Abstract
In this study we tested whether vascular aging is associated with the risk of first stroke in the Kailuan cohort, a community-based Chinese cohort. For participants aged ≥ 50 years, healthy vascular aging (HVA) was defined as an absence of hypertension and a brachial-ankle pulse wave velocity < the mean + 2 standard deviations, which was determined from a reference sample of healthy participants aged < 30 years. The primary outcome was first stroke (ischemic or hemorrhagic). In total, 11,474 participants were enrolled. The prevalence of HVA decreased from 36.0% in participants aged 50-59 years to 4.7% in those aged ≥ 70 years. During a median follow-up of 3.3 years, the incidence of first stroke was 0.5% in the HVA group but was 2.6% in the Non-HVA group. After adjusting for confounding variables, HVA was associated with a 0.32-fold lower risk of first stroke compared to the Non-HVA group (95% confidence interval, 0.18-0.56; p < 0.001). It thus appears that HVA reduced the risk of first stroke in a community-based Chinese population. This suggests that evaluation of vascular aging as part of public health screening may be useful for stroke risk assessment.
Keywords: healthy vascular aging, stroke, arterial stiffness, pulse wave velocity
INTRODUCTION
Age-related alterations in blood pressure related to vascular aging is attributable primarily to increases in the stiffness of the large elastic arteries and to endothelial dysfunction [1–3]. Although such vascular aging may seem inevitable, several studies have shown that it is uncommon in some populations leading healthy lifestyles, indicating that it can be avoided [4–6]. Moreover, various pharmacological interventions and lifestyle modifications that can influence blood pressure and arterial stiffness are promising de-stiffening therapies that may prevent or delay vascular aging [7–11].
Healthy vascular aging (HVA), a new concept defined as the absence of age-related hypertension and increased arterial stiffness, has been shown to be associated with a reduced risk of cardiovascular disease in Western populations [12, 13]. We therefore speculated that HVA may be related to a reduced risk of first stroke. To test that idea, we investigated the association between HVA and the risk of first stroke in community-based Chinese cohort.
RESULTS
Study participants
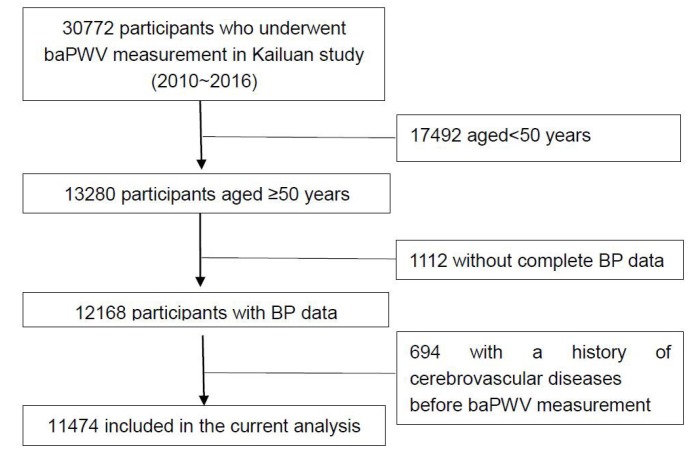
From January 1, 2010 to December 31, 2016, brachial-ankle pulse wave velocity (baPWV) measurements were made in 30,772 participants in the Kailuan cohort. Among them, 13,280 were older than 50 years. After excluding participants for whom blood pressure data was lacking (N=1112) and those with a history of cerebrovascular diseases before the baPWV measurement (N=694), a total of 11,474 participants were included in the study (Figure 1). Among that group, 3003 (26.2%) exhibited HVA at baseline, whereas the remaining 8471 (73.8%) did not. A comparison of demographic and clinical information between the HVA and Non-HVA groups is shown in Table 1.
Figure 1.
Flow diagram of the participant selection in the current analysis. Abbreviations: BP, blood pressure; baPWV, brachial-ankle pulse wave velocity.
Table 1. Baseline characteristics of participants included in the study.
| Variable | Non-HVA group (n=8 471) | HVA group (n=3 003) | p |
| Age(y) | 61.8±8.9 | 56.3±5.7 | <0.001 |
| 50y≤age<60y | 4136(48.8) | 2324(77.4) | |
| 60y≤age<70y | 2679(31.6) | 597(19.9) | |
| age≥70y | 1656(19.5) | 82(2.7) | |
| Female | 1981(23.4) | 895(29.8) | <0.001 |
| SBP(mmHg) | 140.7±18.3 | 118.8±10.6 | <0.001 |
| DBP(mmHg) | 87.0±11.0 | 77.2±6.7 | <0.001 |
| Heart rate(bpm) | 75.0±11.5 | 71.4±10.1 | <0.001 |
| baPWV(m/s) | 18.0±3.5 | 13.4±1.1 | <0.001 |
| Cardiovascular risk factors | |||
| BMI(kg/m2) | 25.5±3.3 | 24.5±3.0 | <0.001 |
| Current smoking | 2640(31.2) | 1005(33.5) | 0.020 |
| Current alcohol drinking | 880(10.4) | 260(8.7) | 0.020 |
| Inactive physical activity | 1668(19.7) | 500(16.7) | 0.001 |
| History of hypertension | 6035(71.2) | 0(0) | <0.001 |
| History of diabetes | 2349(27.7) | 320(10.7) | <0.001 |
| History of hyperlipidemia | 4797(56.6) | 1482(49.4) | <0.001 |
| Monthly income ≥ $120 | 490(5.8) | 154(5.1) | 0.310 |
| Education (≥High school) | 1703(20.1) | 789(26.3) | <0.001 |
| Laboratory results | |||
| FBG(mmol/L) | 6.5±2.7 | 5.7±1.6 | <0.001 |
| TC(mmol/) | 5.3±1.8 | 5.2±1.4 | <0.001 |
| LDL-C(mmol/L) | 2.7±1.0 | 2.6±1.0 | 0.002 |
| HDL-C(mmol/L) | 1.5±0.8 | 1.5±0.5 | <0.001 |
| Hs-CRP(mg/L) | 1.4(0.7-2.9) | 1.10(0.6-2.2) | <0.001 |
| Medication use | |||
| Antihypertensive drugs | 2464(29.1) | 0(0) | <0.001 |
| Antidiabetic drugs | 903(10.7) | 109(3.6) | <0.001 |
| Lipid-lowering drugs | 129(1.5) | 20(0.7) | 0.010 |
Abbreviations: HVA: healthy vascular aging; SBP: systolic blood pressure; DBP: diastolic blood pressure; baPWV: brachial-ankle pulse wave velocity; BMI: body mass index; FBG: fasting blood glucose; TC: total cholesterol; LDL-C: low-density lipoprotein cholesterol; HDL-C: high-density lipoprotein cholesterol; hs-CRP: high-sensitive C-reactive protein.
The prevalence and determinants of HVA
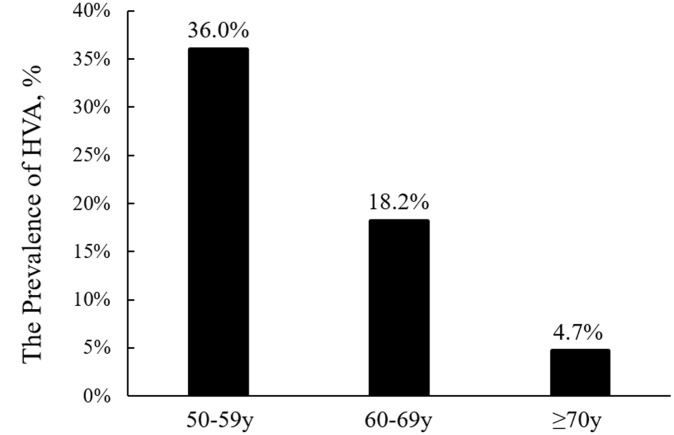
The prevalence of HVA was 36.0% among participants aged 50 to 59 years, 18.2% among participants aged 60 to 69 years, and 4.7% among those aged ≥70 years (Figure 2). Using a multivariable analysis, we found that younger age, female sex, lower body mass index (BMI), lower high-sensitive C-reactive protein (hs-CRP), lower fasting blood glucose (FBG), higher education level, active physical activity, absence of current alcohol drinking, and the absence of diabetes mellitus and hyperlipidemia were significantly associated with HVA (p<0.05) (Table 2).
Figure 2.
Comparison of the prevalence of healthy vascular aging (HVA) among different age groups.
Table 2. Multivariate logistic regression HVA vs. Non-HVA group.
| Variable | OR for HVA (95%CI) | p |
| Age | 0.90(0.89–0.91) | <0.001 |
| Male | 0.685(0.61–0.77) | <0.001 |
| BMI | 0.91(0.90–0.93) | <0.001 |
| Hs-CRP | 0.99(0.97–1.00) | 0.013 |
| FBG | 0.92(0.88–0.96) | <0.001 |
| Inactive physical activity | 0.87(0.77–0.99) | 0.031 |
| Education (≥High school) | 1.22(1.10–1.36) | <0.001 |
| Current alcohol consumption | 0.78(0.66–0.91) | 0.002 |
| History of diabetes | 0.61(0.50–0.74) | <0.001 |
| History of hyperlipidemia | 0.84(0.75–0.93) | 0.007 |
Abbreviations: HVA: healthy vascular aging; BMI: body mass index; Hs-CRP: high-sensitive C-reactive protein; FBG: fasting blood glucose; OR: odds ratio; CI: confidence interval.
Association between HVA and the risk of first-ever stroke
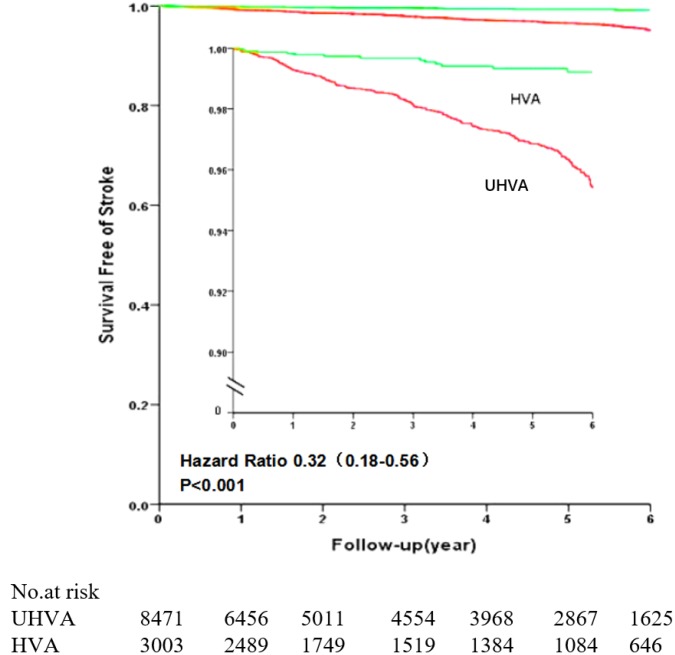
The follow-up times were similar in the two groups (HVA group, 3.37 ± 2.25 years; Non-HVA group, 3.29 ± 2.31 years). During a median follow-up of 3.3 years, first stroke occurred in 0.5% (15/3003) of participants in HVA group, as compared to 2.6% (217/8471) of those in the Non-HVA group (p<0.001). First ischemic stroke occurred in 0.5% (14/3003) of participants in the HVA group, as compared to 2.3% (198/8471) of those in the Non-HVA group (p<0.001) (Table 3). Participants with HVA had considerably lower age and sex-adjusted (model 1) risk of stroke (hazard ratio (HR), 0.21; 95% confidence intervals (CI), 0.12-0.36) and ischemic stroke (HR, 0.22; 95% CI, 0.12-0.37) as compared to participants in the Non-HVA group (Table 4). After adjusting for other cardiovascular risk factors (including BMI, smoking, alcohol consumption, physical activity, history of diabetes, history of hyperlipidemia) (model 2), HVA was associated with a 0.23-fold (95% CI, 0.13–0.39) lower risk of stroke and a 0.23-fold lower risk (95% CI, 0.14–0.41) of ischemic stroke. After further adjusting for monthly income, education level, FBG, total cholesterol (TC), low-density lipoprotein cholesterol (LDL), hs-CRP, and use of anti-diabetic and lipid-lowering medications (model 3), HVA was associated with a 0.23-fold (95% CI, 0.14-0.40) lower risk of stroke and a 0.24-fold lower risk (95% CI, 0.14–0.42) of ischemic stroke. After additionally adjusting for systolic blood pressure (model 4), HVA was associated with a 0.32-fold (95% CI, 0.18-0.56) lower risk of stroke and a 0.32-fold lower risk (95% CI, 0.18-0.58) of ischemic stroke (Figure 3).
Table 3. Outcome incidence during follow-up.
| Outcomes | Non-HVA (n=8471) | HVA (n=3003) | p value |
| Total stroke (%) | 217(2.6) | 15(0.5) | <0.001 |
| Ischemic stroke (%) | 198(2.3) | 14(0.5) | <0.001 |
| Hemorrhagic stroke (%) | 28(0.3) | 1(0) | 0.003 |
Abbreviation: HVA: healthy vascular aging.
Table 4. Risk of first stroke or ischemic stroke related to HVA vs Non-HVA.
| Outcome | HR(95%CI) | p value |
| Stroke | ||
| Model 1 | 0.21(0.12–0.36) | < 0.001 |
| Model 2 | 0.23(0.13–0.39) | < 0.001 |
| Model 3 | 0.23(0.14–0.40) | < 0.001 |
| Model 4 | 0.32(0.18–0.56) | < 0.001 |
| Ischemic stroke | ||
| Model 1 | 0.22(0.12–0.37) | <0.001 |
| Model 2 | 0.23(0.14–0.41) | <0.001 |
| Model 3 | 0.24(0.14–0.42) | <0.001 |
| Model 4 | 0.32(0.18–0.58) | <0.001 |
Model 1: Adjusted for age and sex.
Model 2: Model 1 + adjustment for body mass index, smoking, alcohol drinking, physical activity, history of diabetes, history of hyperlipidemia.
Model 3: Model 2 + adjustment for monthly income, education level, fast blood glucose, total cholesterol, low-density lipoprotein cholesterol, high-sensitive C-reactive protein, use of antidiabetic and lipid-lowering medications.
Model 4: Model 3 + adjustment for systolic blood pressure
Abbreviations: HVA: healthy vascular aging; HR: hazard ratio; CI: confidence interval.
Figure 3.
Comparison of stroke-free survival between the HVA and Non-HVA groups. The inset shows the same data on an enlarged segment of the y-axis. Abbreviations: HVA: healthy vascular aging.
DISCUSSION
The main finding of this study was that HVA was significantly associated with a lower risk of first stroke in a community-based Chinese cohort. Although the relationship between arterial stiffness and blood pressure is complex [14–18], the combination of normal pulse wave velocity and an absence of hypertension may be meaningful for prediction of cardiovascular disease risk. HVA was previously evaluated using two easily obtained metrics of vascular health: blood pressure and pulse wave velocity [12]. Considering the racial and geographic differences between Western and Chinese populations, our study builds a Chinese reference sample and establishes normal baPWV values for defining HVA, instead of adopting the reported references from a Western community [19]. Comparing the results from the Framingham heart study with our findings, we find that the prevalence of HVA was higher in this Chinese population than in the Western population (26.2% vs. 17.7%), particularly for participants aged 60 to 69 years (18.2% vs. 7.4%). Data from both the Framingham and Kailuan studies show that the prevalence of HVA decreases sharply with age. HVA appears achievable but is rare among participants aged ≥ 70 years. This may be attributable to greater arterial stiffness and a higher prevalence of hypertension among elderly participants. Our study also suggests that HVA status may be influenced by both non-modifiable (age and sex) and modifiable (BMI, hs-CRP, FBG, physical activity, alcohol consumption, education level, history of diabetes mellitus and hyperlipidemia) factors [20, 21]. Notably, in a univariate analysis, the fraction of participants currently smoking was higher in the HVA group than in the Non-HVA group. However, we did not observe an independent association between smoking status and HVA after multivariable logistic regression, which is consistent with an earlier study [12]. In addition, some epidemiologic studies have observed that smokers might have lower rates of hypertension and decreased arterial stiffness [22, 23]. Therefore, the impact of smoking on vascular health remains unclear and seems not straightforward.
The large sample size in our study increases its statistical reliability. In addition, all of the participants had access to local medical insurance, which guaranteed that the outcome data could be tracked through the medical insurance system. Nevertheless, several limitations should be noted. First, to evaluate the arterial stiffness, baPWV was adopted instead of carotid-femoral pulse wave velocity (cfPWV) [24–26]. However, because of the simplicity of its measurement and good correlation with cfPWV, we suggest baPWV could be potentially applied in clinical practice [27–29]. Second, participants in the Kailuan cohort volunteered to undergo baPWV measurement, which could potentially introduce selection bias. Third, since the Kailuan Group is a coal energy and chemical enterprise, males make up the majority of employees. All employees (≥ 18 years) were enrolled in the Kailuan cohort, which led to a gender imbalance among the participants in the analysis (males, 75%; females, 25%). Finally, because the numbers of incident strokes in this community-based population were limited, we did not conduct a stratification analysis based on age. In the future, the follow-up will go on to determine whether the effect of HVA is similar in different age groups.
In conclusion, it appears that HVA may have reduced the risk of first stroke in a community-based Chinese cohort. This suggests that evaluation of vascular aging as part of public health screening may be useful for stroke risk assessment and for stroke prevention.
MATERIALS AND METHODS
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
Study participants
The Kailuan cohort is an ongoing prospective cohort based in the Kailuan community in Tangshan City in the north of China [30, 31]. All employees (≥ 18 years) of the Kailuan Group, a coal energy and chemical enterprise, were enrolled with the aim of investigating the risk factors of various diseases (e.g., stroke and cancers). As of December 31, 2016, 171,414 participants were included in the Kailuan cohort. The study’s protocol was approved by the participating hospitals’ ethics committees. All participants provided written informed consent. All participants underwent questionnaire assessments, clinical examinations and laboratory tests at the 11 hospitals responsible for healthcare in the Kailuan community.
To capture a distinct sample of individuals with HVA, in the present study we considered only participants in late middle age or older (aged ≥ 50 years), and we only enrolled participants who underwent baPWV measurement between 2010 and 2016. We excluded participants for whom blood pressure data was lacking as well as those with a history of cerebrovascular diseases before the baPWV measurement.
Assessment of baPWV
BaPWV was measured using a BP-203 RPE III networked arteriosclerosis detection device produced by Omron Health Medical Co., LTD (China) [30, 31]. Participants received baPWV assessment after 5 min of rest in a supine position. Cuffs were applied to both arms and ankles. The lower edge of the arm cuff was positioned 2-3 cm above the cubital fossa transverse striation, and the lower edge of the ankle cuff was positioned 1-2 cm above the medial malleolus. The heartbeat monitor was placed at the left edge of the sternum, and electrocardiogram electrodes were placed on both wrists. BaPWV was calculated as the distance between the two sites divided by the pulse transit time (defined as the time interval between the wave fronts of the brachial and ankle waveforms). The distance between the sampling points was calculated automatically according to the subject’s height. The maximum value of the bilateral baPWV was used in our study. Using the detection device, we were able to read the baPWV value directly. The methodology for baPWV measurement was the same for all participants.
Baseline data on demographics and cardiovascular risk factors were collected through face-face interviews when baPWV was measured. The collected data included age, sex, BMI, smoking, alcohol consumption, physical activity, history of diabetes, history of hyperlipidemia, monthly income, education level, FBG, TC, LDL, hs-CRP, use of antidiabetic or lipid-lowering drugs. Physical activity was evaluated from responses to questions about the type and frequency of physical activity at work and during leisure time. Inactive physical activity was defined as no physical activity or less than 30 minutes every week.
Assessment of HVA
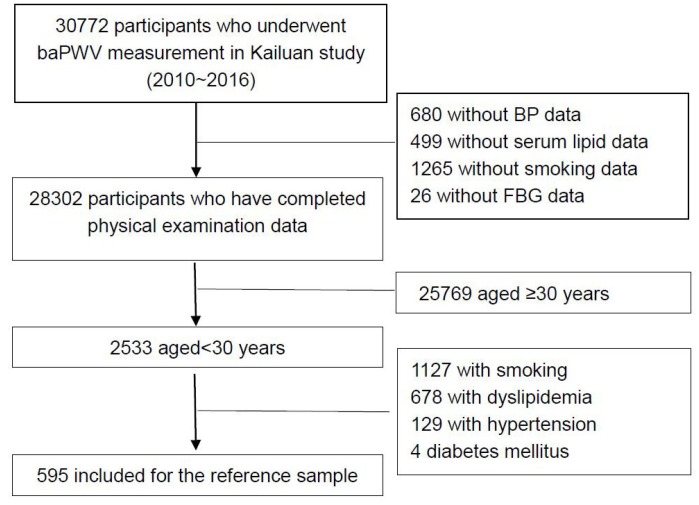
We defined HVA for participants aged ≥ 50 years as follows [12]: 1) absence of hypertension, which was defined as a systolic blood pressure < 140 mmHg, a diastolic blood pressure < 90 mmHg (blood pressure was measured at least twice at different occasions), and an absence of antihypertensive drugs; 2) baPWV < mean + 2 standard deviations (SD), which was determined from a reference sample of healthy participants aged 18-30 years with no cardiovascular risk factors (e.g., hypertension, diabetes, smoking, hyperlipidemia) and without a history of myocardial infarction, stroke, and other cardiovascular disease. To obtain the reference value for baPWV, we enrolled 595 healthy participants aged < 30 years from the Kailuan study (Figure 4). The results showed that the reference value for baPWV was 11.90 ± 1.65 m/s. Thus, HVA was defined as a normotensive blood pressure and baPWV < 15.20 m/s. Based on these data, participants were classified into HVA and Non-HVA groups.
Figure 4.
Flow diagram of the participant selection for determination of the mean baPWV value. Abbreviations: baPWV: brachial-ankle pulse wave velocity; BP: blood pressure; FBG: fasting blood glucose.
Outcome assessment and follow-up
The participants were followed up by face-to-face interviews at every two-year routine medical examination until December 31, 2017 or to death. The outcome information for the participants without face-to-face follow-up was obtained by referring to death certificates from provincial vital statistics offices, discharge summaries from the 11 hospitals, and medical records from medical insurance. The follow-ups were performed by hospital physicians, research physicians, and research nurses who were blinded to the baseline data. All stroke records were reviewed by two independent stroke specialists. If there were instances of disagreement in a single case, the final evaluation was made by the event adjudication committee.
The primary outcome was the first occurrence of stroke (both ischemic and hemorrhagic). These included the first nonfatal stroke event or stroke death without a preceding nonfatal event. Stroke was diagnosed according to the World Health Organization criteria [32] combined with brain computed tomography (CT) or magnetic resonance (MR) imaging for confirmation. The secondary outcome was the first occurrence of ischemic stroke. All study outcomes were validated by the Data Safety Monitoring Board and the Arbitration Committee for Clinical Outcomes.
Statistical analysis
Continuous variables are presented as the mean ± SD or median with interquartile range, while categorical variables are presented as frequencies with percentages. The nonparametric Wilcoxon or t tests were used to compare group differences for continuous variables and χ2 tests were used for categorical variables.
The association between baseline characteristics and HVA was investigated using multivariate backward logistic regression, and variables with p values < 0.05 were selected as confounding variables. Odds ratios (ORs) with the 95% CI were reported. We also examined associations between HVA and first stroke or ischemic stroke using Cox proportional hazards regression models. For each outcome, 4 multivariable models were performed. In model 1, we only adjusted for age and sex. In model 2, we additionally adjusted for other cardiovascular risk factors (including BMI, smoking, alcohol consumption, physical activity, history of diabetes, history of hyperlipidemia). In model 3, we further adjusted for monthly income, education level, FBG, TC, LDL, hs-CRP, and use of antidiabetic or lipid-lowering medications. In model 4, we further adjusted for systolic blood pressure. Life table methods were used to calculate the cumulative rate of stroke-free survival, and a curve was generated.
Two-sided p values < 0.05 were considered significant. All analyses were performed using SAS 9.4 (SAS Institute, Cary, North Carolina).
ACKNOWLEDGMENTS
We gratefully acknowledge all the participating hospitals in the study and thank all enrolled participants and their relatives.
Footnotes
CONFLICTS OF INTEREST: The authors declare that they have no conflicts of interest.
FUNDING: The study was supported by grants from the National Natural Science Foundation of China (81825007), the Ministry of Science and Technology of the People’s Republic of China (2017YFC1307900), Beijing Municipal Education Commission (Excellent Young Scientists Project), the third batch of National Ten Thousand Talents Plan, Young Elite Scientists Sponsorship Program by CAST (2018QNRC001), the Beijing Municipal Science and Technology Commission (Beijing Excellent Talents Training and Supporting Top Youth Team, D171100003017001 and 2016000021223TD03).
REFERENCES
- 1.Brant LC, Hamburg NM, Barreto SM, Benjamin EJ, Ribeiro AL. Relations of digital vascular function, cardiovascular risk factors, and arterial stiffness: the Brazilian Longitudinal Study of Adult Health (ELSA-Brasil) cohort study. J Am Heart Assoc. 2014; 3:e001279–001279. 10.1161/JAHA.114.001279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Trindade M, Oigman W, Fritsch Neves M. Potential Role of Endothelin in Early Vascular Aging. Curr Hypertens Rev. 2017; 13:33–40. 10.2174/1573402113666170414165735 [DOI] [PubMed] [Google Scholar]
- 3.El Assar M, Angulo J, Rodríguez-Mañas L. Oxidative stress and vascular inflammation in aging. Free Radic Biol Med. 2013; 65:380–401. 10.1016/j.freeradbiomed.2013.07.003 [DOI] [PubMed] [Google Scholar]
- 4.Lemogoum D, Ngatchou W, Janssen C, Leeman M, Van Bortel L, Boutouyrie P, Degaute JP, Van de Borne P. Effects of hunter-gatherer subsistence mode on arterial distensibility in Cameroonian pygmies. Hypertension. 2012; 60:123–28. 10.1161/HYPERTENSIONAHA.111.187757 [DOI] [PubMed] [Google Scholar]
- 5.Gurven M, Blackwell AD, Rodríguez DE, Stieglitz J, Kaplan H. Does blood pressure inevitably rise with age?: longitudinal evidence among forager-horticulturalists. Hypertension. 2012; 60:25–33. 10.1161/HYPERTENSIONAHA.111.189100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Najjar SS, Scuteri A, Lakatta EG. Arterial aging: is it an immutable cardiovascular risk factor? Hypertension. 2005; 46:454–62. 10.1161/01.HYP.0000177474.06749.98 [DOI] [PubMed] [Google Scholar]
- 7.Dumor K, Shoemaker-Moyle M, Nistala R, Whaley-Connell A. Arterial Stiffness in Hypertension: an Update. Curr Hypertens Rep. 2018; 20:72. 10.1007/s11906-018-0867-x [DOI] [PubMed] [Google Scholar]
- 8.Barton M, Husmann M, Meyer MR. Accelerated Vascular Aging as a Paradigm for Hypertensive Vascular Disease: prevention and Therapy. Can J Cardiol. 2016; 32:680–686.e4. 10.1016/j.cjca.2016.02.062 [DOI] [PubMed] [Google Scholar]
- 9.Chen Y, Shen F, Liu J, Yang GY. Arterial stiffness and stroke: de-stiffening strategy, a therapeutic target for stroke. Stroke Vasc Neurol. 2017; 2:65–72. 10.1136/svn-2016-000045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nilsson PM. Early vascular aging (EVA): consequences and prevention. Vasc Health Risk Manag. 2008; 4:547–52. 10.2147/VHRM.S1094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.García-Hermoso A, Martínez-Vizcaíno V, Gomez-Marcos MA, Cavero-Redondo I, Recio-Rodriguez JI, García-Ortiz L. Ideal Cardiovascular Health and Arterial Stiffness in Spanish Adults-The EVIDENT Study. J Stroke Cerebrovasc Dis. 2018; 27:1386–94. 10.1016/j.jstrokecerebrovasdis.2017.12.031 [DOI] [PubMed] [Google Scholar]
- 12.Niiranen TJ, Lyass A, Larson MG, Hamburg NM, Benjamin EJ, Mitchell GF, Vasan RS. Prevalence, Correlates, and Prognosis of Healthy Vascular Aging in a Western Community-Dwelling Cohort: The Framingham Heart Study. Hypertension. 2017; 70:267–74. 10.1161/HYPERTENSIONAHA.117.09026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nowak KL, Rossman MJ, Chonchol M, Seals DR. Strategies for Achieving Healthy Vascular Aging. Hypertension. 2018; 71:389–402. 10.1161/HYPERTENSIONAHA.117.10439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaess BM, Rong J, Larson MG, Hamburg NM, Vita JA, Levy D, Benjamin EJ, Vasan RS, Mitchell GF. Aortic stiffness, blood pressure progression, and incident hypertension. JAMA. 2012; 308:875–81. 10.1001/2012.jama.10503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen W, Li S, Fernandez C, Sun D, Lai CC, Zhang T, Bazzano L, Urbina EM, Deng HW. Temporal Relationship Between Elevated Blood Pressure and Arterial Stiffening Among Middle-Aged Black and White Adults: The Bogalusa Heart Study. Am J Epidemiol. 2016; 183:599–608. 10.1093/aje/kwv274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu S, Jin C, Li S, Zheng X, Zhang X, Cui L, Gao X. Aging, Arterial Stiffness, and Blood Pressure Association in Chinese Adults. Hypertension. 2019; 73:893–899. 10.1161/HYPERTENSIONAHA.118.12396 [DOI] [PubMed] [Google Scholar]
- 17.Fortier C, Sidibé A, Desjardins MP, Marquis K, De Serres SA, Mac-Way F, Agharazii M. Aortic–Brachial Pulse Wave Velocity RatioNovelty and Significance. Hypertension. 2017; 69:96–101. 10.1161/HYPERTENSIONAHA.116.08409 [DOI] [PubMed] [Google Scholar]
- 18.Ichikawa K, Sakuragi S, Nishihara T, Tsuji M, Mori A, Yokohama F, Wada T, Hasegawa D, Kawamoto K, Tanakaya M, Katayama Y, Ito H. Influence of arterial stiffness on cardiovascular outcome in patients without high blood pressure. Heart. 2018; 104:318–23. 10.1136/heartjnl-2017-311751 [DOI] [PubMed] [Google Scholar]
- 19.Reference Values for Arterial Stiffness’ Collaboration. Determinants of pulse wave velocity in healthy people and in the presence of cardiovascular risk factors: ‘establishing normal and reference values’. Eur Heart J. 2010; 31:2338–50. 10.1093/eurheartj/ehq165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dengo AL, Dennis EA, Orr JS, Marinik EL, Ehrlich E, Davy BM, Davy KP. Arterial destiffening with weight loss in overweight and obese middle-aged and older adults. Hypertension. 2010; 55:855–61. 10.1161/HYPERTENSIONAHA.109.147850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gottsäter M, Hindy G, Orho-Melander M, Nilsson PM, Melander O. A genetic risk score for fasting plasma glucose is independently associated with arterial stiffness: a Mendelian randomization study. J Hypertens. 2018; 36:809–14. 10.1097/HJH.0000000000001646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Omvik P. How smoking affects blood pressure. Blood Press. 1996; 5:71–77. 10.3109/08037059609062111 [DOI] [PubMed] [Google Scholar]
- 23.Green MS, Jucha E, Luz Y. Blood pressure in smokers and nonsmokers: epidemiologic findings. Am Heart J. 1986; 111:932–40. 10.1016/0002-8703(86)90645-9 [DOI] [PubMed] [Google Scholar]
- 24.Ohyama Y, Ambale-Venkatesh B, Noda C, Kim JY, Tanami Y, Teixido-Tura G, Chugh AR, Redheuil A, Liu CY, Wu CO, Hundley WG, Bluemke DA, Guallar E, Lima JA. Aortic Arch Pulse Wave Velocity Assessed by Magnetic Resonance Imaging as a Predictor of Incident Cardiovascular Events: The MESA (Multi-Ethnic Study of Atherosclerosis). Hypertension. 2017; 70:524–30. 10.1161/HYPERTENSIONAHA.116.08749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Niiranen TJ, Kalesan B, Larson MG, Hamburg NM, Benjamin EJ, Mitchell GF, Vasan RS. Aortic–Brachial Arterial Stiffness Gradient and Cardiovascular Risk in the CommunityNovelty and Significance. Hypertension. 2017; 69:1022–28. 10.1161/HYPERTENSIONAHA.116.08917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nilsson PM. Hemodynamic Aging as the Consequence of Structural Changes Associated with Early Vascular Aging (EVA). Aging Dis. 2014; 5:109–13. 10.14336/AD.2014.0500109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tanaka H, Munakata M, Kawano Y, Ohishi M, Shoji T, Sugawara J, Tomiyama H, Yamashina A, Yasuda H, Sawayama T, Ozawa T. Comparison between carotid-femoral and brachial-ankle pulse wave velocity as measures of arterial stiffness. J Hypertens. 2009; 27:2022–27. 10.1097/HJH.0b013e32832e94e7 [DOI] [PubMed] [Google Scholar]
- 28.Ohkuma T, Ninomiya T, Tomiyama H, Kario K, Hoshide S, Kita Y, Inoguchi T, Maeda Y, Kohara K, Tabara Y, Nakamura M, Ohkubo T, Watada H, et al. Brachial-Ankle Pulse Wave Velocity and the Risk Prediction of Cardiovascular DiseaseNovelty and Significance. Hypertension. 2017; 69:1045–52. 10.1161/HYPERTENSIONAHA.117.09097 [DOI] [PubMed] [Google Scholar]
- 29.Kim J, Song TJ, Song D, Lee KJ, Kim EH, Lee HS, Nam CM, Nam HS, Kim YD, Heo JH. Brachial-ankle pulse wave velocity is a strong predictor for mortality in patients with acute stroke. Hypertension. 2014; 64:240–46. 10.1161/HYPERTENSIONAHA.114.03304 [DOI] [PubMed] [Google Scholar]
- 30.Wu S, Huang Z, Yang X, Zhou Y, Wang A, Chen L, Zhao H, Ruan C, Wu Y, Xin A, Li K, Jin C, Cai J. Prevalence of ideal cardiovascular health and its relationship with the 4-year cardiovascular events in a northern Chinese industrial city. Circ Cardiovasc Qual Outcomes. 2012; 5:487–93. 10.1161/CIRCOUTCOMES.111.963694 [DOI] [PubMed] [Google Scholar]
- 31.Chen S, Li W, Jin C, Vaidya A, Gao J, Yang H, Wu S, Gao X. Resting Heart Rate Trajectory Pattern Predicts Arterial Stiffness in a Community-Based Chinese Cohort. Arterioscler Thromb Vasc Biol. 2017; 37:359–64. 10.1161/ATVBAHA.116.308674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stroke—1989. Recommendations on stroke prevention, diagnosis, and therapy. Report of the WHO Task Force on Stroke and other Cerebrovascular Disorders. Stroke. 1989; 20:1407–31. 10.1161/01.STR.20.10.1407 [DOI] [PubMed] [Google Scholar]