Abstract
Multiplex tandem mass spectrometry (MS/MS)-based enzyme activity assays for newborn screening (NBS) and diagnosis of lysosomal storage diseases (LSDs) in newborns, using dried blood spots (DBS) on newborn screening cards, have garnered much attention due to its sensitivity, high precision, and the capability to screen for an unprecedented number of diseases in a single assay. Herein we report the development of MS/MS-based enzyme assays for the diagnosis of α-mannosidosis and fucosidosis. These new protocols are able to distinguish untreated patients from random newborns, carriers and a post-bone marrow transplant patient. We have successfully multiplexed the α-mannosidosis assay with a multiplex MS/MS assay for the screening and diagnosis of other LSDs, namely Fabry, Pompe, MPS I, Gaucher, Niemann-Pick-A/B, and Krabbe diseases. Additionally, we also multiplexed the fucosidosis NBS assay with a 5-plex assay that tests for MPS-II, MPS-IIIB, MPS-IVA, MPS-VI and MPS-VII.
Graphical abstract
Introduction
Fucosidosis and α-mannosidosis are progressive neurodegenerative lysosomal storage disorders (LSDs). Features that overlap other more common LSDs such as MPS-I include coarsening of facial features over time, recurrent upper respiratory infections, and skeletal changes (dysostosis multiplex). As with most LSDs, the clinical spectrum demonstrates varying severity with the most severely affected patients presenting at the earliest ages, within the first two years of life. Both disorders are ultra-rare with an incidence of <1/200,000.
Bone marrow transplant and now enzyme replacement therapy have been independently used for the treatment of α-mannosidosis.1–3 Human recombinant α-mannosidase was recently approved for the non-neurological features of α-mannosidosis by the European Medical Agency (July 2018). Bone marrow transplantation has been utilized in a small number of patients with fucosidosis with improvements noted in some cases.4, 5 Enzyme replacement therapy is not available for fucosidosis. Earlier transplant appears to be more effective in these conditions than transplant after symptoms fully manifest. These results suggest that newborn screening for these disorders may be warranted once treatment options are improved. However, given the fact that these LSDs are ultra-rare, newborn screening may be an option only if they can be multiplexed with other diseases in the newborn screening environment.
Fucosidosis is an LSD caused by mutations in the FUCA1 gene, which is responsible for the production of L-fucosidase enzyme.6, 7 α-Mannosidosisis caused by the deficiency in the activity of the lysosomal enzyme α-mannosidase.8 We have previously reported several tandem mass spectrometry (MS/MS)-based lysosomal enzymatic activity assays that use dried blood spots (DBS) collected from infants on newborn screening cards.9–13 MS/MS is also useful for the detection of disease-specific biomarkers in DBS and other samples, for example in the detection of elevated sulfatides in DBS in patients with metachromatic leukodystrophy.14 The MS/MS platform is the most flexible analytical method reported for the analysis of inborn errors of metabolism including metabolomic surveys that can detect several diseases in a highly multiplexable fashion.15
Herein, we report MS/MS-based enzyme activity assays for the enzymes relevant to α-mannosidosis and fucosidosis using DBS. The new α-mannosidosis assay was successfully multiplexed with our previously reported assay that screens for Fabry, Pompe, MPS I, Gaucher, Niemann-Pick and Krabbe diseases,16 whereas the fucosidosis assay was combined with the multiplex assay for MPS-II, MPS-IIIB, MPS-IVA, MPS-VI and MPS-VII.10 The work illustrates how additional enzymatic assays can be easily added to the MS/MS platform for newborn screening and biochemical diagnosis.
Experimental Section
All chemicals used were reagent grade, unless specified otherwise. The syntheses of the enzymatic substrates and the heavy atom labelled internal standards are detailed in the Supporting Information section. All experiments with human blood samples were conducted in accordance with the guidelines outlined by the Institutional Review Board (IRB). All LSD patients, whose DBS was used in this study, had been previously diagnosed and confirmed by both enzyme activity and molecular diagnostics. They were all either pediatric or adult patients at the time of DBS collection (In the unaffected healthy samples, there was no significant difference in enzyme activities in DBS from newborns and adults).
Standard Experimental Procedure
α-Mannosidosis Assay:
The α-mannosidosis assay cocktail was prepared by mixing an appropriate amount of α-Mannosidosis Substrate (Figure 1) and internal standard (α- Mannosidosis IS) in 0.25 M sodium succinate (Sigma Aldrich) buffer (pH 4.25) to give a final concentration of 1 mM substrate and 20 μM internal standard. When stored at−20°C, this assay cocktail is stable for up to 1 week (α-Glycosides involving mannose are well documented to undergo relatively rapid cleavage due to a neighbouring group effect). A single 3 mm DBS punch was placed in each well of a 96-deep well assay plate. To each well 30 μL of α-mannosidosis assay cocktail was added, and the plate was sealed with a storage matt (Sigma Cat. CLS3080). The plate was shaken for 6 hours at 37°C using an orbital shaker (~250 rpm) incubator, and this was followed by post-assay sample processing.
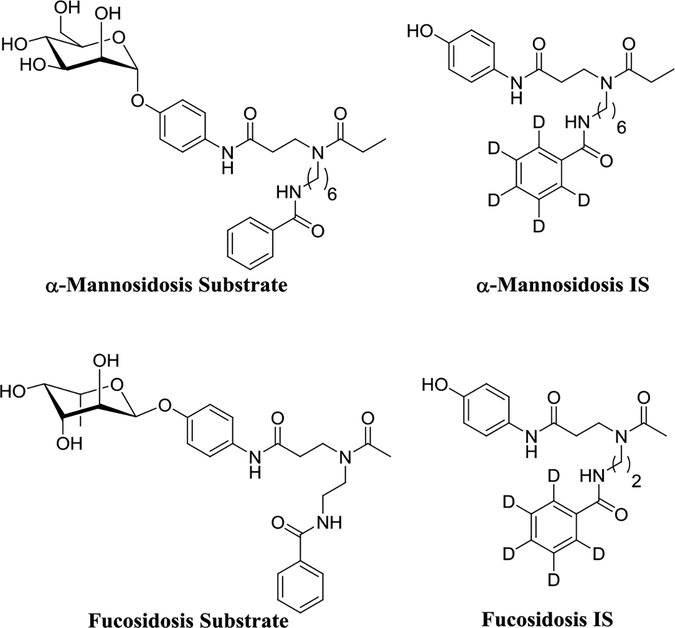
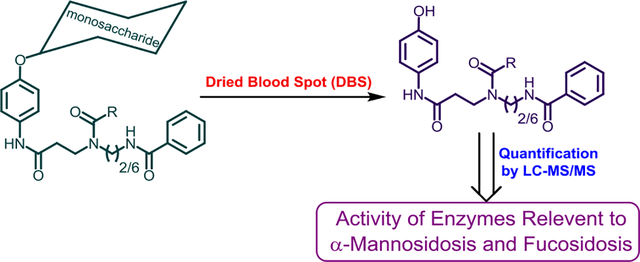
Figure 1.
Substrate and Internal standard for the α-mannosidase and α-L-fucosidase MS/MS assay
α-Mannosidosis, Fabry, Pompe, MPS I, Gaucher, Niemann-Pick-A/B and Krabbe - 7plex Assay:
The 7plex assay cocktail was made by mixing appropriate amount of substrates, internal standards (IS) and sodium oleate in the assay buffer (pH 4.25, succinate and additives as detailed in Supplementary Information) to give a final concentration of reagents as follows: 1) α-mannosidosis (1.0 mM substrate and 20 μM internal standard); 2) Gaucher (0.52 mM substrate and 20.4 μM internal standard); 3) Niemann-Pick-A/B (0.78 mM substrate and 17.9 μM internal standard); 4) Pompe (0.37 mM substrate and 24.5 μM internal standard); 5) Krabbe (1.02 mM substrate and 10.9 μM internal standard); 6) Fabry (1.3 mM substrate and 23.9 μM internal standard); 7) MPS-I (0.22 mM substrate and 14.6 μM internal standard) and sodium oleate (1.3 mg/mL) (When stored at−20°C, this assay cocktail is stable for up to 1 week). These additional reagents were obtained from PerkinElmer.16 A single 3 mm DBS punch was placed in each well in a 96-deep well assay plate. To each well 30 μL of 7plex assay cocktail was added, and the plate was sealed with a storage matt (Sigma Cat. CLS3080). The plate was shaken for 6 hours at 37°C using an orbital sha er rpm incubator, and this was followed by post-assay sample processing.
Fucosidosis Assay:
The fucosidosis assay cocktail was made by mixing 50 mM sodium acetate (Sigma Aldrich) buffer (pH 5.0) with enough substrate and internal standard (IS) to make a final concentration of 1 mM Fucosidosis Substrate (Figure 1) and 20 μM Fucosidosis IS. A single 3 mm DBS punch was placed in each well in a 96-deep well assay plate. To each well 30 μL of fucosidosis assay cocktail was added and the plate was sealed with a storage matt (Sigma Cat. CLS3080). The plate was shaken for 16 hours at 37°C using an orbital shaker (~250 rpm) incubator, and this was followed by post-assay sample processing. Assay cocktails containing fucosidase substrate are stable for at least several months at−20 °C.
Fucosidosis, MPS-II, MPS-IIIB, MPS-IVA, MPS-VI and MPS-VII - 6plex Assay:
The 6plex assay cocktail was made by mixing the appropriate amount of substrates, internal standards (IS) and NAG-thiazoline in the assay buffer (pH 5.0, sodium acetate and additives as detailed in Supplementary Information). Final concentration of reagents are: 1) Fucosidosis (1.0 mM substrate and 20 μM internal standard); 2) MPS-II (1.0 mM substrate and 20 μM internal standard); 3) MPS-IIIB (0.5 mM substrate and 10 μM internal standard); 4) MPS-IVA (1.0 mM substrate and 7.5 μM internal standard); 5) MPS-VI (1.0 mM substrate and 15 μM internal standard); 6) MPS-VII (0.5 mM substrate and 10 μM internal standard), and NAG-thiazoline (100 μM). Reagents for the MPS assays were obtained as described.10 A single 3 mm DBS punch was placed in each well in a 96-deep well assay plate. To each well 30 μL of 6plex assay cocktail was added, and the plate was sealed with a storage matt (Sigma Cat. CLS3080). The plate was then shaken for 16 hours at 37°C using an orbital shaker rpm incubator, and this was followed by post-assay sample processing.
Post-Assay Sample Processing:
The assay was quenched with the addition of 1:1 methanol-ethyl acetate mixture (100 μL), followed by addition of ethyl acetate (400 μL) (J. T. Baker, Cat No. 9282–03) and 0.5 M aqueous sodium chloride (200 μL).16 After mixing up and down ~10 times with the pipettor, the plate was centrifuged (Allegra X-12R, Beckmann) for 5 min at 3000 g to accelerate the solvent layer separation. A 200 μL aliquot of the top layer was pipet transferred to a shallow 96-well plate, and the solvent was dried under a stream of nitrogen at room temperature. The resulting residue was dissolved in an acetonitrile/water mixture (50% acetonitrile in water for α-Mannosidosis assay and 7plex assay, whereas 25% acetonitrile in water was used for the fucosidosis assay and 6plex assay). After mixing up and down ~10 times with the pipettor, samples were subjected to combined liquid chromatography-MS/MS (LC-MS/MS). Detailed LC-MS/MS conditions are given in the Supplementary Information section.
Results and Discussion
In the previous studies blood leukocytes, cultured skin fibroblasts, blood plasma17 and DBS18 have been successfully used to diagnose α-mannosidosis using fluorometric techniques. In the present study we developed a MS/MS assay for α-mannosidosis that can be multiplexed with other LSD assays. Previously we have shown that reagents with a bis-amide functional group in the aglycone tend to ionize well in positive ion mode electrospray MS/MS, which leads to higher ion counts and subsequently better sensitivity.19 We designed and synthesized an enzymatic substrate for α-mannosidase (α-Mannosidosis Substrate) (Supplementary Information Scheme-1) (Figure 1). To quantify the enzymatic product formed by MS/MS, we synthesized the d5-labeled internal standard (α-Mannosidosis IS). Similarly, plasma and serum samples have been successfully used to diagnose fucosidosis by the use of fluorometric methods, but a MS/MS-based diagnosis has not been reported.20, 21 Therefore, we designed and synthesized an enzymatic substrate for L-fucosidase (Fucosidosis Substrate) (Supplementary Information Scheme-2) and the d5-labeled internal standard (Fucosidosis IS).
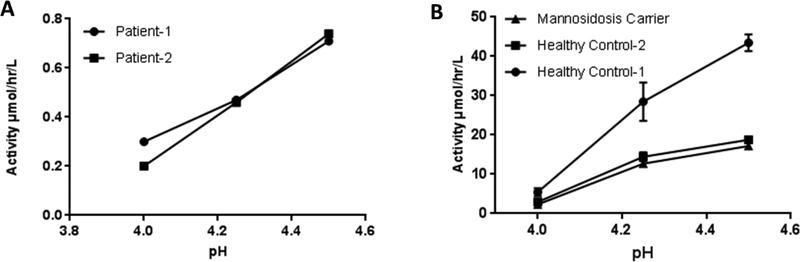
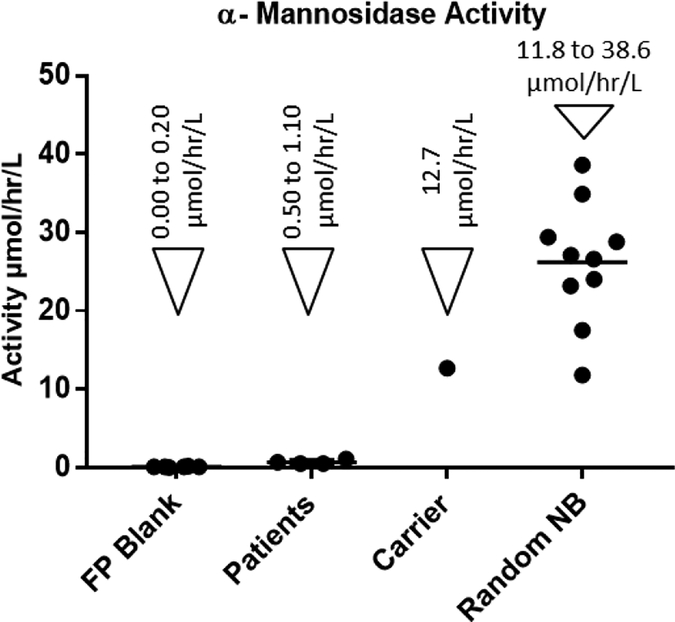
According to the previous studies on the α-mannosidase activity in plasma, when the pH of the assay is raised above 4.5, elevated background activity due to the action of isoenzymes is observed, which could lead to missed diagnosis.17 We found that at pH 4. α-mannosidosis patients had negligible α-mannosidase activity, while the healthy control samples showed significant activity (Figure-2). The linearity of the α-mannosidase activity, in DBS, as a function of time was studied (Supporting Information, Figure-S1). Product formation was linear up to 6 hours and begins to drop gradually beyond that duration. Furthermore, we found that incubation at pH 4.0 or longer incubation time (16 hours) lead to higher background signal due to non-enzymatic breakdown of the substrate. Therefore, we concluded that pH 4.25 and an incubation time of 6 hours would be ideal to avoid any false negatives and still obtain the best signal to noise ratio. Using the DBS samples from a healthy control, we found that the intra assay coefficient of variation is 8.5 %. The tandem mass spectrometry-based assay using this new substrate and DBS sample was able to successfully differentiate the α-mannosidosis patients from healthy and carrier samples (Figure-3). The mean α-mannosidase activity for the random newborns and the patients were 26.2 ± 7.7 μmol/hr/L and .68 ± 0.29 μmol/hr/L respectively.
Figure-2.
(A) DBS samples from α-mannosidosis patients were measured for α-mannosidase activity at various pH (B) DBS samples from healthy control and α-mannosidosis carrier were measured for α-mannosidase activity at various pH
Figure-3.
α-Mannosidase activity in blank filter paper punch (FP Blank) and DBS samples from patients already diagnosed with α-Mannosidosis (4 specimens), α-Mannosidosis carrier (1 specimens) and healthy random newborns (10 specimens)
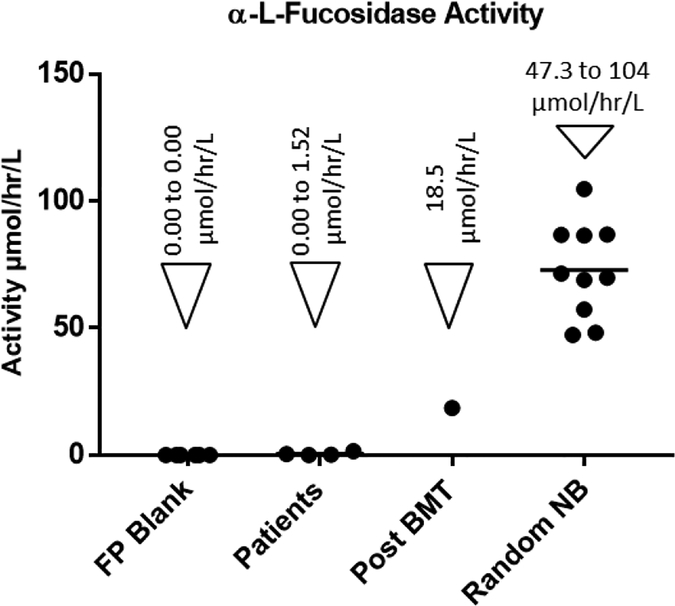
Previous study of L-fucosidase activity in serum and plasma has revealed that the peak enzyme activity is attained at pH 5.0.20 The activity of L-fucosidase in DBS samples was found to be linear at different time intervals over a period of 20 hours (Supporting Information, Figure-S2). Consequently, we standardized our assay for L-fucosidase at pH 5.0 with a DBS incubation time of 16 hours. Upon testing the reproducibility of the assay with DBS samples from a healthy control, it was found that the intra assay coefficient of variation is 7.9 %. Studies with DBS from random presumably healthy newborns, fucosidosis patients and patients treated with bone marrow transplant revealed that our MS/MS based fucosidosis assay is able to well distinguish each group (Figure-4). Random newborns and patients had a mean α-L-fucosidase activity of 72.8 ± 18.6 μmol/hr/L and 0.56 ± 0.67 μmol/hr/L respectively.
Figure-4.
α-L-Fucosidase activity in blank filter paper punch (FP Blank) and DBS samples from patients already diagnosed with Fucosidosis (4 specimens), patients treated with bone marrow transplant (Post BMT) (1 specimen) and healthy random newborns (10 specimens)
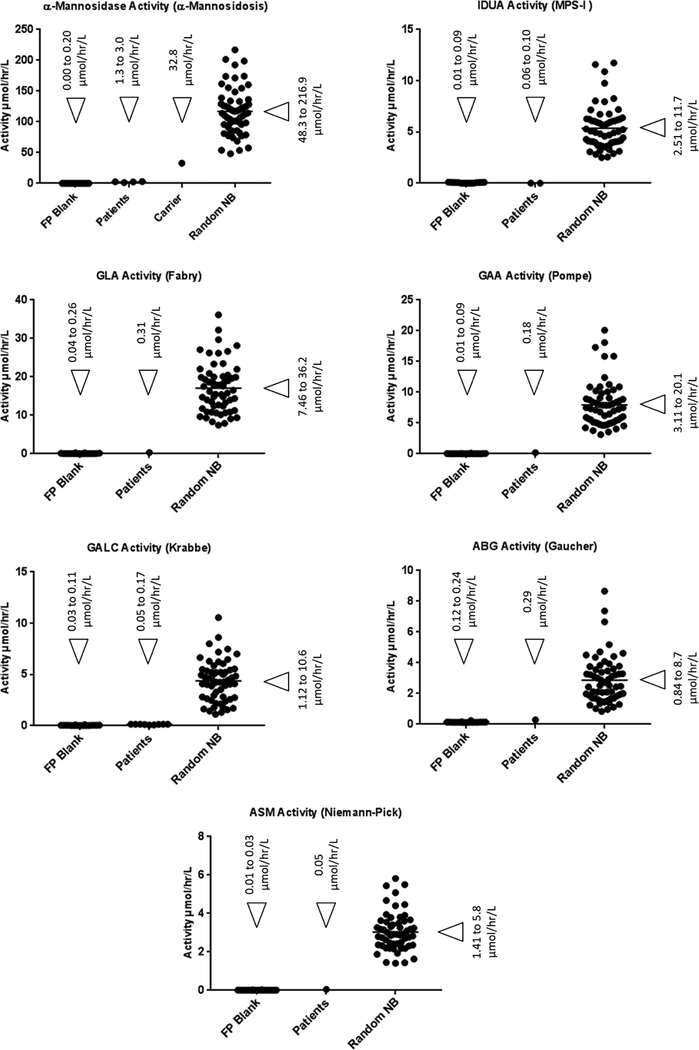
One of the key benefits of using MS/MS based assays is the multiplexability, the ability to assay for multiple LSDs in a single assay. Therefore, we combined the new α-mannosidosis and fucosidosis assay with our previously standardized 6plex assay (Fabry, Pompe, MPS I, Gaucher, Niemann-Pick and Krabbe diseases) and 5plex assay (MPS-II, MPS-IIIB, MPS-IVA, MPS-VI and MPS-VII) respectively.9–11 The 6plex assay was previously standardized to be performed with sodium succinate buffer, pH 4.7, whereas, the α-mannosidosis assay was originally standardized in sodium acetate buffer, pH 4.0. It was previously reported that above pH 4.5 the background α-mannosidase activity in the patient samples increases rapidly, which could result in false negative diagnosis.17 Therefore, adjustment of the pH for both the assays was the only way forward to combine the α-mannosidosis assay in the 6plex assay. Based on our pH study, we found that in sodium succinate buffer up to pH 4.25, the background α-mannosidase activity in α-mannosidosis patient samples is virtually zero, and it increases as the pH is raised over 4.5, due to the activity of an iso-enzyme (Figure 2). Consequently, pH 4.25, sodium succinate buffer, was chosen for the multiplex assay. The 6plex assay was subsequently performed at pH 4.25, sodium succinate buffer, to confirm that none of the individual assay in the multiplex was adversely affected by the small drop in pH. DBS from 60 random newborns and patients were screened with the new 7plex assay α-mannosidosis + 6plex). The results are given in the Figure-5 (see the Supplementary Information Table-S3 for all numerical values). For α-mannosidosis, Fabry, Pompe, MPS I, Gaucher, Niemann-Pick and Krabbe diseases, all the random newborns are clearly separated from the patients (Figure-5). The ratio of the average healthy baby activity to that of affected patient activity is 116 for α-mannosidosis, over 10,000-fold for MPS-I, 68 for Fabry, 18 for Gaucher, 50 for Pompe, 63 for Krabbe and 70-fold for Niemann-Pick A/B.
Figure-5.
Results for the new 7plex assay (α-mannosidosis + 6plex). The relevant enzyme activity in blank filter paper punch (FP Blank) and DBS samples from patients already diagnosed with the disorder and healthy random newborns
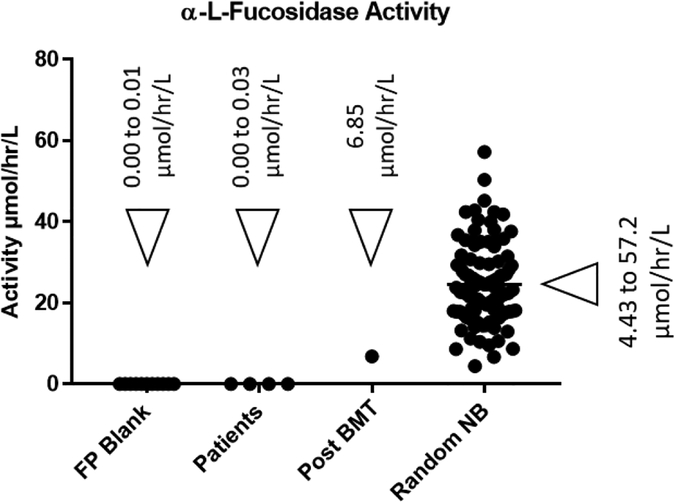
We also multiplexed the fucosidosis assay by combining it with the 5plex assay that is used to assay for MPS-II, MPS-IIIB, MPS-IVA, MPS-VI and MPS-VII.10 Our 5plex assay was originally designed to be performed at pH 5.0, and since the newly developed fucosidosis assay is also done at the same pH, there was no need for any further pH optimization. DBS from 104 random newborns were tested with the new 6plex assay (fucosidosis + 5plex assay). The results indicate that the fucosidosis patients were clearly differentiated from the random newborns and post-bone marrow transplant patient (Figure 6), (see the Supplementary Information Table-S4 for the numerical data). The ratio of average healthy newborns α-L-fucosidase activity to that of fucosidosis patients was found to be over 3,000-fold.
Figure-6.
α-L-Fucosidase activity, as measured by the 6plex assay (fucosidosis + 5plex assay), in blank filter paper punches (FP Blank) and DBS samples from patients already diagnosed with Fucosidosis, patients treated with bone marrow transplant (Post BMT) and healthy random newborns
Conclusions
The MS/MS assays that we reported herein for α-mannosidosis and fucosidosis diagnosis work well on their own or when multiplex with a panel of additional lysosomal enzymes (6plex + α-mannosidosis and 5plex + fucosidosis). Upcoming work will show that these enzymatic assays can be further multiplex with additional enzyme assays and with biomarker assays. The assays well differentiate affected patients from healthy controls using DBS. The designed aglycones of the new substrates ionize well in the mass spectrometry and provide more than adequate assay signal to support robust NBS.
Supplementary Material
Acknowledgments
This study was supported by the funding from the National Institutes of Health (R01 DK067859).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference
- 1.Harmatz P; Cattaneo F; Ardigò D; Geraci S; Hennermann JB; Guffon N; Lund A; Hendriksz CJ; Borgwardt L, Enzyme replacement therapy with velmanase alfa (human recombinant alpha-mannosidase): Novel global treatment response model and outcomes in patients with alpha-mannosidosis. Mol Genet Metab 2018, 124 (2), 152–160. [DOI] [PubMed] [Google Scholar]
- 2.Broomfield AA; Chakrapani A; Wraith JE, The effects of early and late bone marrow transplantation in siblings with alpha-mannosidosis. Is early haematopoietic cell transplantation the preferred treatment option? J Inherit Metab Dis 2010, 33 Suppl 3, S123–7. [DOI] [PubMed] [Google Scholar]
- 3.Wall DA; Grange DK; Goulding P; Daines M; Luisiri A; Kotagal S, Bone marrow transplantation for the treatment of alpha-mannosidosis. J Pediatr 1998, 133 (2), 282–5. [DOI] [PubMed] [Google Scholar]
- 4.Miano M; Lanino E; Gatti R; Morreale G; Fondelli P; Celle ME; Stroppiano M; Crescenzi F; Dini G, Four year follow-up of a case of fucosidosis treated with unrelated donor bone marrow transplantation. Bone Marrow Transplant 2001, 27 (7), 747–51. [DOI] [PubMed] [Google Scholar]
- 5.Vellodi A; Cragg H; Winchester B; Young E; Young J; Downie CJ; Hoare RD; Stocks R; Banerjee GK, Allogeneic bone marrow transplantation for fucosidosis. Bone Marrow Transplant 1995, 15 (1), 153–8. [PubMed] [Google Scholar]
- 6.Willems PJ; Gatti R; Darby JK; Romeo G; Durand P; Dumon JE; O’Brien JS, Fucosidosis revisited: a review of 77 patients. Am J Med Genet 1991, 38 (1), 111–31. [DOI] [PubMed] [Google Scholar]
- 7.Bharati A; Higgins C; Ellis I; Wraith J, Fucosidosis: a therapeutic challenge. Pediatr Dermatol 2007, 24 (4), 442–3. [DOI] [PubMed] [Google Scholar]
- 8.Borgwardt L; Lund AM; Dali CI, Alpha-mannosidosis -a review of genetic, clinical findings and options of treatment. Pediatr Endocrinol Rev 2014, 12 Suppl 1, 185–91. [PubMed] [Google Scholar]
- 9.Kumar AB; Masi S; Ghomashchi F; Chennamaneni NK; Ito M; Scott CR; Turecek F; Gelb MH; Spacil Z, Tandem Mass Spectrometry Has a Larger Analytical Range than Fluorescence Assays of Lysosomal Enzymes: Application to Newborn Screening and Diagnosis of Mucopolysaccharidoses Types II, IVA, and VI. Clin Chem 2015, 61 (11), 1363–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu Y; Yi F; Kumar AB; Kumar Chennamaneni N; Hong X; Scott CR; Gelb MH; Turecek F, Multiplex Tandem Mass Spectrometry Enzymatic Activity Assay for Newborn Screening of the Mucopolysaccharidoses and Type 2 Neuronal Ceroid Lipofuscinosis. Clin Chem 2017, 63 (6), 1118–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spacil Z; Tatipaka H; Barcenas M; Scott CR; Turecek F; Gelb MH, High-throughput assay of 9 lysosomal enzymes for newborn screening. Clin Chem 2013, 59 (3), 502–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hong X; Kumar AB; Ronald Scott C; Gelb MH, Multiplex tandem mass spectrometry assay for newborn screening of X-linked adrenoleukodystrophy, biotinidase deficiency, and galactosemia with flexibility to assay other enzyme assays and biomarkers. Mol Genet Metab 2018, 124 (2), 101–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chennamaneni NK; Kumar AB; Barcenas M; Spáčil Z; Scott CR; Tureče F; Gelb MH, Improved reagents for newborn screening of mucopolysaccharidosis types I, II, and VI by tandem mass spectrometry. Anal Chem 2014, 86 (9), 4508–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Spacil Z; Babu Kumar A; Liao HC; Auray-Blais C; Stark S; Suhr TR; Scott CR; Turecek F; Gelb MH, Sulfatide Analysis by Mass Spectrometry for Screening of Metachromatic Leukodystrophy in Dried Blood and Urine Samples. Clin Chem 2016, 62 (1), 279–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tebani A; Abily-Donval L; Afonso C; Marret S; Bekri S, Clinical Metabolomics: The New Metabolic Window for Inborn Errors of Metabolism Investigations in the Post-Genomic Era. Int J Mol Sci 2016, 17 (7) 1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Elliott S; Buroker N; Cournoyer JJ; Potier AM; Trometer JD; Elbin C; Schermer MJ; Kantola J; Boyce A; Turecek F, Pilot study of newborn screening for six lysosomal storage diseases using Tandem Mass Spectrometry. Molecular genetics and metabolism 2016, 118 (4), 304–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Prence EM; Natowicz MR, Diagnosis of alpha-mannosidosis by measuring alpha-mannosidase in plasma. Clin Chem 1992, 38 (4), 501–3. [PubMed] [Google Scholar]
- 18.Verma J; Thomas DC; Kasper DC; Sharma S; Puri RD; Bijarnia-Mahay S; Mistry PK; Verma IC, Inherited Metabolic Disorders: Efficacy of Enzyme Assays on Dried Blood Spots for the Diagnosis of Lysosomal Storage Disorders. JIMD Rep 2017, 31, 15–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Spáčil Z; Hui R; Gelb MH; Tureče F, Protonation sites and dissociation mechanisms of t-butylcarbamates in tandem mass spectrometric assays for newborn screening. J Mass Spectrom 2011, 46 (10), 1089–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wood S, A sensitive fluorometric assay for alpha-KL-fucosidase. Clin Chim Acta 1975, 58 (3), 251–6. [DOI] [PubMed] [Google Scholar]
- 21.Attia MS; Othman AM; Aboaly MM; Abdel-Mottaleb MS, Novel spectrofluorimetric method for measuring the activity of the enzyme alpha-L-fucosidase using the nano composite optical sensor samarium(III)-doxycycline complex doped in sol-gel matrix. Anal Chem 2010, 82 (14), 6230–6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.