Summary
Bone fractures are the most common traumatic injuries in humans. The repair of bone fractures is a regenerative process that recapitulates many of the biological events of embryonic skeletal development. Most of the time it leads to successful healing and the recovery of the damaged bone. Unfortunately, about 5-10% of fractures will lead to delayed healing or non-union, more so in the case of co-morbidities such as diabetes. In this article, we review the different strategies to heal bone defects using synthetic bone graft substitutes and biologically active substances or stem cells. Our review is different from previous reviews, which focus on strategies that are still at the early stages of development and use mostly in vitro experiments with cell lines or stem cells. Here, we focus on what is already implemented in the clinics, what is currently in clinical trials, and what has been tested in animal models. Treatment approaches can be classified in three major categories: i) synthetic bone graft substitutes (BGS) whose architecture and surface can be optimized; ii) BGS combined with bioactive molecules such as growth factors, peptides or small molecules targeting bone precursor cells, bone formation and metabolism; iii) cell-based strategies with progenitor cells combined or not with active molecules that can be injected or seeded on BGS for improved delivery. We review the major types of adult stromal cells (bone marrow, adipose and periosteum derived) that have been used and compare their properties. Finally, we discuss the remaining challenges that need to be addressed to significantly improve the healing of bone defects.
1. Introduction
1.1. The need for bone repair
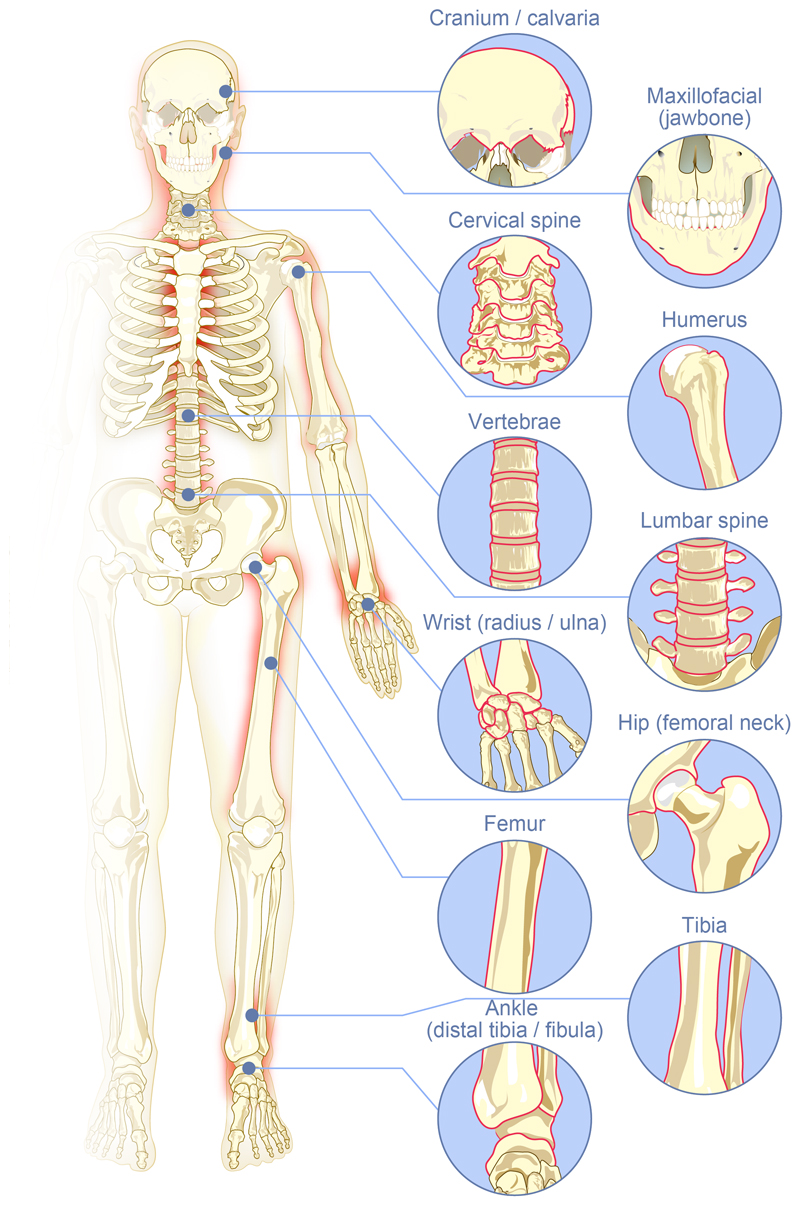
Bone fractures are one of the most common organ injuries that can result from high energy trauma such as car and motorbike accidents or sport injuries (rugby, mountain bike, paraglide...). In developing countries, due to the boom of economic activity and the resulting working conditions, work accidents are also an important cause of fractures [1]. Typically, bone defects can be segmented into different subfields depending on their location: long bones and spine, maxillofacial and craniofacial. The most common bone fracture sites are shown in Figure 1: femur, shoulder (mostly humerus), hip (femoral neck), wrist (radius/ulna), tibia (distal third), ankle (above the joint, distal tibia/fibula fractures) together with vertebral, maxillo- and cranio-facial (jawbone, calvaria) fractures.
Figure 1.
The major fracture sites in the body where strategies using synthetic bone graft substitutes, bioactive molecules and/or stem cells are needed to repair bones in difficult clinical situations.
Under healthy circumstances, bone has a unique healing capacity without inducing scar tissue formation. However, complex or compromised bone fractures (i.e. fractures above critical size, severely damaged surrounding environment) can fail to heal, leading to a non-union fracture (Figure 2). Co-morbidities such as diabetes, genetic factors and poor lifestyle (e.g. smoking or alcohol abuse) increase the risk of delayed healing and non-unions. Moreover, inappropriate initial fracture treatment may result in complications leading to non-unions [2]. Commonly, these health conditions lead to poor and/or disrupted vascularization and an insufficient number of progenitor cells that can form the new bone, resulting in failure of the natural healing process [3].
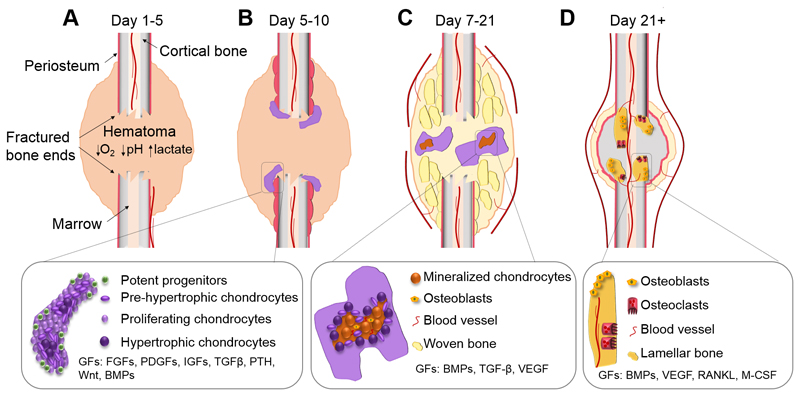
Figure 2.
Healing of a non-stabilized long bone fracture through the formation of a cartilaginous callus. The major biological phases during healthy fracture healing go through the chronological stages of inflammation, the formation of a cartilaginous callus and remodeling of the callus into bone. The primary cell types that are found at each stage include inflammatory cells, chondrocytes, osteoblasts, osteoclasts, hematopoietic cells and osteocytes. (A) Upon fracture, the hematoma forms, associated with reduced O2 and pH levels as well as increased lactate. At this stage, the inflammatory cells remove injured tissue and secrete stimulatory factors to recruit cells from the environment including the periosteum. (B) A callus forms due to the massive progenitor cell expansion leading to cellular condensation and initiation of chondrogenic differentiation. (C) Hypertrophic chondrocytes in the callus mineralize and osteoblasts enter and subsequently form woven bone. The woven bone remodels through osteoclast-osteoblast coupling and the lamellar bone eventually bridges the fracture (D).
Additional indications that require bone healing include bone defects resulting from the resection of bone tumors, from infection or, increasingly, in the context of prosthetic revisions. Moreover, low back pain has become a common burden of western societies, often associated with degenerative vertebral disc disease and osteoarthritis. Severely damaged joints and degenerative disease may require arthrodesis, an artificial induction of joint bridging between two bones, also known as joint fusion. Arthrodesis is most commonly performed on joints in the spine, hand, ankle and foot. All of these conditions require bone defect filling and bony bridging.
In terms of industrial markets, fracture treatments and bone bridging/repair solutions are classified in different application fields generating important revenues. The worldwide orthopaedic product sales are segmented as fracture repair, a market estimated at $5.5 billion that includes all products used to repair fractures internally or externally: plates, screws, intramedullary nails, pins, wires, staples, and external fixators;; spinal implants and instrumentation a $~7 billion market that includes spinal fusion; and orthobiologics a $4.7 billion market that includes different strategies used to repair bone or fuse joints [1]. To note, orthobiologics are biological substances, either active molecules, stem cells or demineralized bone grafts that are used to help bone defects heal more quickly. The term orthobiologics is specific to bone, while tissue engineering is a more generic term that can apply to the repair of all tissues, including bones.
1.2. Mechanism of bone repair
Bone fracture healing is a complex, orchestrated, regenerative process that involves a crucial number of progenitor cells as well as inflammatory, endothelial and hematopoietic cells. The cellular and molecular events are strictly regulated during the healing cascade which includes the initial inflammatory phase, hematoma formation and progenitor cell recruitment (Figure 2A), formation of an intermediate callus (Figure 2B), maturation of the callus (Figure 2C) and the final remodeling of the bony callus to the original bone’s structure and shape (Figure 2D) [2]. The concerted action of the cells is strictly regulated by a crucial interplay of biochemical, physical and mechanical factors [4], largely recapitulating phenomenological events of endochondral bone development during embryogenesis [5]. As a result, many of the homeotic genes and primary morphogenetic pathways that are active during skeletal development also play a role during fracture healing [6].
The initial fracture causes a local disruption of the vascular network and surrounding tissues, which leads to hematoma formation, closely followed by the acute inflammatory phase [7]. The hematoma is formed by cells from the peripheral blood and the intramedullary hematopoietic compartment [8]. This process occurs due to the plasma coagulation and platelet exposure to the extravascular environment, which together provide a fibrin network as a first provisional matrix. As a result, the hematoma has a high concentration of angiogenic growth factors, which explains its strong pro-angiogenic activity. The importance of the hematoma has been confirmed; its removal attenuates repair, whereas transplantation stimulates new bone formation [9]. The soft matrix of the hematoma allows recruitment and infiltration of the first inflammatory cells, neutrophils, within 24 hours post fracture. By secreting inflammatory and chemotactic mediators such as Interleukin 6 (IL-6) and Chemokine ligand 2 (CCL2), the neutrophils recruit the second wave of inflammatory cells, the monocytes and macrophages [5, 7, 10]. From this inflammatory milieu, macrophages are polarized to a predominantly pro-inflammatory M1 phenotype, a specific type of macrophages [11].
Next, the inflammatory cells that migrated to the site remove the provisional fibrin matrix and necrotic cells and resorb necrotic bone fragments. In addition, macrophages, increasingly assuming the anti-inflammatory phenotype, known as M2 macrophages [11], secrete a repertoire of inflammatory, chemotactic and progenitor mediators including stromal derived factor-1α (SDF-1α), tumor necrosis factor alpha (TNF-α), IL1β, IL-6, CCL2, bone morphogenetic proteins (BMP), fibroblast growth factors (FGF) and Wingless-type MMTV integration site family of proteins (Wnt) to initiate the recruitment of progenitor cells from the bone marrow, periosteum and the cortical bone [5]. As a result, the hematoma and acute inflammatory reaction are cleared after a week and the hematoma is then replaced by granulation tissue, which consists of proliferating progenitor cells and neovasculature embedded in an unorganized ECM. Of note, a balanced acute inflammatory response has been shown to be crucial for healthy fracture healing, since macrophage depletion or knock out of inflammatory cytokines impairs the healing cascade [11–14]. In addition, the important switch between the pro-inflammatory M1 and anti-inflammatory M2 macrophage phenotypes is likely mediated by both macrophage autocrine signaling as well as paracrine signaling from other cells at the fracture site, including the recruited progenitors [11].
After the inflammatory stage, the following fracture healing process largely recapitulates the process of long bone development in the embryo, including: 1) migration of skeletal precursors to the site of skeletogenesis followed by 2) cellular condensation, leading to the subsequent 3) differentiation towards chondrocytes and/or osteoblasts [15–17]. Skeletal progenitor cells have been found to be recruited locally and concurrently from periosteum, bone marrow/endosteum and/or dura mater during bone repair. All tissue sources give rise to osteoblasts, whereas the periosteum is the major source of chondrocytes [18, 19]. Importantly, intrinsic and environmental signals modulate cell fate decisions within these tissues. It is believed that depending on the relative distance to the blood vessels, combined with the release profiles of cytokines and growth factors, progenitor cells either first differentiate into chondrocytes, or directly mature into bone-forming osteoblasts. Due to the adapted metabolism in chondrocytes, designed to survive and function in poorly vascularized environments, these cells are located furthest away from the blood vessels [5, 20]. The local hypoxia at the fracture site induces production of angiogenic factors such as VEGF to stimulate neo-angiogenesis [21]. Osteoblasts are dependent on oxidative metabolism and require a constant and substantial supply of oxygen and nutrients. Therefore, they accumulate in the vicinity of the newly formed blood vessels near the fracture extremities [22].
The success of fracture healing, bone integration and remodeling is also highly dependent on the biomechanics of the fracture site. Stabilized fractures in which the bone ends are closely opposed, mechanically stabilized and with limited vascular disruption, mainly heal through intramembranous ossification [23]. Partially stabilized or non-stabilized fractures on the other hand, with damaged vasculature, heal through rapid formation of a cartilaginous intermediate to provide initial stabilization. This stabilization allows sufficient rigidity for blood vessel ingrowth, closely followed by invasion of bone forming osteoblasts that transform the soft callus to bone in collaboration with chondroclasts [24].
Similarly to embryonic bone development, fracture healing is directly regulated by crucial factors from - and related to - the BMPs, transforming growth factor-beta (TGF-β), FGFs, parathyroid hormone (PTH), Wnt, platelet-derived growth factors (PDGFs) and insulin-like growth factor 1 (IGF-1) families [6]. During the fracture healing cascade, TGF-β and BMPs are secreted to help recruit progenitor cells. Thereafter, together with FGF, PDGF and IGF, they induce proliferation that is followed by cell differentiation, the latter being largely driven by BMPs [21, 25–28]. Several of these morphogenetic processes form interactive feedback loops, including co-regulation and regulation between different cell and tissue types during fracture repair. This elegant balance of complex interplay can be interrupted in a compromised biological environment due to severe damage, co-morbidities or a large defect size. This impaired signalling and or lack of progenitors leads to insufficient regenerative potential and the need to boost bone repair using other means.
1.3. Current strategies to repair bones
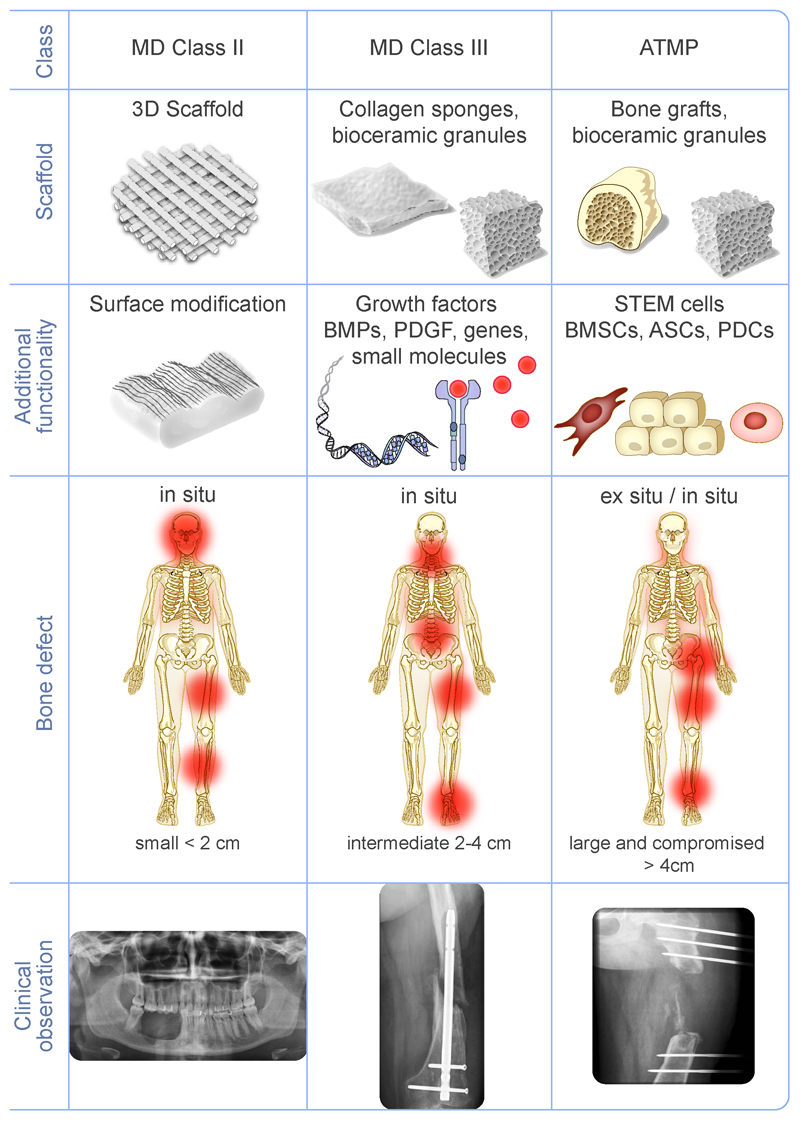
Currently, the “gold standard” treatment of patients suffering from slow or incomplete bone healing is to perform bone grafting, using either an autograft or an allograft. However, there are drawbacks to bone grafting. Autograft treatment is limited by the volume of bone that can be harvested from the iliac crest and subsequently transplanted to the defect site. Complications include morbidity at the harvest site, local hematoma and remodeling issues of the implanted bone [29, 30]. Allograft is hampered by bone tissue integration from the host and vascularization issues. A last resort treatment strategy is distraction osteogenesis, which is largely successful, but long, painful and dependent on the availability of a competent medical team. This technique has also been associated with poor healing and re-fracture [31, 32]. Consequently, a more sustainable, long term treatment strategy is required. To that end, bone graft substitutes (BGS) are being engineered to help impaired fracture healing. Depending on the severity of the trauma, we can distinguish three main strategies for bone repair (Figure 3): i) synthetic scaffolds alone, ii) scaffolds combined with active molecules and iii) cell-based combination products with cells from various sources. As we will review below, the first generation of these strategies are currently in clinical trials at different phases (I, II, or III) and some have even made it to the clinic. In addition, the next generation of implants are currently being developed at the pre-clinical stage. Traditionally, scaffolds are used in trauma or spine fusion markets, while the combination of scaffolds with active molecules and/or stem cells is typically classified in the “orthobiologics” field.
Figure 3.
The three major strategies currently used and developed to repair bone. The first (left column) relies on using only synthetic bone graft substitutes (BGS). The second (middle column) relies on combining bioactive molecules with a carrier that is mostly an extracellular matrix protein or a ceramic-based carrier. The third (right column) consists of combining stem cells with a carrier, possibly with the use of additional bioactive molecules. Each of these approaches is more appropriate for the healing of bone defects depending on their severity. When healing of a defect is compromised, there is a need to have a biological functionality in addition to the BGS, which is provided either by bioactive molecules, stem cells, or a combination of both.
1.4. Important steps in clinical translation
Researchers and scientists face many barriers to the translation of innovative bone healing strategies to clinical and commercial applications. These barriers have been reviewed by others [33–35] and will not be reviewed here. Cell therapies are particularly difficult to translate [36] due to the more complex regulatory challenges and additional steps required to prepare the cells before implantation and to evaluate their fate once implanted. In brief, the known important steps for clinical translation follow:
-Establish specific regulatory path. The most challenging point for effective translation is understanding the complex regulatory environment, medical device classification and advanced therapeutic medical products. In fact, different agencies, especially in the USA versus EU, have different classification systems:
In the USA, a medical device product must receive authorization by the Food and Drug administration (FDA) prior to being marketed. The FDA classifies medical devices in three categories: Class I, Class II, Class III depending on the risks associated with the devices. For instance, a medical device combined with a drug belong to class III. For combination devices, i.e. devices that contain drugs and/or stem cells, the FDA determines which of three centers, namely the Center for Devices and Radiological Health (CDRH), the Center for Drug Evaluation and Research (CDER) or the Center for Biologics Evaluation and Research (CBER), has primary jurisdiction on the authorization process. The FDA has a specific regulation for Human Cellular and Tissue Products (HCTP). Generally, the CBER has primary jurisdiction on Regenerative Medicine Advanced Therapy (RMAT) products when cells are used.
In Europe, the situation is different. Medical devices do not need to be evaluated by a regulatory agency. Classified as class I, II and III depending on their intended use and risk level, they are simply CE marked by certified notification bodies, a CE mark being the manufacturer’s declaration that the product meets the requirement of a European directive. In contrast, drugs are considered as pharmaceutical products and need to have authorization from the European Medicine Agency (EMA). Stem cells also belong to a specific category, the advanced therapeutic medicinal products (ATMP). The more complex the product, the longer the path toward clinical translation and the higher the product will cost. The most complex products are by far the stem-cell based products.
- Define the specific clinical application to address. Who is the patient to be treated? The answer to this question will guide the clinical development steps including implantation site and animal model.
- Select the most appropriate animal models for the pre-clinical experiments. Pre-clinical trials require animal care facilities and veterinary schools or labs. Later, clinical trials involve patients in clinics and hospitals. There are several recent reviews on animal models for bone repair from ectopic bone induction (mice), small bone defects (rats) and larger bone defects (rabbit, dog, sheep, goat, mini-pigs and pigs) [37–39]. The selection criteria need to be carefully considered, since each model has pros and cons [40] and each study uses a specific set of parameters for bone scaffold design, which makes comparisons between studies difficult. Furthermore, there is no current consensus about the pre-clinical assessment strategies, including animal implantation site [41]. The efficacy of bone repair, including the amount of regenerated bone, the kinetics of bone formation and mechanical properties of regenerated bone, needs to be assessed as well as, importantly, the safety of the product, including the possible presence of toxic degradation products in the different organs, and the possibility of chronic inflammatory reaction, as examples.
- Consider the cost-effectiveness of the product and the potential reimbursement by medical care systems. These depend on the specific country. Of note, in Europe, a large part of medical expenses is paid by government bodies, while in the USA, it is paid by private insurances.
- Implement the industrialization of the product. In view of industrialization, aspects such as large-scale manufacturing, sterilization processes, and mode of storage need to be considered. Last but not least, the quality of both the process and the product needs to be assessed. This includes the sourcing of the raw components, good manufacturing practice (GMP) grade, batch-to-batch reproducibility, the manufacturing process and the respect of standards for production (ISO norms).
In the following sections, we will review the current state and recent advances for bone healing strategies with a focus on products that are at the clinical or pre-clinical trial stage.
2. Bone graft substitutes: Optimization of implant design, structure and surface properties
The large variety of biomaterials that have been used for in vivo bone tissue engineering have already been reviewed by others [42, 43]. Here, we focus on clinical and pre-clinical research from the past 5 years (2013-2017) that shows efforts to optimize the architecture, chemistry and/or surface properties of rigid BGS for the enhancement of bone regeneration and/or osteointegration in bone defects. The literature search focused on BGS without active biomolecules and excluded studies where BGS are implanted in animals or in subjects affected by systemic diseases, such as diabetes and osteoporosis that might compromise bone healing.
Indicative of the vast efforts in optimizing BGS materials and structure is the number of clinical trials recently completed, or currently in progress. We conducted a search on clinicaltrials.gov, for “bone scaffolds” and “bone implants”, and selected 23 studies out of 895 total hits, that were relevant to custom-designing BGS structure by 3D printing (7 studies), improving the design of BGS material properties such as porosity and mechanical properties (8 studies) and optimizing the surface properties such as chemistry and microtexture (9 studies). Of note, the results of the clinical trials, even if they have been concluded for several years, are not often accessible to the scientific community; for most of the clinical trials examined, the results have not been published in scientific or clinical journals. For that reason, we often cite, below, the clinicaltrials.gov identifier, the NCT number, and, if the results are published in peer-reviewed journals, we cite the corresponding papers. At the pre-clinical level, several studies with BGS implanted in small or large animals show efforts to optimize BGS architecture, material chemistry and surface properties to enhance bone regeneration without the use of biologics or exogenous cells.
2.1. 3D printing to customize the design of patient-specific BGS
Three-dimensional (3D) printing uses 3D images of the patient’s anatomy, generally obtained from Computed Tomography (CT) scans, with the calculating power of a software, to design a BGS shape that corresponds to a patient’s bony defect. The customized BGS shape is then fabricated through additive manufacturing using a 3D printer. This fabrication technique allows a better control of the BGS mechanical properties and structural parameters. The structure optimization ensures a better correspondence between the BGS and the patient’s anatomy, allowing the recovery of form and function immediately following the surgery. In addition, a custom-designed BGS better fits into the defect, which lowers the likelihood of implant movement in the defect and reduces the need to manipulate or modify the BGS intraoperatively. Hence, a better fitting BGS could decrease the duration of the surgery and improve the surgical outcome (NCT03312491). In clinical trials, additive manufacturing is mostly applied for craniofacial and maxillofacial applications, such as mandibular reconstruction, and dental implants. Metallic implants made of titanium are the most widely used (NCT03292679, NCT03057223, NCT03242330) since the fabrication techniques (selective laser melting and electron beam laser melting) are now mature [44]. Titanium plates are routinely used to immobilize bone segments in jaw surgeries. Generally, these plates are commercialized with standard construction specifications and should be deformed by the surgeon to match the jaw contour before being positioned and fastened in place by screws. This causes fatigue stress on the BGS, potentially affecting its mechanical properties, and offers less accurate correspondence between the BGS and the patient’s skeletal contours and dimensions.
Clinical trial NCT03292679, which will begin in 2018 (60 participants), aims to show that the contouring of titanium BGS plates for facial fracture reconstruction can be improved with the use of 3D printed plastic models of patient facial anatomy. On-site fabricated 3D printed models can be used to pre- or intra-operatively shape and bend titanium BGS. NCT03057223 (48 participants) investigates the feasibility, efficacy and safety of 3D printed titanium BGS plates for mandibular reconstruction. The outcome measures include the success rate, potential adverse events and accuracy. NCT03242330 (ongoing, 156 participants) investigates whether there is a difference between single-piece and two-piece titanium patient-specific mandibular subperiosteal implants in terms of implant survival, patient satisfaction, and soft tissue dehiscence (i.e. a rupture of the wound along a surgical incision). This is to enhance both surgical and prosthetic outcomes for the sake of improved implant longevity, soft tissue health and patient well-being.
3D printing is also being investigated for orthopedic applications: for acetabular fractures (NCT03312491, completed in October 2017 but no results posted), ankle bone defects (NCT03185286) and other bone defects resulting from bone fracture, burst fracture of spine, bone tumor or non-union (NCT03166917).
The results of a clinical study in 22 patients showing the safety and efficacy of titanium mesh for orbital floor reconstruction have been published [45]. More recently, printing of titanium was proposed for reconstructing multilevel cervical spine (C2-C4) after resection of metastatic papillary thyroid carcinoma [46]. The personalized porous implant printed in Ti6Al4V provided excellent physicochemical properties and biological performance, including biocompatibility, osteogenic activity, and bone ingrowth. At a more fundamental level, researchers are now working to optimize BGS pore size [47]. A pore size of 350 μm provided an optimal mechanical shielding to the surrounding bone and osteoconduction of the implant itself. Other materials such as bioceramics [48] and polymers like polyetheretherketon (PEEK) [49] can now be custom-designed, but are currently being studied only at the pre-clinical stage. Polycaprolactone/hydroxyapatite (PCL/HAP) scaffolds (NCT03232788, 20 patients in test group, currently recruiting) will be explored for the treatment of gingival recession associated with bone and gingival tissue deficiency.
The reported drawbacks of BGS 3D printing are related to the delay associated with shipping the BGS before surgery if it is fabricated off-site, and the difficulty of using BGS in urgent surgery due to the time required for obtaining a 3D model and fabricating the custom-designed BGS. Also, the costs of printing and additional scans generally increase the overall cost of the procedure.
In their recent review, Annemans and coworkers [50] conclude that further research is needed to determine whether the increased intervention costs can be balanced with the observable advantages of this new technology.
2.2. Novel design of material properties
Optimization of BGS mechanical properties
The optimization of the mechanical properties of BGS is important to restore mechanical function by matching, or mimicking, the mechanical properties of the natural tissues. Also, the closer the mechanical properties of the BGS are to those of the natural tissue, the higher the chances of avoiding adverse effects to the surrounding anatomy. Indeed, bone tissue surrounding a metallic implant, such as a hip prosthesis, degrade over time due to stress shielding [51]. This is due to the redistribution of mechanical loads after implantation, whereby the stiffer metal implant bears most of the mechanical stresses, while the surrounding bone tissue is not mechanically solicited, which inhibits the integration and regenerative potential. Similarly, if the BGS material properties are weaker than the surrounding tissue, the tissue will compensate, which may hinder bone regeneration in the BGS and/or cause damage and fatigue to the surrounding tissue. It is therefore important to optimize the mechanical properties of BGS and studies are being conducted to evaluate the effects on bone healing of BGS structural rigidity and design, especially at the interface with native tissue (NCT01441999, NCT01269840, NCT03218150).
New formulations of material architecture and chemistry to control porosity and degradability of ceramic BGS
Studies are also investigating the effects of architecture, including porosity, pore interconnectivity, specific surface area and resorbability on the efficacy of ceramic BGS (NCT02639572, NCT02613663, NCT01770574, NCT01982045, NCT02250248). Optimization of BGS architecture aims at replicating, or mimicking, bone structure to provide an environment similar to the natural in vivo environment for bone cells to colonize and regenerate healthy bone tissue [52]. Architecture optimization in rigid BGS includes the optimization of macro- and micro-porosity, pore size and pore interconnectivity to replicate the porosity of cancellous or cortical bone [53]. Mumith et al. [54] showed increased osteointegration in endoprostheses with porous (700-1500 µm pores) collars implanted in sheep tibia compared to current solid designs with grooves. Microporosity (<10 microns) was shown to improve bone healing in scaffolds with 400 µm pores implanted in pig mandibles [55], and to provide additional space for bone growth leading to better load transfer. Rustom et al. [56] showed that combining regions with different architectures in the same scaffold allows to direct bone growth in a pig mandible model, demonstrating that BGS architecture influenced bone growth within a single pig mandibular defect. Combining collagen and other biocompatible polymers such as polycaprolactone [57] to a rigid BGS provides a soft ECM-like interface intended to enhance the activity of endogenous bone cells. The composition of the material may also enhance the osteogenic properties. This is what is targeted with a new synthetic “nanobone” made of nanocrystalline HAP embedded in a porous silica matrix (NCT02613663).
The control of scaffold degradability is a key challenge for ceramic BGS designs. Indeed, the BGS must first serve as a temporary scaffold, and must gradually be replaced by new bone in order to restore form and function until newly grown bone is mechanically competent. Biodegradability and porosity are related in ceramic BGS because the increased surface area provided by pores favors the dissolution of ionic compounds in the BGS material. The increased pore surface also favors interactions between the BGS material and surrounding cells and molecules. Thus, bone cells involved in the remodeling of newly formed bone, and specifically osteoclasts, have more access to resorb the material. The resorbability of the BGS may be controlled by material selection, for example by including a more resorbable tricalcium phosphate (β-TCP) phase compared to the less resorbable hydroxyapatite (HAP) or a new composition for a fully resorbable BGS, such as Siloss® (Azurebio) (dicalcium phosphate anhydrous, HAP, amorphous silica and trace amounts of zinc) studied in a clinical trial (NCT02639572). The degradation of the material might enhance bone regeneration, by providing increased space for bone to grow into. Furthermore, the dissolution and reprecipitation of ceramic compounds can provide building materials for the fabrication of new bone: the release of ions (Ca, P, Si and Zn) may also stimulate the action of osteogenic cells. Indeed, at the preclinical level, the incorporation of silicon or magnesium in implant materials improved bone regeneration in BGS [58]. β-TCP scaffolds doped with Si and Mg ions showed increased bone formation and angiogenesis compared to β-TCP scaffolds without added silicon and magnesium when implanted in rat femurs [59]. The addition of bioactive glass fibers to titanium implants in rabbit tibia showed increased bone regeneration [60]. Silicon incorporation in α-TCP scaffolds implanted in rat femurs promoted osteogenesis and delayed scaffold degradation [61].
AttraX® putty (CE-557130), a bioresorbable β-TCP mixed with a fast resorbing polymer carrier to improve surgical handling, is currently being evaluated in NCT01982045 and NCT02250248 in patients undergoing posterolateral spine fusion. Synthetic bone grafts may also be used in pediatric foot surgery as is currently studied using ReproBone™ (NCT01770574).
2.3. Optimization of surface properties
Surface properties of BGS are an important consideration for the attachment of endogenous osteogenic cells and for new bone tissue growth on the BGS surface. They can be altered by surface treatment or by coating the BGS structure with a material that promotes bone regeneration. For instance, titanium and tantalum are currently used in clinical orthopedics and dentistry because of their excellent mechanical properties, but their bioactivity is low, leading to poor osteointegration [62]. Therefore, surface treatments are needed to enhance BGS adherence and stability in the surrounding anatomy.
Surface optimization aims to avoid the use of a cement (NCT00253838). Pre-clinical and clinical studies may involve coating a metallic BGS with a porous ceramic or metallic layer to improve osteoconductivity (NCT00253838, NCT00116038, NCT01936415) or modifying the BGS surface chemistry, for example through acid etching (NCT03242330, NCT01641198, NCT01529801). Braem et al. [60] reported bone ingrowth in pores with diameters under 10 microns in the microporous titanium coatings of titanium BGS implanted in rabbit tibia.
Roughened surfaces are thought to promote osteointegration by increasing the apposition of osseous tissue and favoring epithelial attachment to the implant, in comparison with smooth surfaces. Better osteointegration may increase implant survival/durability, improve implant function and reduce the need for additional surgeries. One study evaluated osteointegration around a BGS with two parts, one smooth and the other roughened, compared to the same BGS with all its surface roughened (NCT01821417). Micro-nanostructured titanium implants [63] also showed higher bonding strength at the bone interface compared to titanium implants with a polished surface. Immobilizing gold nanoparticles on titanium implants [64] also improved bone-implant contact in rabbit femurs. Enhanced bone-implant contact was reported in titanium dental implants surface-treated with micro-arc oxidation in mini-pig mandibles [65]; the treatment generates a layer of titanium oxide with micro-nanostructured roughness at the surface of the implant. The chemical nature of the surface material may also affect surface roughness (NCT00116051).
Coating can be used to modify the surface chemistry of BGS. Titanium implants coated with Mg-doped hydroxyapatite [66] showed a higher volume of ingrown bone compared to uncoated implants in rabbit femurs. Nanoporous silica coating loaded with bioactive glass nanoparticles on titanium implants [67] accelerated bone formation and improved osteointegration in rat tibia. Thermo-chemical treatment of titanium foams with NaOH and heat treatment at 600oC enhanced their bioactivity by promoting the formation of an apatite layer on the BGS outer surface and on the pore inner walls [68]. This bioactivation significantly enhanced bone ingrowth compared to untreated titanium foams in rabbit femurs. Surface treatment is also interesting for ceramics: tricalcium silicate bone cements with nanostructured surfaces [58] significantly enhanced bone regeneration in comparison to the same bone cements without surface modification.
To conclude, the next generation of implants will have an improved material chemistry and architecture, while the surfaces will also be optimized to enable more efficient bone integration. Custom-made implants may be used for specific clinical indications.
3. Bone graft substitutes combined with active biomolecules
As described above, the mechanism of bone repair is complex and involves several important growth factors. In this part, we review the strategies to boost bone repair using active biomolecules based on a search on clinicaltrials.gov and scientific databases (Table 1). The strategies can be classified in three sub-categories: i) use of recombinant growth factors or a mixture of these growth factors that are associated with a natural matrix or calcium phosphate material carrier, ii) use of peptides, derived from extra-cellular matrix proteins, to target other cellular receptors, and iii) use of small molecules that target the pathways influencing bone mass, such as the Wnt signalling pathway [69]. These molecules can act directly to positively influence the bone mass, or indirectly, by acting on negative regulators (i.e. inhibitors) of the bone mass. In this second case, they are designed to inhibit the action of inhibitors and thereby have a positive effect on bone mass.
Table 1.
List of products associating bioactive molecules (growth factors, peptides or small molecules) with a material carrier and their stage of development. M: medical use; W: withdrawn; Phase I, II or III (P-I; P-II, P-III); TGA: therapeutic good administration; IDE: investigational device exemption. The countries are listed. COLL: collagen
| Company | Product Name | Application | Stage | Country | Active molecule | Material carrier | Mechanism of action | References |
|---|---|---|---|---|---|---|---|---|
| Medtronic, Inc. | Infuse® Bone Graft | Anterior lumbar interbody fusion (ALIF) Spinal fusion Open tibial shaft fractures | M | It, D, ESP, UK Withdrawn: EU |
rhBMP-2 (powder) |
COLL type 1 Titanium or PEEK cage |
Interaction with BMP receptors, initiate stem cell differentiation in chondrocytes and osteoblasts | http://www.infusebonegraft.com/healthcare-providers/about-infuse-bonegraft/index.htm |
| Olympus Biotech | Osigraft | Recalcitrant long bone nonunions Spine surgery |
W | EU | rhBMP-7/OP-1 (powder) |
COLL type 1 (powder, from cow) |
Similar mechanism as BMP-2 | http://www.stryker.com/cn/products/Orthobiologicals/Osteoinductive/OP-1/OP-1Implant/020210 |
| Wright Medical Group | Augment® Bone Graft | Hindfoot/ankle fusion Distal radius fractures |
M | Can Aus NZ, USA |
rhPDGF-BB (solution) |
ß-TCP particles | Interaction with PDGF receptors, stimulates recruitment and proliferation of cells, promotes revascularization |
http://www.augmentbonegraft.com/ [85, 199] |
| Biopharm DePuy Spine |
MD05-P MD05-I BB-1 rhGDF-5 |
Sinus floor augmentation Periodental bone augmentation Fracture healing/ long bone reconstruction Lumbar disc generation |
P-III P-II POC P-I/II |
US USA USA |
rhGDF-5 (solution) BB-1 solution rhGDF-5 solution |
ß-TCP COLL none |
Mutant of GDF-5 (BMP family), interacts with BMP receptors, regulates skeletal development. BB-1 combines GDF-5 and BMP-2 features [91] |
NCT00520377 NCT00519155 [200] [201] [90] http://www.biopharm.deNCT00813813 |
| NextGen Company Limited | Nucleostim Neovasculgen |
Maxillofacial bone defects | P- II | US | DNA plasmid (Solution) |
COLL/HAP composite Octacalcium phosphate |
Plasmid DNA with VEGF gene to induce VEGF secretion by cells and promote angiogenesis |
NCT02293031 NCT03076138 |
| Bioventus | Osteoamp | Spinal fusion Spondylolisthesis |
Unknown | Unknown | Allograft-derived growth factor | Granules/ putty/sponge | Osteoinductive, angiogenic, and mitogenic proteins acting on stem cells [93] |
http://www.bioventussurgical.com/our-products/osteoamp/ NCT02225444 |
| Genera Research Ltd | Osteogrow | Distal radius fracture | CT | Bosnia Herzegovina Croatia |
rhBMP-6 + whole blood coagulum |
Paste | Cocktail of active biomolecules acting on stem cells | Eudra CT 2014-005101-21/HR |
| Ferring pharmaceuticals | Amplex Prefix |
Foot and ankle arthrodesis Lumbar fusion |
P- III | US | B2A (Powder) |
HAP/β-TCP granules | Synthetic multi-domain peptide inducing stem cell growth, via cell-type specific activation of BMP receptor, leading to ERK activation [96] |
https://www.ferring.com/en/research-development/pipeline/ NCT00798902, NCT01224119, NCT03028415 [97] [202] |
| Cerapedics Inc. |
i-Factor Bone Graft i-Factor Flex FR |
Intervertebral disc degeneration Non-union or traumatic fractures Joint reconstruction |
IDE trial CE Mark TGA |
US EU Aus |
P-15 (Hydrogel) |
Anorganic bone mineral Silk fibers |
P-15 is a collagen-derived peptide triggering stem cell adhesion and also enhancing cell differentiation [99] [100] [101] |
[203] [102] [103] ; [104]; http://cerapedics.com/i-factor-technology-platform/ NCT00310440 NCT01618435 NCT02895555 |
| Kuros (with Baxter) | KUR-111 KUR-112 KUR-113 |
Tibial plateau fractures Tibial shaft fractures Solitary bone cysts |
P-II Orphan Drug |
EU, Aus EU |
PTH (Paste) |
Fibrin matrix + HA/TCP granules |
PTH interacts with PTH-related protein receptor, a G protein that can activate the cAMP-dependent protein kinase A (PKA) and calcium- dependent PKC. [109] |
http://www.kuros.ch/kur-111/ NCT00533793 [110] [111] |
3.1. Growth factors: BMP-2, BMP-7 and PDGF
Three growth factors (GFs) have already been approved in clinics: BMP-2 (Infuse bone graft) since 2003, BMP-7 (OP-1 putty) from 2003 until 2014 when it was withdrawn from the market, and rhPGDF-BB (Augment bone graft ®) more recently since 2015. The growth factors have a direct action on bone progenitors by interacting with their respective receptors, which initiate the biochemical signalling in stem cells leading to bone formation [5, 70].
A large amount of literature has already been published on BMP-2 combined with a type I collagen sponge as carrier, for use in open tibial shaft fractures and in spinal fusion. In this case, it is associated with a titanium or PEEK cage for use in interbody fusion (Anterior lumbar interbody fusion, ALIF). A controversy related to this product emerged in 2011 after adverse effects were reported in response to the supra-physiological doses delivered (1.5 mg/mL of BMP-2) for human use, and off-label use (~85%), including inflammation and pain[71, 72]. It is now known that bone repair in response to BMP-2 is dose-dependent [73] and that high doses can lead to osteolysis. However, to date, it is also acknowledged that BMP-2 remains a powerful activator of bone repair, whose delivery needs to be further optimized. Alternative sources of BMP-2 combined with ceramics are emerging, such as those produced in CHO-cells [74] or in E-coli [75, 76].
At the research stage, intensive studies are carried out in order to better understand how growth factors, especially BMP-2, can be efficiently trapped by materials or immobilized at their surface [77] and how they interact [78], in order to improve the in vivo delivery and release profiles. Once in vivo, the pathophysiological context can greatly influence the release of bioactive molecules from the biomaterial [79]. Recent data in a rat femoral bone defect, using clinical grade PLGA and a biopolymeric carrier as a nanoreservoir for BMP-2 [80], showed that it is possible to tune the dose of BMP-2 delivered in vivo. This dose control impacted the volume of newly formed bone, and accelerated the kinetics of bone repair via surface-medicated delivery of BMP-2.
BMP-7 is another osteoinductive growth factor investigated for the clinical setting. BMP-7 was associated with type I collagen in the form of a paste (OP-1 putty, Olympus Biotech) and used under a humanitarian device exemption (< 4000 patients) for recalcitrant long bone non-unions and spine surgery. When the production stopped, the reports about its efficacy and safety were satisfactory [81]. Interestingly, a recent review highlighted the important role of kidneys in bone metabolism and BMP-7 emerged as one of the important active molecules produced in the kidneys that is involved in different pathways associated with bone formation [82]. Experiments in a sheep model showed that the BMP-7 paste can be combined with an architectured scaffold to trigger the repair of long bones [83].
rhPDGF-BB was approved as a Class III combination medical device/drug product in 2015 for hindfoot and ankle fusion in specific categories of patients suffering from different types of arthritis with operative evidence indicating the need for a supplemental graft material. PDGF, by acting on PDGF receptors, stimulates the recruitment and proliferation of cells, including mesenchymal stem cells [84]. It also promotes the formation of new blood vessels at the site of healing, an important aspect of bone repair (Figure 2), by increasing vascular endothelial cells, pericyte and smooth muscle responses. Currently, a solution of PDGF is associated to β-TCP particles, a scaffold that provides osteoconductivity for new bone formation. A recent clinical study on 434 patients dedicated to hindfoot or ankle arthrodesis, treated with rhPDGF-BB/β-TCP, resulted in comparable fusion rates, less pain, and fewer side effects as compared to treatment with autograft [85]. In particular, the safety profile was improved compared to autograft due to the elimination of harvest site pain and morbidity, since there is no need to graft bone. More recently, a comparative study between PDGF-BB/TCP and autograft showed that there is a relation between the amount of graft material and successful hindfoot and ankle arthrodesis. Graft material filling of > 50% of the fusion space at 9 weeks, regardless of type or origin, was associated with significantly higher fusion rates at 24 weeks [86].
3.2. Other growth factors and gene delivery
GDF-5 is another member of the BMP family that induces bone, cartilage and tendon/ligament formation [87, 88]. Various pre-clinical studies have supported the use of rhGDF-5 for clinical applications including bone induction and soft tissue growth. Biopharm Gmbh developed MD05, a bone substitute associating rhGDF-5 and the inorganic carrier (β-TCP) for dental implants (MD05-I) and surgical treatment of periodontal disease (MD05-P). The project has entered clinical phase III for the treatment of severe periodontitis in 2007 and a phase II clinical trial in which MD05-I was examined for the treatment for dental implants was completed in 2008. Also in 2008, DePuySpine launched a phase I/II trial on 32 patients who had degenerative disc disease and received intradiscal rhGDF-5 injection. The study was based on in vitro experiments showing that rhGDF-5 can stimulate gene expression and synthesis of the extracellular matrix proteins collagen type II and aggrecan [89]. In addition, in vivo experiments in rabbit models of disc degeneration have shown that intradiscal injections of rhGDF-5 can stimulate an increase in disc height and hydration (unpublished data). The study ended in 2013 but so far no publication is available. For bone repair, a mutant growth factor called BB-1 (GDF-5V453/V456) with elevated BMP receptor-IA binding was developed. It was shown that it enhanced the reconstruction of long bone architecture [90]. Besides, both GDF-5 and BB-1 had high angiogenicity but BB-1 outperformed GDF-5 in terms of bone growth [91]. No further studies have been published since 2014, but other teams are investigating BB-1 in the context of surface modification of dental titanium screws [92].
Another strategy to deliver growth factors is to use a gene-activated matrix: Nucleostim aims to regenerate bone tissue in the maxillofacial area by promoting angiogenesis. It consists of a collagen-hydroxyapatite composite scaffold and DNA plasmids with gene encoding vascular endothelial growth factor (VEGF-A165) in concentration of 100-120 ng/mg, which is an active substance of gene-therapeutic drug "Neovasculgen"®. A study on 12 patients with congenital and acquired maxillofacial defects or alveolar bone atrophy was conducted to evaluate the safety and efficacy of gene-activated matrix (NCT02293031). It ended in December 2017 with no results published yet.
Finally, two products are using growth factor cocktails or growth factors associated with a carrier: OsteoAMP from Bioventus is an allograft-derived growth factor cocktail in granules, putty and sponge form that is rich in osteoinductive, angiogenic, and mitogenic proteins. It is being evaluated in transforaminal and lateral lumbar interbody fusion procedures as an alternative to rhBMP-2. In a clinical study, 226 patients received OsteoAMP with autologous local bone, while 95 patients received Infuse with autologous local bone [93]. The fusion rates were systematically higher and the time required to achieve fusion was approximately 40% less than rhBMP-2 with about 70% fewer complications. In addition, the supply costs were 80% lower for patients treated with OsteoAMP as compared to those treated with rhBMP-2. A clinical study was launched in 2015 (NCT NCT02225444, ending in 2019) to evaluate the long term efficacy of OsteoAMP in patients requiring 1 to 2 adjacent levels, instrumented posterolateral spinal fusion procedure of the lumbar or lumbosacral spine. In this study, OsteoAMP in spinal fusion procedures was evaluated based on fusion results, adverse event rates, and pain and health scores. The study is ongoing.
Osteogrow from GeneraResearch Ltd is based on rhBMP-6 and whole blood coagulum [94]. A European clinical trial (EudraCT Number: 2014-005101-21) including 100 patients is ongoing to evaluate its safety, efficacy, tolerability and pharmacokinetics when locally delivered to a fracture site.
3.3. Peptides derived from the extracellular matrix
An alternative strategy to recombinant growth factors are peptides that can be produced easily using peptide synthesizers. If properly designed, peptides can target cellular receptors. Several products are reviewed below.
B2A (B2A2-K-NS). B2A is a bioactive synthetic multi-domain peptide designed to augment spinal fusion. It is associated with HAP/β-TCP granules in the form of Amplex® for foot and ankle fusion and Prefix® for lumbar fusion (Ferring Pharmaceuticals). In vitro, B2A induces chondrogenic differentiation and enhances the in vivo repair of damaged cartilage in an osteoarthritis model [95]. The working mechanism is not fully identified [96] but it was found to induce cell proliferation and cell-type-specific activation of bone morphogenetic protein receptor(s), leading to increased ERK activity. ERK is a hallmark of non-smad signalling pathway leading to bone differentiation. A pre-clinical study in lumbar interbody spinal fusion in sheep [97] concluded that peptide-coated granules produced more bone than in the control group with no heterotopic ossification. The biocompatibility and lack of toxicity and inflammation was also assessed [98]. A pilot study on 22 patients was completed in 2012 to assess the safety and preliminary effectiveness of prefix ® in subjects with degenerative disc disease undergoing spine fusion surgery (NCT00798902). Another study in 24 patients completed in 2012 aimed to assess its safety and effectiveness in comparison to autograft (NCT01224119). More recently in 2017, a multi-center, randomized, pivotal study (NCT03028415, lasting until 2020) was launched to compare Amplex® to autologous bone graft in subjects (480 participants) indicated for hindfoot and ankle arthrodesis surgery.
P-15. P-15 is a 15 amino-acid peptide derived from collagen, a major protein of the bone extracellular matrix, that plays a role in osteoblast attachment and activity [99, 100] and improves the differentiation of mesenchymal stem cells [101]. P-15 was initially used in dental applications and to treat delayed union fractures in 22 patients [102]. Histological assessment of the fracture callus in five of the patients confirmed the encouraging clinical and radiographic results. It was assessed in a pre-clinical study of lumbar spine fusion in an ovine model using PEEK interbody rings filled with autologous bone at one level or with ABM/P-15 at another level [103]. There was no statistical difference between the two treatment groups at 6 months. P-15 is used in combination with bone mineral for spinal fusion, non-union fractures and joint reconstruction. I-FACTOR™ peptide enhanced bone graft (Cerapedics, Inc.) is a composite bone graft material containing the P-15 peptide adsorbed onto calcium phosphate particles, which are suspended in a sodium carboxymethylcellulose hydrogel carrier. It aims to treat low back pain, spinal stenosis, and intervertebral disc degeneration.
A first clinical trial in 2006 (NCT00310440) included 319 participants to evaluate bone putty containing P-15 for effectiveness and safety compared to local autologous bone. P-15 bone putty was applied in instrumented anterior cervical discectomy for fusion in patients with degenerative cervical disc disease, in combination with a structural allograft ring. A second trial in 2012 (NCT01618435) included 108 participants to compare its clinical effect versus allograft in non-instrumented posterolateral spondylodesis operation. In 2015, a study in 40 patients examined its efficacy and safety for use in posterior lumbar interbody fusion [104]. The results showed that ABM/P-15 has equal or greater efficacy at 6 and 12 months than autograft (97% vs 59% intra-cage bridging bone at 6 months). Pain also decreased while function increased. The results of a prospective multicentre pivotal FDA investigational device exemption (IDE) trial in anterior discectomy and cervical fusion were published in 2016 [105]. The overall success rate consisting of fusion, neck disability index, neurological success and safety success was statistically higher in I-FACTOR™ subjects than in autograft subjects (68% and 56%).
The I-Factor™ product received pre-market approval by the FDA in 2016. It is supplied to the clinician as a sterile device in a single-use, pre-filled syringe containing the graft material. It is indicated for use in skeletally mature patients for reconstruction of a degenerated cervical disc and must be used inside an allograft bone ring with supplemental anterior plate fixation. An ongoing clinical trial including 102 participants (NCT02895555 begun in 2016) aims to investigate whether the clinical outcome of I-Factor™ is superior to that of allograft in spinal fusion in elderly people.
To note, although peptides aimed to target BMP receptors are studied at the research level [106, 107], to our knowledge, there is no convincing evidence that they may be used for a clinical purpose. The difficulty resides in the complexity of BMP receptors and associated signaling [108]; these are difficult to reproduce solely by peptides, which are only short fragments of the growth factors.
3.4. Small molecules as regulators of bone mass: PTH, NELL-1, LIMP-1
The last option is to use small molecules that are regulators of bone mass [109].
Parathyroid hormone (PTH). PTH plays a central role in regulating calcium-phosphate metabolism. Its production increases in response to low serum calcium levels. Moreover PTH enhances the Wnt-beta catenin pathway that is central to osteogenesis and bone formation. It is also used as a drug to treat osteoporosis. The products developed by Kuros (KUR-111/112/113) contain PTH trapped in a natural fibrin matrix combined with a structural ceramic component (HAP/TCP granules), to provide mechanical stability during healing. To date, only their MagnetOs granules (granules containing magnetic particles) have obtained CE mark approval in the EU and 510(k) approval in the US. The bioactive products are based on an engineered active fragment of human parathyroid hormone (PTH(1-34)), linked to a transglutaminase substrate for binding to fibrin as a delivery mechanism, and cell-invasion matrix with an intervening plasmin-sensitive link. It was initially tested in femur and humerus defects of female sheep [110], where it was both osteoconductive and osteoinductive. KUR-111 is a bone graft substitute and was initially developed for the treatment of tibial plateau fractures, where success was reported in a phase IIb clinical study (NCT00533793, ended in 2011). This study assessed the safety and efficacy of KUR-111 in 183 patients across 30 centers in Europe and Australia. At 16 weeks, 84% of autograft treated patients and 84% of patients treated with the higher dose of KUR-111 had radiological fracture healing, but no results were published in a peer-reviewed article. KUR-113 was developed for fractures at risk for incomplete healing. It was initially tested in tibial shaft fractures in a phase II clinical study and is now being tested for spinal fusion in patients with degenerative disc disease. KUR-112 is a candidate product for patients with solitary bone cysts and was tested pre-clinically in a horse model [111].
Nel-like molecule-1 (NELL-1). NELL-1 is a secreted osteoinductive protein whose expression controls the amount of bone formed [112] and promoted cartilage regeneration in an in vivo rabbit model [113]. It induced pericyte proliferation and had pro-angiogenic effects, both in vitro and in vivo [114]. A short isoform NELL-1570 induced significant regeneration in a rat calvarial defect accompanied by increased proliferation of bone marrow derived stem cells (BMSCs) [115]. In a model of osteoporotic rats, a combination of human perivascular stem cells and NELL-1 synergistically enhanced spinal fusion [116]. Mechanistic studies were performed in animals (nonhuman primate lumbar spine fusion model), using a peptide coated β-TCP as a carrier. The study revealed an influx of antigen-1 positive mesenchymal progeny cells and complete bone fusion across all samples (100% spinal fusion rate) [117]. Recently, it was tested by systemic administration of PEGylated NELL-1 using a mouse fracture healing model with positive results [118]. All of these pre-clinical data indicate that NELL-1-based therapy for local or systemic bone formation can be further pursued for clinical translation.
LIM mineralization protein-1 (LMP-1). LMP-1 plays a role in the BMP pathway via activation of ERK, and regulates BMP-2 responsiveness [119, 120] and bone development. In 2008, a preliminary study evaluated the possibility of LMP-1-based retroviral gene therapy to stimulate osteoblast differentiation in vitro and fracture repair in vivo [121].
There have also been trials to inhibit sclerostin that is a Wnt inhibitor. This strategy has already been used for treating osteoporosis. Romosozumab, a humanised anti-sclerostin antibody, is developed by Amgen and has been advanced to phase III for osteoporosis. It was also studied at different doses for fracture healing (hip fracture in NCT01081678 on 332 participants) and in tibial diaphyseal fracture post definitive fracture fixation with an intramedullary nail (NCT00907296, 402 participants, ended in 2013), but the results were not as expected and the study was stopped for these applications. In fact, from a mechanistic point of view, it was shown recently that inhibiting the Wnt inhibitor sclerostin leads to a negative feedback mechanism and to the increase in expression of another Wnt inhibitor, Dickkopf-1 (DKK-1) [122]. As a consequence, Wnt-driven bone formation is limited.
In summary, to date, several bioactive molecules are used to enhance bone repair. Full-length growth factors or peptides derived from proteins appear to be the most promising strategies. Of note, the carriers used so far to deliver these bioactive molecules are mainly extra-cellular matrix proteins (collagen, fibrin), or biomimetic calcium phosphate, which provides osteoconduction and mechanical support.
4. Bone graft substitutes and stem cells
The third major strategy relies on using stem cells in a cell-based construct [32, 123]. For this approach, three essential building blocks are typically required: i) progenitor cells to form tissues together with available host cells, ii) stimulatory factors to direct cellular processes, and iii) a biomaterial template to provide cells with 3D cues to form de novo tissue upon implantation in vivo [123, 124]. Three major cell types used as progenitors are bone marrow stromal cells (BMSCs), adipose-derived mesenchymal cells (ASCs) and periosteum-derived stem cells (PDSCs), whose properties are compared in Table 2.
Table 2.
Types of stem cells used in pre-clinical and clinical trials. The different parameters are assessed for each cell type. +, ++, +++ gives an indication of the intensity level of each parameters.
| Cell type | Harvest numbers | Ease of harvest | In vitro proliferation | Osteogenic differentiation | Chondrogenic differentiation |
|---|---|---|---|---|---|
| hBMSCs | ++ | + | ++ | ++ | + |
| hASCs | +++ | +++ | ++ | ++ | + |
| hPDSCs | + | + | ++ | +++ | +++ |
As a first step, autologous or allogenic cells are isolated and expanded in vitro to ensure a sufficient cell population for the therapy. Thereafter, cells can be stimulated in order to induce osteogenic and/or chondrogenic differentiation followed by encapsulation/seeding into/onto a biomaterial. Alternatively, the expanded cells can also be encapsulated/seeded into/onto a biomaterial containing stimulatory molecules (i.e. growth factors), or a combination of both approaches (e.g. stimulation of expanded cells in a 3D environment). Next, the prepared construct can be further cultured in the laboratory in a bioreactor system to reach a more mature stage. Alternatively, the construct can be implanted so that the primed construct can further develop in vivo. Ideally, the chosen combination of mentioned factors should then create an environment that drives and stimulates the cells to form new, functional tissue that subsequently integrates into the existing tissue at the defect site [125–127]. Even though substantial research efforts have been made since the first implantation of cells for bone formation/regeneration was reported over 50 years ago [128, 129], only a few therapies have actually made it to the clinical setting to date.
4.1. Cell-based therapies in clinical trials
By searching on “clinicaltrials.gov” with the keywords “nonunion” and “cell”, 17 registered trials were identified (Table 3). From this search, a variety of cell-based strategies are or have been investigated. In terms of cell source, readily differentiated cells such as osteoblasts or chondrocytes can be used, but are restricted due to their limited accessibility and the lack of a self-renewal capacity. In addition, their heterogeneous nature makes them less attractive from regulatory and efficacy perspectives [130]. Consequently, only 1 of 17 registered clinical trials for cell-based treatment of non-union bone fractures uses osteoblast-like cells (NCT01756326) (Table 3).
Table 3.
Research on clinicaltrials.gov with the keywords “nonunion” and “cell”, resulting in 17 studies
| Reference | Status | Phase | Country | Title |
|---|---|---|---|---|
| NCT03031509 | Not yet recruiting | I | China | Human Amniotic Epithelial Cells of 50 million transplant to nonunion site after debridement surgery |
| NCT02230514 | Recruiting | II | Spain | In vitro expanded autologous mesenchymal stromal cells fixed in allogenic bone tissue in association with open surgery |
| NCT01756326 | Active | II | Belgium | Autologous Osteoblastic Cells (PREOB®) Implantation |
| NCT03325504 | Recruiting | III | Spain | Culture-expanded autologous bone marrow derived cells combined with biphasic calcium phosphate biomaterial granules |
| NCT02815423 | Not yet recruiting | II | China | Transplantation of umbilical cord mesenchymal stem cells |
| NCT01958502 | Unknown | II | Iran | Surgical treatment of non union with mononuclear bone marrow mesenchymal stem cells with BMP2 within a 3-D tissue engineered scaffold |
| NCT01206179 | Completed | I | Iran | Injection of bone marrow derived mesenchymal cells in fractured zone |
| NCT01581892 | Completed | I/II | Spain | Administration of mononuclear bone marrow stem cells combined with an osteogenic matrix |
| NCT01788059 | Completed | II | Iran | Stem cells derived from iliac bone marrow injected to non-union site nonunion site of the bone fracture |
| NCT02177565 | Completed | N/D | United Kingdom | In vitro expanded bone marrow stromal cells on a carrier |
| NCT01429012 | Unknown | II | Belgium | Injection of autologous bone marrow derived mesenchymal stem cells |
| NCT02307435 | Unknown | I | Indonesia | In vitro expanded allogenic mesenchymal stem cells from umbilical cord/ bone marrow/ adipose are implanted combined with HA-CaSo4 |
| NCT01435434 | Unknown | N/D | Israel | Injection of autologous, isolated, sterile centrifuged nucleated bone marrow stromal cells, and Ignite® demineralize bone matrix in the treatment of high risk non-union/delayed fractures |
| NCT00916981 | Completed | I/II | Belgium | Injection of in vitro expanded and stimulated bone marrow stromal cells |
| NCT02448849 | Unknown | II/III | Iran | Percutaneous implantation of autologous bone marrow derived mesenchymal stromal/stem cell in combination with platelet lysate |
| NCT00424567 | Terminated | I/II | US | Freshly isolated bone marrow stromal cells mixed with commercially available bone matrix during surgery |
| NCT01626625 | Unknown | I | Indonesia | In vitro expanded bone marrow stromal cells in combination with hydroxyapatite |
Progenitor cells represent a more suitable cell population that can be harvested and expanded in vitro if required to reach sufficient cell numbers. The required number of cells needed for fracture repair depends on the specific fracture characteristics, cell source, stimulation method and biomaterial. For example, the required number of progenitor cells for a large long bone defect of 4 cm is estimated at 600 million. As a progenitor cell source, two of the registered trials use amniotic epithelial cells (NCT03031509) and umbilical cord mesenchymal stem cells (NCT02815423), respectively. However, a study confirmed that these cells are in an intermediate state of pluripotency, between embryonic and somatic stem cells [131]. Accordingly, they can differentiate into mesoderm, ectoderm and endoderm lineages, but can also give rise to teratoma formation, which raises important safety issues [132]. In one study (NCT02307435, ended in 2017, no result published), umbilical cord derived progenitors, ASCs and BMSCs were compared. In this study, the progenitor cells of autologous origin were expanded in vitro. Thereafter, their efficacy was evaluated upon implantation in combination with a synthetic scaffold (HA-CaSO4). Unfortunately, here again, the results were not published.
Apart from these studies, it is clear that BMSCs remain the most widely used progenitor cell population since the discovery of this multipotent cell population over 50 years ago [129]. Consequently, they are also frequently used for bone repair strategies, as reflected by their use in 14 of the 17 registered clinical trials (Table 2), either in their as-harvested state, or after expansion in vitro. In only one of these studies, the in vitro expanded cells have also been stimulated for differentiation. Unfortunately, no information regarding stimulatory factors, timing or characterization is mentioned (NCT01756326). In all other trials, non-stimulated cells alone, or in combination with stimulatory factors (i.e. BMP-2, platelet lysates) are implanted.
Cells are implanted either via direct injection in a medium or after seeding them onto an osteogenic matrix. The majority of scaffolds are allogenic bone grafts, demineralized bone tissue, hydroxyapatite, granules made of biphasic calcium phosphate or sulfates, and collagen scaffolds. Unfortunately, no results are reported in any of the trials, including the five completed studies and the terminated study (i.e. a study that was terminated earlier and the patients are no longer being examined or treated).
4.2. A strategic approach to non-union treatment - the developmental engineering paradigm
Cell-based strategies have emerged as promising treatment strategies when the intrinsic regenerative potential is diminished by age, health or other factors [133]. Yet, the majority of these strategies still face difficulties in the translation of in vitro findings to more clinically relevant in vivo studies. It has been suggested that one of the additional major reasons for this is the limited attention paid to the mechanism of action in the natural healing process of the particular fracture [133, 134]. Generally, the success of an implant relies on the choice of a potent progenitor cell population able to form the required tissue, together with the subsequent stimulatory cues to frame and support these cells. As a result, the lack of convincing success in current available therapies is still today an issue related to design quality. The developmental engineering paradigm (DEP) has gained attention by fusing engineering principles with concepts from developmental biology [125–127].
The key in the DEP-approach when designing a regenerative construct is having basic knowledge in regard to the anatomical and physiological properties of the original tissue to be replaced. Thereafter, crucial factors required for the regeneration should be combined to develop a functional, engineered solution for a clinical problem. With this in mind, the chosen factors (i.e. cells, stimulatory factors, 3D matrix) used in the development of a cell-based construct for bone regeneration should therefore be inspired by the essential or even crucial factors from the natural healing process. Initially, the clinician defines the characteristics of the fracture. This includes the size, stability, severity of damaged surrounding tissue, vascularization issues and which treatment strategy is required; e.g. for a cartilage intermediate, a primed implant has an immature matrix, which allows an easier integration, while a differentiated implant has a mature matrix that can provide better stability but raises problems with integration/adaptation. Next, biologists can determine which crucial biological processes are involved and can define which factors and molecular events are required to recapitulate these crucial processes.
As an example, we need to define a treatment strategy for a patient with a critical size (> 4 cm) long bone fracture (Figure 3), which is partly unstable due to its large size. This kind of fracture would normally heal through the formation of an intermediate avascular cartilaginous callus, where cells from the periosteum are the main contributing cell population. Consequently, progenitor cells from the periosteum would be the ideal cell candidate for a cell-based construct to treat this fracture.
In terms of stimulatory cues for the construct, BMP-2 has been shown to be essential in periosteum-mediated fracture healing, and is therefore a suitable candidate in terms of stimulatory factors. Next, crucial extra-cellular matrix components in the avascular cartilaginous callus are collagens and sulfated glycosaminoglycans (sGAG), as well as calcium in the more mature callus. Thus, depending on the differentiation maturity of the BMP-2 stimulated progenitors, a scaffold based on collagens, sGAG or calcium may be of interest. In summary, an interesting construct for the treatment of a non-stabilized long bone fracture non-union could be based on in vitro expanded periosteum derived cells. These cells could then be stimulated with BMP-2, and combined with a 3D environment providing a collagen-inspired cell-binding and growth factor-binding material, functionalized with sGAG or calcium ions. The material should also be adapted to provide mechanical stability for the cells, resembling the natural ECM found in the callus.
Next, computational researchers can then simulate cell, growth factor and biomaterial compositions and provide a design for a 3D tissue intermediate. Subsequently, engineers can develop the methods required to create the building blocks, technologies for final tissue assembly and perform a detailed analysis. Once this is achieved, the developed in vitro tissue needs to be validated in suitable in vitro and in vivo pre-clinical studies. Consequently, in the cases of consistent outcomes, these are followed by exploratory clinical trials, and further tailored by refined translation or iterative improvements [35].
4.3. Different cell sources for different fracture repairs
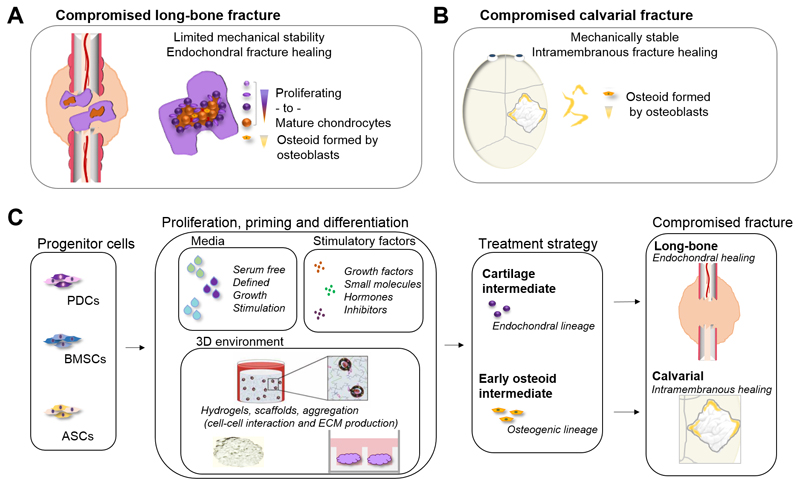
With this in mind, the first step in developing a successful cell-based construct for bone fracture regeneration is to determine the characteristics of the fracture the implant aims to heal (i.e. if it is for endochondral fracture healing or intramembranous fracture healing) (Figure 4A and B). Second, the type of fracture will then define what kind of cells should be used as the driving force for the construct’s regenerative potential (Figure 4C). This is crucial since the specific tissue forming potential of a certain cell population is highly influenced by the stem cell niche [135]. In addition, genetic variability, and/or epigenetic alterations also play a crucial role since adult stem cells are regulated on a genetic as well as epigenetic level during regeneration [136]. The next step is then to define: i) an optimal cell expansion- and/or differentiation medium, ii) suitable stimulatory factors iii) a well-defined 3D environment to further support and steer cell differentiation and iv) an appropriate in vitro priming period. When combined together, these parts define the cell-based construct that will be further developing after in vivo implantation. An example illustrates in Figure 4D two different strategies depending on the nature of the facture: a compromised long bone fracture that is mechanically less stable requires a cartilaginous-intermediate-strategy, while a compromised but mechanically stable defect, such as the calvarial defect, can be better treated with an early osteoid-primed intermediate (Figure 4D). As a first step, a short overview of clinically relevant cell populations for the treatment of compromised bone fractures will be reviewed.
Figure 4.
Biology as a guide in the development of a cell-based construct for the treatment of compromised bone fractures. The natural healing process of a bone fracture is largely dependent on the mechanical environment. (A) A mechanically unstable long bone fracture heals through the formation of an intermediate cartilaginous callus that subsequently remodels into bone and the native bone structure and shape. The initial cartilaginous callus is mainly formed by cells recruited from the periosteum, and provides initial stabilization to the fracture. This allows blood vessel ingrowth closely followed by remodeling by cartilage-resorbing chondroclasts. Thereafter, progenitor cells recruited from the periosteum and bone marrow differentiate into osteoblasts that deposit new bone. (B) A compromised calvarial fracture in a mechanically stable environment mainly heals through direct ossification. In this process, cells from the periosteum, bone marrow (long bones) and dura mater (calvarial) contribute to the defect healing. (C) Consequently, the type of fracture to be repaired/healed determines the cell source, media, stimulatory factors and 3D environment that should be used in the design of a cell-based construct.
4.3.1. Bone marrow stromal cells (BMSCs)
BMSCs represent a heterogeneous cell population that can be harvested from the stromal fraction of the bone marrow [137]. This harvest is generally carried out through bone marrow aspiration from the wing of the ilium, proximal medial tibia, and/or the proximal humerus. Since there is little extracellular matrix present in marrow, gentle mechanical disruption can readily dissociate stroma and hematopoietic cells from the bone marrow harvested into a single-cell suspension [138]. From an aspirate of 400-500 mL of bone marrow, approximately 100,000-130,000 BMSCs can be obtained [139]. Upon injection of BMSCs into a stabilized fracture, they appear to contribute to direct ossification [140]. For expansion, BMSCs are characterized by their ability to adhere to plastic surfaces with a fibroblast-like morphology [138]. Upon plating BMSCs at low density onto plastic culture flasks, they adhere and can be separated from the non-adherent hematopoietic cells by repeated washing. With appropriate culture conditions, distinct colonies form, each of which derives from a single precursor cell, the human fibroblast colony forming units (CFU-F) [141]. The population doubling time for BMSCs is about 79 ± 16 h, a number that increases with each passage [142]. The in vitro expanded BMSC population can differentiate into the osteogenic, chondrogenic and adipogenic lineages, even though their differentiation potential is largely reduced upon in vitro expansion [143]. This has been associated with the relatively fast activation of senescence [144]. Similarly to their role in bone fracture healing, research has shown that BMSCs possess greater osteogenic potential than either chondrogenic or adipogenic potential [144]. Moreover, their osteogenic potential appears to be one of the last lineage commitment phenotypes to be lost upon in vitro expansion [144, 145]; in vivo studies of long bone fracture healing showed that implanted BMSCs did not actively contribute to the chondrogenic and osteogenic lineages during fracture healing, but rather contributed to steer inflammatory and bone resorbing cells [146]. Large donor variability has been reported with respect to the in vivo bone forming capacity of these cells.
4.3.2. Adipose-derived progenitor cells
Adipose-derived stem cells (ASCs) are a heterogeneous cell population that has gained attention for regenerative purposes [147]. This progenitor cell population has shown multi-lineage differentiation potential including adipogenic, osteogenic, chondrogenic and myogenic lineages [148]. Attractive attributes with ASCs are that adipose tissue is abundant, easy to harvest and present in several types of adipose tissue including visceral fat, subcutaneous fat and organ fat. Adipose obtained by liposuction is especially convenient since the procedure provides homogenous finely minced fragments that can easily be digested enzymatically in a short time. About 1 g of adipose tissue can yield roughly 2 million cells, where 10% are confirmed as ASCs [149, 150]. Upon isolation, ASCs are adherent to plastic and can be expanded in vitro with a 10-fold higher CFU-F unit than BMSCs, with a faster population doubling time of 59.8 ± 3.3 h [142]. In addition, they are genetically more stable upon in vitro expansion [150]. This interesting cell population appears to be immunoprivileged since it was found to be protective against acute graft-versus-host disease [150]. ASCs also exert an immunosuppressive effect by inhibiting proliferation of activated allogenic lymphocytes, which could be an attractive feature if used for allogenic implants [151, 152]. In preclinical studies, ASCs were seeded onto scaffolds for critical size mouse calvarial defects, with significant intramembranous bone formation and areas of complete bone regeneration being reported with 84-99% contribution of the implanted cells [153]. ASCs have also shown promising results for spinal fusion, with reduced infiltration of inflammatory cells together with superior fusion as compared to scaffolds without ASCs [154, 155]. However, ASCs should be used with care if the target is endochondral fracture healing, since ASCs produce factors that inhibit chondrocyte proliferation and stimulate chondrocyte apoptosis [156]. A first clinical study with autologous ASCs for microvascular reconstruction showed promising results in terms of outcome and initial safety [157]. A microvascular flap was created consisting of autologous ASCs and a β-TCP scaffold combined with BMP-2 [157]. Mature bone structure and vasculature developed in the flap after 8 weeks of in vitro culture followed by implantation, leading to defect healing. Even though these are promising reports, sporadic reports on the tumor-enhancing properties of ASCs call for caution in the use of ASCs in clinical applications. In addition, significant differences were reported in the differentiation capacity of ASCs isolated at different anatomical sites, and for differences in donor age and gender [158, 159]. Moreover, key transcription factors and molecular events steering ASC differentiation are largely unknown. Thus, even though ASCs may have potential as an attractive cell source for bone regenerative purposes, it seems like they might be suitable for stabilized fractures with intramembranous bone healing (Figure 4). As for all expanded cells, extensive and systematic evaluation of the safety, reproducibility and clinical quality of in vitro expanded ASCs is necessary.
4.3.3. Periosteum derived progenitor cells
The periosteum is a 100 µm thin membrane enveloping all outer bone surfaces not covered by cartilage [160]. This membrane comprises an outer fibrous layer (70 µm) adjacent to the surrounding soft fibrous and muscular tissue and an inner cambium layer (30 µm) [161]. The inner cambium layer is directly connected to the outer bone cortex, is highly vascularized and serves as a host for osteo-chondro progenitor cells with a unique tissue-building capacity [162]. In healthy bone development and homeostasis, cells from the cambium layer give rise to osteoblasts, allowing appositional bone growth as well as remodeling in concert with osteoclasts [163]. During fracture, progenitor cells from the periosteum undergo massive expansion and subsequent chondrogenic and/or osteogenic differentiation [18]. This leads to the development of the callus, which further drives fracture healing. Periosteal tissue can be surgically obtained from a patient by the use of a periosteal elevator, which cuts the fibers that anchor the periosteum to the bone [164]. From a one-gram biopsy of periosteum, roughly 150,000 skeletal progenitor cells can be isolated for in vitro expansion (unpublished data). Periosteum-derived cells (PDSCs) can then be enzymatically released from the periosteal biopsy onto plastic culture dishes where they have shown single-cell-derived clonal populations [165]. Under serum containing conditions, hPDSCs have shown in vitro expansion potential for up to 30 population doublings, with cells exhibiting a fibroblast-like morphology and a population doubling time of 55 h [166]. In addition, their multi-lineage potential upon expansion has been proven in vitro as well as in vivo [123, 124, 165, 167, 168]. Interestingly, cultured hPDSCs do not express telomerase, but possess relatively long telomeres, a potential explanation for their stability upon culture expansion [165]. To note, native or expanded hPDSCs do not express chondrogenic or osteogenic characteristics, but require specific conditions to differentiate towards these lineages [165, 167, 168]. Even though these research findings are interesting, they do not yet meet clinical expectations, potentially due to the use of media containing serum [169]. Serum, which is commonly used in general cell culture protocols, contains an undefined and variable range of factors such as cytokines and inhibitors that bind to cell surface receptors. As a consequence, batch-to-batch variability significantly affects the characteristics of cell-based implants, leading to unpredictable behavior and outcomes [170, 171]. As a result, a serum free pre-conditioning regime of hPDSCs was developed [123]. Interestingly, the serum free conditions led to improve in vitro and in vivo potential of in vitro expanded hPDSCs. Notably, only six days of in vitro BMP-2 stimulation was sufficient to induce fracture healing of a critical size tibial defect in mice by the implanted cells [123]. Unpublished data suggest that this may be related to the selection of a specific skeletal progenitor cell population with elevated expression of BMP receptors, which responds more potently to in vitro priming. These issues will undoubtedly be solved by ongoing and future research, where the focus of biomimetic cell expansion protocols is anticipated to be critical.
4.4. Fate of implanted cells
The fate of implanted cells is likely to depend on the cell type, differentiation stage and stimulatory factors used together with biomaterials. In a study in which hBMSCs were seeded onto biphasic calcium phosphate scaffolds implanted ectopically in nude mice, only 1.5% of the implanted cells remained in the scaffold after 37 days [172]. After 8 weeks, the human cells attached along the periphery of the implant and embedded in the osteocyte lacunae in the newly formed bone. In contrast, a similar construct where hPDSCs where seeded onto a CaP-matrix consisting of 65% HAP and 35% βTCP, 71% of the cells in the implant where of human origin [173]. Since bone formed in both studies solely in the scaffold seeded with cells, it is clear that implanted cells can both produce the newly formed bone, and also steer and/or recruit inflammatory cells in the host environment [174]. This was confirmed for an implant with in vitro primed hPDSCs implanted in a critical size tibial defect in mice [123]. Immunohistochemical analysis with human-specific antigens revealed that the implanted cells gave rise to the majority of the cartilaginous callus intermediate and the early osteoid, but cells from the host environment were recruited for the remodeling into more mature bone. Ideally, this is also how an implant should behave upon implantation into the fracture environment in the patient, in order to integrate with the host tissue.
Current guidelines for clinical studies [175–177] state that cell populations should be characterized in terms of phenotypic and/or genotypic profiles, by relevant markers based on gene expression, antigen presentation and biochemical activity [177]. The previously established minimal set of standard criteria for stem cells was aimed to foster a more uniform characterization of stem cells and to facilitate the exchange of data among investigators [178]. Instead, recent data rather suggest some of these characteristics to be the effect of in vitro culture [179–182]. In terms of human stem cells, in vitro expanded hPDSCs have been partly characterized at the single cell level [165] and in whole genome studies [167], but additional work in characterization of native hPDSCs and ASCs is required. Even though the above discussed stem cell-populations have been used as part of auto- or allogenic bone grafts for the treatment of bone defects [183, 184], little is known regarding how the in vitro expanded populations represent the original native cell population. As a consequence, it is unclear whether in vitro expanded progenitor cells fully or only partly represent the native population [185].
4.5. 3D bioprinting of hydrogels and cells or bioactive molecules
We searched for clinical trials on clinicaltrials.gov using the keywords “bioprinting bone regeneration”, “3D printed bone” and explored the full list of ongoing trials on “bone regeneration.” We did not find ongoing clinical studies using bioprinted implants. All the studies are still at the research stage. However, in view of the increased research in this field, it is interesting and worthwhile to mention them.
Among the current additive manufacturing (AM) technologies emerging for bone tissue engineering, bioprinting techniques enable production of biomimetic bone. Although research is not yet at the stage of clinical trials, there are ongoing intensive research studies. Bioprinting is based on the precise deposition of a composite biomaterial made of an assembly of hydrogels, cells and, in some cases, growth factors [186]. This assembly is generally defined as a “bioink” and the three major AM techniques used for bioprinting are either extrusion-based, drop-based or laser-assisted bioprinting [187]. Each technique consists of the deposition of the bioink onto a substrate, presented in filament or droplet form, to generate either 2D or 3D structures. Then, the structure is generally crosslinked either by UV light, chemically or thermally to increase mechanical stability [188]. Hydrogels such as collagen, alginate or hyaluronic acid are the most common materials used as bioinks since they provide an environment similar to the extracellular matrix for the incorporated cells and also present the advantage of being biodegradable [187, 189]. In the case of bone regeneration, the type of cells used for the bioinks are BMSCs, oral cavity mesenchymal stem cells and ASCs. Umbilical vein stem cells and endothelial progenitor stem cells are used for neovascularization purposes [189, 190]. Even if the hydrogels used for bioprinting provide an adequate extracellular environment to the cells, maintaining their viability remains a key challenge of this technique [187]. Another important limitation of the bioprinted constructs is the lack of mechanical strength of the hydrogels. In addition to the hydrogel crosslinking, which can reduce cell viability and functionality, stronger materials can be added to the hydrogels to enhance their properties [187]. For instance, Lin et al. [191] printed a composite porous bone scaffold composed of collagen and hydroxyapatite at a ratio of 1 to 2 (w/w) for large bone defects. Bioinks may fully mimic native tissues by adding bioactive molecules. In order to help the vascularization, Park et al. printed a structure containing the vascular endothelial growth factor (VEGF) to promote vascularization [192]. Bioprinting bone is interesting in terms of spatial control, but it remains to be proven that printed bone can be produced at a large scale, and can be safe and used in clinics [193]. The clinical translation of printed constructs will most probably take a long time.
5. Remaining challenges for improved clinical efficacy
5.1. Detailed characterization of the local microenvironment
Recent literature has highlighted the importance of the local microenvironment for the outcome of regenerative constructs. Several factors that constrain regeneration including the early inflammatory response, extracellular matrix composition, patient age, injury type, physiological adaptation, and angiogenic capacity will largely affect the short- and long-term success of the fracture treatment. In the field of bone tissue engineering, the impact of the inflammatory response/environment on the regenerative capacity of constructs has partly been neglected. There is increasing evidence supporting the importance of the inflammatory response on successful post-natal fracture healing. Attenuation of the inflammatory/immune response can lead to delayed fracture healing or even non-union, where both immune cells and secreted inflammatory factors play a role [11, 12] [13, 14]. In contrast, abrogation of the bone forming capacity was seen using a cell-based construct in immunocompetent mice, as compared to immunocompromised mice [194]. As such, there is clearly a close interconnectivity between the immune and skeletal regeneration systems, in which the balance largely affects the regenerative outcome. Consequently, future research requires detailed characterization of the microenvironment at the fracture site and how this affects and interacts with cells in, as well as recruited by, the engineered implant. Specifically, the goal for engineered regenerative constructs should not only be to form or develop into bone, but to function in an explicit manner in a patient-specific environment.
5.2. Better understanding of the biological mechanisms
Regarding the active biomolecules, it is clear that there is a need to precisely understand what their mode of action is and whether they affect inflammatory or bone precursor cell response. As already suggested by Martino [195], the role of inflammatory cytokines and immune cells regulated in bone regeneration should be further investigated and exploited. The action of bioactive molecules on osteoblasts versus osteoclasts should also be better understood. In the future, combined strategies targeting growth factor receptors and cellular adhesion receptors may be developed to induce cooperative signalling and optimize bone repair. The specificity of the mechanism of action is key.
5.3. Better control of the spatial and temporal doses and delivery of bioactive molecules in vivo
It is also important to precisely control and confine the bioactive molecules in vivo to avoid undesirable side effects. Their dose should be optimized to avoid over-dosing, in order to attain a safe but efficient working range to form new bone. Of note, a major difficulty arises from the fact that bioactive molecule delivery is not an intrinsic parameter, but depends on the biodegradability (i.e. kinetics and degradation products) of the biomaterial carrier. Ideally, these two parameters should be uncoupled.
5.4. Modulating the material/bioactivity couple for each specific clinical application
To date, as seen in Section 3, the carriers used to deliver the bioactive molecules have typically been biodegradable (matrix proteins, HAP/β–TCP ceramics). In the future, it may be beneficial to take advantage of the mechanical properties of the engineered scaffold, which can be non-biodegradable polymeric materials such as PEEK or biodegradable such as PCL and PLGA. Metallic meshes made of titanium may also benefit from an added bioactivity. Depending on each specific clinical situation, the coupled material/bioactive molecule may be adapted in order to provide proper structural, biodegradable and surface characteristics depending on the indication. As mentioned in Section 1 above, the stability of the bone regeneration site is also crucial for long bone healing. Improvement in scaffold design and production techniques, such as 3D printing and fixation techniques, may improve the bone repair efficiency. Such developments require collaborative work between clinicians, engineers and biologists/biochemists to improve the BGS scaffold, the efficacy of incorporated drugs and the surgical procedure itself.
In the field of vascular stents, the combination of metallic or polymeric scaffolds and active molecules has already been implemented in clinics since 2003, where the tubular mesh provides a mechanical support and the anti-proliferative drug embedded in a surface coating, acts on the cells in the vascular wall [196]. Thus, it will be interesting, by taking examples from the vascular field, to further expand the potential of material carriers. For instance, instead of adding ceramic granules inside a PEEK cage, as done currently in clinics, the bioactive molecules may directly be coated at the surface of the carrier, using a spacer or a “cushion”. Among the numerous technical strategies that are currently developed by academic teams to trap BMPs at the surface of materials, some may well emerge in the near future, provided that they are sufficiently robust to meet the industrial requirements (large-scale industrialization, sterilization, storage…). In terms of regulation and development time, combining an already approved medical device with an already approved active molecule could speed up the translation to clinic.
5.5. Future requirements regarding progenitor cell characterization
Generally, skeletal precursors such as stromal cells represent a diverse population of cells with a wide range of potential therapeutic applications. Despite considerable interest and effort, there are currently quite a few challenges including the established regulatory-defined guidelines regarding detailed characterization for stromal cell-based products [175–177]. Current guidelines for clinical studies state that cell populations should be characterized in terms of phenotypic and/or genotypic profiles, by relevant markers based on gene expression, antigen presentation, biochemical activity [177]. The previously established minimal set of standard criteria for stem cells was aimed to foster a more uniform characterization of SCs and to facilitate the exchange of data among investigators [178]. Plastic-adherent cells, when maintained in standard culture conditions, must express surface receptors such as CD105, CD73 and CD90, and lack expression of CD45, CD34, CD14 or CD11b, CD79alpha or CD19 and HLA-DR surface molecules, and have the ability to differentiate to osteoblasts, adipocytes and chondroblasts in vitro. However, these criteria were based on findings from partly in vitro expanded populations and they have shown limited relevance in terms of “stemness.” Most importantly, they do not correlate or predict in vivo bone forming potency. Instead, recent data rather suggest that their differentiation ability is the effect of in vitro culture [179].
Consequently, stem cell researchers are currently limited in terms of validated markers for human skeletal progenitor cells. The previously suggested cell surface marker characterization together with three-lineage potential is neither distinctive nor sufficient for defining the cellular composition and biological function(s) of stromal cells or stromal cell-based products. Research efforts are largely driven by local regulatory advisors, subsequently leading to inconsistent characterization between cell populations and therapeutic constructs. In terms of SC research, BMSCs are the classical and most investigated SC population, partly due to their history, relative availability, documented characterization and extended investigation [180]. However, based on perceived advantages such as ease of harvest of ASCs, and the critical role of PDSCs in fracture healing together with their proven regenerative potential in animal models, the use of both these cell sources brings the potential of cell-based strategies to new dimensions. The continuous expansion of tools and techniques has allowed better insight into the specific role and regulation of human stromal cells.
Currently, in vivo studies in rodents are starting to confirm the existence of a structured hierarchy of skeletal progenitor cells [181, 182]. In terms of human stromal cells, in vitro expanded hPDSCs have been partly characterized at the single-cell level [165] and in whole genome studies [167], but additional work in terms of characterization of native hPDSCs and ASCs is required. Specifically, this is crucial in order to evaluate the effect, efficacy and optimization of in vitro expansion and stimulatory conditions prior to clinical translation. Even though the above discussed SC-populations have been used as part of auto- or allogenic bone grafts for the treatment of bone defects [183, 184], little is still known regarding how the in vitro expanded populations represent the original native cell population. In consequence, it is unclear whether in vitro expanded progenitor cells fully or only partly represent the native population [185]. Therefore, future studies are needed to identify these, as well as to critically evaluate the impact of in vitro expansion on the preservation of the true progenitor cell identity. These findings will also be a crucial contribution in the development of induced pluripotent stem cells (iPSCs). Tremendous research efforts have been made since the discovery of iPSCs, and their reported in vitro and in vivo potency is impressive [197]. The potential use of iPSCs derived from the patient provide great hope in terms of the development of personalized cell-based treatments. This is based on the hypothesis that the availability of potent progenitors may be less restricted and harmed by in vitro expansion. However, further optimization in terms of safety, reprogramming, stimulation and integration is needed before these cells can be used in the clinic. Moreover, iPSCs generated from patients have been shown to maintain their genetic memory, thus iPSC technology can provide crucial in vitro models in the development of personalized treatment strategies [198].
6. Conclusions
We focused our review on the different strategies for bone repair that are already implemented in the clinics, that are in clinical trials, or that have been tested in animal models. We found that, unfortunately, the results of the clinical trials are not always accessible to researchers and most of these results have not been published in peer-reviewed journals. However, those that are published in peer-reviewed scientific journals provide insightful information for researchers who are currently working on the development of new biomaterials-based products. Thus, there is an urgent need to publish the results of all clinical trials, regardless of whether the trials were successful.
There is a large range of bone repair strategies that are adapted to different clinical needs. Bone graft substitutes, i.e. implantable materials, are currently being optimized in terms of 3D structure and surface properties. Such optimization offers the convenience of a less cumbersome approval procedure by regulating authorities because of the absence of biological materials, but may be limited to small size defects in view of their lack of bone inductive properties. Bioactive molecules are needed when the defects become larger (> 2 cm). For this purpose, growth factors, peptides and small molecules are currently being evaluated at the pre-clinical and clinical levels. The development of bioactive molecules combined with new carriers will likely lead to an improved delivery of the active molecules at a safe and efficient dose. Cell-based strategies may be used for large and compromised defects. Intense pre-clinical studies are currently being performed to better understand how these cell therapies can be effectively used in vivo. Their effective clinical translation requires a larger number of regulatory steps and has a higher cost of development. With the panoply of possibilities and future developments, it will be easier to adapt the treatments to patients in the context of precision medicine.
Acknowledgements
CP is a grateful senior member of the Institut Universitaire de France, whose support is greatly acknowledged. The Picart team receives funding from the Fondation Recherche Medicale (Contract DEQ20170336746) Fondation Gueules Cassées (dossier 21-2016) and Fondation de l’Avenir (AP-RM-16-013). The European commission provided financial support in the frame of H2020 / European Research Council (GA790435). JB is a postdoctoral research fellow funded by Research Foundation Flanders (12S6817N). AWJ is grateful to the nanoscience foundation for support via a Chair of Excellence (FCN-2013-02CE). The Centre of Excellence of Multifunctional Architectured Materials "CEMAM" (n° ANR-10-LABX-44-01) is also acknowledged.
References
- [1].Inc O. In: The orthopaedic industry annual report : 2010-2011. Inc O, editor. Ohio, Chagrin Falls: 44 0232010-2011. [Google Scholar]
- [2].Holmes D. Non-union bone fracture: a quicker fix. Nature. 2017;550:S193. doi: 10.1038/550S193a. [DOI] [PubMed] [Google Scholar]
- [3].Holmes D. Closing the gap. Nature. 2017;550:S194–S5. doi: 10.1038/550S194a. [DOI] [PubMed] [Google Scholar]
- [4].Seeman E. Bone quality: the material and structural basis of bone strength. J Bone Miner Metab. 2008;26:1–8. doi: 10.1007/s00774-007-0793-5. [DOI] [PubMed] [Google Scholar]
- [5].Gerstenfeld LC, Cullinane DM, Barnes GL, Graves DT, Einhorn TA. Fracture healing as a post-natal developmental process: molecular, spatial, and temporal aspects of its regulation. J Cell Biochem. 2003;88:873–84. doi: 10.1002/jcb.10435. [DOI] [PubMed] [Google Scholar]
- [6].Phillips AM. Overview of the fracture healing cascade. Injury. 2005;36(Suppl 3):S5–7. doi: 10.1016/j.injury.2005.07.027. [DOI] [PubMed] [Google Scholar]
- [7].Claes L, Recknagel S, Ignatius A. Fracture healing under healthy and inflammatory conditions. Nat Rev Rheumatol. 2012;8:133–43. doi: 10.1038/nrrheum.2012.1. [DOI] [PubMed] [Google Scholar]
- [8].Kolar P, Schmidt-Bleek K, Schell H, Gaber T, Toben D, Schmidmaier G, Perka C, Buttgereit F, Duda GN. The early fracture hematoma and its potential role in fracture healing. Tissue Eng Part B, Reviews. 2010;16:427–34. doi: 10.1089/ten.TEB.2009.0687. [DOI] [PubMed] [Google Scholar]
- [9].Street J, Winter D, Wang JH, Wakai A, McGuinness A, Redmond HP. Is human fracture hematoma inherently angiogenic? Clinical orthopaedics and related research. 2000:224–37. doi: 10.1097/00003086-200009000-00033. [DOI] [PubMed] [Google Scholar]
- [10].Prystaz K, Kaiser K, Kovtun A, Haffner-Luntzer M, Fischer V, Rapp AE, Liedert A, Strauss G, Waetzig GH, Rose-John S, Ignatius A. Distinct effects of IL-6 classic and trans-signaling in bone fracture healing. Am J Pathol. 2018;188:474–90. doi: 10.1016/j.ajpath.2017.10.011. [DOI] [PubMed] [Google Scholar]
- [11].Schlundt C, El Khassawna T, Serra A, Dienelt A, Wendler S, Schell H, van Rooijen N, Radbruch A, Lucius R, Hartmann S, Duda GN, et al. Macrophages in bone fracture healing: Their essential role in endochondral ossification. Bone. 2018;106:78–89. doi: 10.1016/j.bone.2015.10.019. [DOI] [PubMed] [Google Scholar]
- [12].Gerstenfeld LC, Cho TJ, Kon T, Aizawa T, Tsay A, Fitch J, Barnes GL, Graves DT, Einhorn TA. Impaired fracture healing in the absence of TNF-alpha signaling: the role of TNF-alpha in endochondral cartilage resorption. Journal of Bone Mineralization Research. 2003;18:1584–92. doi: 10.1359/jbmr.2003.18.9.1584. [DOI] [PubMed] [Google Scholar]
- [13].Yang X, Ricciardi BF, Hernandez-Soria A, Shi Y, Pleshko Camacho N, Bostrom MP. Callus mineralization and maturation are delayed during fracture healing in interleukin-6 knockout mice. Bone. 2007;41:928–36. doi: 10.1016/j.bone.2007.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Raggatt LJ, Wullschleger ME, Alexander KA, Wu AC, Millard SM, Kaur S, Maugham ML, Gregory LS, Steck R, Pettit AR. Fracture healing via periosteal callus formation requires macrophages for both initiation and progression of early endochondral ossification. Americal Journal of Pathology. 2014;184:3192–204. doi: 10.1016/j.ajpath.2014.08.017. [DOI] [PubMed] [Google Scholar]
- [15].Hall BK, Miyake T. All for one and one for all: condensations and the initiation of skeletal development. Bioessays. 2000;22:138–47. doi: 10.1002/(SICI)1521-1878(200002)22:2<138::AID-BIES5>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- [16].Hall BK, Miyake T. Divide, accumulate, differentiate: cell condensation in skeletal development revisited. Int J Dev Biol. 1995;39:881–93. [PubMed] [Google Scholar]
- [17].Dunlop LL, Hall BK. Relationships between cellular condensation, preosteoblast formation and epithelial-mesenchymal interactions in initiation of osteogenesis. Int J Dev Biol. 1995;39:357–71. [PubMed] [Google Scholar]
- [18].Colnot C. Skeletal cell fate decisions within periosteum and bone marrow during bone regeneration. J Bone Miner Res. 2009;24:274–82. doi: 10.1359/jbmr.081003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Duchamp de Lageneste O, Julien A, Abou-Khalil R, Frangi G, Carvalho C, Cagnard N, Cordier C, Conway SJ, Colnot C. Periosteum contains skeletal stem cells with high bone regenerative potential controlled by Periostin. Nature communications. 2018;9 doi: 10.1038/s41467-018-03124-z. 773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Schindeler A, McDonald MM, Bokko P, Little DG. Bone remodeling during fracture repair: The cellular picture. Semin Cell Dev Biol. 2008;19:459–66. doi: 10.1016/j.semcdb.2008.07.004. [DOI] [PubMed] [Google Scholar]
- [21].Ai-Aql ZS, Alagl AS, Graves DT, Gerstenfeld LC, Einhorn TA. Molecular mechanisms controlling bone formation during fracture healing and distraction osteogenesis. Journal of dental research. 2008;87:107–18. doi: 10.1177/154405910808700215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Riddle RC, Clemens TL. Bone cell bioenergetics and skeletal energy homeostasis. Physiol Rev. 2017;97:667–98. doi: 10.1152/physrev.00022.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Thompson Z, Miclau T, Hu D, Helms JA. A model for intramembranous ossification during fracture healing. Journal of Orthopedic Research. 2002;20:1091–8. doi: 10.1016/S0736-0266(02)00017-7. [DOI] [PubMed] [Google Scholar]
- [24].Shen X, Wan C, Ramaswamy G, Mavalli M, Wang Y, Duvall CL, Deng LF, Guldberg RE, Eberhart A, Clemens TL, Gilbert SR. Prolyl hydroxylase inhibitors increase neoangiogenesis and callus formation following femur fracture in mice. Journal of Orthopedic Research. 2009;27:1298–305. doi: 10.1002/jor.20886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Barnes GL, Kostenuik PJ, Gerstenfeld LC, Einhorn TA. Growth factor regulation of fracture repair. J Bone Miner Res. 1999;14:1805–15. doi: 10.1359/jbmr.1999.14.11.1805. [DOI] [PubMed] [Google Scholar]
- [26].Gerstenfeld LC, Cho TJ, Kon T, Aizawa T, Cruceta J, Graves BD, Einhorn TA. Impaired intramembranous bone formation during bone repair in the absence of tumor necrosis factor-alpha signaling. Cells, tissues, organs. 2001;169:285–94. doi: 10.1159/000047893. [DOI] [PubMed] [Google Scholar]
- [27].Schmid GJ, Kobayashi C, Sandell LJ, Ornitz DM. Fibroblast growth factor expression during skeletal fracture healing in mice. Dev Dyn. 2009;238:766–74. doi: 10.1002/dvdy.21882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Yu YY, Lieu S, Lu C, Miclau T, Marcucio RS, Colnot C. Immunolocalization of BMPs, BMP antagonists, receptors, and effectors during fracture repair. Bone. 2010;46:841–51. doi: 10.1016/j.bone.2009.11.005. [DOI] [PubMed] [Google Scholar]
- [29].Giannoudis PV, Dinopoulos H, Tsiridis E. Bone substitutes: an update. Injury. 2005;36(Suppl 3):S20–7. doi: 10.1016/j.injury.2005.07.029. [DOI] [PubMed] [Google Scholar]
- [30].Laurencin C, Khan Y, El-Amin SF. Bone graft substitutes. Expert Rev Med Devices. 2006;3:49–57. doi: 10.1586/17434440.3.1.49. [DOI] [PubMed] [Google Scholar]
- [31].Vanderstappen J, Lammens J, Berger P, Laumen A. Ilizarov bone transport as a treatment of congenital pseudarthrosis of the tibia: a long-term follow-up study. Journal of children's orthopaedics. 2015;9:319–24. doi: 10.1007/s11832-015-0675-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Einhorn TA, Gerstenfeld LC. Fracture healing: mechanisms and interventions. Nat Rev Rheumatol. 2015;11:45–54. doi: 10.1038/nrrheum.2014.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Eaton MAW, Levy L, Fontaine OMA. Delivering nanomedicines to patients: A practical guide. Nanomed-Nanotechnol Biol Med. 2015;11:983–92. doi: 10.1016/j.nano.2015.02.004. [DOI] [PubMed] [Google Scholar]
- [34].Hollister SJ, Murphy WL. Scaffold translation: barriers between concept and clinic. Tissue Eng Part B, Reviews. 2011;17:459–74. doi: 10.1089/ten.teb.2011.0251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Volk HD, Stevens MM, Mooney DJ, Grainger DW, Duda GN. Key elements for nourishing the translational research environment. Sci Transl Med. 2015;7:4. doi: 10.1126/scitranslmed.aaa2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Bara JJ, Herrmann M, Evans CH, Miclau T, Ratcliffe A, Richards RG. Improving translation success of cell-based therapies in orthopaedics. J Orthop Res. 2016;34:17–21. doi: 10.1002/jor.23055. [DOI] [PubMed] [Google Scholar]
- [37].Reichert JC, Saifzadeh S, Wullschleger ME, Epari DR, Schutz MA, Duda GN, Schell H, van Griensven M, Redl H, Hutmacher DW. The challenge of establishing preclinical models for segmental bone defect research. Biomaterials. 2009;30:2149–63. doi: 10.1016/j.biomaterials.2008.12.050. [DOI] [PubMed] [Google Scholar]
- [38].Li Y, Chen SK, Li L, Qin L, Wang XL, Lai YX. Bone defect animal models for testing efficacy of bone substitute biomaterials. J Orthop Transl. 2015;3:95–104. doi: 10.1016/j.jot.2015.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Wancket LM. Animal models for evaluation of bone implants and devices: comparative bone structure and common model uses. Vet Pathol. 2015;52:842–50. doi: 10.1177/0300985815593124. [DOI] [PubMed] [Google Scholar]
- [40].Peric M, Dumic-Cule I, Grcevic D, Matijasic M, Verbanac D, Paul R, Grgurevic L, Trkulja V, Bagi CM, Vukicevic S. The rational use of animal models in the evaluation of novel bone regenerative therapies. Bone. 2015;70:73–86. doi: 10.1016/j.bone.2014.07.010. [DOI] [PubMed] [Google Scholar]
- [41].Lammens J, Marechal M, Geris L, Van der Aa J, Van Hauwermeiren H, Luyten FP, Delport H. Warning about the use of critical-size defects for the translational study of bone repair: Analysis of a sheep tibial model. Tissue Eng Part C-Methods. 2017;23:694–9. doi: 10.1089/ten.TEC.2017.0147. [DOI] [PubMed] [Google Scholar]
- [42].Szpalski C, Wetterau M, Barr J, Warren SM. Bone tissue engineering: current Strategies and Techniques-Part I: Scaffolds. Tissue Eng Part B-Rev. 2012;18:246–57. doi: 10.1089/ten.TEB.2011.0427. [DOI] [PubMed] [Google Scholar]
- [43].Amini AR, Adams DJ, Laurencin CT, Nukavarapu SP. Optimally porous and biomechanically compatible scaffolds for large-area bone regeneration. Tissue Eng Part A. 2012;18:1376–88. doi: 10.1089/ten.tea.2011.0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Tan XP, Tan YJ, Chow CSL, Tor SB, Yeong WY. Metallic powder-bed based 3D printing of cellular scaffolds for orthopaedic implants: A state-of-the-art review on manufacturing, topological design, mechanical properties and biocompatibility. Material Science Engineering C Mater Biol Appl. 2017;76:1328–43. doi: 10.1016/j.msec.2017.02.094. [DOI] [PubMed] [Google Scholar]
- [45].Mustafa SF, Evans PL, Bocca A, Patton DW, Sugar AW, Baxter PW. Customized titanium reconstruction of post-traumatic orbital wall defects: a review of 22 cases. Int J Oral Maxillofac Surg. 2011;40:1357–62. doi: 10.1016/j.ijom.2011.04.020. [DOI] [PubMed] [Google Scholar]
- [46].Li X, Wang Y, Zhao Y, Liu J, Xiao S, Mao K. Multilevel 3D printing implant for reconstructing cervical spine with metastatic papillary thyroid carcinoma. Spine (Phila Pa 1976) 2017;42:E1326–E30. doi: 10.1097/BRS.0000000000002229. [DOI] [PubMed] [Google Scholar]
- [47].Yang F, Chen C, Zhou Q, Gong Y, Li R, Li C, Klampfl F, Freund S, Wu X, Sun Y, Li X, et al. Laser beam melting 3D printing of Ti6Al4V based porous structured dental implants: fabrication, biocompatibility analysis and photoelastic study. Scientific Report. 2017;7 doi: 10.1038/srep45360. 45360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Xie Y, Rustom LE, McDermott AM, Boerckel JD, Johnson AJ, Alleyne AG, Hoelzle DJ. Net shape fabrication of calcium phosphate scaffolds with multiple material domains. Biofabrication. 2016;8:015005. doi: 10.1088/1758-5090/8/1/015005. [DOI] [PubMed] [Google Scholar]
- [49].Ng ZY, Nawaz I. Computer-designed PEEK implants: a peek into the future of cranioplasty? The Journal of craniofacial surgery. 2014;25:e55–8. doi: 10.1097/SCS.0b013e3182a2f7b6. [DOI] [PubMed] [Google Scholar]
- [50].Tack P, Victor J, Gemmel P, Annemans L. 3D-printing techniques in a medical setting: a systematic literature review. Biomed Eng Online. 2016;15:115. doi: 10.1186/s12938-016-0236-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Colquhoun R, Tanner KE. Mechanical behaviour of degradable phosphate glass fibres and composites-a review. Biomed Mater. 2016;11:18. doi: 10.1088/1748-6041/11/1/014105. [DOI] [PubMed] [Google Scholar]
- [52].Fernandez-Yague MA, Abbah SA, McNamara L, Zeugolis DI, Pandit A, Biggs MJ. Biomimetic approaches in bone tissue engineering: Integrating biological and physicomechanical strategies. Advances in Drug Delivery Reviews. 2015;84:1–29. doi: 10.1016/j.addr.2014.09.005. [DOI] [PubMed] [Google Scholar]
- [53].Babaie E, Bhaduri SB. Fabrication aspects of porous biomaterials in orthopedic applications: A review. ACS Biomater Sci Eng. 2018;4:1–39. doi: 10.1021/acsbiomaterials.7b00615. [DOI] [PubMed] [Google Scholar]
- [54].Mumith A, Coathup M, Chimutengwende-Gordon M, Aston W, Briggs T, Blunn G. Augmenting the osseointegration of endoprostheses using laser-sintered porous collars: an in vivo study. Bone and Joint Journal. 2017;99B:276–82. doi: 10.1302/0301-620X.99B2.BJJ-2016-0584.R1. [DOI] [PubMed] [Google Scholar]
- [55].Polak SJ, Levengood SK, Wheeler MB, Maki AJ, Clark SG, Johnson AJ. Analysis of the roles of microporosity and BMP-2 on multiple measures of bone regeneration and healing in calcium phosphate scaffolds. Acta Biomater. 2011;7:1760–71. doi: 10.1016/j.actbio.2010.12.030. [DOI] [PubMed] [Google Scholar]
- [56].Rustom LE, Boudou T, Nemke BW, Lu Y, Hoelzle DJ, Markel MD, Picart C, Johnson AJW. Multiscale porosity directs bone regeneration in biphasic calcium phosphate scaffolds. ACS Biomater Sci Eng. 2017;3:2768–78. doi: 10.1021/acsbiomaterials.6b00632. [DOI] [PubMed] [Google Scholar]
- [57].Ciocca L, Lesci IG, Mezini O, Parrilli A, Ragazzini S, Rinnovati R, Romagnoli N, Roveri N, Scotti R. Customized hybrid biomimetic hydroxyapatite scaffold for bone tissue regeneration. J Biomed Mater Res Part B. 2017;105:723–34. doi: 10.1002/jbm.b.33597. [DOI] [PubMed] [Google Scholar]
- [58].Yang C, Wang XY, Ma B, Zhu HB, Huan ZG, Ma N, Wu CT, Chang J. 3D-printed bioactive Ca3SiO5 bone cement scaffolds with nano surface structure for bone regeneration. ACS Appl Mater Interfaces. 2017;9:5757–67. doi: 10.1021/acsami.6b14297. [DOI] [PubMed] [Google Scholar]
- [59].Bandyopadhyay A, Shivaram A, Tarafder S, Sahasrabudhe H, Banerjee D, Bose S. In vivo response of laser processed porous titanium implants for load-bearing implants. Ann Biomed Eng. 2017;45:249–60. doi: 10.1007/s10439-016-1673-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Braem A, Chaudhari A, Cardoso MV, Schrooten J, Duyck J, Vleugels J. Peri- and intra-implant bone response to microporous Ti coatings with surface modification. Acta Biomater. 2014;10:986–95. doi: 10.1016/j.actbio.2013.10.017. [DOI] [PubMed] [Google Scholar]
- [61].Kamitakahara M, Tatsukawa E, Shibata Y, Umemoto S, Yokoi T, Ioku K, Ikeda T. Effect of silicate incorporation on in vivo responses of alpha-tricalcium phosphate ceramics. J Mater Sci-Mater Med. 2016;27:9. doi: 10.1007/s10856-016-5706-5. [DOI] [PubMed] [Google Scholar]
- [62].Garcia-Gareta E, Hua J, Orera A, Kohli N, Knowles JC, Blunn GW. Biomimetic surface functionalization of clinically relevant metals used as orthopaedic and dental implants. Biomed Mater. 2018;13:14. doi: 10.1088/1748-605X/aa87e6. [DOI] [PubMed] [Google Scholar]
- [63].Hao JZ, Li Y, Li BE, Wang XL, Li HP, Liu SM, Liang CY, Wang HS. Biological and mechanical effects of micro-nanostructured titanium surface on an osteoblastic cell Line in vitro and osteointegration in vivo. Appl Biochem Biotechnol. 2017;183:280–92. doi: 10.1007/s12010-017-2444-1. [DOI] [PubMed] [Google Scholar]
- [64].Heo DN, Ko WK, Lee HR, Lee SJ, Lee D, Um SH, Lee JH, Woo YH, Zhang LG, Lee DW, Kwon IK. Titanium dental implants surface-immobilized with gold nanoparticles as osteoinductive agents for rapid osseointegration. J Colloid Interface Sci. 2016;469:129–37. doi: 10.1016/j.jcis.2016.02.022. [DOI] [PubMed] [Google Scholar]
- [65].Hou PJ, Ou KL, Wang CC, Huang CF, Ruslin M, Sugiatno E, Yang TS, Chou HH. Hybrid micro/nanostructural surface offering improved stress distribution and enhanced osseointegration properties of the biomedical titanium implant. Journal of the Mechanical Behavior of Biomedical Materials. 2018;79:173–80. doi: 10.1016/j.jmbbm.2017.11.042. [DOI] [PubMed] [Google Scholar]
- [66].Mroz W, Budner B, Syroka R, Niedzielski K, Golanski G, Slosarczyk A, Schwarze D, Douglas TEL. In vivo implantation of porous titanium alloy implants coated with magnesium-doped octacalcium phosphate and hydroxyapatite thin films using pulsed laser depostion. J Biomed Mater Res Part B. 2015;103:151–8. doi: 10.1002/jbm.b.33170. [DOI] [PubMed] [Google Scholar]
- [67].Covarrubias C, Mattmann M, Von Marttens A, Caviedes P, Arriagada C, Valenzuela F, Rodriguez JP, Corral C. Osseointegration properties of titanium dental implants modified with a nanostructured coating based on ordered porous silica and bioactive glass nanoparticles. Appl Surf Sci. 2016;363:286–95. [Google Scholar]
- [68].Caparros C, Ortiz-Hernandez M, Molmeneu M, Punset M, Calero JA, Aparicio C, Fernandez-Fairen M, Perez R, Gil FJ. Bioactive macroporous titanium implants highly interconnected. J Mater Sci-Mater Med. 2016;27:11. doi: 10.1007/s10856-016-5764-8. [DOI] [PubMed] [Google Scholar]
- [69].Krishnan V, Bryant HU, MacDougald OA. Regulation of bone mass by Wnt signaling. J Clin Invest. 2006;116:1202–9. doi: 10.1172/JCI28551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Santo VE, Gomes ME, Mano JF, Reis RL. Controlled release strategies for bone, cartilage, and osteochondral engineering--Part I: recapitulation of native tissue healing and variables for the design of delivery systems. Tissue Eng Part B, Reviews. 2013;19:308–26. doi: 10.1089/ten.teb.2012.0138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Carragee EJ, Hurwitz EL, Weiner BK. A critical review of recombinant human bone morphogenetic protein-2 trials in spinal surgery: emerging safety concerns and lessons learned. Spine J. 2011;11:471–91. doi: 10.1016/j.spinee.2011.04.023. [DOI] [PubMed] [Google Scholar]
- [72].Simmonds MC. FDA, Food and Drug Administration executive summary for P050036 Medtronic's AMPLIFY TM rhBMP-2 matrix orthopaedic and rehabilitation devices advisory panel. 2013 2010. [Google Scholar]
- [73].Zara JN, Siu RK, Zhang X, Shen J, Ngo R, Lee M, Li W, Chiang M, Chung J, Kwak J, Wu BM, et al. High doses of bone morphogenetic protein 2 induce structurally abnormal bone and inflammation in vivo. Tissue engineering Part A. 2011;17:1389–99. doi: 10.1089/ten.tea.2010.0555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Kim JW, Jung IH, Lee KI, Jung UW, Kim CS, Choi SH, Cho KS, Yun JH. Volumetric bone regenerative efficacy of biphasic calcium phosphate-collagen composite block loaded with rhBMP-2 in vertical bone augmentation model of a rabbit calvarium. J Biomed Mater Res A. 2012;100:3304–13. doi: 10.1002/jbm.a.34278. [DOI] [PubMed] [Google Scholar]
- [75].Hwang DY, On SW, Song SI. Bone regenerative effect of recombinant human bone morphogenetic protein-2 after cyst enucleation. Maxillofac Plast Reconstr Surg. 2016;38:22. doi: 10.1186/s40902-016-0070-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Cho JH, Lee JH, Yeom JS, Chang BS, Yang JJ, Koo KH, Hwang CJ, Lee KB, Kim HJ, Lee CK, Kim H, et al. Efficacy of Escherichia coli-derived recombinant human bone morphogenetic protein-2 in posterolateral lumbar fusion: an open, active-controlled, randomized, multicenter trial. Spine J. 2017;17:1866–74. doi: 10.1016/j.spinee.2017.06.023. [DOI] [PubMed] [Google Scholar]
- [77].King WJ, Krebsbach PH. Growth factor delivery: how surface interactions modulate release in vitro and in vivo. Adv Drug Deliv Rev. 2012;64:1239–56. doi: 10.1016/j.addr.2012.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].El Bialy I, Jiskoot W, Nejadnik MR. Formulation, delivery and stability of bone morphogenetic proteins for effective bone regeneration. Pharm Res. 2017;34:1152–70. doi: 10.1007/s11095-017-2147-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Yu XH, Suarez-Gonzalez D, Khalil AS, Murphy WL. How does the pathophysiological context influence delivery of bone growth factors? Adv Drug Deliv Rev. 2015;84:68–84. doi: 10.1016/j.addr.2014.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Bouyer M, Guillot R, Lavaud J, Plettinx C, Olivier C, Curry V, Boutonnat J, Coll JL, Peyrin F, Josserand V, Bettega G, et al. Surface delivery of tunable doses of BMP-2 from an adaptable polymeric scaffold induces volumetric bone regeneration. Biomaterials. 2016;104:168–81. doi: 10.1016/j.biomaterials.2016.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Cecchi S, Bennet SJ, Arora M. Bone morphogenetic protein-7: Review of signalling and efficacy in fracture healing. J Orthop Transl. 2016;4:28–34. doi: 10.1016/j.jot.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Wei K, Yin ZW, Xie YS. Roles of the kidney in the formation, remodeling and repair of bone. J Nephrol. 2016;29:349–57. doi: 10.1007/s40620-016-0284-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Reichert JC, Cipitria A, Epari DR, Saifzadeh S, Krishnakanth P, Berner A, Woodruff MA, Schell H, Mehta M, Schuetz MA, Duda GN, et al. A tissue engineering solution for segmental defect regeneration in load-bearing long bones. Sci Transl Med. 2012;4:141ra93. doi: 10.1126/scitranslmed.3003720. [DOI] [PubMed] [Google Scholar]
- [84].Ozaki Y, Nishimura M, Sekiya K, Suehiro F, Kanawa M, Nikawa H, Hamada T, Kato Y. Comprehensive analysis of chemotactic factors for bone marrow mesenchymal stem cells. Stem Cells Dev. 2007;16:119–29. doi: 10.1089/scd.2006.0032. [DOI] [PubMed] [Google Scholar]
- [85].DiGiovanni CW, Lin SS, Baumhauer JE, Daniels T, Younger A, Glazebrook M, Anderson J, Anderson R, Evangelista P, Lynch SE. North Amer Orthopedic Foot Ankle S. Recombinant human platelet-derived growth factor-BB and beta-tricalcium phosphate (rhPDGF-BB/beta-TCP): an alternative to autogenous bone graft. J Bone Joint Surg-Am Vol. 2013;95A:1184–92. doi: 10.2106/JBJS.K.01422. [DOI] [PubMed] [Google Scholar]
- [86].DiGiovanni CW, Lin SS, Daniels TR, Glazebrook M, Evangelista P, Donahue R, Beasley W, Baumhauer JF. The importance of sufficient graft material in achieving foot or ankle fusion. J Bone Joint Surg-Am Vol. 2016;98:1260–7. doi: 10.2106/JBJS.15.00879. [DOI] [PubMed] [Google Scholar]
- [87].Jin L, Li X. Growth differentiation factor 5 regulation in bone regeneration. Curr Pharm Des. 2013;19:3364–73. doi: 10.2174/1381612811319190003. [DOI] [PubMed] [Google Scholar]
- [88].Erlacher L, McCartney J, Piek E, ten Dijke P, Yanagishita M, Oppermann H, Luyten FP. Cartilage-derived morphogenetic proteins and osteogenic protein-1 differentially regulate osteogenesis. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 1998;13:383–92. doi: 10.1359/jbmr.1998.13.3.383. [DOI] [PubMed] [Google Scholar]
- [89].Cui M, Wan YQ, Anderson DG, Shen FH, Leo BM, Laurencin CT, Balian G, Li XD. Mouse growth and differentiation factor-5 protein and DNA therapy potentiates intervertebral disc cell aggregation and chondrogenic gene expression. Spine J. 2008;8:287–95. doi: 10.1016/j.spinee.2007.05.012. [DOI] [PubMed] [Google Scholar]
- [90].Kleinschmidt K, Ploeger F, Nickel J, Glockenmeier J, Kunz P, Richter W. Enhanced reconstruction of long bone architecture by a growth factor mutant combining positive features of GDF-5 and BMP-2. Biomaterials. 2013;34:5926–36. doi: 10.1016/j.biomaterials.2013.04.029. [DOI] [PubMed] [Google Scholar]
- [91].Kleinschmidt K, Wagner-Ecker M, Bartek B, Holschbach J, Richter W. Superior angiogenic potential of GDF-5 and GDF-5(V453/V456) compared with BMP-2 in a rabbit long-bone defect model. J Bone Joint Surg Am. 2014;96:1699–707. doi: 10.2106/JBJS.M.01462. [DOI] [PubMed] [Google Scholar]
- [92].Yang DH, Moon SW, Lee DW. Surface modification of titanium with BMP-2/GDF-5 by a heparin linker and its efficacy as a dental implant. Int J Mol Sci. 2017;18:16. doi: 10.3390/ijms18010229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Roh JS, Yeung CA, Field JS, McClellan RT. Allogeneic morphogenetic protein vs. recombinant human bone morphogenetic protein-2 in lumbar interbody fusion procedures: a radiographic and economic analysis. Journal of orthopaedic surgery and research. 2013;8:49. doi: 10.1186/1749-799X-8-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Vukicevic S, Oppermann H, Verbanac D, Jankolija M, Popek I, Curak J, Brkljacic J, Pauk M, Erjavec I, Francetic I, Dumic-Cule I, et al. The clinical use of bone morphogenetic proteins revisited: a novel biocompatible carrier device OSTEOGROW for bone healing. Int Orthop. 2014;38:635–47. doi: 10.1007/s00264-013-2201-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Lin XH, Guo H, Takahashi K, Liu Y, Zamora PO. B2A as a positive BMP receptor modulator. Growth Factors. 2012;30:149–57. doi: 10.3109/08977194.2012.671310. [DOI] [PubMed] [Google Scholar]
- [96].Liu Y, Lin X, Takahashi K, Zamora PO. B2A, a receptor modulator, increases the growth of pluripotent and preosteoblast cells through bone morphogenetic protein receptors. Growth Factors. 2012;30:410–7. doi: 10.3109/08977194.2012.745520. [DOI] [PubMed] [Google Scholar]
- [97].Cunningham BW, Atkinson BL, Hu N, Kikkawa J, Jenis L, Bryant J, Zamora PO, McAfee PC. Ceramic granules enhanced with B2A peptide for lumbar interbody spine fusion: an experimental study using an instrumented model in sheep Laboratory investigation. J Neurosurg-Spine. 2009;10:300–7. doi: 10.3171/2009.1.SPINE08565. [DOI] [PubMed] [Google Scholar]
- [98].Zamora PO, Liu Y, Guo H, Lin XH. Biocompatibility and inflammation profile of B2A-coated granules used in arthrodesis. Int J Toxicol. 2013;32:154–61. doi: 10.1177/1091581813476960. [DOI] [PubMed] [Google Scholar]
- [99].Qian JJ, Bhatnagar RS. Enhanced cell attachment to anorganic bone mineral in the presence of a synthetic peptide related to collagen. J Biomed Mater Res. 1996;31:545–54. doi: 10.1002/(SICI)1097-4636(199608)31:4<545::AID-JBM15>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- [100].Nguyen H, Qian JJ, Bhatnagar RS, Li S. Enhanced cell attachment and osteoblastic activity by P-15 peptide-coated matrix in hydrogels. Biochem Biophys Res Commun. 2003;311:179–86. doi: 10.1016/j.bbrc.2003.09.192. [DOI] [PubMed] [Google Scholar]
- [101].Hennessy KM, Pollot BE, Clem WC, Phipps MC, Sawyer AA, Culpepper BK, Bellis SL. The effect of collagen I mimetic peptides on mesenchymal stem cell adhesion and differentiation, and on bone formation at hydroxyapatite surfaces. Biomaterials. 2009;30:1898–909. doi: 10.1016/j.biomaterials.2008.12.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Gomar F, Orozco R, Villar JL, Arrizabalaga F. P-15 small peptide bone graft substitute in the treatment of non-unions and delayed union. A pilot clinical trial Int Orthop. 2007;31:93–9. doi: 10.1007/s00264-006-0087-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Sherman BP, Lindley EM, Turner AS, Seim HB, 3rd, Benedict J, Burger EL, Patel VV. Evaluation of ABM/P-15 versus autogenous bone in an ovine lumbar interbody fusion model. Eur Spine J. 2010;19:2156–63. doi: 10.1007/s00586-010-1546-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Lauweryns P, Raskin Y. Prospective analysis of a new bone graft in lumbar interbody fusion: results of a 2- year prospective clinical and radiological study. Int J Spine Surg. 2015;9 doi: 10.14444/2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Arnold PM, Sasso RC, Janssen ME, Fehlings MG, Smucker JD, Vaccaro AR, Heary RF, Patel AI, Goulet B, Kalfas IH, Kopjar B. Efficacy of i-Factor bone graft versus autograft in anterior cervical discectomy and fusion: results of the prospective, randomized, single-blinded food and drug administration investigational device exemption study. Spine (Phila Pa 1976) 2016;41:1075–83. doi: 10.1097/BRS.0000000000001466. [DOI] [PubMed] [Google Scholar]
- [106].Zouani OF, Chollet C, Guillotin B, Durrieu MC. Differentiation of pre-osteoblast cells on poly(ethylene terephthalate) grafted with RGD and/or BMPs mimetic peptides. Biomaterials. 2010;31:8245–53. doi: 10.1016/j.biomaterials.2010.07.042. [DOI] [PubMed] [Google Scholar]
- [107].Moeinzadeh S, Jabbari E. Morphogenic peptides in regeneration of load bearing tissues. In: Bertassoni LE, Coelho PG, editors. Engineering Mineralized and Load Bearing Tissues. Berlin: Springer-Verlag Berlin; 2015. pp. 95–110. [DOI] [PubMed] [Google Scholar]
- [108].Mueller TD, Nickel J. Promiscuity and specificity in BMP receptor activation. FEBS letters. 2012;586:1846–59. doi: 10.1016/j.febslet.2012.02.043. [DOI] [PubMed] [Google Scholar]
- [109].Lombardi G, Di Somma C, Rubino M, Faggiano A, Vuolo L, Guerra E, Contaldi P, Savastano S, Colao A. The roles of parathyroid hormone in bone remodeling: prospects for novel therapeutics. Journal of endocrinological investigation. 2011;34:18–22. [PubMed] [Google Scholar]
- [110].Arrighi I, Mark S, Alvisi M, von Rechenberg B, Hubbell JA, Schense JC. Bone healing induced by local delivery of an engineered parathyroid hormone prodrug. Biomaterials. 2009;30:1763–71. doi: 10.1016/j.biomaterials.2008.12.023. [DOI] [PubMed] [Google Scholar]
- [111].Fuerst A, Derungs S, von Rechenberg B, Auer JA, Schense J, Watson J. Use of a parathyroid hormone peptide (PTH(1-34))-enriched fibrin hydrogel for the treatment of a subchondral cystic lesion in the proximal interphalangeal joint of a warmblood filly. Journal of veterinary medicine A, Physiology, pathology, clinical medicine. 2007;54:107–12. doi: 10.1111/j.1439-0442.2007.00890.x. [DOI] [PubMed] [Google Scholar]
- [112].Zhang X, Zara J, Siu RK, Ting K, Soo C. The role of NELL-1, a growth factor associated with craniosynostosis, in promoting bone regeneration. Journal of dental research. 2010;89:865–78. doi: 10.1177/0022034510376401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Siu RK, Zara JN, Hou Y, James AW, Kwak J, Zhang X, Ting K, Wu BM, Soo C, Lee M. NELL-1 promotes cartilage regeneration in an in vivo rabbit model. Tissue engineering Part A. 2012;18:252–61. doi: 10.1089/ten.tea.2011.0142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Askarinam A, James AW, Zara JN, Goyal R, Corselli M, Pan A, Liang P, Chang L, Rackohn T, Stoker D, Zhang X, et al. Human perivascular stem cells show enhanced osteogenesis and vasculogenesis with Nel-like molecule I protein. Tissue engineering Part A. 2013;19:1386–97. doi: 10.1089/ten.tea.2012.0367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Pang S, Shen J, Liu Y, Chen F, Zheng Z, James AW, Hsu CY, Zhang H, Lee KS, Wang C, Li C, et al. Proliferation and osteogenic differentiation of mesenchymal stem cells induced by a short isoform of NELL-1. Stem cells. 2015;33:904–15. doi: 10.1002/stem.1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].James AW, Shen J, Zhang X, Asatrian G, Goyal R, Kwak JH, Jiang L, Bengs B, Culiat CT, Turner AS, Seim Iii HB, et al. NELL-1 in the treatment of osteoporotic bone loss. Nat Commun. 2015;6 doi: 10.1038/ncomms8362. 7362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].James AW, Shen J, Tsuei R, Nguyen A, Khadarian K, Meyers CA, Pan HC, Li W, Kwak JH, Asatrian G, Culiat CT, et al. NELL-1 induces Sca-1+ mesenchymal progenitor cell expansion in models of bone maintenance and repair. JCI insight. 2017;2 doi: 10.1172/jci.insight.92573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Tanjaya J, Lord EL, Wang C, Zhang Y, Kim JK, Nguyen A, Baik L, Pan HC, Chen E, Kwak JH, Zhang X, et al. The effects of systemic therapy of PEGylated NELL-1 on fracture healing in mice. Am J Pathol. 2017 doi: 10.1016/j.ajpath.2017.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Sangadala S, Boden SD, Viggeswarapu M, Liu Y, Titus L. LIM mineralization protein-1 potentiates bone morphogenetic protein responsiveness via a novel interaction with Smurf1 resulting in decreased ubiquitination of Smads. J Biol Chem. 2006;281:17212–9. doi: 10.1074/jbc.M511013200. [DOI] [PubMed] [Google Scholar]
- [120].Pan H, Li X, Wang J, Zhang K, Yang H, Li Z, Zheng Z, Liu H. LIM mineralization protein-1 enhances bone morphogenetic protein-2-mediated osteogenesis through activation of ERK1/2 MAPK pathway and upregulation of runx2 Transactivity. J Bone Miner Res. 2015;30:1523–35. doi: 10.1002/jbmr.2481. [DOI] [PubMed] [Google Scholar]
- [121].Strohbach CA, Rundle CH, Wergedal JE, Chen ST, Linkhart TA, Lau KH, Strong DD. LMP-1 retroviral gene therapy influences osteoblast differentiation and fracture repair: a preliminary study. Calcif Tissue Int. 2008;83:202–11. doi: 10.1007/s00223-008-9163-0. [DOI] [PubMed] [Google Scholar]
- [122].Florio M, Gunasekaran K, Stolina M, Li XD, Liu L, Tipton B, Salimi-Moosavi H, Asuncion FJ, Li CY, Sun BH, Tan HL, et al. A bispecific antibody targeting sclerostin and DKK-1 promotes bone mass accrual and fracture repair. Nat Commun. 2016;7:14. doi: 10.1038/ncomms11505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Bolander J, Ji W, Leijten J, Teixeira LM, Bloemen V, Lambrechts D, Chaklader M, Luyten FP. Healing of a large long-bone defect through serum-free Iin vitro priming of human periosteum-derived cells. Stem cell reports. 2017;8:758–72. doi: 10.1016/j.stemcr.2017.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Bolander J, Ji W, Geris L, Bloemen V, Chai YC, Schrooten J, Luyten FP. The combined mechanism of bone morphogenetic protein- and calcium phosphate-induced skeletal tissue formation by human periosteum derived cells. European Cells and Materials. 2016;31:11–25. doi: 10.22203/ecm.v031a02. [DOI] [PubMed] [Google Scholar]
- [125].Ingber DE, Mow VC, Butler D, Niklason L, Huard J, Mao J, Yannas I, Kaplan D, Vunjak-Novakovic G. Tissue engineering and developmental biology: going biomimetic. Tissue engineering. 2006;12:3265–83. doi: 10.1089/ten.2006.12.3265. [DOI] [PubMed] [Google Scholar]
- [126].Lenas P, Moos M, Luyten FP. Developmental engineering: a new paradigm for the design and manufacturing of cell-based products. Part I: from three-dimensional cell growth to biomimetics of in vivo development. Tissue Eng Part B, Reviews. 2009;15:381–94. doi: 10.1089/ten.TEB.2008.0575. [DOI] [PubMed] [Google Scholar]
- [127].Lenas P, Moos M, Luyten FP. Developmental engineering: a new paradigm for the design and manufacturing of cell-based products. Part II: from genes to networks: tissue engineering from the viewpoint of systems biology and network science. Tissue Eng Part B, Reviews. 2009;15:395–422. doi: 10.1089/ten.TEB.2009.0461. [DOI] [PubMed] [Google Scholar]
- [128].Friedenstein AJ, Piatetzky S, II, Petrakova KV. Osteogenesis in transplants of bone marrow cells. Journal of embryology and experimental morphology. 1966;16:381–90. [PubMed] [Google Scholar]
- [129].Friedenstein AJ, Petrakova KV, Kurolesova AI, Frolova GP. Heterotopic of bone marrow. Analysis of precursor cells for osteogenic and hematopoietic tissues. Transplantation. 1968;6:230–47. [PubMed] [Google Scholar]
- [130].Martinez ME, del Campo MT, Medina S, Sanchez M, Sanchez-Cabezudo MJ, Esbrit P, Martinez P, Moreno I, Rodrigo A, Garces MV, Munuera L. Influence of skeletal site of origin and donor age on osteoblastic cell growth and differentiation. Calcif Tissue Int. 1999;64:280–6. doi: 10.1007/s002239900619. [DOI] [PubMed] [Google Scholar]
- [131].Greco SJ, Liu K, Rameshwar P. Functional similarities among genes regulated by OCT4 in human mesenchymal and embryonic stem cells. Stem cells. 2007;25:3143–54. doi: 10.1634/stemcells.2007-0351. [DOI] [PubMed] [Google Scholar]
- [132].Ding DC, Chang YH, Shyu WC, Lin SZ. Human umbilical cord mesenchymal stem cells: a new era for stem cell therapy. Cell Transplant. 2015;24:339–47. doi: 10.3727/096368915X686841. [DOI] [PubMed] [Google Scholar]
- [133].Bianco P, Cao X, Frenette PS, Mao JJ, Robey PG, Simmons PJ, Wang CY. The meaning, the sense and the significance: translating the science of mesenchymal stem cells into medicine. Nat Med. 2013;19:35–42. doi: 10.1038/nm.3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Heathman TR, Nienow AW, McCall MJ, Coopman K, Kara B, Hewitt CJ. The translation of cell-based therapies: clinical landscape and manufacturing challenges. Regen Med. 2015;10:49–64. doi: 10.2217/rme.14.73. [DOI] [PubMed] [Google Scholar]
- [135].Lin H. The stem-cell niche theory: lessons from flies. Nature Reviews Genetics. 2002;3:931–40. doi: 10.1038/nrg952. [DOI] [PubMed] [Google Scholar]
- [136].Lei J, Levin SA, Nie Q. Mathematical model of adult stem cell regeneration with cross-talk between genetic and epigenetic regulation. Proc Natl Acad Sci U S A. 2014;111:E880–7. doi: 10.1073/pnas.1324267111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Elsafadi M, Manikandan M, Atteya M, Hashmi JA, Iqbal Z, Aldahmash A, Alfayez M, Kassem M, Mahmood A. Characterization of cellular and molecular heterogeneity of bone marrow stromal cells. Stem Cells Int. 2016;2016 doi: 10.1155/2016/9378081. 9378081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Bianco P, Riminucci M, Gronthos S, Robey PG. Bone marrow stromal stem cells: nature, biology, and potential applications. Stem cells. 2001;19:180–92. doi: 10.1634/stemcells.19-3-180. [DOI] [PubMed] [Google Scholar]
- [139].Hernigou P, Mathieu G, Poignard A, Manicom O, Beaujean F, Rouard H. Percutaneous autologous bone-marrow grafting for nonunions. Surgical technique. J Bone Joint Surg Am. 2006;88(Suppl 1 Pt 2):322–7. doi: 10.2106/JBJS.F.00203. [DOI] [PubMed] [Google Scholar]
- [140].Devine MJ, Mierisch CM, Jang E, Anderson PC, Balian G. Transplanted bone marrow cells localize to fracture callus in a mouse model. Journal of Orthopedic Research. 2002;20:1232–9. doi: 10.1016/S0736-0266(02)00051-7. [DOI] [PubMed] [Google Scholar]
- [141].Friedenstein AJ, Deriglasova UF, Kulagina NN, Panasuk AF, Rudakowa SF, Luria EA, Ruadkow IA. Precursors for fibroblasts in different populations of hematopoietic cells as detected by the in vitro colony assay method. Exp Hematol. 1974;2:83–92. [PubMed] [Google Scholar]
- [142].Amable PR, Teixeira MV, Carias RB, Granjeiro JM, Borojevic R. Mesenchymal stromal cell proliferation, gene expression and protein production in human platelet-rich plasma-supplemented media. PloS one. 2014;9:e104662. doi: 10.1371/journal.pone.0104662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Muraglia A, Cancedda R, Quarto R. Clonal mesenchymal progenitors from human bone marrow differentiate in vitro according to a hierarchical model. Jounral of Cell Science. 2000;113(Pt 7):1161–6. doi: 10.1242/jcs.113.7.1161. [DOI] [PubMed] [Google Scholar]
- [144].Minieri V, Saviozzi S, Gambarotta G, Lo Iacono M, Accomasso L, Cibrario Rocchietti E, Gallina C, Turinetto V, Giachino C. Persistent DNA damage-induced premature senescence alters the functional features of human bone marrow mesenchymal stem cells. Journal of cellular and molecular medicine. 2015;19:734–43. doi: 10.1111/jcmm.12387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Szpalski C, Barbaro M, Sagebin F, Warren SM. Bone tissue engineering: Current strategies and techniques-part II: cell types. Tissue Eng Part B-Rev. 2012;18:258–69. doi: 10.1089/ten.TEB.2011.0440. [DOI] [PubMed] [Google Scholar]
- [146].Colnot C, Huang S, Helms J. Analyzing the cellular contribution of bone marrow to fracture healing using bone marrow transplantation in mice. Biochemical Biophyicals Research Communication. 2006;350:557–61. doi: 10.1016/j.bbrc.2006.09.079. [DOI] [PubMed] [Google Scholar]
- [147].Bunnell BA, Flaat M, Gagliardi C, Patel B, Ripoll C. Adipose-derived stem cells: isolation, expansion and differentiation. Methods. 2008;45:115–20. doi: 10.1016/j.ymeth.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].El-Badawy A, Ahmed SM, El-Badri N. Adipose-derived stem cell-based therapies in regenerative medicine. Stem Cells Biol Reg. 2017;117:38. [Google Scholar]
- [149].Aust L, Devlin B, Foster SJ, Halvorsen YD, Hicok K, du Laney T, Sen A, Willingmyre GD, Gimble JM. Yield of human adipose-derived adult stem cells from liposuction aspirates. Cytotherapy. 2004;6:7–14. doi: 10.1080/14653240310004539. [DOI] [PubMed] [Google Scholar]
- [150].Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, Benhaim P, Lorenz HP, Hedrick MH. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng Part A. 2001;7:211–28. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
- [151].Cui L, Yin S, Liu W, Li N, Zhang W, Cao Y. Expanded adipose-derived stem cells suppress mixed lymphocyte reaction by secretion of prostaglandin E2. Tissue Eng Part A. 2007;13:1185–95. doi: 10.1089/ten.2006.0315. [DOI] [PubMed] [Google Scholar]
- [152].Puissant B, Barreau C, Bourin P, Clavel C, Corre J, Bousquet C, Taureau C, Cousin B, Abbal M, Laharrague P, Penicaud L, et al. Immunomodulatory effect of human adipose tissue-derived adult stem cells: comparison with bone marrow mesenchymal stem cells. Br J Haematol. 2005;129:118–29. doi: 10.1111/j.1365-2141.2005.05409.x. [DOI] [PubMed] [Google Scholar]
- [153].Cowan CM, Shi YY, Aalami OO, Chou YF, Mari C, Thomas R, Quarto N, Contag CH, Wu B, Longaker MT. Adipose-derived adult stromal cells heal critical-size mouse calvarial defects. Nat Biotechnol. 2004;22:560–7. doi: 10.1038/nbt958. [DOI] [PubMed] [Google Scholar]
- [154].McIntosh KR, Lopez MJ, Borneman JN, Spencer ND, Anderson PA, Gimble JM. Immunogenicity of allogeneic adipose-derived stem cells in a rat spinal fusion model. Tissue Eng Part A. 2009;15:2677–86. doi: 10.1089/ten.TEA.2008.0566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Lopez MJ, McIntosh KR, Spencer ND, Borneman JN, Horswell R, Anderson P, Yu G, Gaschen L, Gimble JM. Acceleration of spinal fusion using syngeneic and allogeneic adult adipose derived stem cells in a rat model. Journal of Orthopedic Reseach. 2009;27:366–73. doi: 10.1002/jor.20735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Lee CS, Burnsed OA, Raghuram V, Kalisvaart J, Boyan BD, Schwartz Z. Adipose stem cells can secrete angiogenic factors that inhibit hyaline cartilage regeneration. Stem Cell Research and Therapy. 2012;3:35. doi: 10.1186/scrt126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Mesimaki K, Lindroos B, Tornwall J, Mauno J, Lindqvist C, Kontio R, Miettinen S, Suuronen R. Novel maxillary reconstruction with ectopic bone formation by GMP adipose stem cells. Int J Oral Maxillofac Surg. 2009;38:201–9. doi: 10.1016/j.ijom.2009.01.001. [DOI] [PubMed] [Google Scholar]
- [158].Bailey AM, Kapur S, Katz AJ. Characterization of adipose-derived stem cells: an update. Curr Stem Cell Res Ther. 2010;5:95–102. doi: 10.2174/157488810791268555. [DOI] [PubMed] [Google Scholar]
- [159].Oedayrajsingh-Varma MJ, van Ham SM, Knippenberg M, Helder MN, Klein-Nulend J, Schouten TE, Ritt MJ, van Milligen FJ. Adipose tissue-derived mesenchymal stem cell yield and growth characteristics are affected by the tissue-harvesting procedure. Cytotherapy. 2006;8:166–77. doi: 10.1080/14653240600621125. [DOI] [PubMed] [Google Scholar]
- [160].Roberts SJ, van Gastel N, Carmeliet G, Luyten FP. Uncovering the periosteum for skeletal regeneration: the stem cell that lies beneath. Bone. 2015;70:10–8. doi: 10.1016/j.bone.2014.08.007. [DOI] [PubMed] [Google Scholar]
- [161].Moore SR, Milz S, Knothe Tate ML. Periosteal thickness and cellularity in mid-diaphyseal cross-sections from human femora and tibiae of aged donors. J Anat. 2014;224:142–9. doi: 10.1111/joa.12133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Allen MR, Hock JM, Burr DB. Periosteum: biology, regulation, and response to osteoporosis therapies. Bone. 2004;35:1003–12. doi: 10.1016/j.bone.2004.07.014. [DOI] [PubMed] [Google Scholar]
- [163].Clarke B. Normal bone anatomy and physiology. Clin J Am Soc Nephrol. 2008;3(Suppl 3):S131–9. doi: 10.2215/CJN.04151206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Chang H, Knothe Tate ML. Concise review: the periosteum: tapping into a reservoir of clinically useful progenitor cells. Stem Cells Transl Med. 2012;1:480–91. doi: 10.5966/sctm.2011-0056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].De Bari C, Dell' Accio F, Vanlauwe J, Eyckmans J, Khan IM, Archer CW, Jones EA, McGonagle D, Mitsiadis TA, Pitzalis C, Luyten FP. Mesenchymal multipotency of adult human periosteal cells demonstrated by single-cell lineage analysis. Arthritis Rheumatism. 2006;54:1209–21. doi: 10.1002/art.21753. [DOI] [PubMed] [Google Scholar]
- [166].Lambrechts T, Papantoniou I, Rice B, Schrooten J, Luyten FP, Aerts JM. Large-scale progenitor cell expansion for multiple donors in a monitored hollow fibre bioreactor. Cytotherapy. 2016;18:1219–33. doi: 10.1016/j.jcyt.2016.05.013. [DOI] [PubMed] [Google Scholar]
- [167].Eyckmans J, Roberts SJ, Bolander J, Schrooten J, Chen CS, Luyten FP. Mapping calcium phosphate activated gene networks as a strategy for targeted osteoinduction of human progenitors. Biomaterials. 2013;34:4612–21. doi: 10.1016/j.biomaterials.2013.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].Bolander J, Chai YC, Geris L, Schrooten J, Lambrechts D, Roberts SJ, Luyten FP. Early BMP, Wnt and Ca(2+)/PKC pathway activation predicts the bone forming capacity of periosteal cells in combination with calcium phosphates. Biomaterials. 2016;86:106–18. doi: 10.1016/j.biomaterials.2016.01.059. [DOI] [PubMed] [Google Scholar]
- [169].Ryan JM. Effect of different fetal bovine serum concentrations on the replicative life span of cultured chick cells. In Vitro. 1979;15:895–9. doi: 10.1007/BF02618046. [DOI] [PubMed] [Google Scholar]
- [170].Jung S, Panchalingam KM, Rosenberg L, Behie LA. Ex vivo expansion of human mesenchymal stem cells in defined serum-free media. Stem Cells Int. 2012;2012 doi: 10.1155/2012/123030. 123030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [171].Baker M. Reproducibility: Respect your cells! Nature. 2016;537:433–5. doi: 10.1038/537433a. [DOI] [PubMed] [Google Scholar]
- [172].Brennan MA, Renaud A, Amiaud J, Rojewski MT, Schrezenmeier H, Heymann D, Trichet V, Layrolle P. Pre-clinical studies of bone regeneration with human bone marrow stromal cells and biphasic calcium phosphate. Stem Cell Research and Therapy. 2014;5:114. doi: 10.1186/scrt504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [173].Eyckmans J, Roberts SJ, Schrooten J, Luyten FP. A clinically relevant model of osteoinduction: a process requiring calcium phosphate and BMP/Wnt signalling. Journal Cellular and Molecular Medicine. 2010;14:1845–56. doi: 10.1111/j.1582-4934.2009.00807.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [174].Gamblin AL, Brennan MA, Renaud A, Yagita H, Lezot F, Heymann D, Trichet V, Layrolle P. Bone tissue formation with human mesenchymal stem cells and biphasic calcium phosphate ceramics: the local implication of osteoclasts and macrophages. Biomaterials. 2014;35:9660–7. doi: 10.1016/j.biomaterials.2014.08.018. [DOI] [PubMed] [Google Scholar]
- [175].Mendicino M, Bailey AM, Wonnacott K, Puri RK, Bauer SR. MSC-based product characterization for clinical trials: an FDA perspective. Cell stem cell. 2014;14:141–5. doi: 10.1016/j.stem.2014.01.013. [DOI] [PubMed] [Google Scholar]
- [176].Agency EM. ICH Q5D Derivation and characterisation of cell substrates used for production of biotechnological/biological products. 1998 [Google Scholar]
- [177].Agency EM. Guideline on human cell-based medicinal products. 2008 [Google Scholar]
- [178].Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop D, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–7. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- [179].Bara JJ, Richards RG, Alini M, Stoddart MJ. Concise review: Bone marrow-derived mesenchymal stem cells change phenotype following in vitro culture: implications for basic research and the clinic. Stem cells. 2014;32:1713–23. doi: 10.1002/stem.1649. [DOI] [PubMed] [Google Scholar]
- [180].Billing AM, Ben Hamidane H, Dib SS, Cotton RJ, Bhagwat AM, Kumar P, Hayat S, Yousri NA, Goswami N, Suhre K, Rafii A et al. Comprehensive transcriptomic and proteomic characterization of human mesenchymal stem cells reveals source specific cellular markers. Sci Rep. 2016;6 doi: 10.1038/srep21507. 21507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [181].Deveza L, Ortinau L, Lei K, Park D. Comparative analysis of gene expression identifies distinct molecular signatures of bone marrow- and periosteal-skeletal stem/progenitor cells. PloS one. 2018;13:e0190909. doi: 10.1371/journal.pone.0190909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [182].Chan CK, Seo EY, Chen JY, Lo D, McArdle A, Sinha R, Tevlin R, Seita J, Vincent-Tompkins J, Wearda T, Lu WJ, et al. Identification and specification of the mouse skeletal stem cell. Cell. 2015;160:285–98. doi: 10.1016/j.cell.2014.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [183].Doi K, Sakai K. Vascularized periosteal bone graft from the supracondylar region of the femur. Microsurgery. 1994;15:305–15. doi: 10.1002/micr.1920150505. [DOI] [PubMed] [Google Scholar]
- [184].Schmelzeisen R, Schimming R, Sittinger M. Making bone: implant insertion into tissue-engineered bone for maxillary sinus floor augmentation-a preliminary report. Journal of Craniomaxillofacial Surgery. 2003;31:34–9. doi: 10.1016/s1010-5182(02)00163-4. [DOI] [PubMed] [Google Scholar]
- [185].Wagner W, Horn P, Castoldi M, Diehlmann A, Bork S, Saffrich R, Benes V, Blake J, Pfister S, Eckstein V, Ho AD. Replicative senescence of mesenchymal stem cells: a continuous and organized process. PloS one. 2008;3:e2213. doi: 10.1371/journal.pone.0002213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [186].Roseti L, Parisi V, Petretta M, Cavallo C, Desando G, Bartolotti I, Grigolo B. Scaffolds for bone tissue engineering: state of the art and new perspectives. Mater Sci Eng C Mater Biol Appl. 2017;78:1246–62. doi: 10.1016/j.msec.2017.05.017. [DOI] [PubMed] [Google Scholar]
- [187].Tang D, Tare RS, Yang LY, Williams DF, Ou KL, Oreffo RO. Biofabrication of bone tissue: approaches, challenges and translation for bone regeneration. Biomaterials. 2016;83:363–82. doi: 10.1016/j.biomaterials.2016.01.024. [DOI] [PubMed] [Google Scholar]
- [188].Li J, Chen M, Fan X, Zhou H. Recent advances in bioprinting techniques: approaches, applications and future prospects. Journal of translational medicine. 2016;14:271. doi: 10.1186/s12967-016-1028-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [189].Aljohani W, Ullah MW, Zhang X, Yang G. Bioprinting and its applications in tissue engineering and regenerative medicine. Int J Biol Macromol. 2018;107:261–75. doi: 10.1016/j.ijbiomac.2017.08.171. [DOI] [PubMed] [Google Scholar]
- [190].Adepu S, Dhiman N, Laha A, Sharma CS, Ramakrishna S, Khandelwal M. Three-dimensional bioprinting for bone tissue regeneration. Current Opin Biomed Eng. 2017;2:22–8. [Google Scholar]
- [191].Lin KF, He S, Song Y, Wang CM, Gao Y, Li JQ, Tang P, Wang Z, Bi L, Pei GX. Low-temperature additive manufacturing of biomimic three-dimensional hydroxyapatite/collagen scaffolds for bone regeneration. ACS Appl Mater Interfaces. 2016;8:6905–16. doi: 10.1021/acsami.6b00815. [DOI] [PubMed] [Google Scholar]
- [192].Park JY, Shim JH, Choi SA, Jang J, Kim M, Lee SH, Cho DW. 3D printing technology to control BMP-2 and VEGF delivery spatially and temporally to promote large-volume bone regeneration. J Mat Chem B. 2015;3:5415–25. doi: 10.1039/c5tb00637f. [DOI] [PubMed] [Google Scholar]
- [193].Ratheesh G, Venugopal JR, Chinappan A, Ezhilarasu H, Sadiq A, Ramakrishna S. 3D fabrication of polymeric scaffolds for regenerative therapy. ACS Biomater Sci Eng. 2017;3:1175–94. doi: 10.1021/acsbiomaterials.6b00370. [DOI] [PubMed] [Google Scholar]
- [194].Liu Y, Wang L, Kikuiri T, Akiyama K, Chen C, Xu X, Yang R, Chen W, Wang S, Shi S. Mesenchymal stem cell-based tissue regeneration is governed by recipient T lymphocytes via IFN-gamma and TNF-alpha. Nat Med. 2011;17:1594–601. doi: 10.1038/nm.2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [195].Martino MM, Briquez PS, Maruyama K, Hubbell JA. Extracellular matrix-inspired growth factor delivery systems for bone regeneration. Adv Drug Deliv Rev. 2015;94:41–52. doi: 10.1016/j.addr.2015.04.007. [DOI] [PubMed] [Google Scholar]
- [196].Mani G, Feldman MD, Patel D, Agrawal CM. Coronary stents: a materials perspective. Biomaterials. 2007;28:1689–710. doi: 10.1016/j.biomaterials.2006.11.042. [DOI] [PubMed] [Google Scholar]
- [197].Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–72. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- [198].Tsumaki N, Okada M, Yamashita A. iPS cell technologies and cartilage regeneration. Bone. 2015;70:48–54. doi: 10.1016/j.bone.2014.07.011. [DOI] [PubMed] [Google Scholar]
- [199].Krell ES, DiGiovanni CW. The efficacy of platelet-derived growth factor as a bone-stimulating agent. Foot Ankle Clin. 2016;21:763–70. doi: 10.1016/j.fcl.2016.07.002. [DOI] [PubMed] [Google Scholar]
- [200].Simank HG, Manggold J, Sebald W, Ries R, Richter W, Ewerbeck V, Sergi C. Bone morphogenetic protein-2 and growth and differentiation factor-5 enhance the healing of necrotic bone in a sheep model. Growth Factors. 2001;19:247–57. doi: 10.3109/08977190109001090. [DOI] [PubMed] [Google Scholar]
- [201].Kasten P, Beyen I, Bormann D, Luginbuhl R, Ploger F, Richter W. The effect of two point mutations in GDF-5 on ectopic bone formation in a beta-tricalciumphosphate scaffold. Biomaterials. 2010;31:3878–84. doi: 10.1016/j.biomaterials.2010.01.109. [DOI] [PubMed] [Google Scholar]
- [202].Lin XH, Shanmugasundaram S, Liu Y, Derrien A, Nurminskaya M, Zamora PO. B2A peptide induces chondrogenic differentiation in vitro and enhances cartilage repair in rats. J Orthop Res. 2012;30:1221–8. doi: 10.1002/jor.22078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [203].Yukna RA, Callan DP, Krauser JT, Evans GH, Aichelmann-Reidy ME, Moore K, Cruz R, Scott JB. Multi-center clinical evaluation of combination anorganic bovine-derived hydroxyapatite matrix (ABM)/cell binding peptide (P-15) as a bone replacement graft material in human periodontal osseous defects. 6-month results. Journal of periodontology. 1998;69:655–63. doi: 10.1902/jop.1998.69.6.655. [DOI] [PubMed] [Google Scholar]