Abstract
Background and Purpose:
Stroke-induced acute severe body weight (BW) loss is associated with a high rate of mortality during a critical post-stroke period. Several interventions to reduce the weight loss, however, have not been successful. Currently, the biological significance of this extraordinary catabolic process is not well understood. Spleen-derived monocytes/macrophages (MMs) are the major immune cells recruited to the injured brain. The trafficking of MMs has been shown to be important for tissue repair and recovery. The purpose of the study is to investigate whether the BW reduction is essential for MM-mediated immune response for mice to survive and whether a corticosterone-mediated catabolic event underlies the processes.
Methods:
C57BL/6 male mice (12-week-old) were subjected to transient middle cerebral artery occlusion. BW, total MMs, and their Ly-6Chigh and Ly-6Clow subsets were determined in the spleen, blood, and the brain in post-stroke mice. Post-stroke survival rate and MM subsets were determined in mice with adrenalectomy (ADX), sham-ADX and ADX mice supplemented with corticosterone.
Results:
Stroke reduced BW with a maximum reduction at 3D post-stroke (17.2±5.2%). The reduction at 3D was positively linked to injury severity and selective depletion of MMs, but no other types of immune cells, in the spleen. Notably, the splenic MM depletion was significantly greater in mice with severe BW reduction (≥18% at 3D). In the blood, stroke depleted circulating MMs to a similar degree in animals with moderate and severe BW loss. Ly-6C+ monocyte infiltration in the post-stroke brain was greater in mice with severe BW loss. Blocking the catabolic process by ADX significantly increased post-stroke mortality, but the mortality was partially rescued by corticosterone supplement in ADX mice.
Conclusions:
Stroke-induced BW loss facilitates MM-mediated immune response and the adrenal corticosterone-mediated catabolic process is necessary for post-stroke survival.
Keywords: stroke, immunity, monocyte/macrophages mobilization, corticosterone, mortality
Graphical Abstract

A proposed neuroimmune interaction to depict stroke-induced catabolic status and post-stoke outcome. The severity of stroke causes proportional body weight reduction during an acute phase of stroke (3D) and deployment of monocyte/macrophages (MMs). In mice with moderate body weight reduction, circulating MMs infiltrate to the injured brain. In mice with severe body weight reduction, additional MMs come from the spleen, seen as a significant depletion of MMs in this organ. The broken arrow indicates reduced spleen MM deployment into the circulation. Adrenalectomy (ADX) blocks catabolic processes and causes high mortality in stroke, which is partially rescued by corticosterone (CT) treatment.
Introduction
Stroke-induced body weight (BW) loss has been recognized as an adverse event in both clinical and animal studies.1-4 There are numerous preclinical studies reporting stroke-induced severe body weight loss.2-4 However, information on acute BW change in stroke patients is relatively limited besides a report that shows an association between short-term body weight loss during the initial hospital stay and unfavorable outcomes at 3 months.5 Neither the underlying severe acute catabolic event nor its functional significance has been clearly defined. In our previous study, ischemic stroke caused more than 20% of BW reduction in mice during the acute phase, ~10% at 2-4 weeks, which was gradually regained toward baseline at 2 months.2 Considering that the rodent metabolic rate is 7-8 times higher than in humans,6 2-4 weeks of post-stroke in rodents is roughly equivalent to 4-8 months in humans. It has been reported that animals with severe BW loss during acute post-stroke period have higher mortality.7, 8 In a population-based study, stroke patients who experienced weight loss greater than 3 kg at 4 months and 1 year had higher mortality compared to the patients with less than 3 kg of weight loss at the same time points,1 indicating the clinical relevance of a stroke-induced acute catabolic event on stroke outcome.
Post-stroke BW reduction is derived from the loss of body fat and lean body mass including skeletal and cardiac muscle.3 Stroke leads to muscle phenotype shift and atrophy as early as 4 hours after an ictus. Muscle atrophy is observed even in the unaffected limb in stroke patients.4, 9 In addition to sarcopenia, decreased anabolic and increased catabolic pathways, malnutrition, pain, stress, and immobilization have been suggested for potential causes for the weight loss.1, 4 Accumulating evidence indicates that inflammation plays a key role in stroke-induced weight loss/sarcopenia.10-12 Since stroke triggers multiple adaptive responses in the periphery to cope with the primary injury, the entire body’s reactions in response to the primary insult are likely to converge in BW reduction.
Stroke induces a profound immune response in the periphery and brain. Systemic immunological change occurs in bone marrow, blood, and spleen.13 In the brain, early activation of resident microglia triggers a post-ischemic inflammatory response, which is exacerbated by immune cells infiltrating from circulation.14, 15 Within hours of primary injury, neutrophils migrate into the brain, followed by macrophages and NK cells. T and B lymphocytes infiltration during injury development.16, 17 Both preclinical and clinical studies have shown that the inflammatory response correlates with injury size.18, 19 Among peripheral immune cells, monocytes/macrophages (MMs) is the major cell type that elicits post-ischemic inflammation in the brain.15 Deployed from the spleen, pro-inflammatory Ly-6Chigh subset has a short half-life and is recruited to the lesion acutely in a CCR2-dependent manner, while anti-inflammatory Ly-6Clow subset has a long half-life and is predominant in the resolution phase.20 We previously reported that CCR2 mRNA level in the brain is significantly correlated with infarct volume,21 suggesting a potential involvement of the peripheral MMs in BW loss.
In response to various physiological and psychological stressors, tissue trauma, and stroke, the hypothalamic-pituitary-adrenal (HPA) axis is activated and releases glucocorticoids from the adrenal gland. Clinical studies showed increased plasma glucocorticoid levels in acute stroke patients.22, 23 Known actions of glucocorticoids are their regulation of immune function during acute inflammation, immune cell mobilization, and inflammation resolution.24 Catabolic effects of glucocorticoids have been well-established. Treatment of glucocorticoids in rats causes muscle atrophy by augmenting myofibril protein proteolysis and blocking protein synthesis.25, 26 Based on the regulatory role of glucocorticoids in immunity and catabolism, the current study investigates the biological significance of post-stroke BW reduction by determining whether glucocorticoid-mediated immune regulation serves as an underlying mechanism for stroke-induced sarcopenia. Here, we provide experimental evidence that BW reduction at 3D predicts stroke injury severity and that a corticosterone-mediated catabolic process is necessary for post-stroke immunity and survival.
Materials and Methods
Detailed materials and Methods are described in the Supplementary Materials and Methods.
Animals
The use of animals and procedures was approved by the Institutional Animal Care and Use Committee (IACUC) of Weill Cornell Medicine and in accordance with the IACUC, National Institutes of Health, and Animal Research: Reporting of In Vivo Experiments guidelines. Experiments were performed in 12-week-old C57BL/6 mice (Jackson Lab, Bar Harbor, ME). Only male mice were used in this proof of concept study due to recognized sex differences in stroke-induced injury size and body fat/muscle composition.
MCAO and stroke outcome analyses
Procedures for transient focal ischemia and infarct /swelling measurement were performed as previously described15, 21
Bilateral Adrenalectomy and Corticosterone treatment
Adrenalectomy was performed just prior to MCAO procedure. A small dorsal midline skin incision was made in isoflurane-anesthetized mice. A small incision was made on the muscle layer below the rib cage on each flank to access and remove both glands. Adrenalectomized mice were either treated with saline or subcutaneous injections of 10 mg/kg corticosterone (Cat#27840, Millipore Sigma, St. Louis, MO) at the time of reperfusion and 2 mg/kg at 2h of reperfusion.
Plasma Corticosterone Measurement
Blood was collected from the tail vein at different time points and the plasma was stored at −80⁰C until use. The plasma was diluted 1:20 or 1:50 and corticosterone level was measured using a corticosterone ELISA kit (Enzo Life Sciences) according to the manufacturer’s instructions.
Flow Cytometry Analysis
Flow cytometry analysis for immune cells in the periphery and the brain was performed according to the methods previously described.15 The single cells from the spleen and blood were incubated with a cocktail of antibodies against T and B cells, NK cells and granulocytes, CD11b antibody and Ly-6C antibody. Brain mononuclear cells were stained with CD45 antibody, CD11b antibody, and Ly-6C antibody. Flow cytometry data were analyzed using FlowJo software.
Statistics
Sample size (n=13/group) was calculated based on predicting detectable differences to reach a power of 0.80 at a significance level of <0.05, assuming a 33% difference in mean and a 25% SD at the 95% confidence level for brain injury size measurement. Animals’ identity and treatment were blinded to the persons who assessed outcomes. Student’s t-test was used to compare between two groups. One-way ANOVA followed by post hoc Bonferroni tests were used for multiple comparisons. Correlation analysis was performed using Pearson’s test. Statistical analyses were conducted using GraphPad Prism software. Infarct volume and hemispheric swelling were expressed as mean±95% confidence interval and all other data are reported as mean±s.e.m. Cluster analysis was performed using NCSS statistical software.
Results
Stroke-induced BW loss at 3D is associated with stroke severity
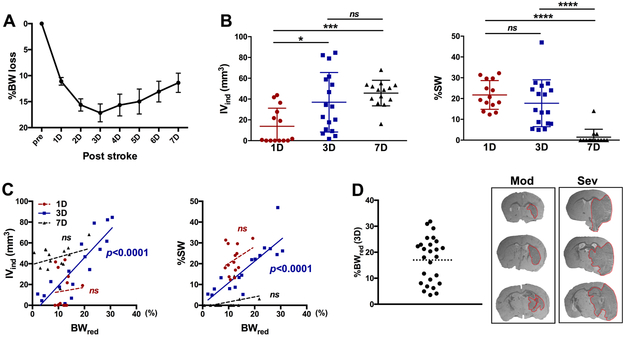
Stroke reduced BW during the first 1-3 days (D), and the BW did not fully return to a pre-ischemic level at 7D (Figure 1A). Infarct appeared at 1D and matured over 3-7D (Figure 1B). Stroke-induced brain edema was apparent at 1D and 3D but mostly resolved by 7D (Figure 1B). The results show that infarct and edema formation after ischemic stroke evolves over time and that 3D post-stroke is an optimal time point to assess both infarct volume and edema in our animal model of stroke. Analyses between BW reduction and stroke severity showed a significant positive correlation only at 3D, but not at 1D and 7D (Figure 1C). Retrospective analysis with a total of 91 C57BL/6 male mice in a preclinical animal bank in our laboratory further confirmed the significant association between weight loss and the extent of stroke injury at 3D (Supplementary Figure IA and B). Fuzzy cluster data analysis based on BW reduction revealed two groups of mice representing moderate (≤18%) and severe (>18%) reduction of BW (Figure 1D, Supplementary Figure IC). The brain injury in mice with moderate weight reduction (Mod) was mostly confined to the subcortical area, while severe (Sev) mice showed additional cortical injuries (Figure 1D).
Figure 1. Association between stroke-induced body weight loss and injury severity.
A, Acute post-stroke body weight reduction at 1-7 days(D). n=26. B, Assessment of IVind and %SW after stroke, n=14-17/group. C, Correlation between %BWred and infarct volume or brain swelling. n=14-17/group. D, Distribution of BW loss at 3D post-stroke. The dotted line indicates 18% of BW loss (left). Cross-sections (+0.4 mm, −0.8 mm and −2.0 mm from bregma) from a representative mouse with moderate (Mod) weight reduction (%BWred 10.4 at 3D, IVind 17.7, %SW 8.0) and mouse with severe (Sev) reduction (%BWred 25.5 at 3D, IVind 61.9, %SW 41.5). n=26. Pre, pre-stroke baseline; IVind, indirect infarct volume; %SW, Percent hemispheric swelling; BWred (3D), body weight reduction at 3D; *, ***, ****p<0.01, 0.001, 0.0001. ns, not significant.
Stroke induces selective MM mobilization from the spleen only in mice with severe weight loss
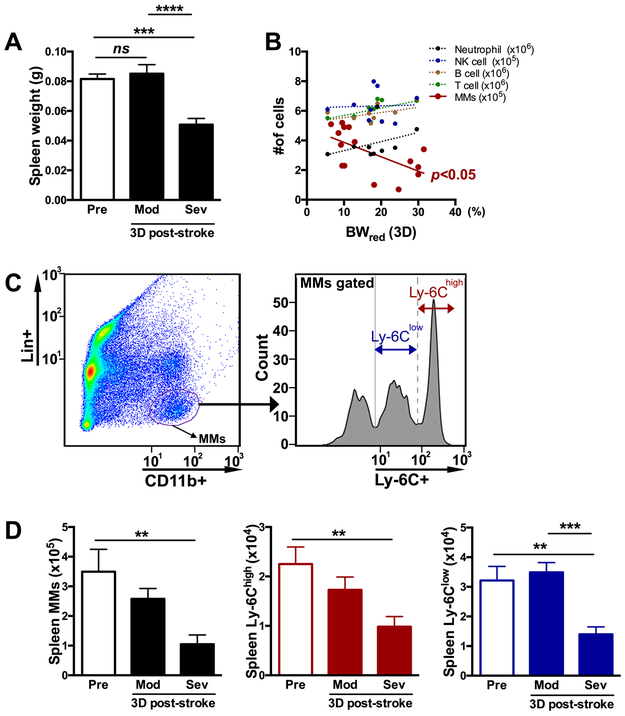
Stroke induces spleen contraction and deploys immune cells into circulation.15 Compared to pre-stroke (pre) mice, significant spleen contraction was observed in Sev group at 3D post-stroke (Figure 2A). To address which type of spleen-derived immune cell deployment is associated with post-stroke BW loss, the number of neutrophils, NK cell, B cell, T cell, and MMs was assessed in the spleen at 3D, a time point that reflects injury severity (Figure 1B). Stroke-induced BW reduction was negatively correlated with the number of MMs in the spleen, but not with neutrophil, NK cell, B cell or T cell (Figure 2B). The link between the selective MM depletion and BW reduction was further addressed by assessing the total number of MMs and their subsets based on Ly-6C expression (Figure 2C). The total number of MMs and Ly-6Chigh and Ly-6Clow subsets in the spleen was significantly depleted in Sev group (Figure 2D). Unlike MMs, other immune cells did not show differences between Mod and Sev groups except that the neutrophil was rather increased in the Sev group (Supplementary Figure II). These results provide a close association between BW reduction and MM mobilization in the spleen after ischemic stroke.
Figure 2. Post-stroke BW loss and spleen immune cell mobilization.
A, Spleen weight before (Pre) and 3D after stroke in mice with moderate (Mod) and severe (Sev) weight loss. n=10-15/group. B, Correlation between %BWred at 3D after stroke and the number of immune cells in the spleen. C, Gating strategy to identify MM subsets: pro-inflammatory Ly-6Chigh and anti-inflammatory Ly-6Clow subset. D, total MMs and Ly-6Chigh and Ly-6Clow MM subsets n=6-11/group. ns, not significant; pre, pre-stroke; BWred (3D), body weight reduction at 3D post-stroke, **, ***, p<0.01, 0.001.
Stroke depletes circulating MMs in both moderate and severe weight loss mice
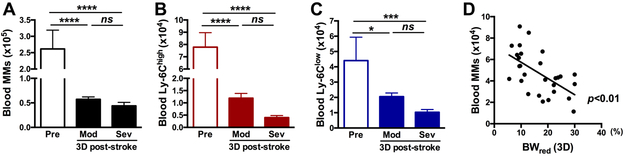
We next investigated the effect of BW reduction on circulating monocyte populations. Stroke reduced the number of total MMs, Ly-6Chigh and Ly-6Clow subsets in the blood of both Mod and Sev groups, without showing any significant difference between the two groups (Figure 3A-C). Despite lacking group differences, the number of MMs in the blood showed a negative correlation with %BWred at 3D (p<0.01, Figure 3D). Taken together, the results showed that circulating monocytes are the primary MMs to be mobilized proportionately to the extent of BW loss with additional depletion from splenic monocytes only in Sev injury group.
Figure 3. Effect of post-stroke BW loss on MM population in blood.
A, Circulating total MMs in the blood 3D before (pre) and 3D after stroke in mice with moderate (Mod) and severe (Sev) body weight loss. B-C, Determination of Ly-6Chigh and Ly-6Clow monocyte subset n=6-11/group. D, Correlation between BW reduction at 3D after stroke and the number of total MMs in the blood. n=28. R2=0.3392. *, ***, **** p<0.05, 0.001, 0.0001.
Stroke-induced weight reduction is associated with the degree of peripheral monocyte infiltration in the post-ischemic brain.
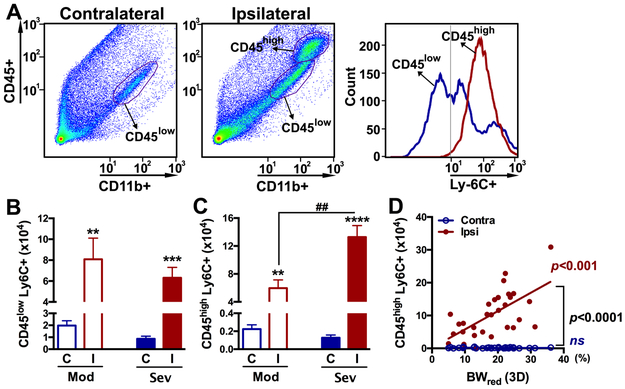
Stroke causes a massive infiltration of MMs into injured brain tissue. The effect of post-stroke BW reduction on the infiltration of MMs in the brain was assessed by determining the leukocytes antigen Ly-6C+ subset in the CD11b+ population at 3D post-stroke (Figure 4A). The presence of CD45low population in both hemispheres and an additional CD45high population in the ipsilateral hemisphere indicates that CD45low and CD45high population represent resident microglia and infiltrated MM, respectively, at this time.15 Unlike periphery, brain CD11b+ cells did not show distinct Ly-6Chigh and Ly-6Clow population due to down-regulation of Ly-6C in monocytes as they differentiated into tissue macrophages.27 Stroke increased the number of Ly6C+ cell in the CD45low subset in both Mod and Sev groups but the extent of increase was similar between the groups (Figure 4B). Stroke, however, significantly increased Ly-6C+ cells in the CD45high subset in Sev group compared to Mod animals (Figure 4C). Furthermore, greater BW reduction is positively correlated with a higher number of CD45high/Ly-6C+ cells in the ipsilateral hemisphere (Figure 4D). Taken together, the results show BW reduction has a significant link to monocyte trafficking from the periphery to injured brain tissue.
Figure 4. Effect of post-stroke BW loss on the infiltration of MMs in the brain at 3D post-stroke.
A, Gating of brain immune cells by CD45+/CD11b+ expression and leukocyte antigen Ly-6C for identification of infiltrated peripheral monocyte in the brain. B,C, Number of Ly-6C+ cells in CD45low and CD45high population in the post-stroke brain in mice with moderate (Mod) and severe (Sev) BW loss. n=7-17/group. D, Correlation between BW reduction at 3D after stroke and the number of Ly-6C+ cells in the CD45high population. n=28. R2=0.3392. **, ***, **** p<0.05, 0.01, and 0.0001 C vs. I; ##p<0.01 Contra (C), contralateral; Ipsi (I), ipsilateral
Blocking the catabolic process causes high mortality in acute stroke
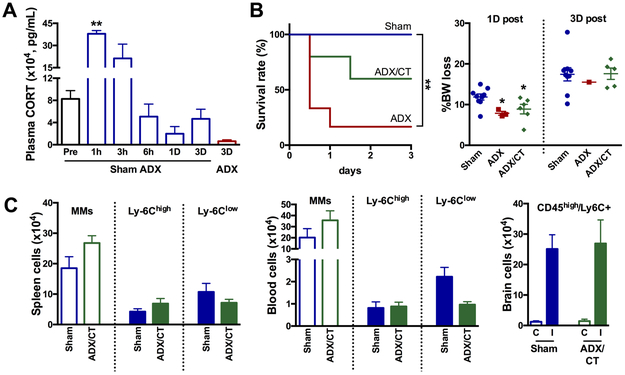
To address the biological significance of BW loss and immune cell mobilization, we next investigated the involvement of glucocorticoids in this process. Corticosterone (CT) is a major catabolic steroid hormone produced from the adrenal gland in rodents (cortisol in humans). In mice subjected to stroke, corticosterone levels in the plasma sharply increased around 1-3h and decreased back to the pre-stroke level around 6h after stroke (Figure 5A). Bilateral adrenalectomy (ADX) causes a profound reduction of plasma corticosterone levels at 3D post-stroke (Figure 5A). There was a striking increase in mortality in stroked mice following ADX (7 out of 8) by 3D while all 9 mice with sham-ADX survived. Treating ADX mice corticosterone partially rescued the high mortality (4 out of 10 at 3D). At 1D, surviving ADX and ADX/CT mice showed less BW reduction compared to the sham-ADX/stroke mice (Figure 5B), indicating a role of corticosterone in medicating post-stroke BW loss. Moreover, all animals surviving up to 3D showed a similar degree of BW reduction regardless of group (Figure 5B), suggesting that the corticosterone-mediated catabolic process is necessary for post-stroke survival. Due to the high mortality in ADX mice, total and MM subsets were analyzed only in sham-ADX and survived ADX/CT group. The total number of MMs and MM subsets in the spleen and blood, as well as CD45high/Ly-6C+ MMs in the brain, were similar in the surviving animals in the two groups. Taken together, the results show the importance of a corticosterone-mediated catabolic event for post-stroke survival and MM mobilization.
Figure 5. Effect of corticosterone on BW reduction, post-stroke survival, and MM mobilization.
A, Sham-adenectomy (Sham) or adenectomy (ADX) was performed immediately before stroke. Plasma corticosterone levels were measured before (pre) and several post-stroke time points n=2-4/time point. **p<0.01 vs pre. B, post-stroke survival rate and %BW loss at 1D and 3D after stroke in mice with sham-ADX (n=9), ADX (n=8), or ADX with corticosterone treatment (n=10). *, **p<0.01 vs. Sham. C, Number of total MMs and MM subsets in the spleen and the blood, and Ly-6C+ cells in the CD45high population of the post-stroke brain. n=9 (sham-ADX) and n=5 (ADX/CT animals). ADX, adrenalectomy; Sham, sham ADX; CT, corticosterone; C, contralateral; I, ipsilateral.
Discussion
Acute post-stroke BW loss is a recognized catabolic event that has a negative influence on stroke outcome and recovery in preclinical and clinical studies.28, 29 With limited clinical literature on acute BW change in patients, this preclinical study defined the biological significance of the observed catabolic event during the acute critical stage to provide insights in human stroke pathology. In addressing mechanisms underlying this catabolic process for its functional significance, we have several important observations. Post-stroke BW loss at 3D, but not in 1D and 7D, estimates the severity of the stroke by both infarct size and hemispheric swelling in our animal model of ischemic stroke. The weight loss at 3D is accompanied by MM deployment from the spleen (source), their mobilization in the blood (transit), and infiltration into the injured brain (destination). Blocking this catabolic process by removing adrenal gland profoundly increased post-stroke mortality, which partially rescued by corticosterone treatment. Notably, the MM mobilization from the spleen to brain occurred similar extent in all surviving mice regardless of whether the mice received sham-adrenalectomy or adrenalectomy with corticosterone replacement. The study demonstrates the importance of stroke-induced BW loss in facilitating MM mobilization from the periphery to the central nervous system and suggests the process is a critical event for post-stroke survival.
As reported by others 8, 30, BW loss was evident in mice subjected to transient focal ischemia in this study. An interesting observation in this study is the pattern of weight loss at 3D. Instead of a normal distribution, there was an apparent clustering divided around 18%, (Figure 1D, Supplementary Figure IC), suggesting the presence of a threshold in BW reduction following the stroke. Studies have indicated potential cause(s) of stroke-induced BW loss: impaired feeding, sympathetic over-activation, and infection3.1, 4, 31 However, inability to prevent stroke-induced sarcopenia by counteracting these causes 3 argues for the involvement of other factors. In agreement with literature that showed greater immune response by severe stroke 18, 19, the current study provided experimental evidence that links between the extent of monocytes/macrophages (MM) mobilization and BW reduction. Thus, immune cell mobilization is likely one of the events that underlie the observed BW reduction following ischemic stroke. A noted difference between the literature and current study is that while we found the correlation between stroke severity with BW loss only at 3D, the other study showed the link at 1D.32. Type and duration of the stroke, species, genetic background, sex, and age are likely contributing factors that influence the time points for the reported association.
Post-ischemic inflammation leads to proportional infiltration of peripheral MM to the injured brain. 15 Our working hypothesis is that stroke-induced MM-specific mobilization is a potential trigger for BW loss after stroke. Significant spleen shrinkage and reduced spleen MM numbers after stroke in our study are in line with previous reports.15, 33 Selective MM deployment from the periphery to the brain after ischemic stroke is reported as a key factor, as monocyte-depleted animals did not show neuroprotection after the intervention.34 Forty percent of stroke patients also show spleen volume reduction, which further supports the acute splenic response after ischemic stroke.35 Another intriguing finding relevant to the weight loss is that the spleen shrinkage and MM number reduction occur only in the severe weight loss group. Since the pattern of BW reduction suggested a threshold effect at around 18% reduction, we speculate the threshold effect in BW reduction also occurs in MM mobilization from the spleen. Although stroke reduces other types of immune cells from the spleen after stroke, 33, 36 these studies did not address whether the depletion of other immune cells depends on BW reduction. Furthermore, we observed that the mobilization of MMs, but T and B lymphocytes, neutrophils or NK cells from the spleen, was influenced by the extent of BW loss at 3D (Figure 2B). Thus, the study suggests the specificity of MM depletion/ mobilization relevant to weight loss at 3D, a time point where the injury severity is best reflected.
Unlike the spleen where the MM depletion occurs only in the Sev weight loss group, the depletion of circulating MMs occurred in both moderate and severe weight loss groups. The findings indicate that MMs in the blood are likely to mobilize to the brain first to meet the initial immune challenge upon stroke, but additional MM deployment from the spleen is required to mount sustained immune response in mice with bigger brain injury, thereby larger weight loss. Since the number of MMs in the spleen far exceeded the number of MMs in the blood,37 MM deployment from the spleen likely replenishes depleted monocytes in circulation to maintain peripheral immunity against post-stroke infection. While the possibility of MM mobilization from bone marrow has not been addressed in this study, a report showing increased MMs in bone marrow at 3D after stroke15 does not indicate a direct link between BW reduction and the egress of MMs from bone marrow at this acute stage.
The presence of MM heterogeneity consisting of Ly-6Chigh and Ly-6Clow subsets has been a challenge to define the role for each subset. Approximately equal mobilization/depletion of both subsets from the spleen and blood suggests the involvement of both subsets to elicit a post-stroke immune response. Unlike the periphery, where we observe two distinct MM subsets, the brain did not have distinct Ly-6C peaks. There was an apparent shift of mean fluorescence from Ly-6Chigh toward Ly-6Clow population in the post-stroke brain (mean fluorescence of brain versus spleen Ly-6Chigh 68,446 vs. 99,257 Ly-6Clow 12,670 vs. 22,805). The shift is consistent with reports that Ly-6C loses its expression during the transition of recruited monocytes in a sterile wound and in S. mansoni infected mouse model.38, 39 It also has been shown that Ly-6Chigh monocytes can transform into Ly-6Clow macrophages, 27 supporting the loss of Ly-6C expression as the infiltrated Ly-6Chigh monocytes differentiate into tissue macrophage in the stroked brain and potential conversion to a less inflammatory MMs in the brain milieu.40, 41 While the significance of this event is unknown and remains to be investigated, infiltration of MMs to the injured brain is closely associated with BW reduction. Analyses of MMs in the post-ischemic brain showed the number of CD45high/Ly-6C+ MMs in the stroked hemisphere was significantly correlated with BW reduction at 3D after stroke (Figure 4D). Notably, CD45low/Ly-6C+ MMs did not significantly correlate with BW reduction (ns, n=18, not shown), suggesting the involvement of infiltrating MMs, rather than resident microglia, in the weight reduction/injury size in the stroked brain. The specific involvement of MMs mobilization in the context of stroke severity is supported in other disease context since the extent of infiltration of MMs, but not CD4+ lymphocytes, was strongly associated with the severity of experimental autoimmune encephalitis.42
Corticosterone is a major glucocorticoid produced in rodents in response to stress. As reported in clinical literature,22 there was an acute increase in plasma corticosterone in mice following stroke (Figure 5A). The role of CORT in ischemic stroke is controversial.43-45 In the current study, we observed increased post-stroke mortality at 3D (87.5%) in mice with bilateral adrenalectomy while all sham-adrenalectomized animals survived. Partial rescue by corticosterone treatment in adrenalectomized mice further indicates a critical role of corticosterone in post-stroke survival. Although the dose and time of corticosterone treatment used in the current study was based on the profile of corticosterone levels after stroke (Figure 5A), it is possible to decrease the mortality with higher corticosterone dose in ADX/CORT mice. Notably, all surviving animals among groups showed a similar degree of BW reduction (Figure 5B), and MM mobilization (Figure 5C) at 3D. A recent study reported the role of corticosterone in mediating stress-induced MM mobilization into the circulation,46 indicating that a corticosterone-mediated catabolic event likely underlies post-stroke MM mobilization and survival. We speculated that the other 7 dead mice of ADX group may have undergone this catabolic process to a lesser extent than the one that survived.
In summary, the study revealed that the degree of weight loss at 3D, which estimates stroke severity, determines the extent of MM-specific deployment in the spleen, their depletion in blood, and the infiltration into the post-ischemic brain. MM infiltration into the stroke brain is initially from the blood and additionally recruited from the spleen in mice with severe weight reduction/brain injury. Furthermore, corticosterone is a critical catabolic hormone for acute BW loss and survival after stroke. The study indicates that stroke-induced BW loss is an essential catabolic event for immune regulation and post-stroke survival.
Supplementary Material
Acknowledgments
JY performed experiments, analyzed data and wrote the manuscript. EK performed experiments and CB generated animal stroke models. SC designed the study and wrote the manuscript.
Sources of Funding
This work was supported by NIH grants HL082511, NS095359, and NS077897 (SC), Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Education 2013R1A6A3A03059436 (JY), and American Heart Association Postdoctoral Fellowship 15POST25680020 (JY).
Footnotes
Disclosures
None
Social media handle: @BurkeNeuroSci
References
- 1.Jonsson AC, Lindgren I, Norrving B, Lindgren A. Weight loss after stroke: A population-based study from the lund stroke register. Stroke; a journal of cerebral circulation. 2008;39:918–923 [DOI] [PubMed] [Google Scholar]
- 2.Qin L, Jing D, Parauda S, Carmel J, Ratan RR, Lee FS, et al. An adaptive role for bdnf val66met polymorphism in motor recovery in chronic stroke. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2014;34:2493–2502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Springer J, Schust S, Peske K, Tschirner A, Rex A, Engel O, et al. Catabolic signaling and muscle wasting after acute ischemic stroke in mice: Indication for a stroke-specific sarcopenia. Stroke. 2014;45:3675–3683 [DOI] [PubMed] [Google Scholar]
- 4.Scherbakov N, von Haehling S, Anker SD, Dirnagl U, Doehner W. Stroke induced sarcopenia: Muscle wasting and disability after stroke. International journal of cardiology. 2013;170:89–94 [DOI] [PubMed] [Google Scholar]
- 5.Kim Y, Kim CK, Jung S, Ko SB, Lee SH, Yoon BW. Prognostic importance of weight change on short-term functional outcome in acute ischemic stroke. Int J Stroke. 2015;10 Suppl A100:62–68 [DOI] [PubMed] [Google Scholar]
- 6.Terpstra AH. Differences between humans and mice in efficacy of the body fat lowering effect of conjugated linoleic acid: Role of metabolic rate. J Nutr. 2001;131:2067–2068 [DOI] [PubMed] [Google Scholar]
- 7.Verma R, Harris NM, Friedler BD, Crapser J, Patel AR, Venna V, et al. Reversal of the detrimental effects of post-stroke social isolation by pair-housing is mediated by activation of bdnf-mapk/erk in aged mice. Sci Rep. 2016;6:25176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meisel C, Prass K, Braun J, Victorov I, Wolf T, Megow D, et al. Preventive antibacterial treatment improves the general medical and neurological outcome in a mouse model of stroke. Stroke. 2004;35:2–6 [DOI] [PubMed] [Google Scholar]
- 9.Harris ML, Polkey MI, Bath PM, Moxham J. Quadriceps muscle weakness following acute hemiplegic stroke. Clin Rehabil. 2001;15:274–281 [DOI] [PubMed] [Google Scholar]
- 10.Beyer I, Mets T, Bautmans I. Chronic low-grade inflammation and age-related sarcopenia. Curr Opin Clin Nutr Metab Care. 2012;15:12–22 [DOI] [PubMed] [Google Scholar]
- 11.Carter CS, Hofer T, Seo AY, Leeuwenburgh C. Molecular mechanisms of life- and health-span extension: Role of calorie restriction and exercise intervention. Appl Physiol Nutr Metab. 2007;32:954–966 [DOI] [PubMed] [Google Scholar]
- 12.Jensen GL. Inflammation: Roles in aging and sarcopenia. JPEN J Parenter Enteral Nutr. 2008;32:656–659 [DOI] [PubMed] [Google Scholar]
- 13.Offner H, Vandenbark AA, Hurn PD. Effect of experimental stroke on peripheral immunity: Cns ischemia induces profound immunosuppression. Neuroscience. 2009;158:1098–1111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gelderblom M, Leypoldt F, Steinbach K, Behrens D, Choe CU, Siler DA, et al. Temporal and spatial dynamics of cerebral immune cell accumulation in stroke. Stroke; a journal of cerebral circulation. 2009;40:1849–1857 [DOI] [PubMed] [Google Scholar]
- 15.Kim E, Yang J, Beltran CD, Cho S. Role of spleen-derived monocytes/macrophages in acute ischemic brain injury. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2014;34:1411–1419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chamorro A, Meisel A, Planas AM, Urra X, van de Beek D, Veltkamp R. The immunology of acute stroke. Nat Rev Neurol. 2012;8:401–410 [DOI] [PubMed] [Google Scholar]
- 17.Gan Y, Liu Q, Wu W, Yin JX, Bai XF, Shen R, et al. Ischemic neurons recruit natural killer cells that accelerate brain infarction. Proc Natl Acad Sci U S A. 2014;111:2704–2709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Audebert HJ, Rott MM, Eck T, Haberl RL. Systemic inflammatory response depends on initial stroke severity but is attenuated by successful thrombolysis. Stroke; a journal of cerebral circulation. 2004;35:2128–2133 [DOI] [PubMed] [Google Scholar]
- 19.Kim EH, Tolhurst AT, Szeto HH, Cho SH. Targeting cd36-mediated inflammation reduces acute brain injury in transient, but not permanent, ischemic stroke. CNS Neurosci Ther. 2015;21:385–391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Auffray C, Fogg DK, Narni-Mancinelli E, Senechal B, Trouillet C, Saederup N, et al. Cx3cr1+ cd115+ cd135+ common macrophage/dc precursors and the role of cx3cr1 in their response to inflammation. The Journal of experimental medicine. 2009;206:595–606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim E, Febbraio M, Bao Y, Tolhurst AT, Epstein JM, Cho S. Cd36 in the periphery and brain synergizes in stroke injury in hyperlipidemia. Annals of neurology. 2012;71:753–764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Olsson T, Marklund N, Gustafson Y, Nasman B. Abnormalities at different levels of the hypothalamic-pituitary-adrenocortical axis early after stroke. Stroke. 1992;23:1573–1576 [DOI] [PubMed] [Google Scholar]
- 23.Feibel JH, Hardy PM, Campbell RG, Goldstein MN, Joynt RJ. Prognostic value of the stress response following stroke. JAMA. 1977;238:1374–1376 [PubMed] [Google Scholar]
- 24.Cain DW, Cidlowski JA. Immune regulation by glucocorticoids. Nature reviews. 2017;17:233–247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shoji S, Pennington RJ. The effect of cortisone on protein breakdown and synthesis in rat skeletal muscle. Mol Cell Endocrinol. 1977;6:159–169 [DOI] [PubMed] [Google Scholar]
- 26.Kayali AG, Young VR, Goodman MN. Sensitivity of myofibrillar proteins to glucocorticoid-induced muscle proteolysis. Am J Physiol. 1987;252:E621–626 [DOI] [PubMed] [Google Scholar]
- 27.Gordon S Macrophage heterogeneity and tissue lipids. J Clin Invest. 2007;117:89–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martin JMH, Elliot D, editors. The prevalence of disability among adults. London: HMSO; 1998 [Google Scholar]
- 29.Writing Group M, Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, et al. Heart disease and stroke statistics-2016 update: A report from the american heart association. Circulation. 2016;133:e38–360 [DOI] [PubMed] [Google Scholar]
- 30.Bacigaluppi M, Pluchino S, Peruzzotti-Jametti L, Kilic E, Kilic U, Salani G, et al. Delayed post-ischaemic neuroprotection following systemic neural stem cell transplantation involves multiple mechanisms. Brain : a journal of neurology. 2009;132:2239–2251 [DOI] [PubMed] [Google Scholar]
- 31.Scherbakov N, Dirnagl U, Doehner W. Body weight after stroke: Lessons from the obesity paradox. Stroke; a journal of cerebral circulation. 2011;42:3646–3650 [DOI] [PubMed] [Google Scholar]
- 32.Cai L, Geng X, Hussain M, Liu Z, Gao Z, Liu S, et al. Weight loss: Indication of brain damage and effect of combined normobaric oxygen and ethanol therapy after stroke. Neurol Res. 2015;37:441–446 [DOI] [PubMed] [Google Scholar]
- 33.Offner H, Subramanian S, Parker SM, Wang C, Afentoulis ME, Lewis A, et al. Splenic atrophy in experimental stroke is accompanied by increased regulatory t cells and circulating macrophages. J Immunol. 2006;176:6523–6531 [DOI] [PubMed] [Google Scholar]
- 34.Zhang Y, Ma L, Ren C, Liu K, Tian X, Wu D, et al. Immediate remote ischemic postconditioning reduces cerebral damage in ischemic stroke mice by enhancing leptomeningeal collateral circulation. J Cell Physiol. 2018;234:12637–12645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vahidy FS, Parsha KN, Rahbar MH, Lee M, Bui TT, Nguyen C, et al. Acute splenic responses in patients with ischemic stroke and intracerebral hemorrhage. J Cereb Blood Flow Metab. 2016;36:1012–1021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Seifert HA, Hall AA, Chapman CB, Collier LA, Willing AE, Pennypacker KR. A transient decrease in spleen size following stroke corresponds to splenocyte release into systemic circulation. J Neuroimmune Pharmacol. 2012;7:1017–1024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nahrendorf M, Swirski FK, Aikawa E, Stangenberg L, Wurdinger T, Figueiredo JL, et al. The healing myocardium sequentially mobilizes two monocyte subsets with divergent and complementary functions. The Journal of experimental medicine. 2007;204:3037–3047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Girgis NM, Gundra UM, Ward LN, Cabrera M, Frevert U, Loke P. Ly6c(high) monocytes become alternatively activated macrophages in schistosome granulomas with help from cd4+ cells. PLoS Pathog. 2014;10:e1004080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Crane MJ, Daley JM, van Houtte O, Brancato SK, Henry WL Jr., , Albina JE. The monocyte to macrophage transition in the murine sterile wound. PLoS ONE. 2014;9:e86660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Miro-Mur F, Perez-de-Puig I, Ferrer-Ferrer M, Urra X, Justicia C, Chamorro A, et al. Immature monocytes recruited to the ischemic mouse brain differentiate into macrophages with features of alternative activation. Brain Behav Immun. 2016;53:18–33 [DOI] [PubMed] [Google Scholar]
- 41.Garcia-Bonilla L, Faraco G, Moore J, Murphy M, Racchumi G, Srinivasan J, et al. Spatio-temporal profile, phenotypic diversity, and fate of recruited monocytes into the post-ischemic brain. J Neuroinflammation. 2016;13:285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ajami B, Bennett JL, Krieger C, McNagny KM, Rossi FM. Infiltrating monocytes trigger eae progression, but do not contribute to the resident microglia pool. Nature neuroscience. 2011;14:1142–1149 [DOI] [PubMed] [Google Scholar]
- 43.Balkaya M, Prinz V, Custodis F, Gertz K, Kronenberg G, Kroeber J, et al. Stress worsens endothelial function and ischemic stroke via glucocorticoids. Stroke. 2011;42:3258–3264 [DOI] [PubMed] [Google Scholar]
- 44.Faraji J, Lehmann H, Metz GA, Sutherland RJ. Stress and corticosterone enhance cognitive recovery from hippocampal stroke in rats. Neurosci Lett. 2009;462:248–252 [DOI] [PubMed] [Google Scholar]
- 45.Borlongan CV, Stahl CE, Redei E, Wang Y. Prepro-thyrotropin-releasing hormone 178-199 exerts partial protection against cerebral ischemia in adult rats. Neuroreport. 1999;10:3501–3505 [DOI] [PubMed] [Google Scholar]
- 46.Niraula A, Wang Y, Godbout JP, Sheridan JF. Corticosterone production during repeated social defeat causes monocyte mobilization from the bone marrow, glucocorticoid resistance, and neurovascular adhesion molecule expression. J Neurosci. 2018;38:2328–2340 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.