Abstract
Background:
The projected increase in the aging population raises concerns about how to manage the healthcare needs in a cost effective way. Within hearing healthcare, there are currently too few audiologists to meet the expected demand, and training more professionals may not be a feasible way of addressing this problem. For this reason, there is a need to develop different ways of assessing hearing sensitivity that can be conducted accurately and inexpensively when a certified audiologist and/or sound-attenuated booth is unavailable. More specifically, there is a need to determine if the HHT can yield accurate and reliable data from older adults with varying degrees of hearing loss.
Purpose:
To compare audiometric thresholds obtained using the Etymotic ‘Home Hearing Test’ (HHT), an automated pure-tone air-conduction test, to those obtained using manual audiometry, among older adults with varying degrees of hearing loss.
Study Sample:
Participants were 112 English-speaking adults (58% Female), aged 60 years and older. Participants were excluded from this study if otoscopy revealed cerumen impaction and/or suspected ear pathology.
Intervention:
All participants completed the HHT on tablet computers in a carpeted classroom and MA in a double-walled sound attenuated booth using insert earphones for both measures. Both measures were completed in the same test session and the order of testing (MA vs. HHT) was counterbalanced.
Data Collection and Analysis:
Absolute differences in thresholds measurements (in dBHL) were calculated across all ears (n = 224 ears) and for all frequencies (octave frequencies from 0.5 – 8 kHz). Correlation and multiple linear regression analyses were conducted to determine if thresholds obtained using the HHT significantly correlated with thresholds using MA. Mean thresholds for each method (HHT and MA) were compared using correlation analyses for each test frequency. Multiple linear regression analysis was used to examine the relationship between the four-frequency PTA (average threshold at 0.5, 1, 2, and 4 kHz) in the better-hearing ear measured using the HHT and a set of seven independent factors: four-frequency PTA in the better-hearing ear measured via MA, treatment group (HHT vs. MA), age, gender, and degree of hearing loss (mild, moderate, > moderate).
Results:
Correlation analyses revealed frequency-specific correlations, ranging from 0.91 – 0.97, for air-conduction thresholds obtained using the HHT and MA. Mean HHT thresholds were significantly correlated with mean MA thresholds in both ears across the frequency range. This relationship held true across different degrees of hearing loss. The regression model accounted for a significant amount of variance in the HHT better ear PTA, with MA better-ear PTA being the only significant predictor in our final model, with no effect of degree of loss, age or gender.
Conclusions:
The HHT is an accurate and cost effective method of establishing pure-tone air conduction thresholds, when compared to manual audiometry. Therefore, the HHT can be used as a tool to acquire accurate hearing thresholds from older adults, in-group settings, without the use of a sound-attenuated booth or a certified audiologist.
Keywords: Home Hearing Test, audiometry, self-test, public health, telehealth, teleaudiology, Etymotic, hearing healthcare, hearing healthcare uptake, hearing health care, hearing health care uptake, presbycusis, age-related hearing loss, aging, older adults
INTRODUCTION
Older people are a rapidly growing proportion of the world’s population. In the United States alone, the Census Bureau projects that individuals over the age of 65 will comprise nearly a quarter of the population by the year 2050 (He et al, 2016). This projected increase presents many opportunities but also several public health challenges because there will be a growing need to address age-related chronic health conditions.
Hearing loss is one of the top chronic conditions contributing to Disability-Adjusted Life Years (DALYs) in older adults (Vos et al, 2015). DALYs can be thought of as a measurement of the gap between a population’s health status and an ideal situation in which the specified population would live free of disease and disability, where one DALY can be considered one lost year of “healthy life” (World Health Organization, c2017.). Mathers, Smith, and Concha (2000) estimated that adult-onset hearing loss yields 1,286 DALYs in the USA and 24,915 DALYs globally.
Despite the growing need for professionals to assist with the auditory needs of our aging society, workforce analyses have indicated that the need for hearing healthcare services will outweigh available capacity. For example, Goulios and Patuzzi (2008) surveyed professional organizations that oversee audiology services in 64 countries worldwide. Eighty-six percent of respondents indicated that insufficient numbers of audiologists were available to meet community needs. While there is a lack of data to accurately project the upcoming global demand for hearing health care professionals, data from the USA can be used as an example. According to Windmill and Freeman (2013), the need for full time audiologists entering the workforce will increase by 50–100% between 2011 – 2040. A compounding problem is that early retirement in this profession is high. Approximately 40% of Audiologists who graduated between the years 1984–1993 have already retired (Windmill & Freeman, 2013). If this 40% attrition rate continues, a negative growth rate for audiology in the USA can be predicted over the next 30 years. A potential decline in the number of practicing audiologists combined with the growing population of older adults contributes to an even smaller capacity of audiologists to provide necessary services over the next several decades (Margolis & Morgan, 2008; Windmill & Freeman, 2013). Therefore, there is a need to address and improve access, uptake, and delivery of hearing healthcare services to facilitate healthy aging in this growing portion of the population (Fagan & Jacobs, 2009; WHO, 2013; Davis et al, 2016). Should these estimated trends in the USA be indicative of global trends, then hearing healthcare planning needs to be addressed globally.
One way to address the shortage of audiology professionals is the use of self-assessment technologies, such as the development of automated hearing assessment tools (Swanepoel & Hall, 2010; Swanepoel et al, 2014). There is a surge of interest in identifying reliable and inexpensive ways of measuring hearing loss that do not require highly specialized professionals or expensive clinical equipment. Some examples include: internet-based hearing screenings which are accessed online (Krumm et al, 2007; Bexelius et al, 2008), computer-based tests which measure hearing sensitivity using software downloaded onto a desktop, laptop, or tablet computer (iHear Medical, 2016; Folmer et al, 2017), and screening tests that can be completed over a landline telephone (Smits et al, 2004; Watson et al, 2012). Other examples include mobile testing and using smartphone applications (Szudek et al, 2012; Handzel et al, 2013; Clark & Swanepoel, 2014; Bright & Pallawela, 2016). One advantage of these newer methods is that they are less expensive than professional diagnostic hearing tests (Margolis & Morgan, 2008), providing a cost-effective effective approach to assessing populations with limited access to traditional clinical services. Further, computer and mobile technology is widely available, where nearly three-quarters of the world’s population has access to mobile phones (Kelly & Minges, 2012), making hearing testing more accessible to the general public. Despite the clear benefits of such solutions, their limitations should be addressed prior to being introduced as part of an integrated system of healthcare. In particular, the use of portable technology and testing in uncontrolled acoustic environments can be difficult to ensure and maintain appropriate background noise levels and calibrated sound systems (including sound cards and transducers). Also, many currently available tools do not enable frequency specific, pure tone air and/or bone conducted audiometric thresholds to be obtained, limiting the diagnostic capability of such devices.
A recent approach to managing background noise levels in the testing environment has been to use specifically designed sound attenuating headphones. For example, Shoebox Audiometry (Clearwater Clinical Limited, Ottawa, Canada) is an FDA-approved iPad audiometer that is compatible with numerous air- and bone-conduction transducers, including the Sennheiser HD 280 noise attenuating headphones. While this tool is compatible with various transducers and can reduce the effects of background noise to provide reliable threshold information, it is cost prohibitive for accessibility of the wider population.
Another recent example is the Etymotic Home Hearing Test (HHT). The HHT can be administered using a PC or tablet, contains a calibrated sound card and sound attenuated insert ear transducers, and is considerably less expensive than some of the previous mentioned alternatives (e.g., Shoebox Audiometry). The HHT is an automated hearing-screening test that measures ear-specific, air-conduction thresholds, at octave frequencies from 0.5 – 8 kHz. It was designed for home use, community-based testing, and telehealth practice. To determine if the HHT is a valid assessment tool, Margolis and colleagues (2016) compared thresholds obtained in the homes of twenty-eight participants, aged 44–88 years, to audiometric data obtained manually, obtained on separate day, in an audiology clinic. They reported that mean threshold measurements at octave frequencies from 0.5 – 8 kHz obtained using the HHT were slightly higher (2.8 dBHL) than those obtained using MA; however this difference was not statistically significant. What is not yet known is how the HHT compares to MA when: older adults with varying degrees of hearing loss are tested, or when the HHT is administered outside of the participant’s home. It is possible that the HHT procedure may pose more difficulty for older adults, and be less reliable depending on the degree of hearing loss. Therefore, the purpose of this study was to assess the parallel reliability of the HHT vs. MA for older adults with varying degrees of hearing loss. It was hypothesized that the HHT would provide reliable threshold information when compared to the gold standard MA procedures (ASHA, 1997). To test this hypothesis, we tested 112 participants aged 60 years and older, with varying degrees of hearing loss, and examined the relationship between hearing thresholds obtained using the HHT vs. MA obtained within the same day.
METHODS
Participants
A total of 112 adults (58% female), ages 60 years and older, participated in the present study. Participants were recruited from the Seattle, WA area using radio advertisements and the University of Washington Communication Studies Participant Pool. All participants were able to communicate using the English language and all testing materials were presented in English. Age distributions are listed in Table 1.
Table 1.
Age and Gender Distribution of Participants
| Age (years) | |||||||
|---|---|---|---|---|---|---|---|
| n | Sex (% Female) | 60–64 | 65–69 | 70–74 | 75–79 | 80–84 | 85+ |
| 112 | 65 (58%) |
20 (17.9%) |
38 (33.9%) |
34 (30.4%) |
15 (13.4%) |
4 (3.6%) |
1 (0.9%) |
Procedure
Participants were assigned to one of two treatment groups: H- completing the HHT before manual audiometry (n = 56) or A- completing manual audiometry before the HHT (n = 56). After explaining the purpose of the study, Doctorate of Audiology (Au.D.) students performed otoscopic examinations bilaterally to rule out cerumen obstruction, and to visualize landmarks on the tympanic membrane. Participants with suspected pathology or significant cerumen impaction (>75% occlusion) were not included in this study. All participants were tested on both measures within the same visit.
Home Hearing Test (HHT)
Microsoft Surface Pro 4 tablets were used to administer the HHT. Testing took place in a carpeted classroom with up to 8 participants completing the assessment simultaneously. Room sound levels were not strictly regulated with the exception of asking people to avoid talking during testing, as well as being conscientious when closing the room door. The HHT soundcard, which moderates the system’s output frequency and amplitude, was inserted into the tablets’ USB drive. ER-3 foam insert earphone tips were attached to the wired headphones.
Participants were informed that the HHT would present sounds varying in loudness and pitch in each ear individually and in a random order. They were instructed to read the tablet screen, which prompted them to listen for the tone and asked them to press “Yes” if they had heard the tone and “No” if they had not (Figure 1). After test instructions were provided, ER-3 insert earphones were placed into each ear. The HHT provides pulsed pure tones, varying in sound amplitude at octave frequencies from 0.5 to 8 kHz; the maximum amplitude output was 85 dB HL. Test time approximated 10 minutes per participant.
Figure 1.
Participant instructions for the HHT.
Manual Audiometry (MA)
AuD students administered MA assessments in a double-walled soundproof booth in the University of Washington Speech and Hearing Clinic. The modified Hughson-Westlake procedure was employed and thresholds were recorded at octave frequencies from 0.5 – 8 kHz in each ear (ASHA, 1997). Pure tone air conduction thresholds were obtained using ER-3 insert earphones.
Statistical Analysis
All statistical tests were performed with the Statistical Program for the Social Sciences (SPSS) version 24 (SPSS Inc., Chicago, IL). Data were analyzed using correlation and multiple linear regressions with sequential predictor entry analyses. Correlation analyses were conducted to determine how closely the HHT replicated ear-specific threshold measurements obtained using MA across octave frequencies from 0.5 – 8 kHz, as well as to examine the relationships between variables included in the regression models. Multiple linear regression analyses were conducted to determine if: 1) the relationship between the HHT and MA in determining Pure Tone Average (PTA; hearing threshold averaged at 0.5, 1, 2, 4 kHz in the better-hearing ear) for each participant and 2) the degree of hearing loss affected the relationship between PTA derived from the HHT versus MA. Sequential predictor entry was used to test the incremental variance accounted for as independent variables were added to the model. Because participants were randomly selected, there were no dependence issues. Normality, linearity, and homoscedasticity of residuals were examined for each model to ensure that linear regression model assumptions were tenable.
For ease of interpretation, age and group were effect coded. Age data were collected categorically rather than on a metrical scale, hence the effect coding. Gender was dummy coded with female as the focal group. Degree of hearing loss (normal, mild, moderate, > moderate) was coded into a set of 3 predictors, with the normal hearing group acting as a fixed-effect. Degrees of hearing loss were defined as follows (Clark, 1981): (1) Normal – −10–25 dB HL, (2) Mild – 26–40 dB HL, (3) Moderate – 51–55 dB HL, (4) > Moderate– 56+ dB HL. Due to the small n in participants with moderately-severe, severe, and profound hearing loss, the three degrees of hearing loss were grouped into one category, “Greater than moderate”. Better-ear PTA was standardized for the HHT and MA. Block 1 included group, age, and gender variables; Block 2 included the MA better-ear PTA variable; Block 3 included degrees of hearing loss variables. The final model was as follows:
HHT better-ear PTA = b0 + b1*Group + b2*Gender + b3*Age
+ b4*MA better-ear PTA
+ b5 *MildHL + b6 *ModerateHL + b7 * >ModerateHL
RESULTS
Correlation and multiple linear regression analyses were conducted to examine the relationship between hearing thresholds obtained using the HHT and thresholds obtained using MA. The distribution of absolute differences between thresholds obtained using the HHT and MA thresholds for 224 ears of 112 participants, at octave frequencies from 0.5 – 8 kHz, is shown in Figure 2. Threshold differences were −5 to 5 dBHL for 89.1% of measured thresholds, −10 to 10 dBHL for 96.9% of measured thresholds, and −15 to 15 dBHL for 98.4% of measured thresholds.
Figure 2.
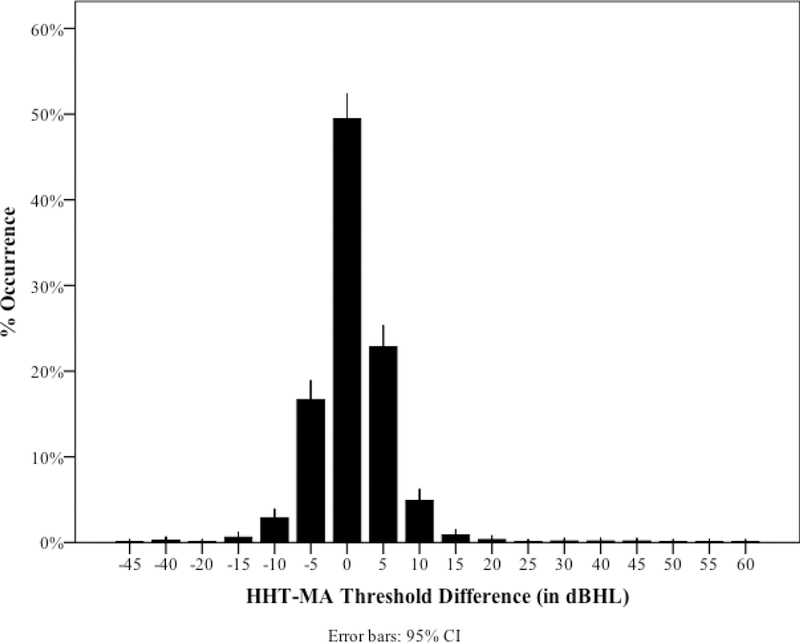
Distribution of differences between air-conduction thresholds measured using the HHT and manual audiometry for 224 ears of 112 participants tested at five frequencies.
Correlation Analyses
Threshold measurements (in dB HL) at octave frequencies from 0.5 – 8 kHz averaged across all participants are shown in Figure 3a for the right ear and Figure 3b for the left ear Pearson’s r correlations were calculated to determine the relationship between HHT and MA thresholds at each frequency (Table 2). All correlations were significant at p < 0.001.
Figure 3a.
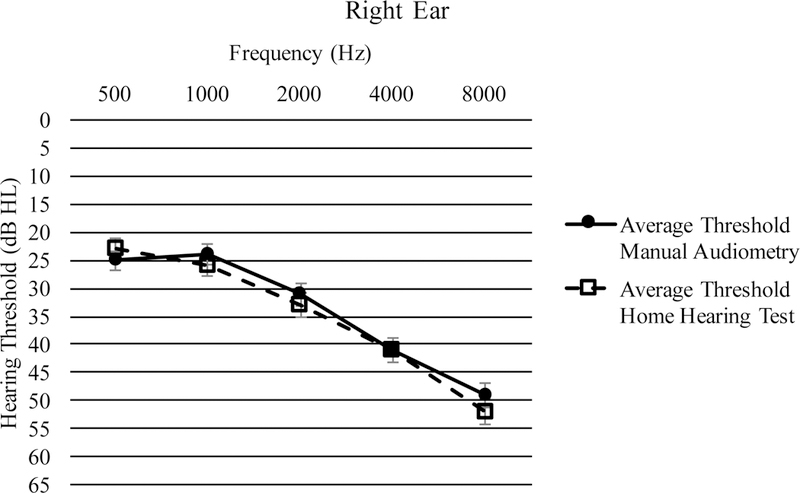
Average thresholds from 500 – 8000 Hz in the right ear with standard error bars.
Figure 3b.
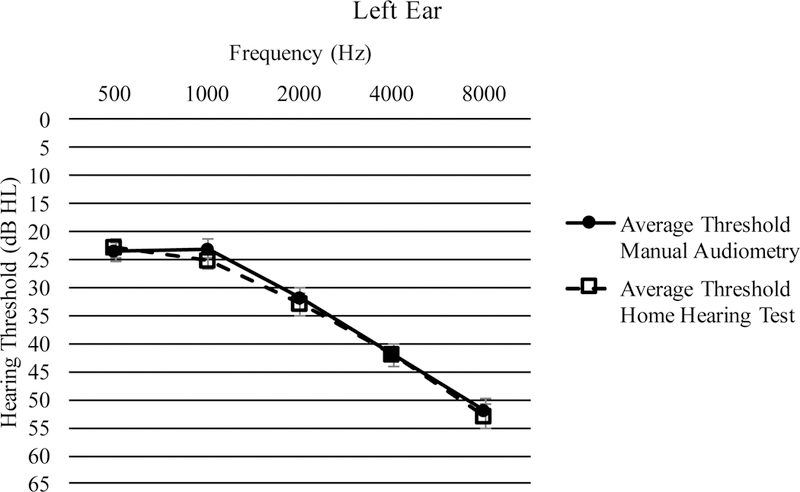
Average thresholds from 500 – 8000 Hz in the left ear with standard error bars.
Table 2.
Pearson’s Correlations Between HHT and MA Thresholds by Frequency in the Right (top) and Left (bottom) Ears, Averaged Across All Participants
| RIGHT EAR: | ||||||
|---|---|---|---|---|---|---|
| Test | MA | |||||
| Hz | 500 | 1000 | 2000 | 4000 | 8000 | |
| 500 | .909 | .849 | .684 | .481 | .414 | |
| 1000 | .833 | .924 | .763 | .569 | .510 | |
| HHT | 2000 | .662 | .773 | .960 | .749 | .655 |
| 4000 | .463 | .575 | .739 | .969 | .781 | |
| 8000 | .366 | .470 | .634 | .770 | .953 | |
| LEFT EAR: | ||||||
|---|---|---|---|---|---|---|
| Test | MA | |||||
| Hz | 500 | 1000 | 2000 | 4000 | 8000 | |
| 500 | .917 | .866 | .659 | .514 | .423 | |
| 1000 | .856 | .945 | .783 | .561 | .477 | |
| HHT | 2000 | .671 | .780 | .961 | .762 | .626 |
| 4000 | .529 | .570 | .765 | .970 | .785 | |
| 8000 | .405 | .487 | .644 | .812 | .968 | |
Bolding indicates correlation between same-frequency thresholds across each measure (e.g., 500 Hz on HHT vs. 500 Hz on MA).
Means, standard deviations, and zero-order correlations among all variables in the final regression model are provided in Table 3. Treatment group was not significantly correlated with any of the variables in the model (p > 0.05 across all comparisons). MA better-ear PTA was significantly correlated with HHT better-ear PTA and all three degrees of hearing loss (p < 0.001 across all comparisons). All three degrees of hearing loss were significantly correlated with HHT better-ear PTA (p < 0.001 across all comparisons), MA better-ear PTA (p < 0.001 across all comparisons), and age (p < 0.01 across all comparisons).
Table 3.
Zero-Order Correlations for Regression Model
| Measure | M | (SD) | 1. | 2. | 3. | 4. | 5. | 6. | 7. |
|---|---|---|---|---|---|---|---|---|---|
| Outcome | |||||||||
| 1. HHT PTA | 27.03 | (16.52) | -- | ||||||
| Block 1 Variables | |||||||||
| 2. Group | 0.50 | (0.50) | (0.10) | -- | |||||
| 3. Age | 3.54 | (1.10) | .38 * | −.11 | -- | ||||
| 4. Gender | 1.42 | (0.50) | -.19 *** | −.05 | -.01 | -- | |||
| Block 2 Variables | |||||||||
| 5. MA PTA | 27.21 | (15.98) | .99 *** | .08 | .39 *** | ### | -- | ||
| Block 3 Variables | |||||||||
| 6. Mild HL | 32.61 | (4.14) | .51 *** | -.50 | .29 ** | ### | .53 *** | -- | |
| 7. Moderate HL | 45.00 | (4.54) | .74 *** | .03 | .36 *** | ### | .74 *** | .71 *** | -- |
| 8. Severe-Profound HL | 68.93 | (10.11) | .87 *** | .65 | .34 *** | ### * | .89 *** | .78 *** | .80 *** |
Note. N=112. HHT= Home Hearing Test; PTA= 4-frequency Pure-Tone Average; MA= Manual Audiometry; HL= Hearing Loss; Group effect coded: −1= MA, HHT 1=HHT, MA; Age effect coded: −1= 60–69 years 1= 70+ years; Gender effect coded: Male=0 Female=1
p < .05,
p < .01,
p < .001.
Regression Models
As shown in Table 4 Block 1, group, gender, and age accounted for a significant variation in HHT better-ear PTA, R2 = 0.20, p < 0.001. However, when MA better-ear PTA was added into the model (Block 2) none of the variables from Block 1 were significant (p > 0.05 across all comparisons). Block 2 accounted for a significant variation in HHT better-ear PTA, R2change = 0.77, p < 0.001. The relationship between MA better-ear PTA and HHT better-ear PTA is illustrated in Figure 4. Finally, Block 3, which added the three degrees of hearing loss variables into the model, was not significantly different from Block 2, R2change < 0.01, p = 0.189. The lack of a statistically significant difference between Block 2 and Block 3 indicates that degree of hearing loss did not uniquely account for a significant variance in HHT better-ear PTA. In other words, the HHT provided PTA thresholds as accurately for participants with mild hearing loss as it did for participants with severe hearing loss. Figure 5 illustrates the relationship between MA better-ear PTA and HHT better-ear PTA by degree of hearing loss.
Table 4.
Summary of Multiple Linear Regression Model with Sequential Predictor Entry
| Block 1 | Block 2 | Block 3 | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| R 2 change | R 2 total | R 2 adj | b | sr 2 | R 2 change | R 2 total | R 2 adj | b | sr 2 | R 2 change | R 2 total | R 2 adj | b | sr 2 | |
| Model Fit | 0.20 *** | 0.20 *** | 0.18 | 0.77 *** | 0.97 *** | 0.97 | 0.00 | 0.97 *** | 0.97 | ||||||
| Coefficients | |||||||||||||||
| Intercept | 0.22 | 0.02 | 0.07 | ||||||||||||
| Group | 0.14 | 0.02 | 0.03 | < 0.01 | 0.02 | < 0.01 | |||||||||
| Gender | −0.35 * | 0.15 | −0.04 | < 0.01 | 0.01 | < 0.01 | |||||||||
| Age | 0.39 *** | 0.03 | 0.01 | < 0.01 | −0.04 | < 0.01 | |||||||||
| MA PTA | 0.98 *** | 0.77 | 0.94*** | 0.10 | |||||||||||
| Mild HL | −0.07 | < 0.01 | |||||||||||||
| Moderate HL | 0.07 | < 0.01 | |||||||||||||
| Severe-Profound HL | 0.06 | < 0.01 | |||||||||||||
Note. N =112. Block 1 F -change test df = 3,108; Block 2 df = 4, 107; Block 3 df = 7, 104. HHT= Home Hearing Test; PTA= 4-frequency Pure-Tone Average; MA= Manual Audiometry; HL= Hearing Loss; Group effect coded: −1= MA, HHT 1=HHT, MA; Age effect coded: −1= 60–69 years 1= 70+ years; Gender effect coded: Male=0 Female=1
p < 0.05,
p < 0.01,
p < 0.001.
Figure 4.
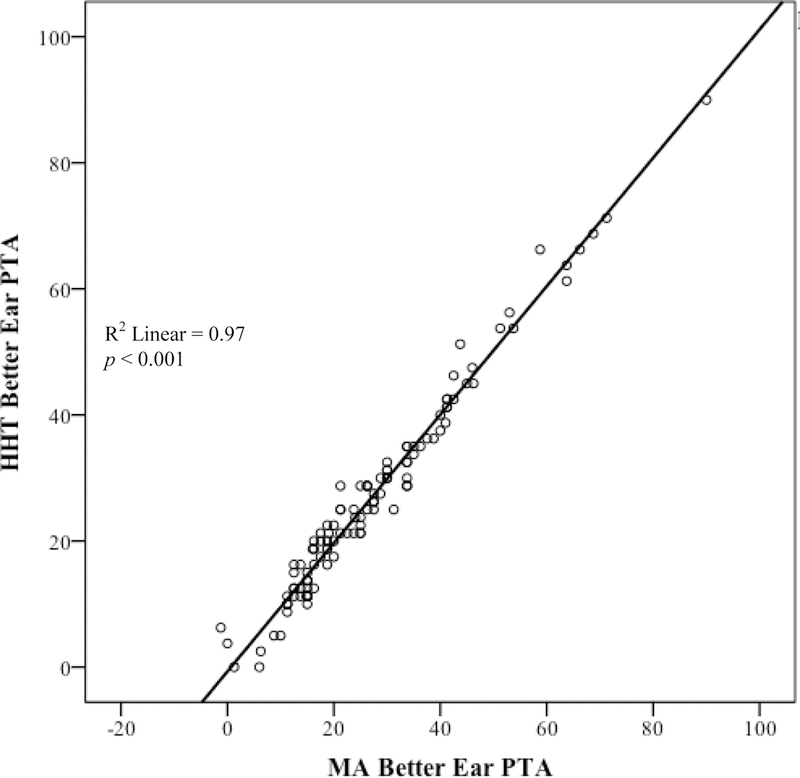
Relationship between 4-frequency PTA in the better-hearing ear as derived by manual audiometry vs. the HHT in N = 112 participants.
Figure 5.
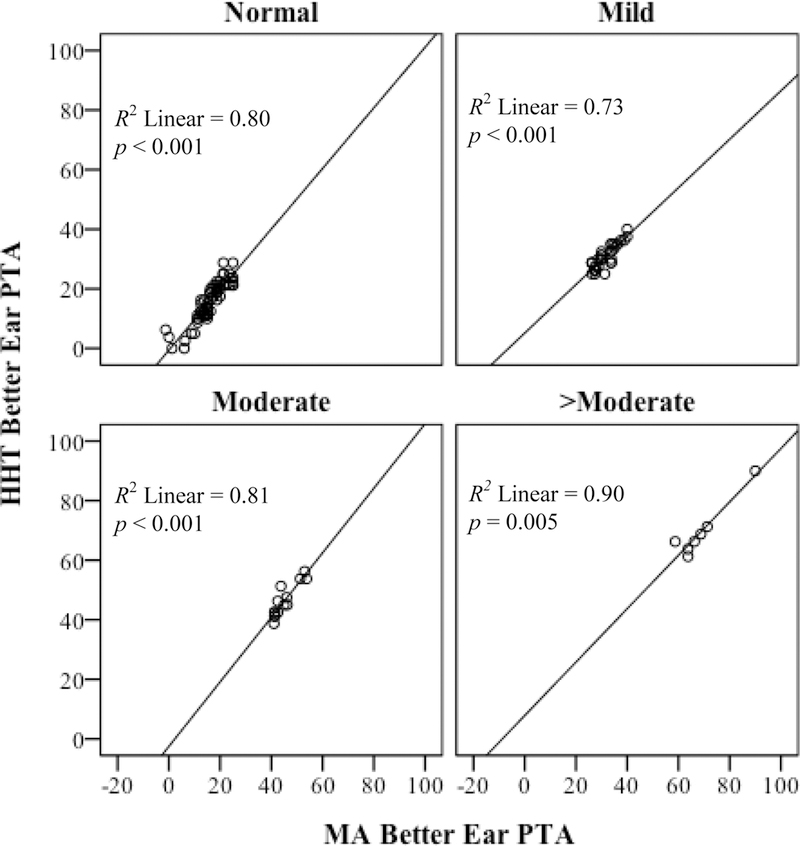
Comparison of 4-frequency pure-tone average (0.5 – 4 kHz) in the better-hearing ear as derived by MA vs. the HHT. Stratified by degree of hearing loss, where Normal: 0–25 dBHL (n = 60), Mild: 26–40 dBHL (n = 31), Moderate: 41–55 dBHL (n = 14), >Moderate: > 55 dBHL (n = 7).
Discussion
Air conduction thresholds can be reliably obtained using the Etymotic HHT in adults ages 60 years and older. Thresholds obtained using the HHT significantly correlated with thresholds using manual audiometry at octave frequencies from 0.5 – 8 kHz in each ear. This relationship held true across different degrees of hearing loss, despite the fact that background noise was not strictly regulated and multiple (up to 8) people completed testing at the same time, in the same room. Results from the current study support and expand the findings previously reported by Margolis et al. (2016), in that reliability of the HHT can be said to be significant across different degrees of hearing, for older participants, measured in a group setting.
Clinical Implications
Results obtained from this experiment demonstrate that it is possible to obtain accurate air conduction thresholds outside of a sound attenuated booth. When put into the context of improving accessibility to hearing healthcare, the HHT can be regarded as one tool that is capable of providing reliable frequency-specific pure tone air conducted threshold information. The ability to derive reliable hearing thresholds in a non-clinical setting could potentially promote the awareness of hearing loss and improve the uptake of hearing healthcare services in older adults. For example, portable tools like HHT could be used in many community centers, retirement facilities, and for administering hearing healthcare in remote locations using teleaudiology. Because an audiologist or sound attenuated booth is not necessary to obtain this audiometric information, the HHT could help mitigate the demand for professional audiologists without compromising the quality of data collected (Swanepoel et al., 2014). Also, because the device does not require an internet connection, the HHT can be used in remote locations as long as there is access to a tablet and the HHT kit.
Limitations
It is important to acknowledge that access to convenient and affordable hearing tests may inform older adults about their own hearing status, but this does not necessarily lead to entry into the hearing healthcare system (Davis et al, 2007; Yueh et al, 2010). When Meyer et al. (2011) examined hearing healthcare seeking behaviors in 193 individuals who had been informed they had failed a telephone hearing screening; only 36% had sought professional help in a follow-up telephone survey. Thus, although products like the HHT may help to identify the presence and degree of hearing loss, use of such tools, on their own, does not fully address the need for improving the uptake of hearing health services. Rather, it is a first step in the hearing healthcare pathway that should be followed by audiological/medical referrals when necessary as well as the intervention (Davis & Smith, 2013).
Another limitation is the HHT is not equipped with a bone oscillator or supra-aural headphones. Therefore, type of hearing loss (conductive versus sensorineural) cannot be determined and people with external ear abnormalities (e.g., stenosis, atresia, cerumen impaction, exostosis) cannot be accurately assessed. Also, the use of insert earphones may be contraindicated if the person has cerumen impaction or other forms of obstruction. Finally, background noise levels, if loud enough, can still potentially interfere. We purposefully did not systematically monitor or restrict noise levels and so they are likely similar to many environments within professional settings, but this does not guarantee that individuals taking this test at home with the television playing the background, or near a kitchen at an assistive living facility, will yield similar results.
Conclusion
The HHT is a reliable way to obtain ear-specific air-conduction threshold information in groups of older adults and when conducted in an environment that is not sound treated, but sufficiently quiet.
Acknowledgements
We thank the National Institute of Health -National Institute on Deafness and Other Communication Disorders for funding this project (NIDCD R21DC013161) awarded to Dr K.Tremblay, as well as Dr. Kathy Pichora-Fuller for providing thoughtful insight and feedback during the preparation of this manuscript, and members of the Brain and Behavior Laboratory for assisting with data collection.
NIH NIDCD R21DC013161 awarded to Kelly Tremblay (PI)
References
- American Speech-Language Hearing Association. (1997) Guidelines for audiologic screening. ASHA, 1–63. [Google Scholar]
- Bexelius C, Honeth L, Ekman A, Eriksson M, Sandin S, Bagger-Sjöbäck D, Litton JE. (2008) Evaluation of an internet-based hearing test—Comparison with established methods for detection of hearing loss. J MIR Med Educ, 10(4), e32 10.2196/jmir.1065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bright T & Pallawela D (2016) Validated smartphone-based apps for ear and hearing assessments: A review. JMIR Rehabil Assist Technol, 3(2), e13 10.2196/rehab.6074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cappuccio FP, Kerry SM, Forbes L, Donald A. (2004) Blood pressure control by home monitoring: meta-analysis of randomised trials. BMJ, 329(7458), 145–145. 10.1136/bmj.38121.684410.AE [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark JG. (1981) Uses and abuses of hearing loss classification. ASHA, 23, 493–500. [PubMed] [Google Scholar]
- Clark JL & Swanepoel DW. (2014) Technology for hearing loss: As we know it and as we dream it. Disabil Rehabil Assist Technol, 9, 408–413. [DOI] [PubMed] [Google Scholar]
- Davis A, McMahon CM, Pichora-Fuller KM, Russ S, Lin F, Olusanya BO, et al. (2016) Aging and hearing health: The life-course approach. Gerontologist, 56(Suppl. 2), S256–S267. 10.1093/geront/gnw033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis A, Smith P. (2013) Adult hearing screening: Health policy issues—What happens next? Am J Audiol, 22(1), 167–170. 10.1044/1059-0889(2013/12-0062). [DOI] [PubMed] [Google Scholar]
- Davis A, Smith P, Ferguson M, Stephens D, Gianopoulos I. (2007) Acceptability, benefit, and costs of early screening for hearing disability: A study of potential screening tests and models. Health Technol Assess, 11(42), 1–294. [DOI] [PubMed] [Google Scholar]
- Etymotic Research, Inc. (2016) Available at https://www.etymotic.com/consumer/accessories/er4.html. Accessed June, 20, 2017.
- Fagan JJ, Jacobs M. (2009) Survey of ENT services in Africa: Need for a comprehensive intervention. Glob Health Action, 2, 10.3402/gha.v2i0.1932. 10.3402/gha.v2i0.1932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folmer RL, Vachhani J, McMillan GP, Watson C, Kidd GR, Feeny MP. (2017) Validation of a computer-administered version of the digits-in-noise test for hearing screening in the United States. J Am Acad Audiol, 28(2): 161–169. doi: 10.3766/jaaa.16038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goulios H, Patuzzi RB. (2008) Audiology education and practice from an international perspective, Int J Audiol, 47:10, 647–664, DOI: 10.1080/14992020802203322 [DOI] [PubMed] [Google Scholar]
- Handzel O, Ben-Ari O, Damian D, Priel MM, Cohen J. et al. (2013) Smartphone-based hearing test as an aid in the initial evaluation of unilateral sudden sensorineural hearing loss. Audiol Neurotol, 18, 201–207. [DOI] [PubMed] [Google Scholar]
- He W, Goodkind D, Kowal P. (2016) An aging world: 2015 U.S. Census Bureau, International Population Reports, P95/16–1, Washington, DC: U.S. Government Publishing Office. [Google Scholar]
- Hussein SY, Swanepoel DW, Biagio de Jager L, Myburgh HC, Eikelboom RH, Hugo J. (2015) Smartphone hearing screening in health assisted community-based primary care. J Telemed Telecare, 22(7), 405–412. 10.1177/1357633X15610721 [DOI] [PubMed] [Google Scholar]
- iHear Test, iHEAR Medical. (2016) Available at http://www.ihearmedical.com/ihear-test/. Accessed April 21, 2017.
- Kelly T, Minges M. (2012) Exclusive Summary. Washington: World Bank. [Google Scholar]
- Krumm M, Ribera J, Klich R. (2007) Providing basic hearing tests using remote computing technology. J Telemed Telecare, 13(8), 406–410. 10.1258/135763307783064395 [DOI] [PubMed] [Google Scholar]
- Linssen AM, Anteunis LJC, Joore MA. (2015) The cost-effectiveness of different hearing screening strategies for 50- to 70-year-old adults: A Markov model. Value Health, 18(5), 560–569. 10.1016/j.jval.2015.03.1789 [DOI] [PubMed] [Google Scholar]
- Margolis RH, Killion MC, Bratt GW, Saly GL. (2016) Validation of the Home Hearing Test. J Am Acad Audiol, 27(5), 416–420. [DOI] [PubMed] [Google Scholar]
- Margolis RH, Morgan DE. (2008) Automated pure-tone audiometry: An analysis of capacity, need, and benefit. Am J Audiol, 17(2), 109–113. 10.1044/1059-0889(2008/07-0047) [DOI] [PubMed] [Google Scholar]
- Mathers CD, Loncar D. (2006) Projections of global mortality and burden of disease from 2002 to 2030. PLOS Med, 3(11), e442 10.1371/journal.pmed.0030442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer C, Hickson L, Khan A, Hartley D, Dillon H, Seymour J. (2011) Investigation of the actions taken by adults who failed a telephone-based hearing screen. Ear Hear, 32, 720–731. doi: 10.1097/AUD.0b013e318220d973 [DOI] [PubMed] [Google Scholar]
- Smits C, Kapteyn T, Houtgast T. (2004) Development and validation of an automatic speech-in-noise screening test by telephone. Int J Audiol, 43(1), 15–28. [DOI] [PubMed] [Google Scholar]
- Swanepoel DW, Hall J (2010) A systematic review of telehealth applications in audiology. Telemed J E Health, 16(2), 181–200. doi: 10.1089/tmj.2009.0111. [DOI] [PubMed] [Google Scholar]
- Swanepoel DW, Myburgh HC, Howe DM, Mahomed F, Eikelboom RH. (2014) Smartphone hearing screening with integrated quality control and data management, Int J Audiol, 53(12), 841 10.3109/14992027.2014.920965 [DOI] [PubMed] [Google Scholar]
- Szudek J, Ostevik A, Dziegielewski P, Robinson-Anagor J, Gomaa N. et al. (2012) Can u hear me? Validation of an iPod-based hearing loss screening test. J Otolaryngol Head Neck Surg, 41, S78–S84. [PubMed] [Google Scholar]
- Tunis SL, Minshall ME. (2008) Self-monitoring of blood glucose in type 2 diabetes: Cost-effectiveness in the United States. Am J Manag Care, 14(3), 131–140. [PubMed] [Google Scholar]
- Vos T, Allen C, Arora M, Barber RM, Bhutta ZA, Brown A, et al. (2016) Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: A systematic analysis for the Global Burden of Disease Study 2015. The Lancet, 388(10053), 1545–1602. 10.1016/S0140-6736(16)31678-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson CS, Kidd GR, Miller JD, Smits C, Humes LE (2012) Telephone screening tests for functionally impaired hearing: Current use in seven countries and development of a US version. J Am Acad Audiol, 23(10), 757–767. [DOI] [PubMed] [Google Scholar]
- Windmill IM, Freeman BA. (2013) Demand for audiology services: 30-yr projections and impact on academic programs, J Am Acad Audiol, 24(5), 407 10.3766/jaaa.24.5.7 [DOI] [PubMed] [Google Scholar]
- World Health Organization; (c2017) Metrics: Disability-Adjusted Life Year (DALY). Geneva, Switzerland: Available from http://www.who.int/healthinfo/global_burden_disease/metrics_daly/en/ [Google Scholar]
- World Health Organization. (2013) Millions of people in the world have a hearing loss that can be treated or prevented. Geneva, Switzerland: Available from: http://0-www.who.int.innopac.up.ac.za/pbd/deafness/news/Millionslivewithhearingloss.pdf?ua [Google Scholar]
- Yueh B, Collins MP, Souza PE, Boyko EJ, Loovis CF, Heagerty PJ, Lui CF, Hedrick SC. (2010) Long-term effectiveness of screening for hearing loss: The screening for auditory impairment--which hearing assessment test (SAI-WHAT) randomized trial. J Am Geriatr Soc, 58(3), 427–434. [DOI] [PubMed] [Google Scholar]


