Abstract
Objective
Compared to typically developing (TD) peers, children with attention-deficit/hyperactivity disorder (ADHD) consistently demonstrate impaired transcranial magnetic stimulation (TMS)-evoked short interval cortical inhibition (SICI) of motor evoked potentials (MEPs) in resting motor cortex (M1). To determine whether perturbed M1 physiology also reflects clinically relevant behavioral dysfunction, we evaluated M1 physiology during a cognitive control task taxing motor response selection/inhibition.
Methods
In this case-control study, behavioral ratings, motor skill (assessed using standardized examination), and left M1 physiology were evaluated in 131 right-handed, 8- to 12-year-old children (66 ADHD: mean 10.5 years, 43 male; 65 TD: mean 10.6 years, 42 male). The primary outcomes were MEP amplitudes and SICI, evaluated during rest and during a modified “racecar” Slater-Hammel stop signal reaction task, with TMS pulses administered 150 ms prior to the target go action and after the dynamic stop cue.
Results
Go responses were significantly slower (p = 0.01) and more variable (p = 0.002) in ADHD. Children with ADHD showed less M1 SICI at rest (p = 0.02) and during go (p = 0.03) and stop trials (p = 0.02). Rest M1 excitability increased during response inhibition task engagement (p < 0.0001). This Task-Related Up-Modulation (TRUM) was less robust across and within groups, with diminished task upmodulation associated with significantly more severe ADHD behavioral ratings and slower stop signal reaction times.
Conclusion
Children with ADHD show anomalous motor cortex physiology, with deficient SICI across behavioral states and less TRUM from rest to action selection. Associations of these physiologic measures with ADHD symptoms and cognitive control measures support further investigation into biological mechanisms.
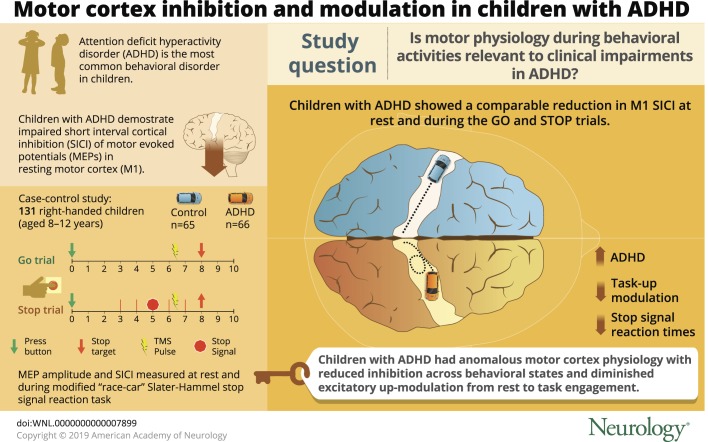
Attention-deficit/hyperactivity disorder (ADHD), the most common behavioral diagnosis in children, incurs high medical costs and often portends low academic achievement and poor adult outcomes.1 Improving our understanding of mechanisms of ADHD must involve development of reliable methods to quantify outputs of biologically relevant neurobehavioral systems. Ideally, these outputs should reflect neurodevelopment, clinical symptoms, and dimensions of impaired function; for example, impaired development of motor control and inefficient response inhibition.2–4
To characterize physiology underlying this atypical motor and behavioral development,5,6 we previously compared transcranial magnetic stimulation (TMS) measures in dominant resting motor cortex (M1) in children with ADHD vs typically developing (TD) children. We7 and others8–10 have reported reduced M1 short interval cortical inhibition (SICI) at rest in ADHD. SICI has been shown in pharmacologic studies to increase in response to both γ-aminobutyric acid (GABA)-A and dopamine receptor agonists.11 To replicate these initial findings and, further, to develop a physiologic understanding of inefficient response inhibition, the primary objective of this study was to determine, in extensively phenotyped 8- to 12-year-old children with ADHD and TD controls, whether SICI differs during action selection and suppression. Our second objective was to determine whether modulation of brain activation during response inhibition differs in ADHD, which could place TMS findings in the context of prior functional imaging research.12 The overarching aim was to understand motor physiology during behavioral activities relevant to clinical impairments in ADHD.
Methods
This is a case-control study of motor development, motor cortex physiology, response inhibition, and clinical/behavioral symptoms in 8- to 12-year-old children recruited at 2 urban medical centers.
Standard protocol approvals, registrations, and patient consents
All children were recruited concurrently via advertisements and mailed solicitations from 2011 to 2017. Written informed consent was obtained from the legal guardians of study participants. The study was approved by the Johns Hopkins Medicine and Cincinnati Children's Hospital Medical Center Institutional Review Boards.
For participant screening, the ADHD Rating Scale IV13 and the Conners' Parent Rating Scale–Revised14 or third edition15 were used. Children with ADHD were included based on diagnostic thresholds on both scales, confirmed using both the Kiddie Schedule for Affective Disorders and Schizophrenia for School-Aged Children (K-SADS)16 and the clinical impression of a board-certified pediatric neurologist with extensive experience with ADHD. Children were included in the TD group only if they did not meet diagnostic criteria on either rating scale or the K-SADS. In addition, children were excluded if they had histories of neurologic illness or injury, genetic disorders, seizures, intellectual disability, or left-handedness/mixed dominance, as assessed by the Edinburgh Handedness Inventory.17 Children with a full-scale IQ (FSIQ) <80 on the Wechsler Intelligence Scale for Children (WISC)–IV18 or WISC-V19 were excluded. Between-group comparisons in IQ were made using the General Ability Index.20 Home environment was evaluated with the Hollingshead21 Parent History Questionnaire assessment of socioeconomic status. Psychostimulants, but no other medications, were allowed, but temporarily discontinued the day prior to and day of testing.
Motor skill assessments of children with ADHD and TD children
Study personnel at both sites trained together for consistency of motor assessments using the Physical and Neurological Examination for Subtle Signs (PANESS).7,22
Motor cortex physiology: Resting (baseline)
Study personnel at both sites trained together for consistency of motor physiology assessments. Both sites used Magstim 200 TMS (Magstim, New York, NY) connected through a Bistim module to a round 90-mm coil, as well as identical amplifiers, filter settings, and signal processing software. TMS utilizes magnetic fields to generate an electric field that can induce depolarization in neurons within range of the coil. Single suprathreshold intensity pulses over M1 can generate a motor evoked potential (MEP) measurable in anatomically localized muscles, measureable with surface EMG. Pairing suprathreshold pulses with preceding subthreshold TMS pulses can consistently inhibit or activate motor cortex interneurons, reducing or increasing the amplitude of the MEP.
TMS coil placement was flat at the vertex, with the handle directly posterior. This was done to enhance stability, compared to the figure of 8 coil, while performing TMS in hyperkinetic children. All protocols for active and resting motor thresholds (AMT, RMT),23 cortical silent periods (CSP),24 and paired pulse TMS for SICI and intracortical facilitation (ICF)25 are in standard use, implemented by our laboratories as previously described.7 Threshold measures were performed first, to habituate children, starting with pulses at 10% maximal stimulator output, increasing by 10% until a consistent MEP was observed, then decreasing the intensity until a point was reached where 3 of 6 pulses produced no MEP and 3 produced a MEP of approximately 50 μV at rest (RMT) and during low force tonic muscle contraction. Pulse intensities were, for CSP, 1.5 × AMT, for SICI (at 3 ms pair) and ICF (at 10 ms pair), 0.6 × RMT conditioning and 1.2 × RMT test pulse.
Response inhibition measure
Response inhibition was assessed using the Slater-Hammel task stop-signal reaction time (SSRT) paradigm,26,27 which we modified into a child-friendly racecar version (figure 1).28 In brief, participants faced a computer monitor running the task in Presentation (v. 10.0; Neurobehavioral Systems, Albany, CA; figure 1) while seated in a comfortable chair. Ulnar aspects of both arms and hands rested fully on a body-surrounding pillow (The Boppy Company, LLC, Golden, CO) so the palmar surface faced medially. The dominant hand operated a game controller with a fully extended index finger. Surface EMG electrodes recorded the first dorsal interosseous (FDI) muscle.
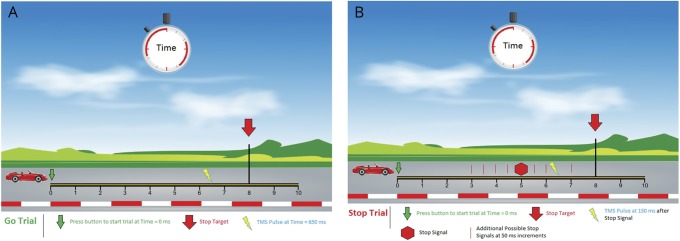
Figure 1. “Racecar Slater Hammel” with transcranial magnetic stimulation (TMS), as described28.
(A) Go trial schematic. Dominant index finger adduction (see Methods for hand position) onto a game-player button activates the car to move across the screen. Participants are instructed to lift the finger, activating the first dorsal interosseous where the surface EMG is recording, between 700 and 800 ms after start of the trial so that the car arrives just prior to the 800 ms “target” indicated by the red down arrow. The TMS pulse (lightning) is given at 650 ms after trial onset. (B) Interspersed at random among go trials are stop trials (3:1 ratio). Participants were instructed if they saw the car stop spontaneously, for example, at site of the red stop sign, to prevent their finger lift at the red arrow and keep the button pushed until the car completely crosses the track and they see a checkered flag appear on the right side of the screen. TMS pulses were delivered 150 ms after the car stop signal. Feedback was provided for each trial. For go: “Too early!” for action at <700 ms; “Great job!” for action from 700 to 800 ms; “Too late!” for action at >800 ms. For stop: “Too early!” for action at <1,000 ms (failed stop); “Great job!” for no action or a finger lift action at >1,000 ms; truncated at 1,250 ms. Early finger lift prior to a TMS pulse is a null trial, repeated later to make the number consistent. Image reprinted with permission from JoVE.
The participant initiated each trial by adducting (pushing down) this finger on the game controller button, activating the finger flexors, antagonistic to the FDI, causing a racecar at the left side of the screen to audibly start its “engine” and then traverse a straight “racetrack” across the screen in 1,000 ms (figure 1). The car keeps going only as long as the finger is pushed down. The “go action” of this task requires lifting the finger, i.e., activating FDI, just prior to the 800 ms mark. Lifting the finger “cuts the engine,” immediately arresting the forward progress of the car. The goal is to get the car as close as possible to the 800 ms mark without going past it. However, in 25% of trials, at random, the car stops itself spontaneously between the 300 and 700 ms mark, i.e., 100–500 ms prior to the finish line. The child is instructed that if the car stops itself early, they should maintain their finger pressed down until they see a checkered flag (which occurs at 1,000 ms). Thus, the spontaneous stopping of the car is a stop cue. Successful stopping is “not lifting the finger at the 800 ms mark,” and maintaining finger adduction for greater than 1,000 ms (figure 1). This stop cue occurs initially 300 ms prior to the finish line, then shifts by 50 ms increments depending on success or failure, allowing the stop trial times to converge to indicate response inhibition efficiency.28
Before testing, participants practiced with 10 go, 10 stop, and 20 mixed go and stop trials. If the study team judged that the child understood instructions, then the game was played with three 40 trial blocks (30 go with 10 stop randomly intermixed). During the first block, the TMS intensity was set at 20% maximum stimulator output (subthreshold). This familiarized participants with the stimulation procedure and the auditory artifact. During the second 2 blocks, single (at 1.2 × RMT) and 3-ms-paired (at 0.6 and 1.2 × RMT) pulses were delivered randomly. For go trials, these occurred 150 ms prior to the expected finger lift; for stop trials, these occurred 150 ms after the car stop.
Critically, our experimental design and statistical analysis allowed for assessment of single and paired pulse evoked MEP amplitudes as well as SICI during action selection, accounting for each individual's action times on a trial by trial basis and adjusting for the confounding effects of movement preparation. In addition, TMS pulses are linked during each stop trial to a dynamically shifting stop cue time, allowing assessment of M1 physiology during successful stopping while adjusting for each individual's stopping efficiency. Finally, this approach allows for comparison of global, task-related modulation of M1, such that any ADHD vs TD differences would be adjusted for intraindividual and interindividual variations in response times and could be evaluated related to behavioral scales, motor control maturation, and stop performance.
Behavioral and EMG data files were extracted, blinded to diagnosis and all other clinical data, then combined with phenotype data for analysis.
Statistical analyses
Univariate analyses of behavior, motor function, motor physiology, and clinical/demographic variables
Motor, physiologic, behavioral, and demographic data were compared across diagnostic groups and sites using t tests and χ2 as appropriate.
Replication: Means
Replicability of prior findings7 for SICI differences between children with ADHD and TD children was assessed using the standard “method of means,” where for each participant SICI was calculated as a ratio of the mean of the paired pulse to mean of the single pulse trial MEP amplitudes.29
Sample size calculation
To replicate prior rest M1 SICI findings,7 we calculated that a sample of 22 children per group would yield 80% power to detect a comparable group difference. For response inhibition,27 a study published using a standard go/no-go task with TMS in children with ADHD (n = 43) vs TD children (n = 29) identified diagnostic group differences of 0.19 (SD 0.35) in successful stop trials.30 On this basis, a balanced sample of 43 per group was calculated as sufficient.
Repeated measures analyses of MEP data
The primary planned analysis for response inhibition M1 physiology was performed on all trials using repeated measures mixed models with participant as a random effect and MEP amplitude as the dependent variable. Increased MEP amplitudes may result from increased excitatory, reduced inhibitory (disinhibition), or some combination of inputs into M1. The primary experimental variables were diagnostic group (ADHD vs TD) and PulseType (paired vs single). Models were analyzed secondarily by log-transformation. All results are given in the original, untransformed scale, except as noted. The following factors were included in all models: age, sex, site (city/institution), and artifact. All models were analyzed using SAS statistical software version 9.4 (SAS Institute Inc, Cary, NC). From the diagnosis × PulseType interaction, SICI is estimated based on ratios of the least squares means. A p value of 0.05 was considered significant. Secondary (dimensional diagnostic) analysis was performed substituting ADHD scores as a covariate across and within groups.
M1 modulation during task engagement
The global effects of task participation on M1 physiology was performed with MEP amplitude as the dependent variable and experimental variables diagnosis (ADHD, TD), PulseType (paired, single), and action (rest, task). Dimensional analysis was again performed.
SICI during response inhibition
The physiology of successful go and stop were evaluated with MEP as the dependent variable and experimental variables diagnosis (ADHD, TD) and PulseType (paired, single). For go trials (only), to account for known effects of motor preparation,31 movement time was included as a covariate.
Additional exploratory analyses were performed by including in the models baseline physiologic measures (RMT, AMT, CSP, ICF), motor measures (PANESS), behavioral data (SSRT), and other data (e.g., FSIQ, Hollingshead).
A comparison of all (early go, optimal go, late go, failed stop, successful stop) trial types' MEP amplitudes by PulseType and diagnosis was performed. Estimates were “normalized” to each diagnostic group's rest values and plotted.
Data sharing
Additional analysis is ongoing of EEG, MRI, goniometer, and clinical data relevant to the analyses performed with funding from the parent grant. Data sharing will occur following the conclusion of those analyses.
Results
Participants
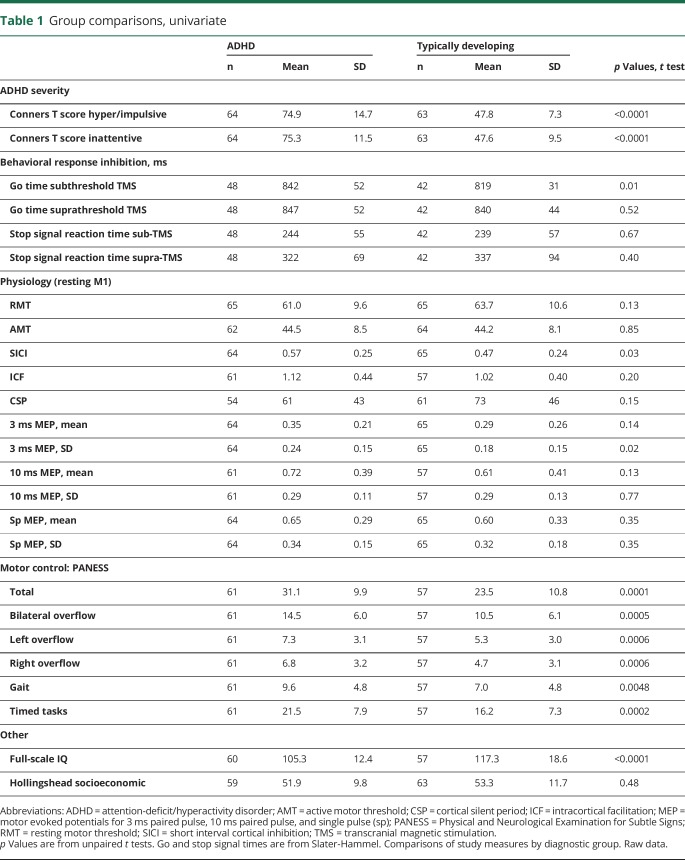
Demographic, clinical, physiologic, and behavioral variables were compared by group (table 1). The recruited sample included 131 children (66 children with ADHD, 65 TD controls; 55 Cincinnati; 76 Baltimore). Boys comprised 65% of both diagnostic groups. Mean age was 10.5 years (SD 1.4) in the ADHD group and 10.6 years (SD 1.3) in the TD group. Racial mix was balanced overall, with Caucasian 61% ADHD vs 63% TD; African American 24% ADHD vs 17% TD; Asian 2% ADHD vs 11% TD; and biracial 14% ADHD vs 9% TD (p = 0.11). Ethnicity mix was Hispanic 17% of ADHD vs 3% of TD (p = 0.02). As expected, ratings for ADHD symptoms were significantly greater in ADHD for all scales (p < 0.0001). All PANESS subscores were higher (worse) in ADHD (p ≤ 0.005). The number completing Slater-Hammel-TMS (n = 48 ADHD, n = 42 TD) was consistent with the planned sample size but less than the total sample due primarily to excessive muscle artifact and high thresholds.
Table 1.
Group comparisons, univariate
Replication in new cohort of prior finding of reduced M1 rest SICI in ADHD, method of means
At rest, children with ADHD showed significantly less M1 SICI than did TD children (table 1). The resting, baseline SICI ratio in the ADHD group was 0.57 (43% inhibition) (95% CI 0.51 to 0.63); in the TD group it was 0.47 (53% inhibition) (95% CI 0.41 to 0.53) (table 1). Thus, M1 SICI was 19% reduced in the ADHD group compared to the TD group (t128 = 2.32; p = 0.028).
Replication in new cohort of finding of reduced M1 rest SICI in ADHD, mixed model
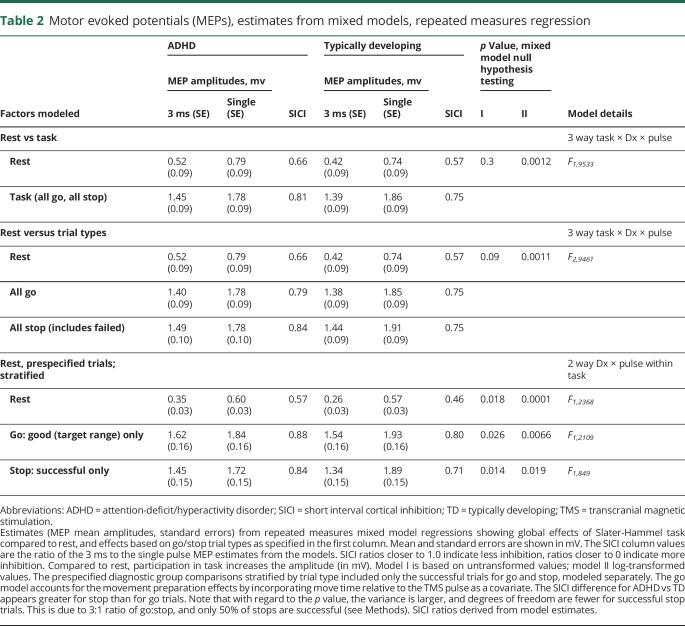
Including all trials, mixed modeling generated SICI estimates similar to the method of means, with less rest M1 SICI in children with ADHD (LSMeans estimate SICI = 0.57) than in TD children (SICI = 0.46) (table 2 and figure 2).
Table 2.
Motor evoked potentials (MEPs), estimates from mixed models, repeated measures regression
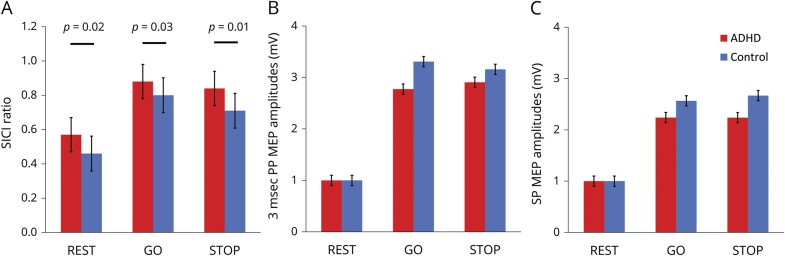
Figure 2. Transcranial magnetic stimulation (TMS) results.
(A) M1, TMS-evoked short interval cortical inhibition (SICI) ratios at rest, during optimal go and successful stop trials. For each, the ratios are significantly greater (i.e., there is less inhibition) in attention-deficit/hyperactivity disorder (table 2). A ratio of 1.0 indicates no inhibition; higher ratios therefore indicate less inhibition. From mixed models, LSMeans estimates: p values are from diagnosis by pulse-type interaction terms; error bars are standard errors of the mean (SEM). (B) 3-ms paired pulse TMS-evoked motor evoked potential (MEP) amplitudes, normalized to rest. (C) Single-pulse TMS-evoked MEP amplitudes, normalized to rest.
Response inhibition behaviors
Response inhibition performance was comparable between children with ADHD and TD children (table 1). The average percentage of successful go trials was 40% in children with ADHD, 40% in TD children. The average percentage of successful stop trials was 47% in children with ADHD, 46% in TD children. The average total number of premature (prior to TMS) finger lift errors, leading to repeated trials, was 13 for children with ADHD and 11 for TD children (all p > 0.1).
During the subthreshold TMS block, go times averaged 23 ms (SE 9.2 ms) slower (worse) in children with ADHD (F1,82 = 6.3, p = 0.014). Go time variability was significantly greater in the children with ADHD (p = 0.0017; Satterthwaite method). As expected, go times were 8.2 ms (SE 3.4) faster per each 1 year older (p = 0.018). No significant bivariate correlations were identified with MEP amplitude variability (not shown).
Extension of finding of reduced M1 rest SICI in ADHD to response inhibition, mixed model
There was significantly less M1 SICI in children with ADHD during both successful go trials and successful stop trials (table 2 and figure 2).
M1 modulation during task engagement
MEP amplitudes, shown normalized to resting MEP amplitudes (figure 2), are larger during the response inhibition task (F2,9535 = 866, p < 0.0001). Task-Related Up-Modulation (TRUM) occurred for all trial subtypes. Normalized to rest, TRUM was 2.5- to 4-fold greater for paired pulse and 2- to 3-fold higher for single pulse. TRUM also occurred during successful stop trials, in which there is no FDI activation (figure 2 and table 2).
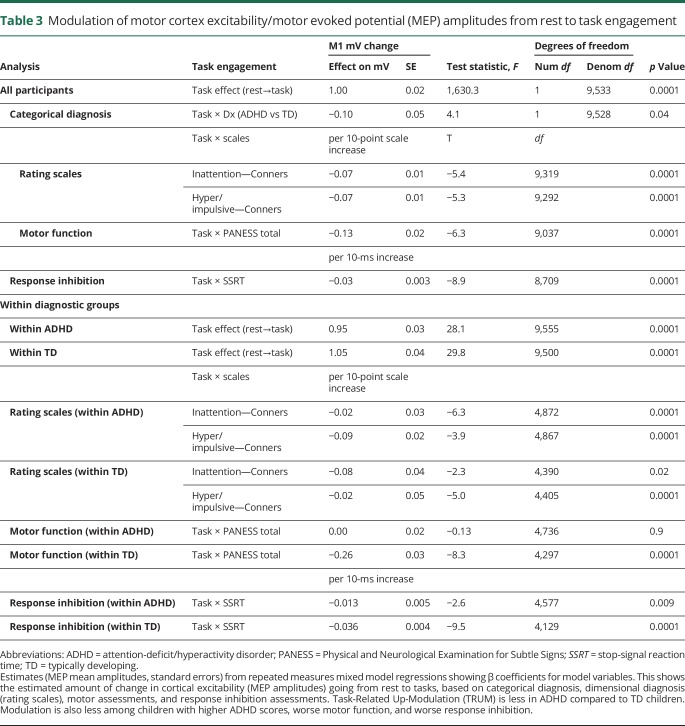
The categorical diagnosis of ADHD was associated with less TRUM of M1 MEP amplitudes (table 3). Modulation of M1 during the response inhibition task showed consistent relationships with dimensional data. Children with higher (worse) ADHD rating scale scores had less TRUM (figure 3 and table 3). Children with less efficient response inhibition, i.e., slower (worse) SSRTs, also had less TRUM (table 3). Importantly, these relationships held across the full cohort as well as within the ADHD and TD groups separately. Children with worse motor development, i.e., higher PANESS scores, also had diminished TRUM. However, stratifying by groups, this relationship remained in the TD children but not the children with ADHD (table 3).
Table 3.
Modulation of motor cortex excitability/motor evoked potential (MEP) amplitudes from rest to task engagement
Figure 3. Modulation as a function of attention-deficit/hyperactivity disorder (ADHD) score severity based on Connor T scores for inattention and hyperactivity/impulsivity, stratified by diagnosis.
(A) Inattention. (B) Hyperactivity/impulsivity. The lower, solid line indicates the transcranial magnetic stimulation (TMS)–evoked motor evoked potential (MEP) amplitudes at rest, 20 rest trials per participant. The upper dashed line indicates the TMS-evoked MEP amplitude during the response inhibition task, 80 task trials per participant. Task-Related Up-Modulation (TRUM) is the vertical “distance” from the rest line to the task line. Worse ADHD severity, as assessed by Conners T scores for inattentiveness and hyperactivity/impulsivity, is statistically associated with less TRUM (p < 0.001 for both). This relationship holds for the entire cohort as well as within diagnosis groups (table 3).
Exploratory analyses and correlations
Univariate
Higher (worse) PANESS scores correlated with younger age and with greater ADHD symptom severity as assessed with all inattention and hyperactivity-impulsivity rating scales (p < 0.001 for all, data not shown). No significant bivariate correlations were identified between ADHD scales and either SICI (method of means) or SSRT.
Mixed models
Overall, and in contrast to our prior study,7 we found no association overall between higher ADHD severity scores and M1 SICI at rest (p > 0.1). However, post hoc analysis of this particular finding identified an effect of city. Restricting the analysis to the Cincinnati site data, less SICI did correspond to more severe ADHD ratings (Conners Inattentive T score F1,953.4 = 8.0, p = 0.005; hyper/impulsive T score F1,953.5 = 9.7, p = 0.002).
Including baseline TMS measures (RMT, AMT, CSP, ICF), PANESS totals or subscales, or mean go times in the models identified either marginal or no statistical associations (not shown). However, including SSRT as a covariate in the mixed models demonstrated an association between shorter (better) SSRTs and larger MEPs during go (F1,85.1 = 10.4, p = 0.002) and stop (F1,88 = 7.3, p = 0.008) but not rest (F1,82.6 = 2.6, p = 0.11) trials.
Discussion
This study comparing carefully matched and comprehensively characterized cohorts of children ages 8–12 years with typical development and with ADHD extends prior findings of significantly reduced TMS-evoked SICI in resting motor cortex (M1) in children with ADHD7,10,32 by demonstrating a comparable reduction in M1 SICI during both response selection (go) and response inhibition (stop). Thus, significantly reduced baseline “resting” SICI in ADHD, which can be conceptualized as “faulty brakes,”33 persists during a clinically relevant behavioral domain of both response selection and response inhibition.34
A second, highly significant finding is a robust association between ADHD symptoms and diminished TRUM during engagement in response selection and inhibition. This diminished TRUM robustly correlated within and across diagnostic groups with higher clinical ADHD severity scores and slower stop signal reaction times. This might seem paradoxical: if neural inhibition in the M1 circuit probed by the SICI paradigm were mechanistically related to the inhibition of behavior, one might have anticipated that children with disinhibited behavior would show disinhibition in M1 physiology during task performance. That is, larger, and not smaller, MEP amplitudes would have been evoked in children with more inattention, hyperactivity, and impulsivity. However, our findings are consistent with functional MRI studies showing reduced cortical activation during stop task performance.35 Further exploration with complementary modalities and TMS during other relevant behavioral tasks may help clarify the relationships between these physiologic measures in M1 and domains of clinical symptoms or impaired response inhibition.
Single-pulse TMS-evoked MEP amplitudes reflect the state of M1. Paired pulse 3 ms interstimulus interval TMS MEP amplitudes reflect the state of local interneurons activated by the first, subthreshold conditioning pulse. A predominantly inhibitory effect is expected for interstimulus intervals of less than 7 ms; however, short interval facilitation (SICF), generated in resting M1 with higher conditioning pulse intensities, has been described related to low dopamine in Parkinson disease.36 The high ratios in some participants during go and stop trials suggest further research evaluating both SICI and SICF in ADHD may be useful. The consistency of SICI ADHD findings in the present study, as well as multiple prior studies across a variety of laboratories and despite some differences in TMS technique, suggests that M1 physiology reflects dysfunction in prefrontal or subcortical regions responsible for clinically impairing inattention, hyperactivity, and impulsivity.
Combining TMS with performance of behaviorally relevant tasks takes advantage of features of motor circuits and creates opportunities for evaluation of behavioral disorders. Upmodulation of M1 net excitability is readily observed during even slight muscle activation at the instant of the TMS pulse. This ramping up of M1 physiology is also observed when TMS pulses are administered 100–150 ms prior to movements,31 as this study was designed to do. This explains the large amplitudes we found during go trials. However, this task engagement effect is not explained solely by a relationship to movement, as upmodulation occurred even during successful stop trials, where there is no movement of the target muscle. Differentiating and quantifying mechanisms involving other cortical and subcortical inputs into M1 and how those contribute to diagnosis-related differences in both reduced M1 SICI and reduced M1 TRUM merits further investigation. In particular, as supported by prior pharmacology/TMS studies,11 understanding relationships between M1 SICI and ICF and their relationship to the neurobiology of inattention, hyperactivity, and impulsivity might be enhanced by studies involving magnetic resonance spectroscopy and GABA or glutamate.37
The Slater-Hammel response inhibition task had previously been shown to be TMS-compatible in adults.27 Our modifications enhanced the validity of this task as children remained engaged with no decline in performance during the task. This experimental paradigm also allowed us to evaluate M1 in all participants at a variety of intervals prior to the target action converging at 800 ms. In contrast, in a standard go/no-go task, this requires indexing TMS pulse times individually based on mean reaction times, a time-consuming exercise that may be problematic given higher reaction time variability in ADHD.
Another important difference between this and most prior studies is the statistical method we employed. The standard method of means, in which each individual's trials are averaged to generate a single SICI ratio, would fail to account for differences in dispersion of amplitudes that might differentially affect diagnostic groups, single vs paired pulse trials, or action vs suppression trials. We therefore employed mixed model, repeated measures regression as our primary analytic method. This also allows in the go trials for an accounting of the “move time” on a trial by trial basis. Indexing the TMS pulse to the dynamic stop cue also allows for automatic adjustment for individual performance. Importantly, this technique insures that physiologic differences during go and stop trials between children with ADHD and TD children are not due primarily to differences in performance. Of interest, using this method, at rest and in go trials, the logarithmic transformation generated far more statistically significant associations.
Comparing our findings to previously published experiments is challenging due to many variations in study populations and experimental measures. One finding, consistent across multiple studies,27,31,38–40 is that MEP amplitudes increase and SICI decreases (ratios increase) immediately prior to movements. There is less consistency across studies, however, regarding MEP change associated with stop trials. This may be due to populations studied, as most experiments involve 20 or fewer healthy adults27,39,41 or technical variation, such as intensity of the test pulse.
In comparing the results of our study to the most similar pediatric TMS ADHD study, which used go/no-go TMS in 29 TD children and 43 children with ADHD, it is important to note some methodologic differences. First, single and paired pulse TMS were administered at fixed intervals after cues without any method of generating consistent action/TMS timing. Second, the 1:1 ratio of go to no-go may not have engaged response inhibition mechanisms robustly. Finally, participants were rewarded as a performance incentive.30 This could be problematic, as reward expectation seems to modify M1 physiology.42,43 These authors reported that TD children had, as expected, greater resting M1 SICI and that this diminished prior to action during go trials.30 However, SICI did not diminish or increase during no-go trials. In contrast, hyperactive/impulsive children with ADHD had diminished SICI at rest, which showed no change prior to actions, but increased during no-go trials.30
Limitations of this study include the possibility of diagnostic misclassification. This is inevitable, since the diagnosis of ADHD does not have independent biological validation. However, our dimensional analyses of clinical symptoms support further investigation of the M1 TRUM as a potential biomarker in the large variety of neurologic and psychiatric diagnoses where patients have impulsivity, hyperactivity, or inattention. As in nearly all other studies of ADHD, many children in the ADHD group were prescribed stimulant medications. Even temporary discontinuation does not eliminate the possibility of some drug effects. Intersite variations in diagnosis or experimental technique can also introduce imprecision. This would tend to yield type 2 rather than type 1 errors. The factor “site” was included in all analyses and only appeared to affect results in one assessment of rating scales. Ultimately, our team's recruiting at multiple sites may enhance results' generalizability.
School-age children with ADHD manifest anomalous patterns of motor cortex physiology at rest7,10,32,38 and across behavioral states requiring response selection and inhibition. Upmodulation of M1 excitability, manifest by substantially larger TMS-evoked MEPs, occurs during the task engagement. The observed muting of this effect, with shallower upmodulation robustly correlating with greater ADHD clinical severity and deficient response inhibition, suggests that ADHD symptoms may be associated with a diminished capacity for cerebral engagement that is readily measurable in M1. Taken together, the SICI and TRUM findings in M1 appear to be robust candidates for further study as brain-based, TMS biomarkers relevant to idiopathic ADHD and related research domain criteria for cognitive and motor control3 in a variety of other diagnoses.
Acknowledgment
The authors thank Dr. Martha Denckla for inspiration, guidance, and comments on the manuscript; and the research coordinators and technicians and the participating families. Data presented in part at the 15th International Child Neurology Congress, November 18, 2018, Mumbai, India; the 2nd International Brain Stimulation Conference, March 8, 2017, Barcelona, Spain; the 46th annual International Neuropsychological Society Meeting, Washington, DC, February 14, 2018; and the 3rd International Brain Stimulation Conference, February 25, 2019, Vancouver, Canada.
Glossary
- ADHD
attention-deficit/hyperactivity disorder
- AMT
active motor threshold
- CSP
cortical silent periods
- FDI
first dorsal interosseous
- FSIQ
full-scale IQ
- GABA
γ-aminobutyric acid
- ICF
intracortical facilitation
- K-SADS
Kiddie Schedule for Affective Disorders and Schizophrenia for School-Aged Children
- M1
dominant resting motor cortex
- MEP
motor evoked potential
- PANESS
Physical and Neurological Examination for Subtle Signs
- RMT
resting motor threshold
- SICF
short interval facilitation
- SICI
short interval cortical inhibition
- SSRT
stop-signal reaction time
- TD
typically developing
- TMS
transcranial magnetic stimulation
- TRUM
Task-Related Up-Modulation
- WISC
Wechsler Intelligence Scale for Children
Author contributions
D.L. Gilbert: drafting/revising the manuscript, data acquisition, study concept or design, analysis or interpretation of data, accepts responsibility for conduct of research and final approval, acquisition of data, statistical analysis, study supervision, obtaining funding. D.A. Huddleston: data acquisition, study concept or design, analysis or interpretation of data, accepts responsibility for conduct of research and final approval, acquisition of data, statistical analysis, study supervision. S.W. Wu: data acquisition, analysis or interpretation of data, accepts responsibility for conduct of research and final approval, acquisition of data, study supervision. E.V. Pedapati: drafting/revising the manuscript, data acquisition, analysis or interpretation of data, accepts responsibility for conduct of research and final approval, developed the software code for inhibition paradigm. P.S. Horn: drafting/revising the manuscript, analysis or interpretation of data, accepts responsibility for conduct of research and final approval, statistical analysis. K. Hirabayashi: data acquisition, accepts responsibility for conduct of research and final approval. D. Crocetti: data acquisition, accepts responsibility for conduct of research and final approval, acquisition of data, study supervision. E.M. Wassermann: study concept or design, analysis or interpretation of data, accepts responsibility for conduct of research and final approval. S.H. Mostofsky: drafting/revising the manuscript, data acquisition, study concept or design, analysis or interpretation of data, accepts responsibility for conduct of research and final approval, study supervision, obtaining funding.
Study funding
This research was funded by R01 MH095014 and R01 MH085328.
Disclosure
D.L. Gilbert has received honoraria and/or travel support from the Tourette Association of America/Centers for Disease Control and Prevention, the American Academy of Pediatrics, and the Child Neurology Society; compensation for expert testimony for the US National Vaccine Injury Compensation Program, through the Department of Health and Human Services; research support from the NIH (NIMH, NINDS); funding for work as a clinical trial site investigator from Ecopipam Pharmaceuticals (clinical trial, Tourette Syndrome) and EryDel (clinical trial, Ataxia Telangiectasia); and has received book royalties from Elsevier and Wolters Kluwer. D.A. Huddleston reports no disclosures. S.W. Wu receives research support from NIH, Tourette Association of America, and Cincinnati Children's Hospital Medical Center; is the site principal investigator for an ataxia-telangiectasia clinical trial sponsored by EryDel S.p.A.; and is a consultant for Medtronic plc. Ernest Pedapati has received research support by the NIH (NIMH), American Academy of Child and Adolescent Psychiatry, and Cincinnati Children's Hospital Research Foundation; is a clinical trial site investigator for the Marcus Autism Center (clinical trial, Autism); receives compensation for consulting for Proctor & Gamble; and receives book royalties from Springer; and reports no disclosures relevant to the manuscript. P.S. Horn received book royalties from the American Association for Clinical Chemistry (AACC) Press. K. Hirabayashi, D. Crocetti, E.M. Wassermann, and S.H. Mostofsky report no disclosures relevant to the manuscript. Go to Neurology.org/N for full disclosures.
References
- 1.Molina BS, Hinshaw SP, Swanson JM, et al. The MTA at 8 years: prospective follow-up of children treated for combined-type ADHD in a multisite study. J Am Acad Child Adolesc Psychiatry 2009;48:484–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Musser ED, Raiker JS Jr. Attention-deficit/hyperactivity disorder: an integrated developmental psychopathology and research domain criteria (RDoC) approach. Compr Psychiatry 2019;90:65–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Garvey MA, Cuthbert BN. Developing a motor systems domain for the NIMH RDoC Program. Schizophr Bull 2017;43:935–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McTeague LM, Huemer J, Carreon DM, Jiang Y, Eickhoff SB, Etkin A. Identification of common neural circuit disruptions in cognitive control across psychiatric disorders. Am J Psychiatry 2017;174:676–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Denckla MB. Revised neurological examination for subtle signs (1985). Psychopharmacol Bull 1985;21:773–800. [PubMed] [Google Scholar]
- 6.Macneil LK, Xavier P, Garvey MA, Gilbert ME, Denckla MB, Mostofsky SH. Quantifying excessive mirror overflow in children with attention-deficit/hyperactivity disorder. Neurology 2011;76:622–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gilbert DL, Isaacs KM, Augusta M, Macneil LK, Mostofsky SH. Motor cortex inhibition: a marker of ADHD behavior and motor development in children. Neurology 2011;76:615–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moll GH, Heinrich H, Trott GE, Wirth S, Bock N, Rothenberger A. Children with comorbid attention-deficit-hyperactivity disorder and tic disorder: evidence for additive inhibitory deficits within the motor system. Ann Neurol 2001;49:393–396. [PubMed] [Google Scholar]
- 9.Gilbert DL, Sallee FR, Zhang J, Lipps TD, Wassermann EM. TMS-evoked cortical inhibition: a consistent marker of ADHD scores in Tourette syndrome. Biol Psychiatry 2005;57:1597–1600. [DOI] [PubMed] [Google Scholar]
- 10.Buchmann J, Gierow W, Weber S, et al. Restoration of disturbed intracortical motor inhibition and facilitation in attention deficit hyperactivity disorder children by methylphenidate. Biol Psychiatry 2007;62:963–969. [DOI] [PubMed] [Google Scholar]
- 11.Ziemann U, Reis J, Schwenkreis P, Rosanova A, Badawy R, Müller-Dahlhaus F. TMS and drugs revisited 2014. Clin Neurophysiol 2015;126:1847–1868. [DOI] [PubMed] [Google Scholar]
- 12.Rubia K. Cognitive neuroscience of attention deficit hyperactivity disorder (ADHD) and its clinical translation. Front Hum Neurosci 2018;12:100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.DuPaul GJ, Power TJ, Anastopoulos AD, Reid R. ADHD Rating Scale-IV: Checklist, Norms, and Clinical Interpretations. New York: Guilford Press; 1998. [Google Scholar]
- 14.Conners CK. Conners' Rating Scales–Revised. North Tonawanda: Multi-Health Systems; 1997. [Google Scholar]
- 15.Conners CK. Conners. 3rd ed. Toronto: Multi-Health Systems; 2008. [Google Scholar]
- 16.Kaufman J, Birmaher B, Axelson D, Perepletchikova F, Brent D, Ryan N. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL 2013, DSM-5). Western Psychiatric Institute and Yale University; 2013. [Google Scholar]
- 17.Oldfield RC. The assessment and analysis of handedness: the Edinburgh Inventory. Neuropsychologia 1971;9:97–113. [DOI] [PubMed] [Google Scholar]
- 18.Wechsler D. Wechsler Intelligence Scale for Children. 4th ed. San Antonio: The Psychological Corporation; 2003. [Google Scholar]
- 19.Wechsler D. Wechsler Intelligence Scale for Children. 5th ed. Bloomington: PsychCorp; 2014. [Google Scholar]
- 20.Thaler NS, Bello DT, Etcoff LM. WISC-IV profiles are associated with differences in symptomatology and outcome in children with ADHD. J Atten Disord 2013;17:291–301. [DOI] [PubMed] [Google Scholar]
- 21.Hollingshead AA. Four-factor Index of Social Status. New Haven; CT1975. [Google Scholar]
- 22.Cole WR, Mostofsky SH, Larson JC, Denckla MB, Mahone EM. Age-related changes in motor subtle signs among girls and boys with ADHD. Neurology 2008;71:1514–1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mills KR, Nithi KA. Corticomotor threshold to magnetic stimulation: normal values and repeatability. Muscle Nerve 1997;20:570–576. [DOI] [PubMed] [Google Scholar]
- 24.Cantello R, Gianelli M, Civardi C, Mutani R. Magnetic brain stimulation: the silent period after the motor evoked potential. Neurology 1992;42:1951–1959. [DOI] [PubMed] [Google Scholar]
- 25.Rothwell JC, Day BL, Thompson PD, Kujirai T. Short latency intracortical inhibition: one of the most popular tools in human motor neurophysiology. J Physiol 2009;587:11–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Slater-Hammel AT. Reliability, accuracy, and refractoriness of a transit reaction. Res Q 1960;31:217–228. [Google Scholar]
- 27.Coxon JP, Stinear CM, Byblow WD. Intracortical inhibition during volitional inhibition of prepared action. J Neurophysiol 2006;95:3371–3383. [DOI] [PubMed] [Google Scholar]
- 28.Guthrie MD, Gilbert DL, Huddleston DA, et al. Online transcranial magnetic stimulation protocol for measuring cortical physiology associated with response inhibition. J Vis Experimentation 2018:e56789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kujirai T, Caramia MD, Rothwell JC, et al. Corticocortical inhibition in human motor cortex. J Physiol 1993;471:501–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hoegl T, Heinrich H, Barth W, Losel F, Moll GH, Kratz O. Time course analysis of motor excitability in a response inhibition task according to the level of hyperactivity and impulsivity in children with ADHD. PLoS One 2012;7:e46066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen R, Yaseen Z, Cohen LG, Hallett M. Time course of corticospinal excitability in reaction time and self-paced movements. Ann Neurol 1998;44:317–325. [DOI] [PubMed] [Google Scholar]
- 32.Moll GH, Heinrich H, Trott G, Wirth S, Rothenberger A. Deficient intracortical inhibition in drug-naive children with attention-deficit hyperactivity disorder is enhanced by methylphenidate. Neurosci Lett 2000;284:121–125. [DOI] [PubMed] [Google Scholar]
- 33.Mink JW. Faulty brakes? Inhibitory processes in attention-deficit/hyperactivity disorder. Neurology 2011;76:592–593. [DOI] [PubMed] [Google Scholar]
- 34.Mostofsky SH, Simmonds DJ. Response inhibition and response selection: two sides of the same coin. J Cogn Neurosci 2008;20:751–761. [DOI] [PubMed] [Google Scholar]
- 35.van Rooij D, Hoekstra PJ, Mennes M, et al. Distinguishing adolescents with ADHD from their unaffected siblings and healthy comparison subjects by neural activation patterns during response inhibition. Am J Psychiatry 2015;172:674–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ni Z, Bahl N, Gunraj CA, Mazzella F, Chen R. Increased motor cortical facilitation and decreased inhibition in Parkinson disease. Neurology 2013;80:1746–1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Greenhouse I, King M, Noah S, Maddock RJ, Ivry RB. Individual differences in resting corticospinal excitability are correlated with reaction time and GABA content in motor cortex. J Neurosci 2017;37:2686–2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Heise KF, Steven B, Liuzzi G, et al. Altered modulation of intracortical excitability during movement preparation in Gilles de la Tourette syndrome. Brain 2010;133:580–590. [DOI] [PubMed] [Google Scholar]
- 39.Fujiyama H, Tandonnet C, Summers JJ. Age-related differences in corticospinal excitability during a Go/NoGo task. Psychophysiology 2011;48:1448–1455. [DOI] [PubMed] [Google Scholar]
- 40.Bruckmann S, Hauk D, Roessner V, et al. Cortical inhibition in attention deficit hyperactivity disorder: new insights from the electroencephalographic response to transcranial magnetic stimulation. Brain 2012;135:2215–2230. [DOI] [PubMed] [Google Scholar]
- 41.Leocani L, Cohen LG, Wassermann EM, Ikoma K, Hallett M. Human corticospinal excitability evaluated with transcranial magnetic stimulation during different reaction time paradigms. Brain 2000;123(pt 6):1161–1173. [DOI] [PubMed] [Google Scholar]
- 42.Thabit MN, Nakatsuka M, Koganemaru S, Fawi G, Fukuyama H, Mima T. Momentary reward induce changes in excitability of primary motor cortex. Clin Neurophysiol 2011;122:1764–1770. [DOI] [PubMed] [Google Scholar]
- 43.Chiu YC, Cools R, Aron AR. Opposing effects of appetitive and aversive cues on go/no-go behavior and motor excitability. J Cogn Neurosci 2014;26:1851–1860. [DOI] [PubMed] [Google Scholar]