Structured Abstract
Background and Purpose
The PILLAR study (ExtracorPoreal FILtration of Subarachnoid Hemorrhage via SpinaL CAtheteR) is a first-in-human trial of cerebrospinal fluid (CSF) filtration in aneurysmal subarachnoid hemorrhage (aSAH). The study evaluates the safety and feasibility of a novel filtration system to rapidly remove blood and blood breakdown products from CSF following securement of a ruptured aneurysm.
Methods
aSAH patients had a dual-lumen lumbar, intrathecal catheter placed following aneurysm securement, and received up to 24 hours of CSF filtration (Neurapheresis therapy). The catheter aspirated blood-contaminated CSF from the lumbar cistern and returned filtered CSF to the thoracic subarachnoid space (SAS). Neuro checks were performed q2hrs and CSF samples were collected for cell counts, total protein, and gram stain. CT scans were acquired at baseline and post-filtration. Clinical follow-up occurred at 2 weeks and 30 days.
Results
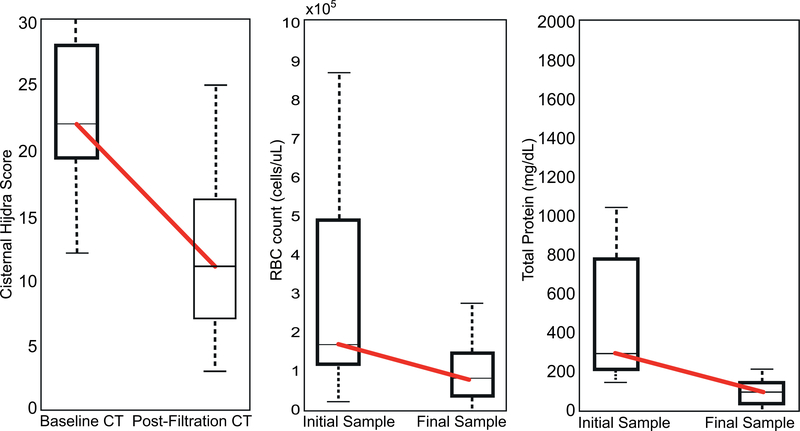
Thirteen patients had a catheter placed (mean time 24:13 hours after ictus). The system processed 632.0 mL [180.6–1447.6 ml] CSF in 15:07 hours [5:32–24:00 hours] of filtration. The mean initial CSF red blood cell count, 2.78×105 cells/μL, reduced to 1.17×105 cells/μL after filtration (52.9% reduction), and total protein reduced 71%. Independent analysis of baseline and post-filtration CTs found notable cisternal blood decrease, with 46.5% mean Hijdra Score reduction. Three mild, anticipated adverse events (AEs) were reported.
Conclusions
The initial safety and feasibility of Neurapheresis therapy in aSAH demonstrated the potential to safely filter CSF and remove blood and blood byproducts. Future studies are warranted.
Clinical Trial Registration
URL: http://www.clinicaltrials.gov. Unique identifier:
Keywords: subarachnoid hemorrhage, cerebrovascular, catheter, Neurapheresis, red blood cell, Clinical Studies, Cerebral Aneurysm, Intracranial Hemorrhage
Introduction
Aneurysmal subarachnoid hemorrhage (aSAH) is a catastrophic neurological condition occurring after the rupture of a cerebral aneurysm. There are roughly 30,000 cases per year in the US, with an estimated worldwide incidence between 4.2 and 22.7 people per 100,000 depending on the region1. Following securement, patients typically stay in the hospital for approximately 14 days to monitor for secondary complications such as hydrocephalus and delayed cerebral ischemia (DCI).
Red blood cells (RBCs) in the SAS break down over several days releasing hemoglobin and other cytotoxic, pro-inflammatory cytokines considered to be instigators of downstream events resulting in delayed ischemia2,3. Rapid and early removal of RBCs and blood byproducts (e.g. hemoglobin) from the cerebrospinal fluid (CSF) may improve poor outcomes associated with secondary brain injury. A number of studies previously evaluated removal of blood via external ventricular drain (EVD) or lumbar drain (LD) in this population4–8. These studies demonstrated potential benefit of early blood removal, particularly via LD, though drainage is limited by the natural production and circulation of CSF9,10. A filtration system that returns “clean” CSF to the patient would allow for more rapid and controlled blood removal.
The PILLAR study (ExtracorPoreal FILtration of Subarachnoid Hemorrhage via SpinaL CAtheteR) is a recently completed first-in-human trial designed to evaluate the safety, tolerability, and feasibility of a new therapy, Neurapheresis™ CSF filtration, that targets blood and blood byproducts for rapid removal from CSF following securement of a ruptured aneurysm. In this paper, we report the results of the PILLAR study.
Materials and Methods
Study Design and Patient Population
The data supporting these study findings are available from the corresponding author upon reasonable request. The PILLAR study was an industry sponsored prospective, single-arm, first-in-human feasibility trial investigating the safety of Neurapheresis CSF filtration at four centers in the United States. The study was FDA approved and IRB approved at all participating centers (NCT02872636). A total of 15 patients diagnosed with aSAH were enrolled in the study after informed consent was obtained from the patient or legally authorized representative. A full list of inclusion and exclusion criteria can be found in Supplemental Table I.
The PILLAR study was overseen by a Data Safety Monitoring Board (DSMB) consisting of an independent Neurologist, Interventional Neuroradiologist, and Medical Ethicist. All DSMB members reviewed adverse events (AE) and submitted a written report with study recommendations following DSMB meetings.
Study System
The Neurapheresis system (Figure 1) is composed of a dual lumen catheter placed under fluoroscopy using an over-the-wire technique, previously described11. The catheter is then connected to an extracorporeal filtration unit that controls the rate at which CSF is filtered, allowing waste products to be removed. Following confirmation of flow patency, the catheter is secured. Patients were then transported to the PACU or ICU prior to the initiation of the Neurapheresis procedure.
Figure 1:
Schematic of the Neurapheresis System.
Neurapheresis Protocol
The procedure time was limited per protocol to 24 hours of aggregate pump time (filtration time) or 36 hours of catheter-indwelling time. During filtration, the system pressures and flow rates were monitored continuously by study personnel. The system was typically maintained at a waste rate (concentrated blood and CSF removed from the patient) between 12–15 mL/hr during system operation.
Neuro checks were performed every 2 hrs. (±1 hr) during catheter indwelling and included Glasgow Coma Scale (GCS), World Federation of Neurosurgeons (WFNS), Hunt & Hess, and peripheral checks. CSF samples were collected from the lumbar cistern via the catheter q4 hrs. (±1 hr). CSF samples were sent to the hospital lab for cell counts, glucose, total protein, and gram stain. Prior to catheter removal a head CT was obtained.
Patients were followed at 2 weeks (±3 days) and 30 days (± 7 days) post-catheter removal. Data collected included disposition, ICU and hospital length of stay, mRS, Glasgow Outcome Scale (GOS), Barthel Index, all applicable images, and AEs.
Endpoint Analysis
The primary safety endpoint was based on an assessment of adverse events associated with placement and removal of the catheter and operation of the Neurapheresis system. The secondary safety endpoint captured any unanticipated adverse device effects (UADE) related to the system. Baseline and post-Neurapheresis CT images were scored by an independent neuroradiologist using the Hijdra scale12. A statistical analysis was not performed due to lack of controls and small enrollment number.
Results
Fifteen patients were consented for the study (Supplemental Table II). One subject was consented but catheter placement was not attempted, and one subject was consented and catheter placement was unsuccessful. The remaining thirteen subjects had a device placed, underwent Neurapheresis therapy and were followed for 30 days.
Mean catheter placement time was 24:13 hours after aneurysm rupture with the catheter remaining in situ for 30:23 hours. The aggregate Neurapheresis pump time was a mean of 15:07 hours (range 5:32–24:00 hours). One patient achieved the maximum 24 hours of pump time and two other patients achieved more than 22 of the allotted 24 hours. A mean of 632.0 mL (median 530.0 mL, range 180.6–1447.6) of CSF was filtered, with 439.0 mL of CSF returned to the patient and mean 193.0 mL (range 75.8–328.1 mL) of concentrated waste removed. This reflects a waste rate between 12–15 ml/hr and a pump flow rate between 30–96 ml/hr.
In 92.3% of patients (12/13), the neurological (GCS, WFNS, Hunt & Hess) and peripheral checks remained stable or improved during Neurapheresis therapy. In one patient, there was a decline in GCS score secondary to the development of focal cerebral edema after clipping of an MCA aneurysm (no AE per DSMB).
In 53.8% of patients, total protein levels were ≤100 mg/dL at the end of therapy (normal range 15–60 mg/dL). The mean initial concentration of RBCs in the CSF was 2.78×105 cells/μL, which decreased to 1.17×105 cells/μL after filtration (Figure 2). CSF glucose levels remained normal throughout the Neurapheresis procedure without evidence of hypoglycorrhachia.
Figure 2:
Demonstrated reduction of blood in the CSF after Neurapheresis therapy based on imaging analysis (A) and CSF sample analysis for red blood cell counts (B) and total protein (C). Box plots indicate the median, 25th and 75th percentile.
Independent analysis of baseline and post-Neurapheresis CT scans revealed a decrease in blood from a mean initial Hijdra score of 22 (out of 30) to 12 (46.5% mean reduction).
At 30 days, one patient was in an extended care facility and 3 patients were in rehabilitation. GOS score ranged from 3–5 and 61.5% (8/13) of patients had a GOS score of 5 at 30-days. mRS score ranged from 0 to 4. 69.2% (9/13) of patients had a mRS between 0–2 and a Barthel Index greater than 90 at 30 days. The mean duration of ICU stay was 12 days (range 6–18 days).
Three AEs were reported; one each in three patients. All were single events that were minor and considered anticipated. Back and leg pain was noted at the two-week follow-up in one patient but was resolved by the 30-day follow-up. One patient reported transient vomiting with concurrent headache during the timeframe of Neurapheresis therapy. A head CT was obtained and was unremarkable. Another patient reported transient vomiting shortly after CSF sampling. No device related serious AEs or UADEs occurred. However, in one patient, Neurapheresis was terminated prematurely due to neurological decline, focal cerebral edema and midline shift, determined to be a related to the surgical clipping procedure.
Discussion
There is a significant unmet need to reduce complications and improve outcomes for patients with aSAH. Early removal of blood and blood byproducts from the CSF via LD has been shown to reduce the incidence of vasospasm, hydrocephalus and shunting, and result in shorter hospital course6. However, removal of blood via LD is limited by CSF production rate. By returning filtered CSF, the closed loop Neurapheresis system is capable of processing CSF and removing blood volume at a faster rate.
This is the first study evaluating the investigational Neurapheresis system in aSAH patients. The safety and feasibility of using an extracorporeal filtration system and catheter to filter hemorrhagic CSF post-SAH treatment and reintroduce the filtered CSF via the same catheter was demonstrated. The primary safety endpoint for the study was the assessment of AEs associated with catheter placement and operation of the system. No AEs were associated with catheter placement and only three minor, non-serious AEs known to occur in aSAH patients were reported.
Several factors impacted pump time during this study, as a result only one subject received the complete 24 hour allotted time for study therapy. The pump was stopped for any intervening SOC or protocol-required activity, including transfer from the OR/IR to the Neuro ICU, transport to imaging and during sampling, which impacted the total aggregate pump time. By study design, there was a catheter indwelling time limit of 36 hours which also impeded the ability to reach the goal of 24 hours of pump time.
A reduction of blood in the CSF was evident after Neurapheresis therapy, from both the cranial and spinal subarachnoid space, despite shorter than anticipated pump time and variable processed volume, initial bleed size, bleed location, and time from ictus to catheter placement. Cranially, the reduction in Hijdra score is initial evidence that the Neurapheresis system may be mobilizing blood in the cisterns and fissures of the brain. In the 3 patients with greater than 22 hours of filtration, complete RBC clearance was not achieved (mean RBC reduction: 79.7%). To that end, the PILLAR-XT study (NCT03607825) will employ longer pump times and will further characterize the treatment curve of Neurapheresis therapy.
Interpretation of the study results is limited by several factors. First, the sample size is small and the pump time for each patient was quite variable. Thus, while outcome data is presented and appeared promising, conclusions about potential benefits cannot be drawn. However, it is the first study evaluating this technology and the purpose was safety and feasibility. Future studies will continue to establish safety of the Neurapheresis system and explore longer pump time and the ability of the system to more completely clear blood from the cranial and spinal subarachnoid space in aSAH.
Conclusions
The initial safety, tolerability, and feasibility of Neurapheresis therapy in aSAH was demonstrated in this 15-patient study along with the capability to rapidly remove blood from the CSF. The therapy is promising, and future studies are warranted.
Supplementary Material
Acknowledgments
The authors would like to thank Dr. Blake Johnson for review and Hijdra score of all CT scans.
Sources of Funding: Minnetronix Neuro, Inc.
Footnotes
Conflict(s)-of-Interest/Disclosure(s):
The PILLAR clinical trial is supported by Minnetronix Inc and all authors received support for approved study related costs. Dr. Blackburn has no relevant conflicts; his effort is supported through NIH K23NS106054. Dr. Grande, Dr. Hauck, and Dr. Provencio have no conflicts. Dr. Swisher receives speaker’s honorarium from USB and Eisai Pharmaceuticals. Dr. Jagadeesan is a consultant for Microvention and Medtronic.
References
- 1.de Rooij NK, Linn FHH, van der Plas JA, Algra A, Rinkel GJE. Incidence of subarachnoid haemorrhage: a systematic review with emphasis on region, age, gender and time trends. J. Neurol. Neurosurg. Psychiatry 2007;78:1365–1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blackburn SL, Kumar PT, McBride D, Zeineddine HA, Leclerc J, Choi HA, et al. Unique Contribution of Haptoglobin and Haptoglobin Genotype in Aneurysmal Subarachnoid Hemorrhage. Front. Physiol [Internet]. 2018. [cited 2018 Sep 5];9 Available from: https://www.frontiersin.org/articles/10.3389/fphys.2018.00592/full [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bulters D, Gaastra B, Zolnourian A, Alexander S, Ren D, Blackburn SL, et al. Haemoglobin scavenging in intracranial bleeding: biology and clinical implications. Nat. Rev. Neurol 2018;14(7):416–432 [DOI] [PubMed] [Google Scholar]
- 4.Al-Tamimi YZ, Bhargava D, Feltbower RG, Hall G, Goddard AJP, Quinn AC, et al. Lumbar drainage of cerebrospinal fluid after aneurysmal subarachnoid hemorrhage: a prospective, randomized, controlled trial (LUMAS). Stroke J. Cereb. Circ 2012;43:677–682. [DOI] [PubMed] [Google Scholar]
- 5.Borkar SA, Singh M, Kale SS, Suri A, Chandra PS, Kumar R, et al. Spinal Cerebrospinal Fluid Drainage for prevention of Vasospasm in Aneurysmal Subarachnoid Hemorrhage: A Prospective, Randomized controlled study. Asian J. Neurosurg 2018;13:238–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Klimo P Jr, Kestle JRW, MacDonald JD, Schmidt RH. Marked reduction of cerebral vasospasm with lumbar drainage of cerebrospinal fluid after subarachnoid hemorrhage. J. Neurosurg. 2004;100:215–224. [DOI] [PubMed] [Google Scholar]
- 7.Kwon OY, Kim Y-J, Kim YJ, Cho CS, Lee SK, Cho MK. The Utility and Benefits of External Lumbar CSF Drainage after Endovascular Coiling on Aneurysmal Subarachnoid Hemorrhage. J. Korean Neurosurg. Soc 2008;43:281–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roelz R, Coenen VA, Scheiwe C, Niesen W-D, Egger K, Csok I, et al. Stereotactic Catheter Ventriculocisternostomy for Clearance of Subarachnoid Hemorrhage: A Matched Cohort Study. Stroke. 2017;48:2704–2709. [DOI] [PubMed] [Google Scholar]
- 9.Alcala-Cerra G, Paternina-Caicedo A, Diaz-Becerra C, Moscote-Salazar LR, Gutierrez-Paternina JJ, Nino-Hernandez LM. External lumbar cerebrospinal fluid drainage in patients with aneurysmal subarachnoid haemorrhage: A systematic review and meta-analysis of controlled trials. Neurologia. 2016;31:431–444. [DOI] [PubMed] [Google Scholar]
- 10.Qian C, Yu X, Chen J, Gu C, Wang L, Chen G, et al. Effect of the drainage of cerebrospinal fluid in patients with aneurismal subarachnoid hemorrhage: A meta-analysis. Medicine (Baltimore). 2016;95:e5140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blackburn SL, Swisher CB, Grande AW, Rubi A, Verbick LZ, McCabe A, et al. Novel Dual Lumen Catheter and Filtration Device for Removal of Subarachnoid hemorrhage: First Case Report. Oper. Neurosurg 2019;16(5):E148–E153. [DOI] [PubMed] [Google Scholar]
- 12.Hijdra A, Brouwers P, Vermeulen M, Van Gijn J. Grading the amount of blood on computed tomograms after subarachnoid hemorrhage. Stroke. 1990;21:1156–1161. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.