Abstract
Objective
To evaluate the effect of cenobamate in patients with photoparoxysmal-EEG response (PPR) to intermittent photic stimulation (IPS) as proof of principle of efficacy in patients with epilepsy.
Methods
In this multicenter, single-blind study, adults with photosensitive epilepsy, with/without concomitant antiepileptic drug therapy, underwent IPS under 3 eye conditions after a single dose of placebo (day −1, day 2) or cenobamate (day 1; 100, 250, or 400 mg). Complete suppression was a standardized photosensitivity range reduction to 0 over ≥1 time points for all eye conditions. Partial suppression was a ≥3-point reduction over ≥3 testing times vs the same time points on day −1 in ≥1 eye condition. Pharmacokinetics and safety were assessed.
Results
Of 6 evaluable patients, 5 reentered to receive higher doses. Cenobamate 100 mg produced partial suppression in 1 of 3 patients; 250 mg produced complete suppression in 1 of 4 and partial suppression in 4 of 4 patients; and 400 mg produced complete suppression in 1 of 4 and partial suppression in 2 of 4 patients. PPR was consistently reduced on days 1 and 2 (>24 hours after cenobamate) vs day −1 (placebo) with the 250- and 400-mg doses. Area under the plasma concentration-time curve (before dose to last measurable concentration) values between 201 and 400 μg/h/mL resulted in partial suppression in 4 of 6 (66%) patients. Most common adverse events were dizziness and somnolence.
Conclusions
This proof-of-principle study demonstrated that cenobamate is a potentially effective product for epilepsy.
ClinicalTrials.gov identifier
Classification of evidence
This study provides Class III evidence that, for patients with photosensitive epilepsy, cenobamate suppresses IPS-induced PPR.
In patients with photosensitive epilepsy, intermittent photic stimulation (IPS) typically results in a characteristic epileptiform response on EEG.1,2 Photosensitivity or the IPS-induced photoparoxysmal response (PPR) on EEG is commonly associated with generalized epilepsies but can also occur in patients with focal epilepsy.3,4 Standardized assessment of the PPR can be used as a proof-of-principle model for the assessment of investigational antiepileptic drugs (AEDs) in early clinical development.1,5,6
In the model, photosensitive patients are exposed to IPS, and a reduction in sensitivity to the number of standard visual stimulation frequencies after a single, acute dose of a potential AED is used as an endpoint.7 The standardized photosensitivity range (SPR) is a reliable screening method for assessing AED efficacy and safety2 and can inform dose selection for larger clinical trials.7
Cenobamate (YKP3089) is a novel AED under investigation for use in patients with focal (partial-onset) seizures. It has been shown to modulate several properties of voltage-gated sodium channels and to preferentially inhibit persistent sodium currents.8 Preclinical data indicate a broad spectrum of anticonvulsant activity in rodent seizure and epilepsy models.9–11 The purpose of this study was to evaluate the effect of cenobamate on the IPS-induced EEG PPR in patients with photosensitive epilepsy as proof of concept of its antiepileptic effects.
Methods
Patients
Male or female patients 18 to 60 years of age with a body mass index of 18 to 35 kg/m2 and diagnosis of epilepsy who were stabilized on 0 to 2 concomitant AEDs for at least 4 weeks before the start of the study were eligible for study inclusion. If a patient was taking 2 concomitant medications, the second drug had to be levetiracetam, gabapentin, or pregabalin. Eligible patients also had to have a reproducible IPS-induced PPR on EEG of ≥3 points on a frequency assessment scale in ≥1 eye conditions (eyes open, eye closure, and/or eyes closed) and no change of >3 frequencies in 2 repeated measurements recorded over the 2 months before study entry in ≥1 eye conditions. Patients were excluded from the study if they had a history of nonepileptic seizures; were pregnant or lactating or were of reproductive potential but chose not to adhere to effective birth control methods; had CNS, neurologic, or psychiatric conditions considered to be progressive during the course of the study; or were at risk for liver adverse events (AEs), including drug-drug interactions (cytochrome p450).
Study design
This phase 2a, single-blind, single-dose, multicenter study enrolled and evaluated adult patients with photosensitive epilepsy in 4 medical centers in the United States from August 20, 2007, to January 26, 2009, under the auspices of the Epilepsy Study Consortium. The clinical portion was single-blinded in that only the patients were blinded to the study treatments. However, after the clinical portion was completed, EEG data were sent to a blinded clinical expert for interpretation.
Four sequential doses of cenobamate were intended to be evaluated per the initial protocol: 100, 250, 400, and 600 mg. Three to 6 patients were recruited sequentially for each dose cohort. Once a cohort was completed, the next higher dose cohort was recruited. Patients who completed a lower dose could be recruited into a higher dose cohort. Patients must have received a 100-mg dose followed by an observation period of 48 hours to be eligible to receive the next sequential dose (250 mg), and so on with each subsequent dose cohort (250-mg dose before 400-mg dose, etc). A washout period of at least 2 weeks was also required before a patient could receive a higher dose (2-week washout after an observation period and 4-week washout after hospitalization and IPS). Patients screened within 21 days were admitted for IPS testing and remained onsite for at least 4 days, including a 48-hour observation period after administration of the first cenobamate dose (100 mg). Patients received a dose of placebo on day −1 (baseline), a single oral dose of cenobamate on day 1, and placebo again on day 2 to maintain the single blind and to allow assessment of the duration of effect after cenobamate.12 If there was a continued treatment effect on the IPS response after the 30-hour post–cenobamate dose IPS assessment, the patient underwent additional daily IPS evaluations with simultaneous blood draws for pharmacokinetic testing and AED levels until there was no treatment effect and the IPS response returned to baseline. All medication was administered under direct supervision.
A Data Safety Monitoring Board reviewed cenobamate plasma levels after exposure to the lower dose level before patients were exposed to the higher dose level. Patients who achieved cenobamate plasma levels consistent with those observed in previous studies of phase 1 volunteers were permitted to participate at the higher dose level, provided that they had not experienced any intolerable side effects. This safeguarded against exposing patients to higher dose levels who may have experienced pharmacokinetic interactions between their AEDs and cenobamate.
Dosing was not to be increased to the next dose level if 2 of 3 patients experienced a moderate AE at the prior cenobamate dose level or if any patient experienced a serious AE that was considered related to treatment. Patients could withdraw from the study at any time. In addition, patients would be withdrawn if they experienced a predefined widening of the photosensitivity range (PR), unexpected tonic-clonic seizures during IPS, or evidence of proconvulsive activity on EEG.
Photic stimulation procedures
The photosensitivity model has been described extensively.1–3,12,13 In this model, patients are exposed to IPS before and after placebo/AED intake at hourly intervals over a 3-day period. A standardized stimulation protocol is used to establish photosensitivity thresholds (in Hertz), which are translated into an SPR value in points.
PPR step ranges were determined for 3 eye conditions (eye closure, eyes closed, and eyes open) before and 1, 2, 3.5, 5, 6.5, and 8 hours after dose on days −1, 1, and 2, and before and 2 and 6 hours after dose on day 3. Flashes were administered at 14 standard frequencies of 2, 5, 8, 10, 13, 15, 18, 20, 23, 25, 30, 40, 50, and 60 Hz that were converted to SPRs. SPR was expressed as the categorical number of standard frequencies for which a PPR was elicited, ranging from 0 to 14, with 0 defined as no PPR elicited at any flash frequencies and 14 defined as PPR elicited in all flash frequencies. Upper and lower limit frequencies triggering a PPR and derived SPR were recorded by eye condition, patient number, visit, and time point for each treatment.
Pharmacokinetics
Blood samples for pharmacokinetic evaluations of cenobamate were collected before dose on day 1 and at 1, 2, 3.5, 5, 6.5, 8, 23.5, 24, 25, 26, 27.5, 29, 30.5, 32, 48, 50, and 54 hours after dose. The following pharmacokinetic parameters were calculated for cenobamate: the area under the plasma concentration vs time curve (AUC) from time 0 to 24 hours after dose (AUC0-24) and the AUC from time 0 on day 1 to the last measurable concentration (AUC0-t) using the linear trapezoidal method; the AUC from time 0 on day 1 to infinity (AUC0-inf); the AUC0-t/AUC0-inf ratio; the maximum measured drug plasma concentration (Cmax); the time of maximum measured plasma concentration (tmax); the apparent first-order terminal elimination rate constant (kel); and the apparent elimination half-life (t1/2).
AED treatment was recorded throughout the study, and blood samples were collected at each visit to measure concomitant AED levels. Specifically, blood samples were collected at the screening visit and on day −1, day 1, and day 2 before dose (trough levels) and 1, 2, 3.5, 5, 6.5, 8, and 24 hours after dose. Additional samples were drawn on day 3 at 26 and 30 hours after dose. Pharmacokinetic parameters calculated for plasma AEDs were AUC0-24, Cmax, and tmax.
Safety
AEs, as reported spontaneously by the patient or observed by the investigator, were recorded over the course of the trial. Safety was also monitored via vital signs, clinical laboratory values, changes in physical examination, EEG, and administration of the Bond and Lader14 Mood Visual Analog Scale.
Statistical methods
The intent-to-treat (ITT) population included all patients who received at least 1 dose of study medication (placebo or cenobamate) and had at least 1 postbaseline assessment. The safety population included all patients who received at least 1 dose of study medication.
Upper and lower limit frequencies triggering a PPR and derived SPR were summarized with descriptive statistics. Specifically, the IPS-induced PPR quantified in terms of a PR (also referred to as PPR step ranges) were determined for the 3 eye conditions. Complete and partial suppression of PPR, defined as change of SPR, was determined for each eye condition, in addition to determination of the most sensitive eye (defined as the eye condition with the largest PR on day −1 across all time points) for each patient at each dose level on days 1 and 2 and overall. The difference in SPR for days 1 to 3 vs the corresponding time points on day −1 (placebo) was also summarized with descriptive statistics.
Complete suppression was defined as the reduction of the SPR to 0 over ≥1 testing time points for all 3 eye conditions within the same day if the baseline PPR at the same time point for each eye condition was >0. Partial suppression was defined as a reduction in SPR by ≥ 3 points over ≥3 testing times within 1 day compared to the range at the same time points on placebo day (day −1). If a patient had a tie for the largest average value of SPR, the priority for the most sensitive eye condition was eye closure > eyes closed > eyes opened.
Plasma concentration and pharmacokinetic data for cenobamate and the AEDs were summarized by cohort with descriptive statistics (sample size, arithmetic mean, SD, percent coefficient of variation, and geometric mean, median, minimum, and maximum values). In addition, geometric percent coefficient of variation was presented for the pharmacokinetic parameter data.
Standard protocol approvals, registrations, and patient consents
The study protocol and informed consent forms were reviewed and approved by the institutional review boards at each investigational center. Before initiation of any study-specific procedures, patients were provided written informed consent that summarized the purpose of the study, procedures of the study, and potential risks of the study (ClinicalTrials.gov identifier NCT00616148). This study was conducted in accordance with the principles and requirements described in the International Conference on Harmonization Consolidated Guidelines on Good Clinical Practice and the Declaration of Helsinki.
Classification of evidence
The primary research question of this phase 2, single-blind, controlled study was whether single-dose cenobamate has an effect on the IPS-induced PPR EEG response in patients with photosensitive epilepsy as proof of principle for cenobamate clinical activity in epilepsy. The nonrandomized study provided Class III evidence that cenobamate (100, 250, and 400 mg) suppressed IPS response compared with baseline, with greater suppression observed at the 250- and 400-mg doses.
Data availability
The data for the analyses described in this article are available by request from the author investigators or SK Life Sciences, Inc, the company sponsoring the clinical development of cenobamate for the treatment of focal epilepsy.
Results
Overall, 7 patients were enrolled and received ≥1 dose of study medication during the trial (figure 1). Of the 7 patients, 5 reentered the study to participate at a higher dose level. Patients were given new identifiers when they reentered the study. After the sequential assessments of the 250-mg and 400-mg dose groups, the study was stopped early, before enrollment of patients at the 600-mg dose, because overall patient enrollment was low and this dose group was no longer deemed necessary. One patient was discontinued from the study after withdrawal of consent. This patient discontinued after a single dose of 100-mg cenobamate but did not undergo IPS assessment and therefore was not included in the ITT analysis. In the ITT population, there were 3 evaluable patients at the 100-mg dose level and 4 evaluable patients in each of the 250- and 400-mg dose levels.
Figure 1. Patient disposition.
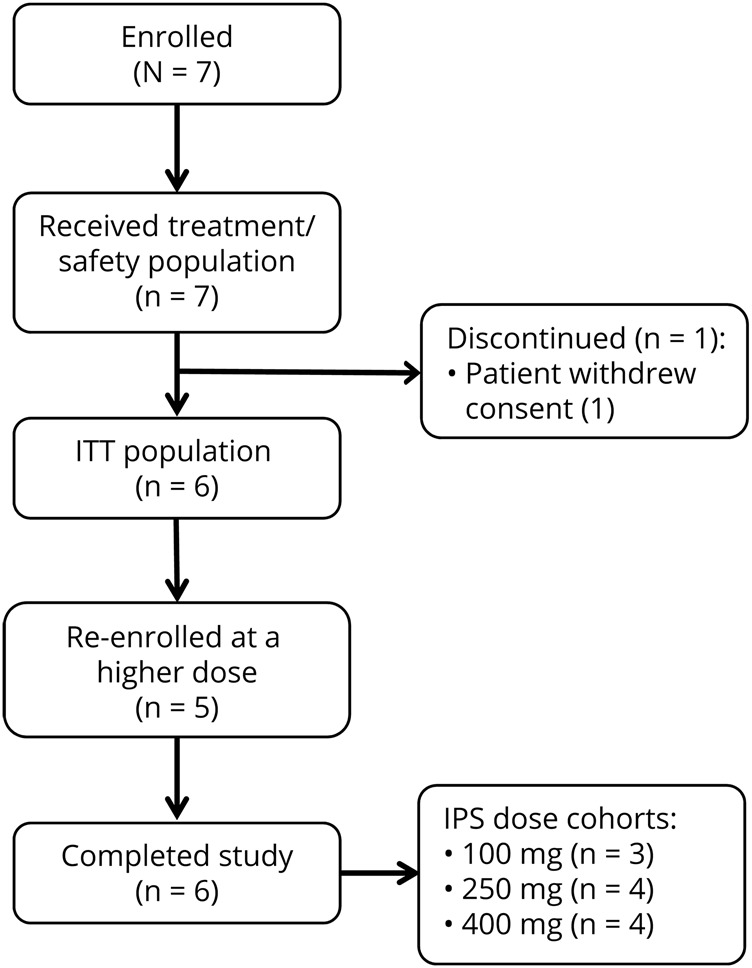
Seven patients enrolled in the study and received treatment. One patient withdrew consent after a 100-mg dose of cenobamate before intermittent photic stimulation (IPS) assessment. Five patients re-enrolled and progressed to a higher dose. ITT = intent to treat.
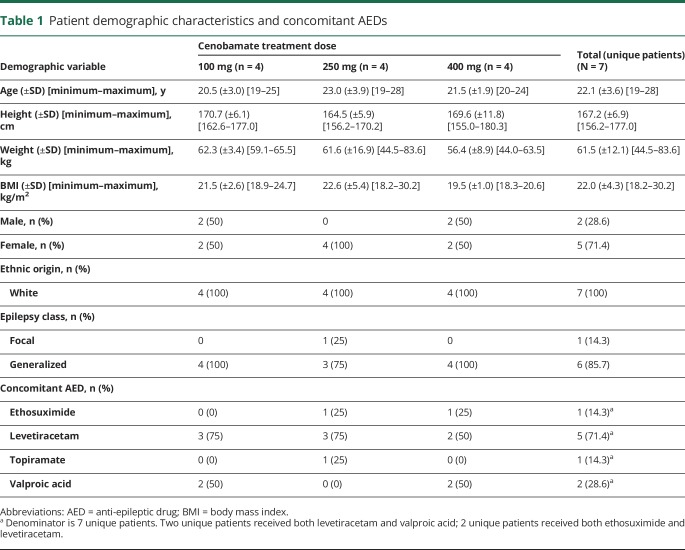
Demographics were similar for patients in the 3 dosing cohorts (table 1). The patient ages ranged from 19 to 28 years. All were white; most patients (5 of 7) were female. Of the 7 patients, 6 had a history of generalized seizures, and 1 patient had a history of focal seizures.15 All but 1 patient took ongoing concomitant AEDs during this study (table 1).
Table 1.
Patient demographic characteristics and concomitant AEDs
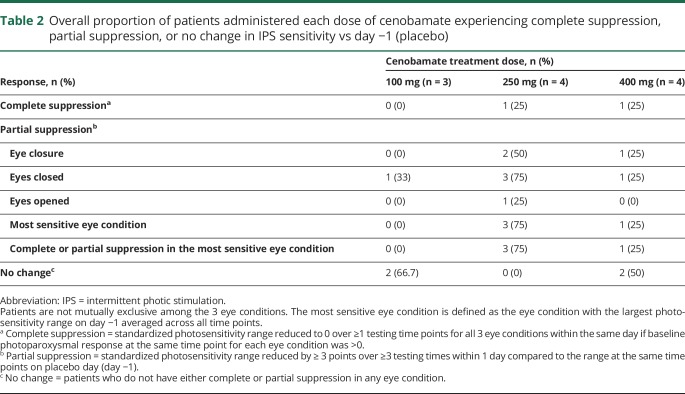
The results of photic stimulation in each eye condition are summarized in table 2. At the 100-mg dose level (n = 3), cenobamate produced partial suppression of IPS sensitivity in 1 (33%) patient in the eyes closed condition (patient 2, day 1). At the 250-mg dose level (n = 4), cenobamate produced complete suppression of IPS sensitivity in 1 (25%) patient (patient 5, day 2). In addition, at this dose, partial suppression of IPS sensitivity was observed in all patients (n = 4) in at least 1 eye condition, including 3 (75%) patients with partial suppression of IPS in the most sensitive eye condition (patients 5 and 6, day 2; patient 7, days 1 and 2) (table 2). At the 400-mg dose level (n = 4), cenobamate produced complete suppression of IPS sensitivity in 1 (25%) patient (patient 12, days 1 and 2), and partial suppression was observed in 2 (50%) patients in at least 1 eye condition (patient 12, days 1 and 2; patient 11, day 2) (table 2).
Table 2.
Overall proportion of patients administered each dose of cenobamate experiencing complete suppression, partial suppression, or no change in IPS sensitivity vs day −1 (placebo)
At 1 hour after dosing, PPR was consistently reduced with 250- and 400-mg cenobamate on days 1 and 2 compared with placebo (day −1) for the most sensitive eye condition (figure 2).
Figure 2. One-hour SPR (mean ± SD) on day −1 and changes in SPR on days 1 and 2 vs day −1.
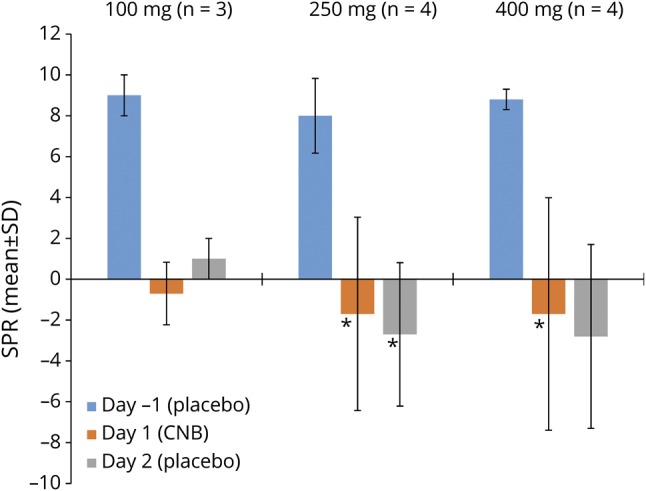
One-hour standardized photosensitivity range (SPR) (mean ± SD) on day −1 (placebo) and mean ± SD changes in SPR on days 1 and 2 (after cenobamate [CNB]) vs day −1 for the most sensitive eye condition. *n = 3.
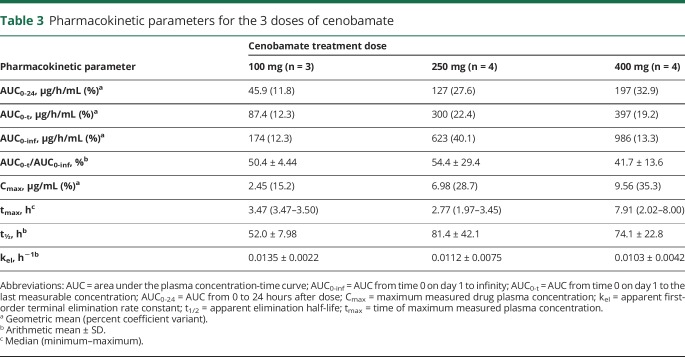
The mean plasma cenobamate concentration-time profile for each dose cohort is shown in figure 3. On the basis of the geometric mean values, the AUC0-24 and Cmax of cenobamate appeared to increase proportionate to dose across the 3 dose levels (table 3). The AUC0-t of cenobamate increased with increasing dose level but not in linear proportion to the dose administered. The AUC0-inf of cenobamate appeared to increase in a more than dose-proportional fashion from the 100-mg to the 250-mg dose level but dose-proportionally from the 250-mg to the 400-mg dose level. The t½ of cenobamate was shorter at the 100-mg dose level than at the 250- and 400-mg dose levels, with these 2 dose levels exhibiting a similar t½. The AUC0-t/AUC0-inf ratio was low for all 3 doses, indicating that a large portion of the overall exposure (AUC0-inf) was extrapolated. The median tmax of cenobamate was different for all 3 dose levels and ranged from 2.77 to 7.91 hours.
Figure 3. Plasma concentration over time (mean ± SD) for 3 ascending doses of cenobamate.
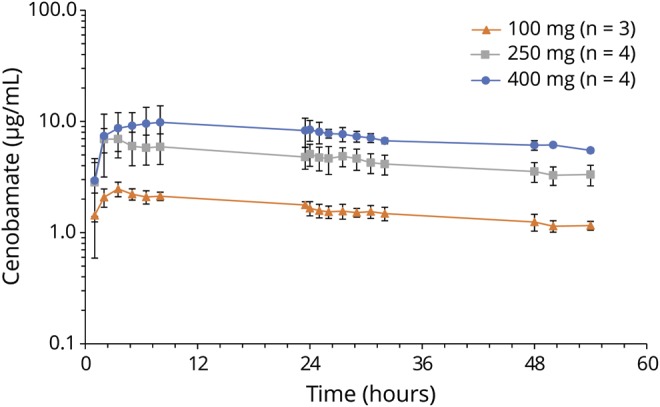
Table 3.
Pharmacokinetic parameters for the 3 doses of cenobamate
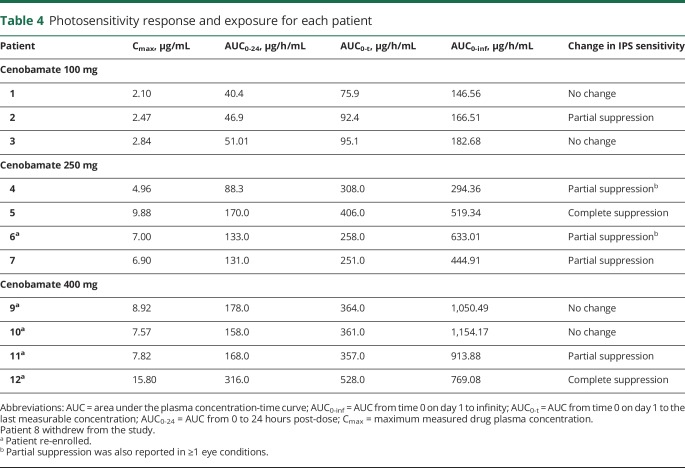
Higher cenobamate Cmax concentrations and AUC0-t resulted in greater suppression of IPS response (table 4), with the caveat that only a small number of patients were evaluated. For example, Cmax values in the range of 1.0 to 4.0 μg/mL resulted in partial suppression of IPS sensitivity in 1 of 3 (33%) patients; Cmax values in the range of 4.1 to 9.0 μg/mL resulted in partial suppression of IPS sensitivity in 4 of 6 (66%) patients; and Cmax values in the range of 9.1 to 16.0 μg/mL resulted in complete suppression in 2 of 2 (100%) patients. Similarly, AUC0-t values in the range of 1 to 200 μg/h/mL resulted in partial suppression of IPS sensitivity in 1 of 3 patients (33%); AUC0-t values in the range of 201 to 400 μg/h/mL resulted in partial suppression of IPS sensitivity in 4 of 6 patients (66%); and AUC0-t values in the range of 401 to 600 μg/h/mL resulted in complete suppression in 2 of 2 patients (100%).
Table 4.
Photosensitivity response and exposure for each patient
Two patients received comedication with ethosuximide, 5 with levetiracetam, 1 with topiramate, and 4 with valproic acid. There did not appear to be any clinically significant changes in serum concentration for any comedication.
Safety
No deaths, serious treatment-emergent AEs (TEAEs), or TEAEs leading to discontinuation occurred during this study. A total of 29 TEAEs were reported in 6 patients, and 24 TEAEs reported in 5 patients were considered treatment related by the investigator. Two severe TEAEs (blood pressure orthostatic decrease and syncope) occurred in 1 patient after administration of 400-mg cenobamate. Nervous system disorders were the most reported class of TEAEs, with the most frequent TEAEs, postural dizziness and somnolence, both reported in 3 patients each. All TEAEs resolved by the end of the study. One patient had 13 of 29 (44.8%) total TEAEs reported during this study. There did not appear to be any trends with regard to TEAEs increasing with dose level. There were no clinically meaningful changes from baseline in laboratory values, vital signs, physical examinations, 12-lead ECG, or mood assessment. Clinically significant postdose neurologic findings were reported in 2 patients, including gait disturbance, mild cognitive impairment, postural dizziness, mild/slight imbalance, inability to perform, and mild limited cognition.
Discussion
The current study used the photosensitivity proof-of-principle model to establish the potential for efficacy of cenobamate in patients with epilepsy. In this study, cenobamate decreased IPS sensitivity, with greater suppression of IPS-induced PPR response observed with higher doses (250 and 400 mg). In particular, the 250-mg dose (n = 4) produced complete or partial suppression of IPS sensitivity in all 4 evaluable patients.
When planned, this study was designed to include a 600-mg dose level to be administered to patients who completed the 400-mg dose level. The study was stopped after the 400-mg dose level because of reduced photosensitivity at doses of 100 mg and because reduced and complete suppression of IPS at doses of 250 and 400 mg had already been demonstrated. There was little reason to dose at the 600-mg dose level because of difficulty recruiting patients and the fact that reduced photosensitivity was already established at lower dose levels.
The photosensitivity model has been successful in identifying AEDs in early-phase development.3,7 Previous studies have shown a reduction in photosensitivity with almost all effective AEDs tested, despite differences in mechanisms of action.1,3,6,7,16,17 In this model, demonstrating a change from placebo confirms delivery of drug to the brain; therefore, it can be used as initial evidence of drug efficacy.
In the current study, on the basis of the geometric mean values, the AUC0-24 and Cmax parameters for each cohort appeared dose-proportional across a dose range of 100 to 400 mg, whereas nonproportional increases in the AUC0-t of cenobamate were exhibited. However, in this study, the small number of patients within each dosing cohort may have precluded a robust assessment of these parameters, as indicated by the low AUC0-t/AUC0-inf ratio.
Pharmacokinetic characteristics of single (up to 750 mg) and multiple (50–300 mg) doses of cenobamate have been explored in studies in healthy volunteers.18 In these studies, cenobamate was associated with more than dose-proportional increases in AUC, especially after single-dose administration, and a long half-life (55–60 hours) was exhibited within the 200- to 300-mg/d dose range.
When evaluated by dosing cohort, it appeared that, although the mean peak and extent of exposure to cenobamate were highest at the 400-mg dose level, the 250-mg dose level seemed to produce the greatest magnitude of suppression in IPS sensitivity. However, when evaluated by individual patients, higher cenobamate plasma concentrations produced greater suppression of IPS response. For example, Cmax values in the range of 1.0 to 4.0 μg/mL and AUC0-t values in the range of 1 to 200 μg/h/mL resulted in partial suppression of IPS sensitivity in 1 of 3 patients (33%). Cmax values in the range of 4.1 to 9.0 μg/mL and AUC0-t values in the range of 201 to 400 μg/h/mL resulted in partial suppression of IPS sensitivity in 4 of 6 (66%) patients. Cmax values in the range of 9.1 to 16.0 μg/mL and AUC0-t values in the range of 401 to 600 μg/h/mL resulted in complete suppression in 2 of 2 patients (100%).
Single doses of cenobamate up to 750 mg were found to be generally well tolerated in previous studies in healthy volunteers.18 In the current study, with doses up to 400 mg, there were no deaths, serious TEAEs, or TEAEs leading to discontinuation. Ongoing clinical trials in patients with focal seizures will help to further characterize the safety and tolerability profile of cenobamate. Similarly, the potential for drug-drug interactions with cenobamate and other AEDs will need further evaluation because the number of patients taking concomitant AEDs was small.
Although the results of this study should be interpreted cautiously because of the small number of patients and lack of formal statistical analysis, the findings shown in this clinical epilepsy model support a proof of principle for cenobamate in epilepsy. That most patients in this study also had a history of generalized epilepsy suggests that the photosensitivity model appears to screen for broad-spectrum antiseizure activity. Furthermore, a reduction in PPR demonstrated in the photosensitivity model, along with measurement of plasma concentrations of compounds, can help establish the pharmacokinetic/pharmacodynamic relationship and identify optimal doses and duration of treatment effects for investigational AEDs.2,7 In a meta-analysis, doses corresponding to 50% to 100% response in proof-of-concept photosensitivity trials were predictive, within 2-fold, of the minimally efficacious doses of the AED.7 On the basis of the pharmacokinetic/pharmacodynamic findings from the current proof-of-principle study of cenobamate, a dose of 200 mg was included for further assessment in large phase 2 clinical trials in patients with focal seizures (ClinicalTrials.gov NCT01397968 and NCT01866111).
Cenobamate suppressed IPS-PPR response in patients with photosensitive epilepsy and was well tolerated in single doses up to 400 mg. These results indicate an efficacy signal for the treatment of patients with epilepsy and support the evaluation of cenobamate efficacy and safety in large clinical trials.
Acknowledgment
The authors thank Janet Peterson, PhD, of MedVal Scientific Information Services, LLC (Princeton, NJ, USA) for her medical writing contributions to the first draft. Medical writing and editing assistance were provided by MedVal Scientific Information Services, LLC (Princeton, NJ), which was funded by SK Life Science, Inc. SK Life Science, Inc performed the statistical analysis. This manuscript was prepared according to the International Society for Medical Publication Professionals' “Good Publication Practice for Communicating Company-Sponsored Medical Research: GPP3.”
Glossary
- AE
adverse event
- AED
antiepileptic drug
- AUC
area under the plasma concentration vs time curve
- IPS
intermittent photic stimulation
- ITT
intent-to-treat
- PPR
photoparoxysmal response
- PR
photosensitivity range
- SPR
standardized photosensitivity range
- TEAE
treatment-emergent adverse event
Appendix. Authors
Footnotes
Class of Evidence: NPub.org/coe
Study funding
This study was funded by SK Life Science, Inc (Fair Lawn, NJ).
Disclosure
D.G.A. Kasteleijn-Nolst Trenite is a consultant/advisor for Idorsia, Otsuka, Ovid, and UCB. B.D. DiVentura is an employee of the Epilepsy Study Consortium, which has received research support, meeting support, and services from Acadia, Acorda, Adamas, Addex, Aeonian, Alexza, Anavex, Axcella, Axovant, Biogen, BioPharm Solutions, BioMotiv/Koutif, Blackfynn, Bloom Science, Bridge Valley Ventures, Cavion, Cerebral, Cerevel, Clinilabs, Concert, Covance, Crossject, CuroNZ, Eisai, Empatica, Engage, Epilepsy Foundation, Epitel, GlaxoSmithKline, GW Pharmaceuticals, Idorsia, Impax, Ionis, Johnson & Johnson, Marinus, Medtronic, MonoSol Rx/Aquestive, Monteris, Nestle-Health Science, Neurelis, NeuroPace, Novartis, Otsuka, Ovid, Pfizer, Pfizer-Neusentis, Praxis, Redpin, Sage, Sancilio, Shire, SK Life Science, Inc, SpringWorks, Stoke, Sunovion, Supernus, Takeda, UCB, Ultragenyx, Upsher-Smith, Vyera, West Therapeutic Development, Xenon, Xeris, Zogenix, and Zynerba. J. Pollard is a co-owner of Cognizance Biomarkers. G. Krauss is a consultant/advisor for Adamas, Eisai, Otsuka, Shire, and Vertex and has received research support from SK Life Science, Inc and UCB. S. Mizne is an employee of MedVal Scientific Information Services, which was contracted by SK Life Science, Inc for medical writing support. J. French receives New York University salary support from the Epilepsy Foundation and for consulting work and/or attending scientific advisory boards on behalf of the Epilepsy Study Consortium for Acadia, Adamas, Addex, Aeonian, Alexza, Anavex, Axcella Health, Axovant, Biogen, BioMotiv/Koutif, Blackfynn, Bloom Science, Bridge Valley Ventures, Cavion, Cerebral Therapeutics, Cerevel, Clinilabs, Concert Pharmaceuticals, Covance, Crossject, CuroNZ, Eisai, Empatica, Engage Therapeutics, Epitel, GW Pharmaceuticals, Idorsia, Impax, Ionis, J&J Pharmaceuticals, Marinus, MonoSol Rx, Neurelis, Novartis, Otsuka Pharmaceutical Development, Ovid Therapeutics Inc., Pfizer, Pfizer-Neusentis, Praxis, Redpin, Sage, Sancilio, Shire, SK Life Science, Inc, SpringWorks, Stoke, Sunovion, Supernus, Takeda, UCB, Ultragenyx, Upsher-Smith, Vyera, West Therapeutic Development, Xenon, Xeris, Zogenix, and Zynerba. J. French has also received research grants from Biogen, Cavion, Engage, Neurelis, Ovid, SK Life Science, Inc, UCB, and Zogenix, as well as grants from the Epilepsy Research Foundation, Epilepsy Study Consortium, and National Institute of Neurological Disorders and Stroke. She is on the editorial board of Lancet Neurology and Neurology Today. She is scientific officer for the Epilepsy Foundation, for which New York University receives salary support. She has received travel reimbursement related to research, advisory meetings, or presentation of results at scientific meetings from the Epilepsy Study Consortium, the Epilepsy Foundation, Adamas, Axovant, Biogen, Blackfynn, CuroNZ, Eisai, Engage, Idorsia, Neurelis, Novartis, Otsuka, Ovid, Pfizer, Redpin, Sage, SK Life Science, Inc, Sunovion, Takeda, UCB, Ultragenyx, and Zynerba. Go to Neurology.org/N for full disclosures.
References
- 1.Binnie CD, Kasteleijn-Nolst Trenite DG, De Korte R. Photosensitivity as a model for acute antiepileptic drug studies. Electroencephalogr Clin Neurophysiol 1986;63:35–41. [DOI] [PubMed] [Google Scholar]
- 2.Di Prospero NA, Gambale JJ, Pandina G, et al. Evaluation of JNJ-26489112 in patients with photosensitive epilepsy: a placebo-controlled, exploratory study. Epilepsy Res 2014;108:709–716. [DOI] [PubMed] [Google Scholar]
- 3.Kasteleijn-Nolst Trenite D, Genton P, Brandt C, Reed RC. The “photosensitivity model” is (also) a model for focal (partial) seizures. Epilepsy Res 2017;133:113–120. [DOI] [PubMed] [Google Scholar]
- 4.Lu Y, Waltz S, Stenzel K, Muhle H, Stephani U. Photosensitivity in epileptic syndromes of childhood and adolescence. Epileptic Disord 2008;10:136–143. [DOI] [PubMed] [Google Scholar]
- 5.Kasteleijn-Nolst Trenite DG, van Emde Boas W, Groenhout CM, Meinardi H. Preliminary assessment of the efficacy of Org 6370 in photosensitive epileptic patients: paradoxical enhancement of photosensitivity and provocation of myoclonic seizures. Epilepsia 1992;33:135–141. [DOI] [PubMed] [Google Scholar]
- 6.Kasteleijn-Nolst Trenite DG, Marescaux C, Stodieck S, Edelbroek PM, Oosting J. Photosensitive epilepsy: a model to study the effects of antiepileptic drugs: evaluation of the piracetam analogue, levetiracetam. Epilepsy Res 1996;25:225–230. [DOI] [PubMed] [Google Scholar]
- 7.Yuen ES, Sims JR. How predictive are photosensitive epilepsy models as proof of principle trials for epilepsy? Seizure 2014;23:490–493. [DOI] [PubMed] [Google Scholar]
- 8.Nakamura M, Shin H, Jang IS. Mechanism of action of cenobamate: preferential inhibition of the persistent sodium current. Neurology 2018;90(suppl 15):P5.278. Abstract. [Google Scholar]
- 9.Younus I, Reddy DS. A resurging boom in new drugs for epilepsy and brain disorders. Expert Rev Clin Pharmacol 2018;11:27–45. [DOI] [PubMed] [Google Scholar]
- 10.Zaccara G, Schmidt D. Do traditional anti-seizure drugs have a future? A review of potential anti-seizure drugs in clinical development. Pharmacol Res 2016;104:38–48. [DOI] [PubMed] [Google Scholar]
- 11.Bialer M, Johannessen SI, Levy RH, Perucca E, Tomson T, White HS. Progress report on new antiepileptic drugs: a summary of the Eleventh EILAT Conference (EILAT XI). Epilepsy Res 2013;103:2–30. [DOI] [PubMed] [Google Scholar]
- 12.Kasteleijn-Nolst Trenite D, Brandt C, Mayer T, et al. Dose-dependent suppression of human photoparoxysmal response with the competitive AMPA/kainate receptor antagonist BGG492: clear PK/PD relationship. Epilepsia 2015;56:924–932. [DOI] [PubMed] [Google Scholar]
- 13.French JA, Krauss GL, Kasteleijn D, DiVentura BD, Bagiella E. Effects of marketed antiepileptic drugs and placebo in the human photosensitivity screening protocol. Neurotherapeutics 2014;11:412–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bond A, Lader M. The use of analogue scales in rating subjective feelings. Br J Med Psychol 1974;47:211–218. [Google Scholar]
- 15.Scheffer IE, Berkovic S, Capovilla G, et al. ILAE classification of the epilepsies: position paper of the ILAE Commission for Classification and Terminology. Epilepsia 2017;58:512–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kasteleijn-Nolst Trenite DG, Genton P, Parain D, et al. Evaluation of brivaracetam, a novel SV2A ligand, in the photosensitivity model. Neurology 2007;69:1027–1034. [DOI] [PubMed] [Google Scholar]
- 17.Kasteleijn-Nolst Trenite DG, French JA, Hirsch E, et al. Evaluation of carisbamate, a novel antiepileptic drug, in photosensitive patients: an exploratory, placebo-controlled study. Epilepsy Res 2007;74:193–200. [DOI] [PubMed] [Google Scholar]
- 18.Vernillet L, Kamin M. Pharmacokinetics of cenobamate (YKP3089): results from single and multiple oral rising-dose studies in healthy subjects. Neurology 2018;90(suppl 15):P5.280. Abstract. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data for the analyses described in this article are available by request from the author investigators or SK Life Sciences, Inc, the company sponsoring the clinical development of cenobamate for the treatment of focal epilepsy.