Abstract
Background
Serotonergic system abnormalities are implicated in many psychiatric disorders, including major depression. The temporal lobe receives a high density of serotonergic afferent projections, and responses in the primary auditory cortex to sound are modulated by serotonergic tone. However, the associations between changes in serotonergic tone, disease state and changes in auditory cortical function remain to be clarified.
Methods
We quantified serotonin 1A (5-HT1A) receptor binding, serotonin 2A (5-HT2A) receptor binding, and serotonin transporter (SERT) binding in Brodmann areas (BA) 41/42, 22, 9 and 4 from postmortem brain sections of 40 psychiatrically healthy controls and 39 individuals who had a history of a major depressive episode (MDE).
Results
There was 33% lower 5-HT2A receptor binding in BA 41/42 in individuals who had an MDE than in controls (p = 0.0069). Neither 5-HT1A nor SERT binding in BA 41/42 differed between individuals who had an MDE and controls. We also found 14% higher 5-HT1A receptor binding (p = 0.045) and 21% lower SERT binding in BA 9 of individuals who had an MDE (p = 0.045).
Limitations
The study was limited by the small number of postmortem brain samples including BA 41/42 available for binding assays and the large overlap between suicide and depression in the MDE sample.
Conclusion
Depression may be associated with altered serotonergic function in the auditory cortex involving the 5-HT2A receptor and is part of a wider view of the pathophysiology of mood disorders extending beyond psychopathology.
Introduction
The temporal lobe, including the superior temporal gyrus, receives some of the highest-density serotonergic innervation in the telencephalon, with the primary auditory cortex receiving denser projections than the secondary auditory cortex.1,2 The serotonergic innervation occurs throughout all cortical layers.3 The loudness-dependent auditory evoked potential (LDAEP), an electroencephalography (EEG) measure originating from the primary auditory cortex, is linked to serotonergic tone in both humans and animals.4 Administration of selective serotonin reuptake inhibitors (SSRIs) and other compounds that increase serotonin, both systemically and locally in the auditory cortex, decrease the magnitude of the LDAEP.5–9 Taken together, these findings suggest that serotonin (5-HT) modulates auditory cortex function.
Serotonergic system abnormalities are implicated in a wide variety of mental health disorders, including major depressive disorder (MDD), bipolar disorder, anxiety disorders and schizophrenia. In MDD, the LDAEP correlates with responsiveness to antidepressant treatment.5,6,10 Depressed patients with larger LDAEPs at baseline have a greater response to SSRIs, and LDAEP magnitude correlates with the degree of improvement as measured by clinical ratings such as the Hamilton Rating Scale for Depression.6,11,12 These findings raise the possibility of functional differences in serotonergic function in the auditory cortex in MDD.
Beyond the LDAEP, our understanding of the role that 5-HT plays in the auditory cortex is limited. Animal studies have shown that 5-HT decreases GABAergic neurotransmission pre- and postsynaptically in the primary auditory cortex via 5-HT1A and 5-HT2A receptors, respectively, and decreases firing rate, input resistance and firing rate adaptation of pyramidal cells in juvenile rats.13,14 Although SSRI treatment has been associated with plasticity in the primary visual cortex,15 this association has not been observed in the primary auditory cortex.16 Tryptophan depletion studies in humans suggest that 5-HT affects early, attention-related processing of auditory stimulus information.7,17 Data on the effect of tryptophan depletion on LDAEP are mixed.7,18–20
The association between 5-HT, the neurophysiology of the auditory cortex and psychopathology remains to be understood. To better understand what effect 5-HT may be exerting in the auditory cortex and how this effect may be different in disease, we studied the expression of 5-HT1A receptors, 5-HT2A receptors and the serotonin transporter (SERT) in postmortem brain tissue sections containing auditory cortex of healthy nonpsychiatric controls and individuals who had a mood disorder and met criteria for a major depressive episode (MDE). Receptor binding was quantified in primary and secondary auditory cortical regions (Brodmann areas [BA] 41/42 and 22) compared with the primary motor cortex (BA 4) and prefrontal cortex (BA 9).
Methods
Details on the collection of brain tissue, toxicology, psychological autopsy and receptor autoradiography have been described in detail in previous publications21,22 and are only summarized here for convenience.
Subjects
Written informed consent was obtained from the next of kin of the deceased individuals from whom the samples were obtained. Both the nonpsychiatric controls and the individuals who had an MDE in the present study were chosen based on the availability of tissue samples with auditory cortex.
Collection of brain samples
Brains were collected in New York, NY, Pittsburgh, PA, and the Republic of Macedonia. The collection of brain samples and brain tissue use were approved by the respective institutional review boards. Brain sample collection procedures were standardized across all collection sites. Brain collection took place at the time of autopsy, within 24 hours of the time of death. Upon removal from the cranium, the dura mater was stripped, the brainstem was separated by a transverse cut just anterior to the superior colliculus and the cerebellum was removed. The brain was bisected and the right hemisphere was cut into slabs approximately 2 cm thick in the coronal plane and frozen in liquid Freon. Samples were then flash-frozen to −80°C and stored at that temperature until further processing. The left hemisphere was placed in formalin for neuropathological examination. A psychological autopsy was used to obtain DSM-IV Axis I and II diagnoses. Lifetime information on suicidal behaviour, medical illness, medications, family history and developmental history was obtained in the psychological autopsy.23 The inclusion criteria for controls were death by accident, homicide, or sudden natural causes; no psychiatric disorder as per DSM-IV criteria24 or history of suicide attempts; and negative toxicology for psychoactive drugs. The inclusion criteria for the MDE sample were Axis I diagnosis of MDD or bipolar disorder, with at least 1 lifetime MDE. Individuals who had an MDE and a history of alcohol use disorder (AUD) were included in this study.
Toxicology
Brain samples were screened for more than 30 prescription medications and drugs of abuse, and were quantified if present. Blood and urine were additionally screened for alcohol, antidepressants, barbiturates, benzodiazepines, cannabis, carbon monoxide, cocaine, opiates, methadone, amphetamines, phencyclidine and salicylates.
Brain sectioning and autoradiography
Brain blocks were sectioned at 20 μM thickness. Receptor autoradiography assays were performed on tissue sections as described previously.22,25,26 In this study, we considered sections that contained BA 4, BA 9, BA 41/42 and BA 22. Prior to incubation with radioligand, endogenous ligands were removed by incubation in buffers. Nonspecific binding was determined by examining adjacent sections that had been incubated with a selective displacer. Sections were washed in incubation buffer at 4°C, dipped in water, rapidly dried and transferred to a vacuum desiccator until ready for exposure to film. Sections were exposed for 4–12 weeks depending on the receptor. Dried slides were arranged in an x-ray film cassette with 3H-containing polymer standards (American Radiolabelled Chemicals). Serotonin transporter availability was quantified by incubation with 0.4 nM of [3H]Cyanoimipramine, using 10 μM sertraline to determine nonspecific binding.25 We measured 5-HT1A receptor availability by incubation with 2 nM [3H]8-OH-DPAT, using 1 μM of 5-HT to assess nonspecific binding.25 Finally, we measured 5-HT2A receptor availability by incubation with 2 nM [3H]Ketanserin, using 1 μM prazosin and 1 μM tetrabenazine to block α-1 and tetrabenazine sites. Nonspecific binding was determined using 1 μM mianserin.27
Autoradiograms were sampled using a computer-based image analysis system (MCID, Interfocus Imaging), as described previously.22 Regions were drawn using a pointing device. The sampled regions at this anatomic level included BA4, BA9, BA41/42 and BA22, as defined using the atlas of Byrd (unpublished), as previously described.22,25
Statistical analysis
All data analyses were completed using custom scripts written for MATLAB 2017R. Initially, the binding profiles in the gyri of each BA in the depressed and control samples were compared using a Student t test. To account for the fact that receptor levels can change with age and differ by sex, we also analyzed ligand binding data from each BA using a general linear model. In these models, ligand binding was designated as the dependent variable, and diagnosis, sex and age were independent variables. Binding data from the gyri and sulci of brain tissue samples were modelled separately. However, because findings between gyri and sulci were very similar, only gyrus binding data are presented here, unless otherwise noted. Because 5-HT1A receptor, 5-HT2A receptor and SERT binding has previously been shown to be influenced by alcoholism,28–31 we created separate general linear models using 5-HT1A, 5-HT2A and SERT binding as the dependent variables, and age, sex and AUD as independent variables for the MDE sample only. Similarly, because previously published work has suggested that serotonergic activity may differ between individuals with unipolar and bipolar depression,32 we used a general linear model to examine the effect of unipolar versus bipolar depression diagnosis, age and sex on 5-HT1A, 5-HT2A and SERT binding in the MDE sample only. There were no brain section samples from individuals who had bipolar depression who had binding data for all 3 receptor subtypes from BA 41/42; therefore, this latter general linear model could not be completed for this region.
Results
Demographic characteristics and other sample information
Brain tissue samples from 76 individuals were included in this study: 40 controls and 36 who had an MDE. The demographic characteristics of both groups are listed in Table 1. There were no significant differences between the groups with regard to age, sex, racial/ethnic background and exposure to childhood adversity. Women were underrepresented in both samples, as expected from a medical examiner collection of a population with many accident and suicide decedents. Most of the individuals in the MDE group (> 90%) died by suicide. This sample also included 4 people who met diagnostic criteria for bipolar disorder. Approximately one-third of the individuals with an MDE had a history of AUD. Appendix 1, available at jpn.ca/180190-a1, includes information on age, sex, race/ethnicity, diagnosis, cause of death, postmortem interval, brain pH and brain toxicology for individuals in each group.
Table 1.
Demographic and clinical characteristics of participants in the study
| Characteristic | Group, no. (%)* | p value | ||
|---|---|---|---|---|
|
| ||||
| Control (n = 40) | MDE (n = 36) | Whole sample (n = 76) | ||
| Age, yr, mean ± SD | 48 ± 18.0 | 47 ± 21.3 | 48 ± 19.6 | 0.97 |
| Female sex | 9 (22.5) | 11 (30.5) | 20 (26.3) | 0.27 |
| Suicide | — | 34 (94.4) | 34 (44.7) | |
| Bipolar disorder | — | 4 (11.1) | 4 (5.2) | |
| AUD | — | 12 (33.3) | 12 (15.8) | |
| Childhood adversity | 10 (25) | 10 (27.8) | 20 (26.3) | 0.74 |
| Race/ethnicity | 0.51 | |||
| Asian | 1 (2.5) | 1 (2.7) | 2 (2.6) | |
| African American | 7 (17.5) | 2 (5.5) | 9 (11.8) | |
| White | 28 (70) | 27 (75) | 55 (72.4) | |
| Hispanic | 6 (15) | 5 (13.8) | 11 (14.5) | |
AUD = alcohol use disorder; MDE = major depressive episode; SD = standard deviation.
Unless indicated otherwise.
5-HT2A receptor binding
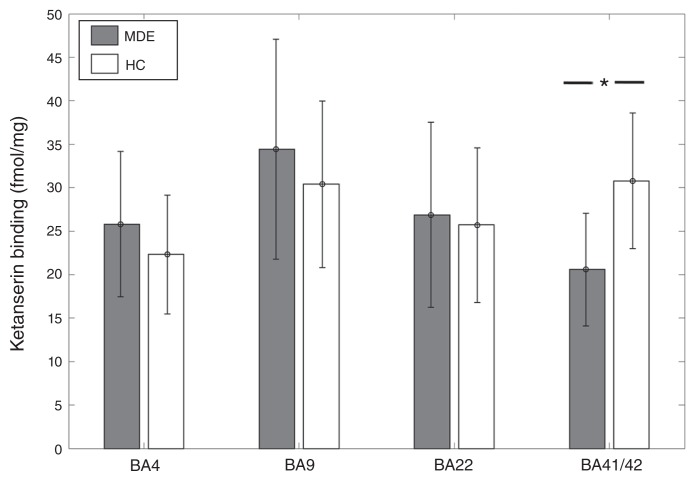
[3H]Ketanserin binding was 33% lower in BA41/42 of individuals who had an MDE (n = 8) than in controls (n = 12) (MDE mean 20.59 ± 6.5 fmol/mg tissue; controls mean 30.78 ± 7.8 fmol/mg tissue; t = −3.05, p = 0.0069; Fig. 1). In the general linear model, when accounting for the effects of age and sex, this difference remained significant with an inverse association between [3H]Ketanserin binding and MDE (t = −2.44, p = 0.027). [3H]Ketanserin binding did not differ significantly between the MDE and control samples in BA 22 (27 MDE, 31 controls, t = 0.23, p = 0.8), BA 4 (24 MDE, 23 controls, t = 1.56, p = 0.13) and BA 9 (28 MDE, 35 controls, t = 1.44, p = 0.15). However, in the general linear model, accounting for age and sex, BA 4 showed a trend-level positive association between [3H]Ketanserin binding and MDE (t = 1.71, p = 0.095).
Fig. 1.
[3H]Ketanserin binding across Brodmann area (BA) 4, BA 9, BA 22, and BA 41/42 in healthy controls (HC) and individuals with a history of major depressive episodes (MDE). [3H]Ketanserin binding was 33% lower in BA41/42 of the MDE compared with the control group (MDE mean 20.59 ± 6.5 fmol/mg, HC mean 30.78 ± 7.8 fmol/mg; t = −3.05, p = 0.0069). There were no significant differences in BA 4 (MDE mean 24.81 ± 8.35 fmol/mg, HC mean 22.32 ± 6.84 fmol/mg), BA 9 (MDE mean 34.44 ± 12.64 fmol/mg, HC mean 30.4 ± 9.57 fmol/mg), and BA 22 (MDE mean 25.86 ± 10.65 fmol/mg, HC mean 24.76 ± 8.75 fmol/mg).
Within the MDE sample, [3H]Ketanserin binding showed a positive association with AUD in BA 41 (t = 3.65, p = 0.022) and BA 22 (t = 2.3, p = 0.025). To account for the effect of AUD in the comparison between the MDE and control samples, AUD was included as an additional independent variable in the general linear model. This did not significantly change the negative association between MDE and [3H]Ketanserin binding (t = −2.86, p = 0.012). [3H]Ketanserin binding did not show any association with bipolar depression in any BA other than a trend-level positive association in BA 9 (t = 1.84, p = 0.078).
The general linear model for BA 22 showed a significant positive association between male sex and [3H]Ketanserin binding (t = 3.3, p = 0.0017), indicating higher binding in males, as well as an inverse association with age (t = −4.15, p = 0.00011). In BA 9 and BA 4, the general linear model showed an inverse association between age and [3H]Ketanserin binding (BA 9: t = −3.93, p = 0.00022; BA 4: t = −3.31, p = 0.0019).
5-HT1A receptor binding
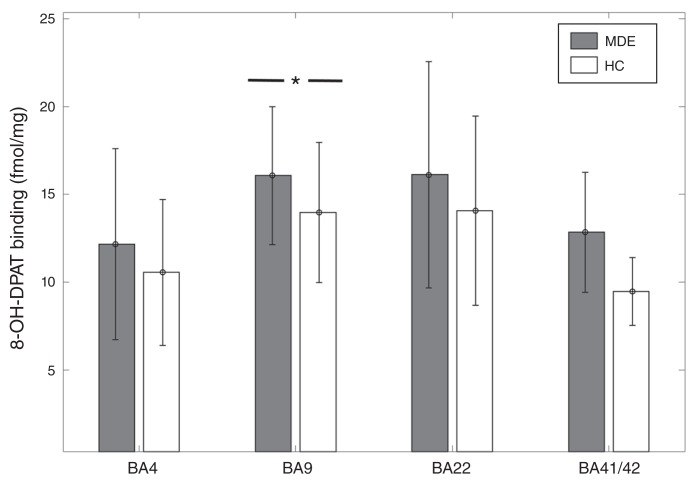
In BA 9, [3H]8-OH-DPAT binding was 14% higher in the MDE sample (n = 29) than the control sample (n = 31) (MDE mean 16.07 ± 3.92 fmol/mg; control mean 13.98 ± 3.99 fmol/mg, t = 2.05, p = 0.045; Fig. 2). However, after accounting for sex and age with the general linear model, this result became a trend-level finding in gyrus data (t = 1.79, p = 0.079). There was no significant difference in [3H]8-OH-DPAT binding in BA 41 (6 MDE, 2 controls, t = 1.28, p = 0.25), BA 22 (33 MDE, 34 controls, t = 1.46, p = 0.15), or BA 4 (30 MDD, 26 controls, t = 1.23, p = 0.22; Fig. 2).
Fig. 2.
[3H]8-OH-DPAT binding across Brodmann area (BA) 4, BA 9, BA 22, and BA 41/42 in healthy controls (HC) and individuals with a history of major depressive episodes (MDE). [3H]8-OH-DPAT binding was 14% higher in BA 9 of the MDE group compared with the control group (MDE mean 16.07 ± 3.92 fmol/mg, HC mean 13.98 + 3.99 fmol/mg, t = 2.05, p = 0.045). However, after accounting for the effects of age and sex, this finding became a trend-level observation (t = 1.79, p = 0.079). There was no significant difference between the MDE group and the control group in BA 4 (MDE mean 12.17 ± 5.45 fmol/mg, HC mean 10.56 ± 4.15 fmol/mg), BA 22 (MDE mean 16.13 ± 6.44 fmol/mg, HC 14.07 ± 5.38 fmol/mg), and BA 41/42 (MDE mean 12.84 ± 3.42 fmol/mg, HC mean 9.47 ± 1.94 fmol/mg).
The generalized linear model for [3H]8-OH-DPAT binding in BA 22 showed a significant inverse association with age in gyrus binding measures (t = −2.93, p = 0.0047), but only a trend-level association was found with sulcus binding measures (t = −1.87, p = 0.066). In BA 9 there was an inverse association between [3H]8-OH-DPAT binding and age (t = −2.18, p = 0.033), and in sulcus binding measures there was an inverse association with male sex, such that males had lower binding (t = −2.14, p = 0.036). In BA 4, there was a positive association between [3H]8-OH-DPAT binding and male sex in sulcus data (t = 2.25, p = 0.029), and there were trend-level associations for age in gyrus and sulcus (t = −1.99, p = 0.051). [3H]8-OH-DPAT binding did not show any association with AUD or bipolar depression in BA 22, BA 9 and BA 4. The data were insufficient to assess the effects of AUD and bipolar depression in BA 41.
SERT binding
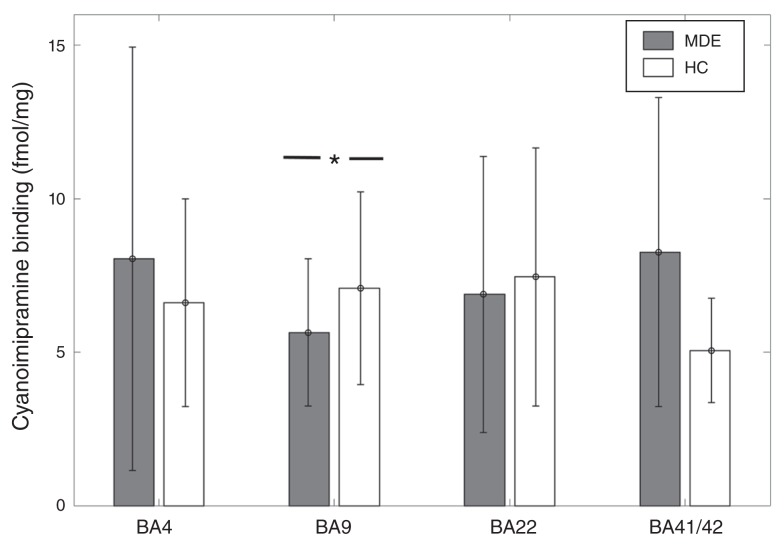
[3H]Cyanoimipramine binding was 21% lower in BA 9 in the MDE sample (n = 30) than in the control sample (n = 35) in sulcus data (MDE mean 5.65 ± 2.4 fmol/mg; controls mean 7.09 ± 3.14 fmol/mg, t = −2.04, p = 0.045; Fig. 3). This finding remained significant after accounting for the effects of age and sex with the general linear model (t = 2.13, p = 0.037). [3H]Cyanoimipramine binding did not differ significantly between the MDE and control samples in BA 41/42 (7 MDE, 6 controls, t = 0.78, p = 0.45), BA 22 (26 MDE, 26 controls, t = −0.89, p = 0.37) and BA 4 (22 MDE, 23 controls, t = 0.61, p = 0.53; Fig. 3).
Fig. 3.
[3H]Cyanoimipramine binding across Brodmann area (BA) 4, BA 9, BA 22, and BA 41/42 in healthy controls (HC) and individuals with a history of major depressive episodes (MDE). [3H]Cyanoimipramine binding was 21% lower in BA 9 in the MDE group compared with the control group in sulcus data (MDE mean 5.65 ± 2.4 fmol/mg, HC mean 7.09 ± 3.14 fmol/mg, t = −2.04, p = 0.045). There was no significant difference between the MDE group and the control group in BA 4 (MDE mean 8.04 ± 6.93 fmol/mg, HC mean 6.61 ± 3.4 fmol/mg), BA 22 (MDE mean 6.89 ± 4.53 fmol/mg, HC mean 7.45 ± 4.25 fmol/mg), and BA 41/42 (MDE mean 8.26 ± 5.04 fmol/mg, HC mean 5.05 ± 1.69 fmol/mg).
[3H]Cyanoimipramine binding showed a negative association with male sex in gyrus data in BA 22 (t = −2.53, p = 0.015) and BA 41/42 (t = −2.26, p = 0.05). Binding also showed a negative association with age in BA 22 gyrus data (t = −2.3, p = 0.026) and both gyrus and sulcus data in BA 9 (t = −2.18, p = 0.033). There was a trend-level positive association between AUD and [3H]Cyanoimipramine binding in BA 9 (t = 1.75, p = 0.092) and BA 4 (t = 1.86, p = 0.079). There was no association between [3H]Cyanoimipramine binding and bipolar depression in any BA.
Discussion
In this study, we found that individuals who had a history of MDE had lower 5-HT2A receptor binding, specifically in BA 41/42, where the primary auditory cortex is located. Although we did not perform measurements to determine whether these changes were due to lower numbers of receptors or changes in affinity of receptors, previous studies using brain homogenates support the former conclusion.33–35 This observation indicates perturbations in serotonergic function in MDD in cortical, early-stage auditory processing areas. This finding is consistent with previous data that indicate that primary auditory cortex responses are modulated by 5-HT availability5,7–9 and that these cortical responses may predict treatment response in MDD.6,10,11 Furthermore, a similar finding in postmortem tissue of patients with schizophrenia has been reported.36
While modulation of auditory responses by 5-HT has been observed as early in the auditory processing pathway as the inferior colliculus,37–40 our finding also suggests that modulation of auditory cortical responses is not merely carried forward from lower processing regions. To date, the receptors and specific cell types that underlie the serotonergic modulation of the loudness-dependent response in the primary auditory cortex remain unclear, though previous work has implicated both 5-HT1A receptors41 and SERT.42 Our work suggests that the role of 5-HT2A receptors should also be examined.
We did not observe significant differences between controls and depressed individuals in 5-HT2A receptor binding in other control regions examined, most notably in the prefrontal cortex (BA 9), which is consistent with the results of some previous studies.43–47 However, other studies have reported increased 5-HT2A receptor levels in the prefrontal cortex of individuals who died by suicide.33–35,48–50 The 5-HT2A receptor binding in these regions may be modulated by aggression and whether the individual died by violent suicide.45,47 As in other studies, the effect of depression is difficult to separate from the effect of suicide in our sample, given that most of the individuals in our MDE sample died by suicide.
In addition, we observed lower levels of SERT binding and higher levels of 5-HT1A receptor binding in BA 9 in the MDE sample. However, the latter finding did not remain significant after accounting for the effect of age and sex. Lower SERT binding in BA 9 is consistent with the findings of previous postmortem studies, which reported lower prefrontal cortex SERT binding in depression and suicide.25,31,51 Lower SERT binding has also been described previously in midbrain,52–55 anterior cingulate and subcortical regions55,56 in positron emission tomography studies of individuals with MDD. However, others have also reported no differences or higher binding in these regions.57–60 Similarly, our finding of higher 5-HT1A receptor binding in BA 9 is consistent with those of some previous postmortem61 and positron emission tomography studies,62–66 though others have differed in their findings.67–73 Overall, these findings may indicate adaptations to changes in serotonergic tone in the prefrontal cortex, suggesting decreased 5-HT availability.
Adaptive changes in depression may be regionally specific, given that lower 5-HT2A receptor binding was observed only in the primary auditory cortex, but not in other regions. This may relate to the specific functional role that these receptors play in various cortical regions. Although the specific role of 5-HT2A receptors in the auditory cortex has yet to be clarified, it has been suggested that they play an important role in postnatal cortical development.74 In mature auditory cortex, 5-HT2A receptors contribute tracking of dynamic tonal structure in sound.75 Several hallucinogens, such as lysergic acid diethylamide (LSD), are 5-HT2A receptor agonists and are known to produce auditory hallucinations and perturbations. It is notable that lower 5-HT2A receptor levels have also been found in the auditory cortex of patients with schizophrenia,36 a disease characterized by hallucinations and in which serotonergic dysfunction is also thought to play an important role given the serotonergic activity of many second-generation antipsychotic medications.
Limitations
Limitations of this study include the small number of postmortem brain samples including BA 41/42 available for binding assays. Despite this limitation, our analysis yielded a significant finding. However, the study was not adequately powered to correct for multiple comparisons, which we did not perform in these analyses. In addition, the effect of depression was confounded by the effect of suicide and aggression, as indicated by violent suicide deaths, in this sample. Therefore, this study should be considered preliminary; further replications of this finding are needed.
Conclusion
We present here a preliminary but novel finding of lower 5-HT2A receptor binding in the primary auditory cortex of individuals in an MDE at the time of death. This finding supports previous functional data that indicate that depression is associated with changes in the primary auditory cortex. Furthermore, our results suggest that adaptive changes in depression may be regionally specific, given that lower 5-HT2A receptor binding was observed only in the primary auditory cortex, whereas changes in SERT binding were confined to BA 9 in this study. Our data support further study of potential biomarkers of depression in auditory processing regions.
Acknowledgements
The authors thank Dr. Steve Ellis for his feedback on the statistical analysis of the data.
Footnotes
Competing interests: J. Mann receives royalties from the Research Foundation of Mental Hygiene for commercial use of the C-SSRS. No other competing interests declared.
Contributors: L. Steinberg, M. Underwood, J. Mann and V. Arango designed the study. M. Underwood, M. Bakalian, S. Kassir, J. Mann and V. Arango acquired the data, which L. Steinberg, M. Underwood, M. Bakalian, J. Mann and V. Arango analyzed. L. Steinberg and M. Bakalian wrote the article, which all authors reviewed. All authors approved the final version to be published and can certify that no other individuals not listed as authors have made substantial contributions to the paper.
References
- 1.Azmitia EC, Gannon PJ. The primate serotonergic system: a review of human and animal studies and a report on Macaca fascicularis. Adv Neurol. 1986;43:407–68. [PubMed] [Google Scholar]
- 2.Lewis DA, Campbell MJ, Foote SL, et al. The monoaminergic innervation of primate neocortex. Hum Neurobiol. 1986;5:181–8. [PubMed] [Google Scholar]
- 3.Campbell MJ, Lewis DA, Foote SL, et al. Distribution of choline acetyltransferase-, serotonin-, dopamine-beta-hydroxylase-, tyrosine hydroxylase-immunoreactive fibers in monkey primary auditory cortex. J Comp Neurol. 1987;261:209–20. doi: 10.1002/cne.902610204. [DOI] [PubMed] [Google Scholar]
- 4.Juckel G, Pogarell O, Agustin H, et al. Serotonin: from sensory processing to schizophrenia using an electrophysiological method. Behav Brain Res. 2015;277:121–4. doi: 10.1016/j.bbr.2014.05.042. [DOI] [PubMed] [Google Scholar]
- 5.Hegerl U, Juckel G. Intensity dependence of auditory evoked potentials as an indicator of central serotonergic neurotransmission: a new hypothesis. Biol Psychiatry. 1993;33:173–87. doi: 10.1016/0006-3223(93)90137-3. [DOI] [PubMed] [Google Scholar]
- 6.Juckel G, Pogarell O, Augustin H, et al. Differential prediction of first clinical response to serotonergic and noradrenergic antidepressants using the loudness dependence of auditory evoked potentials in patients with major depressive disorder. J Clin Psychiatry. 2007;68:1206–12. doi: 10.4088/jcp.v68n0806. [DOI] [PubMed] [Google Scholar]
- 7.Kähkönen S, Jääskeläinen IP, Pennanen S, et al. Acute tryptophan depletion decreases intensity dependence of auditory evoked magnetic N1/P2 dipole source activity. Psychopharmacology (Berl) 2002;164:221–7. doi: 10.1007/s00213-002-1194-z. [DOI] [PubMed] [Google Scholar]
- 8.Nathan PJ, Segrave R, Phan KL, et al. Direct evidence that acutely enhancing serotonin with the selective serotonin reuptake inhibitor citalopram modulates the loudness dependence of the auditory evoked potential (LDAEP) marker of central serotonin function. Hum Psychopharmacol. 2006;21:47–52. doi: 10.1002/hup.740. [DOI] [PubMed] [Google Scholar]
- 9.Wutzler A, Winter C, Kitzrow W, et al. Loudness dependence of auditory evoked potentials as indicator of central serotonergic neurotransmission: simultaneous electrophysiological recordings and in vivo microdialysis in the rat primary auditory cortex. Neuropsychopharmacology. 2008;33:3176–81. doi: 10.1038/npp.2008.42. [DOI] [PubMed] [Google Scholar]
- 10.Jaworska N, Blondeau C, Tessier P, et al. Response prediction to antidepressants using scalp and source-localized loudness dependence of auditory evoked potential (LDAEP) slopes. Prog Neuropsychopharmacol Biol Psychiatry. 2013;44:100–7. doi: 10.1016/j.pnpbp.2013.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee B-H, Park Y-M, Lee S-H, et al. Prediction of long-term treatment response to selective serotonin reuptake inhibitors (SSRIs) using scalp and source loudness dependence of auditory evoked potentials (LDAEP) analysis in patients with major depressive disorder. Int J Mol Sci. 2015;16:6251–65. doi: 10.3390/ijms16036251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Linka T, Müller BW, Bender S, et al. The intensity dependence of the auditory evoked N1 component as a predictor of reponse to citalopram treatment in patients with major depression. Neurosci Lett. 2004;367:375–8. doi: 10.1016/j.neulet.2004.06.038. [DOI] [PubMed] [Google Scholar]
- 13.García-Oscos F, Torres-Ramirez O, Dinh L, et al. Activation of 5-HT receptors inhibits GABAergic transmission by pre-and postsynaptic mechanisms in layer II/III of the juvenile rat auditory cortex: synaptic mechanisms in rat auditory cortex. Synapse. 2015;69:115–27. doi: 10.1002/syn.21794. [DOI] [PubMed] [Google Scholar]
- 14.Rao D, Basura GJ, Roche J, et al. Hearing loss alters serotonergic modulation of intrinsic excitability in auditory cortex. J Neurophysiol. 2010;104:2693–703. doi: 10.1152/jn.01092.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maya Vetencourt JF, Sale A, Viegi A, et al. The antidepressant fluoxetine restores plasticity in the adult visual cortex. Science. 2008;320:385–8. doi: 10.1126/science.1150516. [DOI] [PubMed] [Google Scholar]
- 16.Dringenberg HC, Branfield Day LR, Choi DH. Chronic fluoxetine treatment suppresses plasticity (long-term potentiation) in the mature rodent primary auditory cortex in vivo. Neural Plast. 2014;2014:1–9. doi: 10.1155/2014/571285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ahveninen J, Jääskeläinen IP, Pennanen S, et al. Auditory selective attention modulated by tryptophan depletion in humans. Neurosci Lett. 2003;340:181–4. doi: 10.1016/s0304-3940(03)00102-2. [DOI] [PubMed] [Google Scholar]
- 18.Debener S, Strobel A, Kürschner K, et al. Is auditory evoked potential augmenting/reducing affected by acute tryptophan depletion? Biol Psychol. 2002;59:121–33. doi: 10.1016/s0301-0511(01)00132-6. [DOI] [PubMed] [Google Scholar]
- 19.Dierks T, Barta S, Demisch L, et al. Intensity dependence of auditory evoked potentials (AEPs) as biological marker for cerebral serotonin levels: effects of tryptophan depletion in healthy subjects. Psychopharmacology (Berl) 1999;146:101–7. doi: 10.1007/s002130051094. [DOI] [PubMed] [Google Scholar]
- 20.Massey AE, Marsh VR, McAllister-Williams RH. Lack of effect of tryptophan depletion on the loudness dependency of auditory event related potentials in healthy volunteers. Biol Psychol. 2004;65:137–45. doi: 10.1016/j.biopsycho.2003.03.001. [DOI] [PubMed] [Google Scholar]
- 21.Boldrini M, Underwood MD, Hen R, et al. Antidepressants increase neural progenitor cells in the human hippocampus. Neuropsychopharmacololgy. 2009;34:2376–89. doi: 10.1038/npp.2009.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Underwood MD, Mann JJ, Arango V. Morphometry of dorsal raphe nucleus serotonergic neurons in alcoholism. Alcohol Clin Exp Res. 2007;31:837–45. doi: 10.1111/j.1530-0277.2007.00365.x. [DOI] [PubMed] [Google Scholar]
- 23.Kelly TM, Mann JJ. Validity of DSM-III-R diagnosis by psychological autopsy: a comparison with clinician ante-mortem diagnosis. Acta Psychiatr Scand. 1996;94:337–43. doi: 10.1111/j.1600-0447.1996.tb09869.x. [DOI] [PubMed] [Google Scholar]
- 24.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Washington (DC): APA; 1994. [Google Scholar]
- 25.Arango V, Underwood MD, Gubbi AV, et al. Localized alterations in pre- and postsynaptic serotonin binding sites in the ventrolateral prefrontal cortex of suicide victims. Brain Res. 1995;688:121–33. doi: 10.1016/0006-8993(95)00523-s. [DOI] [PubMed] [Google Scholar]
- 26.Arango V, Underwood MD, Mann JJ. Serotonin brain circuits involved in major depression and suicide. Prog Brain Res. 2002;136:443–53. doi: 10.1016/s0079-6123(02)36037-0. [DOI] [PubMed] [Google Scholar]
- 27.Glennon RA. Serotonin receptors: clinical implications. Neurosci Biobehav Rev. 1990;14:35–47. doi: 10.1016/s0149-7634(05)80158-7. [DOI] [PubMed] [Google Scholar]
- 28.Storvik M, Tiihonen J, Haukijärvi T, et al. Lower serotonin transporter binding in caudate in alcoholics. Synapse. 2006;59:144–51. doi: 10.1002/syn.20228. [DOI] [PubMed] [Google Scholar]
- 29.Storvik M, Tiihonen J, Haukijärvi T, et al. Nucleus accumbens serotonin transporters in alcoholics measured by whole-hemisphere autoradiography. Alcohol. 2006;40:177–84. doi: 10.1016/j.alcohol.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 30.Underwood MD, Mann JJ, Arango V. Serotonergic and noradrenergic neurobiology of alcoholic suicide. Alcohol Clin Exp Res. 2004;28:57S–69S. doi: 10.1097/01.alc.0000127415.15000.ca. [DOI] [PubMed] [Google Scholar]
- 31.Underwood MD, Kassir SA, Bakalian MJ, et al. Serotonin receptors and suicide, major depression, alcohol use disorder and reported early life adversity. Transl Psychiatry. 2018;14:279. doi: 10.1038/s41398-018-0309-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wiste AK, Arango V, Ellis SP, et al. Norepinephrine and serotonin imbalance in the locus coeruleus in bipolar disorder. Bipolar Disord. 2008;10:349–59. doi: 10.1111/j.1399-5618.2007.00528.x. [DOI] [PubMed] [Google Scholar]
- 33.Arora RC, Meltzer HY. Serotonergic measures in the brains of suicide victims: 5-HT2 binding sites in the frontal cortex of suicide victims and control subjects. Am J Psychiatry. 1989;146:730–6. doi: 10.1176/ajp.146.6.730. [DOI] [PubMed] [Google Scholar]
- 34.Arango V, Ernsberger P, Marzuk PM, et al. Autoradiographic demonstration of increased serotonin 5-HT2 and beta-adrenergic receptor binding sites in the brain of suicide victims. Arch Gen Psychiatry. 1990;47:1038–47. doi: 10.1001/archpsyc.1990.01810230054009. [DOI] [PubMed] [Google Scholar]
- 35.Hrdina PD, Demeter E, Vu TB, et al. 5-HT uptake sites and 5-HT2 receptors in brain of antidepressant-free suicide victims/depressives: increase in 5-HT2 sites in cortex and amygdala. Brain Res. 1993;614:37–44. doi: 10.1016/0006-8993(93)91015-k. [DOI] [PubMed] [Google Scholar]
- 36.Kang K, Huang X-F, Wang Q, et al. Decreased density of serotonin 2A receptors in the superior temporal gyrus in schizophrenia — a postmortem study. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:867–71. doi: 10.1016/j.pnpbp.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 37.Ebert U, Ostwald J. Serotonin modulates auditory information processing in the cochlear nucleus of the rat. Neurosci Lett. 1992;145:51–4. doi: 10.1016/0304-3940(92)90201-h. [DOI] [PubMed] [Google Scholar]
- 38.Felix RA, Elde CJ, Nevue AA, et al. Serotonin modulates response properties of neurons in the dorsal cochlear nucleus of the mouse. Hear Res. 2017;344:13–23. doi: 10.1016/j.heares.2016.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hurley LM, Pollak GD. Serotonin differentially modulates responses to tones and frequency-modulated sweeps in the inferior colliculus. J Neurosci. 1999;19:8071–82. doi: 10.1523/JNEUROSCI.19-18-08071.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Papesh MA, Hurley LM. Modulation of auditory brainstem responses by serotonin and specific serotonin receptors. Hear Res. 2016;332:121–36. doi: 10.1016/j.heares.2015.11.014. [DOI] [PubMed] [Google Scholar]
- 41.Guille V, Gogos A, Nathan PJ, et al. Interaction of estrogen with central serotonergic mechanisms in human sensory processing: loudness dependence of the auditory evoked potential and mismatch negativity. J Psychopharmacol. 2011;25:1614–22. doi: 10.1177/0269881110370506. [DOI] [PubMed] [Google Scholar]
- 42.Lee IH, Yang YK, Chen PS, et al. Loudness dependence of auditory evoked potentials (LDAEP) correlates with the availability of dopamine transporters and serotonin transporters in healthy volunteers — a two isotopes SPECT study. Psychopharmacology (Berl) 2011;214:617–24. doi: 10.1007/s00213-010-2064-8. [DOI] [PubMed] [Google Scholar]
- 43.Cheetham SC, Crompton MR, Katona CL, et al. Brain 5-HT2 receptor binding sites in depressed suicide victims. Brain Res. 1988;443:272–80. doi: 10.1016/0006-8993(88)91621-6. [DOI] [PubMed] [Google Scholar]
- 44.Arranz B, Eriksson A, Mellerup E, et al. Brain 5-HT1A, 5-HT1D, and 5-HT2 receptors in suicide victims. Biol Psychiatry. 1994;35:457–63. doi: 10.1016/0006-3223(94)90044-2. [DOI] [PubMed] [Google Scholar]
- 45.Lowther S, De Paermentier F, Crompton MR, et al. Brain 5-HT2 receptors in suicide victims: violence of death, depression and effects of antidepressant treatment. Brain Res. 1994;642:281–9. doi: 10.1016/0006-8993(94)90932-6. [DOI] [PubMed] [Google Scholar]
- 46.Stockmeier CA, Dilley GE, Shapiro LA, et al. Serotonin receptors in suicide victims with major depression. Neuropsychopharmacolology. 1997;16:162–73. doi: 10.1016/S0893-133X(96)00170-4. [DOI] [PubMed] [Google Scholar]
- 47.Oquendo MA, Russo SA, Underwood MD, et al. Higher postmortem prefrontal 5-HT2A receptor binding correlates with lifetime aggression in suicide. Biol Psychiatry. 2006;59:235–43. doi: 10.1016/j.biopsych.2005.06.037. [DOI] [PubMed] [Google Scholar]
- 48.Turecki G, Brière R, Dewar K, et al. Prediction of level of serotonin 2A receptor binding by serotonin receptor 2A genetic variation in postmortem brain samples from subjects who did or did not commit suicide. Am J Psychiatry. 1999;156:1456–8. doi: 10.1176/ajp.156.9.1456. [DOI] [PubMed] [Google Scholar]
- 49.Stanley M, Mann JJ. Increased serotonin-2 binding sites in frontal cortex of suicide victims. Lancet Lond Engl. 1983;1:214–6. doi: 10.1016/s0140-6736(83)92590-4. [DOI] [PubMed] [Google Scholar]
- 50.Mann JJ, Stanley M, McBride PA, et al. Increased serotonin2 and beta-adrenergic receptor binding in the frontal cortices of suicide victims. Arch Gen Psychiatry. 1986;43:954–9. doi: 10.1001/archpsyc.1986.01800100048007. [DOI] [PubMed] [Google Scholar]
- 51.Mann JJ, Huang YY, Underwood MD, et al. A serotonin transporter gene promoter polymorphism (5-HTTLPR) and prefrontal cortical binding in major depression and suicide. Arch Gen Psychiatry. 2000;57:729–38. doi: 10.1001/archpsyc.57.8.729. [DOI] [PubMed] [Google Scholar]
- 52.Hsieh PC, Chen KC, Yeh TL, et al. Lower availability of midbrain serotonin transporter between healthy subjects with and without a family history of major depressive disorder — a preliminary two-ligand SPECT study. Eur Psychiatry. 2014;29:414–8. doi: 10.1016/j.eurpsy.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 53.Parsey RV, Hastings RS, Oquendo MA, et al. Lower serotonin transporter binding potential in the human brain during major depressive episodes. Am J Psychiatry. 2006;163:52–8. doi: 10.1176/appi.ajp.163.1.52. [DOI] [PubMed] [Google Scholar]
- 54.Miller JM, Hesselgrave N, Ogden RT, et al. Positron emission tomography quantification of serotonin transporter in suicide attempters with major depressive disorder. Biol Psychiatry. 2013;74:287–95. doi: 10.1016/j.biopsych.2013.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Miller JM, Kinnally EL, Ogden RT, et al. Reported childhood abuse is associated with low serotonin transporter binding in vivo in major depressive disorder. Synapse. 2009;63:565–73. doi: 10.1002/syn.20637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Staley JK, Sanacora G, Tamagnan G, et al. Sex differences in diencephalon serotonin transporter availability in major depression. Biol Psychiatry. 2006;59:40–7. doi: 10.1016/j.biopsych.2005.06.012. [DOI] [PubMed] [Google Scholar]
- 57.Meyer JH, Houle S, Sagrati S, et al. Brain serotonin transporter binding potential measured with carbon 11-labeled DASB positron emission tomography: effects of major depressive episodes and severity of dysfunctional attitudes. Arch Gen Psychiatry. 2004;61:1271–9. doi: 10.1001/archpsyc.61.12.1271. [DOI] [PubMed] [Google Scholar]
- 58.Cannon DM, Ichise M, Rollis D, et al. Elevated serotonin transporter binding in major depressive disorder assessed using positron emission tomography and [11C]DASB; comparison with bipolar disorder. Biol Psychiatry. 2007;62:870–7. doi: 10.1016/j.biopsych.2007.03.016. [DOI] [PubMed] [Google Scholar]
- 59.Ichimiya T, Suhara T, Sudo Y, et al. Serotonin transporter binding in patients with mood disorders: a PET study with [11C](+) McN5652. Biol Psychiatry. 2002;51:715–22. doi: 10.1016/s0006-3223(01)01351-8. [DOI] [PubMed] [Google Scholar]
- 60.Ruhé HG, Booij J, Reitsma JB, et al. Serotonin transporter binding with [123I]beta-CIT SPECT in major depressive disorder versus controls: effect of season and gender. Eur J Nucl Med Mol Imaging. 2009;36:841–9. doi: 10.1007/s00259-008-1057-x. [DOI] [PubMed] [Google Scholar]
- 61.Underwood MD, Kassir SA, Bakalian MJ, et al. Neuron density and serotonin receptor binding in prefrontal cortex in suicide. Int J Neuropsychopharmacol. 2012;15:435–47. doi: 10.1017/S1461145711000691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hesselgrave N, Parsey RV. Imaging the serotonin 1A receptor using [11C]WAY100635 in healthy controls and major depression. Philos Trans R Soc Lond B Biol Sci. 2013;368 doi: 10.1098/rstb.2012.0004. 20120004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kaufman J, Sullivan GM, Yang J, et al. Quantification of the serotonin 1A receptor using PET: identification of a potential biomarker of major depression in males. Neuropsychopharmacology. 2015;40:1692–9. doi: 10.1038/npp.2015.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Miller JM, Brennan KG, Ogden TR, et al. Elevated serotonin 1A binding in remitted major depressive disorder: evidence for a trait biological abnormality. Neuropsychopharmacology. 2009;34:2275–84. doi: 10.1038/npp.2009.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Miller JM, Hesselgrave N, Ogden RT, et al. Brain serotonin 1A receptor binding as a predictor of treatment outcome in major depressive disorder. Biol Psychiatry. 2013;74:760–7. doi: 10.1016/j.biopsych.2013.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Parsey RV, Oquendo MA, Ogden RT, et al. Altered serotonin 1A binding in major depression: a [carbonyl-C-11]WAY100635 positron emission tomography study. Biol Psychiatry. 2006;59:106–13. doi: 10.1016/j.biopsych.2005.06.016. [DOI] [PubMed] [Google Scholar]
- 67.Lowther S, De Paermentier F, Cheetham SC, et al. 5-HT1A receptor binding sites in post-mortem brain samples from depressed suicides and controls. J Affect Disord. 1997;42:199–207. doi: 10.1016/s0165-0327(96)01413-9. [DOI] [PubMed] [Google Scholar]
- 68.Mann JJ, Metts AV, Ogden RT, et al. Quantification of 5-HT1A and 5-HT2A receptor binding in depressed suicide attempters and non-attempters. Arch Suicide Res. 2017 doi: 10.1080/13811118.2017.1417185. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 69.Yates M, Ferrier IN. 5-HT1A receptors in major depression. J Psychopharmacol. 1990;4:69–74. doi: 10.1177/026988119000400204. [DOI] [PubMed] [Google Scholar]
- 70.Bhagwagar Z, Rabiner EA, Sargent PA, et al. Persistent reduction in brain serotonin1A receptor binding in recovered depressed men measured by positron emission tomography with [11C]WAY-100635. Mol Psychiatry. 2004;9:386–92. doi: 10.1038/sj.mp.4001401. [DOI] [PubMed] [Google Scholar]
- 71.Nugent AC, Bain EE, Carlson PJ, et al. Reduced post-synaptic serotonin type 1A receptor binding in bipolar depression. Eur Neuropsychopharmacol. 2013;23:822–9. doi: 10.1016/j.euroneuro.2012.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hirvonen J, Karlsson H, Kajander J, et al. Decreased brain serotonin 5-HT1A receptor availability in medication-naive patients with major depressive disorder: an in-vivo imaging study using PET and [carbonyl-11C]WAY-100635. Int J Neuropsychopharmacol. 2008;11:465–76. doi: 10.1017/S1461145707008140. [DOI] [PubMed] [Google Scholar]
- 73.Sargent PA, Kjaer KH, Bench CJ, et al. Brain serotonin1A receptor binding measured by positron emission tomography with [11C] WAY-100635: effects of depression and antidepressant treatment. Arch Gen Psychiatry. 2000;57:174–80. doi: 10.1001/archpsyc.57.2.174. [DOI] [PubMed] [Google Scholar]
- 74.Basura GJ, Abbas AI, O’Donohue H, et al. Ontogeny of serotonin and serotonin2A receptors in rat auditory cortex. Hear Res. 2008;244:45–50. doi: 10.1016/j.heares.2008.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Barrett FS, Preller KH, Herdener M, et al. Serotonin 2A receptor signaling underlies LSD-induced alteration of the neural response to dynamic changes in music. Cereb Cortex. 2018;28:3939–50. doi: 10.1093/cercor/bhx257. [DOI] [PMC free article] [PubMed] [Google Scholar]