumin had only minimal effects on the activation. These results indicate that optimizing singlet oxygen/ROS in the cell is critical for cell motility.
To date, six tyrosine phosphor-acceptor sites in FAK have been identified: Tyr397, Tyr407, Tyr576, Tyr577, Tyr861, and Tyr925.36 We only studied phospho-FAK Tyr861. The other phosphorylated sites of FAK require further evaluation. ICG-PDT induces the expression of hundreds of genes and downstream proteins. Low doses of light with a photosensitizer clearly demonstrated the beneficial effects of PDT on wound healing. We examined CFTR as a possible regulator in ICG-PDT-enhanced cell migration and skin wound healing. PDT may activate CFTR, FAK, and paxillin in a synergistic manner during cell migration. A limitation of targeting CFTR is that the ICG-PDT-conditioned medium must be collected before treating a wound; additionally, molecules in the conditioned medium other than CFTR that may regulate wound healing have not been examined. Additional studies are needed to confirm whether our results reflect a general phenomenon in PDT by testing other photosensitizers.
Innovation
Many clinical trials have shown that PDT has positive effects in patients with chronic skin ulcers. In this study, we found that ICG-PDT activated CFTR and eventually enhanced cell migration and wound healing. CFTR is an attractive target in ICG-PDT-conditioned medium. Most, if not all, photosensitizers are costly and PDT light sources are not available in most clinics. ICG-PDT was shown to be safe and inexpensive. The conditioned medium from ICG-PDT is easy to collect. This approach will enable rapid translation to improve wound healing in patients with chronic ulcer.
Key Findings.
ICG-PDT-conditioned medium activates CFTR in keratinocytes.
Activated CFTR regulates other molecules in the focal adhesion, including FAK and paxillin to enhance cell migration.
Direct ICG-PDT treatment of mouse wounds enhanced wound healing, which is related to CFTR activation.
ICG-PDT-conditioned medium may be effective for treating chronic skin ulcer because of its high safety profile and easy accessibility.
Supplementary Material
Abbreviations and Acronyms
- ANOVA
analysis of variance
- CF
cystic fibrosis
- CFTR
cystic fibrosis transmembrane conductance regulator
- DMEM
Dulbecco's modified Eagle's medium
- DMSO
dimethyl sulfoxide
- FAK
focal adhesion kinase
- ICG
indocyanine green
- MTT
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- NIR
near-infrared
- PBS
phosphate-buffered saline
- PDT
photodynamic therapy
- ROS
reactive oxygen species
Acknowledgments and Funding Sources
The authors sincerely thank the Bioimaging Core Facility of the National Core Facility Program for Biopharmaceuticals, Ministry of Science and Technology, Taiwan for technical services. The study was supported by National Cheng Kung University [B106-K028, B107-K048], National Cheng Kung University Hospital [NCKUH107-030-19, NCKUH-10802008], Ministry of Science and Technology (MOST) of Taiwan [107-2321-B-006-008], and the Center of Applied Nanomedicine, National Cheng Kung University from the Featured Areas Research Center Program within the framework of the Higher Education Sprout Project by the Ministry of Education (MOE) in Taiwan to T.W.W. Ministry of Science and Technology of Taiwan [105-2628-B-006-003] to W.T.C.
Author Disclosure and Ghostwriting
The authors declare that no competing financial interests exist. The article was written by the authors listed. No ghostwriters were used to write this article.
About the Authors
Tak-Wah Wong, MD, PhD, is an associate professor in the Departments of Dermatology, Biochemistry, and Molecular Biology, Center of Applied Nanomedicine, Director of Photomedicine, Wound Clinic at National Cheng Kung University (NCKU), where his laboratory studies photomedicine and wound healing. Wen-Tai Chiu, PhD, is an associate professor in the Department of Biomedical Engineering at NCKU, where his laboratory studies the effects of store-operated Ca2+ entry. Ho-Kai Huang, MS, and Ying-Chi Chen, PhD, are students in Dr. Chiu's Laboratory. Thi-Tuong Vi Tran, MD, MS, is a dermatologist from Vietnam who studied in Wong's laboratory. Shin-Chen Pan MD, PhD, is an associate professor in the Department of Plastic Surgery at NCKU works in wound healing research.
Supplementary Material
References
- 1. Sen CK, Gordillo GM, Roy S, et al. Human skin wounds: a major and snowballing threat to public health and the economy. Wound Repair Regen 2009;17:763–771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jarbrink K, Ni G, Sonnergren H, et al. Prevalence and incidence of chronic wounds and related complications: a protocol for a systematic review. Syst Rev 2016;5:152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Singer AJ, Clark RA. Cutaneous wound healing. N Engl J Med 1999;341:738–746 [DOI] [PubMed] [Google Scholar]
- 4. Avishai E, Yeghiazaryan K, Golubnitschaja O. Impaired wound healing: facts and hypotheses for multi-professional considerations in predictive, preventive and personalised medicine. EPMA J 2017;8:23–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nesi-Reis V, Lera-Nonose D, Oyama J, et al. Contribution of photodynamic therapy in wound healing: a systematic review. Photodiagnosis Photodyn Ther 2018;21:294–305 [DOI] [PubMed] [Google Scholar]
- 6. Dougherty TJ. An update on photodynamic therapy applications. J Clin Laser Med Surg 2002;20:3–7 [DOI] [PubMed] [Google Scholar]
- 7. Dabrowski JM, Arnaut LG, Pereira MM, et al. Combined effects of singlet oxygen and hydroxyl radical in photodynamic therapy with photostable bacteriochlorins: evidence from intracellular fluorescence and increased photodynamic efficacy in vitro. Free Radic Biol Med 2012;52:1188–1200 [DOI] [PubMed] [Google Scholar]
- 8. Wong TW, Tracy E, Oseroff AR, Baumann H. Photodynamic therapy mediates immediate loss of cellular responsiveness to cytokines and growth factors. Cancer Res 2003;63:3812–3818 [PubMed] [Google Scholar]
- 9. Wong TW, Cheng CW, Hsieh ZJ, Liang JY. Effects of blue or violet light on the inactivation of Staphylococcus aureus by riboflavin-5'-phosphate photolysis. J Photochem Photobiol B 2017;173:672–680 [DOI] [PubMed] [Google Scholar]
- 10. Grieve R, Guerriero C, Walker J, et al. Verteporfin photodynamic therapy cohort study: report 3: cost effectiveness and lessons for future evaluations. Ophthalmology 2009;116:2471–2477 e2471–2472. [DOI] [PubMed] [Google Scholar]
- 11. Wong TW, Wu EC, Ko WC, Lee CC. Photodynamic inactivation of methicillin-resistant Staphylococcus aureus by indocyanine green and near infrared light. Dermatol Sinica 2018;36:8–15 [Google Scholar]
- 12. Fox IJ, Wood EH. Indocyanine green: physical and physiologic properties. Proc Staff Meet Mayo Clin 1960;35:732–744 [PubMed] [Google Scholar]
- 13. Urbanska K, Romanowska-Dixon B, Matuszak Z, Oszajca J, Nowak-Sliwinska P, Stochel G. Indocyanine green as a prospective sensitizer for photodynamic therapy of melanomas. Acta Biochim Pol 2002;49:387–391 [PubMed] [Google Scholar]
- 14. Omar GS, Wilson M, Nair SP. Lethal photosensitization of wound-associated microbes using indocyanine green and near-infrared light. BMC Microbiol 2008;8:111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Elborn JS. Cystic fibrosis. Lancet 2016;388:2519–2531 [DOI] [PubMed] [Google Scholar]
- 16. Zhao X, Guan JL. Focal adhesion kinase and its signaling pathways in cell migration and angiogenesis. Adv Drug Deliv Rev 2011;63:610–615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. De Pascalis C, Etienne-Manneville S. Single and collective cell migration: the mechanics of adhesions. Mol Biol Cell 2017;28:1833–1846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Marshall WS, Watters KD, Hovdestad LR, Cozzi RR, Katoh F. CFTR Cl- channel functional regulation by phosphorylation of focal adhesion kinase at tyrosine 407 in osmosensitive ion transporting mitochondria rich cells of euryhaline killifish. J Exp Biol 2009;212:2365–2377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Farrell PM, Rosenstein BJ, White TB, et al. Guidelines for diagnosis of cystic fibrosis in newborns through older adults: cystic Fibrosis Foundation consensus report. J Pediatr 2008;153:S4–S14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dong J, Jiang X, Zhang X, et al. Dynamically regulated CFTR expression and its functional role in cutaneous wound healing. J Cell Physiol 2015;230:2049–2058 [DOI] [PubMed] [Google Scholar]
- 21. Shirata C, Kaneko J, Inagaki Y, et al. Near-infrared photothermal/photodynamic therapy with indocyanine green induces apoptosis of hepatocellular carcinoma cells through oxidative stress. Sci Rep 2017;7:13958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ansell DM, Campbell L, Thomason HA, Brass A, Hardman MJ. A statistical analysis of murine incisional and excisional acute wound models. Wound Repair Regen 2014;22:281–287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Li WP, Su CH, Wang SJ, et al. CO2 delivery to accelerate incisional wound healing following single irradiation of near-infrared lamp on the coordinated colloids. ACS Nano 2017;11:5826–5835 [DOI] [PubMed] [Google Scholar]
- 24. Zhang X, Chen Q, Wang Y, Peng W, Cai H. Effects of curcumin on ion channels and transporters. Front Physiol 2014;5:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kelly M, Trudel S, Brouillard F, et al. Cystic fibrosis transmembrane regulator inhibitors CFTR(inh)-172 and GlyH-101 target mitochondrial functions, independently of chloride channel inhibition. J Pharmacol Exp Ther 2010;333:60–69 [DOI] [PubMed] [Google Scholar]
- 26. Golubovskaya V, Curtin L, Groman A, Sexton S, Cance WG. In vivo toxicity, metabolism and pharmacokinetic properties of FAK inhibitor 14 or Y15 (1, 2, 4, 5-benzenetetramine tetrahydrochloride). Arch Toxicol 2015;89:1095–1101 [DOI] [PubMed] [Google Scholar]
- 27. Tedesco A, Jesus P. Low level energy photodynamic therapy for skin processes and regeneration. In: Tanaka Y, ed. Photomedicine—Advances in Clinical Practice. Rijeka: InTech; 2017:Ch. 05 [Google Scholar]
- 28. de Jesus PD, Saeki SI, Tedesco AC. An ex vivo study of photobiostimulation in the treatment of skin pathologies. J Biophotonics 2016;9:1189–1198 [DOI] [PubMed] [Google Scholar]
- 29. Tian C, Chen X, Cao J, Yang L. Application of ICG-enhanced thermocoagulation method and photodynamic therapy in circumscribed choroidal hemangioma. Oncol Lett 2018;15:5760–5766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Martin KR, Barrett JC. Reactive oxygen species as double-edged swords in cellular processes: low-dose cell signaling versus high-dose toxicity. Hum Exp Toxicol 2002;21:71–75 [DOI] [PubMed] [Google Scholar]
- 31. Huang W, Jin A, Zhang J, et al. Upregulation of CFTR in patients with endometriosis and its involvement in NFkappaB-uPAR dependent cell migration. Oncotarget 2017;8:66951–66959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chen J, Chen Y, Chen Y, et al. Epidermal CFTR suppresses MAPK/NF-kappaB to promote cutaneous wound healing. Cell Physiol Biochem 2016;39:2262–2274 [DOI] [PubMed] [Google Scholar]
- 33. Bernard K, Wang W, Narlawar R, Schmidt B, Kirk KL. Curcumin cross-links cystic fibrosis transmembrane conductance regulator (CFTR) polypeptides and potentiates CFTR channel activity by distinct mechanisms. J Biol Chem 2009;284:30754–30765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Egan ME, Pearson M, Weiner SA, et al. Curcumin, a major constituent of turmeric, corrects cystic fibrosis defects. Science 2004;304:600–602 [DOI] [PubMed] [Google Scholar]
- 35. Leu TH, Su SL, Chuang YC, Maa MC. Direct inhibitory effect of curcumin on Src and focal adhesion kinase activity. Biochem Pharmacol 2003;66:2323–2331 [DOI] [PubMed] [Google Scholar]
- 36. Lim Y, Park H, Jeon J, et al. Focal adhesion kinase is negatively regulated by phosphorylation at tyrosine 407. J Biol Chem 2007;282:10398–10404 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.