Abstract
Background:
Rates of depression increase and peak during late adolescence and alterations in immune processes are thought to be both a risk factor and outcome of depression. However, few studies have examined depression-immune dynamics among adolescents. Using a functional genomics approach, the current study examined whether depressive symptoms were associated with activation of a gene expression profile, characterized by upregulated expression of pro-inflammatory-related genes and downregulated expression of antiviral-related genes in a sample of older adolescents (Mage = 18.37, SD = .51).
Method:
Participants (n = 87) reported on their depressive symptoms during the past week using the CES-D, and provided blood samples for genome-wide transcriptional profiling of mRNA.
Results:
Adolescents with clinically-significant levels of depressive symptoms (CES-D ≥ 16) exhibited upregulated expression of inflammation-related genes and downregulated expression of antiviral-related genes compared to their peers with lower levels of depressive symptoms (CES-D < 16). Bioinformatic analyses suggested that this pattern of differential gene expression was mediated by greater activity of the pro-inflammatory transcription factor, nuclear factor-kappa B (NF-κB), and reduced activity of glucocorticoid receptors (GRs) and interferon response factors (IRFs). Additional analyses implicated monocytes, B cells, and dendritic cells as primary cellular sources of the observed gene expression patterns associated with depressive symptoms.
Conclusion:
Results are consistent with past work demonstrating links between depression and altered immunity. They provide a molecular basis for these associations and suggest that the underlying molecular signature may emerge as early as late adolescence when rates of depression tend to increase.
Depression is the leading cause of disability worldwide and is one of the most common health disorders, affecting more than 300 million individuals (World Health Organization, 2017). This is especially concerning because in addition to compromising functioning in psychosocial and behavioral domains, depression is associated with serious health consequences, including cardiovascular disease (Bortolato et al., 2017; Moussavi et al., 2007; B. W. Penninx, 2017). Consequently, significant efforts to understand the etiology of depression and the pathways by which depression confers risk for disease have been made. Alterations in the immune system, particularly in aspects of inflammatory and antiviral processes, have been identified as both a critical precursor to depression and a consequence that increases vulnerability to disease. However, the majority of past work has focused on adults despite the fact that depression increases during adolescence, and underlying signaling pathways remain unclear. Therefore, the purpose of the present investigation was to examine the relation between depressive symptoms and transcriptional control pathways relevant to inflammatory and antiviral processes in a sample of older adolescents.
A substantial body of work has linked elevated levels of depressed mood to higher levels of inflammation, as summarized in several meta-analyses (Dowlati et al., 2010; Haapakoski, Mathieu, Ebmeier, Alenius, & Kivimäki, 2015; Howren, Lamkin, & Suls, 2009; Liu, Ho, & Mak, 2012). Prospective studies, in particular, have shown that depression subsequently predicts heightened levels of inflammation, and vice versa--that initially higher levels of inflammation subsequently predict depressed mood (Bell et al., 2017, Deverts et al., 2010, Huang et al., 2019, Niles et al., 2018, Stewart et al., 2009, Valkanova et al., 2013, Zalli et al., 2016). There is ample evidence for a reciprocal relation between depression and inflammation, though not all studies have observed these links (Herder et al., 2018, Jones et al., 2015), and the depression-inflammation link may be apparent only in a subgroup of individuals (Danese et al., 2008, Glassman and Miller, 2007).
Depression has also been linked to alterations in antiviral processes. For example, in the National Health and Nutrition Examination Survey, elevated levels of depression were associated with greater risk for herpes simplex virus type 2 (Gale, Berrett, Erickson, Brown, & Hedges, 2018). Lifetime prevalence of major depressive disorder has also been linked to greater risk for hepatitis C infection (Carta et al., 2007), and among patients with hepatitis C viral infections, those with more depressive symptoms are less likely to clear the virus in response to treatment (Raison et al., 2005). Other studies have similarly shown that depressed adults compared to non-depressed adults have compromised immune responses to the vaccination for the varicella-zoster virus (Irwin et al., 2013), as well as to the virus itself (Irwin et al., 1998; Irwin et al., 2011). Other viral infections that depression has been associated with include Borna disease virus, herpes simplex virus type 1, Epstein-Barr virus, cytomegalovirus, and chlamydophila trachomatis (Haeri et al., 2011; Miller, Freedland, Duntley, & Carney, 2005; Wang et al., 2014).
Curiously, much of the work on depression and immune functioning has focused on adults. However, it may be crucial to focus on the adolescent period given that depression tends to emerge during this developmental stage. Rates of depression increase dramatically from childhood to adolescence and into young adulthood (Avenevoli et al., 2015, Mojtabai et al., 2016). Up to 3.1 million or 12.8% of adolescents in the US met DSM-IV criteria for clinical depression in 2016 (Substance Abuse and Mental Health Services Administration, 2016, Mojtabai et al., 2016), and some studies suggest that nearly a third of adolescents exhibit subclinical levels of depressive symptomatology (Balázs et al., 2013). Importantly, rates of depression have been rising more rapidly in youth than in adults (Weinberger et al., 2018), suggesting that an increasing number of adolescents may go on to develop depression-related disabilities. Although rates of depression have long been known to increase during adolescence, only relatively recently have immune-depression relations during earlier stages of development been examined.
These studies show that clinical depressive disorders and subclinical symptoms of depression are cross-sectionally associated with higher levels of inflammation in youth (Brambilla, Monteleone, & Maj, 2004; Gabbay et al., 2009; Oddy et al., 2018; Pallavi et al., 2015), though not all cross-sectional studies have observed this association (Caserta, Wyman, Wang, Moynihan, & O’Connor, 2011; Chaiton, O’Loughlin, Karp, & Lambert, 2010). Three prospective studies have also linked depression to higher subsequent levels of C-reactive protein (CRP) in children, adolescents, and young adults (Copeland, Shanahan, Worthman, Angold, & Costello, 2012; Duivis et al., 2015; Elovainio et al., 2006), suggesting that depression may precede elevated inflammation. Other prospective studies of youth have also predicted later depression from previous levels of heightened inflammatory markers (Khandaker et al., 2014, Khandaker et al., 2018, Miller and Cole, 2012), supporting the notion that inflammation contributes to the development of depression (although one study showed that CRP did not prospectively predict depression (Copeland et al., 2012)). It may be that depression and heightened inflammation may cluster together only for certain adolescents, such as those with a history of early adversity, excess adiposity, or low parental support (Beach et al., 2017; J. J. Chiang, Bower, Irwin, Taylor, & Fuligni, 2017; Guan et al., 2016; Miller & Cole, 2012), or for certain subtypes of depression, as has been shown in adults (Kaestner et al., 2005; Lamers et al., 2013). Links between depression and antiviral processes have been much less studied among youth. Nevertheless, similar to results from adult studies, one recent study of 11 to 17-year-old adolescents showed that depressive symptoms were associated with reactivation of the Epstein-Barr virus among female adolescents positive for the virus (Ford and Stowe, 2017). Together, these studies start to provide initial evidence that links between depression and the immune system can become apparent by the second decade of life. However, given the relatively few studies on youth, the molecular bases of these associations are not entirely clear.
One molecular pathway underlying the relation between depression and alterations in inflammatory-related and antiviral-related outcomes may involve activation of a conserved transcriptional response to adversity (CTRA). The CTRA refers to a transcriptomic pattern characterized by upregulation of pro-inflammatory gene expression and downregulation of antiviral gene expression, due in part by increased signaling by nuclear factor-kappa B (NF-κB) and decreased signaling by glucocorticoid receptors (GRs) and interferon response factors (IRFs) (Cole, 2014; Irwin & Cole, 2011; Slavich & Cole, 2013). NF-κB and GRs are critical players of the inflammatory response: whereas increased NF-κB activity can upregulate production of pro-inflammatory cytokines, cortisol bound to GRs in immune cells can inhibit the production of pro-inflammatory cytokines and terminate the inflammatory response. With respect to antiviral processes, IRFs are critically involved in the transcription of interferons, which contributes to host resistance to viral pathogens.
Whether depression is associated with the CTRA pattern during adolescence has been rarely examined, but preliminary evidence points to the possibility that the two may be linked. For instance, in one relatively recent study, adolescents with major depressive disorder compared to healthy controls exhibited greater NF-κB activation in response to cell stimulation (Miklowitz et al., 2016). This study did not examine GR sensitivity, but previous studies of adults have shown that depressed adults, compared to non-depressed adults, exhibit altered GR function, including diminished GR sensitivity (Carvalho et al., 2014; Pace, Hu, & Miller, 2007; Pariante & Miller, 2001; Rohleder, Wolf, & Wolf, 2010). Adult studies have also shown that under various circumstances related to depression, including social isolation, socioeconomic disadvantage, bereavement, and early deprivation, circulating leukocytes exhibit CTRA activation (Cole, 2014; Slavich & Cole, 2013).
The purpose of the current study was to extend these prior findings and determine whether depression relates to the CTRA pattern during adolescence. We examined this in a sub-study of adolescents from a three-wave longitudinal study aimed at understanding the psychosocial contributions to early health risk. Previous analyses of these data showed that greater levels of depressive symptoms were associated with greater circulating inflammatory responses to stress among adolescents with greater adiposity (Chiang et al., 2017) and with higher levels of CRP among those with low parental support (Guan et al., 2016). Here, we take a more in-depth look at the relation between depressive symptoms and immune transcriptional profiles to help elucidate potential underlying molecular signaling pathways of previously reported links between depression and immune alterations. We first identified a set of genes that showed an empirical association with high vs. low levels of depressed mood, and then used bioinformatic analyses of transcription factor activity to infer whether these distinct gene expression profiles were mediated by differences in the activity of transcription factors specifically involved in inflammation (NF-κB, GRs) and/or Type I interferon antiviral responses (IRFs). Based on previous work reviewed above, we hypothesized that gene expression profiles from late adolescents with elevated depressed mood would exhibit a CTRA pattern, including indications of up-regulated NF-κB activity, and down-regulated GR and IRF activity. In additional follow-up analyses, we examined whether any observed patterns were mediated in part by increased output of immature “classical” monocytes from the bone marrow (Heidt et al., 2014; Powell et al., 2013).
Methods
Participants
As described previously (Chiang et al., 2017; Chiang et al., 2019), participants (n = 91; Mage = 18.37, SD = .51; 57% female) of the current study were from a three-wave longitudinal study aimed at understanding the psychosocial contributions to early health risk. In between the second and third wave of data collection, participants who were at least 18 years old and self-identified as Latino or European-American were invited to participate in an experimental study. Other ethnicities were excluded due to insufficient numbers in the larger study. Blood samples were unavailable for two participants, leaving a total of 89 participants with samples available for gene expression assays. Of these participants, two had missing data on covariates, leaving a final analytic sample of 87. Characteristics of the samples are presented in Table 1.
Table 1.
Sample characteristics.
| Variable | CESD < 16 n = 61 |
CESD ≥ 16 n = 26 |
|---|---|---|
| n (%) / Mean (SD) | n (%) / Mean (SD) | |
| Gender | ||
| Female | 35 (58.38%) | 13 (50.00%) |
| Male | 26 (42.62%) | 13 (50.00%) |
| Ethnicity | ||
| European-American | 20 (32.79%) | 10 (38.46%) |
| Latino | 41 (67.21%) | 16 (61.54%) |
| Parent education (mean (SD)) | 7.46 (0.26) | 7.27 (0.42) |
| Less than high school diploma | 8 (13.11%) | 5 (19.23%) |
| High school diploma | 5 (8.20%) | 2 (7.69%) |
| Trade or vocational school or some college, | 25 (40.98%) | 9 (34.62%) |
| College degree or higher | 23 (37.70%) | 10 (38.46%) |
| BMI (mean (SD)) | 25.34 (0.71) | 25.04 (1.35) |
| Smoking history (ever smoked more than 2 puffs) | 15 (24.59%) | 9 (34.62%) |
| Alcohol history (ever had more than a few sips) | 35 (57.38%) | 16 (61.54%) |
Note. Categories for parent education were 1=some elementary school, 2 =completed elementary school, 3=some junior high school, 4=completed junior high school, 5=some high school, 6=graduated high school, 7=trade or vocational school, 8=some college, 9=graduated from college, 10=some medical, law, or graduate school, and 11 = graduated from medical, law, or graduate school. Education was averaged across parents. CESD = Center for Epidemiologic Studies Depression Scale
Procedures
Eligible individuals were contacted via telephone, and those who responded were given information about the experimental study. Individuals expressing interest were scheduled for a laboratory visit and instructed to refrain from eating or drinking anything (except water) the hour prior to the visit. During the laboratory visit, a nurse measured height and weight using an electronic scale and stadiometer, assessed vital signs, and inserted an indwelling intravenous catheter in the antecubital vein of the non-dominant arm. Participants then viewed a neutral-content video for 20 minutes to facilitate acclimation to the testing environment. After this baseline period, blood samples were collected into CPT tubes for the assessment of RNA. Participants then completed a standardized laboratory-based stress task and provided additional blood and saliva samples, which are not part of the current study. They also completed a set of psychosocial questionnaires. All study procedures were approved by the UCLA Institutional Review Board and all study participants provided written consent.
Measures
Depressive symptoms.
During the lab visit, participants reported their depressive symptoms during the past week using the 20-item Center for Epidemiologic Studies Depression Scale (CES-D; Radloff, 1977). On a 4-point scale (0 = rarely or none of the time, 3 = most or all of the time), participants indicated how often they experienced cognitive, affective, and somatic symptoms of depression. Example items include “You were bothered by things that usually don’t bother you,” and “You talked less than usual.” CESD scores of 16 or higher suggest clinical depression, and thus, participants were categorized as likely to have clinical depressed mood (CES-D ≥ 16) and unlikely to have clinical depressed mood (CES-D < 16). The CES-D has demonstrated excellent reliability and validity (Radloff 1977) and in the current sample, internal reliability was good (α = .90).
Gene expression profiling.
Baseline blood samples for RNA were collected during the laboratory visit. Within two hours of sample collection, peripheral blood mononuclear cells (PBMCs) were separated from red blood cells, lysed in RNA-stabilization buffer (RLT, Qiagen Inc.), and stored at −80°C. When all samples were available, total RNA was extracted from PBMC (Qiagen RNeasy) and checked for suitable mass (> 200 ng by NanoDrop ND1000 spectrophotometry) and integrity (RNA integrity number > 3 by Agilent TapeStation capillary electrophoresis). Samples were assayed by RNA sequencing (RNAseq) in the UCLA Neuroscience Genomics Core Laboratory using Lexogen QuantSeq 3’ FWD cDNA library synthesis and multiplex DNA sequencing on an Illumina HiSeq 4000 instrument with single strand 65 nt sequence reads (all following the manufacturer’s standard protocol). Samples yielded >10 million sequence reads, each of which was mapped to the RefSeq human genome sequence using the STAR aligner (Dobin et al., 2013) to generate transcript counts per million total transcripts (TPM). No additional RNAseq read-level QC or sample normalization was required because STAR accounts for variations in read quality in the mapping and transcript quantification process, and TPM normalization controls for variations in library size/sequencing depth. To ensure that this specific form of normalization did not influence substantive results, we repeated analyses with other sample normalizations (e.g., median ratio standardization, and reference gene standardization (Eisenberg & Levanon, 2013) and found similar substantive results.
Covariates.
Sociodemographic characteristics (i.e., gender, ethnicity, parental education), body mass index (BMI), and health behaviors (smoking, alcohol consumption) were included in models as potential confounding factors. Gender was based on participant self-reports, and ethnicity was based on both participant self-reports and parent reports of parents’ own birth countries as well as the birth countries of participants’ grandparents. Family SES was also based on parent reports. Specifically, on an 11-point scale (1 = some elementary school, 11 = graduated from medical, law, or graduate school), primary caregivers reported the highest level of education they and their spouses completed. Responses were then averaged across both parents. BMI was computed from height and weight assessments collected during the laboratory visit, and information on history of smoking (ever taken more than two cigarette puffs) and of alcohol consumption (ever had more than a few sips of alcohol) was collected during Wave 2 of the parent study.
Data analysis
Analyses first involved identifying a set of genes that showed an empirical association with depressive symptoms. In the next stage of analyses, we tested whether any observed empirical transcriptome difference might be attributable to differential activity of inflammatory and interferon-related transcription factors. For empirical transcriptome mapping, TPM values were floored at 1 and log2-transformed for analysis by standard linear statistical models relating transcript abundance to depressive symptom level (CESD ≥ vs. < 16) while controlling for gender, ethnicity, body mass index (kg/m2), smoking history, heavy alcohol consumption history, and parental educational attainment. Genes with minimal level or variation in expression (mean or SD < .5 log2 expression units) were excluded. Genes showing a point estimate of > 1.5-fold difference in average transcript abundance in participants with high vs. low levels of depressive symptoms served as input into the second-stage analysis of transcription factor activity (see below). Individual genes were not tested for statistically significant difference in expression because the goal of this study was solely to assess transcription factor activity. For this application point, estimate-based screening has been shown to provide more reliable results than gene screening based on p-values (Cole, Yan, Galic, Arevalo, & Zack, 2005).
The next phase of analyses involved transcription factor bioinformatics using the TELiS promoter sequence database (Cole et al., 2005) to test three a priori-specified hypotheses regarding activity of transcription control pathways relevant to CTRA biology: 1) the positive CTRA component of inflammation (as indicated by over-representation of NF-κB binding sites in promoters of upregulated genes relative to down-regulated genes, as indicated by the TRANSFAC position-specific weight matrix V$NFKAPPAB_01), 2) the inverse CTRA component of innate antiviral responses (IRFs, as indicated by under-representation of V$ISRE_01 in up-regulated vs. down-regulated genes), and 3) GR desensitization (as indicated by under-representation of the V$GR_Q6 promoter motif). TELiS analyses were conducted using 9 different parametric combinations of core promoter DNA sequence length (−300, −600, and −1000 to +200 nucleotides surrounding the RefSeq-designated transcription start site) and transcription factor-binding motif (TFBM) detection stringency (TRANSFAC mat_sim values of .80, .90, and .95) (Cole et al., 2005). Log2-transformed TFBM ratios (comparing prevalence in promoters of up- vs. down-regulated genes) were averaged across the 9 parametric combinations and tested for statistical significance using standard errors derived from bootstrap resampling of linear model residual vectors (controlling for potential correlation across genes).
Lastly, to identify the specific subtypes of PBMCs that may mediate any observed differences in gene expression, secondary analyses of transcript cellular origin were conducted using the same set of up- and down-regulated genes as input into Transcript Origin Analysis as previously described (Cole, Hawkley, Arevalo, & Cacioppo, 2011), with reference cell type-specific gene expression profiles derived from publicly available data sets (Gene Expression Omnibus GSE1133 and GSE25913).
Results
Descriptive information is displayed in Table 1. Approximately 30% of participants showed clinically significant levels of depressive symptoms (CESD ≥ 16). There were no gender (x2(1) = .29, p = .587) or ethnic (x2(1) = .39, p = .535) differences in adolescents with clinically significant vs. non-clinically significant levels of depressive symptoms. Parent education (t(86) = .50, p = .640), BMI (t(86) = .44, p = .664), smoking behavior (x2(1) = 1.54, p = .215), and drinking behavior (x2(1) = .06, p = .806) also did not differ between these two groups.
Transcription Factor Activity
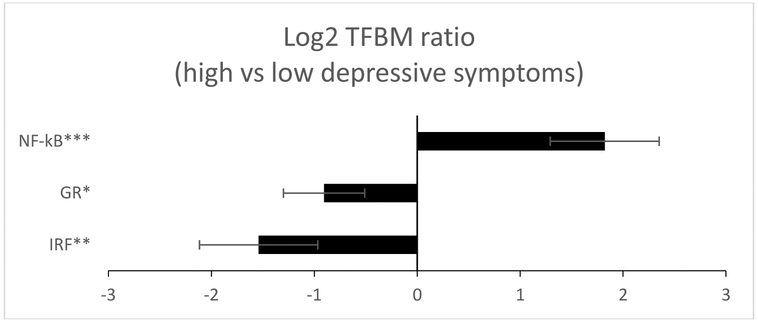
Analyses of PBMC transcriptomes identified 45 gene transcripts that differed in average expression level by > 1.5-fold in adolescents with low vs. clinically significant levels of depressive symptoms after controlling for participant gender, ethnicity, BMI, smoking history, alcohol history, and parental educational attainment (35 up-regulated and 10 down-regulated; listed in Supporting Information Table 1). Bioinformatic analysis of transcription factor-binding motifs (TFBMs) in the promoters of these genes linked clinically significant depressive symptoms to increased activity of NF-κB (mean log2-trasnformed TFBM ratio in up- vs down-regulated genes = 1.822 ± standard error .529, p = .001), reduced GR activity (−.905 ± .395, p = .023), and reduced activity of IRF family factors (−1.541 ± .575, p = .008), as depicted in Figure 1. Similar results emerged when CESD scores were analyzed as a continuous variable, with higher levels of depressive symptoms again associating with increased activity of NF-κB (.922 ± .426, p = .032), and reduced GR activity (−.869 ± .345, p = .013), although variation in IRF activity could no longer be quantified due to an insufficient number of genes bearing promoter sequences for this factor1.
Figure 1.
Bioinformatic analysis of pro-inflammatory and anti-viral transcription control pathways in PBMCs of adolescents with high vs. low levels of depressive symptoms. Data represent log2-transformed ratios of transcription factor-binding motifs (TFBMs) for pro-inflammatory (NF-κB, GR) and antiviral (IRF) transcription factors in the promoters of identified differentially expressed genes (> 1.5-fold greater difference in average transcript abundance in high vs. low levels of depressive symptoms).
*p < .05 **p < .01 ***p ≤ .001
Cellular Origin
Transcript Origin Analysis indicated that genes up-regulated in association with clinically significant depressive symptoms derived preferentially from monocytes in general (mean cell diagnosticity score = .678 ± standard error .211, p = .001), and from the subset of classical monocytes in particular (.561 ± .150, p < .001). We had no hypothesis about the cellular origin of down-regulated gene transcripts, but results of exploratory analyses of major PBMC subsets implicated B cells (1.257 ± .283, p = .001) and dendritic cells (.711 ± .164, p = .001), and analyses of monocyte subsets implicated non-classical monocytes in the derivation of down-regulated gene transcripts (.912 ± .197, p < .001).
Discussion
In a sample of older adolescents from Latino and European-American backgrounds, clinically significant levels of depressive symptoms were tied to expression of the CTRA pattern. Specifically, elevated levels of depressive symptoms were associated with increased expression of genes bearing response elements for NF-κB, and reduced expression of genes bearing response elements for GRs and IRFs. Notably, this pattern of results was independent of demographic and behavioral risk factors known to influence immune processes (gender, ethnicity, parental education, BMI, smoking, alcohol consumption).
Results of the present investigation converge with previous studies linking depression to heightened protein levels of inflammatory markers and altered antiviral responses in adults (e.g., Howren et al., 2009; Wang et al., 2014). They are also consistent with previous gene expression studies showing upregulation of inflammatory-related genes in post-mortem brain tissue of depressed adults relative to healthy controls (Sharma, 2016, Shelton et al., 2011), although another study showed that NF-κB and GR control pathways of differentially expressed genes did not differ between individuals with major depressive disorders and healthy controls (Mellon et al., 2016). Nonetheless, a broader body of work on adults has demonstrated that chronic stress (a construct related to depression) is associated with the CTRA pattern, including increased pro-inflammatory gene expression as well as reduced expression of genes involved in Type I interferon innate antiviral responses, and reduced expression of GR target genes that may be secondary to functional desensitization of the GR protein (Cole, 2014; Slavich & Cole, 2013). Results from the current investigation extend these previous findings to depressed mood in otherwise healthy late adolescents.
To our knowledge, this is one of the first studies connecting elevated depressed mood to divergent immune gene expression profiles during late adolescence. The focus on older adolescents in this study is particularly noteworthy given that depression risk tends to increase during adolescence, peaks during the transition into adulthood, and can have consequences for future mental and physical health in adulthood. Yet, relatively few studies have examined depression-immune dynamics, particularly as they relate to antiviral processes, in young individuals. Furthermore, extant studies focusing on inflammatory processes in particular have yielded inconsistent findings, with some studies linking depressive symptoms to elevated levels of CRP and others showing no associations (e.g., Chaiton et al., 2010; Copeland et al., 2012). Mixed findings may in part be due to limitations of assessing markers of systemic inflammation in young individuals. Most past studies focus on circulating markers of systemic inflammation, but systemic inflammation generally remains low early in life, as the immune system remains relatively intact and may be more effective at terminating inflammatory responses once threats have been resolved (Miller & Chen, 2010). Moreover, circulating markers of inflammation can come from non-immune sources (e.g., adipose tissue), and thus potential effects of depressed mood on inflammatory signaling at the systemic level may be more difficult to detect among young individuals. Our findings point to the possibility that inflammatory dynamics at the genomic level may offer another means of investigating how depressed mood may alter inflammatory activity in young individuals.
Building on previous studies linking depressed mood to heightened systemic inflammation in adolescents, our findings highlight two potential molecular pathways underlying this association. First, we found that depressed mood was associated with increased expression of genes bearing response elements for the pro-inflammatory transcription factor NF-κB, suggesting that upstream pathways that act on NF-κB signaling may also play a role. One such pathway may have to do with imbalanced activity of the sympathetic and parasympathetic branches of the autonomic nervous system. Previous studies indicate that depression is associated with persistent or over-activation of the sympathetic nervous system (SNS) and sometimes reduced or under-activation of the parasympathetic nervous system (PNS) (Headrick et al., 2017; Koschke et al., 2009; B. W. J. H. Penninx, 2017; Schumann, Andrack, & Bär, 2017; Sgoifo, Carnevali, Pico Alfonso, & Amore, 2015). Importantly, SNS activity can increase NF-κB activity and inflammatory gene transcription (Cole et al., 2010; Grebe et al., 2010) whereas PNS activity can inhibit NF-kB activity and inflammatory responses (Martelli, McKinley, & McAllen, 2014; Tracey, 2009). Thus, a proinflammatory gene expression profile related to depressed mood may emerge via elevated SNS and reduced PNS activity.
Second, we found that clinically significant depressed mood was associated with reduced expression of genes bearing response elements for GRs, suggesting decreased glucocorticoid signal transduction and potential failure of the HPA axis to dampen pro-inflammatory processes. This finding converges with previous work in adults demonstrating that GR sensitivity is diminished in depressed individuals compared to non-depressed individuals (Carvalho et al., 2014; Pace et al., 2007; Pariante & Miller, 2001; Rohleder et al., 2010), and points to the possibility that this association may emerge as early as late adolescence. Decreased GR activity related to depressive symptoms may emerge from initially high levels of cortisol. Depression has been associated with heightened levels of cortisol (Stetler and Miller, 2011), which may subsequently contribute to the development of glucocorticoid resistance in immune cells, a state in which immune cells’ glucocorticoid receptors become desensitized to cortisol’s anti-inflammatory signals (Cohen et al., 2012, Miller et al., 2002). In previous analyses, however, we did not find evidence that depressive symptoms were linked to cortisol responses to acute stress in the current sample (Chiang et al., 2017). This may have to do with the fact that in our previous analyses, we focused on HPA reactivity to stress; it may also have to do with the temporal dynamics between depression, HPA activity, and GR sensitivity. Thus, further research is needed to clarify whether cortisol output is a primary pathway linking depressive symptoms to decreased GR sensitivity during late adolescence.
The current study further contributes to previous work linking depressed mood to youth’s immune systems by examining antiviral processes in addition to inflammatory processes. The present data showed that elevated depressed mood was associated with reduced expression of genes bearing response elements for IRFs. This finding is consistent with previous work showing that adults with greater depressive symptoms or clinical diagnosis of depression are more likely to be virally infected and have impaired immune responses to vaccines (e.g., Irwin et al., 2013; Gale et al., 2018; Wang et al., 2014). Recently, this pattern was shown in adolescents (Ford & Stowe, 2017), and the present findings provide a molecular basis for this association. It may be that elevated depressive symptoms act on IRF signaling pathways to decrease host resistance to viruses. That said, in one study, reductions of depressive symptoms after a mindfulness intervention was not associated with changes in expression of the antiviral CTRA subcomponent (Boyle et al., 2019). This discrepant finding with that of the current study may stem from several study differences—for instance, the previous study was based on a smaller sample size of 22 and on a group of breast cancer survivors. Clearly, replication of our findings is necessary.
Using transcript origin analysis, we identified the cellular origins of the observed pattern of upregulated and downregulated genes in relation to depressive symptoms. These analyses implicated monocytes in general, and the classical monocyte subset in particular, as primary sources of up-regulated genes associated with elevated depressive symptoms. This pattern converges with previous CTRA research linking chronic social threat to upregulation of myelopoiesis mediated by SNS activation (Cole et al., 2015; Powell et al., 2013). Increased production and/or activation of monocytes, then, may be one primary contributor to altered inflammatory dynamics related to depression. In exploratory analyses related to downregulated genes, B cells, dendritic cells, and non-classical monocytes were identified as primary sources of downregulated genes associated with elevated depressive symptoms. This suggests that increased production or activation of these particular leukocytes may mediate the observed pattern of depression-related downregulated genes; however, given the exploratory nature of these analyses, future research is needed to solidify these results.
The current study had several limitations that should be considered. First, the cross-sectional study design precludes drawing any conclusion about the causality and directionality of the relation between immune functioning and depressive symptoms. For instance, other factors associated with depressive symptoms or immune processes, like stress, may play an important role, and future work is needed to disentangle the role of depression from that of stress. Second, depressive symptoms were assessed via self-reports as opposed to more formal clinical diagnosis of depression, and thus there may have been misclassification of likelihood of clinical levels of depression. Third, the study’s sample size was limited and thus the current study was not powered for hypothesis-free discovery of statistically significant associations between individual gene transcripts and depressive symptoms. However, power analyses indicated that our sample size was sufficient to detect a moderate effect size of depressive symptoms on gene set-based bioinformatic assessments of CTRA-related transcription factor activity with statistical power of .80. Fourth, this study recruited a localized population of community-dwelling adolescents and the generalizability of these results to other socio-demographic contexts remains to be determined. Future research in larger samples will be required to replicate the current findings, evaluate their generalizability to other settings, and identify additional transcriptional correlates of depression beyond the CTRA-related transcriptional dynamics examined here.
Previous research has linked clinical and subclinical levels of depression to altered immune functioning, as evidenced by heightened circulating levels of systemic inflammatory markers (Dowlati et al., 2010; Howren et al., 2009; Liu et al., 2012) and compromised immunity to viral infections (e.g., Wang et al., 2014). Our findings provide a potential molecular basis of these associations. Importantly, these molecular alterations were observed in late adolescents of a relatively narrow age range that represents the developmental period when depression rates begin to peak. Thus, findings suggest that late adolescents with heightened levels of depressed mood may exhibit an altered immune phenotype characterized by upregulation of monocytes and NF-κB signaling and downregulation of GR and IRF signaling. To the extent that such a phenotype persists over time, older adolescents with elevated levels of depressive symptoms may be at greater risk for later immune-related somatic disease in adulthood. Given the causal role that inflammatory cytokines are thought to play in depression, the inflammatory subcomponent of the CTRA related to depressive symptoms observed here may also be implicated in recurring depressive episodes in adulthood, thus perpetuating a cycle between depression and inflammation. Accordingly, the present investigation further justifies the increasing attention towards the importance of adolescence as a sensitive developmental period for laying the foundations of both physical and mental health (Fuhrmann, Knoll, & Blakemore, 2015; Viner et al., 2012).
Supplementary Material
Highlights.
Depressed mood was linked to upregulated expression of inflammation-related genes.
Depressed mood was linked to downregulated expression of antiviral-related genes.
This pattern was mediated by greater NF-κB activity and reduced GR and IRF activity.
Cellular sources of this pattern included monocytes, B cells, and dendritic cells.
Acknowledgements
Preparation of this manuscript was supported by the National Heart, Lung, and Blood Institute (F32-HL134276 to J.J.C.). Research reported in this publication was supported jointly by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (R01-HD062547, R24-HD041022), National Institute on Aging (U24AG047867, P30-AG028748, P30-AG017265), UCLA Cousins Center for Psychoneuroimmunology, University of California Institute for Mexico and the US, American Psychological Association, and Division 38 of the American Psychological Association, the UK Economic and Social Research Council, and the Biotechnology and Biological Sciences Research Council (ES/M00919X/1).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest: None.
As previously reported, IL-6 was measured but we observed no link between continuous measures CESD scores and circulating levels of IL-6 (Chiang et al., 2017). Dichotomous CESD scores were also unrelated to circulating levels of IL-6 (b(SE) = −.16(.19), p = .191). We may not have observed links between CESD scores and protein levels of IL-6 because the expression of inflammation-related genes and upregulated NF-κB activity may represent a multitude of pro-inflammatory signals, and not just IL-6. Moreover, whereas immune cells were specifically probed in the current study, the cellular origins of circulating IL-6 cannot be determined as multiple tissues release IL-6.
References
- Substance Abuse and Mental Health Services Administration. (2016). 2015–2016 National Survey on Drug Use and Health: Model-based prevalence estimates (50 states and the District of Columbi). https://www.samhsa.gov/data/sites/default/files/NSDUHsaePercents2016/NSDUHsaePercents2016.pdf
- Avenevoli S, Swendsen J, He JP, Burstein M, & Merikangas KR (2015). Major depression in the National Comorbidity Survey–Adolescent Supplement: prevalence, correlates, and treatment. Journal of the American Academy of Child & Adolescent Psychiatry, 54(1), 37–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balázs J, Miklósi M, Keresztény Á, Hoven CW, Carli V, Wasserman C, … Cosman D (2013). Adolescent subthreshold- depression and anxiety: Psychopathology, functional impairment and increased suicide risk. Journal of Child Psychology and Psychiatry, 54(6), 670–677. [DOI] [PubMed] [Google Scholar]
- Beach SRH, Lei MK, Simons RL, Barr AB, Simons LG, Ehrlich KB, … Philibert RA (2017). When inflammation and depression go together: The longitudinal effects of parent–child relationships. Development and Psychopathology, 29(5), 1969–1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell JA, Kivimäki M, Bullmore ET, Steptoe A, Bullmore E, Vértes PE, … Freeman T (2017). Repeated exposure to systemic inflammation and risk of new depressive symptoms among older adults. Translational Psychiatry, 7(8), e1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bortolato B, Hyphantis TN, Valpione S, Perini G, Maes M, Morris G, … Stubbs B (2017). Depression in cancer: the many biobehavioral pathways driving tumor progression. Cancer Treatment Reviews, 52, 58–70. [DOI] [PubMed] [Google Scholar]
- Boyle CC, Cole SW, Dutcher JM, Eisenberger NI, & Bower JE (2019). Changes in eudaimonic well-being and the conserved transcriptional response to adversity in younger breast cancer survivors. Psychoneuroendocrinology, 103, 173–179. [DOI] [PubMed] [Google Scholar]
- Brambilla F, Monteleone P, & Maj M (2004). Interleukin-1β and tumor necrosis factor-α in children with major depressive disorder or dysthymia. Journal of Affective Disorders, 78(3), 273–277. [DOI] [PubMed] [Google Scholar]
- Carta MG, Hardoy MC, Garofalo A, Pisano E, Nonnoi V, Intilla G, … Cauli C (2007). Association of chronic hepatitis C with major depressive disorders: irrespective of interferon-alpha therapy. Clinical Practice and Epidemiology in Mental Health, 3(22). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho LA, Bergink V, Sumaski L, Wijkhuijs J, Hoogendijk WJ, Birkenhager TK, & Drexhage HA (2014). Inflammatory activation is associated with a reduced glucocorticoid receptor alpha/beta expression ratio in monocytes of inpatients with melancholic major depressive disorder. Translational Psychiatry, 4(1), e344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caserta MT, Wyman PA, Wang H, Moynihan J, & O’Connor TG (2011). Associations among depression, perceived self-efficacy, and immune function and health in preadolescent children. Development and Psychopathology, 23(4), 1139–1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaiton M, O’Loughlin J, Karp I, & Lambert M (2010). Depressive symptoms and C-reactive protein are not associated in a population-based sample of adolescents. International Journal of Behavioral Medicine, 17(3), 216–222. [DOI] [PubMed] [Google Scholar]
- Chiang JJ, Bower JE, Irwin MR, Taylor SE, & Fuligni AJ (2017). Adiposity moderates links from early adversity and depressive symptoms to inflammatory reactivity to acute stress during late adolescence. Brain, Behavior, and Immunity, 66, 146–155. doi: 10.1016/j.bbi.2017.06.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang JJ, Ko A, Bower JE, Irwin ME, Taylor SE, & Fuligni AJ (2019). Stress, psychological resources, and HPA and inflammatory reactivity during late adolescence. Development and Psychopathology, 31(2), 699–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S, Janicki-Deverts D, Doyle WJ, Miller GE, Frank E, Rabin BS, & Turner RB (2012). Chronic stress, glucocorticoid receptor resistance, inflammation, and disease risk. Proceedings of the National Academy of Sciences, 109(16), 5995–5999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole SW (2014). Human social genomics. PLoS genetics, 10(8), e1004601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole SW, Arevalo JMG, Takahashi R, Sloan EK, Lutgendorf SK, Sood AK, … Seeman TE (2010). Computational identification of gene–social environment interaction at the human IL6 locus. Proceedings of the National Academy of Sciences, 107(12), 5681–5686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole SW, Capitanio JP, Chun K, Arevalo JMG, Ma J, & Cacioppo JT (2015). Myeloid differentiation architecture of leukocyte transcriptome dynamics in perceived social isolation. Proceedings of the National Academy of Sciences, 112(49), 15142–15147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole SW, Hawkley LC, Arevalo JMG, & Cacioppo JT (2011). Transcript origin analysis identifies antigen-presenting cells as primary targets of socially regulated gene expression in leukocytes. Proceedings of the National Academy of Sciences, 108(7), 3080–3085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole SW, Yan W, Galic Z, Arevalo J, & Zack JA (2005). Expression-based monitoring of transcription factor activity: the TELiS database. Bioinformatics, 21(6), 803–810. [DOI] [PubMed] [Google Scholar]
- Copeland WE, Shanahan L, Worthman C, Angold A, & Costello EJ (2012). Cumulative depression episodes predict later C-reactive protein levels: a prospective analysis. Biological Psychiatry, 71(1), 15–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danese A, Moffitt TE, Pariante CM, Ambler A, Poulton R, & Caspi A (2008). Elevated inflammation levels in depressed adults with a history of childhood maltreatment. Archives of General Psychiatry, 65(4), 409–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deverts DJ, Cohen S, DiLillo VG, Lewis CE, Kiefe C, Whooley M, & Matthews KA (2010). Depressive symptoms, race, and circulating C-reactive protein: the Coronary Artery Risk Development in Young Adults (CARDIA) study. Psychosomatic Medicine, 72(8), 734–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S, … Gingeras TR (2013). STAR: ultrafast universal RNA-seq aligner. Bioinformatics, 29(1), 15–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowlati Y, Herrmann N, Swardfager W, Liu H, Sham L, Reim EK, & Lanctôt KL (2010). A meta-analysis of cytokines in major depression. Biological Psychiatry, 67(5), 446–457. [DOI] [PubMed] [Google Scholar]
- Duivis HE, Kupper N, Vermunt JK, Penninx BW, Bosch NM, Riese H, … de Jonge P (2015). Depression trajectories, inflammation, and lifestyle factors in adolescence: The TRacking Adolescents’ Individual Lives Survey. Health Psychology, 34(11), 1047–1057. doi: 10.1037/hea0000210 [DOI] [PubMed] [Google Scholar]
- Eisenberg E, & Levanon EY (2013). Human housekeeping genes, revisited. Trends in Genetics, 29(10), 569–574. [DOI] [PubMed] [Google Scholar]
- Elovainio M, Keltikangas-Järvinen L, Pulkki-Råback L, Kivimäki M, Puttonen S, Viikari L, … Raitakari OT (2006). Depressive symptoms and C-reactive protein: the Cardiovascular Risk in Young Finns Study. Psychological Medicine, 36(6), 797–805. [DOI] [PubMed] [Google Scholar]
- Ford JL, & Stowe RP (2017). Depressive symptoms are associated with salivary shedding of Epstein-Barr virus in female adolescents: The role of sex differences. Psychoneuroendocrinology, 86, 128–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuhrmann D, Knoll LJ, & Blakemore SJ (2015). Adolescence as a sensitive period of brain development. Trends in Cognitive Sciences, 19(10), 558–566. [DOI] [PubMed] [Google Scholar]
- Gabbay V, Klein RG, Alonso CM, Babb JS, Nishawala M, De Jesus G, … Gonzalez CJ (2009). Immune system dysregulation in adolescent major depressive disorder. Journal of Affective Disorders, 115(1), 177–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale SD, Berrett AN, Erickson LD, Brown BL, & Hedges DW (2018). Association between virus exposure and depression in US adults. Psychiatry Research, 261, 73–79. [DOI] [PubMed] [Google Scholar]
- Glassman AH, & Miller GE (2007). Where there is depression, there is inflammation… sometimes! Biological Psychiatry. [DOI] [PubMed] [Google Scholar]
- Grebe KM, Takeda K, Hickman HD, Bailey AM, Embry AC, Bennink JR, & Yewdell JW (2010). Cutting edge: Sympathetic nervous system increases proinflammatory cytokines and exacerbates influenza A virus pathogenesis. The Journal of Immunology, 184(2), 540–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan SSA, Bower JE, Almeida DM, Cole SW, Dahl RE, Irwin MR, … Fuligni AJ (2016). Parental support buffers the association of depressive symptoms with cortisol and C-reactive protein during adolescence. Brain, Behavior, and Immunity, 57, 134–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haapakoski R, Mathieu J, Ebmeier KP, Alenius H, & Kivimäki M (2015). Cumulative meta-analysis of interleukins 6 and 1β, tumour necrosis factor α and C-reactive protein in patients with major depressive disorder. Brain, Behavior, and Immunity, 49, 206–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haeri S, Johnson N, Baker AM, Stuebe AM, Raines C, Barrow DA, & Boggess KA (2011). Maternal depression and Epstein-Barr virus reactivation in early pregnancy. Obstetrics & Gynecology, 117(4), 862–866. [DOI] [PubMed] [Google Scholar]
- Headrick JP, Peart JN, Budiono BP, Shum DHK, Neumann DL, & Stapelberg NJC (2017). The heartbreak of depression:’Psycho-cardiac’coupling in myocardial infarction. Journal of Molecular and Cellular Cardiology, 106, 14–28. [DOI] [PubMed] [Google Scholar]
- Heidt T, Sager HB, Courties G, Dutta P, Iwamoto Y, Zaltsman A, … Denninger J (2014). Chronic variable stress activates hematopoietic stem cells. Nature Medicine, 20(7), 754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herder C, Schmitt A, Budden F, Reimer A, Kulzer B, Roden M, … Hermanns N (2018). Longitudinal associations between biomarkers of inflammation and changes in depressive symptoms in patients with type 1 and type 2 diabetes. Psychoneuroendocrinology, 91, 216–225. [DOI] [PubMed] [Google Scholar]
- Howren MB, Lamkin DM, & Suls J (2009). Associations of depression with C-reactive protein, IL-1, and IL-6: A meta-analysis. Psychosomatic Medicine, 71(2), 171–186. [DOI] [PubMed] [Google Scholar]
- Huang M, Su S, Goldberg J, Miller AH, Levantsevych OM, Shallenberger L, … Vaccarino V (2019). Longitudinal association of inflammation with depressive symptoms: A 7-year cross-lagged twin difference study. Brain, Behavior, & Immunity, 75, 200–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin MR, & Cole SW (2011). Reciprocal regulation of the neural and innate immune systems. Nature Reviews Immunology, 11(9), 625–632. doi: 10.1038/nri3042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin MR, Costlow C, Williams H, Artin KH, Chan CY, Stinson DL, … Oxman MNJ (1998). Cellular immunity to varicella-zoster virus in patients with major depression. The Journal of Infectious Diseases, 178(Supplement_1), S104–S108. [DOI] [PubMed] [Google Scholar]
- Irwin MR, Levin MJ, Carrillo C, Olmstead R, Lucko A, Lang N, … Clair J (2011). Major depressive disorder and immunity to varicella-zoster virus in the elderly. Brain, Behavior, and Immunity, 25(4), 759–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin MR, Levin MJ, Laudenslager ML, Olmstead R, Lucko A, Lang N, … Weinberg A (2013). Varicella zoster virus–specific immune responses to a herpes zoster vaccine in elderly recipients with major depression and the impact of antidepressant medications. Clinical Infectious Diseases, 56(8), 1085–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones SMW, Weitlauf J, Danhauer SC, Qi L, Zaslavsky O, Wassertheil-Smoller S, … LaCroix AZ (2015). Prospective data from the Women’s Health Initiative on depressive symptoms, stress, and inflammation. Journal of Health Psychology, 22(4), 457–464. [DOI] [PubMed] [Google Scholar]
- Kaestner F, Hettich M, Peters M, Sibrowski W, Hetzel G, Ponath G, … Rothermundt M (2005). Different activation patterns of proinflammatory cytokines in melancholic and non-melancholic major depression are associated with HPA axis activity. Journal of Affective Disorders, 87(2), 305–311. [DOI] [PubMed] [Google Scholar]
- Khandaker GM, Pearson RM, Zammit S, Lewis G, & Jones PB (2014). Association of serum interleukin 6 and C-reactive protein in childhood with depression and psychosis in young adult life: a population-based longitudinal study. JAMA Psychiatry, 71(10), 1121–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khandaker GM, Stochl J, Zammit S, Goodyer I, Lewis G, & Jones PB (2018). Childhood inflammatory markers and intelligence as predictors of subsequent persistent depressive symptoms: a longitudinal cohort study. Psychological Medicine, 48(9), 1514–1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koschke M, Boettger MK, Schulz S, Berger S, Terhaar J, Voss A, … Bär KJ (2009). Autonomy of autonomic dysfunction in major depression. Psychosomatic Medicine, 71(8), 852–860. [DOI] [PubMed] [Google Scholar]
- Lamers F, Vogelzangs N, Merikangas KR, De Jonge P, Beekman ATF, & Penninx BWJH (2013). Evidence for a differential role of HPA-axis function, inflammation and metabolic syndrome in melancholic versus atypical depression. Molecular Psychiatry, 18(6), 692. [DOI] [PubMed] [Google Scholar]
- Liu Y, Ho RCM, & Mak A (2012). Interleukin (IL)-6, tumour necrosis factor alpha (TNF-α) and soluble interleukin-2 receptors (sIL-2R) are elevated in patients with major depressive disorder: a meta-analysis and meta-regression. Journal of Affective Disorders, 139(3), 230–239. [DOI] [PubMed] [Google Scholar]
- Martelli D, McKinley MJ, & McAllen RM (2014). The cholinergic anti-inflammatory pathway: a critical review. Autonomic Neuroscience, 182, 65–69. [DOI] [PubMed] [Google Scholar]
- Mellon SH, Wolkowitz OM, Schonemann MD, Epel ES, Rosser R, Burke HB, … Liew CC (2016). Alterations in leukocyte transcriptional control pathway activity associated with major depressive disorder and antidepressant treatment. Translational Psychiatry, 6(5), e821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miklowitz DJ, Portnoff LC, Armstrong CC, Keenan-Miller D, Breen EC, Muscatell KA, … Irwin MR (2016). Inflammatory cytokines and nuclear factor-kappa B activation in adolescents with bipolar and major depressive disorders. Psychiatry Research, 241, 315–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, & Chen E (2010). Harsh family climate in early life presages the emergence of a proinflammatory phenotype in adolescence. Psychological Science, 21(6), 848–856. doi: 10.1177/0956797610370161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Cohen S, & Ritchey AK (2002). Chronic psychological stress and the regulation of pro-inflammatory cytokines: a glucocorticoid-resistance model. Health Psychology, 21(6), 531–541. [DOI] [PubMed] [Google Scholar]
- Miller GE, & Cole SW (2012). Clustering of depression and inflammation in adolescents previously exposed to childhood adversity. Biological Psychiatry, 72(1), 34–40. doi: 10.1016/j.biopsych.2012.02.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Freedland KE, Duntley S, & Carney RM (2005). Relation of depressive symptoms to C-reactive protein and pathogen burden (cytomegalovirus, herpes simplex virus, Epstein-Barr virus) in patients with earlier acute coronary syndromes. The American Journal of Cardiology, 95(3), 317–321. [DOI] [PubMed] [Google Scholar]
- Mojtabai R, Olfson M, & Han B (2016). National trends in the prevalence and treatment of depression in adolescents and young adults. Pediatrics, e20161878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moussavi S, Chatterji S, Verdes E, Tandon A, Patel V, & Ustun B (2007). Depression, chronic diseases, and decrements in health: results from the World Health Surveys. The Lancet, 370(9590), 851–858. [DOI] [PubMed] [Google Scholar]
- Niles AN, Smirnova M, Lin J, & O’Donovan A (2018). Gender Differences in Longitudinal Relationships between Depression and Anxiety Symptoms and Inflammation in the Health and Retirement Study. Psychoneuroendocrinology, 95, 149–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oddy WH, Allen KL, Trapp GSA, Ambrosini GL, Black LJ, Huang RC, … Beilin L (2018). Dietary patterns, body mass index and inflammation: Pathways to depression and mental health problems in adolescents. Brain, Behavior, & Immunity, 69, 428–439. [DOI] [PubMed] [Google Scholar]
- Organization, W. H. (2017). Depression and other common mental disorders: global health estimates.
- Pace TWW, Hu F, & Miller AH (2007). Cytokine-effects on glucocorticoid receptor function: relevance to glucocorticoid resistance and the pathophysiology and treatment of major depression. Brain, Behavior, and Immunity, 21(1), 9–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallavi P, Sagar R, Mehta M, Sharma S, Subramanium A, Shamshi F, … Mukhopadhyay AK (2015). Serum cytokines and anxiety in adolescent depression patients: Gender effect. Psychiatry Research, 229(1–2), 374–380. [DOI] [PubMed] [Google Scholar]
- Pariante CM, & Miller AH (2001). Glucocorticoid receptors in major depression: relevance to pathophysiology and treatment. Biological Psychiatry, 49(5), 391–404. [DOI] [PubMed] [Google Scholar]
- Penninx BW (2017). Depression and cardiovascular disease: epidemiological evidence on their linking mechanisms. Neuroscience & Biobehavioral Reviews, 74, 277–286. [DOI] [PubMed] [Google Scholar]
- Penninx BWJH (2017). Depression and cardiovascular disease: epidemiological evidence on their linking mechanisms. Neuroscience & Biobehavioral Reviews, 74, 277–286. [DOI] [PubMed] [Google Scholar]
- Powell ND, Sloan EK, Bailey MT, Arevalo JMG, Miller GE, Chen E, … Cole SW (2013). Social stress up-regulates inflammatory gene expression in the leukocyte transcriptome via β-adrenergic induction of myelopoiesis. Proceedings of the National Academy of Sciences, 110(41), 16574–16579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radloff LS. (1977). The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas, 1(3), 385–401. [Google Scholar]
- Raison CL, Broadwell SD, Borisov AS, Manatunga AK, Capuron L, Woolwine BJ, … Miller AH (2005). Depressive symptoms and viral clearance in patients receiving interferon-α and ribavirin for hepatitis C. Brain, Behavior, and Immunity, 19(1), 23–27. [DOI] [PubMed] [Google Scholar]
- Rohleder N, Wolf JM, & Wolf OT (2010). Glucocorticoid sensitivity of cognitive and inflammatory processes in depression and posttraumatic stress disorder. Neuroscience & Biobehavioral Reviews, 35(1), 104–114. [DOI] [PubMed] [Google Scholar]
- Schumann A, Andrack C, & Bär KJ (2017). Differences of sympathetic and parasympathetic modulation in major depression. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 79, 324–331. [DOI] [PubMed] [Google Scholar]
- Sgoifo A, Carnevali L, Pico Alfonso MDLA, & Amore M (2015). Autonomic dysfunction and heart rate variability in depression. Stress, 18(3), 343–352. [DOI] [PubMed] [Google Scholar]
- Sharma A (2016). Systems genomics support for immune and inflammation hypothesis of depression. Current Neuropharmacology, 14(7), 749–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelton RC, Claiborne J, Sidoryk-Wegrzynowicz M, Reddy R, Aschner M, Lewis DA, & Mirnics K (2011). Altered expression of genes involved in inflammation and apoptosis in frontal cortex in major depression. Molecular Psychiatry, 16(7), 751–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavich GM, & Cole SW (2013). The emerging field of human social genomics. Clinical Psychological Science, 1(3), 331–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stetler C, & Miller GE (2011). Depression and hypothalamic-pituitary-adrenal activation: A quantitative summary of four decades of research. Psychosomatic Medicine, 73(2), 114–126. [DOI] [PubMed] [Google Scholar]
- Stewart JC, Rand KL, Muldoon MF, & Kamarck TW (2009). A prospective evaluation of the directionality of the depression–inflammation relationship. Brain, Behavior, & Immunity, 23(7), 936–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracey KJ (2009). Reflex control of immunity. Nature Reviews Immunology, 9(6), 418–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valkanova V, Ebmeier KP, & Allan CL (2013). CRP, IL-6 and depression: a systematic review and meta-analysis of longitudinal studies. Journal of Affective Disorders, 150(3), 736–744. [DOI] [PubMed] [Google Scholar]
- Viner RM, Ozer EM, Denny S, Marmot M, Resnick M, Fatusi A, & Currie C (2012). Adolescence and the social determinants of health. The Lancet, 379(9826), 1641–1652. doi: 10.1016/S0140-6736(12)60149-4 [DOI] [PubMed] [Google Scholar]
- Wang X, Zhang L, Lei Y, Liu X, Zhou X, Liu Y, … Fan S (2014). Meta-analysis of infectious agents and depression. Scientific Resports, 4, 4530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger AH, Gbedemah M, Martinez AM, Nash D, Galea S, & Goodwin RD (2018). Trends in depression prevalence in the USA from 2005 to 2015: widening disparities in vulnerable groups. Psychological Medicine, 48(8), 1308–1315. [DOI] [PubMed] [Google Scholar]
- Zalli A, Jovanova O, Hoogendijk WJG, Tiemeier H, & Carvalho LA (2016). Low-grade inflammation predicts persistence of depressive symptoms. Psychopharmacology, 233(9), 1669–1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.