Abstract
Objective
Frequent administration of gadolinium-based contrast agents in multiple sclerosis (MS) may increase signal intensity (SI) unenhanced T1-weighted imaging MRI throughout the brain. We evaluated the association between lifetime cumulative doses of gadodiamide administration and increased SI within the dentate nucleus (DN), globus pallidus (GP), and thalamus in patients with early MS.
Methods
A total of 203 patients with MS (107 with baseline and follow-up MRI assessments) and 262 age- and sex-matched controls were included in this retrospective, longitudinal, 3T MRI-reader-blinded study. Patients with MS had disease duration <2 years at baseline and received exclusively gadodiamide at all MRI time points. SI ratio (SIR) to pons and CSF of lateral ventricle volume (CSF-LVV) were assessed. Analysis of covariance and correlation analyses, adjusted for age, sex, and region of interest volume, were used.
Results
The mean follow-up time was 55.4 months, and the mean number of gadolinium-based contrast agents administrations was 9.2. At follow-up, 49.3% of patients with MS and no controls showed DN T1 hyperintensity (p < 0.001). The mean SIR of DN (p < 0.001) and of GP (p = 0.005) to pons and the mean SIR of DN, GP, and thalamus to CSF-LVV were higher in patients with MS compared to controls (p < 0.001). SIR of DN to pons was associated with number of gadodiamide doses (p < 0.001). No associations between SIR of DN, GP, and thalamus and clinical and MRI outcomes of disease severity were detected over the follow-up.
Conclusions
DN, GP, and thalamus gadolinium deposition in early MS is associated with lifetime cumulative gadodiamide administration without clinical or radiologic correlates of more aggressive disease.
MRI protocols used for detection of acute inflammatory lesions for diagnosing and monitoring patients with multiple sclerosis (MS), and in the context of clinical trials, require frequent IV administrations of gadolinium-based contrast agents (GBCA).1,2 Recently, increased concern has been raised regarding the deposition of gadolinium in the brain3–7 and potentially other organs,8 generating considerable controversy in the scientific community and among patients and families, health institutions, industries, and regulatory agencies.9–11 Consequently, recommendations for the clinical and research use of GBCAs have been revised,12,13 which may continue to evolve as new evidence becomes available.
In 2014, the development of increased signal intensity (SI) in the dentate nucleus (DN) and globus pallidus (GP) on unenhanced T1-weighted images (WI) was reported in patients who had undergone multiple MRIs with GBCAs.4 Since then, several studies found increased SI within the DN on unenhanced T1-WI, as a possible consequence of multiple applications of GBCAs in patients with MS.14–22
Against this background, the aim of this study was to evaluate the association between lifetime cumulative doses of GBCA administration and increased SI within the DN, GP, and thalamus on MRI unenhanced T1-WI in patients with early MS, using rigorous selection criteria, to overcome major pitfalls encountered in the previous research studies on the same topic.14–20,23–26 An additional objective was to investigate the association between increased SI within these structures on unenhanced T1-WI and clinical and MRI outcomes of disease severity over the follow-up.
Methods
Study design and population
This was a retrospective, longitudinal, MRI-reader-blinded study in patients with early phase of MS. The study considered all patients with MS with available MRI examination who were followed in the specialized MS center in the period between July 2003 and October 2015. The most recent follow-up (MRF) MRI examination, on which the SI ratio (SIR) was evaluated, was performed in the period between June 2007 and November 2016. The baseline for this study was defined as the date of the time of diagnosis when the first lifetime MRI scan with gadodiamide was performed, whereas the follow-up was defined as the date of the MRF MRI examination, using a standardized 3T MRI protocol.
The inclusion criteria for the study were (1) diagnosis of MS according to the McDonald criteria,27 (2) patients with MS followed in a specialized MS center from the time of diagnosis, (3) disease duration (from disease onset) at the time of diagnosis <2 years, (4) age at time of diagnosis between 18 and 65 years old, (5) all lifetime MRI scans performed on scanners within our specialized MS center, (6) all patients with MS received exclusively 0.1 mMol/kg of gadodiamide (Omniscan, GE Healthcare, Piscataway, NJ) GBCA at all MRI time points, (7) minimum follow-up period between the time of diagnosis and the MRF MRI scan of 12 months, and (8) MRF MRI examination, on which SIR was evaluated, performed on the same 3T scanner, using a standardized MRI protocol that included unenhanced 3D T1-WI. Exclusion criteria were (1) presence of relapse and steroid treatment in the 30 days preceding MRF MRI examination for patients with MS, (2) preexisting medical conditions known to be associated with brain pathology (cerebrovascular disease, positive history of alcohol abuse), (3) significantly reduced estimated glomerular filtration rate of <60 mL/min in patients with MS, and (4) pregnancy.
Controls included nonfamilial relatives of patients with MS, as well as participants recruited through local advertisements. Control participants were enrolled in the study if they presented with normal neurologic and age-compatible MRI examinations. They never received GBCA and were scanned on the same 3T scanner, using the same MRI protocol in the period between years 2007 and 2016. In total, 203 patients with MS and 262 controls fulfilled inclusion and exclusion criteria and were included in the study (figure e-1, doi.org/10.5061/dryad.04df893). Of those 203 patients with MS, 107 (52.7%) had 3T examination at baseline and follow-up using the same MRI hardware, software, and imaging protocol.
Standard protocol approvals, registrations, and patient consents
The study was approved by the Human Subjects Institutional Review Board of the University at Buffalo.
MRI acquisition
All MRI scans, on which SIR was evaluated, were acquired on the same 3T GE Signa Excite HD 12.0 MRI scanner (General Electric, Milwaukee, WI) with an 8-channel head and neck coil. The imaging system did not undergo any major hardware or software upgrades in the period of the study follow-up (10 years), and the MRI acquisition protocol was kept standardized.
MRI sequences included multiplanar dual fast spin-echo proton density and T2-WI, fluid-attenuated inversion recovery (FLAIR), spin-echo T1-WI, and unenhanced 3D T1-WI using a magnetization-prepared fast spoiled single-echo spoiled gradient echo sequence.
SIR was evaluated on 3D T1-WI, acquired with echo time (TE)/inversion time (TI)/repetition time (TR) = 2.8/900/5.9 ms, flip angle = 10°, with a 256 × 256 × 180 matrix and 1 mm isotropic resolution. Other pulse sequence characteristics for 3T MRI were as follows: all scans were acquired with a 256 × 256 matrix and a 25.6 cm field of view (FOV) for an in-plane resolution of 1 × 1 mm2 with a phase FOV of 75% and 1 average. Sequence-specific measures were as follows: for proton density/T2: 3-mm-thick slices with no gap, TE1/TE2/TR = 12/95/3,000 ms, echo train length = 14, flip angle = 90°; for the FLAIR scans, 3-mm-thick slices with no gap, TE/TI/TR = 120/2,100/8,500 ms, flip angle = 90°; and for spin-echo T1-WI, 3-mm-thick slices with no gap, TE/TR = 16/600 ms, flip angle = 90°.
MRI analyses
MRI analyses were performed rater-blinded to the participant’s disease or gadodiamide administration status.
Estimation of DN, GP, and thalamus SIR to pons and CSF of lateral ventricle volume (CSF-LVV)
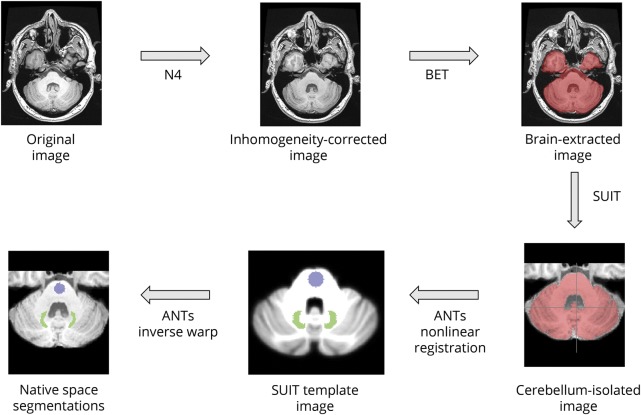
To calculate the SIR of DN, GP, and thalamus to pons, we implemented an automated pipeline as follows (figure 1). We used the spatially unbiased atlas template of the cerebellum and brainstem (SUIT) toolbox28 to isolate the cerebellum from the 3D T1-WI, which was first corrected for intensity inhomogeneity using the N4 algorithm.29 The cerebellum was then warped to the SUIT cerebellar template using advanced normalization tools (ANTs),30 rather than the default SPM-based algorithm implemented in SUIT. We opted for this approach as we found that it yielded improved spatial alignment. Moreover, ANTs were previously ranked the highest in terms of volumetric nonlinear image registration techniques.31 We also warped the whole brain image to Montreal Neurological Institute 152 space. The inverse of the calculated warps was then used to bring the SUIT-defined DN mask and Harvard-Oxford structural atlas–defined GP, thalamus, and CSF-LVV masks, thresholded at 50%, and a pons mask back into the native space of the 3D T1 image. The pons mask was defined on the SUIT template by centering a 7-mm sphere at the voxel coordinate X = 70, Y = 76, Z = 43. The average SI was then calculated for the DN, GP, thalamus, and the pons on the N4-corrected 3D T1-WI, and the ratio between the 2 was calculated to yield the SIR of these structures. All analyses were quality controlled by a single operator. The scan–rescan reproducibility of the method was tested using the intraclass correlation coefficient (ICC) and 95% confidence intervals (CIs) in 5 patients with MS and 5 controls.
Figure 1. Flowchart of the automated pipeline for calculating the signal intensity ratio (SIR) of dentate nucleus (DN) to pons.
The original 3D T1 image was inhomogeneity corrected using the N4 algorithm, which was subsequently processed with the brain extraction tool (BET). Next, the spatially unbiased atlas template of the cerebellum and brainstem (SUIT) toolbox was used to isolate the cerebellum from the brain-extracted image. Advanced normalization tools (ANTs) were then used to nonlinearly register the cerebellum to the SUIT template image. The dentate (shown in green) and pons (shown in blue), defined in SUIT space, were then brought back to the native space, inhomogeneity-corrected image, and the mean signal intensity was calculated for each region of interest. Finally, the signal intensity ratio of dentate nucleus to pons was calculated.
To evaluate the presence or absence of DN hyperintensity on the unenhanced 3D T1-WI, 2 experienced neuroimagers evaluated qualitatively all MRI scans. An additional expert neuroimager served on a panel to reach a consensus when there were discrepancies by the 2 readers. The reproducibility for detection of DN T1 hyperintensity was obtained by 2 raters in 50 patients with MS and 50 controls using Cohen kappa agreement.
Lesion and tissue volumetry
T2-weighted hyperintense lesion volumes (LVs) were assessed using a semiautomated reliable edge detection contouring/thresholding technique with JIM software (version 6.0, Xinapse Systems, Northants, UK; xinapse.com).32 3D T1-WI were lesion filled prior to tissue volumetry analysis using the “lesion_filling” tool from FSL, which replaces lesional voxels with values drawn from the normal-appearing white matter (WM). Normalized whole brain, total gray matter (GM), and total WM volumes were calculated using SIENAX,33 and their changes over the follow-up were assessed using SIENA33 and SIENAX-MTP.34 The volume of DN, GP, and thalamus region of interest (ROI) was also derived.
Data availability statement
The data that support the findings of this study are available on reasonable request to the corresponding author (R.Z.). The data are not publicly available due to containing information that could compromise the privacy of research participants.
Statistical analyses
Statistical analyses were performed using SPSS (version 24; IBM Corp., Armonk, NY). Differences in demographic and clinical characteristics between the study groups were assessed using the χ2, Mann-Whitney rank-sum test, and Student t test, as appropriate. The Shapiro-Wilk test was used to assess normality of the data. Comparisons between MS and controls, and patients with MS who received ≥8 and <8 gadodiamide doses (median number of GBCA administrations: 8), were made using analysis of covariance, corrected for age, sex, and volume of DN, GP, and thalamus ROIs. Spearman, Pearson, and point-biserial correlation coefficients were used to investigate the correlations between SIR of DN, GP and thalamus, and demographic, clinical, and MRI outcomes of disease severity over the follow-up. Using partial correlation analysis adjusted for age and sex, we also investigated the relationship between the number of gadodiamide doses and SIR of DN over the follow-up. The changes of the SIR of DN, GP, and thalamus over the follow-up were examined using paired t test. Effect sizes between patients with MS and healthy controls were calculated using Cohen d. A nominal p value of ≤0.05 was considered statistically significant, using 2-tailed tests.
Results
Demographic and clinical characteristics
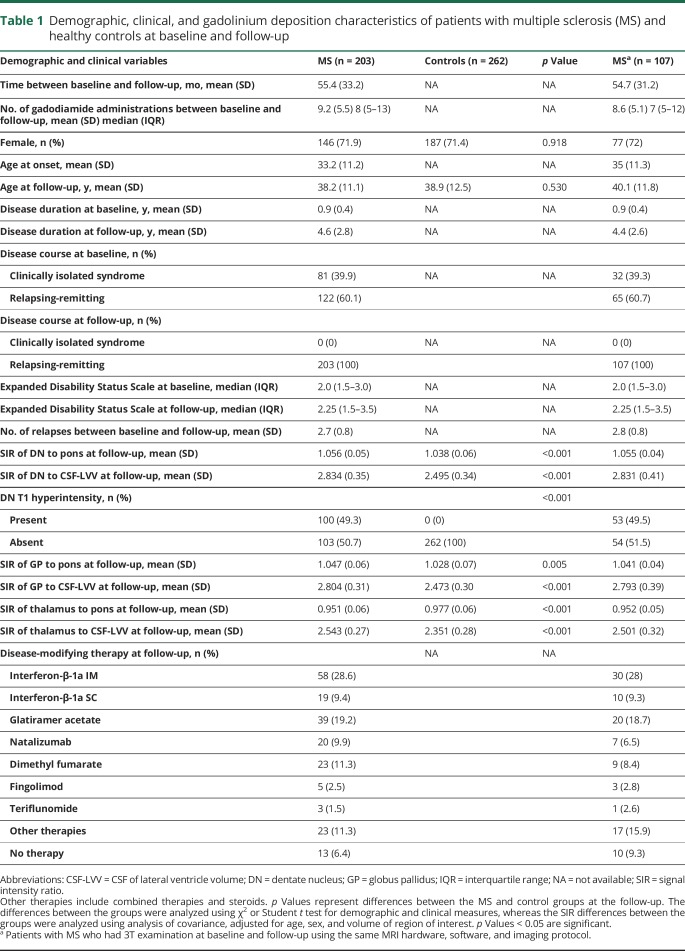
Demographic and clinical characteristics of patients with MS and controls are shown in table 1. The mean time of follow-up was 55.4 months (SD 33.2) and the mean number of gadodiamide administrations was 9.2 (median 8, interquartile range 5–13). At baseline, 81 patients had clinically isolated syndrome (CIS) and 122 (60.1%) had relapsing-remitting MS (RRMS). At follow-up, all patients converted to RRMS. The mean disease duration at baseline was 0.9 (0.4) years, and 4.6 (SD 2.8) years at the follow-up. Over the follow-up, the mean number of relapses was 2.7 (SD 0.8) and 190 (93.6%) patients were on disease-modifying treatment. Similar characteristics were detected in the subgroup of patients with MS (n = 107) who had MRI at baseline and follow-up, confirming that this cohort was representative of the overall study population (table 1).
Table 1.
Demographic, clinical, and gadolinium deposition characteristics of patients with multiple sclerosis (MS) and healthy controls at baseline and follow-up
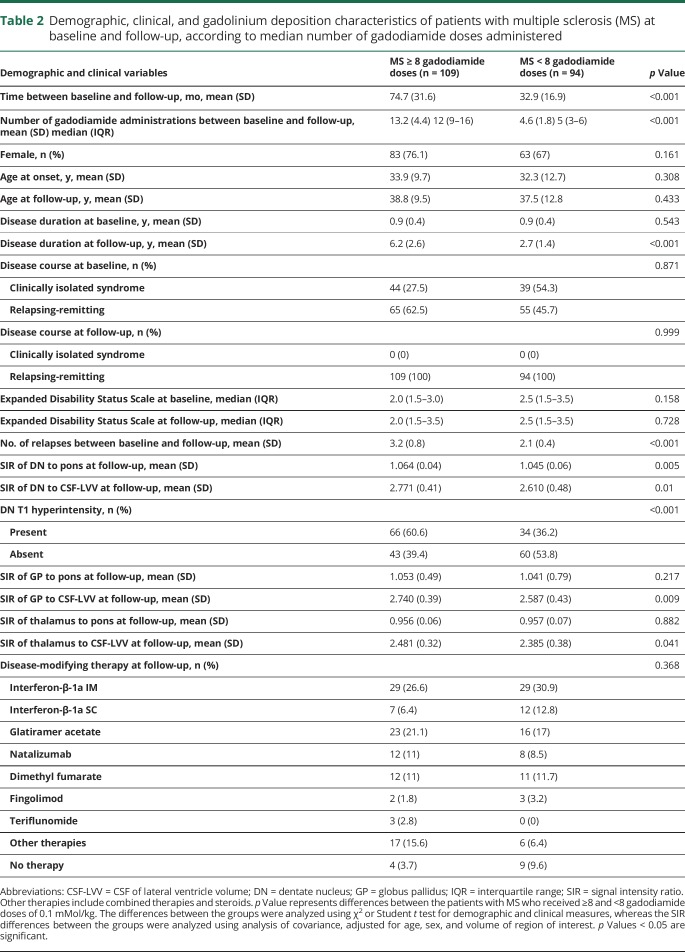
Demographic and clinical characteristics of patients with MS, split by the median number of gadodiamide administrations, are shown in table 2. As expected, patients with MS who received ≥8 gadodiamide doses had longer time of follow-up (74.7 vs 32.9 months, p < 0.001), higher number of gadodiamide administrations (13.2 vs 4.6, p < 0.001), longer disease duration at the follow-up (6.2 vs 2.7 years, p < 0.001), and higher number of relapses (3.2 vs 2.1, p < 0.001), compared to those who received <8 doses.
Table 2.
Demographic, clinical, and gadolinium deposition characteristics of patients with multiple sclerosis (MS) at baseline and follow-up, according to median number of gadodiamide doses administered
Demographic and clinical characteristics of patients with MS, according to sex, are available in table e-1 (doi.org/10.5061/dryad.04df893).
Reproducibility of SIR assessments
The ICC for scan–rescan reproducibility of examined ROIs in participants was between 0.91 and 0.94 (95% CI 0.65–0.98, p < 0.001). Cohen kappa agreement for the presence of DN T1 hyperintensity (yes/no) was 0.88, p < 0.001. There was correlation between SIR of DN to pons quantitative and DN T1 hyperintensity qualitative analyses in patients with MS (r = 0.69, p < 0.001).
MRI differences in the study groups at follow-up
Table 1 shows MRI characteristics of patients with MS and controls at the follow-up. The mean SIR of DN to pons in patients with MS was 1.056 vs 1.038 in controls (p < 0.001, d = −0.33) (figure e-2, doi.org/10.5061/dryad.04df893), whereas the mean SIR of DN to CSF-LVV was 2.834 vs 2.495 (p < 0.001, d = −0.70), respectively. We also found that 100 (49.3%) patients with MS had DN T1 hyperintensity, while none of the controls did (p < 0.001). The mean SIR of GP to pons (p = 0.005, d = −0.22), GP to CSF-LVV (p < 0.001, d = −0.77), and thalamus to CSF-LVV (p < 0.001, d = −0.50) was higher in MS compared to controls. The mean SIR of thalamus to pons in patients with MS was lower in patients with MS compared to controls (p < 0.001, d = 0.31).
Table 2 shows MRI characteristics of patients with MS, split by the median number of gadodiamide administrations. The mean SIR of DN to pons (p = 0.005, figure 2), and DN, GP, and thalamus to CSF-LVV in 109 patients with MS who received ≥8 gadodiamide doses vs in 94 patients who received <8 gadodiamide doses was higher.
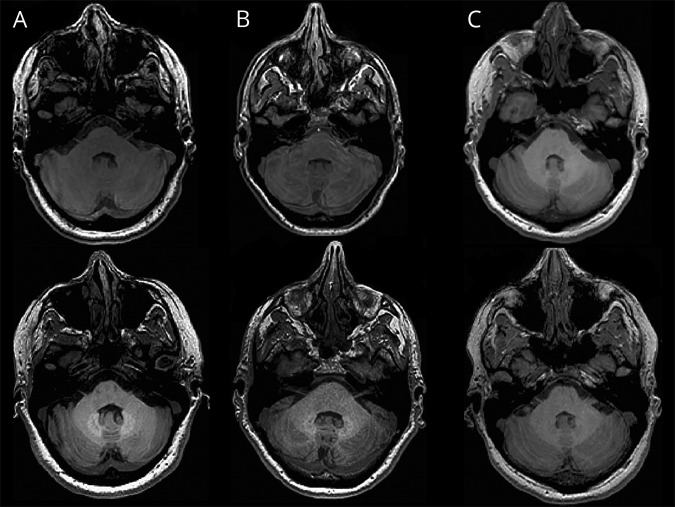
Figure 2. Dentate nucleus (DN) hyperintensity on 3D-T1-weighted imaging in patients with multiple sclerosis (MS).
DN hyperintensity on 3D-T1-weighted imaging in patients with MS with 15 (A), 8 (B), and 4 (C) gadodiamide administered doses at baseline (top row) and at the follow-up (bottom row). (A) A 38-year-old man with relapsing-remitting MS (RRMS) whose signal intensity ratio (SIR) of DN to pons was 1.05 at baseline and 1.15 at the follow-up and shows bilateral hyperintensities in the DN region. (B) A 39-year-old woman with RRMS whose SIR was 1.01 at baseline and 1.07 at the follow-up and shows bilateral hyperintensities in the DN region. (C) A 41-year-old woman with RRMS whose SIR was 1.00 at baseline and is 1.01 at the follow-up and but does not show hyperintensities in the DN region.
MRI characteristics of patients with MS according to sex are available in table e-1 (doi.org/10.5061/dryad.04df893). The mean SIR of DN to pons (p = 0.007) and of DN to CSF-LVV (p = 0.027) was higher in male participants. No sex effect was observed for gadolinium deposition in GP or thalamus.
Because it has been reported that the development of DN T1 hyperintensity becomes visible on MRI examination only after 5 GBCA administrations,9,11,35 we compared patients with MS who received ≥5 (n = 143) or <5 (n = 60) gadodiamide doses to controls. We found higher mean SIR of DN to pons in patients with MS with ≥5 gadodiamide doses compared to controls (p < 0.001) and to patients with MS who received <5 gadodiamide doses (p = 0.002).
Conventional and nonconventional MRI characteristics in patients with MS and controls are available in table e-2 (doi.org/10.5061/dryad.04df893). As expected, due to longer disease duration and time of follow-up, patients with MS who received ≥8 gadodiamide doses showed higher T2-LV (p = 0.046) and lower normalized GM volume (p = 0.047), compared to patients who received <8 gadodiamide doses.
MRI differences in patients with MS over the follow-up
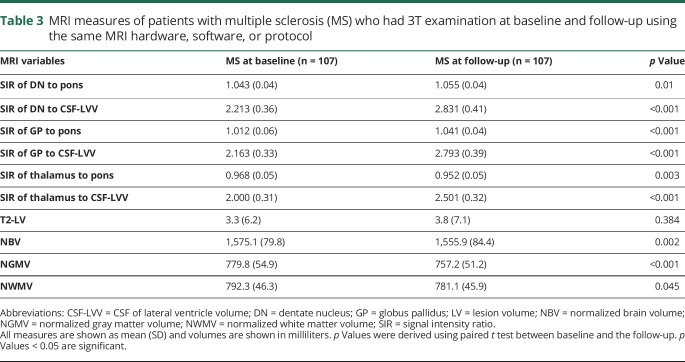
Table 3 shows characteristics of the MRI outcomes between baseline and the follow-up. The mean SIR of DN to pons (p = 0.01), DN to CSF-LVV (p < 0.001), GP to pons (p < 0.001), GP to CSF-LVV (p < 0.001), and thalamus to CSF-LVV (p < 0.001) increased over the follow-up, while the thalamus to pons decreased (p = 0.003). None of the patients with MS had DN T1 hyperintensity at baseline, while 53 (49.5%) showed it at the follow-up (p < 0.001, table 1). As expected, patients with MS had significantly lower whole brain (p = 0.002), GM (p < 0.001), and WM (p = 0.045) volumes at the follow-up (table 3).
Table 3.
MRI measures of patients with multiple sclerosis (MS) who had 3T examination at baseline and follow-up using the same MRI hardware, software, or protocol
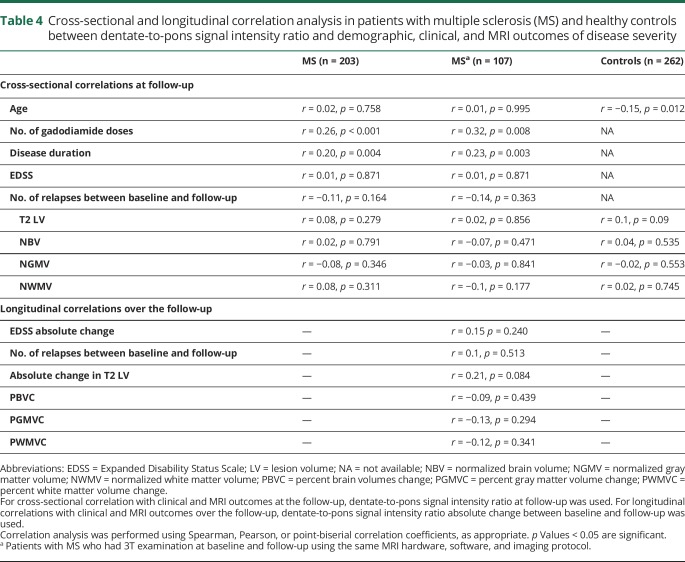
Cross-sectional and longitudinal correlations between SIR and demographic, clinical, and MRI outcomes of disease severity
Table 4 shows MRI cross-sectional (at the follow-up) and longitudinal (over the follow-up) correlations between SIR of DN to pons and demographic, clinical, and MRI outcomes of disease severity. In controls, there was an inverse correlation between age and SIR of DN to pons (r = −0.15, p = 0.012), which was not observed in patients with MS.
Table 4.
Cross-sectional and longitudinal correlation analysis in patients with multiple sclerosis (MS) and healthy controls between dentate-to-pons signal intensity ratio and demographic, clinical, and MRI outcomes of disease severity
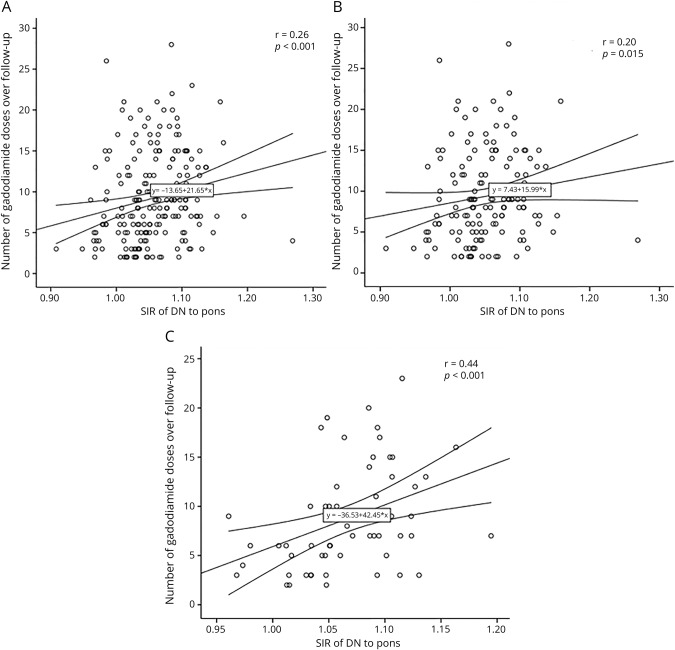
In Spearman correlation analysis, there was a correlation between higher number of gadodiamide administrations and higher SIR of DN to pons in total MS population (r = 0.26, p < 0.001, figure 3A), as well as in women (r = 0.20, p = 0.015, figure 3B) and men (r = 0.44, p < 0.001, figure 3C). In partial correlation analysis, adjusted for age and sex, we found similar correlation between higher number of gadodiamide administrations and higher SIR of DN to pons (r = 0.21, p = 0.004) in the total MS population. These results were confirmed in the longitudinal cohort of patients with MS (table 4). As expected, there was an association between longer disease duration and higher SIR of DN to pons (r = 0.2, p = 0.004), but not to CSF-LVV (r = −0.03, p = 0.686) at the follow-up.
Figure 3. Spearman correlation analysis between number of gadodiamide doses administered between baseline and follow-up and signal intensity ratio (SIR) of dentate nucleus (DN) to pons at follow-up.
Total multiple sclerosis population (A), women (B), men (C). The middle line represents the regression line; outside lines are 95% confidence intervals. Spearman correlation coefficients and p values are displayed.
Over the follow-up, we did not find any significant associations between the absolute change in mean SIR of DN to pons and clinical and MRI outcomes of disease progression (table 4) in the total MS population or when patients with MS who received ≥5 or <5 gadodiamide doses were considered.9,11,35
In addition, annualized number of gadolinium scans received over the lifetime was not associated with the mean SIR in the examined structures.
Discussion
Our study sheds further light on the dynamics of gadolinium deposition in the early stage of MS, after repeated usage of gadodiamide. We showed that SIR of DN and GP to pons and of all 3 structures to CSF-LVV in patients with early MS is associated with the lifetime cumulative gadodiamide administration from disease onset. The findings from our quantitative analysis were in agreement with those from previous qualitative DN T1 hyperintensity analyses, although SIR may be more sensitive to depict subtle SI changes than the qualitative assessment.
Free gadolinium is highly toxic, and for clinical use, it has to be complexed with chelating molecules.36 Recently, a general consensus has emerged that linear, but to a lesser extent macrocyclic GBCAs are associated with the development of gadolinium deposition in the DN on unenhanced T1-WI.6,7,14,15,17–19,21,37 Because macrocyclic GBCAs are more thermodynamically stable than linear, larger effects of gadolinium deposition were found with the latter.3,6,7,14–20,22,35,37,38 The discrepancies in the reported findings between different gadolinium deposition rates in MS studies can be attributed to differences in patient population, disease duration, type of GBCAs used, length of follow-up, use of scanners with inhomogeneous protocols, and strength fields, not accounting for lifetime cumulative dose of GBCA administered, and use of different methodologic approaches for assessment of gadolinium deposition, among others.14–20,22–26 Moreover, the interpretation of putative gadolinium deposition in the DN of patients with MS on MRI is complicated by the fact that a previous study reported that DN hyperintensity on unenhanced T1-WI is more frequently related to disease progression.39
The major strength of the present study is the selection of a homogenous patient population with 0.9 years of disease duration from onset at baseline that has been followed in our center from the time of diagnosis, and has been assessed using the same GBCA with identical dose at all time points. Moreover, all baseline (in more than half of the MS cohort) and MRF (all the MS cohort) MRI examinations, on which SIR was evaluated, were performed on the same 3T scanner that did not undergo any hardware or software changes during the 10-year period of the study, and standardized MRI protocol was applied in all participants. In addition, we used a large cohort of age- and sex-matched controls as a comparator group, who obtained MRI examination in the same time period and were scanned on the same 3T scanner.
Patients with MS are potentially more susceptible to accumulate gadolinium in their brains, due to more frequent administration and blood-brain barrier disruption, compared to other neurologic disorders.12,15,16 In a previous study, 217 patients with CIS who had never received GBCA of any type before the study and underwent at least one follow-up examination after baseline using macrocyclic agent gadobutrol were examined.25 Using voxel-based whole brain analysis, no increased SI in the GM and WM was found in relation to gadobutrol administration; however, no qualitative or quantitative evaluation or DN ROI-based approach was used in that study. In the present study, the length of the follow-up was 4.5 years, and more than 70% of the patients with MS received ≥5 doses of gadodiamide, a minimum cutoff for visualization of T1 hyperintensity in the DN.9,11,35 We showed similar SIR of DN values in patients with MS with ≥5 (minimum cutoff) or ≥8 (median cutoff) doses of gadodiamide, and both cutoffs differentiated MS from controls, whereas patients with MS with <5 doses of gadodiamide behaved similarly to controls. Still, 18 (8.9%) patients with MS who received <5 doses of gadodiamide had DN T1 hyperintensity, whereas none of controls did. Because we found an inverse correlation between age and SIR of DN to pons in controls (possibly related to less iron content in the DN in younger controls),20,40 and positive association with male sex in patients with MS, we adjusted all analyses for age and sex in patients with MS; however, we showed similar relationship between SIR and lifetime cumulative administration of gadodiamide. To our knowledge, this is the first study to detect greater gadolinium deposition of gadodiamide in DN in male patients with MS compared to female patients, and while this finding is of interest, it should be interpreted with caution, as no preclinical or clinical studies have reported a similar observation. One possible explanation for this unexpected finding could be related simply to the higher dose of gadodiamide administered in male patients with MS, related to greater body weight.
Examining the longitudinal cohort (n = 107) of patients with MS who had baseline and follow-up standardized MRI assessments, we detected significant increase in SIR of DN and GP to pons, and SIR of DN, GP, and thalamus to CSF-LVV, which were significantly associated with the lifetime cumulative gadodiamide administration from disease onset. Although we could not assess SIR at all serial MRI timepoints of the study when gadodiamide was administered, we calculated the annualized number of gadolinium scans received over the lifetime and the follow-up period. This metric reflects whether a participant had many gadolinium scans/injections close in time or more spread out in time. We did not observe any significant correlation between that measure and SIR of DN, GP, or thalamus, normalized to pons or CSF-LVV.
To our knowledge, previous studies have not investigated whether the region used for normalization plays an important effect for detecting gadolinium deposition in MS. To that end, we utilized the T1-weighted CSF signal from the lateral ventricles as additional region for the normalization purposes. The SIR remained greater in patients with MS compared to controls for all 3 examined structures. We also calculated effect sizes using both methods and showed greater effect size when using the CSF-LVV signal compared to that of the pons. Therefore, these results suggest that normalizing by the pons-derived signal may actually underestimate the full extent of gadolinium deposition in patients with MS. In addition, because the greater atrophy of the examined structures could create an analysis bias, we used the ROI volumes of these structures as covariate in analyses.
The findings from examining gadolinium deposition in the GP and thalamus support findings of the DN in this study. When using the pons for normalization, the SIR was lower in the thalamus of patients with MS compared to controls, while being greater in the GP. The lower thalamic ratio in patients with MS may potentially reflect decreased iron content compared to controls in this structure, as has been recently reported using quantitative susceptibility mapping.41,42 However, when using the CSF-LVV for normalization, the SIR was greater in patients with MS for the thalamus and GP structures. When considering the thalamus, the exact reason for the apparently discrepant results (i.e., lower for MS when normalizing to pons but greater when normalizing to CSF of lateral ventricles) is not exactly clear at this time.
In line with our results, another recent study compared the effect of linear GBCA gadopentetate dimeglumine and macrocyclic GBCA gadobutrol in 97 patients with MS, and reported that SIR of DN to pons increased between the first and the last scan with gadopentetate dimeglumine administration, but not with gadobutrol.16 This is in line with several studies that showed increased gadolinium deposition in patients with MS receiving linear or multiple types of GBCAs.14,15,17,19,20,22 Yet another study, using macrocyclic GBCA, showed increased SIR in the DN of patients with RRMS,18 while a number of other investigations did not.23–26
At this time, the clinical significance of the retained gadolinium in the brain, if any, remains unclear.12 However, in the last couple of years there has been a growing concern among patients with MS and families (“gadolinium-phobia”) about the possible long-term consequences of gadolinium deposits, especially in terms of disease progression. A recent retrospective clinical study showed no de novo clinical cerebellar syndrome following repeated administrations of gadoterate,43 while a self-reported survey among individuals with normal renal function, who received repeated GBCA administrations, showed that most of the participants reported self-described toxicity to GBCA administration, with bone, joint pain, and skin changes as the most common complaints.44 Given that a previous study showed that presence of DN T1 hyperintensity is associated with more severe neurodegeneration in patients with MS,39 the question is whether disease progression is a confounding variable for, or potentiates, gadolinium deposition. Our study is one of the first studies to investigate the longitudinal association between well-established clinical and MRI outcomes of disease severity and gadolinium deposition. We did not find any significant association between accumulation of lesion burden, development of brain atrophy, occurrence of relapses, or increase in disability and gadolinium deposition over the 4.5 years of follow-up, in line with recent study showing that there was no difference in apparent diffusion coefficient values in the T1-hyperintense DN of patients exposed to macrocyclic or linear GBCAs, indicating similar tissue integrity between the two.22 However, we observed that longer disease duration was associated with higher SIR of DN to pons, and patients with MS who received ≥8 gadodiamide doses showed higher lesion burden and more advanced GM atrophy compared to patients who received <8 gadodiamide doses. Although the differences between patients with high and low doses of gadodiamide administration (13.2 vs 4.6) were also linked to different timeline of disease duration, the time of follow-up in our study was relatively short, so we cannot exclude that gadolinium deposition does not contribute to acceleration of clinical or radiologic signs of more aggressive disease in the early phase of MS. Therefore, longer prospective studies should investigate this important issue in more detail. Another recent longitudinal retrospective study found that increased SI in the DN and GP was associated with lower verbal fluency in 23 patients with MS, even after adjustment for confounding variables,15 while other studies found no associations with clinical outcomes.20,21
The Food and Drug Administration recommends that health care professionals should limit GBCA use to circumstances in which additional information provided by the contrast agent is necessary, and assess the necessity of repeated MRIs with GBCAs.10 At this time, more specific recommendations in the MS community with regards to use of GBCA in clinical and research environment are emerging.13 The findings from the present study should be incorporated into a risk-versus-benefit analysis when determining the need for GBCA administration, in particular gadodiamide and other linear agents, in individual patients with MS.
In the past, GBCAs were believed to be exceedingly safe,9 and have been used without particular restrictions in patients with MS, except the concern for nephrogenic systemic fibrosis in patients with kidney dysfunction.1,2,9,12 We did not report renal measures of kidney function, although patients with MS with an estimated glomerular filtration rate of <60 mL/min were excluded.
Our study is not without limitations. While this was a retrospective study in its design, the collection of demographic, clinical, and MRI data was performed prospectively, as all participants underwent MRI scanning in our MS specialized center, and were part of the long-term follow-up institutional review board–approved study, which was initiated back in 2003. In addition, exploring association of GBCAs with other clinical outcomes and quality of life over the long term is recommended in future studies. Moreover, we did not investigate the relationship between accumulation of gadodiamide and subtle alterations in cognitive, motor, and cerebellar domains in patients with MS. Finally, an important limitation of this study is that we were unable to include a matched group of patients with MS who received a nonlinear gadolinium compound, or no gadolinium at all. This would require a prospective study design and it would be very difficult to perform without a properly organized clinical trial.
We showed that accumulation of gadolinium deposition in DN, GP, and thalamus in patients with early MS is associated with lifetime cumulative gadodiamide administration, without apparent clinical or radiologic association with more aggressive disease over a period of 4.5 years from disease onset.
Glossary
- ANT
advanced normalization tool
- CI
confidence interval
- CIS
clinically isolated syndrome
- CSF-LVV
CSF of lateral ventricle volume
- DN
dentate nucleus
- FLAIR
fluid-attenuated inversion recovery
- FOV
field of view
- GBCA
gadolinium-based contrast agents
- GM
gray matter
- GP
globus pallidus
- ICC
intraclass correlation coefficient
- LV
lesion volume
- MRF
most recent follow-up
- MS
multiple sclerosis
- ROI
region of interest
- RRMS
relapsing-remitting multiple sclerosis
- CSI
signal intensity
- SIR
signal intensity ratio
- TE
echo time
- TI
inversion time
- TR
repetition time
- WI
weighted images
- WM
white matter
Footnotes
Editorial, page 239
Podcast: NPub.org/uihdww
Author contributions
R. Zivadinov: study concept and design, analysis and interpretation, critical revision of the manuscript for important intellectual content, study supervision. N. Bergsland: study concept and design, analysis and interpretation, critical revision of the manuscript for important intellectual content, study supervision. J. Hagemeier: study concept and design, analysis and interpretation, critical revision of the manuscript for important intellectual content, study supervision. D. Ramasamy: analysis and interpretation, critical revision of the manuscript for important intellectual content. M.G. Dwyer: analysis and interpretation, critical revision of the manuscript for important intellectual content. F. Schweser: analysis and interpretation, critical revision of the manuscript for important intellectual content. C. Kolb: analysis and interpretation, critical revision of the manuscript for important intellectual content. B. Weinstock-Guttman: analysis and interpretation, critical revision of the manuscript for important intellectual content. D. Hojnacki: study concept and design, analysis and interpretation, critical revision of the manuscript for important intellectual content, study supervision.
Study funding
Research reported in this publication was funded by the National Center for Advancing Translational Sciences of the NIH under award number UL1TR001412. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Disclosure
R. Zivadinov received personal compensation from EMD Serono, Genzyme-Sanofi, Celgene, and Novartis for speaking and consultant fees; and financial support for research activities from Genzyme-Sanofi, Novartis, Celgene, Mapi Pharma, and Protembis. N. Bergsland, J. Hagemeier, and D. Ramasamy report no disclosures relevant to the manuscript. M. Dwyer received personal compensation from EMD Serono and Claret Medical, Inc.; and financial support for research activities from Novartis and Celgene. F. Schweser received personal compensation from Toshiba Canada Medical Systems Limited and Goodwin Procter LLP for speaking and consultant fees; and financial support for research activities from SynchroPET Inc. and travel sponsorship from GE Healthcare and SynchroPET Inc. C. Kolb has received speaker honoraria and consultant fees from EMD Serono, Teva Pharmaceuticals, Acorda, Novartis, Genzyme, and Biogen-Idec. B. Weinstock-Guttman received honoraria as a speaker and as a consultant for Biogen Idec, Teva Pharmaceuticals, EMD Serono, Genzyme & Sanofi, Novartis, and Acorda; and research funds from Biogen Idec, Teva Pharmaceuticals, EMD Serono, Genzyme & Sanofi, Novartis, and Acorda. D. Hojnacki has received speaker honoraria and consultant fees from Biogen Idec, Teva Pharmaceutical Industries Ltd., EMD Serono, Pfizer Inc., and Novartis. Go to Neurology.org/N for full disclosures.
References
- 1.Rovira À, Wattjes MP, Tintoré M, et al. Evidence-based guidelines: MAGNIMS consensus guidelines on the use of MRI in multiple sclerosis-clinical implementation in the diagnostic process. Nat Rev Neurol 2015;11:471–482. [DOI] [PubMed] [Google Scholar]
- 2.Traboulsee A, Simon JH, Stone L, et al. Revised recommendations of the Consortium of MS Centers Task Force for a standardized MRI protocol and clinical guidelines for the diagnosis and follow-up of multiple sclerosis. AJNR Am J Neuroradiol 2016;37:394–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kanda T, Fukusato T, Matsuda M, et al. Gadolinium-based contrast agent accumulates in the brain even in subjects without severe renal dysfunction: evaluation of autopsy brain specimens with inductively coupled plasma mass spectroscopy. Radiology 2015;276:228–232. [DOI] [PubMed] [Google Scholar]
- 4.Kanda T, Ishii K, Kawaguchi H, Kitajima K, Takenaka D. High signal intensity in the dentate nucleus and globus pallidus on unenhanced T1-weighted MR images: relationship with increasing cumulative dose of a gadolinium-based contrast material. Radiology 2014;270:834–841. [DOI] [PubMed] [Google Scholar]
- 5.McDonald RJ, McDonald JS, Kallmes DF, et al. Intracranial gadolinium deposition after contrast-enhanced MR imaging. Radiology 2015;275:772–782. [DOI] [PubMed] [Google Scholar]
- 6.Radbruch A, Weberling LD, Kieslich PJ, et al. High-signal intensity in the dentate nucleus and globus pallidus on unenhanced T1-weighted images: evaluation of the macrocyclic gadolinium-based contrast agent gadobutrol. Invest Radiol 2015;50:805–810. [DOI] [PubMed] [Google Scholar]
- 7.Ramalho J, Castillo M, AlObaidy M, et al. High signal intensity in globus pallidus and dentate nucleus on unenhanced T1-weighted MR images: evaluation of two linear gadolinium-based contrast agents. Radiology 2015;276:836–844. [DOI] [PubMed] [Google Scholar]
- 8.Lord ML, Chettle DR, Gräfe JL, Noseworthy MD, McNeill FE. Observed deposition of gadolinium in bone using a new noninvasive in vivo biomedical device: results of a small pilot feasibility study. Radiology 2018;287:96–103. [DOI] [PubMed] [Google Scholar]
- 9.Quattrocchi CC, van der Molen AJ. Gadolinium retention in the body and brain: is it time for an international joint research effort? Radiology 2017;282:12–16. [DOI] [PubMed] [Google Scholar]
- 10.FDA Drug Safety Communication. FDA identifies no harmful effects to date with brain retention of gadolinium-based contrast agents for MRIs; review to continue. Available at: www.fda.gov/Drugs/DrugSafety/ucm589213.htm. Accessed December 19, 2017.
- 11.Kanda T, Oba H, Toyoda K, Kitajima K, Furui S. Brain gadolinium deposition after administration of gadolinium-based contrast agents. Jpn J Radiol 2016;34:3–9. [DOI] [PubMed] [Google Scholar]
- 12.Gulani V, Calamante F, Shellock FG, Kanal E, Reeder SB; International Society for Magnetic Resonance in Medicine. Gadolinium deposition in the brain: summary of evidence and recommendations. Lancet Neurol 2017;16:564–570. [DOI] [PubMed] [Google Scholar]
- 13.Traboulsee A, Li D. Addressing concerns regarding the use of gadolinium in a standardized MRI protocol for the diagnosis and follow-up of multiple sclerosis. AJNR Am J Neuroradiol 2016;37:E82–E83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Errante Y, Cirimele V, Mallio CA, Di Lazzaro V, Zobel BB, Quattrocchi CC. Progressive increase of T1 signal intensity of the dentate nucleus on unenhanced magnetic resonance images is associated with cumulative doses of intravenously administered gadodiamide in patients with normal renal function, suggesting dechelation. Invest Radiol 2014;49:685–690. [DOI] [PubMed] [Google Scholar]
- 15.Forslin Y, Shams S, Hashim F, et al. Retention of gadolinium-based contrast agents in multiple sclerosis: retrospective analysis of an 18-year longitudinal study. AJNR Am J Neuroradiol 2017;38:1311–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schlemm L, Chien C, Bellmann-Strobl J, et al. Gadopentetate but not gadobutrol accumulates in the dentate nucleus of multiple sclerosis patients. Mult Scler 2017;23:963–972. [DOI] [PubMed] [Google Scholar]
- 17.Splendiani A, Perri M, Marsecano C, et al. Effects of serial macrocyclic-based contrast materials gadoterate meglumine and gadobutrol administrations on gadolinium-related dentate nuclei signal increases in unenhanced T1-weighted brain: a retrospective study in 158 multiple sclerosis (MS) patients. Radiol Med 2018;123:125–134. [DOI] [PubMed] [Google Scholar]
- 18.Stojanov DA, Aracki-Trenkic A, Vojinovic S, Benedeto-Stojanov D, Ljubisavljevic S. Increasing signal intensity within the dentate nucleus and globus pallidus on unenhanced T1W magnetic resonance images in patients with relapsing-remitting multiple sclerosis: correlation with cumulative dose of a macrocyclic gadolinium-based contrast agent, gadobutrol. Eur Radiol 2016;26:807–815. [DOI] [PubMed] [Google Scholar]
- 19.Tanaka M, Nakahara K, Kinoshita M. Increased signal intensity in the dentate nucleus of patients with multiple sclerosis in comparison with neuromyelitis optica spectrum disorder after multiple doses of gadolinium contrast. Eur Neurol 2016;75:195–198. [DOI] [PubMed] [Google Scholar]
- 20.Tedeschi E, Palma G, Canna A, et al. In vivo dentate nucleus MRI relaxometry correlates with previous administration of Gadolinium-based contrast agents. Eur Radiol 2016;26:4577–4584. [DOI] [PubMed] [Google Scholar]
- 21.Cocozza S, Pontillo G, Lanzillo R, et al. MRI features suggestive of gadolinium retention do not correlate with Expanded Disability Status Scale worsening in multiple sclerosis. Neuroradiology 2019;61:155–162. [DOI] [PubMed] [Google Scholar]
- 22.Eisele P, Szabo K, Ebert A, et al. Diffusion-weighted imaging of the dentate nucleus after repeated application of gadolinium-based contrast agents in multiple sclerosis. Magn Reson Imaging 2019;58:1–5. [DOI] [PubMed] [Google Scholar]
- 23.Eisele P, Alonso A, Szabo K, et al. Lack of increased signal intensity in the dentate nucleus after repeated administration of a macrocyclic contrast agent in multiple sclerosis: an observational study. Medicine 2016;95:e4624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eisele P, Konstandin S, Szabo K, et al. Sodium MRI of T1 high signal intensity in the dentate nucleus due to gadolinium deposition in multiple sclerosis. J Neuroimaging 2017;27:372–375. [DOI] [PubMed] [Google Scholar]
- 25.Langner S, Kromrey ML, Kuehn JP, Grothe M, Domin M. Repeated intravenous administration of gadobutrol does not lead to increased signal intensity on unenhanced T1-weighted images-a voxel-based whole brain analysis. Eur Radiol 2017;27:3687–3693. [DOI] [PubMed] [Google Scholar]
- 26.Tedeschi E, Cocozza S, Borrelli P, Ugga L, Morra VB, Palma G. Longitudinal assessment of dentate nuclei relaxometry during massive gadobutrol exposure. Magn Reson Med Sci 2018;17:100–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Polman CH, Reingold SC, Banwell B, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol 2011;69:292–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Diedrichsen J. A spatially unbiased atlas template of the human cerebellum. Neuroimage 2006;33:127–138. [DOI] [PubMed] [Google Scholar]
- 29.Tustison NJ, Avants BB, Cook PA, et al. N4ITK: improved N3 bias correction. IEEE Trans Med Imaging 2010;29:1310–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Avants BB, Epstein CL, Grossman M, Gee JC. Symmetric diffeomorphic image registration with cross-correlation: evaluating automated labeling of elderly and neurodegenerative brain. Med Image Anal 2008;12:26–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Klein A, Andersson J, Ardekani BA, et al. Evaluation of 14 nonlinear deformation algorithms applied to human brain MRI registration. Neuroimage 2009;46:786–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zivadinov R, Heininen-Brown M, Schirda CV, et al. Abnormal subcortical deep-gray matter susceptibility-weighted imaging filtered phase measurements in patients with multiple sclerosis: a case-control study. Neuroimage 2012;59:331–339. [DOI] [PubMed] [Google Scholar]
- 33.Smith SM, Zhang Y, Jenkinson M, et al. Accurate, robust, and automated longitudinal and cross-sectional brain change analysis. Neuroimage 2002;17:479–489. [DOI] [PubMed] [Google Scholar]
- 34.Dwyer MG, Bergsland N, Zivadinov R. Improved longitudinal gray and white matter atrophy assessment via application of a 4-dimensional hidden Markov random field model. Neuroimage 2014;90:207–217. [DOI] [PubMed] [Google Scholar]
- 35.Zhang Y, Cao Y, Shih GL, Hecht EM, Prince MR. Extent of signal hyperintensity on unenhanced T1-weighted brain MR images after more than 35 administrations of linear gadolinium-based contrast agents. Radiology 2017;282:516–525. [DOI] [PubMed] [Google Scholar]
- 36.Port M, Idée JM, Medina C, Robic C, Sabatou M, Corot C. Efficiency, thermodynamic and kinetic stability of marketed gadolinium chelates and their possible clinical consequences: a critical review. Biometals 2008;21:469–490. [DOI] [PubMed] [Google Scholar]
- 37.Kanda T, Osawa M, Oba H, et al. High signal intensity in dentate nucleus on unenhanced T1-weighted MR images: association with linear versus macrocyclic gadolinium chelate administration. Radiology 2015;275:803–809. [DOI] [PubMed] [Google Scholar]
- 38.Boyken J, Frenzel T, Lohrke J, Jost G, Pietsch H. Gadolinium accumulation in the deep cerebellar nuclei and globus pallidus after exposure to linear but not macrocyclic gadolinium-based contrast agents in a retrospective pig study with high similarity to clinical conditions. Invest Radiol 2018;53:278–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roccatagliata L, Vuolo L, Bonzano L, Pichiecchio A, Mancardi GL. Multiple sclerosis: hyperintense dentate nucleus on unenhanced T1-weighted MR images is associated with the secondary progressive subtype. Radiology 2009;251:503–510. [DOI] [PubMed] [Google Scholar]
- 40.Hinoda T, Fushimi Y, Okada T, et al. Quantitative assessment of gadolinium deposition in dentate nucleus using quantitative susceptibility mapping. J Magn Reson Imaging 2017;45:1352–1358. [DOI] [PubMed] [Google Scholar]
- 41.Schweser F, Raffaini Duarte Martins AL, Hagemeier J, et al. Mapping of thalamic magnetic susceptibility in multiple sclerosis indicates decreasing iron with disease duration: a proposed mechanistic relationship between inflammation and oligodendrocyte vitality. Neuroimage 2018;167:438–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zivadinov R, Tavazzi E, Bergsland N, et al. Brain iron at quantitative MRI is associated with disability in multiple sclerosis. Radiology 2018;289:487–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Perrotta G, Metens T, Absil J, Lemort M, Manto M. Absence of clinical cerebellar syndrome after serial injections of more than 20 doses of gadoterate, a macrocyclic GBCA: a monocenter retrospective study. J Neurol 2017;264:2277–2283. [DOI] [PubMed] [Google Scholar]
- 44.Burke LM, Ramalho M, AlObaidy M, Chang E, Jay M, Semelka RC. Self-reported gadolinium toxicity: a survey of patients with chronic symptoms. Magn Reson Imaging 2016;34:1078–1080. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on reasonable request to the corresponding author (R.Z.). The data are not publicly available due to containing information that could compromise the privacy of research participants.