Abstract
Various commensal enteric and pathogenic bacteria may be involved in the pathogenesis of inflammatory bowel diseases (IBDs), a chronic condition with a pathogenic background that involves both immunogenetic and environmental factors. IBDs comprising of Crohn’s disease, and ulcerative colitis, and pauchitis are chronic inflammatory conditions, and known for causing disturbed homeostatic balance among the intestinal immune compartment, gut epithelium and microbiome. An increasing trend of IBDs in incidence, prevalence, and severity has been reported during recent years. Probiotic strains have been reported to manage the IBDs and related pathologies, and hence are current hot topics of research for their potential to manage metabolic diseases as well as various immunopathologies. However, the probiotics industry will need to undergo a transformation, with increased focus on stringent manufacturing guidelines and high-quality clinical trials. This article reviews the present state of art of role of probiotic bacteria in reducing inflammation and strengthening the host immune system with reference to the management of IBDs. We infer that t healthcare will move beyond its prevailing focus on human physiology, and embrace the superorganism as a paradigm to understand and ameliorate IBDs.
Keywords: Gut Microbiome, Immunomodulation, Inflammatory bowel diseases, Probiotics
INTRODUCTION
The mammalian gut contains 300–500 different bacterial species, and overall concentration of microbial species inhabiting the human gastrointestinal (GI) tract may reach 1011–1012 cells per gram of luminal contents (Guarner and Malagelada, 2003). Under normal circumstances, majority of the GI symbionts are protective and some are neutral, but a few can turn pathogenic if gut physiology become abrupt (Sartor, 2004). This dynamic community of GI microbiome plays a critical role in maintaining intestinal health serving metabolic, digestive, trophic as well as protective functions (Guarner and Malagelada 2003). Probiotics when administered or ingested in specific quantities, exert beneficial physiologic and therapeutic health benefits besides their normal metabolic properties (Sartor 2004; Fioramonti et al., 2003). Examples of probiotic bacteria demonstrated to have beneficial effects include lactic acid bacteria (LAB), Bifidobacterium sp., Escherichia coli Nissle 1917, Streptococcus thermophilus and a non-pathogenic yeast, Saccharomyces boulardii etc. (Fioramonti et al., 2003; Kumar et al., 2009; 2010; 2011; 2012a, b, c; Nagpal et al., 2007; 2010; 2012a, b, c; Nagpal and Kaur, 2011). Potential mechanisms of probiotic action (Fig. 1) include competitive interactions, production of antimicrobial metabolites, detoxification of certain carcinogens reaching GI tract, influences on the epithelium, and immunomodulation (Table 1) (Sartor 2004, 2005; Dotan and Rachmilewitz 2005; Kumar et al., 2009; 2010; 2011; 2012a, b, c; Nagpal et al., 2007; 2010; 2012a, b, c; Nagpal and Kaur, 2011). such as Salmonella enteritica and Typhimurium induce host inflammation to facilitate their fitness. This species has recently been shown to proliferate under conditions of GI inflammation
FIGURE 1.
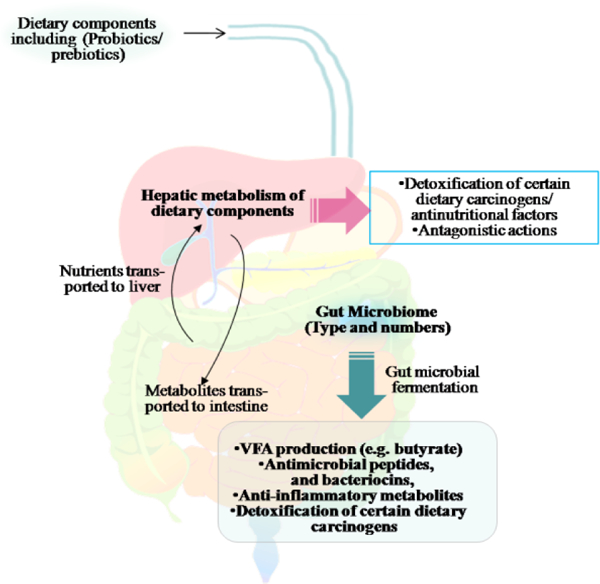
A dynamic relationship exists among the gut microbiome and dietary ingredients. Fermentation of food components by microbes occurs both during certain food production processes and in the gastrointestinal tract. Hepatic as well as gut microbial metabolisms play a crucial role in detoxification of anti-nutritional phyto-metabolites and certain dietary mutagens. In addition, hepatic as well as gut microbial metabolome can influence colon cancer risk and tumour behaviour through inhibition of microbes that could possibly contribute to inflammation or genotoxicity in colon cells.
TABLE 1.
Proposed mechanisms of action of probiotics against IBDs.
| Inhibition of pathogenic enteric bacteria by: |
Improvement in epithelial and mucosal barrier function by: |
Alteration of immuno-regulation by: |
|---|---|---|
| • decreasing luminal pH • secretion of bacteriocidal proteins • resisting colonization • blocking epithelial binding |
• production of short-chain fatty acids • enhancing mucus production • increasing barrier integrity |
• increasing interleukin-10 and TGFp, and decreasing TNF levels • increasing IgA production |
GUT MICROBIOME IN HEALTH AND GASTRIC DISEASES
The association between gut microbiota composition and various aspects of host health, including physiological development, metabolism, and immunological responses is well established (Amaral et al., 2008; Bauer et al., 2006; Nagalingam and Lynch, 2012). Clearly, early development of the mammalian system is highly dependent on microbial colonization. It has been found that specific GI bacterial species activate host immune responses to facilitate their own survival and exclusion of other competing microorganisms. For instance, Bacteriodetes thetaiotaomicron induces host production of defensins, presumably to eliminate potential competing organisms (including pathogens) and reduce competition for available space; however, in doing so it also plays a key role in regulation of postnatal angiogenesis (Stappenbeck et al., 2002). On the other hand, certain pathogenic GI microbes due to its ability to use the unusual terminal electron acceptor, tetrathionate, generated upon the interaction of reactive oxygen species and luminal thiosulfate (Winter et al., 2010). Ohkusa et al., (2009), has also been postulated that resident bacteria may cause colitis by compromising the colonic epithelial barrier.
IBDs is an emerging disease that affects 20 out of 100,000 genetically susceptible people in Europe and North America. Approximately 5–20% of the global human population is estimated to suffer from inflammatory bowel diseases (IBDs) (Drossman et al., 2002; Hillilä MT, Färkkilä, 2004), and the situation seems to worsen in future. The major clinical symptoms include abdominal discomfort or pain, diarrhea, constipation, bloating, and flatulence. As the conventional therapies for IBDs are considered to be only moderately effective, new approaches in treatment are being sought.
Though the pathogenesis of IBDs remains unclear, available evidences suggest that altered gut motility, visceral hypersensitivity, and dysregulation of the brain–gut axis are some of the well studied mechanisms (Drossman et al., 2002). Clinical evidences suggest that an imbalanced gut microbiome and enteric bacteria-mediated mucosal inflammation may be associated with IBDs (Linskens et al., 2001; Chadwick et al., 2002). The chronic idiopathic IBDs such as Crohn’s disease (CD), ulcerative colitis (UC), and pouchitis may be caused by a hyper-responsive cell-mediated immune response to intestinal commensal bacteria or microbial aberrancies in susceptible individuals (Podolsky 2002; Sartor 2004). Therefore, it is rational to consider therapeutic approaches that correct the aberrancies in microbiota and eliminate the inflammation- inducing bacteria and adjuvants for the treatment of IBDs in conjunction with anti-inflammatory and immunosuppressant agents (Sartor 2004). Some probiotic strains applied either singly or in combination appeared to be effective in relieving some of the IBDs symptoms, such as constipation, flatulence, and borborygmi. However, the effect and efficacy varies widely between studies and between strains of probiotics.
The relation between a dysregulated bacterial ecosystem and mucosal inflammation in IBDs has been demonstrated in a variety of clinical and basic studies (Tamboli et al., 2004; Mahida and Rolfe 2004). The CD and UC are chronic aggressive disorders with a prevalence of 0.1–0.5%, and the both disorders have distinct features. The UC is characterized by inflammation with superficial ulcerations limited to the mucosa of the colon, and inflammation normally starts in the rectum and continuously spreads throughout the large intestine. Enteric microflora varies considerably between active IBD patients and healthy conditions, and the studies comparing enteric bacteria profiles in active and non-active IBD have shown a decrease in bifidobacteria and LAB in patients with active diseases, but normal in patients with remission (Giaffer et al., 1991). Even though no specific pathogen has been identified as the cause of IBDs, populations of some pathogenic and potentially harmful enteric bacteria are observed to be higher in patients suffering from IBDs (Seksik et al., 2003; Cummings et al., 2003). The pathogenic bacteria trigger intestinal inflammation by secreting enterotoxins that increase epithelial cell permeability (Robertson and Sandler 2001) producing immunosuppressive proteins that dysregulate the mucosal immune system, and may impair the epithelial cellular metabolome.
The distal intestinal epithelia depend on butyrate as the primary energy source (Singh et al., 2008; Kumar et al., 2012a, Macfarlane and Macfarlane, 2012. Interestingly, H2S and sulphate-reducing bacterial counts are found to increase in patients with UC (Pitcher and Cummings 1996), and the block of butyrate metabolism by H2S has detrimental effects on mucosal permeability and healing. In the intestinal lumen of IBD patients, balance between commensal and detrimental seems to be interrupted with secondary harm on immune system activities, though the changes in microbial composition may be transient with a limited applicability (Sartor, 2005; Dotan and Rachmilewitz 2005).
The rationale behind their potential effect is linked to the presumed dysregulation of immune responses to commensal bacteria in IBD patients. Furthermore, the risk of IBDs among first-degree relatives is known to be higher (Abraham and Cho, 2009). Environmental triggers, such as stress (Konturek et al., 2011), air pollution (Beamish et al., 2011), and smoking are considered to have an impact on the disease. This is evidenced by a recent report that patients with CD who smoke have a more complicated disease course (Leung et al., 2012). Evidences are there that stress may have a profound effect on bacterial gut microbes leading to increased adhesion and translocation of bacteria due to increased barrier permeability. Hence, the gut microbes that play a major role in overall health of host, will play altered role under stressful milieu. But the one area where research has greatly evolved is in the concept of gut immunity. The studies on interleukin-10 (IL-10) gene-deficient mouse have revealed the development of colitis when they were exposed to commensal bacteria, while no colitis was observed in germfree mice (Kuhn et al., 1993). These findings support the role of an abnormal or dysregulated immune response to commensal bacteria in the pathogenesis of IBDs. In humans, the colon and the distal ileum are the regions containing very high microbial densities and, these are areas mostly affected in IBDs.
TYPES OF GUT INFLAMMATORY DISEASES
Crohn’s Disease
CD is a common idiopathic IBDs of unknown etiology that affects the GI tract, and is believed to develop as a result of an aberrant immune response to intestinal microbes in a genetically susceptible host. The pathogenesis of CD is multifactorial, and several factors have been implicated in its development including genetic and environmental ones (Lakatos et al., 2006). In adult CD, most studies have failed to show any beneficial effect of probiotics both in induction or maintenance therapy. In terms of induction therapy, only two placebo-controlled studies are available (Malchow et al., 1997; Schultz et al., 2004). Both studies found no significant difference in the time needed to induce therapy in the two groups. As for studies in maintenance therapy, one small-randomized trial with Saccharomyces boulardii showed a significant reduction in the number of patients suffering from relapse in the combined mesalamine–probiotic group (Guslandi et al., 2000). However, these promising findings have not been confirmed by other trials, and there is no evidence to suggest that probiotics are beneficial for the maintenance of remission in CD (Rolfe et al., 2006). Gionchetti et al., (2003) performed a randomized trial to evaluate the efficacy of a combination of rifaximin and the probiotic preparation VSL#3 (Vivomixx in Europe and Visbiome in USA) in the prevention of postoperative recurrence of CD. Rifaximin (1.8g/day) for three months followed by VSL#3 (6g/day; 1,800 billion bacteria) for nine months was compared with mesalazine (4g/day) for 12 months in 40 patients after curative resection for CD.
Ulcerative colitis
Reports are available that highlight the protective role of probiotics in UC. Venturi et al. (1999) showed that VSL#3, a special probiotic preparation containing 5 ×1011/g of viable lyophilized bifidobacteria (B. infantis, B. longum and B. breve), four strains of lactobacilli (L. acidophilus, L. delbrueckii subsp. bulgaricus, L. plantarum and L. casei) and one strain of Streptococcus salivarius subsp. thermophilus significantly increased fecal populations of probiotic bacteria as long as patients were taking the above preparation. A randomized controlled trial demonstrated equivalence of non-pathogenic Escherichia coli to mesalamine for maintenance of remission in UC (Rembacken et al., 1999). In 2001, E. coli Nissle 1917 was shown in a multi-center, double-blind, mesalamine-controlled trial to be equivalent to mesalamine for maintenance of remission in UC. In this study, 327 patients with UC in remission were followed over a 12-month period. Both clinical and endoscopic estimates of disease severity were performed using the Crohn’s activity index and endoscopic activity index (Kruis et al., 2001). Relapse rates were 45.1% for the E. coli group and 36.4% for the mesalamine group by intention to treat analysis. Fedorak et al., (2003) studied 30 patients with mild to moderately active UC in a small, open-label trial of VSL#3 for efficacy of induction of remission or response. Clinical symptoms and sigmoidoscopic ratings were performed at baseline and at week 6, where 19 of 30 (63%) patients achieved remission, 7 of 19 (23%) achieved a response, and 4 of 19 (13%) had no response (Fedorak et al., (2003). An uncontrolled trial of S. boulardii was suggestive of benefit in maintaining clinical remission (using Rachmilewitz’s activity index) in patients with mild to moderate UC in combination with a stable dose of mesalamine 1 g daily. Kruis et al., (2012) have concluded that probiotic E. coli Nissle 1917 shows effects in IBDs, especially in patients with altered enteric microflora, e.g. after gastroenterocolitis or administration of antibiotics. In addition, high vegetable intake reduces the risk of UC, whereas increased fruit and/or dietary fiber intake appears protective against CD. Lacking or reduced availability of certain micronutrients, especially vitamin D, may increase the risk of both diseases (Gentschew and Ferguson, 2012).
Pouchitis
Proctocolectomy with ileal pouch-anal anastomosis may be required in some UC patients because their disease is medically intractable or they develop secondary dysplasia or cancer. Pouchitis or inflammation of the ileal reservoir created during the procedure may develop in 15 to 50% patients (Mac, 2011). Clinically, pouchitis is characterized by variable symptoms, including increased stool frequency and fluidity, abdominal cramping, pelvic discomfort, bleeding, tenesmus, fever and weight loss, and extra-intestinal manifestations in more severe cases (Madden et al., 1990). It is the most common complication of the surgery and although the exact etiology is not clear, host genetic factors, local pouch issues and the microbiota contained within the pouch are thought to be involved (Elahi et al., 2008; Pardi et al., 2009).
Trials for treating mild/ moderate pouchitis are few with small number of adult patient trials. For an unequivocal diagnosis, endoscopic examination and histologic investigation are mandatory (Shen et al., 2001). Pouchitis disease activity index (PDAI) is the most commonly used diagnostic instrument and represents an objective and reproducible scoring system for pouchitis (Sandborn et al., 1994). Active pouchitis is defined as a score ≥ 7 and remission is defined as a score < 7. Studies have suggested that altering the microbiota in the pouch by administering probiotic bacteria can be effective in maintaining remission and reducing the incidence of flare-ups in chronic pouchitis (Gionchetti et al., 2000, Mimura et al., 2004). Moreover, the efficacy of probiotic therapy as prophylaxis to delay the first onset of pouchitis after pouch-surgery has been demonstrated (Gionchetti et al., 2003; Gosselink et al., 2004). Mimura et al. (2004) proved the efficacy ofVSL#3 in maintaining remission in antibiotic-sensible pouchitis. Therefore, a strong body of evidence supports the use of probiotics as a therapeutic option in adult pouch. As information is currently scarce on this aspect in infants, studies are warranted to advocate role of probiotics in managing pouchitis in infants.
A molecular overview of IBDs- involvement of genes identified
The precise molecular mechanisms behind the development of IBDs are still not clearly understood. Genome- wide association studies are considerably improving our understanding of genetic susceptibility to IBDs (Budarfet al., 2009). IBDs, including CD and UC, result from an exaggerated mucosal immune response to bacterial antigens in genetically susceptible individuals (Sartor et al., 2006; Xavier and Podolsky, 2007), and consequently there a many genes which are involved therein (Table 2).
TABLE 2.
Genes responsible for IBDs.
| Gene | Function |
|---|---|
| Crohn’s Disease | |
| CARD | NFκB activation and/or regulation, killing of intracellular pathogens, Paneth-cell function,(α-defensin production) (Hugot et al., 2001; Ogura et al., 2001) |
| SLC22A4 and SLC22A5 | Organic cation, carnitine transporters, possibly transport xenobiotic substances (Peltikova et al., 2004; Torok et al., 2005) |
| DLG5 | Epithelial scaffolding protein |
| PPARG | Intracellular inhibitor of NFκB and cellular activation (Sugawara et al., 2008) |
| Ulcerative colitis | |
| MDR1 | Efflux transporter for drugs and, possibly, xenobiotic compounds (Panwala, 1998; Maroo and Farrell, 2006) |
Considering epidemiological, genetic and immunological data, it is concluded that the IBDs are heterogeneous disorders of multifactorial etiology in which hereditability and environment interact to produce the disease. It is probable that patients have a genetic predisposition for the development of the disease coupled with disturbances in immunoregulation (Tsianos et al., 2012). At the moment, several genes have been so far related to the diagnosis of CD. The first gene to be identified with CD was CARD15 (caspase recruitment domain family member 15, previously known as NOD2) (Hugot et al., 2001; Ogura et al., 2001). There are three mutations (causing amino-acid substitutions Arg702Trp and Gly908Arg and the frameshift 1007fs) found within the region of CARD15 that encodes a leucine-rich repeat, which is responsible for bacterial recognition. At least one of these mutations is present in 25–35% of CD patients of European ancestry, but not in Asian or African American CD patients.
A number of studies have identified several other CD susceptibility genes such as SLC22A4 and SLC22A5 in the IBDs locus (Peltekova et al., 2004; Török et al., 2005). Two functional variants of the organic cation transporters OCTN1 and OCTN2 have been associated with CD in association with CARD15 mutations (Peltekova et al., 2004). Mutations in the transcribed region of SLC22A4, which encodes OCTN1, and the promoter region of SLC22A5, which encodes OCTN2, affect the transcription and function of these carnitine and organic cation transporters. These variants are most actively expressed in the intestinal epithelium, macrophages and T-cells, and cause decreased carnitine transport. Two haplotypes of DLG5, which encodes a scaffolding protein that helps to maintain epithelial integrity, have been associated with CD and combined UC and CD populations (Stoll et al., 2004). Like the OCTN1 and OCTN2 variants, the 113G>A substitution in DLG5 is associated with CARD15 mutations in patients with CD. P-glycoprotein 170 might also function as a ‘flippase’ that moves amphipathic substrates from the inner to the outer leaflet of the cell membrane. MDR1 variants have been associated with UC and CD (Brant et al., 2003). MDR1 is of particular interest, because it has been associated with treatment refractory IBDs (Maroo and Farrell, 2006), and because mice in which Mdr1 has been deleted develop colitis (Panwala et al., 1998).
PPARG (peroxisome proliferative-activated receptor γ) variants have been linked with susceptibility in the SAMP1/ YitFc mouse model of spontaneous chronic ileitis, and rare PPARG polymorphisms were found to be associated with human CD (Sugawara et al., 2005). PPARγ is a nuclear receptor that inhibits NFκB activity: its expression is decreased in patients with active UC (Dubuquoy et al., 2003) and its expression is upregulated by 5-aminosalicylic acid (Rousseaux et al., 2005). In addition to a potential role in protecting against intestinal inflammation, treatment with the PPARγ ligand rosiglitazone was effective in an open-label trial involving UC patients (Lewis et al., 2001) as well as in mouse experimental colitis.
Large-scale genome-wide association studies have become technically feasible given the introduction of high-throughput sequencing, and technologies and development of gene chip- based technologies, development of software to manage data leading to the detection of several additional CD susceptibility genes including IL23R (Duerr et al., 2006; Glas et al., 2007), ATG16L1 (Hampe et al., 2007; Glas et al., 2007), NELL1 (Franke et al., 2007) and IRGM (Parkes et al., 2007) as well as an intergenic region on 5p 13.1 that modulates PTGER4 expression (Libioulle et al., 2007). In addition, there are a large number of genes modifying the CD phenotype such as variants in the genes of the TLR-4 (Brand et al., 2005), the fractalkine receptor (CX3CR1) (Brand et al., 2006), the macrophage migration inhibitory factor (MIF) (Dambacher et al., 2007) and C-reactive protein (CRP) (Thalmaier et al., 2006). While most of these genes modify only susceptibility and phenotype of CD, the IL23R gene has been shown to also be associated with UC, although this association is far less pronounced than that with CD (Duerr et al., 2006; Glas et al., 2007).
ROLE OF CYTOKINES IN IBDs
Cytokines and chemokines are small (4–15 kDa), inducible, pro-inflammatory and immune-regulatory proteins that play a central role in the development and homeostasis of immune system. Both CD and UC patients have activated innate (macrophage, neutrophil) and acquired (T and B cell) immune responses and are more susceptible to enteric pathogens (Mow et al., 2004). Tolerance in normal hosts is mediated by regulatory T cells, B lymphocytes, natural killer T cells and dendritic cells that secrete transforming growth factor (TGF)-P, interleukin (IL)-10, interferon (IFN)-α/β and prostaglandin J2. Antibody-neutralization studies have implicated tumor necrosis factor (TNF) and IL-12 p40 in the pathogenesis of CD (Targan et al., 1997; Mannon et al., 2004); while T-cells have been linked to UC by the effectiveness of T-cell-ablative therapies (Sawada K et al., 2005), cyclosporine and tacrolimus (Lichtiger et al., 1994).
A network of cytokines has been extensively investigated in IBDs, and an imbalance of pro-inflammatory and counter-regulatory molecules has been demonstrated in both CD and UC tissues. The pro-inflammatory cytokines IL-1 β, IL-6, IL- 8, IL-18, IFN- γ and TNF-α are produced in excess (Fiocchi, 1998) while the immuno-suppressive IL-10 is reduced and the inhibitory activity of TGF- β1 signaling is paradoxically inefficient. Both the excess of proinflammatory cytokines and the relative inefficiency of counter-regulatory molecules are required for maintaining, amplifying and perpetuating chronic inflammation in IBDs (MacDonald and Monteleone 2005). Different secretory patterns and pathways have been identified within the cytokines network in IBDs. A general consensus has been reached for a predominant role of T-helperl (Th1) cytokines in CD.
Evidence also indicates that in the gut mucosa the IL-12- induced Th1 polarization can be expanded by other cytokines, such as IL-15 and IL-18. IL-21, a newly T-cell-derived cytokine of the IL-2 family, has also been found to associate with IBDs. The expression of this molecule is enhanced in IBDs, in direct correlation with disease activity, and a functional evaluation suggests that IL-21 contributes to stabilize the Th1 phenotype at the site of inflammation (Monteleone et al., 2005).
Mucosal immune response in UC differs from that occurring in CD. Several observations indicate a predominant humoral immunity characterized by a high level of IgG released by mucosal plasma cells. T-cells isolated from colonic mucosa of UC patients were found to release high IL-5 and IFN- γ while IL-4 is relatively low. The Th1 cytokine profile, which includes IFN-y and IL-12 p40, is dominant in patients with CD. Traditional Th1 responses are mediated by IFN-γ, the production of which is stimulated by IL-12, produced by antigen-presenting cells (APCs). Most experimental colitis models also have a dominant Th1 response (Sartor 2004), although in several models, Th1 responses can change into Th2 (type-2 T-helper lymphocyte) responses as the inflammatory process matures (Spencer et al., 2002; Bamias et al., 2005); whereas in UC, the T-cell profile is not properly understood.
In Innate immune responses, macrophages and dendritic cells in the lamina propria are increased in number and have an activated phenotype in both forms of IBDs, but have been studied in greater detail in CD. Production of proinflammatory cytokines and chemokines is enhanced in IBDs (Table 3), and expression of adhesion molecules and co-stimulatory molecules is increased (Sartor and Hoentjen 2005). Cells involved in innate immune responses are activated and the expression of most proinflammatory cytokines and chemokines is upregulated in both CD and UC. Th1 and Th1- related cytokines involved in innate immunity (e.g. IL-12, IL- 23 and IL-27) are, however, selectively activated in CD.
TABLE 3.
Cytokines associated with IBDs.
| Cytokine | Crohn’s Disease | Ulcerative colitis |
|---|---|---|
| Non-specific immune responses | ||
| IL-1 β | Elevate; Source macrophages | Elevate; Source macrophages |
| TNF | Elevate; Source macrophages | Elevate; Source macrophages |
| IL-6 | Elevate; Source macrophages | Elevate; Source macrophages dendritic cells |
| IL-8 | Elevate; Source macrophages | Elevate; Source Epithelial cells |
| IL-12 | Elevate; Source macrophages | Normal; Source Macrophages |
| IL-18 | Elevate; Source macrophages | Elevate; Source APCs |
| IL-23 | Elevate; Source macrophages and T Helper cell involved in innate immunity | Normal; Source T Helper cell involved in innate immunity |
| IL-27 | Elevate; Source T Helper cell involved in innate immunity | Normal; Source T Helper cell involved in innate immunity |
| T-cell responses | ||
| IFN-γ | Elevate; antigen-presenting cells (APCs) | Normal; antigen-presenting cells (APCs) |
| IL-5 | Normal; CD4 + Lymphocytes | Elevate; CD4 + Lymphocytes |
| IL-13 | Normal; NK T cells | Elevate; NK T cells |
| IL-17 | Elevate; CD4 + Lymphocytes | Normal; CD4 + Lymphocytes |
| IL-21 | Elevate | Normal |
GUT MICROBIAL METABOLOME IN GUT DISEASES
The colonic microbiome plays an important role in digestive physiology and makes a significant contribution to homeostasis in the large bowel (Macfarlane and Macfarlane, 2012). The therapeutically important end products of microbial fermentation of undigested hydrocarbons reaching the colon include short chain fatty acids (SCFAs), principally acetate, propionate and butyrate and gases such as carbon dioxide, hydrogen and methane (Samuel et al., 2008). Mounting evidence suggests an important role for the intestinal microbiota in the chronic mucosal inflammation that occurs in IBDs, and novel molecular approaches have further identified a dysbiosis in these patients. Several mechanisms of action of probiotic products that may interfere with possible etiological factors in IBDs have been postulated (Jonkers et al., 2012; Siciliano and Mazzeo, 2012). Probiotics and their metabolic products have been proposed as food supplements for a healthier intestinal homeostasis, and also as therapeutic aids in IBDs with, however, very little clinical benefit. This may be due to the lack of reliable preclinical models for testing the efficacy of different strains (Tsilingiri et al., 2012). The mechanisms by which intestinal bacteria achieve their associated health benefits can be complex and multi-faceted. The gut symbionts participate in the barrier effect by developing antimicrobial activity against enterovirulent bacteria and the antimicrobial activity is related to a number of bioactive biomolecules including antimicrobial peptides (Hassan et al., 2012; Nagpal et al., 2012; Siciliano and Mazzeo, 2012 ) organic acids, hydrogen peroxide, diacetyl and bacteriocins (Singh et al., 2008; O’Shea et al., 2011, 2012).
Vero-cytotoxin produced by E. coli O157:H7 is associated with hemorrhagic colitis and hemolytic uremic syndrome in humans. Kim et al. (2001) showed that some substance in the culture supernatant of Bifidobacterium longum HY8001 could neutralize the virulence of vero-cytotoxin successfully. In this study, control mice were inoculated intragastrically with E. coli O157:H7, and B. longum HY8001 culture supernatant were inoculated intragastrically to mice simultaneously. The TNFα and IL-1 levels in sera were decreased in mice treated with B. longum culture supernatant compared with those control mice. The concomitant experiment in vitro showed the culture supernatant of B. longum primarily bound vero- cytotoxin to interfere attachment and invasion of E. coli to epithelia. These results suggest that soluble substance in B. longum HY8001 culture supernatant may have inhibitory activity on the virulence of E. coli (Liévin et al., 2000; Flynn et al., 2002). Inhibition of Salmonella typhimurium for cell association and cell invasion was investigated using Caco-2 cells in a study in vitro (Liévin et al., 2000). Two strains of bifidobacteria expressed antagonistic activity against pathogens in vitro, inhibited cell entry, and found to attenuate or kill intracellular S. typhimurium in Caco-2 cells. The antibacterial component produced was found to be a lipophilic molecule with a molecular weight less than 3500 Da. Data have shown a small heat stable bacteriocin, ABP- 118, which was a Class Ilb two-peptide bacteriocin produced by L. salivarius. Heterologous expression of ABP-118 was also achieved in L. plantarum, L. lactis, and Bacillus cereus (Flynn et al., 2002; Siciliano and Mazzeo, 2012).
CD is also characterized by an impaired induction of human b-defensins-2 and −3 (hBD), and a decrease or lack of mucosal peptide antibiotics may play a central part in the etiopathogenesis of CD (Wehkamp et al., 2005). E. coli strain Nissle 1917 and a variety of other probiotics can induce the expression of the antimicrobial peptide hBD- 2 in Caco-2 intestinal epithelial cells in a time and dose dependent-manner (Wehkamp et al., 2004). The induction of hBD-2 may contribute to an increased mucosal barrier to the luminal bacteria, or affect adhesion of pathogens to intestinal epithelial cells (Schierack et al., 2011). Nevertheless, polarized administration of bacteria is critical to control the ensuing immune response as it mimics the physiological entrance of bacteria. Further well designed studies based on intention-to-treat analyses by several independent research groups are still warranted to support the promising results for E. coli Nissle in inactive UC and the multispecies product VSL#3 in active UC and inactive pouch patients. So far, no evidence is available to support the use of probiotics in CD (Jonkers et al., 2012).
PROBIOTICS IN MANAGING IBDs
The gut microflora is a community that has co-evolved with the host and confers beneficial effects, including nutrient metabolism, immunomodulation and defenses against invading pathogens. However, dysregulation of normal co-evolved homeostatic relationships between gut bacteria and host immune responses can lead to intestinal inflammation, suggesting that luminal flora is a requisite, or perhaps a central factor, in the development of IBDs (Khan et al., 2012). There is abundant evidence that commensal bacteria are involved in the pathogenesis of human IBDs and in experimental colitis (Sartor 2004). The most convincing mechanistic studies have been done in gnotobiotic animal models. An altered balance of beneficial versus spoilage and pathogenic microbial species could lead to a pro-inflammatory luminal milieu that drives chronic intestinal inflammation. Numerous studies have implicated several commensal organisms, such as E. coli, Bacteroides, Enterococcus and Klebsiella species, in the pathogenesis of experimental intestinal inflammation and human IBDs (Sartor 2004). By contrast, various Lactobacillus and Bifidobacterium species have predominantly protective effects and have been used therapeutically as probiotics (Sartor 2004; Kumar et al., 2009; 2010; 2011; 2012a, b, c; Nagpal et al., 2007; 2010; 2012a, b, c; Nagpal and Kaur, 2011; Foye et al., 2012) (Table 3). Several groups have documented alterations in luminal or adherent microbial commensal flora in patients with CD, UC and pouchitis (Swidsinski et al., 2002; Sartor 2004).
An alternative mean of changing the microenvironment in such a way as to stimulate aggressive immune responses is the acquisition of virulence factors by commensal bacteria. As discussed above, enteroadherent and invasive E. coli have been found in the neoterminal ileum of patients with post-operative recurrence of CD (Darfeuille-Michauxs, 2002). Flagellin from Clostridium (sub-phylum cluster XlVa) has been shown to be a dominant antigen in experimental colitis, and to elicit serologic responses in approximately 50% of CD patients (Lodes et al., 2004). The presence of superoxide dismutase activity alters the pathogenicity of E. faecalis. Dietary components can alter the composition and virulence of enteric commensal bacteria, providing one potential explanation for the marked increase in the incidence of IBDs in Western countries in the second half of the twentieth century, and more recently in Eastern countries, as they adopt Western dietary practices.
It is evident that short-chain fatty acids (SCFAs), predominantly acetate, propionate, and butyrate, are the principal metabolites generated during the catabolism of dietary carbohydrates and proteins. In contrast, protein digestion yields a greater diversity of end products, including SCFAs, amines, phenols, indoles, thiols, CO2, H2, and H2S, many of which have toxic properties. The majority of SCFAs are absorbed from the gut and metabolized in various body tissues, making a relatively small but significant contribution to the body’s daily energy requirements (Macferlane and Macferlane, 2012). Prebiotics, such as non-absorbed carbohydrates promote the growth of probiotic bifidobacteria and lactobacilli, and provide a substrate for the production of SCFA during gut fermentation. Probiotic L. acidophilus, prebiotic inulin or symbiotic may promote host protective immunity and attenuate Cr-induced intestinal inflammation through mechanisms affecting NF- κB and Smad 7 signaling (Foye et al., 2012).
Dynamic community of intestinal microflora plays a critical role in maintaining intestinal health serving metabolic, digestive, trophic as well as protective functions (Guarner et al., 2003). Increased numbers of adherent invasive Escherichia coli (AIEC) have been found in CD patients. Probiotics are viable microorganisms with beneficial physiologic and therapeutic properties that when ingested in specific numbers exert health benefits beyond those of basic nutrition (Huebner et al., 2011; Kumar et al., 2009; 2010; 2011; 2012a, b, c; Nagpal et al., 2007; 2010; 2012a, b, c; Nagpal and Kaur, 2011). Examples of bacteria demonstrated to have beneficial effects include Lactic acid bacteria, Lactobacillus, Bifidobacterium, E. coli Nissle 1917, Streptococcus salivarius subsp. thermophilus and a non-pathogenic yeast Saccharomyces boulardii (Fioramonti et al., 2003; Nagpal et al, 2007; 2012). Levels of commensal bacteria appear to be altered in IBDs patients with increased numbers of bacteroides, adherent/ invasive E. coli, enterococci and decreased Bifidobacterium and Lactobacillus species (Neut et al., 2002).
MODULATING IMMUNE RESPONSE OF INTESTINAL EPITHELIA AND MUCOSAL IMMUNE CELLS
Intestinal macrophages and dendritic cells act in a synergistic fashion with intestinal epithelial cells and microbiota to initiate the triad that governs the intestinal immune responses (whether inflammatory or regulatory) (Khan et al., 2012). The adaptive immune system allows individual organisms to mount defensive reactions against unanticipated pathogens by developmentally creating a diverse repertoire of clonally distributed receptors capable of recognizing a multitude of antigens and then expanding as effector cell populations comparable those that can recognize signals from the invading or endogenous pathogens (Schwartz, 2005). Probiotic treatment can down-regulate the expression of proinflammatory cytokines such as TNFα, IL1 β, and IFN-γ, inducible nitric oxide synthase, and matrix metalloproteinase activity in inflamed mucosa of active IBDs or experimental colitis (Tables 1 and 4) (Dieleman et al., 2003; Furrie et al., 2005; Mileti et al., 2009). Lavasani et al., (2010) have shown that therapeutic effect of probiotic lactobacilli is associated with induction of transferable tolerogenic Tregs in MLNs, but in the periphery and the central nervous system, mediated through an IL-10-dependent mechanism. These findings indicate a therapeutic potential of orally administered probiotics, and provide a platform to understand host-commensal interactions that contribute to beneficial effects in autoimmune diseases. In inflammatory conditions of IBDs, immune cells secrete excessive inflammatory products such as cytokines chemokines and active oxides. Over-production of cytokines affect the biological action of epithelial cells, for instance, TNFα induces epithelial cells secreting IL8, expressing membrane toll-like receptors (TLR)-4 excessively (Hausmann et al., 2002).
TABLE 4.
Some potential probiotics proposed for their efficacy against IBDs.
| Probiotic starins | References |
|---|---|
| Lactobacillus reuteri (R2LC) | Madsen et al., (1999) |
| L. salivarius UCC118 | O’Mahony et al., (2001) |
| L. casei GG (L. GG) | Malin et al., (1996) |
| VSL#3 (Vivomixx in Eurpoe and Visbiome in USA) | Madsen (2001) |
| L. plantarum 299v; L. plantarum HY115; L. plantarum DSM 9843 | Schultz et al., (2002); Osman et al., (2004); Lee, et al., (2008) |
| Bifidobacterium Infantis; Bifidobacterium Bb-12 | Tanabe et al., (2008) |
| L. rhamnosus GG | Schultz et al., (2003) |
| S. boulardii | Guslandi et al., (2000) |
| L. fermentum ACA-DC179 | Zoumpopoulou et al., (2008) |
| L. brevis HY7401 | Lee, et al., (2008) |
| L. johnsonii NCC 533 | Pridmore et al., (2008) |
| Lactococcus Lactis (engineered) | Steidler et al., (2000) |
| E. coli Nissle 1917 | Malchow (1997); Kruis et al., (2004) |
| L. salivarius 433118 | McCarthy et al., (2003) |
| Bifidobacterium infantis 1222; Bifidobacteria sp. 3B1; B. infantis DSM 15158 | Shiba et al., (2003); Osman et al., (2004) |
Probiotic strains interact with intestinal epithelia, and attenuate synthesis of inflammatory effector molecules elicited by diverse proinflammatory stimuli (Otte et al., 2004; Bai et al., 2004; Lammers et al., 2002). This immunosuppressive effect involves inhibition of the inhibitor kB/nuclear factor kB (IkB/NF-kB) pathway by block of IkB-α degradation, which prevents nuclear translocation of active NF-kB dimmer and subsequent relevant gene expression (Neish et al., 2000; Neish et al., 2004). Hence, the probiotics can be responsible for the unique tolerance of the GI mucosa to proinflammatory stimuli. The intestinal apical di-/tripeptide transporter PepT1, responsible for the uptake of a broad array of small peptides transports bacterial proteoglycan derived muramyl dipeptide (MDP). PepT1 expression in chronic colonic inflammation is increased substantially (Vavricka et al., 2004). In colonic epithelial cells, MDP taken up by hPepT1 activates NF-kB and chemokine production. In this way, PepT1 may play an important part in promoting colonocyte participation in host defense and pathogen clearance through increased uptake of MDP. Probiotic LAB such as L. casei can change the function of intestinal PepT1 (Neudeck et al., 2004). In view of scarce data on this topic, more studies should be carried out to explore probiotic-intestinal transporter interactions, and to find out how probiotics participate in host defense and pathogen clearance from the gut.
Some probiotic strains (Table 4) may modify immune response of the intestinal immune cells (Borruel et al., 2002). Mucosal specimens from CD patients and controls were cultured for 24 hours alone or with probiotic strains as non- pathogenic E. coli, L. casei DN-114001, L. bulgaricus LB10, or L. crispatus. Release of TNFα by inflamed CD mucosa was significantly reduced by co-culture with L. casei or L. bulgaricus, whereas changes induced by L. crispatus or E. coli were not significant. The co-culture with L. casei and L. bulgaricus reduced the number of CD4 cells as well as TNFα expression among intraepithelial lymphocytes from CD mucosa. None of the bacteria induced changes in non-inflamed mucosa. Probiotics interact with immunocompetent cells using the mucosal interface and modulate locally the production of proinflammatory cytokines. Toll-like receptors (TLRs) are mediators of pathogen recognition in innate immune system (Andreakos et al., 2004). Preliminary data from experimental colitis show that TLR9 signaling is essential in mediating the anti-inflammatory effect of probiotics (Rachmilewitz et al., 2004). Such recognition results in the initiation of an inflammatory immune response and subsequent instruction of the adaptive immune system, both of which are designed to provide protection against invading pathogens.
Probiotics can also modify mucosal immune function via TLR signaling (Rachmilewitz et al., 2004). The protective effect of probiotics is mediated by their own genome via TLR9 signaling pathway, rather than by their metabolites or ability to colonize the colon (Rachmilewitz et al., 2004). Sturm et al. (2005) have demonstrated that one probiotic strain, E. coli Nissle 1917, could affect cell cycling and apoptosis of peripheral blood T-cells (PBT). When anti-CD3 stimulated PBT are treated with E. coli Nissle 1917 conditioned medium, E. coli Nissle 1917 medium inhibit cell cycling and expansion of peripheral blood T-cells, decrease cyclin D2, B1 and retinoblastoma protein expression contributing to the reduction of T cell proliferation, and regulate the decreased expression of IL2, TNFα and interferon gamma but increased IL-10 production in PBT. The inhibition of PBT proliferation by E. coli Nissle 1917 conditioned medium is TLR2- dependent, as shown by using TLR-2 knockout mice.
The peroxisome proliferator-activated receptors (PPARs, namely PPARα, γ and δ) are transcription factors that translate nutritional signals into specific gene-expression patterns that control cellular bioenergetics. The receptors act as nutritional sensors, regulating metabolism across organs to modify systemic metabolism (Dubuquoy et al., 2006; Ament et al., 2012). Probiotics can modulate the inflammatory response in inflamed mucosa of IBDs patients through PPAR pathway that has been proposed as a key inhibitor of colitis through attenuation of NF-kB activity (Nencioni et al., 2003). However, PPAR expression is impaired in patients with ulcerative colitis, compared with normal controls (Dubuquoy et al., 2003).
FUTURE DIRECTIONS AND CONCLUSION
There is a rapid increase in the understanding of how microbes can be employed to deliver health benefits. Gut bacteria play an important role in the pathogenesis of IBDs, its complications and symptoms. While the exact causes are unclear, IBDs, including the most common manifestations i.e. CD and UC, are known to be the result of an overactive immune response that is linked to an imbalance of the normal types of bacteria inhabiting the gut. With recent evidences implicating disruption in the balance of the gut microbiome and gut immunity as a potential trigger for IBDs and related pathologies, there has been growing interest in developing and utilizing gut microbes as probiotics as an adjunct to antiinflammatory and immune suppressing therapies. Probiotics can have inflammatory activities in both healthy and IBDs tissue, and are regarded as safer alternative for the treatment of patients with IBDs in acute inflammatory phase. Recent advances deciphering the complex gut microbiome will highlight novel insights into the cellular and molecular bases of IBDs, and will provide opportunities to manipulate gut microbiome through intake of potential probiotics to manage pathologies associated with IBDs and related diseases. Future studies should focus on specific disease subtypes and disease location. New knowledge about the role of microbial communities in the microbiota of the GI tract and their microbiome, in chronic diseases opens new opportunities for therapeutic interventions. Insight into the etiology of IBDs and the cellular and molecular mechanisms of probiotic actions will aid in selecting probiotic strains for specific disease entities and disease locations. Valid preclinical data on proper model systems should, therefore, be generated before specific probiotic strains are recommended for clinics, especially if administered during acute inflammatory responses. As for the potential of a gut-flora analysis to drive a more tentatively specific probiotics intervention in IBDs, it can be noted the recent availability of the next-generation sequencing (NGS) methodology that by combining multiple samples in a single sequencing run, allows the analyte of the whole microbial community within a minute sample. This powerful technology uses clonal amplification and sequencing with simultaneously targeting of DNA bases emitting a unique fluorescent signal.
Although the massive amount of data still needs to be fully unfolded and hierarchically prioritized, it is worth mentioning that a multi-team approach to these data may already start be amenable to clinical practice. In particular, this has been quite recently achieved by a spin off of dedicated genetists and biologist (Next Genomics, Prato, Italy) which avail itself of a kit allowing small sampling and remaining stable for 14 days at room temperature and with a detection accuracy of 0.0001% of the original sample. The report gathers all the above mentioned expertise so to make it also a tool of potential clinical use, with all due limitation of exploring this new field, to possibly unveil subtle disease risk-prone conditions, early signs of IBDs flare ups and to tentatively shape up a tailored intervention and an objective follow up monitoring.
FIGURE 2.
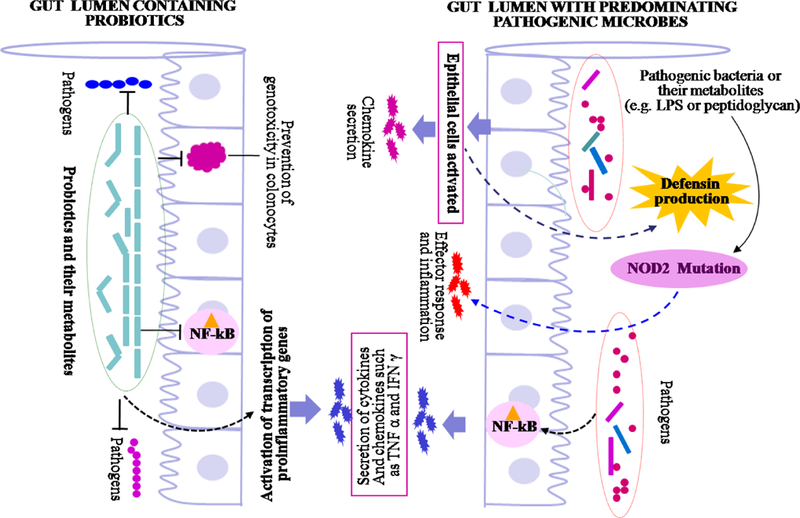
Possible mechanisms involved in beneficial effects of probiotics, and inhibition of gut inflammation. The probiotics inhibit invading pathogenic microbes through various mechanisms including competitive inhibition, production of antagonistic compounds including antimicrobial peptides, bacteriocins, organic acids, hydrogen peroxide, diacetyl etc. The disturbance in population of probiotics can lead to enhance number of pathogenic microorganisms which leads to inflammatory responsed and pathological conditions. The dotted arrows represent multistep metabolic pathways.
REFERENCES
- Abraham C, Cho JH (2009). Inflammatory bowel disease. New England Journal of Medicine. 361: 2066–2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaral F, Sachs D, Costa V (2008). Commensal microbiota is fundamental for the development of inflammatory pain. Proceedings of National Academy of Science USA 105:2193–2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ament Z, Masoodi M, Griffin JL (2012). Applications of metabolomics for understanding the action of peroxisome proliferator-activated receptors (PPARs) in diabetes, obesity and cancer. Genome Medicine, 4: 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreakos E, Foxwell B, Feldmann M(2004). Is targeting Toll-like receptors and their signaling pathway a useful therapeutic approach to modulating cytokine-driven inflammation? Immunology Review, 202:250–265. [DOI] [PubMed] [Google Scholar]
- Bai AP, Ouyang Q, Zhang W, Wang CH, Li SF (2004). Probiotics inhibit TNF-alpha-induced interleukin-8 secretion of HT29 cells. World Journal of Gastroenterology, 10:455–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamias G, Martin C, Mishina M, Ross WG, Rivera-Nieves J, Marini M, Cominelli F (2005). Proinflammatory effects of TH2 cytokines in a murine model of chronic small intestinal inflammation. Gastroenterology, 128: 654–666. [DOI] [PubMed] [Google Scholar]
- Bauer E, Williams B, Smidt H (2006). Influence of the gastrointestinal microbiota on development of the immune system in young animals. Current Issues Intestinal Microbiology, 7:35–51. [PubMed] [Google Scholar]
- Beamish LA, Osornio-Vargas AR, Wine E (2011). Air pollution: An environmental factor contributing to intestinal disease. Journal of Crohns Colitis, 5:279–286. [DOI] [PubMed] [Google Scholar]
- Borruel N, Carol M, Casellas F, Antolín M, de Lara F, Espín E, Naval J, Guarner F, Malagelada JR (2002). Increased mucosal tumour necrosis factor alpha production in Crohn’s disease can be down regulated ex vivo by probiotic bacteria. Gut, 51:659–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand S, Hofbauer K, Dambacher J, Schnitzler F, Staudinger T, Pfennig S, Seiderer J, Tillack C, Konrad A, Göke B, Ochsenkühn T, Lohse P (2006). Increased expression of the chemokine fractalkine in Crohn’s disease and association of the fractalkine receptor T280M polymorphism with a fibrostenosing disease phenotype. American Journal of Gastroenterology, 101: 99–106. [DOI] [PubMed] [Google Scholar]
- Brand S, Staudinger T., Schnitzler F, Pfennig S, Hofbauer K, Dambacher J, Seiderer J, Tillack C, Konrad A, Crispin A, Göke B, Lohse P, Ochsenkühn T (2005). The role of Toll-like receptor 4 Asp299Gly and Thr399Ile polymorphisms and CARD15/NOD2 mutations in the susceptibility and phenotype of Crohn’s disease. Inflammatory Bowel Diseases, 11:645–652. [DOI] [PubMed] [Google Scholar]
- Brant SR, Panhuysen CI, Nicolae D, Reddy DM, Bonen DK, Karaliukas R, Zhang L, Swanson E, Datta LW, Moran T, Ravenhill G, Duerr RH, Achkar JP, Karban AS, Cho JH (2003). MDR1 Ala893 polymorphism is associated with inflammatory bowel disease. American Journal of Human Genetics, 73:1282–1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budarf ML, Labbé C, David G, Rioux JD, GWA studies: rewriting the story of IBD. Trends in Genetics, 25:137–146. [DOI] [PubMed] [Google Scholar]
- Chadwick VS, Chen W, Shu D, Paulus B, Bethwaite P, Tie A, Wilson I (2002). Activation of the mucosal immune system in irritable bowel syndrome. Gastroenterology, 122: 1778–1783. [DOI] [PubMed] [Google Scholar]
- Cummings JH, Macfarlane GT, Macfarlane S (2003). Intestinal bacteria and ulcerative colitis. Current Issues in Intestinal Microbiology, 4:9–20. [PubMed] [Google Scholar]
- Dambacher J, Staudinger T, Seiderer J, Sisic Z, Schnitzler F,Pfennig S, Hofbauer K, Konrad A, Tillack C, Otte JM, Diebold J, Göke B, Ochsenkühn T, Lohse P, Brand S (2007). Macrophage migration inhibitory factor (MIF)-173G/C promoter polymorphism influences upper gastrointestinal tract involvement and disease activity in patients with Crohn’s disease. Inflammatory Bowel Diseases, 13:71–82. [DOI] [PubMed] [Google Scholar]
- Dieleman LA, Goerres MS, Arends A, Sprengers D, Torrice C, Hoentjen F, Grenther WB, Sartor RB. (2003). Lactobacillus GG prevents recurrence of colitis in HLA-B27 transgenic rats after antibiotic treatment. Gut, 52:370–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dotan I, Rachmilewitz D (2005). Probiotics in inflammatory bowel disease: possible mechanisms of action. Current Opinion in Gastroenterology, 21:426–430. [PubMed] [Google Scholar]
- Drossman DA, Camilleri M, Mayer EA, and Whitehead WE (2002). AGA technical review on irritable bowel syndrome. Gastroenterology, 123:2108–2131. [DOI] [PubMed] [Google Scholar]
- Dubuquoy L, Jansson EA, Deeb S, Rakotobe S, Karoui M, Colombel JF, Auwerx J, Pettersson S, Desreumaux P (2003). Impaired expression of peroxisome proliferator- activated receptor gamma in ulcerative colitis. Gastroenterology, 124:1265–1276. [DOI] [PubMed] [Google Scholar]
- Dubuquoy L, Rousseaux C, Thuru X, Peyrin-Biroulet L, Romano O, Chavatte P, Chamaillard M, Desreumaux P 2006. PPARgamma as a new therapeutic target in inflammatory bowel diseases. Gut, 55:1341–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duerr RH, Taylor KD, Brant SR, Rioux JD, Silverberg MS, Daly MJ, Steinhart AH, Abraham C, Regueiro M, Griffiths A, Dassopoulos T, Bitton A, Yang H, Targan S, Datta LW, Kistner EO, Schumm LP, Lee AT, Gregersen PK, Barmada MM, Rotter JI, Nicolae DL, Cho JH (2006). A genome-wide association study identifies IL23R as an inflammatory bowel disease gene. Science, 314: 461–1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elahi B, Nikfar S, Derakhshani S, Vafaie M, Abdollahi M (2008) . On the benefit of probiotics in the management of pouchitis in patients underwent ileal pouch anal anastomosis: A meta-analysis of controlled clinical trials. Digestive Diseases and Sciences, 53:1278–1284. [DOI] [PubMed] [Google Scholar]
- Fedorak RN, Gionchetti P, Campieri M (1998). VSL3 probiotic mixture induces remission in patients with active ulcerative colitis (abstr). Gastroenterology, 124:A377. [DOI] [PubMed] [Google Scholar]
- Fiocchi C (1998). Inflammatory bowel disease: etiology and pathogenesis. Gastroenterology, 115: 182–205. [DOI] [PubMed] [Google Scholar]
- Fioramonti J, Theodorou V, Bueno L (2003). Probiotics: what are they? What are their effects on gut physiology? Best Practice Research Clinics in Gastroenterology, 17: 711–724. [DOI] [PubMed] [Google Scholar]
- Flynn S, van Sinderen D, Thornton GM, Holo H, Nes IF, Collins JK (2002). Characterization of the genetic locus responsible for the production of ABP-118, a novel bacteriocin produced by the probiotic bacterium Lactobacillus salivarius subsp. salivarius UCC118. Microbiology, 148:73–84. [DOI] [PubMed] [Google Scholar]
- Foye OT, Huang IF, Chiou CC, Allan Walker W, Shi HN (2012). Early Administration of probiotic Lactobacillus acidophilus and/or prebiotic inulin attenuates pathogen-mediated intestinal inflammation and Smad 7 cell signaling. FEMS Immunology & Medical Microbiology, 65:467–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke A, Hampe J, Rosenstiel P, Becker C, Wagner F, Hasler R, Little RD, Huse K, Ruether A, Balschun T, Wittig M, Elsharawy A, Mayr G, Albrecht M, Prescott NJ, Onnie CM, Fournier H, Keith T, Radelof U, Platzer M, Mathew CG, Stoll M, Krawczak M, Nürnberg P, Schreiber S (2007). Systematic association mapping identifies NELL1 as a novel IBD disease gene. PLosOne, 2: e691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furrie E, Macfarlane S, Kennedy A, Cummings JH, Walsh SV, O’neil DA, Macfarlane GT (2005). Synbiotic therapy (Bifidobacterium longum/’Synergy 1) initiates resolution of inflammation in patients with active ulcerative colitis: a randomized controlled pilot trial. Gut, 54:242–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentschew L, Ferguson LR (2012). Role of nutrition and microbiota in susceptibility to inflammatory bowel diseases. Molecular Nutrition Food Research, 56:524–535. [DOI] [PubMed] [Google Scholar]
- Giaffer MH, Holdsworth CD, Duerden BI (1991).The assessment of faecal flora in patients with inflammatory bowel disease by a simplified bacteriological technique. Journal of Medical Microbiology, 35:238–43. [DOI] [PubMed] [Google Scholar]
- Gionchetti P, Rizzello F, Helwig U, Venturi A, Lammers KM, Brigidi P, Vitali B, Poggioli G, Miglioli M, Campieri M (2003). Prophylaxis of pouchitis onset with probiotic therapy: a double blind, placebo-controlled trial. Gastroenterology, 124:1202–1209. [DOI] [PubMed] [Google Scholar]
- Gionchetti P, Rizzello F, Venturi A, Brigidi P, Matteuzzi D, Bazzocchi G, Poggioli G, Miglioli M, Campieri M. (2000). Oral bacteriotherapy as maintenance treatment in patients with chronic pouchitis: a double-blind, placebo-controlled trial. Gastroenterology, 119:305–309. [DOI] [PubMed] [Google Scholar]
- Glas J, Seiderer J, Wetzke M., Konrad A, Török HP, Schmechel S, Tonenchi L, Grassl C, Dambacher J, Pfennig S,Maier K, Griga T, Klein W, Epplen JT, Schiemann U, Folwaczny C, Lohse P, Göke B, Ochsenkühn T, Müller- Myhsok B, Folwaczny M, Mussack T, Brand S (2007). rs1004819 is the main disease-associated IL23R variant in German Crohn’s disease patients: combined analysis of IL23R, CARD15, and OCTN1/2 variants. PLoSOne, 2:e819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosselink MP, Schouten WR, van Lieshout LM, Hop WC, Laman JD,. Ruseler-van Embden JG (2004). Delay of the first onset of pouchitis by oral intake of the probiotic strain Lactobacillus rhamnosus GG. Diseases of the Colon & Rectum, 47:876–884. [DOI] [PubMed] [Google Scholar]
- Guarner F, Malagelada JR (2003). Gut flora in health and disease. Lancet, 361:512–519. [DOI] [PubMed] [Google Scholar]
- Guslandi M, Mezzi G, Sorghi M, Testoni PA. (2000). Saccharomyces boulardii in maintenance treatment of Crohn’s disease. Digestive Disease Science, 45:1462–1464. [DOI] [PubMed] [Google Scholar]
- Hampe J, Franke A, Rosenstiel P, Till A, Teuber M, Huse K, Albrecht M, Mayr G, De La Vega FM, Briggs J, Günther S,Prescott NJ, Onnie CM, Häsler R, Sipos B, Fölsch UR, Lengauer T, Platzer M, Mathew CG, Krawczak M, Schreiber SA (2007). Genome-wide association scan of non- synonymous SNPs identifies a susceptibility variant for Crohn disease in ATG16L1. Nature Genetics, 39:207–211. [DOI] [PubMed] [Google Scholar]
- Hassan M, Kjos M, Nes IF, Diep DB, Lotfipour F (2012). Natural antimicrobial peptides from bacteria: characteristics and potential applications to fight against antibiotic resistance. Journal of Applied Microbiology, 113:723–36. [DOI] [PubMed] [Google Scholar]
- Hausmann M, Kiessling S, Mstermann S (2002). Toll-like receptors 2 and 4 are up-regulated during intestinal inflammation. Gastroenterology, 122:1987–2000. [DOI] [PubMed] [Google Scholar]
- Hillilä MT, Färkkilä MA(2004). Prevalence of irritable bowel syndrome according to different diagnostic criteria in a non-selected adult population. Alimentary Pharmacology Therapeutics, 20: 339–345. [DOI] [PubMed] [Google Scholar]
- Huebner C, Ding Y, Petermann I, Knapp C, Ferguson LR (2011). The probiotic Escherichia coli Nissle 1917 reduces pathogen invasion and modulates cytokine expression in Caco-2 Cells infected with Crohn’s Disease-associated E. coli LF82. Applied Environmental Microbiology, 77:2541–2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hugot JP, Chamaillard M, Zouali H, Lesage S, Cezard JP, Belaiche J, Almer S, Tysk C, O’Morain CA, Gassull M, Binder V, Finkel Y, Cortot A, Modigliani R, Laurent-Puig P, Gower-Rousseau C, Macry J, Colombel JF, Sahbatou M, Thomas G (2001). Associations of NOD2 leucine-rich repeat variants with susceptibility to Crohn’s disease. Nature, 411:599–603. [DOI] [PubMed] [Google Scholar]
- Jonkers D, Penders J, Masclee A, Pierik M (2012). Probiotics in the management of inflammatory bowel disease: a systematic review of intervention studies in adult patients. Drugs, 72:803–823. [DOI] [PubMed] [Google Scholar]
- Khan MW, Kale AA, Bere P, Vajjala S, Gounaris E, Pakanati KC (2012). Microbes, intestinal inflammation and probiotics. Expert Review Gastroenterology and Hepatology, 6:81–94. [DOI] [PubMed] [Google Scholar]
- Kim SH, Yang SJ, Koo HC, Bae WK, Kim JY, Park JH, Baek YJ, Park YH(2001). Inhibitory activity of Bifidobacterium longum HY8001 against Vero cytotoxin of Escherichia coli O157:H7. Journal of Food Protection, 64:667–1673. [DOI] [PubMed] [Google Scholar]
- Konturek PC, Brzozowski T, Konturek SJ (2011). Stress and the gut: pathophysiology, clinical consequences, diagnostic approach and treatment options. Journal of Physiology and Pharmacology, 62: 591–599. [PubMed] [Google Scholar]
- Kruis W, Chrubasik S, Boehm S, Stange C, Schulze J (2012). A double-blind placebo-controlled trial to study therapeutic effects of probiotic Escherichia coli Nissle 1917 in subgroups of patients with irritable bowel syndrome. International Journal of Colorectal Diseases, 27:467–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruis W, Fric P, Pokrotnieks J, Lukas M, Fixa B, Kascak M, Kamm MA, Weismueller J, Beglinger C, Stolte M, Wolff C, Schulze J (2004). Maintaining remission of ulcerative colitis with the probiotic Escherichia coli Nissle 1917 is as effective as with standard mesalazine. Gut, 53:1617–1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruis W, Kalk EK, Fric P (2001). Maintenance of remission in ulcerative colitis is equally effective with Escherichia coli Nissle 1917 and with standard mesalamine (abstr). Gastroenterology, 120:A127. [Google Scholar]
- Kühn R, Löhler J, Rennick D, Rajewsky K, Müller W(1993). Interleukin-10-deficient mice develop chronic enterocolitis. Cell, 75:263–274. [DOI] [PubMed] [Google Scholar]
- Kumar M, Verma V, Nagpal R, Kumar A, Gautam SK, Behare PV, Grover CR and Aggarwal PK (2011). Effect of Probiotic Fermented Milk and Chlorophyllin on Gene Expressions and Genotoxicity during AFB1-induced Hepatocellular Carcinoma. Gene, 490: 54–59. [DOI] [PubMed] [Google Scholar]
- Kumar M, Verma V, Nagpal R, Kumar A, Behare PV, Singh B, Aggarwal PK (2012b). Anti-carcinogenic effect of probiotic fermented milk and Chlorophyllin on aflatoxin-B1 induced liver carcinogenesis in rats. British Journal of Nutrition, 107:1006–1016. [DOI] [PubMed] [Google Scholar]
- Kumar M, Nagpal R, Verma V, Kumar A, Kaur N, Hemalatha R, Gautam S, Singh B. (2012c). The Probiotic metabolites as epigenetic target to control colon cancer. Nutrition Reviews, 71:23–34. [DOI] [PubMed] [Google Scholar]
- Kumar M, Nagpal R., Kumar R, Hemalatha R, Verma V, Kumar A, Chakraborty C, Singh B, Marotta F, Jain S, Yadav H (2012a). Cholesterol lowering Probiotics as potential biotherapeutics for metabolic diseases. Experimental Diabetes Research, 2012:902917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar M, Kumar A, Nagpal R, Mohania D, Behare P, Verma V, Kumar P, Poddar D, Aggarwal PK, Henry CJ, Jain S and Yadav H (2010). Cancer-preventing attributes of probiotics: an update. International Journal of Food Science & Nutrition, 61:473–496. [DOI] [PubMed] [Google Scholar]
- Kumar M, Mohania D, Poddar D, Behare PV, Nagpal R, Kumar A, Aggarwal PK (2009). A probiotic-fermented milk prepared by mixed culture combination reduces pathogen shedding and alleviates disease signs in rats challenged with pathogens. International Journal of Probiotics and Prebiotics, 4:211–218. [Google Scholar]
- Lakatos PL, Fischer S, Lakatos L, Gal I, Papp J (2006). Current concept on the pathogenesis of inflammatory bowel disease-crosstalk between genetic and microbial factors: pathogenic bacteria and altered bacterial sensing or changes in mucosal integrity take “toll”? World Journal of Gastroenterology, 12:829–1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammers KM, Helwig U, Swennen E, Rizzello F, Venturi A, Caramelli E, Kamm MA, Brigidi P, Gionchetti P, Campieri M (2002). Effect of probiotic strains on interleukin 8 production by HT29/19A cells. American Journal of Gastroenterology, 97:1182–1186. [DOI] [PubMed] [Google Scholar]
- Lavasani S, Dzhambazov B, Nouri M, Fak F, Buske S, Molin G, Thorlacius H, Alenfall J, Jeppsson B, Weström B (2010). A novel probiotic mixture exerts a therapeutic effect on experimental autoimmune encephalomyelitis mediated by IL-10 producing regulatory T cells. PLoSOne, 2:5:e9009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HS, Han SY, Bae EA, Huh CS, Ahn YT, Lee JH, Kim DH (2008). Lactic acid bacteria inhibit proinflammatory cytokine expression and bacterial glycosaminoglycan degradation activity in dextran sulfate sodium-induced colitic mice. InternationalImmunopharmacology, 8:574–580. [DOI] [PubMed] [Google Scholar]
- Leung Y, Kaplan GG, Rioux KP, Hubbard J, Kamhawi S, Stasiak L, Cohen RD, Devlin SM, Panaccione R, Hanauer SB, Rubin DT (2012). Assessment of variables associated with smoking cessation in Crohn’s disease. Digestive Diseases and Sciences. 57:1026–1032. [DOI] [PubMed] [Google Scholar]
- Lewis JD, Lichtenstein GR, Stein RB, Deren JJ, Judge TA, Fogt F, Furth EE, Demissie EJ, Hurd LB, Su CG, Keilbaugh SA, Lazar MA, Wu GD. (2001). An open-label trial of the PPAR-gamma ligand rosiglitazone for active ulcerative colitis. American Journal of Gastroenterology, 96:3323–3328. [DOI] [PubMed] [Google Scholar]
- Libioulle C, Louis E, Hansoul S, Sandor C, Farnir F, Franchimont D, Vermeire S, Dewit O, de Vos M, Dixon A, Demarche B, Gut I, Heath S, Foglio M, Liang L, Laukens D, Mni M, Zelenika D, Van Gossum A, Rutgeerts P, Belaiche J, Lathrop M, Georges M (2007). Novel Crohn disease locus identified by genome-wide association maps to a gene desert on 5p13.1 and modulates expression of PTGER4. PLoS Genetics, 3:e58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtiger S, Present DH, Kornbluth A, Gelernt I, Bauer J, Galler G, Michelassi F, Hanauer S (1994). Cyclosporine in severe ulcerative colitis refractory to steroid therapy. New England Journal of Medicine, 330:1841–1845. [DOI] [PubMed] [Google Scholar]
- Lievin V, Peiffer I, Hudault S, Rochat F, Brassart D, Neeser JR, Servin AL (2000). Bifidobacterium strains from resident infant human gastrointestinal microflora exert antimicrobial activity. Gut, 47:646–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linskens RK, Huijsdens XW, Savelkoul PH, Vandenbroucke-Grauls CM, Meuwissen SG, (2001). The bacterial flora in inflammatory bowel disease: current insights in pathogenesis and the influence of antibiotics and probiotics. Scandinavian Journal of Gastroenterology. Suppl., 36:29–40. [DOI] [PubMed] [Google Scholar]
- Lodes MJ, Cong Y, Elson CO, Mohamath R, Landers CJ, Targan SR, Fort M, Hershberg RM(2004). Bacterial flagellin is a dominant antigen in Crohn’s disease. Journal of Clinical Investigation, 113:1296–1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mac DR (2011).Probiotics in inflammatory bowel diseases and associated conditions. Nutrients, 3: 245–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald TT, Monteleone G (2005). Immunity, inflammation, and allergy in the gut. Science, 307:1920 −1925. [DOI] [PubMed] [Google Scholar]
- Macfarlane GT and Macfarlane S (2012). Bacteria, colonic fermentation, and gastrointestinal health. Journal of AOAC International, 95: 50–60. [DOI] [PubMed] [Google Scholar]
- Madden MV, Farthing MJ, Nicholls RJ (1990). Inflammation in ileal reservoirs: ‘pouchitis’. Gut, 31: 247–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madsen KL, Doyle JS, Jewell LD, Taverini MM, Fedorak RN. (1999). Lactobacillus species prevents colitis in interleukin-10 gene-deficient mice. Gastroenterology. 116:1107–1114. [DOI] [PubMed] [Google Scholar]
- Madsen KL (2001). Inflammatory bowel disease: lessons from the IL-10 gene deficient mouse. Clinical & Investigative Medicine, 24:250–257. [PubMed] [Google Scholar]
- Mahida YR, Rolfe VE. (2004). Host-bacterial interactions in inflammatory bowel disease. Clinical Science (Lond). 107:331–341. [DOI] [PubMed] [Google Scholar]
- Malchow HA. (1997). Crohn’s disease and Escherichia coli, a new approach in therapy to maintain remission of colonic Crohn’s disease? Journal of Clinical Gastroenterology 25:653–658. [DOI] [PubMed] [Google Scholar]
- Malin M, Suolalainen H, Saxelin M, Isolauri E (1996). Promotion of IgA immune response in patients with Crohn’s disease by oral bacteriotherapy with Lactobacillus GG. Annanls of Nutrition and Metabolism, 40:137–45. [DOI] [PubMed] [Google Scholar]
- Mannon PJ, Fuss IJ, Mayer L, Elson CO, Sandborn WJ, Present D, Dolin B, Goodman N, Groden C, Hornung RL, Quezado M, Yang Z, Neurath MF, Salfeld J, Veldman GM,(2004). Schwertschlag U, Strober W. Anti-IL-12 Crohn’s Disease Study Group. Anti-interleukin-12 antibody for active Crohn’s disease. New England Journal of Medicine, 351:2069–2079. [DOI] [PubMed] [Google Scholar]
- Maroo S, Farrell RJ (2006). From SNPs to haplotypes: unraveling the role of MDR1 in IBD. Inflammatory Bowel Diseases, 12:74–76. [DOI] [PubMed] [Google Scholar]
- McCarthy J, O’Mahony L, O’Callaghan L, Sheil B, Vaughan EE, Fitzsimons N, Fitzgibbon J, O’Sullivan GC, Kiely B, Collins JK, Shanahan F. (2003). Double blind, placebo controlled trial of two probiotic strains in interleukin 10 knockout mice and mechanistic link with cytokine balance. Gut, 52:975–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mileti E, Matteoli G, Iliev ID, Rescigno M (2009). Comparison of the immunomodulatory properties of three probiotic strains of Lactobacilli using complex culture systems: prediction for in vivo efficacy. PLoS One, 16: 4:e7056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mimura T, Rizzello F, Helwig U, Poggioli G, Schreiber S, Talbot IC, Nicholls RJ, Gionchetti P, Campieri M, Kamm MA (2004). Once daily high dose probiotic therapy (VSL#3) for maintaining remission in recurrent or refractory pouchitis. Gut, 53:108–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteleone G, Monteleone I, Fina D, Vavassori P, Del Vecchio Blanco G, Caruso R, Tersigni R, Alessandroni L, Biancone L, Naccari GC, MacDonald TT, Pallone F (2005). Interleukin-21 enhances T-helper cell type I signaling and interferon-gamma production in Crohn’s disease. Gastroenterology, 128:687–694. [DOI] [PubMed] [Google Scholar]
- Mow WS, Vasiliauskas EA, Lin YC, Fleshner PR, Papadakis KA, Taylor KD, Landers CJ, Abreu-Martin MT, Rotter JI, Yang H, Targan SR (2004). Association of antibody responses to microbial antigens and complications of small bowel Crohn’s disease. Gastroenterology, 126:414–424. [DOI] [PubMed] [Google Scholar]
- Nagalingam NA, Lynch SV (2012). Role of the Microbiota in inflammatory bowel diseases. Inflammatory Bowel Diseases, 18:968–980. [DOI] [PubMed] [Google Scholar]
- Nagpal R, Kumar A, Kumar M, Behare PV, Jain S, Yadav H(2012c). Probiotics, their health benefits and applications for developing healthier foods: a review. FEMS Microbiology Letters, 334:1–15. [DOI] [PubMed] [Google Scholar]
- Nagpal R, Kumar A, Kumar M (2012b). Fortification and fermentation of fruit juices by probiotic lactobacilli. Annals of Microbiology, 62: 1573–1578. [Google Scholar]
- Nagpal R, Behare PV, Kumar M, Mohania D, Yadav M, Jain S, Menon S, Parkash O, Marotta F, Minelli E, Henry CJK and Yadav H (2012a). Milk, milk products and disease free health: an updated overview. Critical Reviews in Food Science & Nutrition, 52: 321–333. [DOI] [PubMed] [Google Scholar]
- Nagpal R, Kaur A (2011). Synbiotic effect of various prebiotics on in-vitro activities of probiotic lactobacilli. Ecology of Food & Nutrition, 50:63–68. [DOI] [PubMed] [Google Scholar]
- Nagpal R, Kumar A and Arora S (2010). In vitro probiotic potential of lactobacilli from indigenous milk products. International Journal of Probiotics and Prebiotics, 5: 103–110. [Google Scholar]
- Nagpal R, Yadav H, Puniya AK, Singh K, Jain S and Marotta F (2007). Potential of probiotics and prebiotics for synbiotic functional dairy foods. International Journal of Probiotics and Prebiotics, 2:75–84. [Google Scholar]
- Neish AS, Gewirtz AT, Zeng H, Young AN, Hobert ME, Karmali V, Rao AS, Madara JL (2000). Prokaryotic regulation of epithelial responses by inhibition of IkappaB- alpha ubiquitination. Science, 289:1560–1563. [DOI] [PubMed] [Google Scholar]
- Neish AS Bacterial inhibition of eukaryotic pro-inflammatory pathways. (2004). Immunology Research 29:175–86. [DOI] [PubMed] [Google Scholar]
- Nencioni A, Wesselborg S, Brossart P (2003). Role of peroxisome proliferatoractivated receptor gamma and its ligands in the control of immune responses. Critical Review in Immunology, 23:1–13. [DOI] [PubMed] [Google Scholar]
- Neudeck BL, Loeb JM, Faith NG (2004). Lactobacillus casei alters hPEPT1-mediated glycylsarcosine uptake in Caco- 2 cells. Journal of Nutrition, 134:1120–3. [DOI] [PubMed] [Google Scholar]
- Neut C, Bulois P., Desreumaux P, Membre JM, Lederman E,, Gambiez L, Cortot A, Quandalle P, van Kruiningen H, Colombel JF (2002). Changes in the bacterial flora of the neoterminal ileum after ileocolonic resection for Crohn’s disease. American Journal of Gastroenterology, 97:939–946. [DOI] [PubMed] [Google Scholar]
- Ogura Y, Bonen DK, Inohara N, Nicolae DL, Chen FF, Ramos R, Britton H, Moran T, Karaliuskas R, Duerr RH, Achkar JP, Brant SR, Bayless TM, Kirschner BS, Hanauer SB, Nuñez G, Cho JH (2001). A frameshift mutation in NOD2 associated with susceptibility to Crohn’s disease. Nature, 411: 603–606. [DOI] [PubMed] [Google Scholar]
- Ohkusa T, Yoshida T, Sato N (2009). Commensal bacteria can enter colonic epithelial cells and induce proinflammatory cytokine secretion: a possible pathogenic mechanism of ulcerative colitis. Journal of Medical Microbiology, 58:535–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Mahony L, Feeney M, O’Halloran S, Murphy L, Kiely B,Fitzgibbon J, Lee G, O’Sullivan G, Shanahan F, Collins JK. (2001). Probiotic impact on microbial flora, inflammation and tumour development in IL10 knockout mice. Alimentary Pharmacology Therapeutics, 15:1219–1225. [DOI] [PubMed] [Google Scholar]
- O’Shea EF, Cotter PD, Stanton C, Ross RP, Hill C (2012). Production of bioactive substances by intestinal bacteria as a basis for explaining probiotic mechanisms: bacteriocins and conjugated linoleic acid. International Journal of Food Microbiology, 152:189–205. [DOI] [PubMed] [Google Scholar]
- O’Shea EF, O’Connor PM, Raftis EJ, O’Toole PW, Stanton C, Cotter PD, Ross RP, Hill C (2011). Production of multiple bacteriocins from a single locus by gastrointestinal strains of Lactobacillus salivarius. Journal of Bacteriology, 193:6973–6982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osman N, Adawi D, Ahrne S, Jeppsson B, Molin G (2004). Modulation of the effect of dextran sulfate odium-induced acute colitis by the administration of different probiotic strains of Lactobacillus and Bifidobacterium. Digestive Diseases and Science, 49:320–327. [DOI] [PubMed] [Google Scholar]
- Otte JM, Podolsky DK (2004). Functional modulation of enterocytes by gram-positive and gram-negative microorganisms. American Journal of Physiology and Gastrointestinal Liver Physiology, 286: G613–26. [DOI] [PubMed] [Google Scholar]
- Panwala CM, Jones JC, Viney JL (1998). A novel model of inflammatory bowel disease: mice deficient for the multiple drug resistance gene, mdrla, spontaneously develop colitis. Journal of Immunology, 161:5733–5744. [PubMed] [Google Scholar]
- Pardi DS, D’Haens G, Shen B, Campbell S, Gionchetti P (2009). Clinical guidelines for the management of pouchitis. Inflammatory Bowel Diseases, 15:1424–1431. [DOI] [PubMed] [Google Scholar]
- Parkes M, Barrett JC, Prescott NJ, Tremelling M, Anderson CA, Fisher SA, Roberts RG, Nimmo ER, Cummings FR, Soars D, Drummond H, Lees CW, Khawaja SA, Bagnall R, Burke DA, Todhunter CE, Ahmad T, Onnie CM, McArdle W, Strachan D, Bethel G, Bryan C, Lewis CM, Deloukas P, Forbes A, Sanderson J, Jewell DP, Satsangi J, Mansfield JC Wellcome Trust Case Control Consortium, Cardon L, Mathew CG. (2007). Sequence variants in the autophagy gene IRGM and multiple other replicating loci contribute to Crohn’s disease susceptibility. Nature Genetics, 39:830–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peltekova VD, Wintle RF, Rubin LA, Amos CI, Huang Q, Gu X, Newman B, Van Oene M, Cescon D, Greenberg G, Griffiths AM, St George-Hyslop PH, Siminovitch KA (2004). Functional variants of OCTN cation transporter genes are associated with Crohn disease. Nature Genetics, 36:471–475. [DOI] [PubMed] [Google Scholar]
- Pitcher MC, Cummings JH (1996). Hydrogen sulphide: a bacterial toxin in ulcerative colitis? Gut, 39:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podolsky DK (2002). Inflammatory bowel disease. New England Journal of Medicine, 347: 417–429 [DOI] [PubMed] [Google Scholar]
- Pridmore RD, Pittet AC, Praplan F, Cavadini C (2008). Hydrogen peroxide production by Lactobacillus johnsonii NCC 533 and its role in anti-Salmonella activity. FEMS Microbiology Letter, 283:210–215. [DOI] [PubMed] [Google Scholar]
- Rachmilewitz D, Katakura K, Karmeli F, Hayashi T, Reinus C, Rudensky B, Akira S, Takeda K, Lee J, Takabayashi K, Raz E (2004). Toll-like receptor 9 signaling mediates the anti-inflammatory effects of probiotics in murine experimental colitis. Gastroenterology, 126:520–528. [DOI] [PubMed] [Google Scholar]
- Rembacken BJ, Snelling AM, Hawkey PM, Chalmers DM,Axon AT (1999). Non-pathogenic Escherichia coli versus mesalazine for the treatment of ulcerative colitis: a randomized trial. Lancet, 354:635–639. [DOI] [PubMed] [Google Scholar]
- Robertson DJ, Sandler RS (2001). Measles virus and Crohn’s disease: a critical appraisal of the current literature. Inflammatory Bowel Diseases, 7:51–57. [DOI] [PubMed] [Google Scholar]
- Rolfe VE, Fortun PJ, Hawkey CJ, Bath-Hextall F (2006). Probiotics for maintenance of remission in Crohn’s disease, Cochrane Database. Systematic Review, 4, CD004826. [DOI] [PubMed] [Google Scholar]
- Rousseaux C, Lefebvre B, Dubuquoy L, Lefebvre P, Romano O, Auwerx J, Metzger D, Wahli W, Desvergne B, Naccari GC, Chavatte P, Farce A, Bulois P, Cortot A, Colombel JF, Desreumaux P (2005). Intestinal antiinflammatory effect of 5-aminosalicylic acid is dependent on peroxisome proliferator-activated receptor- gamma. Journal of Experimental Medicine, 201:1205–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuel BS, Shaito A, Motoike T, Rey FE, Backhed F, Manchester JK, Hammer RE, Williams SC, Crowley J, Yanagisawa M (2008). Effects of the gut microbiota on host adiposity are modulated by the short-chain fatty-acid binding protein-coupled receptor Gpr41. Proceedings of National Academy of Sciences USA, 105:16767–16772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandborn WJ, Tremaine WJ, Batts KP, Pemberton JH, Phillips SF (1994). Pouchitis after ileal pouch-anal anastomosis: a Pouchitis Disease Activity Index. Mayo Clinic Proceedings, 69: 409–415. [DOI] [PubMed] [Google Scholar]
- Sartor RB and Hoentjen F (2005). Proinflammatory cytokines and signaling pathways in intestinal innate immune cells In: Mucosal Immunology, 681–701 (Eds Mestecky J et al. ) Philadelphia: Elsevier. [Google Scholar]
- Sartor RB (2006). Mechanisms of disease: pathogenesis of Crohn’s disease and ulcerative colitis. Nature Clinicao Practice in Gastroenterology and Hepatology, 3:390–407. [DOI] [PubMed] [Google Scholar]
- Sartor RB (2005). Probiotic therapy ofintestinal inflammation and infections. Current Opinion in Gastroenterology, 21:44–50. [PubMed] [Google Scholar]
- Sartor RB (2004).Therapeutic manipulation of the enteric microflora in inflammatory bowel diseases: antibiotics, probiotics, and prebiotics. Gastroenterology, 126:1620–1633. [DOI] [PubMed] [Google Scholar]
- Sawada K, Kusugami K, Suzuki Y, Bamba T, Munakata A, Hibi T, Shimoyama T (2005). Leukocytapheresis in ulcerative colitis: results of a multicenter double-blind prospective case— control study with sham apheresis as placebo treatment. American Journal of Gastroenterology, 100:1362–1369. [DOI] [PubMed] [Google Scholar]
- Schierack P, Kleta S, Tedin K, Babila JT, Oswald S, Oelschlaeger TA, Hiemann R, Paetzold S, Wieler LH (2011). E. coli Nissle 1917 Affects Salmonella adhesion to porcine intestinal epithelial cells. PLoS One, 6: e14712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz M, Linde HJ, Lehn N, Zimmermann K, Grossmann J, Falk W, Schölmerich J (2003). Immunostimulatory consequences of oral administration of Lactobacillus rhamnosus strain GG in healthy volunteers. Journal of Dairy Research, 70: 165–73. [DOI] [PubMed] [Google Scholar]
- Schultz M, Timmer A, Herfarth HH, Sartor RB, Vanderhoof JA, Rath HC (2004). Lactobacillus GG in inducing and maintaining remission of Crohn’s disease, BMC Gastroenterology, 4:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz M, Veltkamp C, Dieleman LA, Grenther WB, Wyrick PB, Tonkonogy SL, Sartor RB (2002). Lactobacillus plantarum 299V in the treatment and prevention of spontaneous colitis in interleukin-10-deficient mice. Inflammatory Bowel Diseases, 8: 71–80. [DOI] [PubMed] [Google Scholar]
- Schwartz RH (2005). Natural regulatory T cells and self-tolerance. Nature Immunology, 6: 327–330. [DOI] [PubMed] [Google Scholar]
- Seksik P, Rigottier-Gois L, Gramet G, Sutren M, Pochart P, Marteau P, Jian R, Doré J (2003). Alterations of the dominant faecal bacterial groups in patients with Crohn’s disease of the colon. Gut, 52: 237–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen B, Achkar JP, Lashner BA, Ormsby AH, Remzi FH, Bevins CL, Brzezinski A, Petras RE, Fazio VW (2001). Endoscopic and histologic evaluation together with symptom assessment are required to diagnose pouchitis. Gastroenterology 121:261–267. [DOI] [PubMed] [Google Scholar]
- Shiba T, Aiba Y, Ishikawa H, Ushiyama A, Takagi A, Mine T, Koga Y (2003). The suppressive effect of bifidobacteria on Bacteroides vulgatus, a putative pathogenic microbe in inflammatory bowel disease. Microbiology and Immunology, 47:371–378. [DOI] [PubMed] [Google Scholar]
- Siciliano RA, Mazzeo MF (2012). Molecular mechanisms of probiotic action: a proteomic perspective. Current Opinion in Microbiology, 15:390–396. [DOI] [PubMed] [Google Scholar]
- Singh B, Bhat TK, Kurade NP, Sharma OP (2008). Metagenomics in animals gastrointestinal ecosystem: a microbiological and biotechnolgical perspective. Indian Journal of Microbiology 48:216–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh B, Bhat TK, Singh B (2003). Potential therapeutic applications of some antinutritional plant secondary metabolites. Review. Journal of Agriculture Food Chemistry, 51:5579–5597. [DOI] [PubMed] [Google Scholar]
- Spencer DM, Veldman GM, Banerjee S, Willis J, Levine AD (2002). Distinct inflammatory mechanisms mediate early versus late colitis in mice. Gastroenterology, 122:94–105. [DOI] [PubMed] [Google Scholar]
- Stappenbeck T, Hooper L, Gordon J (2002). Developmental regulation of intestinal angiogenesis by indigenous microbes via Paneth cells. Proceeding of National Academy of Sciences USA, 99:15451–15455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steidler L, Hans L, Schotte W, Neirynck S, Obermeier F, Falk W, Remaut E (2000). Treatment of murine colitis by Lactococcus lactis secreting interleukin-10. Science, 289:1352–1355. [DOI] [PubMed] [Google Scholar]
- Stoll M, Corneliussen B, Costello CM, Waetzig GH, Mellgard B, Koch WA, Rosenstiel P, Albrecht M, Croucher PJ, Seegert D, Nikolaus S, Hampe J, Lengauer T, Pierrou S,Foelsch UR, Mathew CG, Lagerstrom-Fermer M, Schreiber S (2004). Genetic variation in DLG5 is associated with inflammatory bowel disease. Nature Genetics, 36:476–480. [DOI] [PubMed] [Google Scholar]
- Sturm A, Rilling K, Baumgart DC, Gargas K, Abou- Ghazalé T, Raupach B, Eckert J, Schumann RR, Enders C, Sonnenborn U, Wiedenmann B, Dignass AU (2005). Escherichia coli Nissle 1917 Distinctively Modulates T-Cell Cycling and Expansion via Toll-Like Receptor 2 Signaling. Infection and Immunity, 73:1452–1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugawara K, Olson TS, Moskaluk CA, Stevens BK, Hoang S, Kozaiwa K, Cominelli F, Ley KF, McDuffie M (2005). Linkage to peroxisome proliferator-activated receptor- gamma in SAMP1/YitFc mice and in human Crohn’s disease. Gastroenterology, 128:351–360. [DOI] [PubMed] [Google Scholar]
- Tamboli CP, Neut C, Desreumaux P, Colombel JF (2004). Dysbiosis in inflammatory bowel disease. Gut, 53:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanabe S, Kinuta Y, Saito Y (2008). Bifidobacterium infantis suppresses proinflammatory interleukin-17 production in murine splenocytes and dextran sodium sulfate-induced intestinal inflammation. International Journal of Molecular Medicine, 22:181–185. [PubMed] [Google Scholar]
- Targan SR, Hanauer SB, van Deventer SJ, Mayer L, Present DH,Braakman T, DeWoody KL, Schaible TF, Rutgeerts PJ (1997). A short-term study of chimeric monoclonal antibody cA2 to tumor necrosis factor a for Crohn’s disease. Crohn’s Disease cA2 Study Group. New England Journal of Medicine, 337:1029–1035. [DOI] [PubMed] [Google Scholar]
- Thalmaier D, Dambacher J, Seiderer J, Konrad A, Schachinger V, Pfennig S, Otte JM, Crispin A, Göke B, Ochsenkühn T, Lohse P, Brand S (2006). The +1059G/C polymorphism in the C-reactive protein (CRP) gene is associated with involvement of the terminal ileum and decreased serum CRP levels in patients with Crohn’s disease. Alimentary Pharmacology Therapeutics, 24:1105–1115. [DOI] [PubMed] [Google Scholar]
- Török HP Glas J, Tonenchi L, Lohse P, Müller-Myhsok B, Limbersky O, Neugebauer C, Schnitzler F, Seiderer J, Tillack C, Brand S, Brünnler G, Jagiello P, Epplen JT, Griga T, Klein W, Schiemann U, Folwaczny M, Ochsenkühn T, Folwaczny C (2005). Polymorphisms in the DLG5 and OCTN cation transporter genes in Crohn’s disease. Gut, 54:1421–1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsianos EV, Katsanos KH, Tsianos VE(2012). Role of genetics in the diagnosis and prognosis of Crohn’s disease. World Journal of Gastroenterology, 18:105–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsilingiri K, Barbosa T, Penna G, Caprioli F, Sonzogni A, Viale G, Rescigno M (2012). Probiotic and postbiotic activity in health and disease: comparison on a novel polarised ex-vivo organ culture model. Gut, 61:1007–1015. [DOI] [PubMed] [Google Scholar]
- Vavricka SR, Musch MW, Chang JE, Nakagawa Y, Phanvijhitsiri K, Waypa TS, Merlin D, Schneewind O, Chang EB (2004). hPepT1 transports muramyl dipeptide, activating NF-kappaB and stimulating IL-8 secretion in human colonic Caco2/bbe cells. Gastroenterology, 127:1401–1419. [DOI] [PubMed] [Google Scholar]
- Venturi A, Gionchetti P, Rizzello F, Johansson R, Zucconi E, Brigidi P, Matteuzzi D, Campieri M (1999). Impact on the composition of the fecal flora by a new probiotic preparation: preliminary data on the maintenance treatment of patients with ulcerative colitis. Alimentary Pharmacology Therapeutics, 13:1103–1108. [DOI] [PubMed] [Google Scholar]
- Wehkamp J., Fellermann K., Stange EF. (2005). Human defensins in Crohn’s disease. Chemical Immunology and Allergy, 86:42–-54.. [DOI] [PubMed] [Google Scholar]
- Wehkamp J, Harder J, Wehkamp K, Wehkamp-von Meissner B, Schlee M, Enders C, Sonnenborn U, Nuding S,Bengmark S, Fellermann K, Schröder JM, Stange EF (2004). NF-kappaB- and AP-1-mediated induction of human beta defensin-2 in intestinal epithelial cells by Escherichia coli Nissle 1917: a novel effect of a probiotic bacterium. Infection and Immunity, 72:5750–5758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter SE, Thiennimitr P, Winter MG (2010). Gut inflammation provides a respiratory electron acceptor for Salmonella. Nature, 467:426–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xavier RJ, Podolsky DK (2007). Unraveling the pathogenesis of inflammatory bowel disease. Nature, 448:427–434. [DOI] [PubMed] [Google Scholar]
- Zoumpopoulou G, Foligne B, Christodoulou K, Grangette C, Pot B, Tsakalidou E (2008). Lactobacillus fermentum ACA-DC 179 displays probiotic potential in vitro and protects against trinitrobenzene sulfonic acid (TNBS)-induced colitis and Salmonella infection in murine models. International Journal of Food Microbiology, 121:18–26. [DOI] [PubMed] [Google Scholar]


