Abstract
Nonalcoholic steatohepatitis (NASH) is one of the top 3 indications for liver transplantation (LT) in Western countries. It is unknown whether renal dysfunction at the time of LT has any effect on post-LT outcomes in recipients with NASH. From the United Network for Organ Sharing-Standard Transplant Analysis and Research data set, we identified 4088 NASH recipients who received deceased donor LT. We divided our recipients a priori into 3 categories: group 1 with estimated glomerular filtration rate (eGFR) <30 mL/minute/1.73 m2 at the time of LT and/or received dialysis within 2 weeks preceding LT (n = 937); group 2 with recipients who had eGFR ≥30 mL/minute/1.73 m2 and who did not receive renal replacement therapy prior to LT (n = 2812); and group 3 with recipients who underwent simultaneous liver-kidney transplantation (n = 339). We examined the association of pre-transplant renal dysfunction with death with a functioning graft, all-cause mortality, and graft loss using competing risk regression and Cox proportional hazards models. The mean ± standard deviation age of the cohort at baseline was 58 ± 8 years, 55% were male, 80% were Caucasian, and average exception Model for End-Stage Liver Disease score was 24 ± 9. The median follow-up period was 5 years (median, 1816 days; interquartile range, 1090–2723 days). Compared with group 1 recipients, group 2 recipients had 19% reduced trend for risk for death with a functioning graft (subhazard ratio [SHR], 0.81; 95% confidence interval [CI], 0.64–1.02) and similar risk for graft loss (SHR, 1.25; 95% CI, 0.59–2.62), whereas group 3 recipients had similar risk for death with a functioning graft (SHR, 1.23; 95% CI, 0.96–1.57) and graft loss (SHR, 0.18; 95% CI, 0.02–1.37) using an adjusted competing risk regression model. In conclusion, recipients with preserved renal function before LT showed a trend toward lower risk of death with a functioning graft compared with SLKT recipients and those with pretransplant severe renal dysfunction in patients with NASH.
It is estimated that 1 in 4 liver transplantation (LT) recipients has an estimated glomerular filtration rate (eGFR) of <60 mL/minute/1.73 m2 at the time of LT.(1) Renal dysfunction, both before or after LT, is an important comorbidity associated with an increased risk of death, morbidity, and cost.(2) Serum creatinine, a major component of the Model for End-Stage Liver Disease (MELD) score, has driven the increased incidence of renal dysfunction among patients undergoing LT since the introduction of MELD in 2002.(3) Moreover, end-stage liver failure patients with preserved renal function and unremarkable urinalysis may be noted to have histologic abnormalities on kidney biopsy.(4) More than 50% of the patients with end-stage liver disease and preserved renal function have morphological renal abnormalities, mainly immunoglobulin A nephropathy and diabetic changes, which are evident on the renal biopsy.(4) As a result, the frequency of simultaneous liver-kidney transplantation (SLKT) compared with LT alone has increased(3)
Preexisting renal dysfunction before LT is associated with an increased risk of development of end-stage renal disease (ESRD) as well as death after transplantation.(1,5) The more perplexing clinical question is being able to determine which recipients with renal dysfunction will have recovery of their kidney function versus those recipients who continue to experience a worsening renal dysfunction after LT. Most of these LT recipients will continue to worsen due to calcineurin inhibitor toxicity and lack of recovery from hepatorenal syndrome (HRS),(6) necessitating renal replacement therapy. Several guidelines have attempted to address this question, and all of them use the preexisting renal dysfunction before LT(7–10) for allocation of SLKT.
Nonalcoholic fatty liver disease (NAFLD) is the most common cause of liver disease with a prevalence ranging between 20% and 30% in the Western society(11,12) Nonalcoholic steatohepatitis (NASH) is the subset of NAFLD with progressive histologic damage that can lead to end-stage liver failure.(13) Patients with NASH are at higher risk for developing renal dysfunction as a result of obesity, diabetes mellitus, and hypertension-related chronic kidney disease (CKD).(11,14) Patients in a large observational study showed a strong association between the presence of NAFLD and the development of incidences of CKD.(15) Consequently, the prevalence of CKD in patients with end-stage liver failure secondary to NASH is even higher compared with patients with other etiologies of end-stage liver failure, and NASH is associated with a higher risk of kidney graft loss even after SLKT.(16) However, it is unknown whether the renal dysfunction at the time of LT has any effect on post-LT survival or graft loss in recipients with NASH.
To address this knowledge gap, we aimed to investigate the association of pretransplant renal dysfunction with posttransplant death with a functioning graft, all-cause mortality, and graft loss using a large nationally representative cohort of patients with liver failure secondary to NASH in the United States. We hypothesized that the recipients with preserved renal function versus renal dysfunction had a significantly lower risk of death with a functioning graft, all-cause mortality risk after LT, similar risk for graft loss, and longer kidney transplantation-free survival after LT. We also hypothesized that recipients with SLKT had significantly higher risk for death with a functioning graft and all-cause mortality, but they had a similar risk for graft loss after LT compared with recipients with severe renal dysfunction.
Patients and Methods
DATA source AND COHORT DEFINITION
A total of 60,394 LT recipients (January 2002 through June 2013) were identified from the United Network for Organ Sharing (UNOS)-Standard Transplant Analysis and Research (STAR) data set as the population. NASH LT recipients were determined by primary or secondary indication for LT as reported to UNOS. Only individuals who had NASH as a cause of liver failure and who had data regarding renal dys-function or renal replacement therapy were included in the study. The algorithm for the cohort definition is shown in Fig. 1. We excluded patients with a diagnosis of hepatocellular carcinoma (HCC; n = 12,068) or those who received a living donor LT (n = 2516) or transplantation from split-liver donors (n = 3212), non-heart-beating donors (n = 2607), and multiorgan transplants (n = 688; except SLKT). Some of the excluded patients had more than 1 exclusion criterion. Furthermore, after the exclusion of recipients with non-NASH etiology of chronic liver disease (n = 36,075), 4088 NASH-related LT recipients were included in the final study cohort: 3749 were liver-only recipients, and 339 were SLKT recipients. We also linked the LT data to the kidney transplant data in the UNOS-STAR database using encrypted recipient identifier numbers to identify those patients who received a kidney transplant after their LT or SLKT. The institutional review boards of the University of Tennessee Health Science Center and the University of Memphis approved the study with exemption from informed consent.
FIG. 1.
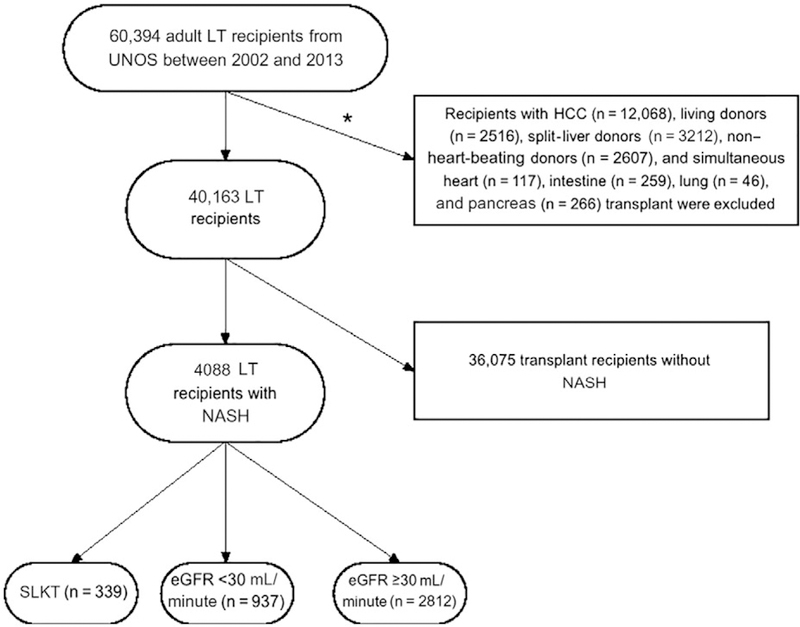
A flowchart of the study population. *Some patients had more than 1 reason for exclusion.
exposure variable
The eGFR was calculated using the abbreviated Modification of Diet in Renal Disease study equation: eGFR (mL/minute/1.73 m2) = 175 × (serum creatinine [Scr])−L154 × (age)−0.203 × (0.742 if female) × (1.212 if African American).(17) We divided our recipients a priori into 3 categories according to their renal dysfunction before LT. Group 1 included recipients who had eGFR <30 mL/minute/1.73 m2 at the time of LT and/or received dialysis within 2 weeks preceding LT (n = 937) and served as the reference group; group 2 included recipients who had eGFR ≥30 mL/minute/1.73 m2 and did not receive renal replacement therapy prior to LT (n = 2812); and group 3 included recipients who underwent SLKT (n = 339).
covariates
The UNOS-STAR database was used to determine baseline demographic characteristics at the time of LT, information on comorbidities, laboratory data at the time of LT, and donor-related data.
outcome assessment
The primary outcomes of interest were death with a functioning graft, all-cause mortality, and graft loss after LT. Mortality and graft loss data, censoring events, and associated dates were obtained from UNOS-STAR data source.
These outcomes were defined as follows:
For the death with a functioning graft analysis, the start of the follow-up period was the date of transplantation, and patients were followed up until death or other events, including graft loss, lost to follow-up, or end of follow-up period. For this analysis, we used a competing risk regression, where the primary outcome was death and the competing outcome was graft loss. Data were censored for loss to follow-up or end of follow-up period.
For the all-cause death analysis, the start of the follow-up period was the date of transplantation, and patients were followed up until death or other censoring events, including lost to follow-up or end of follow-up period. For this analysis, we used Cox proportional hazards regression.
For the graft loss analysis, the start of the follow-up period was the date of transplantation, and patients were followed up until graft loss or other events, including death, lost to follow-up, or end of follow-up period. For this analysis, we used competing risk regression, where the primary outcome was graft loss and the competing outcome was death. Data were censored for loss to follow-up or end of follow-up period.
For the kidney transplant-free analysis, the start of the follow-up period was the date of transplantation, and patients were followed up until kidney transplantation or other events, including liver graft loss, death, lost to follow-up, or end of follow-up period. For this analysis, we used the Kaplan-Meier method only.
statistical analysis
Baseline recipient characteristics were summarized according to the renal dysfunction at the time of LT and presented as percentages for categorical variables and mean ± standard deviation (SD) or median and interquartile range (IQR) for continuous variables. Continuous and categorical variables were compared using P for trend test.
The associations between different renal dysfunction categories and outcomes after LT were assessed using competing risk regression using the Fine and Gray model(18) for death with a functioning graft and graft loss, and the Kaplan-Meier method with log-rank test and Cox proportional hazards models for all-cause mortality.
Independent variables were included in the multivariate models based on theoretical considerations. Variance influence factors were used to indicate collinearity between independent variables. Proportional hazards assumptions were tested using scaled Schoenfeld residuals in the Cox proportional hazards models. Models were incrementally adjusted for the following potential confounders based on theoretical considerations and their availability in this study: model 1 was unadjusted; model 2 was adjusted for age, sex, and race/ethnicity; and model 3 was additionally adjusted for comorbidities (malignancy and diabetes), exception MELD score at the time of transplantation, presence of ascites at the time of transplantation, history of transjugular intrahepatic portosystemic shunt (TIPS) placement, functional status and laboratory data (serum albumin [mg/dL], international normalized ratio [INR], and serum bilirubin [mg/dL]); and model 4 was additionally adjusted for donor-related (age, sex, race, and body mass index [BMI] of the donor) and transplantation-related (cold ischemia time and cytomegalovirus [CMV] mismatches) data and characteristics.
We conducted several sensitivity analyses to evaluate the robustness of our main findings. The association between the absence or degree of underlying renal dysfunction and outcomes after LT were examined in subgroups of patients stratified by age, sex, race, presence or absence of diabetes, and exception MELD score. Potential interactions were formally tested by inclusion of relevant interaction terms. There were 3323 (81%) recipients who had complete data for analysis in the final model (model 4). Missing values were not imputed in primary analyses but were substituted by multiple imputation (n = 5, data set) procedures using the Stata (StataCorp, College Station, TX) “mi” set of commands in sensitivity analyses.(19,20)
Reported P values were 2-sided and reported as significant at <0.05 for all analyses. All analyses were conducted using Stata/MP, version 13.1 (StataCorp, College Station, TX).
Results
BASELINE CHARACTERISTICS
The mean ± SD age of the cohort at baseline was 58 ± 8 years, 55% were male, and 80% were Caucasian. The average MELD score was 24 ± 9, and 52% of the patients were diabetic. Baseline characteristics of recipients categorized by renal dysfunction are shown in Table 1. The recipients with preserved renal function (group 2) were more likely to be male and Caucasian and had lower prevalence of diabetes and lower exception MELD score compared with recipients in groups 1 and 3.
TABLE 1.
Baseline Characteristics of the Study Population
| All Patients (n = 4088) |
Group 1: eGFR <30 mL/minute/ 1.73 m2 or Dialysis (n = 937) |
Group 2: eGFR ≥30 mL/minute/ 1.73 m2 (n = 2812) |
Group 3: SLKT (n = 339) |
PValue for Trend |
|
|---|---|---|---|---|---|
| Sociodemographic data | |||||
| Age, years | 58±8 | 58±8 | 58±8 | 59±8 | 0.02 |
| Sex, male | 2236 (55) | 407 (43) | 1656 (59) | 173 (51) | <0.001 |
| Race/ethnicity | <0.001 | ||||
| Caucasian | 3420 (84) | 735 (78) | 2422 (86) | 263 (78) | |
| African American | 94 (2) | 24 (3) | 53 (2) | 17 (5) | |
| Hispanic | 455 (11) | 144 (15) | 261 (9) | 50 (15) | |
| Others | 88 (2) | 15 (2) | 64 (2) | 9 (3) | |
| Education level | 0.46 | ||||
| Completion of high school or lower | 1764 (43) | 401 (43) | 1210 (43) | 153 (45) | |
| Attendance of college or technical school | 810 (20) | 168 (18) | 576 (20) | 66 (19) | |
| Bachelor’s degree or higher | 903 (22) | 215 (23) | 604 (21) | 84 (25) | |
| Comorbidities | |||||
| Presence of diabetes mellitus | 2116 (52) | 483 (52) | 1403 (50) | 230 (68) | <0.001 |
| Presence of ascites | 3565 (87) | 847 (90) | 2414 (86) | 304 (90) | <0.001 |
| Malignancy | 0.02 | ||||
| No malignancy | 3577 (88) | 835 (89) | 2439 (87) | 303 (90) | |
| Malignancy present | 309 (8) | 58 (6) | 230 (8) | 21 (6) | |
| Uncertain or missing data on malignancy | 171 (4) | 25 (3) | 131 (5) | 15 (4) | |
| Functional status | <0.001 | ||||
| Able to perform normal activities | 955 (23) | 77 (8) | 818 (29) | 60 (18) | |
| Unable to perform normal activities | 2907 (71) | 788 (84) | 1848 (66) | 271 (80) | |
| Presence of TIPS | 446 (11) | 88 (9) | 325 (12) | 33 (10) | 0.43 |
| BMI, kg/m2 | 33±6 | 33±6 | 33±5 | 32±6 | 0.04 |
| Exception MELD score | 24±9 | 33±8 | 20±7 | 30±8 | <0.001 |
| Laboratory data | |||||
| Serum creatinine, mg/dL | 1.3 (1.0–2.1) | 2.7 (2.1–3.7) | 1.1 (0.9–1.4) | 3.4 (2.3–4.7) | <0.001 |
| Serum albumin, g/dL | 3.0 ± 0.7 | 3.1 ± 0.8 | 2.9 ± 0.6 | 3.0 ± 0.8 | <0.001 |
| Serum bilirubin, mg/dL | 4.1 (2.3–8.3) | 6.5 (3.2–19.1) | 3.8 (2.2–6.9) | 3.2 (1.7–7.6) | <0.001 |
| INR | 1.7 (1.4–2.2) | 1.9 (1.6–2.4) | 1.7 (1.4–2.1) | 1.7 (1.4–2.1) | <0.001 |
| Transplantation-related data | |||||
| Cold ischemia time, hours | 6.7 (5.0–8.3) | 6.5 (5.0–8.4) | 6.0 (5.0–7.7) | 6.0 (5.0–7.7) | 0.05 |
| Donor age, years | 43±17 | 41±17 | 44±17 | 37±15 | 0.67 |
| Donor sex, male | 2407 (59) | 532 (57) | 1662 (59) | 213 (63) | 0.29 |
| Donor race/ethnicity | 0.001 | ||||
| Caucasian | 2749 (67) | 609 (65) | 1903 (68) | 237 (70) | |
| African American | 769 (19) | 155 (17) | 562 (20) | 52 (15) | |
| Hispanic | 404 (10) | 118 (13) | 244 (9) | 42 (12) | |
| Others | 135 (3) | 36 (4) | 91 (3) | 8 (2) | |
| Donor BMI, kg/m2 | 28±6 | 27±6 | 28±6 | 27±5 | 0.05 |
| CMV mismatch | 0.047 | ||||
| Donor–/recipient– | 443 (11) | 98 (10) | 307 (11) | 38 (11) | |
| Donor– or donor+/recipient+ | 2485 (61) | 588 (63) | 1677 (60) | 220 (65) | |
| Donor+/recipient– | 862 (21) | 172 (18) | 630 (22) | 60 (18) | |
NOTE: Data are given as n (%), mean ± SD, and median (IQR).
DEATH WITH A FUNCTIONING GRAFT AND ALL-CAUSE MORTALITY IN THE STUDY COHORT
During the entire follow-up period (median, 1816 days [5 years]; IQR, 1090–2723 days [3–7.5 years]) following transplantation, a total of 1065 (26%) deaths occurred (crude incidence rate, 50 per 1000 patient-years; 95% confidence interval [CI], 47–53). The crude mortality rate was the highest in recipients who underwent SLKT, 118 (35%) deaths (77 per 1000 patient-years, 95% CI, 64–92), followed by recipients with group 1, 284 (30%) deaths (66 per 1000 patient-years, 95% CI, 59–74), whereas the lowest mortality rate was observed in recipients in group 2, 663 (24%) deaths (43 per 1000 patient-years, 95% CI, 40–47) as shown in the KaplanMeier survival curve (Fig. 2).
FIG. 2.
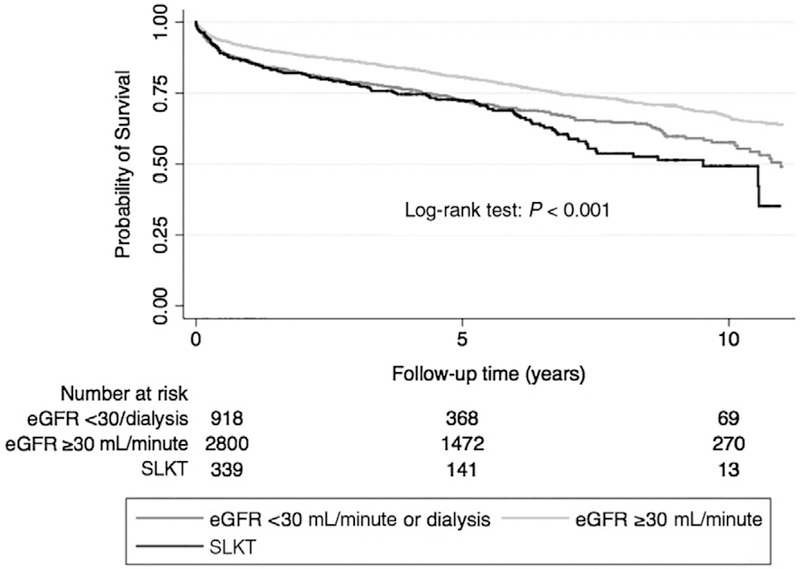
The probability of all-cause mortality of recipients with different kidney function.
Compared with recipients in group 1, recipients in group 2 had 33% lower risk for death with a functioning graft (subhazard ratio [SHR], 0.67; 95% CI, 0.58–0.77), whereas recipients in group 3 had a similar risk for death with a functioning graft (SHR, 1.18; 95% CI, 0.96–1.46) using an unadjusted competing risk regression model (Table 2). A similar trend was observed after adjustment in our adjusted model (Table 2) and also for all-cause mortality using Cox proportional regression models (Supporting Table 1). Additionally, compared with group 1 recipients, recipients in group 2 had 24% lower risk for death with a functioning graft (SHR, 0.76; 95% CI, 0.62–0.93), whereas group 3 recipients had a similar risk for death with a functioning graft (SHR, 1.21; 95% CI, 0.97–1.50) in our multiple-imputed adjusted competing risk regression model (Table 2). Qualitatively, similar results were found for all-cause mortality using multiple-imputed adjusted Cox proportional regression models (Supporting Table 1). Finally, similar to the entire cohort, group 2 recipients had lower risk for death with a functioning graft, whereas group 3 recipients had a similar risk for death with a functioning graft compared with group 1 recipients in most of the subgroups (Fig. 3). Similar qualitative results were found for all-cause mortality using Cox proportional regression models (Supporting Fig. 1).
TABLE 2.
Association Between Renal Function and Death With a Functioning Graft Using a Competing Risk Regression Model With Different Levels of Adjustment
| SHR | 95% CI of SHR | P Value | |
|---|---|---|---|
| Model 1 (n = 4057)* | |||
| eGFR ≥30 mL/minute/1.73 m2 | 0.67 | 0.58–0.77 | <0.001 |
| SLKT | 1.18 | 0.96–1.46 | 0.12 |
| Model 2 (n = 4057)† | |||
| eGFR ≥30 mL/minute/1.73 m2 | 0.66 | 0.57–0.76 | <0.001 |
| SLKT | 1.11 | 0.90–1.38 | 0.32 |
| Model 3 (n = 3793)‡ | |||
| eGFR ≥30 mL/minute/1.73 m2 | 0.75 | 0.60–0.93 | 0.01 |
| SLKT | 1.14 | 0.91–1.43 | 0.25 |
| Model 4 (n = 3323)§ | |||
| eGFR ≥30 mL/minute/1.73 m2 | 0.81 | 0.64–1.02 | 0.08 |
| SLKT | 1.23 | 0.96–1.57 | 0.11 |
| Multiple imputation model (n = 4057) | |||
| eGFR ≥30 mL/minute/1.73 m2 | 0.76 | 0.62–0.93 | 0.009 |
| SLKT | 1.21 | 0.97–1.50 | 0.10 |
NOTE: Reference: recipients who had eGFR <30 mL/minute/1.73 m2 and/or received dialysis.
Unadjusted.
Adjusted for age, sex, and race/ethnicity.
Adjusted for variables included in model 2 and additionally adjusted for comorbidities (malignancy and diabetes), MELD score at the time of transplantation, presence of ascites at the time of transplantation, history of TIPS placement, functional status, and laboratory data (albumin, INR, and serum bilirubin).
Adjusted for variables included in model 3 and additionally adjusted for donor-related data (age, sex, race, and BMI of the donor) and transplantation-related data (cold ischemia time and CMV mismatches).
Fig. 3.
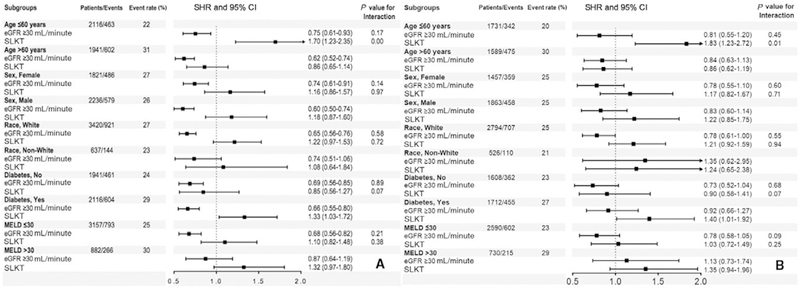
Association of different renal functions and death with a functioning graft in (A) unadjusted and (B) adjusted competing risk regression models in selected subgroups (reference category: recipients who had eGFR <30 mL/minute/1.73 m2 and/or received dialysis). The adjusted value is adjusted for age, sex, race/ethnicity, comorbidities (malignancy and diabetes), MELD score at the time of transplantation, presence of ascites at the time of transplantation, history of TIPS placement, functional status and laboratory data (albumin, INR, and serum bilirubin), donor-related data (age, sex, race, and BMI of the donor), and transplantation-related data (cold ischemia time and CMV mismatches).
GRAFT LOSS IN THE STUDY COHORT
During the entire follow-up period following transplantation, a total of 113 (3%) graft losses occurred (crude incidence rate, 5.4 per 1000 patient-years; 95% CI, 4.5–6.4). The crude graft loss rate was the lowest in group 3 recipients with 3 (1%) graft losses (2.0 per 1000 patient-years, 95% CI, 0.6–6.1), followed by group 1 recipients with 21 (2%) graft losses (4.9 per 1000 patient-years, 95% CI, 3.2–7.5), whereas the highest graft loss rate was observed in group 2 recipients with 89 (3%) graft losses (5.8 per 1000 patient-years, 95% CI, 4.7–7.2).
Compared with group 1 recipients, recipients in group 2 (SHR, 1.39; 95% CI, 0.86–2.23) and in group 3 (SHR, 0.38; 95% CI, 0.11–1.29) had similar graft loss risk, respectively, using an unadjusted competing risk regression model (Table 3). Similar results were observed after adjustment in our adjusted model and also in our multiple-imputed adjusted competing risk regression model (Table 3). Finally, similar to the entire cohort, group 2 and group 3 recipients had similar graft loss risk compared with group 1 recipients in most of the subgroups (Fig. 4).
TABLE 3.
Association Between Renal Function and Graft Loss Using a Competing Risk Regression Model With Different Levels of Adjustment
| SHR | 95% CI of SHR | P Value | |
|---|---|---|---|
| Model 1 (n = 4057)* | |||
| eGFR ≥30 mL/minute/1.73 m2 | 1.39 | 0.86–2.23 | 0.18 |
| SLKT | 0.38 | 0.11–1.29 | 0.12 |
| Model 2 (n = 4057)† | |||
| eGFR ≥30 mL/minute/1.73 m2 | 1.34 | 0.83–2.14 | 0.23 |
| SLKT | 0.41 | 0.12–1.38 | 0.15 |
| Model 3 (n = 3793)‡ | |||
| eGFR ≥30 mL/minute/1.73 m2 | 1.29 | 0.65–2.57 | 0.47 |
| SLKT | 0.45 | 0.13–1.51 | 0.19 |
| Model 4 (n = 3323)§ | |||
| eGFR ≥30 mL/minute/1.73 m2 | 1.25 | 0.59–2.62 | 0.56 |
| SLKT | 0.18 | 0.02–1.37 | 0.10 |
| Multiple imputation model (n = 4057) | |||
| eGFR ≥30 mL/minute/1.73 m2 | 1.40 | 0.70–2.80 | 0.34 |
| SLKT | 0.47 | 0.14–1.58 | 0.23 |
NOTE: Reference: recipients who had eGFR <30 mL/minute/1.73 m2 and/or received dialysis.
Unadjusted.
Adjusted for age, sex, and race/ethnicity.
Adjusted for variables included in model 2 and additionally adjusted for comorbidities (malignancy and diabetes), MELD score at the time of transplantation, presence of ascites at the time of transplantation, history of TIPS placement, functional status, and laboratory data (albumin, INR, and serum bilirubin).
Adjusted for variables included in model 3 and additionally adjusted for donor-related data (age, sex, race, and BMI of the donor) and transplantation-related data (cold ischemia time and CMV mismatches).
FIG. 4.
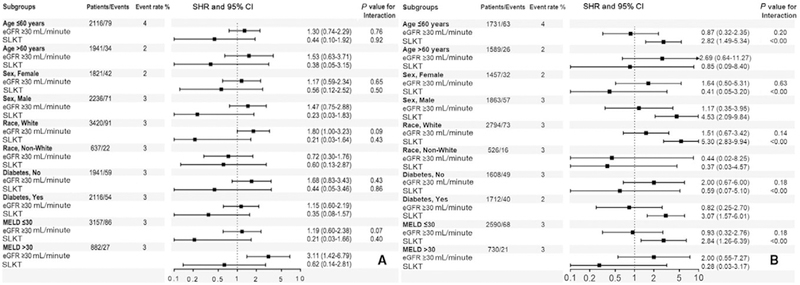
Association of different renal functions and graft loss in (A) unadjusted and (B) adjusted competing risk regression models in selected subgroups (reference category: recipients who had eGFR <30 mL/minute/1.73 m2 and/or received dialysis). The adjusted value is adjusted for age, sex, race/ethnicity, comorbidities (malignancy and diabetes), MELD score at the time of transplantation, presence of ascites at the time of transplantation, history of TIPS placement, functional status and laboratory data (albumin, INR, and serum bilirubin), donor-related data (age, sex, race, and BMI of the donor), and transplantation-related data (cold ischemia time and CMV mismatches).
kidney transplantation-free survival in the study cohort
Group 1 recipients had the lowest probability for kidney transplant-free survival, whereas group 2 recipients had the highest probability during the 8-year follow-up period as shown in Fig. 5.
FIG. 5.
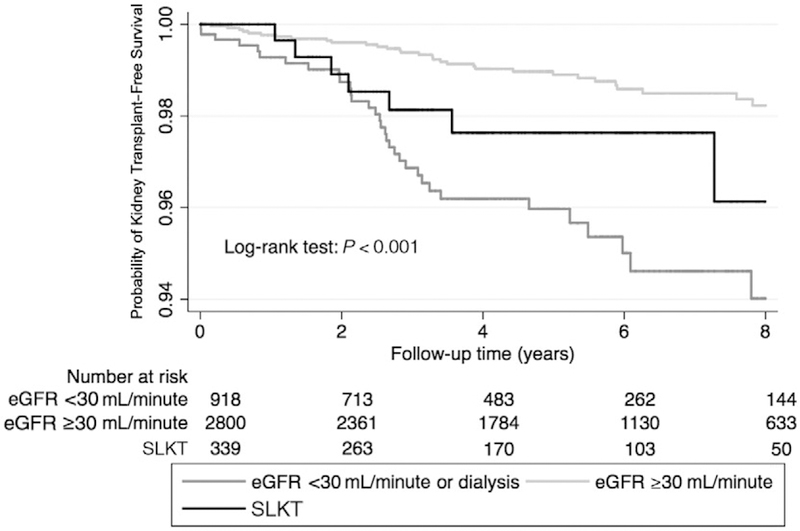
Probability of kidney transplant–free survival of recipients with different kidney functions.
Discussion
In this large national cohort of US LT recipients with NASH, we found an association between pretransplant renal dysfunction with death with a functioning graft and all-cause mortality following LT, independent of demographics, comorbidities, and donor-related variables. Although patients with better renal function (eGFR ≥30 mL/minute/1.73 m2) at the time of LT experienced a trend for lower risk for posttransplant death with a functioning graft and all-cause mortality, recipients who underwent SLKT experienced a comparable risk of death with a functioning graft and all-cause mortality versus recipients with renal dysfunction independent of other relevant risk factors. These associations were robust and present in almost all subgroups of the recipients. Additionally, we could not find any association between pretransplant renal dysfunction and risk of graft loss in this cohort.
The presence and severity of NASH is associated with an increased risk and severity of CKD.(21) Therefore, it is not surprising to note the growing indication for SLKT in NASH patients in the United States.(16) NASH remains an independent risk factor for renal dysfunction after LT.(22) Although several published studies have reported the negative impact of renal failure on survival of patients undergoing LT,(23–27) the specific mechanism of the degree or severity of renal dysfunction and its relationship to survival probability following LT in recipients with NASH have not been well characterized. Studies are needed to examine the mechanisms of these findings and to develop strategies to improve renal outcomes in recipients for NASH.
The current study highlights the importance of pretransplant renal dysfunction as an important predictor of posttransplant survival in LT recipients with NASH. Our findings are further reinforced by an earlier study using UNOS-STAR data that reported that the presence of pretransplant renal dysfunction was independently associated with lower survival following LT in alcohol-related liver disease and NASH patients.(28) We, however, did not detect any survival difference between the recipients with renal dysfunction before LT and recipients who received SLKT. Similar results have been shown in non-NASH recipients as well.(29)
Several factors could have contributed to the poor survival outcomes in NASH patients with renal dysfunction at the time of LT (eGFR <30 mL/minute/1.73 m2/dialysis and/or underwent SLKT) compared with LT recipients with better renal function (eGFR ≥30 mL/minute/1.73 m2). First, LT recipients with pretransplant CKD have a substantial burden of posttransplant renal dysfunction and high short-term mortality.(30) The presence of pretransplant CKD in LT candidates with NASH may have contributed to the higher percentage of nonrecovery of the renal insult after LT.(21) Second, NASH recipients have an increased risk of cardiovascular disease (CVD) mortality after LT explained by a high prevalence of comorbid cardiometabolic risk factors such as renal dysfunction or presence of diabetes.(31) In fact, pretransplant renal dysfunction was the strongest predictor of post-LT CVD mortality in NASH recipients.(31) In addition, diabetes, either alone or in comorbid association with obesity, is linked with significantly greater posttransplant mortality.(32,33) The burden of diabetes could be even higher in NASH recipients receiving SLKT. A higher proportion of SLKT recipients with NASH have diabetes in the UNOS-STAR cohort possibly due to long-standing CKD related to diabetes.(31,34) Hence, pretransplant renal dysfunction along with presence of diabetes in LT candidates with NASH might result in additive deleterious consequences leading to lower overall survival.(31) Future studies should prospectively evaluate the identification of other factors associated with outcomes in patients with NASH and pretransplant renal dysfunction.
Our study identifies an inferior survival outcome in NASH patients with renal dysfunction undergoing LT (eGFR <30 mL/minute/1.73 m2 or needing dialysis), but their outcome is no different than those with SLKT. This study raises questions regarding the current allocation policy in NASH candidates for LT in a resource-poor setting for optimal utilization of the kidney allograft. It can be argued that considering similarly poor survival in NASH patients with eGFR <30 mL/minute/1.73 m2 or those needing short-term dialysis compared with those who received SLKT, consideration should be given for a kidney after LT, particularly for those with shorter duration on renal replacement therapy (as opposed to established ESRD). Although this notion has been argued against in previously published studies,(35) an improved immunosuppression regimen (early use of mammalian target of rapamycin inhibitors in patients with renal impairment) in recent years might confer a better longterm outcome. Sharma et al. have reported among recipients on renal replacement therapy before LT who survived after LT alone that the majority recovered their renal function within 6 months of LT. Longer pretransplantation renal replacement therapy duration, advanced age, diabetes, and retransplantation were significantly associated with an increased risk of renal nonrecovery.(36) Habib et al. have reported that SLKT improved 1-year survival only in low MELD (16–20) recipients but not in other groups.(37) The authors concluded that performance of SLKT should be limited to patients in whom a benefit in survival and posttransplant out-comes can be demonstrated. However, in our study, MELD score did not modify the association between renal dysfunction and survival. MELD prioritization of liver recipients with renal dysfunction has significantly increased utilization of SLKT. With 20% short-term loss of kidney grafts after SLKT, Lunsford et al. have suggested that renal transplantation should be deferred in liver recipients at high risk for renal allograft futility.(38) Consideration for a kidney allocation variance to allow for delayed renal transplantation after LT may prevent the loss of scarce renal allografts. Without well-established listing guidelines, SLKT potentially wastes renal allografts in both cases for high-acuity liver recipients at risk for early mortality and recipients who may regain native kidney function. On the basis of our study, despite these theoretical concerns, current UNOS allocation policy allowed similar survival outcomes (all-cause mortality) in SLKT patients with NASH compared with those with severe renal dysfunction receiving LT alone. In addition, group 3 (SLKT) patients have superior kidney transplant-free survival (kidney retransplant-free survival in this group) compared with group 2 (eGFR <30 mL/minute/1.73 m2) recipients with application of the current allocation policy, reaffirming the validity of current UNOS policy in NASH patients.
Our study is unable to specifically address this question of renal allograft allocation in LT candidates with NASH. Future studies should be directed to specifically address which subgroup of LT candidates with NASH most benefits from SLKT.
Our study is notable for its large sample size and event numbers and for being representative of US LT recipients. We also used a statistical approach with counts for competing events in the case of graft loss or death with a functioning graft. To our knowledge, this is the first study to assess the association between renal dysfunction before LT and death with a functioning graft, all-cause mortality, and graft loss after transplantation in recipients, specifically in recipients with NASH. We used multiple imputation to increase the power of our analysis. Although our main result showed only a trend for a lower mortality risk for patients with preserved renal function, the imputation increased the power, and the result became significant without a major change in the value of the point estimate.
This study also has several limitations that need to be acknowledged. First, because this was an observational study, only associations, but no cause-effect relationships, can be established. Second, our patients were US deceased donor LT recipients; hence, the results may not be generalizable to other recipient populations outside of the United States. Third, we were unable to assess the duration of renal dysfunction and preexisting CKD before LT using the UNOS-STAR data. Fourth, the etiology of death was not uniformly available in all patients, so we were unable to perform death cause-specific analyses. Fifth, lack of data on immunosuppression and incidence of renal dysfunction after LT is also another glaring deficiency significantly limiting our ability to evaluate the cause of renal nonrecovery. Sixth, we used estimated GFR in our analysis as renal data, and we did not have more granular, detailed data about the patient’s underlying renal disease prior to their listing. Finally, as with all observational studies, we were not able to eliminate the possibility of unmeasured confounders.
In conclusion, in this large national cohort of US LT recipients with NASH, we found that recipients with more preserved renal function had lower mortality risk but similar liver allograft loss risk after transplantation whereas recipients who received SLKT had similar mortality and liver allograft loss risk but superior kidney transplant-free survival compared with recipients with severe renal dysfunction independent of demographics, comorbidities, and donor-related data.
Supplementary Material
Acknowledgment:
The authors thank Dr. Abduzhappar Gaipov for help in creating the figures.
Miklos Z. Molnar served as a consultant for Merck and Abbvie, which was unrelated to this work. Sanjaya K. Satapathy has received grant/research support from Biotest, Conatus, Genfit, Gilead Sciences, Intercept, and Shire; has served on the advisory board or as consultant for Abbvie, Gilead Sciences, and Intercept; and has served on the speaker’s bureau for Intercept and Alexion. Vincent Wai-Sun Wong has consulted for Abbvie, Allergan, Echosens, Gilead Sciences, and Janssen and serves on the speakers bureau for Bristol-Myers Squibb, Echosena, Gilead Sciences, and Merck. Kalyan Ram Bhamidimarri has advisory agreements with Gilead, Merck, Abbvie, and Intercept; serves on the speaker’s bureaufor Alexion; and has received grants and contracts from Vital Therapies, Gilead, Mallinckrodt, Allergan, and Contus. Satheesh Nair served as a consultant for Gilead.
Abbreviations:
- BMI
body mass index
- CI
confidence interval
- CKD
chronic kidney disease
- CMV
cytomegalovirus
- CVD
cardiovascular disease
- eGFR
estimated glomerular filtration rate
- ESRD
end-stage renal disease
- HCC
hepatocellular carcinoma
- HRS
hepatorenal syndrome
- INR
international normalized ratio
- IQR
interquartile range
- LT
liver transplantation
- MELD
Model for End-Stage Liver Disease
- NAFLD
nonalcoholic fatty liver disease
- NASH
nonalcoholic steatohepatitis
- Scr
serum creatinine
- SD
standard deviation
- SHR
subhazard ratio
- SLKT
simultaneous liver-kidney transplantation
- STAR
Standard Transplant Analysis and Research
- TIPS
transjugular intrahepatic portosystemic shunt
- UNOS
United Network for Organ Sharing
Footnotes
Additional supporting information may befound in the online version of this article.
references
- 1).Ojo AO, Held PJ, Port FK, Wolfe RA, Leichtman AB, Young EW, et al. Chronic renal failure after transplantation of a nonrenal organ. N Engl J Med 2003;349:931–940. [DOI] [PubMed] [Google Scholar]
- 2).Weber ML, Ibrahim HN, Lake JR. Renal dysfunction in liver transplant recipients: evaluation of the critical issues. Liver Transpl 2012;18:1290–1301. [DOI] [PubMed] [Google Scholar]
- 3).Parajuli S, Foley D, Djamali A, Mandelbrot D. Renal function and transplantation in liver disease. Transplantation 2015;99:1756–1764. [DOI] [PubMed] [Google Scholar]
- 4).Calmus Y, Conti F, Cluzel P, Hill G, Antoine C, Scatton O, et al. Prospective assessment of renal histopathological lesions in patients with end-stage liver disease: effects on long-term renal function after liver transplantation. J Hepatol 2012;57:572–576. [DOI] [PubMed] [Google Scholar]
- 5).Gonwa TA, McBride MA, Anderson K, Mai ML, Wadei H, Ahsan N. Continued influence of preoperative renal function on outcome of orthotopic liver transplant (OLTX) in the US: where will MELD lead us? Am J Transplant 2006;6:2651–2659. [DOI] [PubMed] [Google Scholar]
- 6).Gonwa TA, Mai ML, Melton LB, Hays SR, Goldstein RM, Levy MF, Klintmalm GB. End-stage renal disease (ESRD) after orthotopic liver transplantation (OLTX) using calcineurin-based immunotherapy: risk of development and treatment. Transplantation 2001;72:1934–1939. [DOI] [PubMed] [Google Scholar]
- 7).Davis CL, Feng S, Sung R, Wong F, Goodrich NP, Melton LB, et al. Simultaneous liver-kidney transplantation: evaluation to decision making. Am J Transplant 2007;7:1702–1709. [DOI] [PubMed] [Google Scholar]
- 8).Eason JD, Gonwa TA, Davis CL, Sung RS, Gerber D, Bloom RD. Proceedings of consensus conference on simultaneous liver kidney transplantation (SLKT). Am J Transplant 2008;8:2243–2251. [DOI] [PubMed] [Google Scholar]
- 9).Chang Y, Gallon L, Shetty K, Chang Y, Jay C, Levitsky J, et al. Simulation modeling of the impact of proposed new simultaneous liver and kidney transplantation policies. Transplantation 2015;99:424–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10).OPTN/UNOS Kidney Transplantation Committee. Simultaneous liver kidney (SLK) allocation policy. https://optn.transplant.hrsa.gov/media/1192/0815-12_SLK_Allocation.pdf. Accessed December 10, 2018.
- 11).Rinella ME. Nonalcoholic fatty liver disease: a systematic review. JAMA 2015;313:2263–2273. [DOI] [PubMed] [Google Scholar]
- 12).Bedogni G, Miglioli L, Masutti F, Tiribelli C, Marchesini G, Bellentani S. Prevalence of and risk factors for nonalcoholic fatty liver disease: the Dionysos nutrition and liver study. Hepatology 2005;42:44–52. [DOI] [PubMed] [Google Scholar]
- 13).Clark JM, Diehl AM. Nonalcoholic fatty liver disease: an underrecognized cause of cryptogenic cirrhosis. JAMA 2003;289:3000–3004. [DOI] [PubMed] [Google Scholar]
- 14).Orlić L, Mikolasevic I, Bagic Z, Racki S, Stimac D, Milic S. Chronic kidney disease and nonalcoholic fatty liver disease-is there a link? Gastroenterol Res Pract 2014;2014:847539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15).Sinn DH, Kang D, Jang HR, Gu S, Cho SJ, Paik SW, et al. Development of chronic kidney disease in patients with non-alcoholic fatty liver disease: a cohort study. J Hepatol 2017;67:1274–1280. [DOI] [PubMed] [Google Scholar]
- 16).Singal AK, Hasanin M, Kaif M, Wiesner R, Kuo YF. Nonalcoholic steatohepatitis is the most rapidly growing indication for simultaneous liver kidney transplantation in the United States. Transplantation 2016;100:607–612. [DOI] [PubMed] [Google Scholar]
- 17).Levey AS, Greene T, Kusek JW, Beck GJ. A simplified equation to predict glomerular filtration rate from serum creatinine. J Am Soc Nephrol 2000;11:155A. [Google Scholar]
- 18).Fine JP, Gray RJ. A proportional hazards model for subdistribution of a competing risk. J Am Stat Assoc 1999;94:496–509. [Google Scholar]
- 19).Rubin DB. Multiple Imputation for Nonresponse in Surveys. New York: John Wiley & Sons; 1987. [Google Scholar]
- 20).Schafer JL. Analysis of Incomplete Multivariate Data. London, UK: Chapman and Hall; 1997. [Google Scholar]
- 21).Musso G, Gambino R, Tabibian JH, Ekstedt M, Kechagias S, Hamaguchi M, et al. Association of non-alcoholic fatty liver disease with chronic kidney disease: a systematic review and meta-analysis. PLoS Medicine 2014;11:e1001680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22).Houlihan DD, Armstrong MJ, Davidov Y, Hodson J, Nightingale P, Rowe IA, et al. Renal function in patients under-going transplantation for nonalcoholic steatohepatitis cirrhosis: time to reconsider immunosuppression regimens? Liver Transpl 2011;17:1292–1298. [DOI] [PubMed] [Google Scholar]
- 23).Nadim MK, Genyk YS, Tokin C, Fieber J, Ananthapanyasut W, Ye W, Selby R. Impact of the etiology of acute kidney injury on outcomes following liver transplantation: acute tubular necrosis versus hepatorenal syndrome. Liver Transpl 2012;18:539–548. [DOI] [PubMed] [Google Scholar]
- 24).Fraley DS, Burr R, Bernardini J, Angus D, Kramer DJ, Johnson JP. Impact of acute renal failure on mortality in end-stage liver disease with or without transplantation. Kidney Int 1998;54:518–524. [DOI] [PubMed] [Google Scholar]
- 25).Northup PG, Argo CK, Bakhru MR, Schmitt TM, Berg CL, Rosner MH. Pretransplant predictors of recovery of renal function after liver transplantation. Liver Transpl 2010;16: 440–446. [DOI] [PubMed] [Google Scholar]
- 26).Campbell MS, Kotlyar DS, Brensinger CM, Lewis JD, Shetty K, Bloom RD, et al. Renal function after orthotopic liver transplantation is predicted by duration of pretransplantation creatinine elevation. Liver Transpl 2005;11:1048–1055. [DOI] [PubMed] [Google Scholar]
- 27).Barri YM, Sanchez EQ, Jennings LW, Melton LB, Hays S, Levy MF, Klintmalm GB. Acute kidney injury following liver transplantation: definition and outcome. Liver Transpl 2009;15:475–483. [DOI] [PubMed] [Google Scholar]
- 28).Cheong J, Galanko JA, Arora S, Cabezas J, Ndugga NJ, Lucey MR, et al. Reduced impact of renal failure on the outcome of patients with alcoholic liver disease undergoing liver transplantation. Liver Int 2017;37:290–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29).Brennan TV, Lunsford KE, Vagefi PA, Bostrom A, Ma M, Feng S. Renal outcomes of simultaneous liver-kidney transplantation compared to liver transplant alone for candidates with renal dys-function. Clin Transplant 2015;29:34–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30).Bahirwani R, Forde KA, Mu Y, Lin F, Reese P, Goldberg D, et al. End-stage renal disease after liver transplantation in patients with pretransplant chronic kidney disease. Clin Transplant 2014;28:205–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31).VanWagner LB, Lapin B, Skaro AI, Lloyd-Jones DM, Rinella ME. Impact of renal impairment on cardiovascular disease mortality after liver transplantation for nonalcoholic steatohepatitis cirrhosis. Liver Int 2015;35:2575–2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32).Wong RJ, Cheung R, Perumpail RB, Holt EW, Ahmed A. Diabetes mellitus, and not obesity, is associated with lower survival following liver transplantation. Dig Dis Sci 2015;60:1036–1044. [DOI] [PubMed] [Google Scholar]
- 33).Barritt AS 4th, Dellon ES, Kozlowski T, Gerber DA, Hayashi P. The influence of nonalcoholic fatty liver disease and its associated comorbidities on liver transplant outcomes. J Clin Gastroenterol 2011;45:372–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34).Zelnick LR, Weiss NS, Kestenbaum BR, Robinson-Cohen C, Heagerty PJ, Tuttle K, et al. Diabetes and CKD in the United States Population, 2009–2014. Clin J Am Soc Nephrol 2017;12:1984–1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35).Simpson N, Cho YW, Cicciarelli JC, Selby RR, Fong TL. Comparison of renal allograft outcomes in combined liver-kid-ney transplantation versus subsequent kidney transplantation in liver transplant recipients: analysis of UNOS database. Transplantation 2006;82:1298–1303. [DOI] [PubMed] [Google Scholar]
- 36).Sharma P, Goodrich NP, Zhang M, Guidinger MK, Schaubel DE, Merion RM. Short-term pretransplant renal replacement therapy and renal nonrecovery after liver transplantation alone. Clin J Am Soc Nephrol 2013;8:1135–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37).Habib S, Khan K, Hsu CH, Meister E, Rana A, Boyer T. Differential simultaneous liver and kidney transplant benefit based on severity of liver damage at the time of transplantation. Gastroenterology Res 2017;10:106–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38).Lunsford KE, Bodzin AS, Markovic D, Zarrinpar A, Kaldas FM, Gritsch HA, et al. Avoiding futility in simultaneous liver-kidney transplantation: analysis of 331 consecutive patients listed for dual organ replacement. Ann Surg 2017;265: 1016–1024. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


