Abstract
BACKGROUND:
Two vaccines against herpes zoster are currently authorized for use in Canada: the recombinant subunit zoster vaccine and live attenuated zoster vaccine. We compared the effectiveness and cost-effectiveness of these 2 vaccines.
METHODS:
We used a decision analytic static cohort model parametrized with Canadian epidemiologic and economic data. We performed the economic analysis from the health care system perspective, using a lifetime horizon and a 3% discount rate for costs and benefits. The primary outcome was the incremental cost per quality-adjusted life-year (QALY) gained, relative to no vaccination. We ran 30 000 simulations varying all model parameters, including vaccine costs, efficacy and waning.
RESULTS:
The number needed to vaccinate (NNV) was higher for the live attenuated zoster vaccine than for the recombinant subunit zoster vaccine for all herpes zoster–related events at all ages. For example, in persons exactly 65 years old, for herpes zoster, median NNV was 21 (90% uncertainty interval [UI] 13–31) versus 8 (90% UI 6–18), and for postherpetic neuralgia, NNV was 64 (90% UI 33–93) versus 31 (90% UI 23–73). For the recombinant vaccine, the median cost-effectiveness ratios varied between cost-saving and $25 881 per QALY gained for adults aged 50 years or older. For the live vaccine, the cost-effectiveness ratios varied between cost-saving and $130 587 per QALY gained and were less than $45 000 per QALY gained only for those 65 to 75 years old. Given its higher efficacy, we estimated that the cost for the complete series of the recombinant vaccine could be $150 to $200 more than the cost of the live vaccine and still be considered cost-effective.
INTERPRETATION:
Our model predicted that the recombinant subunit zoster vaccine is likely cost-effective in Canada for adults 60 years or older, and is likely more cost-effective than live attenuated zoster vaccine. These results have informed updated national and provincial recommendations on herpes zoster vaccination.
Herpes zoster, characterized by dermatomal pain and rash,1,2 affects about 1 of every 3 persons during their lifetime.3–5 The most common complication is long-lasting debilitating pain, known as postherpetic neuralgia, which occurs in about 8% to 27% of individuals with herpes zoster.6–10 Given that postherpetic neuralgia has a substantial negative impact on health-related quality of life11 and that therapeutic options are only partially effective,12 the best option remains the prevention of herpes zoster and thus postherpetic neuralgia.13
Two herpes zoster vaccines are currently authorized for use in Canada among adults aged 50 years or older: the recombinant subunit zoster vaccine (Shingrix) and the live attenuated zoster vaccine (Zostavax). The recombinant vaccine was approved recently (October 2017), whereas the live vaccine has been available since 2008. Clinical trials have shown that the recombinant vaccine is highly effective against herpes zoster and postherpetic neuralgia for adults aged 50 years or older (vaccine efficacy against herpes zoster 96.6% for those 50–59 yr and 97.9% for those > 70 yr) with no evidence of waning protection after 4 years.14 Recent immunogenicity data also suggest that the immune response is maintained up to 9 years after vaccination.15
Conversely, clinical trials and observational data have suggested that the efficacy of the live vaccine against herpes zoster decreases with older age at vaccination (from 65.5% for those 60–69 yr to 55.4% for those ≥ 70 yr10) and wanes with increasing time since vaccination.16–18
Although the recombinant vaccine appears to be more effective, particularly among older adults, a 2-dose schedule is recommended, compared with a 1-dose schedule for the live vaccine; this difference has implications for costs and vaccination logistics. Furthermore, although both vaccines have been shown to be safe, a significantly higher proportion of adults vaccinated with the recombinant vaccine experienced grade 3 adverse events (e.g., injection-site pain, redness or swelling, myalgia, fatigue, headache), relative to those receiving placebo (17% v. 3%).14 These adverse events could affect completion of the vaccination schedule and vaccine efficacy.
Clinicians and policy-makers in various jurisdictions are currently making recommendations about the choice of herpes zoster vaccine to use and the age cohorts to be vaccinated. The criteria considered in such decisions include cost-effectiveness. The aims of this study were to evaluate the effectiveness and cost-effectiveness of vaccinating adults 50 years of age or older against herpes zoster in Canada, using 1 of the 2 currently available vaccines (live attenuated zoster vaccine or recombinant subunit zoster vaccine), relative to the absence of vaccination, and then to compare the 2 vaccines in terms of effectiveness and cost-effectiveness. This work informed the 2018 updated recommendations on the use of herpes zoster vaccines by the National Advisory Committee on Immunization (NACI)19 and the Comité d’immunisation du Québec.20
Methods
Model structure
We used a previously published decision analytic static cohort model.4,21 The model structure for the current study was the same as previously published, but we updated all parameter values. Briefly, the model followed a cohort of adults through different phases of herpes zoster (no herpes zoster, herpes zoster, postherpetic neuralgia) (Figure A1 in Appendix 1, available at www.cmaj.ca/lookup/suppl/doi:10.1503/cmaj.190274/-/DC1). The model compared the incidence of herpes zoster and postherpetic neuralgia, mortality rate, use of health care resources (in terms of hospitalization, consultations and length of hospital stay), costs and quality-adjusted life-years (QALYs) lost between vaccinated and unvaccinated cohorts of adults.
Vaccine efficacy parameters
Vaccine efficacy comprises 2 elements: the degree to which adults are protected shortly after vaccination (initial vaccine efficacy) and the loss of vaccine protection over time (waning of vaccine efficacy). We estimated parameter values for the efficacy of the 2 vaccines by fitting the age-specific annual incidence of herpes zoster predicted by the model with that observed in the vaccination arm of randomized clinical trials,10,14,22,23 using 6 different functions of waning efficacy over time (for a formal description of these functions, see Table A1 in Appendix 1). This method, based on previous modelling studies,21,24 makes it possible to estimate both short-term vaccine efficacy and waning efficacy, as well as to capture the uncertainty surrounding the long-term efficacy of the 2 vaccines (Figure A2 in Appendix 1).
Epidemiologic and economic parameters
We updated the epidemiologic and economic parameters used by Brisson and colleagues21 through literature reviews and analyses of available data sources (Table 1).3,4,10,11,21,25–31 More specifically, we updated the epidemiologic parameters and health care resource use associated with herpes zoster through a systematic review of the literature and data extraction from Quebec administrative databases, previously published by Letellier and colleagues. 25 The parameters presented in Table 1 represent the minimum and maximum values identified in the literature (including the article by Brisson and colleagues21) and obtained from analysis of Quebec administrative databases. We also updated costs related to herpes zoster and postherpetic neuralgia through a literature review. We identified a recent study conducted in Manitoba that specifically estimated the costs associated with herpes zoster and postherpetic neuralgia;30 we used the values from this study as our base values. The costs presented in Table 1 also represent the minimum and maximum values identified in the literature. All costs were adjusted to 2018 Canadian dollars according to the Consumer Price Index.32 We varied the cost of a complete vaccination series between $100 and $200 (including both the vaccine price and administration costs).
Table 1:
Epidemiologic, health care resource use and economic parameters
| Parameter by age category, yr | Base value | Minimum | Maximum | References |
|---|---|---|---|---|
| Epidemiologic | ||||
| Herpes zoster incidence, per 1000 person-years | Brisson et al.,21 Letellier et al.,25 Russell et al.,26 Tanuseputro et al.,27 Marra et al.28 | |||
| 50–54 | 3.8 | 3.525¶ | 4.226 | |
| 55–64 | 6 | 5.125¶ | 6.926 | |
| 65–74 | 8.6 | 7.325¶ | 10.026 | |
| ≥ 75 | 9.9 | 8.025¶ | 11.826 | |
| Postherpetic neuralgia,* % of herpes zoster cases | Oxman et al.,10 Brisson et al.21 | |||
| 50–54 | 9.4 | 6.921 | 11.921 | |
| 55–64 | 9.4 | 6.921 | 11.921 | |
| 65–74 | 26 | 18.521 | 33.421 | |
| ≥ 75 | 27.7 | 22.021 | 33.421 | |
| Case-fatality rate, %† | Edmunds et al., 4 Brisson et al.21 | |||
| 50–54 | 0 | 0.00021 | 0.00221 | |
| 55–64 | 0 | 0.00021 | 0.00221 | |
| 65–74 | 0.012 | 0.01221 | 0.08321 | |
| ≥ 75 | 0.076 | 0.04021 | 0.08321 | |
| Health care resource use | ||||
| Hospitalization, % of herpes zoster cases | Brisson et al.,3 Brisson et al.,21 Letellier et al.,25 Tanuseputro et al.27 | |||
| 50–54 | 1.1 | 0.521 | 1.621 | |
| 55–64 | 1.6 | 0.721 | 2.521 | |
| 65–74 | 3.3 | 1.521 | 5.121 | |
| ≥ 75 | 9.9 | 4.121 | 15.621 | |
| Consultations, per herpes zoster case | Brisson et al.,21 Letellier et al.,25 Najafzadeh et al.29 | |||
| 50–54 | 1.7 | 1.021 | 2.421 | |
| 55–64 | 2 | 1.021 | 2.921 | |
| 65–74 | 2.3 | 1.021 | 3.521 | |
| ≥ 75 | 2.6 | 1.021 | 4.221 | |
| Length of hospital stay, d, mean | Brisson et al.,21 Letellier et al.,25 Najafzadeh et al.29 | |||
| 50–54 | 9.3 | 5.821 | 12.721 | |
| 55–64 | 11.1 | 6.221 | 14.729 | |
| 65–74 | 12.6 | 8.321 | 16.529 | |
| ≥ 75 | 18 | 12.421 | 23.729 | |
| Costs,‡ in 2018 Can$ | ||||
| Herpes zoster–related hospitalization, per day | 91830 | 49521 | 148321 | Brisson et al.,21 Najafzadeh et al.,29 Friesen et al.30 |
| Herpes zoster–related consultations | 2830 | 2430 | 11329 | Brisson et al.,21 Najafzadeh et al.,29 Friesen et al.30 |
| Treatment per herpes zoster episode | 13630 | 5521 | 25529 | Brisson et al.,21 Najafzadeh et al.,29 Friesen et al.30 |
| Treatment per postherpetic neuralgia episode | 158830 | 96930 | 270721 | Brisson et al.,21 Najafzadeh et al.,29 Friesen et al.30 |
| QALYs lost§ | ||||
| Herpes zoster | Drolet et al.,11 Brisson et al.,21 Brisson et al.31 | |||
| 50–59 | 0.009 | 0.00631 | 0.01231 | |
| 60–69 | 0.01 | 0.00631 | 0.01331 | |
| ≥ 70 | 0.01 | 0.00731 | 0.01431 | |
| Postherpetic neuralgia | Drolet et al.,11 Brisson et al.,21 Brisson et al.31 | |||
| 50–59 | 0.041 | 0.03231 | 0.05231 | |
| 60–69 | 0.192 | 0.10331 | 0.29031 | |
| ≥ 70 | 0.234 | 0.19131 | 0.29031 |
Note: base value = mean of minimum and maximum values identified in literature, maximum = maximum values identified in literature, minimum = minimum values identified in literature, QALY = quality-adjusted life-year.
Postherpetic neuralgia was defined as clinically significant pain persisting for more than 90 days after onset of rash.
Given the scarcity of data on herpes zoster–related mortality in Canada, we used case-fatality values estimated in a previous study in England and Wales.4
Values from Friesen and colleagues30 were used as the base values.
This variable captures, in a single measure, morbidity and mortality associated with a disease. Data for QALYs lost were obtained by measuring QALY-weight (or disutility), ranging from 0 to 1, where a weight of 1 corresponds to optimal health and a weight of 0 corresponds to a health state judged as equivalent to death. The QALY lost per case is the difference in QALY weights with and without the disease, multiplied by duration of the disease. The QALY weights were taken from MASTER, a pan-Canadian, multicentre 6-month prospective study, which recruited patients aged ≥ 50 years who presented with herpes zoster or postherpetic neuralgia, as described by Drolet and colleagues11 and Brisson and colleagues.31 Calculation of QALY lost is explained in detail by Brisson and colleagues.21
Letellier and colleages25 did not present data by specific age groups, but we had access to the original data from Quebec administrative databases (2001 to 2015); for the purposes of our analysis, we estimated the incidence of herpes zoster by age groups.
We performed the analysis from a health care system perspective, on the basis of discussions with Canadian decision-makers, and therefore did not include indirect costs (e.g., wages lost).
Outcomes
We estimated the following 3 outcomes: pre-vaccination burden of herpes zoster in Canada, effectiveness of herpes zoster vaccination and cost-effectiveness of herpes zoster vaccination.
For the pre-vaccination burden of herpes zoster, we estimated the yearly number of herpes zoster–related events (cases of herpes zoster, ophthalmic herpes zoster, postherpetic neuralgia, hospital admissions and deaths).
For vaccination effectiveness, we used the number needed to vaccinate (NNV), calculating the NNV values as number of people vaccinated divided by number of herpes zoster–related events prevented over a lifetime.
For the cost-effectiveness of herpes zoster vaccination, we used 2 comparisons: vaccination versus no vaccination and recombinant vaccine versus live vaccine. As the primary outcome, we used the incremental cost per QALY gained of herpes zoster vaccination compared with no vaccination. Although there is no recommended cost-effectiveness threshold in Canada, we used a threshold of $45 000 per QALY gained, which corresponds to the gross domestic product per capita (as suggested by the World Health Organization33).
Because the complete series of the recombinant vaccine (2 doses) will likely be more costly than the live vaccine (1 dose), our secondary cost-effectiveness outcome was the additional cost of a complete series of the recombinant vaccine, to obtain an incremental cost-effectiveness ratio under the $45 000 per QALY gained threshold (v. the live vaccine).
Statistical analysis
We performed the economic analysis from the health care perspective, used a lifetime time horizon and assumed a 3% discount rate for both costs and benefits (as traditionally used in Canada when assessing the cost-effectiveness of vaccines).
To illustrate results across different cost-effectiveness thresholds, we produced acceptability curves for the vaccination of adults aged 65 years and for vaccine costs of $140 and $200.
Sensitivity analyses
We performed a probability sensitivity analysis by assigning a triangular probability distribution to each parameter and then drawing 30 000 combinations of these parameter values using Latin hypercube sampling. The minimum and maximum values of the distribution were the minimum and maximum value identified from the literature, and the median or mode is the base value presented in Table 1. We present all model predictions as the median and 90% uncertainty interval (UI; the 5th and 95th percentiles taken from the distribution of 30 000 simulation results).
We also performed univariable sensitivity analyses for the key model parameters (e.g., percentage of herpes zoster cases with development of postherpetic neuralgia, QALYs lost to postherpetic neuralgia). To do so, we fixed 1 key parameter value to its minimum or maximum value and varied all other parameters using the same probability distributions as for the main analysis (Table A4 in Appendix 1). In addition, we examined the potential impact of a single dose of the recombinant vaccine and of vaccination limited to immunocompetent adults (Table A2 in Appendix 1).
Ethics approval
For this modelling study, no ethics approval was required or obtained.
Results
In total, 90 623 cases of herpes zoster, 13 575 cases of ophthalmic herpes zoster and 17 502 cases of postherpetic neuralgia were predicted to occur each year in Canada among adults aged 50 years or older (Table 2). Most of the burden of disease would occur in adults aged 70 years or older.
Table 2:
Yearly burden of illness in Canada*
| Variable | Age group; no. (%) of population | Total, no. (90% UI) | ||
|---|---|---|---|---|
| 50–59 yr | 60–69 yr | ≥ 70 yr | ||
| Herpes zoster | 25 629 (28) | 29 188 (32) | 35 765 (39) | 90 623 (85 375–95 812) |
| Ophthalmic herpes zoster | 3831 (28) | 4355 (32) | 5354 (39) | 13 575 (10 403–16 972) |
| Postherpetic neuralgia | 2405 (14) | 5398 (31) | 9681 (55) | 17 502 (15 512–19 707) |
| Hospital admission | 362 (9) | 738 (19) | 2751 (71) | 3867 (2829–4937) |
| Death | 0 (1) | 5 (21) | 20 (78) | 26 (18–36) |
Note: 90% UI = uncertainty interval (based on 5th and 95th percentiles of 30 000 simulation results).
Total population 35 million, according to 2016 population structure.
Effectiveness of vaccination
The NNV was higher for the live vaccine than for the recombinant vaccine for all herpes zoster–related events that we investigated (Table 3). The difference in NNV between the 2 vaccines increased with increasing age at vaccination, mainly because of the decline in vaccine efficacy by age with the live vaccine. For example, for adults exactly 60 years of age, the median NNV to prevent 1 case of herpes zoster was 18 (90% UI 9–28) for the live vaccine versus 8 (90% UI 6–21) for the recombinant vaccine, and the median NNV to prevent 1 case of postherpetic neuralgia was 78 (95% UI 31–150) for the live vaccine versus 33 (90% UI 23–128) for the recombinant vaccine. In contrast, for adults exactly 75 years of age, the median NNV for herpes zoster was 42 (90% UI 32–63) for the live vaccine versus 11 (90% UI 9–19) for the recombinant vaccine, whereas for postherpetic neuralgia, the median NNV was 78 (90% UI 51–102) for the live vaccine versus 40 (90% UI 31–71) for the recombinant vaccine.
Table 3:
Estimated NNV with herpes zoster vaccines to prevent herpes zoster–related events, by age at vaccination
| Herpes zoster–related event, by age at vaccination* | Type of vaccine; median NNV (90% UI) | |
|---|---|---|
| Live attenuated vaccine | Recombinant subunit vaccine | |
| 50 yr | ||
| Herpes zoster | 15 (6–28) | 7 (5–26) |
| Ophthalmic herpes zoster | 103 (34–230) | 49 (29–193) |
| Postherpetic neuralgia | 106 (26–341) | 36 (22–288) |
| Hospital admission | 638 (103–2419) | 171 (84–2061) |
| Death | 71 898 (13 407–2 610 325) | 19 473 (11 148–2 200 338) |
| 60 yr | ||
| Herpes zoster | 18 (9–28) | 8 (6–21) |
| Ophthalmic herpes zoster | 122 (56–229) | 54 (34–150) |
| Postherpetic neuralgia | 78 (31–150) | 33 (23–128) |
| Hospital admission | 463 (119–1136) | 149 (84–947) |
| Death | 39 072 (14 670–91 623) | 15 915 (10 625–8621) |
| 65 yr | ||
| Herpes zoster | 21 (13–31) | 8 (6–18) |
| Ophthalmic herpes zoster | 138 (76–285) | 57 (37–138) |
| Postherpetic neuralgia | 64 (33–93) | 31 (23–73) |
| Hospital admission | 335 (124–700) | 137 (82–613) |
| Death | 27 828 (14 354–41 590) | 13 672 (9865–32 620) |
| 70 yr | ||
| Herpes zoster | 28 (21–43) | 9 (7–19) |
| Ophthalmic herpes zoster | 196 (123–362) | 65 (43–139) |
| Postherpetic neuralgia | 73 (42–96) | 35 (27–73) |
| Hospital admission | 289 (133–494) | 130 (83–385) |
| Death | 32 120 (18 131–43 416) | 15 753 (11 630–32 797) |
| 75 yr | ||
| Herpes zoster | 42 (32–63) | 11 (9–19) |
| Ophthalmic herpes zoster | 295 (171–586) | 77 (48–161) |
| Postherpetic neuralgia | 78 (51–102) | 40 (31–71) |
| Hospital admission | 215 (126–378) | 116 (75–226) |
| Death | 34 638 (21 718–50 059) | 18 139 (13 206–32 667) |
| 80 yr | ||
| Herpes zoster | 75 (47–125) | 14 (11–22) |
| Ophthalmic herpes zoster | 523 (257–1136) | 96 (60–190) |
| Postherpetic neuralgia | 97 (68–126) | 50 (40–80) |
| Hospital admission | 269 (169–466) | 145 (95–267) |
| Death | 43 125 (29 410–61 470) | 22 811 (16 801–37 057) |
| 85 yr | ||
| Herpes zoster | 142 (67–380) | 18 (15–25) |
| Ophthalmic herpes zoster | 983 (377–3133) | 124 (79–237) |
| Postherpetic neuralgia | 124 (94–164) | 66 (52–94) |
| Hospital admission | 351 (229–604) | 188 (125–328) |
| Death | 55 957 (40 079–79 288) | 29 816 (22 096–44 152) |
Note: 90% UI = uncertainty interval (based on 5th and 95th percentiles of 30 000 simulation results), NNV = number needed to vaccinate.
Ages shown are individuals’ exact age.
Cost-effectiveness
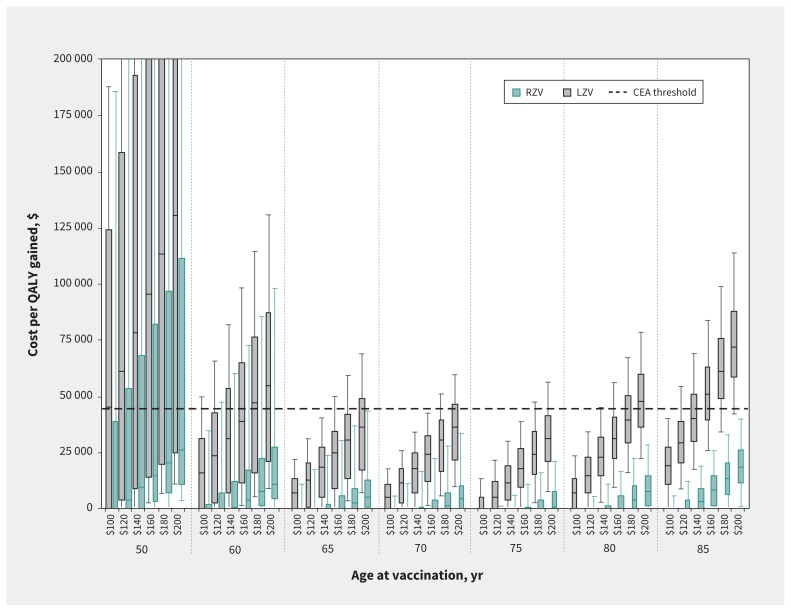
Vaccinating adults aged 65 to 75 years against herpes zoster was predicted to result in cost-effectiveness ratios below $45 000 per QALY gained, for both vaccines and under all scenarios investigated (Figure 1; Table A3 in Appendix 1). However, there were considerable differences in cost-effectiveness ratios between the 2 vaccines. For the recombinant vaccine, the median cost-effectiveness ratio predictions varied between cost-saving and $25 881 per QALY gained. Above 60 years, the cost-effectiveness ratios were relatively stable by age at vaccination, with the variability mainly due to vaccination cost. For the live vaccine, the median cost-effectiveness ratio predictions varied between cost-saving and $130 587 per QALY gained. The cost-effectiveness ratios for the live vaccine were highly sensitive to age at vaccination, but remained below $45 000 per QALY gained for those between 65 and 75 years. Cost-effectiveness ratios were higher among adults older than 75 years, because of lower vaccine efficacy, and among adults younger than 65 years, because of waning vaccine efficacy (Figure A2 in Appendix 1).
Figure 1:
Cost per quality-adjusted life-year (QALY) gained of vaccination with the recombinant subunit zoster vaccine (RZV) and the live attenuated zoster vaccine (LZV) compared with no vaccination, by age at vaccination and vaccine cost (complete series). Box plots represent the 5th, 25th, 50th, 75th and 95th percentiles from 30 000 simulation results. Costs are reported in 2018 Canadian dollars. CEA = cost-effectiveness analysis.
Finally, the recombinant vaccine was estimated to be more cost-effective than the live vaccine for all ages at vaccination. We estimated that, depending on the age at vaccination, the cost for the complete series of recombinant vaccine could be $150 to $200 more than the live vaccine and still be considered cost-effective using the threshold of $45 000 per QALY gained (Figure A4 in Appendix 1). The cost-effectiveness acceptability curves for the vaccination of adults aged 65 years indicated that for the recombinant vaccine, most of our simulations (> 70%) would be cost-effective for cost-effectiveness thresholds of $15 000 or more per QALY gained (assuming vaccine costs of $140 or $200). For the live vaccine, most of our simulations (> 70%) would be cost-effective for cost-effectiveness thresholds of $30 000 or more per QALY gained (assuming vaccine costs of $140) and $50 000 or more per QALY gained (assuming vaccine costs of $200) (Figure A3 in Appendix 1).
In the sensitivity analysis, the median cost-effectiveness ratios for the recombinant vaccine remained below the threshold of $45 000 per QALY gained for all scenarios investigated (Table A4, Table A5 and Figure A5 in Appendix 1). However, the median cost-effectiveness ratios for the live vaccine were highly sensitive to the parameters that determined the burden of herpes zoster and postherpetic neuralgia (e.g., incidence of herpes zoster, proportion of herpes zoster cases leading to postherpetic neuralgia and QALYs lost to postherpetic neuralgia). Of note, our results remained robust when we used discount rates of 0% and 5%. The choice of discount rate has less impact for herpes zoster vaccines (relative to other vaccines) because the benefits accrue shortly after vaccination. Finally, when assuming that 2 doses were necessary for the recombinant vaccine to provide efficacy, our model predicted that the compliance with the second dose of the recombinant would have to be less than 50% to produce health benefits lower than using the live vaccine.
Interpretation
Our model predicted that the recombinant subunit zoster vaccine is likely cost-effective in Canada for adults 60 years or older and that it provides greater health benefits than the live attenuated zoster vaccine for all age groups. Thus, at a similar cost per series, the recombinant vaccine is likely a more cost-effective option than the live vaccine. The cost per series for the live vaccine would have to be $150 to $200 lower than for the recombinant vaccine for it to be considered a cost-effective alternative.
These results are consistent with other economic analyses of vaccination against herpes zoster conducted in the United States and the Netherlands, which predicted that vaccination with either vaccine is highly likely to be cost-effective, but at the same vaccine price, vaccination with the recombinant vaccine is more cost-effective.34–36
On the basis of the cost-effectiveness analysis and results presented here, NACI recommended that adults 50 years or older receive vaccination with the recombinant vaccine.19 Although that vaccine is predicted to be cost-effective for adults aged 60 years or older, it may not be feasible to vaccinate all of these individuals. Hence, in accordance with our cost-effectiveness results, NACI indicated that, for publicly funded programs, vaccination of adults aged between 65 and 79 years would be the most cost-effective option.
Provincial immunization committees have made different recommendations in terms of the age cohorts to be targeted. The Comité d’immunisation du Québec recommended vaccination with the recombinant vaccine for adults aged 65 years or older, but noted that if it was not economically feasible to target all adults in this age group, adults 70 years or older should be prioritized, because of the greater incidence of herpes zoster and postherpetic neuralgia in this age group.20 Conversely, in Ontario, the live vaccine is publicly funded for adults aged 65 to 70 years, but physicians are obliged to offer both NACI-recommended vaccines to their patients.37 In British Columbia, vaccination against herpes zoster is recommended for adults aged 50 years or older, but there is currently no publicly funded vaccination program.38
Our study had several strengths. It represents a unique examination of the effectiveness and cost-effectiveness of both the live and recombinant vaccines in a Canadian context, and our results are consistent with other economic analyses of herpes zoster vaccines from other counties.34–36,39 To capture the uncertainty around the duration of protection with herpes zoster vaccines, our predictions are based on simulations using 6 different functions for waning of vaccine efficacy (Figure A2 in Appendix 1). We have presented all predictions with 90% UIs, which captures the variability in the estimates of incidence and burden of disease of herpes zoster across Canada. Finally, the conclusions remained robust in our sensitivity analyses.
Limitations
The limitations of this study were mainly related to the availability of empiric data. First, a key factor influencing the cost-effectiveness of both vaccines is the duration of protection. Although both trial and postlicensure studies suggest that the efficacy of the live vaccine declines substantially over time,16–18 there are no long-term efficacy data for the recombinant vaccine. We captured the uncertainty in the duration of the recombinant vaccine by means of 90% UIs and predicted that although waning can affect the cost-effectiveness ratio value, it does not affect the conclusion that this vaccine is likely cost-effective for adults aged 60 years or older.
There may be lower compliance with the second dose of the recombinant vaccine because of grade 3 adverse events described in the trial.14 There are reports from the US that some health care providers are deciding not to administer the second dose after observation of adverse effects following the first dose.40 We examined an extreme scenario in which there would be no vaccine efficacy for adults vaccinated with only 1 dose. Our model predicted that compliance with the second dose had to be less than 50% to produce health benefits lower than would be achieved using the live vaccine. Preliminary data from the US have suggested that compliance with the second dose is about 75% to 85%.41
The randomized trials assessing the efficacy of both vaccines were conducted in healthy, immunocompetent populations. Although some recent unpublished data have suggested that the recombinant vaccine may be slightly less effective against herpes zoster in immunosuppressed populations,42,43 there is no information on whether vaccine efficacy changes if vaccinated adults become immunosuppressed. In our study, we assumed that vaccine efficacy did not change among vaccinated adults who become immunosuppressed. This assumption could lead to overestimation of the effectiveness of herpes zoster vaccination, depending on the proportion of adults who become immunosuppressed over time and on the extent of the decline in vaccine efficacy after they become immunosuppressed.
Conclusion
Our modelling analysis suggests that vaccination against herpes zoster is most likely a cost-effective intervention in Canada. However, vaccination with the recombinant subunit zoster vaccine is predicted to provide greater effectiveness for all age groups and is likely to be more cost-effective than the live attenuated zoster vaccine. Future research should focus on assessing the long-term durability of 2 doses of the recombinant vaccine, compliance with the second dose and efficacy of a single dose of the vaccine.
Footnotes
Competing interests: Philippe DeWals has received research grants and reimbursement for travel expenses from vaccine manufacturers, including the GSK group of companies, Novartis, Pfizer and Sanofi Pasteur. The quality-adjusted life-year estimates were partially derived from MASTER, a study conducted in 2005–2006 and funded by Merck Frosst Canada Ltd. through a collaborative research agreement between Merck and the study’s scientific steering committee, of which Marc Brisson was a member. No other competing interests were declared.
This article has been peer reviewed.
Contributors: Mélanie Drolet and Marc Brisson were primarily responsible for the conception and design of the study, the acquisition of data, and the analysis and interpretation of results; they also drafted the first version of the manuscript. Zhou Zhou performed the analysis and revised the paper for important intellectual content. Chantal Sauvageau, Philippe DeWals, Vladimir Gilca, Rachid Amini and Élodie Bénard contributed to either the acquisition of the data or the analysis, and critically revised the paper for important intellectual content. All of the authors provided final approval of the version to be published, and agreed to be accountable for all aspects of the work.
Funding: This work was funded by the Public Health Agency of Canada, the Ministère de la Santé et des Services Sociaux du Québec, the Canadian Institutes of Health Research (Foundation scheme grant FDN-143283) and the Fonds de recherche du Québec – Santé (support to Marc Brisson). The funders had no role in the study design, data collection, data analysis and interpretation, or the writing of this article.
Data sharing: The data available from this modelling study are presented in the tables and appendices of this article.
References
- 1.Gnann JW, Jr, Whitley RJ. Clinical practice. Herpes zoster. N Engl J Med 2002;347:340–6. [DOI] [PubMed] [Google Scholar]
- 2.Head H, Campbell AW, Kennedy PG. The pathology of herpes zoster and its bearing on sensory localisation. Rev Med Virol 1997;7:131–43. [DOI] [PubMed] [Google Scholar]
- 3.Brisson M, Edmunds WJ, Law B, et al. Epidemiology of varicella zoster virus infection in Canada and the United Kingdom [published erratum in Epidemiol Infect 2015;143:1332]. Epidemiol Infect 2001;127:305–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Edmunds WJ, Brisson M, Rose JD. The epidemiology of herpes zoster and potential cost-effectiveness of vaccination in England and Wales. Vaccine 2001;19:3076–90. [DOI] [PubMed] [Google Scholar]
- 5.Insinga RP, Itzler RF, Pellissier JM, et al. The incidence of herpes zoster in a United States administrative database. J Gen Intern Med 2005;20:748–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oxman MN. Clinical manifestation of herpes zoster. In: Arvin AM, Gerson AA, editors. Varicella zoster virus: virology and clinical management. Cambridge (UK): Cambridge University Press; 2000:246–75. [Google Scholar]
- 7.Opstelten W, Zuithoff NP, van Essen GA, et al. Predicting postherpetic neuralgia in elderly primary care patients with herpes zoster: prospective prognostic study. Pain 2007;132(Suppl 1):S52–9. [DOI] [PubMed] [Google Scholar]
- 8.Schmader KE. Epidemiology and impact on quality of life of postherpetic neuralgia and painful diabetic neuropathy. Clin J Pain 2002;18:350–4. [DOI] [PubMed] [Google Scholar]
- 9.Drolet M, Brisson M, Schmader K, et al. Predictors of postherpetic neuralgia among patients with herpes zoster: a prospective study. J Pain 2010;11:1211–21. [DOI] [PubMed] [Google Scholar]
- 10.Oxman MN, Levin MJ, Johnson GR, et al. A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. N Engl J Med 2005;352:2271–84. [DOI] [PubMed] [Google Scholar]
- 11.Drolet M, Brisson M, Schmader KE, et al. The impact of herpes zoster and postherpetic neuralgia on health-related quality of life: a prospective study. CMAJ 2010;182:1731–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johnson RW, Wasner G, Saddier P, et al. Herpes zoster and postherpetic neuralgia: optimizing management in the elderly patient. Drugs Aging 2008;25:991–1006. [DOI] [PubMed] [Google Scholar]
- 13.Drolet M, Oxman MN, Levin MJ, et al. Vaccination against herpes zoster in developed countries: state of the evidence. Hum Vaccin Immunother 2013;9:1177–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lal H, Cunningham AL, Godeaux O, et al. Efficacy of an adjuvanted herpes zoster subunit vaccine in older adults. N Engl J Med 2015;372:2087–96. [DOI] [PubMed] [Google Scholar]
- 15.Pauksens K, Volpe S, Schwarz T, et al. Persistence of immune response to an adjuvanted varicella-zoster virus subunit candidate vaccine for up to year 9 in older adults [poster]. IDSA ID Week; 2017 Oct. 4–8; San Diego. [Google Scholar]
- 16.Morrison VA, Johnson GR, Schmader KE, et al. Long-term persistence of zoster vaccine efficacy. Clin Infect Dis 2015;60:900–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tseng HF, Harpaz R, Luo Y, et al. Declining effectiveness of herpes zoster vaccine in adults aged ≥ 60 years. J Infect Dis 2016;213:1872–5. [DOI] [PubMed] [Google Scholar]
- 18.Izurieta HS, Wernecke M, Kelman J, et al. Effectiveness and duration of protection provided by the live-attenuated herpes zoster vaccine in the Medicare population ages 65 years and older. Clin Infect Dis 2017;64:785–93. [DOI] [PubMed] [Google Scholar]
- 19.Updated recommendations on the use of herpes zoster vaccines: an Advisory Committee Statement (ACS) National Advisory Committee on Immunization (NACI). Ottawa: Public Health Agency of Canada; 2018. Available: https://www.canada.ca/en/services/health/publications/healthy-living/updated-recommendations-use-herpes-zoster-vaccines.html (accessed 2019 Feb. 22). [Google Scholar]
- 20.Comité sur l’immunisation du Québec (CIQ). Avis sur la pertinence d’ajouter la vaccination contre le zona au Programme québécois d’immunisation. Québec (QC): Institut national de santé publique du Québec; 2018. Available: https://www.inspq.qc.ca/sites/default/files/publications/2381_pertinence_vaccination_zona_programme_quebecois_immunisation.pdf (accessed 2019 Feb. 22). [Google Scholar]
- 21.Brisson M, Pellissier JM, Camden S, et al. The potential cost-effectiveness of vaccination against herpes zoster and post-herpetic neuralgia. Hum Vaccin 2008;4:238–45. [DOI] [PubMed] [Google Scholar]
- 22.Schmader KE, Oxman MN, Levin MJ, et al. Persistence of the efficacy of zoster vaccine in the shingles prevention study and the short-term persistence substudy. Clin Infect Dis 2012;55:1320–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cunningham AL, Lal H, Kovac M, et al. Efficacy of the herpes zoster subunit vaccine in adults 70 years of age or older. N Engl J Med 2016;375:1019–32. [DOI] [PubMed] [Google Scholar]
- 24.Bilcke J, Ogunjimi B, Hulstaert F, et al. Estimating the age-specific duration of herpes zoster vaccine protection: a matter of model choice? Vaccine 2012;30: 2795–800. [DOI] [PubMed] [Google Scholar]
- 25.Letellier MC, Amini R, Gilca V, et al. Herpes zoster burden in Canadian provinces: a narrative review and comparison with Quebec provincial data. Can J Infect Dis Med Microbiol 2018;2018:3285327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Russell ML, Dover DC, Simmonds KA, et al. Shingles in Alberta: before and after publicly funded varicella vaccination. Vaccine 2014;32:6319–24. [DOI] [PubMed] [Google Scholar]
- 27.Tanuseputro P, Zagorski B, Chan KJ, et al. Population-based incidence of herpes zoster after introduction of a publicly funded varicella vaccination program. Vaccine 2011;29:8580–4. [DOI] [PubMed] [Google Scholar]
- 28.Marra F, Chong M, Najafzadeh M. Increasing incidence associated with herpes zoster infection in British Columbia, Canada. BMC Infect Dis 2016;16:589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Najafzadeh M, Marra CA, Galanis E, et al. Cost effectiveness of herpes zoster vaccine in Canada. Pharmacoeconomics 2009;27:991–1004. [DOI] [PubMed] [Google Scholar]
- 30.Friesen KJ, Chateau D, Falk J, et al. Cost of shingles: population-based burden of disease analysis of herpes zoster and postherpetic neuralgia. BMC Infect Dis 2017;17:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brisson M, Johnson RW, Levin MJ, et al. Measuring herpes zoster (HZ) and postherpetic neuralgia (PHN) associated burden of illness, health care utilization and costs in Canada: a clinical epidemiological study. Can J Infect Dis Med Microbiol 2006;17:381. [Google Scholar]
- 32.Consumer price index, annual average, not seasonally adjusted. Ottawa: Statistics Canada; 2019. Available: https://www150.statcan.gc.ca/t1/tbl1/en/tv.action?pid=1810000501(accessed 2019 May). [Google Scholar]
- 33.Cost-effectivess and strategic planning (WHO-CHOICE). Geneva: World Health Organization; 2014. Available: http://www.who.int/choice/en (accessed 2019 Mar. 6). [Google Scholar]
- 34.Curran D, Patterson B, Varghese L, et al. Cost-effectiveness of an adjuvanted recombinant zoster vaccine in older adults in the United States. Vaccine 2018;36:5037–45. [DOI] [PubMed] [Google Scholar]
- 35.Le P, Rothberg MB. Cost-effectiveness of the adjuvanted herpes zoster subunit vaccine in older adults. JAMA Intern Med 2018;178:248–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.de Boer PT, van Lier A, de Melker H, et al. Cost-effectiveness of vaccination of immunocompetent older adults against herpes zoster in the Netherlands: a comparison between the adjuvanted subunit and live-attenuated vaccines. BMC Med 2018;16:228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Immunization update: NACI recommends RZV (Shingrix®) vaccine in persons aged 50 years and older. Ottawa: Ottawa Public Health; 2018. Available: https://www.ottawapublichealth.ca/Modules/News/blogcomments.aspx?feedId=9fd17063-0b6e-449b-8679-d24788af5476&lang=en&BlogId=ed6092fb-2df9-49f6-a711-978f84f3a2dc (accessed 2019 Mar. 7). [Google Scholar]
- 38.Shingles. Vancouver: BC Centre for Disease Control; Available: http://www.bccdc.ca/health-info/diseases-conditions/shingles (accessed 2019 Mar. 7). [Google Scholar]
- 39.Prosser LA, Harpaz R, Rose AM, et al. A cost-effectiveness analysis of vaccination for prevention of herpes zoster and related complications: input for national recommendations. Ann Intern Med 2019. February 19 10.7326/M18-2347. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 40.Hesse EM, Shimabukuro TT, Su JR, et al. Postlicensure safety surveillance of recombinant zoster vaccine (Shingrix) — United States, October 2017–June 2018. MMWR Morb Mortal Wkly Rep 2019;68:91–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dooling K. Herpes zoster vaccines: update. Meeting of Advisory Committee on Immunization Practices; 2019 Feb. 27–28; Atlanta. [Google Scholar]
- 42.New data supports the safety and efficacy of GSK’s Shingrix in preventing shingles in autologous haematopoietic stem cell transplant patients [media release]. London: GlaxoSmithKline; 2017. December 6 Available: https://www.gsk.com/en-gb/media/press-releases/new-data-supports-the-safety-and-efficacy-of-gsk-s-shingrix-in-preventing-shingles-in-autologous-haematopoietic-stem-cell-transplant-patients/ (accessed 2019 Feb. 27). [Google Scholar]
- 43.Dagnew A, Ilhan O, Lee WS, et al. Immunogenicity, safety and post-hoc efficacy assessment of the adjuvanted recombinant zoster vaccine in adults with hematologic malignancies: a phase 3, randomized clinical trial [abstract]. IDSA ID Week; 2018 Oct. 3–7; San Francisco. [Google Scholar]