Abstract
Importance:
Although quality improvement (QI) interventions can reduce central-line-associated and catheter-related bloodstream infections (CLABSI and CRBSI), their economic value is uncertain.
Objective:
To systematically review economic evaluations of QI interventions designed to prevent CLABSI/CRBSI in acute-care hospitals.
Evidence Review:
Ovid MEDLINE, Econlit, Centre for Reviews & Dissemination, New York Academy of Medicine’s Grey Literature Report, Worldcat (January 2004 to July 2016), IDWeek conference abstracts, and prior systematic reviews.
We included English-language studies of any design that evaluated organizational or structural changes to prevent CLABSI/CRBSI, and reported program and infection-related costs.
Dual reviewers assessed study design, effectiveness, costs, and study quality. For each eligible study, we performed a cost-consequences analysis from the hospital perspective, estimating the incidence rate ratio [IRR] and incremental net savings. Unadjusted weighted regression analyses tested predictors of these measures, weighted by catheter-days per study per year.
Findings:
Of 505 titles, 15 unique studies were eligible, together representing data from 113 hospitals. Thirteen studies compared AHRQ-recommended practices with usual care, including 7 testing insertion checklists. Eleven studies were based on uncontrolled-before-after designs, one on a randomized controlled trial, one on a time-series analysis, and two on modeled estimates. Overall, the weighted mean IRR was 0.43 (95% CI 0.35–0.51) and incremental net savings was $1.85 million (95% CI $1.30 to $2.40 million) per hospital over three years (2015 U.S. dollars). Each $100,000-increase in program cost was associated with $310,000 greater savings (p<0.001). Infections and net costs declined when hospitals already used checklists or had baseline infection rates of 1.7–3.7 per 1,000 catheter-days. Study quality was not associated with effectiveness or costs.
Conclusions and Relevance:
Interventions related to central catheters were, on average, associated with 57% fewer blood stream infections and substantial savings to hospitals. Larger initial investments may be associated with greater savings. Although checklists are now widely used and infections have started to decline, additional improvements and savings can occur at hospitals that have not yet attained very low infection rates.
Prospero Registration Number:
CRD42015014950
Keywords: quality improvement, cost-effectiveness, return on investment, budget impact analysis, business case analysis, cost-benefit analysis, economic evaluation, Healthcare associated infection, Catheter-associated blood-stream infection
INTRODUCTION
About 60,400 primary bloodstream infections related to central catheters occur in U.S. hospitals each year, costing $1.85 billion.1–3 Accordingly, hospitals are implementing various infection-prevention practices, such as insertion checklists or bundles.4 Yet little is known about the economic value of doing so, meaning associated changes in clinical outcomes and costs.5,6 The program costs associated with implementing such interventions have seldom been evaluated systematically, and it is unclear whether hospitals tend to incur net savings or losses.
We sought to systematically review economic evaluations of quality improvement (QI) interventions for the prevention of bloodstream infection related to the use of central catheters in the hospital setting, considering both program costs and changes in infection-related costs. To identify such studies, we searched peer-reviewed and non-peer-reviewed literature. We then examined the nature of interventions that have been evaluated, their clinical effectiveness, the associated costs, and the quality of the economic evaluations.
METHODS
This review is reported in accordance with Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines,7 and a protocol is registered on Prospero (CRD42015014950).8 An eight-member technical expert panel provided input at key stages.
Catheter-related bloodstream infection (CRBSI) is a diagnosis based on specific laboratory testing that identifies a catheter as the source of a bloodstream infection. In contrast, central-line-associated bloodstream infection (CLABSI) is a less specific surveillance definition that reflects a bloodstream infection in the presence of a recent central line without another source of infection.9,10 We included both.
Data Sources and Searches
A reference librarian developed search terms for CLABSI/CRBSI, and expanded on terms related to economic evaluation that have demonstrated sensitivity 11 (Appendix). Databases of peer-reviewed literature included Ovid MEDLINE, Econlit, and the Centre for Reviews & Dissemination Economic Evaluations. To identify grey literature, we searched New York Academy of Medicine’s Grey Literature Report and Worldcat. We searched IDweek conference presentations for unpublished analyses.12 We searched for English-language publications (January 2004 to July 2016), and hand searched citations from previous systematic reviews.4,5,13–17 We excluded earlier studies because infection rates and clinical practices have changed over time.
Study Selection
Eligible studies represented original investigations, addressed QI interventions designed to prevent CLABSI/CRBSI in acute care hospitals, reported or estimated clinical effectiveness, measured or modeled costs of the QI intervention, compared alternatives (e.g., QI intervention vs. usual care), and reported both program and infection-related costs. We excluded studies from low- to middle-income countries,18 but included all ages, hospital settings, clinical study designs, cost evaluation approaches, analytical perspectives, and time horizons. A QI intervention was “an effort to change/improve the clinical structure, process, or outcomes of care by means of an organizational or structural change.”19 Studies were ineligible if they tested novel materials or equipment but omitted costs associated with organizational efforts to support implementation.
Two trained reviewers independently examined titles, abstracts, and full-text publications to determine eligibility; discrepancies were resolved by consensus, or, when necessary, through discussion with the research team.
Data Extraction and Quality Assessment
Pairs of investigators with training in quality of care and economic evaluation extracted data; discrepancies were resolved as described above.
QI Intervention, Context, and Clinical Evaluation
For each study, reviewers extracted the nature of the QI intervention, setting, clinical study design and reporting, funding source, and findings. We identified practices strongly recommended in a recent AHRQ evidence review, including components of insertion checklists.4,20 Contextual variables included academic status (major, minor, non-teaching) and location (urban, suburban/small city, rural). Clinical study designs included randomized controlled trial, non-randomized controlled trial, controlled before-after analysis, uncontrolled before-after analysis, interrupted time series and repeated measures studies, and modeling exercises.21 Reviewers extracted selected items from the Minimum Quality Criteria Set, a tool for critically appraising the reporting of QI interventions.22 Funding sources included government, non-profit, commercial, and none. Finally, reviewers extracted infection rates in intervention and comparison groups.
Economic Evaluation
Reviewers extracted the evaluation approach (cost analyses such as cost-consequences or business-case analyses vs. cost-effectiveness and related analyses); perspective (hospital, health system, payer, society); time horizon; discount rate; year and currency of cost data; and incremental program, infection-related, and net costs.
To identify relevant costs within each paper, we used the Quality-Cost Framework.23 Together, structure and process-related costs comprise an intervention’s program costs. Structure-related costs are fixed costs associated with start-up and maintenance, such as training providers, monitoring adherence, and making capital purchases (e.g., ultrasound machines). Process-related costs are variable, recurring costs associated with the care of individual patients, such as provider time spent on catheter-related care. Outcome-related costs are healthcare expenditures related to infections.
Study Quality
Reviewers assessed whether economic evaluations met basic standards using a modified version of the Quality of Health Economics Studies Checklist (mQHES)..24,25 Questions address whether the study objective is clear, the perspective is stated, cost and effectiveness estimates are from the best sources, and effects of uncertainty and variability are described. We divided each question into subparts for easier scoring and added two questions related to competing alternatives and overall credibility. To calculate total mQHES scores (scale 0–115), we determined the percentage of “yes” responses to subparts of each question, weighted each question’s raw score as per QHES scoring guidelines24 (using estimated weights for new questions), and summed weighted values.
Data Standardization
To facilitate comparisons, we performed a cost-consequences analysis from the hospital perspective for each study, where clinical and economic outcomes included the incidence rate ratio (IRR) and incremental net cost per hospital. If authors did not report an IRR, we calculated it by dividing the infection rate in the intervention group by the rate in the comparison group.
For each study, we standardized program and infection-related costs by converting to 2015 U.S. dollars and discounting recurring costs over a three-year time-horizon (discount rate 3%).26 Infection-related costs were based on numbers of infections averted times the cost per infection. We based the cost per infection on a recent meta-analysis ($51,770 in 2015 U.S. dollars),3 except for 2 studies in which authors reported site-specific estimates. Finally, to yield the incremental net cost, we summed standardized program and incremental infection-related costs (see Appendix).
Analysis
To identify factors potentially associated with greater effectiveness (lower IRR) and savings (lower incremental net cost) among the studies, we conducted 7 sets of unadjusted weighted regression analyses. We separately examined 5 factors potentially associated with effectiveness (study size in central venous catheter [CVC]-days per study-year, measure of infection, baseline infection rate, whether interventions included use of checklists, and program cost) and 7 factors potentially associated with incremental net costs (same factors plus mQHES score and effectiveness). In each analysis (other than study size), we weighted each study by the number of central-venous catheter-days (CVC-days) per study-year.
QI interventions were heterogeneous and generally included multiple components, limiting our ability to perform subgroup analyses. However, we were able to classify studies using three clinically relevant categories: (1) interventions involving checklists vs. usual care (reference group); (2) other practices vs. usual care; and (3) other practices vs. usual care with checklists already in use.
In a series of sensitivity analyses, we sequentially dropped each of the 8 largest studies, and we dropped the two pediatric studies to determine whether results changed. There were too few studies for multivariate regression, and not enough data on variance for inverse variance weighted meta-regression.
RESULTS
Study Selection
We identified 505 records, selecting 63 for full-text review; 16 articles met all eligibility criteria, reflecting 15 unique studies.27–42 Eleven articles focused on CLABSI 27–29,32,33,36,37,39–42, and 5 on CRBSI.30,31,34,35,38 Two articles drew from a study on CLABSI and ventilator-associated pneumonia; we focused on a cost analysis from the hospital perspective,40 rather than a cost-effectiveness analysis from the societal perspective.41 Another study addressed CLABSI, catheter-associated urinary tract infection, and ventilator-associated pneumonia.28 Searches of grey literature did not identify eligible articles. Fifteen excluded studies tested materials or equipment but omitted costs associated with implementation.15,43–56 See Figure 1 for PRISMA diagram.
Figure 1:
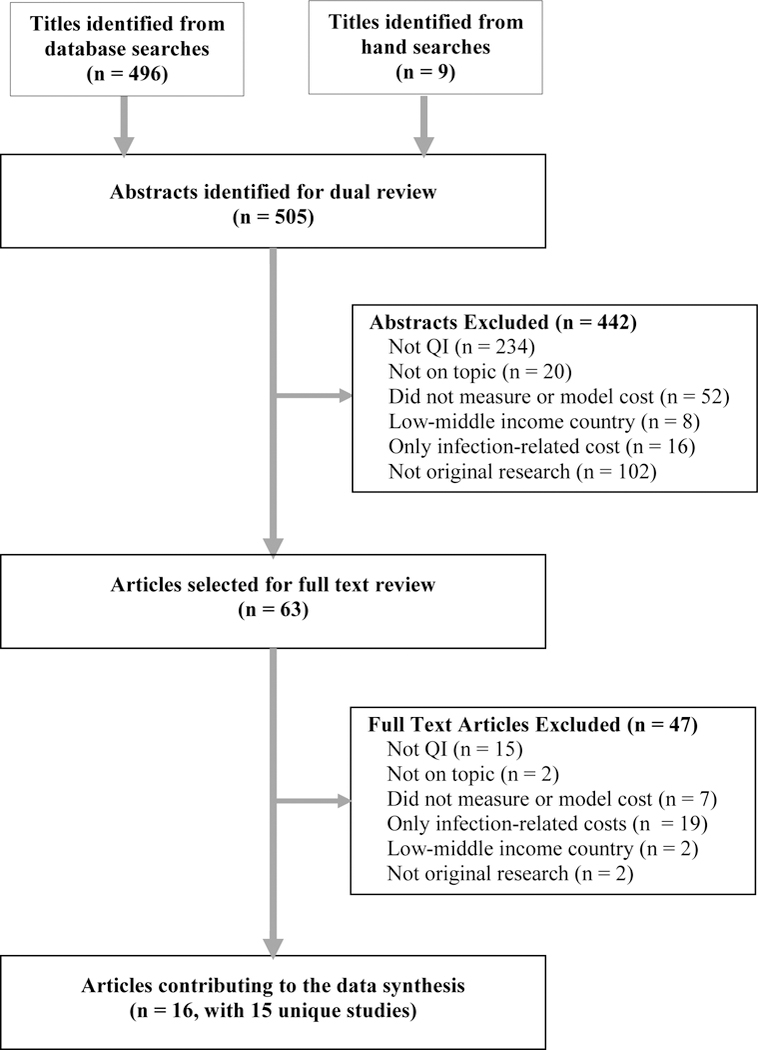
PRISMA Flow Diagram
Study Characteristics and Quality Assessment
QI Interventions
One or more AHRQ-recommended practices were tested in 12 of the 15 unique studies (Table 1).4,27–29,31–36,38–42 These included: insertion checklists with 5 specific components (6 studies, plus 1 study with 4 components),28,29,32,36,38–41 provider education (11 studies),27–29,31,32,34–36,38–41 ultrasound-guided placement (3 studies),29,33,38 all-inclusive catheter kits (5 studies),27,28,35,38,39 sterile dressings (5 studies),27,28,35,38,39chlorhexidine gluconate sponge or antimicrobial dressing (2 studies);28,39 antimicrobial catheters (2 studies, one of which did not specify the antimicrobial agent).28,35
Table 1:
Use of Practices Designed to Prevent CLABSI or CRBSI in Studies with Economic Evaluations
| Allen 2014 27 | Ander-son 2011 28,72 | Bond 201129 | Burden 2012 30 | Cohen 2010 31 | Cooper 2014 32 | Deutsch 2013 33 | Fraher 2009 34,73,74 | Frankel 2005 35 | Herzer 2014 36,75,76 | Kagan 2014 37 | Kamboj 2015 42 | Kim 2011 38 | Miller 2011 39,77 | Waters 2011/ Dick 2015 40,41 |
|
| Practices Strongly Recommended by AHRQ | |||||||||||||||
| CVC checklists a | I, C | I | I | I, C | -- | I | -- | I, C | -- | I | I, C | I, C | I | I | I |
| Hand hygiene prior to catheter insertion a,b | I, C | I | I | I, C | -- | I | -- | I, C | -- | I | I, C | -- | I | I | I |
| Maximal sterile barrier precautions a,b,c | I, C | I | I | I, C | -- | I | -- | I, C | I, C | I | I, C | -- | I | I | I |
| Chlorhexidine skin anti-sepsis a,b,c | I, C | I | I | I, C | -- | I | -- | I, C | -- | I | I, C | -- | I | I | I |
| Avoidance of femoral and jugular sites a,b | I, C | I | I | I, C | -- | I | -- | I, C | I, C | I | I, C | -- | I | -- d | I |
| Remove non-essential catheters a,b | I, C | I | I | I, C | -- | I | -- | I, C | -- | I | I, C | -- | I | I | I |
| Antimicrobial catheters a,c | I | ||||||||||||||
| Chlorhexidine/silver sulfadiazine | -- | -- | - | -- | -- | -- | -- | -- | I | -- | I, C | -- | -- | -- | -- |
| Minocycline/rifampin | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| All inclusive catheter carts or kits a | I | I | -- | I, C | -- | -- | -- | -- | I | -- | -- | -- | -- | I | -- |
| Disinfect hubs and needleless connectorsa | -- | I | -- | -- | -- | -- | -- | -- | -- | -- | -- | I, C | -- | -- | -- |
| Ultrasound guided placement a,b | -- | - | I | -- | -- | -- | I | -- | -- | -- | -- | -- | I | -- | -- |
| Cover catheter with sterile dressing a,b | I | I | -- | -- | -- | -- | -- | I, C | I | -- | -- | -- | I | I | -- |
| Chlorhexidine spongeaor antimicrobial dressingc | -- | I | -- | -- | -- | -- | -- | -- | -- | -- | -- | I, C | -- | I | -- |
| Education a,b | I | I | I | I, C | I | I | -- | I | I | I | -- | -- | I | I | I |
| Specialized catheter insertion teams a | -- | - | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| Other Practices | |||||||||||||||
| Simulation-based training | I | -- | I | I | I | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| Facility-wide surveillance and feedback c | I | I | I | -- | -- | -- | -- | -- | I | -- | -- | -- | I | I | -- |
| Reminders to consider removal | -- | -- | I | I, C | -- | -- | -- | -- | -- | -- | -- | -- | -- | I | -- |
| “Time out” / empowering nurses to stop placement | I | -- | I | I, C | -- | -- | -- | -- | -- | -- | -- | -- | I | I | -- |
| Substitute midline catheter for central line in selected patients | -- | -- | -- | -- | -- | -- | I | -- | -- | -- | -- | -- | -- | -- | -- |
| Reduce frequency of routine catheter changes e | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | I | -- | -- | -- | -- |
| Reduce frequency of routine dressing, tubing, and cap changes | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | I | -- |
| Removal of lines placed in Emergency Department | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | I | -- | -- |
| TPN surveillance by clinical nurse manager | -- | -- | -- | -- | -- | -- | -- | I | -- | -- | -- | -- | -- | -- | -- |
| Six sigma techniques | -- | -- | -- | -- | -- | -- | -- | -- | I | -- | -- | -- | -- | -- | -- |
| Attending supervision of residents | -- | -- | -- | -- | -- | -- | -- | -- | I | -- | -- | -- | -- | -- | -- |
| Specific documentation system | -- | -- | -- | -- | -- | -- | -- | -- | I | -- | -- | -- | -- | -- | -- |
| Disinfectant caps for catheters | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | I | -- | -- | -- |
Strongly recommended in AHRQ Report, Making Healthcare Safer II4
Recommended by CDC guidelines9
Used in at least 25% of hospitals as per national survey69
CDC guidelines recommend against routine replacement of central venous catheters9
Abbreviations: CLABSI, central-line-associated bloodstream infection; CVC, central-venous catheter; AHRQ, Agency for Healthcare Research and Quality; I, practice used in intervention scenario; C, practice used in control / usual care scenario; TPN, total parenteral nutrition; CDC, Centers for Disease Control and Prevention
Other practices tested included: simulation-based training (4 studies);27,29–31,35 facility-wide audit and feedback (5 studies),27–29,35,38,39 time out / empowering nurses to stop placement (4 studies),27,29,38,39 reminders to remove lines (2 studies),29,39 and disinfectant caps for catheter hubs.42 Seven studies had one or more unique practices.33–35,37–39,42 No eligible studies considered daily bathing with chlorhexidine gluconate or intervention sustainability.
Investigators compared interventions involving checklists vs. usual care in 7 studies,28,29,32,36,38–41 other practices vs. usual care in 3 studies31,33,35 (although in one, the usual care scenario included two common components of checklists),35 and other practices vs. usual care with checklists already in use in 5 studies.27,30,34,37,42
For interested readers, the 15 studies excluded because they omitted implementation costs examined: maximum sterile barriers47; antibiotic-impregnated CVCs45,46,49,51,52; antimicrobial dressings,15,44,48,53 1- vs. 2-piece chlorhexidine-gluconate-impregnated dressings,43 chlorhexidine gluconate vs. providone-iodine solutions for insertion site care,49 standardized maintenance kits vs. ad hoc supplies,54 disinfection caps for CVC hubs vs. scrubbing the hubs.55,56
Context
Thirteen of the 15 unique studies (Table 2) were based in the U.S.,27–31,33,35–42 one in the United Kingdom,32 and one in Ireland.34 Most studies were set at a single hospital, although one study included 24 hospitals,28 one study included 37 hospitals,32 one study included 29 pediatric intensive care units (ICUs),39 two studies included data from six hospitals each,36,40,41 and one study was based at two affiliated hospitals.29 In total, data were from 113 hospitals. Ten studies were based at only major academic institutions,27,30,31,33–38,42 two studies were based at only community hospitals,28,32 two studies were based at both,29,40,41 and one study did not state academic status.39
Table 2:
Summary of Data Extracted from Economic Evaluations for QI Interventions Designed to Prevent CLABSI and CRBSI
| Author (Year) |
QI Intervention and Clinical Evaluation | Cost Evaluation | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Intervention Components | Setting | Population | Design & Compar-ator | Baseline Rate of Infection | Effectiveness | Approach & Perspective | Start-up Program Costs in Year 1 | Recurring Program Costs in Year 1 and Beyond | Infection-related Costs | Incremental Net Cost | Year of Costs | mQHES Score | |
| Use of CVC Checklists | |||||||||||||
| Ander-son (2011)28 | Checklists, audit & feedback for CLABSI, CAUTI, VAP, MRSA | U.S., 24 hospitals, Community | Population not reported | UCBA, Usual care | 3.7 CLABSI per 1000 CVC-days | IRR 0.53 | Cost analysis, Hospital | NR | $20,000 to $40,000 per hospital per year | −$82,722 to −$159,902 per hospital per year (literature) | −$7.94 to −$15.4 million over 24 hospitals over 5 years | 2010 | 102 |
| Bond (2011)29 | Checklists, simulation-based training, time out, audit & feedback | U.S., 800-bed hospital system with 1 academic and 1 community hospital | Patients with CVCs placed outside of operating room, including children | Model based on assumption, Usual care | 1 CLABSI per 1000 CVC-days | Authors assumed 50% decline | Cost analysis, Hospital | $106,750 for two-hospital system | $63,610 for two-hospital system per year | −$16,350 per infection averted (literature) | First year: $223 per catheter Later years: $44 per catheter | 2009 | 101 |
| Cooper (2014)32 | Checklists | U.K., 37 ICUs, Community | Patients in ICUs, age not reported | Model based on UCBA, Usual care | 1.31 CLABSI per 100 ICU patients (3.7 per 1000 CVC-days) | IRR 0.40 | CEA, Health system | NR | £1,548 per 100 ICU patients | −£3,940 per infection averted (literature) | −£1,557 per 100 ICU patients; −£573 per QALY | 2011 | 106 |
| Herzer (2014)36 | Checklists | U.S., 6 hospitals, Academic | Adults in ICUs | Model based on RCT, Usual care | CLABSI in 5.2% of 423 ICU patients with catheters per year (literature) | IRR 0.19 (95% CI 0.06 to 0.57) | CEA, Hospital | $83,725 per hospital | $192,291 per hospital per year | −$18,793 per infection averted (literature) | −$249,000 per 1000 ICU patients with catheters | 2013 | 113 |
| Kim (2011)38 | Checklists, audit & feedback, time out | U.S., 1 hospital, Academic | Patients in ICUs, age not reported | UCBA, Usual care | 9.0 CRBSI per 1000 CVC-days | IRR 0.70 (95%-CI 0.59–0.77) | Cost analysis, Hospital | $100 | $0 | −$32,254 per infection averted (literature and site data) | −$32,254 per infection averted | 2009 | 85 |
| Miller (2011)39 | Checklists, catheter kits and care, time out | U.S., 29 hospitals | Children in pediatric ICUs | Time series, Usual care | 5.2 CLABSI per 1000 CVC-days | IRR 0.44 (95%-CI: 0.37–0.53) | Cost analysis, Health system | NR | $75,000 per hospital per year | −$45,000 per infection averted (literature) | −$31 million for 29 hospitals over 3 years | 2009 | 99 |
| Waters (2011)40 | Checklists for CLABSI and VAP | U.S., 6 hospitals, Academic and community | Patients in ICUs, age not reported | UCBA, Usual care | 7.7 CLABSI per 1000 CVC-days | IRR 0.14 | Cost analysis, Hospital | $64,420 per hospital (2004) | $146,973 per hospital per year (2008) | −$36,500 per infection averted (literature) | −$1.1 million per hospital per year | 2004,2008 | 105 |
| Dick (2015)41 | Elderly patients in ICUs | Model based on UCBA, Usual care | 5.0 CLABSI per 1000 CVC-days | IRR 0.24 based on prior literature | CEA, Society | Excluded | 145,000 per hospital per year | Inpatient plus outpatient costs (literature) | +$26,996 per QALY | 2013 | 111 | ||
| Use of Other Practices Strongly Recommended by AHRQ | |||||||||||||
| Allen (2014)27 | Simulation-based training, all-inclusive kits | U.S., 1 hospital, Academic | Patients in medical and surgical ICUs | UCBA, Usual care | 2.0 CLABSI per 1000 CVC-days | IRR 0.37 | Cost analysis, Hospital | NR | $13,043 per hospital per year | −$71,165 per infection averted (site data) | −$1.67 million per hospital over 3 years | 2009 | 93 |
| Frankel (2005)35 | Six Sigma, antimicrobial catheters, kits, others | U.S. 1 hospital, Academic | Patients in a surgical ICU | UCBA, Usual care | 11.0 CRBSI per 1000 CVC-days | IRR 0.15 | Cost analysis, Hospital | NR | $5,000 per hospital per year | −$3,000 per infection averted | −$66,000 per hospital per year | 2002 | 94 |
| Use of Other Practices | |||||||||||||
| Burden (2012)30 | Simulation-based training | U.S., 1 hospital, Academic | Patients in ICUs, age not reported | UCBA, Usual care | 6.5 CRBSI per 1000 CVC-days | IRR 0.38 | Cost analysis, Hospital | NR | $64,487 per hospital per year | −$23,472 per infection averted (literature) | −$539,902 per hospital over 2 years | 2008 | 103 |
| Cohen (2010)31 | Simulation-based training | U.S., 1 hospital, Academic | Medical and surgical patients in an ICU | UCBA, Usual care | 4.2 CRBSI per 100 ICU patients with catheters | IRR 0.10 | Cost analysis, Hospital | $111,916 per hospital | $89,455 per hospital per year | −$82,730 per infection averted (site data) | −$704,034 per hospital per year | 2008 | 111 |
| Deutsch (2013)33 | Substitute midline for central line | U.S., 1 hospital, Academic | Adults in a surgical ICU | Model based on catheter days avoided, Usual care | 1.7 CLABSI per 1000 CVC-days (literature) | −283 CVC-days per ICU per 6 mo (IRR 0.72) | Cost analysis, Hospital | $30,000 | −$1,413 per CVC, 60 CVCs per hospital per year | −$29,156 per infection averted (literature) | NR | 2011 | 87 |
| Fraher (2009)34 | TPN surveillance nurse | Ireland, 1 hospital, Academic | Patients in ICUs and wards, age not reported | UCBA, Usual care | 20.5 CRBSI per 1000 CVC-days | IRR 0.71 | Cost analysis, Hospital | NR | €56,700 per hospital per year | −€13,775 per infection averted | −€78,300 per hospital per year | 2007 | 99 |
| Kagan (2014)37 | Reduced frequency of routine catheter changes | U.S., 1 Children’s hospital, Academic | Children with burns, unit type not reported | UCBA, Usual care | 3.1 CLABSI per 1000 CVC-days | IRR 0.90 (NS) | Cost analysis, Hospital | NR | $100 per CVC change, 280 fewer changes per year | $0 due to lack of effectiveness | −$28,000 per hospital per year | 2009 | 98 |
| Kamboj (2014) 42 | Change from scrubbing CVC hubs to using disinfection caps | U.S., 1 Cancer hospital, Academic | Oncology patients | UCBA, Usual care | 2.46 CLABSI per 1000 CVC-days | IRR 0.711 (95% CI 0.56–0.87) | Cost analysis, Hospital | NR | $202,707 per hospital per year | −$3,471,696 per hospital per year | −$3,268,990 per hospital per year | 2012 | |
Abbreviations: CLABSI, central-line-associated blood-stream infection; CRBSI, catheter-related blood stream infection. CAUTI, catheter-associated urinary tract infection; VAP, ventilator-associated pneumonia; MRSA, methicillin-resistant staph aureus; QI, quality improvement; U.S., United States; U.K., United Kingdom; CVC, central venous catheter; IRR, incidence rate ratio; CEA, includes cost-effectiveness, cost-benefit, and related analyses; RCT, randomized controlled trial; UCBA, uncontrolled before-after analysis; U.S., United States; U.K., United Kingdom; NR, not reported.
All studies included or were limited to intensive care settings. The median estimated number of CVC days per hospital per year was 3,843 (IQR 2,917).27–42 One study based at an oncology hospital had 40,711 CVC days per year.42 Two studies were limited to pediatric populations.37,39 The median baseline rate of CLABSI/CRBSI was 4.0 (interquartile range [IQR] 4.3) per 1000 catheter-days among the 15 unique studies;27–40 this equated to a median of about 18.3 infections per study hospital per year (IQR 17.3).
Clinical Evaluation
The 15 unique studies compared the QI interventions with usual care scenarios (Table 2). Ten studies used uncontrolled-before-after designs (UCBA) 27,28,30,31,34,35,37,38,40–42 and one used a time-series analysis.39 Four of the unique studies reported modeling exercises, including one based on a randomized controlled trial and one based on a UCBA design.29,32,33,36,41
In total, 13 studies, including two of the modeling analyses, used empirical data on changes in infection rates.27,28,30–32,34–42 One modeling study of insertion checklists assumed a 50% decline in infections,29 which is similar to prior literature.57 Another modeling study estimated a decline in infections based on changes in CVC-days.33 Excluding the study that assumed a 50% decline, the median IRR was 0.42 (IQR 0.47),27,28,30–41 which equated to a median of about 2.8 fewer infections per 1,000 CVC-days (IQR 2.6) and 9.8 (IQR 12.2) fewer infections per study hospital per year.
Items from the Minimum Quality Data Set are given in the Appendix.
Cost Evaluation
As noted above, a cost-effectiveness analysis taking the societal perspective41 and a cost analysis taking the hospital perspective were based on the same study.40 Two other studies were cost-effectiveness analyses;32,36 one considered the hospital perspective,27,30,31,34,36,37 and one the health system perspective.32 The remaining 12 studies were cost analyses; 11 used the hospital perspective 27–31,33–35,37,38,40,42 and one used the health system perspective.39
Among the 15 studies, the resources invested in infection prevention and the associated program costs varied. Six studies estimated start-up costs (standardized median $108,000, IQR $92,500),29,31,33,36,38,40 such as the purchase of ultrasound machines,29,31,38 vascular simulators such as mannequins,27,29–31,35 and vascular access carts.31,38,40 All 15 studies estimated annually recurring costs (standardized median $29,600 per year, IQR $37,900),27–40,42 such as catheters and supplies27,30,31,33,35,37–40 and labor costs associated with time that physicians and nurses spent in training, 27–31,35,38,40 catheter-related care,30,32,33,35,37,38,40,42 documentation,27,29,38 data collection and analysis,27–31,35,38–40 and leadership and oversight.28,35,40 Program costs were negative in two studies: one substituted placement of peripheral midline catheters by residents for placement of central lines by interventional radiologists,33 and the other reduced the frequency of routine catheter changes.37
Study Quality
Cost evaluation methods were of moderate to high quality (Table 3), with median mQHES scores of 100.5 (IQR 8.3) among the 16 articles.
Table 3:
Results of Weighted Regression: Associations between Setting, Study, and Intervention Characteristics and Predicted Incidence Rate Ratio (IRR) or Incremental Net Cost per Hospital over Three Years
| Characteristics and Subgroups Being Compared | k * | Incidence Rate Ratio (95% Confidence Interval) | P-value for Characteristic | Incremental Net Cost in Millions (95% Confidence Interval) | P-value for Characteristic |
|---|---|---|---|---|---|
| Results Including All 15 Studies | 15 | 0.43 (0.35 to 0.51) | --- | −$1.85 (−$2.40 to −$1.30) | --- |
| Study Size | |||||
| ≥40,000 CVC Days per Study per Year | 4 | 0.52 (0.27 to 0.77) | 0.606 | −$1.78 (−$3.03 to −$0.53) | 0.951 |
| <40,000 CVC Days per Study per Year | 11 | 0.44 (0.29 to 0.59) | −$1.74 (−$2.49 to −$0.98) | ||
| Measure of Infection | |||||
| CLABSI † | 10 | 0.43 (0.36 to 0.50) | 0.731 | −$1.84 (−$2.36 to −$1.31) | 0.818 |
| CRBSI | 5 | 0.35 (0 to 0.82) ‡ | −$2.24 (−$5.60 to $41.11) | ||
| Baseline Rate of Infection § | |||||
| Weighted mean rate (4.49 per 1,000 CVC Days) | 15 | 0.43 (0.36 to 0.49) | 0.082 | −$1.85 (−$2.35 to −$1.35) | 0.313 |
| 10% higher (4.94 per 1,000 CVC Days) | 0.41 (0.34 to 0.48) | −$1.92 (−$2.44 to −$1.40) | |||
| Program Cost per Hospital over Three Years | |||||
| Weighted mean cost ($290,000) | 15 | 0.43 (0.36 to 0.50) | 0.477 | −$1.85 (−$2.19 to −$1.51) | 0.001 |
| $100,000 higher ($390,000) | 0.42 (0.34 to 0.50) | −$2.16 (−$2.54 to −$1.79) | |||
| Types of Infection-prevention Practices Evaluated | |||||
| Checklists vs. usual care | 7 | 0.40 (0.34 to 0.47) | reference | −$1.66 (−$2.16 to −$1.16) | reference |
| Other practices vs. usual care | 3 | 0.20 (0 to 0.75) ‡ | 0.484 | −$2.76 (−$7.07 to $1.55) | 0.629 |
| Other practices vs. usual care with checklists | 5 | 0.65 (0.47 to 0.83) | 0.026 | −$3.17 (−$4.55 to −$1.79) | 0.067 |
| Effectiveness | |||||
| Weighted mean IRR (0.43) | 15 | not applicable | −$1.85 (−$2.37 to −$1.33) | 0.888 | |
| 10% higher (0.47) | −$1.84 (−$2.38 to −$1.29) | ||||
| Study Quality | |||||
| Weighted mean mQHES score (103) | 15 | not applicable | −$1.84 (−$2.35 to −$1.35) | 0.335 | |
| 10% higher (113) | −$1.29 (−$2.46 to −$0.12) |
Number of studies in group.
Abbreviations: CLABSI, central-line-associated blood-stream infection; CRBSI, catheter-related blood stream infection; CVC-days, central-venous catheter-days; IRR, incidence rate ratio.
IRRs cannot be less than 0; therefore, we truncated any values below zero.
For characteristics that involve continuous variables (baseline rate of infection, program cost, effectiveness, and study quality), we report results for two values, the mean for the variable and, generally, a value 10% higher. P-values reflect the significance of the characteristic overall, not the specific values selected.
Data Standardization
Among the 15 unique studies, the median total program cost per hospital over three years was $271,000 (IQR $417,000), and the median incremental infection-related cost was -$2.27 million (IQR $2.16 million),27–42 relative to usual care. Based on differences between program and incremental infection-related costs, the median net savings was $1.85 million (IQR $1.77 million) 27–42 (Figure 2). These estimates are unweighted. Program costs could be more than 6.8-fold higher than we observed before net savings would be eliminated.
Figure 2:
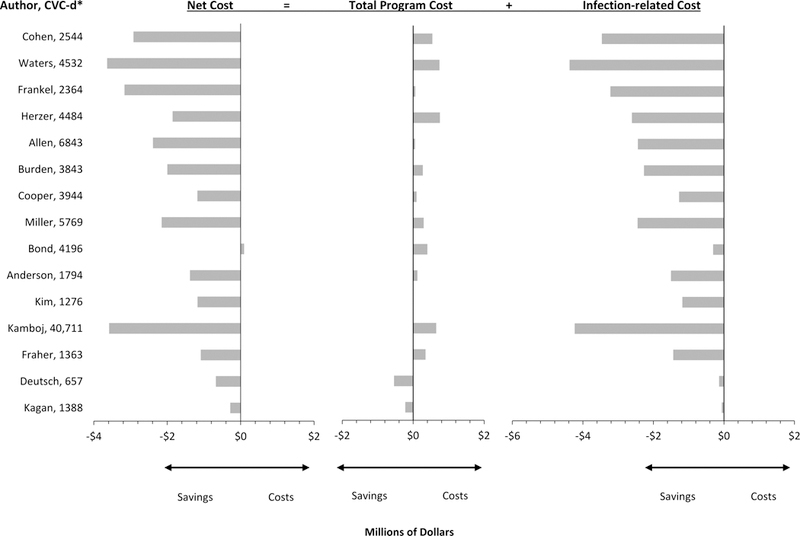
Net Costs Associated with CLABSI/CRBSI-Prevention Interventions from the Hospital Perspective over Three Years (2015 US Dollars)
*Central-venous-catheter-days per hospital per year
Among the 7 studies testing checklists, the median net savings was $1.12 million (IQR $1.31 million).28,29,32,36,38–41 In the study that assumed a 50% decline in infections, there was a net loss of $90,000 due to a low baseline rate of CLABSI (1.0 per 1,000 CVC-days) and relatively high program cost ($400,000).29 Six studies with lower baseline infection rates (1.7 to 3.7 CLABSI per 1,000 CVC-days) were associated with declines in infections as well as net savings.28,29,32,33,37,42
Analysis
In unadjusted regression analyses weighted by CVC-days per study per year, the mean IRR among the 15 studies was 0.43 (95% CI 0.35–0.51, Table 3), reflecting a 57% decline in infections. Compared with studies that tested use of checklists, infections declined less in studies that tested other practices when checklists were already in use (IRR 0.40 vs. 0.65, p=0.026).
The mean incremental net savings was $1.85 million (95% CI $1.30 to $2.40 million) over three years. Larger investments in infection prevention (program costs) were associated with greater net savings (p=0.001): each additional $100,000 invested was associated with $310,000 higher savings ($1.85 vs. $2.16 million).
These results were robust to sequential elimination of the largest studies and the 2 pediatric studies, with one notable exception. The oncology study had a relatively high IRR (0.711) and incremental net savings (-$3.85 million) as well as ten times more CVC days than other hospitals. Excluding this study, the type of infection-prevention practice tested was no longer associated with effectiveness. However, investments in infection prevention were associated with greater effectiveness (p=0.002): each additional $100,000 invested was associated with 4% greater effectiveness (IRR 0.40 vs. 0.36), or approximately 2.4 fewer infections per hospital. In addition, a higher baseline infection rate and greater effectiveness were both associated larger net savings (p=0.014 and p=0.019, respectively). See Appendix.
DISCUSSION
Based on our analysis, QI interventions that are effective at reducing bloodstream infections related to central catheters are generally a good value for hospitals because they are associated with improved clinical outcomes and lower costs. We identified 15 eligible, unique economic evaluations that together included data from 113 hospitals.27–42 Most interventions involved practices strongly recommended by AHRQ.27–29,31–36,38–41,4 On average, these interventions were associated with a 57% decline in infections (IRR 0.43, 95% CI 0.35–0.51) and net savings of $1.85 million (95% CI $1.30 to $2.40 million) per hospital over three years.27–41 Each additional $100,000 invested was associated with $310,000 greater net savings in unadjusted analyses. Larger investments were also associated with greater effectiveness when a study from an oncology hospital was excluded.42
In assessing value, both clinical effectiveness and cost are important.6 The effectiveness of the interventions we studied was similar to prior studies.4,58 One meta-analysis reported pooled odds ratios for CLABSI of 0.34 (95% CI 0.27–0.41) for interventions with checklists vs. usual care, and 0.45 (95% CI 0.36–0.55) for interventions without checklists.58 Another meta-analysis that compared checklists with usual care reported a pooled IRR for CLABSI of 0.44 (95% CI 0.39–0.50) among 79 primary studies.57 (Herein, we refer to CLABSI or CRBSI when the literature cited does).
To determine the total cost of an intervention, both program and infection-related costs should be considered. Yet prior literature has emphasized infection-related costs.3,16 Until now, there has been no synthesis of program costs—meaning the value of the resources that hospitals invest infection prevention, such as equipment, supplies, and time spent by physicians and nurses on planning, training, clinical care, and surveillance. Our results suggest that effective interventions tend to be a good value for hospitals, despite the program costs involved.
Hospitals have come under increasing pressure to invest in preventing healthcare associated infections (HAIs) over the last decade, as federal and state policymakers have partnered together and with stakeholder groups to eliminate HAIs.59–62 The Centers for Medicare and Medicaid Services (CMS) have established multiple incentives to reduce HAIs including CLABSI, including public reporting, non-payment for hospital-associated complications, value-based purchasing, and, starting in 2015, sizeable payment penalties.63–66 Accordingly, the use of prevention practices has risen substantially since 2005, and infection rates have declined.67,68 A 2013 national survey found that 98–99% of hospitals used two common insertion checklist components (maximum barrier precautions and chlorhexidine site antisepsis), 90% monitored rates hospital-wide, 78% used antimicrobial dressings, 34% used antimicrobial catheters.69 According to AHRQ, from 2010 to 2013, rates of CLABSI fell by 49%, averting 8,800 infections as well as $150 million in infection-related costs.61 CLABSI rates in medical and surgical ICUs reached 0.8 to 1.4 per 1,000 CVC-days as of 2013.67 Net savings from these changes may have been somewhat smaller than AHRQ’s estimates, which did not account for program costs.
Now that checklists are used widely and infection rates have declined, what are the prospects for additional reductions in infections and net savings? Hospitals that have already attained very low infection rates would likely see smaller clinical benefits and savings than in the studies we have reviewed. Nonetheless, we found that QI interventions can be associated with declines in CLABSI/CRBSI and net savings when checklists are already in use,27,30,34,37,42 and when hospitals have CLABSI rates as low as 1.7 to 3.7 per 1,000 CVC-days.27,28,32,33,37,42
Despite the possibility of net savings, investing in the prevention of HAIs like CLABSI/CRBSI may be burdensome for hospitals with limited financial resources. HAI prevention is labor-intensive, wages and benefits account for two thirds of all spending by hospitals, and a quarter of hospitals have had negative operating margins in recent years.70 We found that, for CLABSI/CRBSI-prevention interventions, median program costs were about $270,000 per hospital over three years—but reached $500,000 to $750,000 in some studies. Higher program costs were generally associated with greater net savings and possibly larger declines in infection rates. This suggests that both patients and hospitals might benefit when hospitals invest more in effective prevention programs. However, we were unable to control for hospital characteristics. Hospitals with ample financial resources, for example, may both invest more heavily in HAI prevention and have better trained providers who implement interventions more effectively. Even if some hospitals can achieve greater net savings from larger, costlier HAI prevention programs, success is not assured and many hospitals may lack the cash flow or other resources to make sizeable up-front investments.71 Future research should more thoroughly examine the relationships among hospital financial performance, economic investments in QI, and effects on quality of care.
This analysis had several limitations. Only a few studies have examined the cost of QI interventions related to CLABSI/CRBSI, and most of these used weak uncontrolled before-after designs. We could only include interventions for which economic evaluations have been performed. Studies used two different measures of infection; CLABSI is a more sensitive measure, but eligible studies using CRBSI reported relatively high rates of infection (4.0 to 28.3 CVC-days per 1,000 patient days).30,31,34,35,38 We were unable to identify specific practices that are associated with higher value due to the complexity of the interventions, or to assess the role of contextual factors. Nonetheless, these findings reflect more than 100 sites, and the changes in CLABSI rates we observed are consistent with other sources. We were unable to formally test for publication bias, but found no evidence that lower quality studies with greater net savings were published preferentially. Authors may have omitted some program costs; however, a several-fold underestimate would be needed to eliminate the net savings. We attributed all inpatient infection-related costs to the hospital perspective, when private payers may reimburse some of these costs. We did not account for Medicare policies that preclude payment and impose penalties for hospital-acquired infections, which may underestimate benefits to hospitals.
In conclusion, interventions designed to prevent CLABSI were, on average, associated with a 57% decline in infections as well as $1.85 million net savings to hospitals within one to three years, making them of high value to hospitals. Interventions that involve larger initial investments of resources may be associated with greater net savings. Although checklists are now widely used and infection rates have declined, additional improvements and cost savings can occur at hospitals that have not yet attained very low infection rates.
Supplementary Material
Acknowledgements:
We would like to acknowledge the contributions of the following individuals:
Role of the Funding Source in the Present Study
The Agency for Healthcare Research and Quality (AHRQ, R01 HS22644–01) funded the work but did not participate in study design, data acquisition, analysis, or interpretation of results. The views reported are those of the authors, not AHRQ.
Funding Source: Agency for Healthcare Research and Quality
Footnotes
The authors have no conflicts of interest with the work.
References
- 1.2012 National and State Healthcare-Associated Infections Progress Report. Atlanta, GA: Centers for Disease Control and Prevention; March 26 2014. [Google Scholar]
- 2.Magill SS, Edwards JR, Bamberg W, et al. Multistate point-prevalence survey of health care-associated infections. The New England journal of medicine. 2014;370(13):1198–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zimlichman E, Henderson D, Tamir O, et al. Health care-associated infections: a meta-analysis of costs and financial impact on the US health care system. JAMA internal medicine. 2013;173(22):2039–2046. [DOI] [PubMed] [Google Scholar]
- 4.Shekelle PG, Wachter RM, Pronovost PJ, et al. Making Health Care Safer II: An Updated Critical Analysis of the Evidence for Patient Safety Practices. Rockville, MD: Agency for Healthcare Research and Quality;March 2013. [PMC free article] [PubMed] [Google Scholar]
- 5.Etchells E, Koo M, Daneman N, et al. Comparative economic analyses of patient safety improvement strategies in acute care: a systematic review. BMJ quality & safety. 2012;21(6):448–456. [DOI] [PubMed] [Google Scholar]
- 6.Porter ME. What Is Value in Health Care? The New England journal of medicine. 2010;363:2477–2481. [DOI] [PubMed] [Google Scholar]
- 7.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nuckols T, Maglione M, Shekelle P, Morton S, Escarce J, Keeler E. Systematic review of cost outcomes of quality improvement. PROSPERO. 2015;CRD42015014950. Available from http://www.crd.york.ac.uk/PROSPERO/display_record.asp?ID=CRD42015014950. [Google Scholar]
- 9.O’Grady NP, Alexander M, Burns LA, et al. Guidelines for the Prevention of Intravascular Catheter-Related Infections, 2011. Washington, D.C.: U.S. Department of Health & Human Services, Centers for Disease Control;2011. [Google Scholar]
- 10.Chen X-X, Lo Y-C, Su L-H, Chang C-L. Investigation of the case numbers of catheter-related bloodstream infection overestimated by the central line-associated bloodstream infection surveillance definition. Journal of Microbiology, Immunology and Infection. 2015;48(6):625–631. [DOI] [PubMed] [Google Scholar]
- 11.Glanville J How well do search filters perform in identifying economic evaluations in MEDLINE and EMBASE. International Journal of Technology Assessment in Health Care. 2009;25(4):522–529. [DOI] [PubMed] [Google Scholar]
- 12.Infectious Diseases Society of America. IDSA Archived Conference Presentations 2016; https://idsa.confex.com/idsa/archives.cgi. Accessed August 2, 2016. [Google Scholar]
- 13.Mauger Rothenberg B, Marbella A, Pines E, Chopra R, Black ER, Aronson N. Closing the quality gap: revisiting the state of the science (vol. 6: prevention of healthcare-associated infections). Evidence report/technology assessment. 2012(208.6):1–578. [PMC free article] [PubMed] [Google Scholar]
- 14.Ranji SR, Shetty K, Posley KA, et al. Closing the Quality Gap: A Critical Analysis of Quality Improvement Strategies (Vol. 6: Prevention of Healthcare-Associated Infections). Rockville MD: 2007. [PubMed] [Google Scholar]
- 15.Ho KM, Litton E. Use of chlorhexidine-impregnated dressing to prevent vascular and epidural catheter colonization and infection: a meta-analysis. The Journal of antimicrobial chemotherapy. 2006;58(2):281–287. [DOI] [PubMed] [Google Scholar]
- 16.Umscheid CA, Mitchell MD, Doshi JA, Agarwal R, Williams K, Brennan PJ. Estimating the proportion of healthcare-associated infections that are reasonably preventable and the related mortality and costs. Infection control and hospital epidemiology : the official journal of the Society of Hospital Epidemiologists of America. 2011;32(2):101–114. [DOI] [PubMed] [Google Scholar]
- 17.Flodgren G, Conterno LO, Mayhew A, Omar O, Pereira CR, Shepperd S. Interventions to improve professional adherence to guidelines for prevention of device-related infections. The Cochrane database of systematic reviews. 2013;3:CD006559. [DOI] [PubMed] [Google Scholar]
- 18.Goeree R, Burke N, O’Reilly D, Manca A, Blackhouse G, Tarride JE. Transferability of economic evaluations: approaches and factors to consider when using results from one geographic area for another. Curr Med Res Opin. 2007;23(4):671–682. [DOI] [PubMed] [Google Scholar]
- 19.Danz MS, Rubenstein LV, Hempel S, et al. Identifying quality improvement intervention evaluations: is consensus achievable? Qual Saf Health Care. 2010;19(4):279–283. [DOI] [PubMed] [Google Scholar]
- 20.Pronovost P, Needham D, Berenholtz S, et al. An intervention to decrease catheter-related bloodstream infections in the ICU. The New England journal of medicine. 2006;355(26):2725–2732. [DOI] [PubMed] [Google Scholar]
- 21.Cochrane Consumers & Communication Review Group Study Design Guide For Review Authors. The Cochrane Collaboration. June 2013:1–56. http://cccrg.cochrane.org/sites/cccrg.cochrane.org/files/uploads/Study_design_guide2013.pdf. [Google Scholar]
- 22.Hempel S, Shekelle PG, Liu JL, et al. Development of the Quality Improvement Minimum Quality Criteria Set (QI-MQCS): a tool for critical appraisal of quality improvement intervention publications. BMJ quality & safety. 2015;24(12):796–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nuckols TK, Escarce JJ, Asch SM. The effects of quality of care on costs: a conceptual framework. Milbank Q. 2013;91(2):316–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chiou CF, Hay JW, Wallace JF, et al. Development and validation of a grading system for the quality of cost-effectiveness studies. Med Care. 2003;41(1):32–44. [DOI] [PubMed] [Google Scholar]
- 25.Walker DG, Wilson RF, Sharma R, et al. Best Practices for Conducting Economic Evaluations in Health Care: A Systematic Review of Quality Assessment Tools. Methods Research Report (Prepared by Johns Hopkins University Evidence-based Practice Center under contract No. 290–2007-10061-I.). Rockville, MD: Agency for Healthcare Research and Quality;October 2012. [PubMed] [Google Scholar]
- 26.Gold MR, Siegel JE, Russell LB, Weinstein MC, (eds). Cost-effectiveness in Health and Medicine. New York: Oxford University Press; 1996. [Google Scholar]
- 27.Allen GB, Miller V, Nicholas C, et al. A multitiered strategy of simulation training, kit consolidation, and electronic documentation is associated with a reduction in central line-associated bloodstream infections. American journal of infection control. 2014;42(6):643–648. [DOI] [PubMed] [Google Scholar]
- 28.Anderson DJ, Miller BA, Chen LF, et al. The network approach for prevention of healthcare-associated infections: long-term effect of participation in the Duke Infection Control Outreach Network. Infection control and hospital epidemiology : the official journal of the Society of Hospital Epidemiologists of America. 2011;32(4):315–322. [DOI] [PubMed] [Google Scholar]
- 29.Bond WF, King AE. Modeling for the decision process to implement an educational intervention: an example of a central venous catheter insertion course. Journal of Patient Safety. 2011;7(2):85–91. [DOI] [PubMed] [Google Scholar]
- 30.Burden AR, Torjman MC, Dy GE, et al. Prevention of central venous catheter-related bloodstream infections: is it time to add simulation training to the prevention bundle? Journal of clinical anesthesia. 2012;24(7):555–560. [DOI] [PubMed] [Google Scholar]
- 31.Cohen ER, Feinglass J, Barsuk JH, et al. Cost savings from reduced catheter-related bloodstream infection after simulation-based education for residents in a medical intensive care unit. Simulation in healthcare : journal of the Society for Simulation in Healthcare. 2010;5(2):98–102. [DOI] [PubMed] [Google Scholar]
- 32.Cooper K, Frampton G, Harris P, et al. Are educational interventions to prevent catheter-related bloodstream infections in intensive care unit cost-effective? The Journal of hospital infection. 2014;86(1):47–52. [DOI] [PubMed] [Google Scholar]
- 33.Deutsch GB, Sathyanarayana SA, Singh N, Nicastro J. Ultrasound-guided placement of midline catheters in the surgical intensive care unit: a cost-effective proposal for timely central line removal . The Journal of surgical research. 2013. [DOI] [PubMed] [Google Scholar]
- 34.Fraher MH, Collins CJ, Bourke J, Phelan D, Lynch M. Cost-effectiveness of employing a total parenteral nutrition surveillance nurse for the prevention of catheter-related bloodstream infections. The Journal of hospital infection. 2009;73(2):129–134. [DOI] [PubMed] [Google Scholar]
- 35.Frankel HL, Crede WB, Topal JE, Roumanis SA, Devlin MW, Foley AB. Use of corporate Six Sigma performance-improvement strategies to reduce incidence of catheter-related bloodstream infections in a surgical ICU. Journal of the American College of Surgeons. 2005;201(3):349–358. [DOI] [PubMed] [Google Scholar]
- 36.Herzer KR, Niessen L, Constenla DO, Ward WJ, Jr., Pronovost PJ. Cost-effectiveness of a quality improvement programme to reduce central line-associated bloodstream infections in intensive care units in the USA. BMJ open. 2014;4(9):e006065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kagan RJ, Neely AN, Rieman MT, et al. A performance improvement initiative to determine the impact of increasing the time interval between changing centrally placed intravascular catheters. Journal of burn care & research : official publication of the American Burn Association. 2014;35(2):143–147. [DOI] [PubMed] [Google Scholar]
- 38.Kim JS, Holtom P, Vigen C. Reduction of catheter-related bloodstream infections through the use of a central venous line bundle: epidemiologic and economic consequences. American journal of infection control. 2011;39(8):640–646. [DOI] [PubMed] [Google Scholar]
- 39.Miller MR, Niedner MF, Huskins WC, et al. Reducing PICU central line-associated bloodstream infections: 3-year results. Pediatrics. 2011;128(5):e1077–1083. [DOI] [PubMed] [Google Scholar]
- 40.Waters HR, Korn R, Colantuoni E Jr., et al. The business case for quality: economic analysis of the Michigan Keystone Patient Safety Program in ICUs . American journal of medical quality : the official journal of the American College of Medical Quality. 2011;26(5):333–339. [DOI] [PubMed] [Google Scholar]
- 41.Dick AW, Perencevich EN, Pogorzelska-Maziarz M, Zwanziger J, Larson EL, Stone PW. A decade of investment in infection prevention: a cost-effectiveness analysis. American journal of infection control. 2015;43(1):4–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kamboj M, Blair R, Bell N, et al. Use of Disinfection Cap to Reduce Central-Line-Associated Bloodstream Infection and Blood Culture Contamination Among Hematology-Oncology Patients. Infection control and hospital epidemiology : the official journal of the Society of Hospital Epidemiologists of America. 2015;36(12):1401–1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pfaff B, Heithaus T, Emanuelsen M. Use of a 1-piece chlorhexidine gluconate transparent dressing on critically ill patients. Critical care nurse. 2012;32(4):35–40. [DOI] [PubMed] [Google Scholar]
- 44.Ye X, Rupnow M, Bastide P, Lafuma A, Ovington L, Jarvis WR. Economic impact of use of chlorhexidine-impregnated sponge dressing for prevention of central line-associated infections in the United States. American journal of infection control. 2011;39(8):647–654. [DOI] [PubMed] [Google Scholar]
- 45.Halton KA, Cook DA, Whitby M, Paterson DL, Graves N. Cost effectiveness of antimicrobial catheters in the intensive care unit: addressing uncertainty in the decision. Critical care (London, England). 2009;13(2):R35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Borschel DM, Chenoweth CE, Kaufman SR, et al. Are antiseptic-coated central venous catheters effective in a real-world setting? American journal of infection control. 2006;34(6):388–393. [DOI] [PubMed] [Google Scholar]
- 47.Hu KK, Veenstra DL, Lipsky BA, Saint S. Use of maximal sterile barriers during central venous catheter insertion: clinical and economic outcomes. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2004;39(10):1441–1445. [DOI] [PubMed] [Google Scholar]
- 48.Crawford AG, Fuhr JP Jr., Rao B Cost-benefit analysis of chlorhexidine gluconate dressing in the prevention of catheter-related bloodstream infections. Infection control and hospital epidemiology : the official journal of the Society of Hospital Epidemiologists of America. 2004;25(8):668–674. [DOI] [PubMed] [Google Scholar]
- 49.Chaiyakunapruk N, Veenstra DL, Lipsky BA, Sullivan SD, Saint S. Vascular catheter site care: the clinical and economic benefits of chlorhexidine gluconate compared with povidone iodine. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2003;37(6):764–771. [DOI] [PubMed] [Google Scholar]
- 50.Hanna HA, Raad II, Hackett B, et al. Antibiotic-impregnated catheters associated with significant decrease in nosocomial and multidrug-resistant bacteremias in critically ill patients. Chest. 2003;124(3):1030–1038. [DOI] [PubMed] [Google Scholar]
- 51.Shorr AF, Humphreys CW, Helman DL. New choices for central venous catheters: potential financial implications. Chest. 2003;124(1):275–284. [PubMed] [Google Scholar]
- 52.Marciante KD, Veenstra DL, Lipsky BA, Saint S. Which antimicrobial impregnated central venous catheter should we use? Modeling the costs and outcomes of antimicrobial catheter use. American journal of infection control. 2003;31(1):1–8. [DOI] [PubMed] [Google Scholar]
- 53.Schwebel C, Lucet JC, Vesin A, et al. Economic evaluation of chlorhexidine-impregnated sponges for preventing catheter-related infections in critically ill adults in the Dressing Study. Critical care medicine. 2012;40(1):11–17. [DOI] [PubMed] [Google Scholar]
- 54.Nelson RE, Angelovic AW, Nelson SD, Gleed JR, Drews FA. An economic analysis of adherence engineering to improve use of best practices during central line maintenance procedures. Infection control and hospital epidemiology : the official journal of the Society of Hospital Epidemiologists of America. 2015;36(5):550–556. [DOI] [PubMed] [Google Scholar]
- 55.Stango C, Runyan D, Stern J, Macri I, Vacca M. A successful approach to reducing bloodstream infections based on a disinfection device for intravenous needleless connector hubs. Journal of infusion nursing : the official publication of the Infusion Nurses Society. 2014;37(6):462–465. [DOI] [PubMed] [Google Scholar]
- 56.Merrill KC, Sumner S, Linford L, Taylor C, Macintosh C. Impact of universal disinfectant cap implementation on central line-associated bloodstream infections. American journal of infection control. 2014;42(12):1274–1277. [DOI] [PubMed] [Google Scholar]
- 57.Ista E, van der Hoven B, Kornelisse RF, et al. Effectiveness of insertion and maintenance bundles to prevent central-line-associated bloodstream infections in critically ill patients of all ages: a systematic review and meta-analysis. Lancet Infectious Diseases. 2016;[Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 58.Blot K, Bergs J, Vogelaers D, Blot S, Vandijck D. Prevention of central line-associated bloodstream infections through quality improvement interventions: a systematic review and meta-analysis. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2014;59(1):96–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Office of Disease Prevention and Health Promotion, Office of the Assistant Secretary for Health, Office of the Secretary, U.S. Department of Health and Human Services. National Action Plan To Prevent Health Care-Associated Infections: Road Map To Elimination. Part 2: Framework; April 2013; http://www.health.gov/hai/pdfs/hai-action-plan-framework.pdf. Accessed May 19, 2016. [Google Scholar]
- 60.Centers for Medicare & Medicaid Services . Partnership for Patients. 2015; http://innovation.cms.gov/initiatives/partnership-for-patients/. Accessed May 19, 2016.
- 61.Interim Update on 2013 Annual Hospital-Acquired Condition Rate and Estimates of Cost Savings and Deaths Averted From 2010 to 2013. Washington, D.C: Health and Human Services;2014. [Google Scholar]
- 62.Prepared by Clarkwest Andrew, Chen Arnold, Higgins Maureen, et al. Project Evaluation Activity in Support of Partnership for Patients: Task 2 Evaluation Progress Report. Baltimore, MD: Center for Medicare and Medicaid Innovation;2014. [Google Scholar]
- 63.Centers for Medicare & Medicaid Services. Hospital Value-Based Purchasing. 2012; https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/hospital-value-based-purchasing/index.html?redirect=/Hospital-Value-Based-Purchasing/. Accessed May 19, 2016.
- 64.Rajaram R, Barnard C, Bilimoria KY. Concerns about using the patient safety indicator-90 composite in pay-for-performance programs. JAMA : the journal of the American Medical Association. 2015;313(9):897–898. [DOI] [PubMed] [Google Scholar]
- 65.Centers for Medicare & Medicaid Services. Hospital-Acquired Conditions. 2015; http://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/HospitalAcqCond/Hospital-Acquired_Conditions.html. Accessed May 19, 2016.
- 66.Centers for Medicare & Medicaid Services. Hospital-Acquired Condition Reduction Program (HACRP). 2014; https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/AcuteInpatientPPS/HAC-Reduction-Program.html. Accessed May 19, 2016. [DOI] [PubMed]
- 67.Dudeck MA, Edwards JR, Allen-Bridson K, et al. National Healthcare Safety Network (NHSN) Report, data summary for 2013, Device-associated Module. American journal of infection control. 2015;43:206–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Weiner LM, Fridkin SK, Aponte-Torres Z, et al. Vital Signs: Preventing Antibiotic-Resistant Infections in Hospitals — United States, 2014. MMWR. Morbidity and mortality weekly report. 2016;65(9):235–241. [DOI] [PubMed] [Google Scholar]
- 69.Krein SL, Fowler KE, Ratz D, Meddings J, Saint S. Preventing device-associated infections in US hospitals: national surveys from 2005 to 2013. BMJ quality & safety. 2015:1–8. Accessed February 18, 2016. [DOI] [PubMed] [Google Scholar]
- 70.Avalere Health for the American Hospital Association. American Hospital Association Trendwatch Chartbook 2015: Trends Affecting Hospitals and Health Systems. 2015; http://www.aha.org/research/reports/tw/chartbook/ch4.shtml. Accessed May 19, 2016.
- 71.Pratt WR. What does free cash flow tell us about hospital efficiency? A stochastic frontier analysis of cost inefficiency in California hospitals. J Health Care Finance. 2010;37(1):35–44. [PubMed] [Google Scholar]
- 72.Marschall J, Mermel LA, Classen D, et al. Strategies to Prevent Central Line-Associated Bloodstream Infections in Acute Care Hospitals Infection Control and Hospital Epidemiology, A Compendium of Strategies to Prevent Healthcare‐Associated Infections in Acute Care Hospitals. Vol 29, No. S1: The University of Chicago Press on behalf of The Society for Healthcare Epidemiology of America; October 2008:S22–S30. [DOI] [PubMed] [Google Scholar]
- 73.Pratt RJ, Pellowe CM, Wilson JA, et al. epic2: National evidence-based guidelines for preventing healthcare-associated infections in NHS hospitals in England. The Journal of hospital infection. 2007;65 Suppl 1:S1–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.O’Grady NP, Alexander M, Dellinger EP, et al. Guidelines for the prevention of intravascular catheter-related infections. Infection control and hospital epidemiology : the official journal of the Society of Hospital Epidemiologists of America. 2002;23(12):759–769. [DOI] [PubMed] [Google Scholar]
- 75.Pronovost PJ, Berenholtz SM, Goeschel C, et al. Improving patient safety in intensive care units in Michigan. Journal of critical care. 2008;23(2):207–221. [DOI] [PubMed] [Google Scholar]
- 76.Pronovost PJ, Berenholtz SM, Needham DM. Translating evidence into practice: a model for large scale knowledge translation. BMJ (Clinical research ed.). 2008;337:a1714. [DOI] [PubMed] [Google Scholar]
- 77.Miller MR, Griswold M, Harris JM 2nd, et al. Decreasing PICU catheter-associated bloodstream infections: NACHRI’s quality transformation efforts. Pediatrics. 2010;125(2):206–213. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


