Abstract
Background:
Patient and caregiver perspectives on amyloid positron-emission tomography (PET) use are largely unexplored, particularly as compared to clinician views.
Methods:
We surveyed clinicians, patients, caregivers and dementia advocates on topics relating to an evidence-based guideline on amyloid PET use. Topic importance was rated on a 9-point scale. Patient stakeholder and clinician views were compared using the Mann-Whitney U Test.
Results:
Patient representatives (n=107) rated all survey topics as equal to or more important than clinicians (n=114) except one item discussing potential harms of false positive diagnoses. Differences between patient representative and clinician populations were greatest when comparing the competing values of false positive and negative diagnoses and the value of testing asymptomatic individuals.
Conclusions and Relevance:
Patients and caregivers emphasized the importance of having a dementia diagnosis and placed more value on testing and outcomes for asymptomatic populations than clinicians. This underscores the importance of research investigating the effect of amyloid PET results on asymptomatic individuals and the need for amyloid PET ordering and disclosure standards.
Keywords: Amyloid PET imaging, dementia [MeSH term], mild cognitive impairment, preclinical Alzheimer disease, guidelines as topic [MeSH term], patient participation [MeSH term]
INTRODUCTION
In 2011, the National Institute on Aging-Alzheimer’s Association workgroup published recommendations for the diagnosis of dementia1 and mild cognitive impairment (MCI)2 secondary to Alzheimer disease (AD). These frameworks included clinical diagnostic criteria but emphasized the growing role of biomarkers. The 2018 update proposed a research framework focusing on AD as a biological entity with staging based on biomarkers and cognitive symptoms.3 The increasing emphasis on biomarkers highlights the need to understand patient and caregiver preferences concerning biomarker testing, clinical (syndromic) diagnosis, and pathologic/biologic (etiologic) diagnosis. Only 45% of individuals with symptomatic AD and 27% of individuals with other dementing illnesses receive an etiologic diagnosis.4 However, 91% of cognitively normal and 85% of symptomatic individuals favor dementia diagnosis disclosure.5 Public views on dementia screening and diagnostic testing are more variable.6–9
Understanding patient and caregiver preferences is relevant to clinical care, research, and evidence-based guidelines. Seeking and incorporating the views of patients is one standard for high quality and trustworthy guidelines.10,11 Existing consensus-based appropriate use criteria12 and policy statements13,14 provide important insight into amyloid PET use but do not meet Institute of Medicine standards for evidence-based guidelines.11 In addition, these documents were developed by expert panels without clear evidence of patient engagement.
Based on the rapid expansion of evidence since the 2013 appropriate use report,12 the tension between the availability of Food and Drug Administration-approved amyloid PET tracers vs lack of coverage by the Centers for Medicare and Medicaid Services (CMS),15 and a request from American Academy of Neurology (AAN) membership, the AAN guideline subcommittee initiated an amyloid PET guideline in 2016. As one strategy for identifying patient preferences regarding amyloid PET use, guideline developers surveyed individuals with cognitive impairment, families of individuals with cognitive impairment, dementia advocates, and clinicians regarding draft amyloid PET guideline questions to investigate the importance of different guideline questions and identify differences between patient and clinician views.
METHODS
Guideline question development
As part of a larger research project (K08HS24159), two guideline panels drafted amyloid PET guideline questions16 using the Population-Intervention-Comparator-Outcome-Time (PICOT) framework.17 The intervention was amyloid PET by definition. Both guideline panels included physicians; one group also included an individual with MCI and their spouse, the spouse of an individual with dementia, and an advocate from the Alzheimer’s Association.
Populations proposed as relevant to the guideline included individuals over 65 years-old, cognitively normal individuals at increased risk of developing symptomatic AD based on family history or APOEe4 allele carrier status, and those with subjective cognitive complaints, MCI, or typical or atypical dementia. The panels recommended including these populations based on patient preferences and clinical experience, accepting that some of these populations are outside those described for appropriate use.12 Examining the evidence does not imply that recommendations will support use in these populations.
Proposed outcomes relating to amyloid PET use included 1) accurate diagnosis of AD in an individual with dementia; 2) accurate assessment of future risk of dementia in asymptomatic individuals (with/without AD risk factors, e.g. family history, APOEe4 allele carrier status) and individuals with MCI; 3) reliably excluding AD in symptomatic individuals 4) the ability to stop looking for other explanations for an individual’s dementia; and, 5) prediction of rates of decline in individuals with dementia. The guideline panels also suggested that some individuals receiving amyloid PET will query the degree of amyloid burden identified on scans. One panel suggested that it is important to know not only if amyloid PET findings predict future development of AD dementia in asymptomatic patients and those with MCI, but also whether dementia will develop over 1, 3, 5, 10 or 15 years in amyloid positive individuals.
The guideline panels noted that having an etiologic dementia diagnosis (e.g., “dementia due to AD”) is important to prepare for the personal consequences of dementia (e.g. for advance care and financial planning), to understand if treatment might be helpful, to determine disability benefit eligibility, and to improve quality of life.
The panels identified five potential harms related to testing: increased healthcare system costs, patient/family costs, inappropriate test ordering, allergic reactions and radiation exposure. The guideline panels identified ten potential harms related to amyloid PET outcomes and their disclosure including false positive and negative diagnoses, inaccurate prognosis, depression and loss of opportunities.
Study design
The study consisted of a survey investigating the importance placed by patients, family members, dementia advocates and clinicians on the proposed populations, outcomes and harms for the amyloid PET guideline. The survey for patients, family members and advocates (“patient stakeholders”) and the survey for clinicians differed only in introduction and demographic questions (see Supplemental Digital Content 1). The University of Florida institutional review board provided approval (IRB201501210). Participants reviewed an informed consent document and indicated agreement to participate prior to accessing survey questions. Responses were anonymous.
Survey Design
The AAN’s guideline methodology18 uses a modified Grading of Recommendations Assessment, Development, and Evaluation (GRADE) approach for guideline development. GRADE recommends rating outcome importance using a 9-point ordinal scale prior to the guideline systematic review. Ratings of 1–3 identify outcomes of “limited importance,” 4–6 indicates “important” outcomes, and 7–9 indicates outcomes of “critical importance”.17 The proposed populations, outcomes, and harms for the guideline were the basis for survey questions. Participants rated items using the GRADE scale. A single multiple-choice question asked respondents to compare which is worse: a false positive or false negative diagnosis of AD dementia. Respondents were encouraged throughout the survey to use the full range of response options.
Survey item wording was revised iteratively in response to suggestions from co-investigators and AAN survey staff. Investigators pilot tested the final online-only Qualtrics survey prior to production. Apart from questions determining eligibility and consent, survey responses were optional.
Participants
All guideline panelists (physicians and patient representatives) completed the survey. Anyone providing feedback during the online guideline protocol public comment (March 10–April 10, 2017) was invited to participate. Additional clinicians were recruited through the AAN. AAN members were eligible if they were a neurologist or advanced practice provider in current clinical practice in the U.S. with primary or secondary specialization in general neurology or behavioral neurology. Trainees, business managers, members of guideline-associated AAN committees/boards, and members who received a different AAN survey in the last 6 months were excluded. Based on prior survey response rates, the AAN sent invitations to 910 eligible members, anticipating 100 responses. The initial survey link was emailed on 3/28/2017 with reminders sent 4/6/2017 and 4/12/2017.
To recruit patient representatives, flyers with survey information were distributed through the Alzheimer’s Association North Dakota chapter mailings and in Meeting of the Minds (March 2017) conference bags. Recruitment through the University of Maryland PATIENTS Program, a program connecting stakeholders from underserved and minority populations to research, occurred via a newsletter blurb and link. Patient stakeholders were also recruited through Opinions for Good (Op4G, http://op4g.com/), an online data collection company that donates to non-profit organizations when members participate in survey research. Op4G disseminated a survey link to members with cognitive impairment and those identified as caregivers for persons with cognitive impairment. This survey included one additional screening question asking, “Do you or does a close friend or family member have one of these conditions?” If the respondent clicked “mild cognitive impairment or dementia (memory and thinking changes),” they were directed to the survey. If they selected another listed neurologic disorder, they were ineligible.
Analysis
Results were analyzed using SPSS (Version 24.0, Armonk, NY:IBM Corp). Demographic information was analyzed descriptively using means, standard deviation (SD), and percentages. Importance ratings were assessed using medians, interquartile range (IQR), and score ranges. Mann-Whitney U Tests (Wilcoxon Rank-Sum) compared importance ratings between patient stakeholders and clinicians. A chi-square test assessed the multiple-choice question. Using Noether’s method for nonparametric sample size estimates,19 approximately 80 survey responses in each group maximized the ability to detect differences between groups with 80% probability (alpha 0.05). Study recruitment targeted 100 surveys for each group, assuming 20% incomplete surveys. Secondary subgroup analyses compared the responses of (1) patient stakeholders vs clinicians on the guideline panel, public comment respondents, and general survey populations, (2) patients vs caregivers, and (3) specialist physicians vs other neurologists. For the secondary analyses, p<0.001 was considered significant to adjust for multiple comparisons.
RESULTS
Participant characteristics
Two hundred twenty-one individuals (107 patient stakeholders, 114 clinicians) responded to survey questions beyond demographics (Table 1). Patient stakeholders included individuals with MCI or dementia (29, 27%), caregivers (78, 72%) and advocates (2, 2%). Clinician respondents identified as behavioral specialists (76, 67%), other neurologists (28, 25%), or “other” (10, 9%; e.g. advanced practice provider). Responses included 17 guideline panelists (4 patient stakeholders, 13 clinicians), 18 public comment respondents (2 patient stakeholders, 16 clinicians), 101 patient stakeholders from the broader survey, and 85 clinicians through the AAN survey (85/910, 9.3%). Subgroup demographics are presented in Table e-1 in Supplemental Digital Content 2.
Table 1.
Cohort Demographics
| Demographic Category | Clinicians (n=114) |
Patient Stakeholders (n=107) |
|---|---|---|
| Gender | ||
| Male | 74 (65%) | 47 (44%) |
| Female | 39 (34%) | 60 (56%) |
| Other | 1 (1%) | 0 (0%) |
| Age | ||
| <30 | 2 (2%) | 5 (5%) |
| 30–39 | 28 (25%) | 32 (30%) |
| 40–49 | 35 (31%) | 23 (22%) |
| 50–59 | 34 (30%) | 26 (24%) |
| ≥60 | 13 (12%) | 21 (20%) |
| Not provided | 2 (2%) | 0 (0%) |
| Race | ||
| Non-Hispanic White | 80 (71%) | 87 (81%) |
| Black/African-American | 5 (4%) | 11 (10%) |
| Hispanic | 3 (3%) | 6 (6%) |
| Asian | 21 (19%) | 2 (2%) |
| Other or not provided | 5 (5%) | 1 (1%) |
Patient representatives rated all survey items as equal to or more important than clinicians except for the question discussing potential harms of a false positive diagnosis. Even when median importance was identical, between-group differences were sometimes significant owing to the use of a statistical method that accounts for spread (which was often greater for clinicians than patient stakeholders).
Guideline Populations
Clinicians and patient representatives agreed that symptomatic patients who do not meet standard diagnostic criteria represent an important population warranting additional testing (Table 2, question 6). Patient stakeholders rated inclusion of all other proposed guideline populations as more important than clinicians. The most prominent differences in median scores occurred when considering asymptomatic populations with or without subjective cognitive complaints (Table 2, questions 1–3).
Table 2.
Importance of Different Populations when Considering Amyloid PET Use
| Survey question: How important would it be to test for Alzheimer disease in: | Clinicians (n=103) |
Patients Stakeholders (n=102) |
P-value |
|---|---|---|---|
| 1: All individuals over 65 years-old, regardless of whether or not they complain of memory problems? | 2 (IQR 1–5) Range 1–9 |
7 (IQR 6–9) Range 1–9 |
<0.001 |
| 2: People at increased risk for Alzheimer disease (e.g. due to family history) who do not complain of any memory or thinking problems? | 4 (IQR 2–7) Range 1–9 |
8 (IQR 7–9) Range 2–9 |
<0.001 |
| 3: People who complain of trouble with memory and thinking but who do not have any problems found on memory testing? | 5 (IQR 3–7) Range 1–9 |
8 (IQR 7–9) Range 2–9 |
<0.001 |
| 4: People with mild difficulties in memory (mild cognitive impairment), which puts them at a higher risk of developing dementia (more severe problems with memory and thinking)? | 7 (IQR 6–8) Range 1–9 |
8 (IQR 7–9)* Range 4–9 |
0.003 |
| 5. People with trouble with memory and thinking impacting activities in daily life (dementia) that fits a typical Alzheimer disease dementia pattern? | 7 (IQR 5–8) Range 1–9 |
8 (IQR 7–9) Range 4–9 |
<0.001 |
| 6: People with trouble with memory and thinking impacting activities in daily life (dementia) that does not fit a typical dementia pattern, where doctors are having difficulty giving a clear diagnosis? | 8 (IQR 7–9) Range 1–9 |
8 (IQR 7–9) Range 1–9 |
0.937 |
Responses: Limited importance 1–3, important 4–6, critical importance 7–9. Data presented as median (interquartile range, IQR).
n=101 for this question
Outcomes relating to diagnosis and diagnostic benefits
Both clinician and patient stakeholders rated the outcomes of future development of dementia in individuals with MCI and accurate diagnosis in individuals with dementia as critically important (Table 3). Patient stakeholders felt that critically important outcomes included prediction of future development of dementia in asymptomatic individuals and identifying the degree of amyloid burden (regardless of symptoms or diagnosis).These outcomes were rated as “important” by clinicians (Table 3, questions 1 & 7).
Table 3.
Importance of Outcomes Relating to Diagnosis
| Survey Question | Clinicians (n=106) |
Patient Stakeholders (n=106) |
P-value |
|---|---|---|---|
| 1: In a person with NO memory/thinking problems, how important is it to predict whether someone will develop memory and thinking problems due to Alzheimer disease in the future? | 5 (IQR 2–7) Range 1–9 |
7 (IQR 6–9) Range 1–9 |
<0.001 |
| 2: In a person with mild cognitive impairment, how important is it to predict whether that person will develop more severe problems with memory and thinking (dementia) due to Alzheimer disease in the future? | 8 (IQR 7–9) Range 1–9 |
8 (IQR 7–9) Range 3–9 |
0.015 |
| 3: In a person with dementia (memory and thinking problems affecting daily life), how important is it to get an accurate diagnosis of Alzheimer disease? | 8 (IQR 6–9) Range 2–9 |
9 (IQR 8–9) Range 3–9 |
<0.001 |
| 4: In a person with dementia (memory and thinking problems affecting daily life), how important is it to exclude a diagnosis of Alzheimer disease? | 8 (IQR 6–9) Range 1–9 |
8 (IQR 6–9) Range 1–9 |
0.821 |
| 5: In a person with memory and thinking problems (dementia), how important is it to have a certain diagnosis of Alzheimer disease so that patients, families, and physicians can stop looking for other explanations/causes? | 8 (IQR 7–9) Range 1–9 |
8 (IQR 7–9) Range 2–9 |
0.432 |
| 6: In a person with memory and thinking problems (dementia) due to Alzheimer disease, how important is it to predict how fast he or she will worsen? | 8 (IQR 6–9) Range 1–9 |
8 (IQR 7–9) Range 3–9 |
0.003 |
| 7: In people with Alzheimer disease, a protein (amyloid) clumps in the brain. How important is it to know how much protein (amyloid) a person has built up in the brain, irrespective of symptoms/diagnosis? | 5 (IQR 2–6) Range 1–9 |
9 (IQR 7–9) Range 3–9 |
<0.001 |
Responses: Limited importance 1–3, important 4–6, critical importance 7–9. Data presented as median (interquartile range, IQR).
If testing an asymptomatic individual, clinicians and patient stakeholders agreed that it would be critically important to know if an individual would develop dementia at 1 year (clinicians 8 [IQR 7–9] vs patients 9 [IQR 7–9], p=0.265) and 3 years (clinicians 7 [IQR 6–8] vs patients 8 [IQR 7–9], p=0.05). Clinician-assigned importance dropped as intervals increased, but patient stakeholders continued to place high value on future diagnosis (5 years: clinicians 7 [IQR 5–8] vs patients 7 [IQR 6–9], p=0.010; 10 years: clinicians 5 [IQR 3–7] vs patients 7 [IQR 5–8], p<0.001; 15 years: clinicians 4 [IQR 2–7] vs patients 7 [IQR 5–9], p<0.001). Reponses were similar when considering a person with MCI progressing to dementia. Clinicians and patient stakeholders agreed that it would be critically important to know if an individual would progress to dementia at 1 year (clinicians 8 [IQR 7–9] vs patients 9 [IQR 7–9], p=0.712) and 3 years (clinicians 7 [IQR 6–8] vs patients 8 [IQR 7–9], p=0.05). Patient representatives placed a higher value on future diagnosis at subsequent time points (5 years: clinicians 7 [IQR 5–8] vs patients 8 [IQR 6–9], p=0.031; 10 years: clinicians 6 [IQR 4–8] vs patients 7 [IQR 5–8], p<0.001; 15 years: clinicians 5 [IQR 2–7] vs patients 7 [IQR 5–9], p<0.001).
Clinicians and patient stakeholders rated benefits of a dementia diagnosis as critically important (preparing for personal consequences: clinicians 8 [IQR 7–9] vs patients 9 [IQR 8–9], p=0.008; identifying treatments: clinicians 8 [IQR 6–9] vs patients 9 [IQR 7–9], p<0.001; determining disability eligibility: clinicians 8 [IQR 6–9] vs patients 9 [IQR 7–9], p<0.001; improving quality of life: clinicians 8 [IQR 6–9] vs patients 8 [IQR 7–9], p=0.004).
Outcomes relating to testing and diagnostic harms
There were no differences between groups concerning the importance of healthcare system expense (clinicians 7 [IQR 5–8] vs patients 7 [IQR 5–9], p=0.38). Patient stakeholders rated other potentially adverse outcomes as more important than clinicians, including patient/family costs (clinicians 7 [IQR 5–8] vs patients 8 [IQR 7–9], p=0.011), inappropriate ordering (clinicians 7 [IQR 5–8] vs patients 8 [IQR 7–9], p=0.005), allergic reactions (clinicians 5 [IQR 4–7] vs patients 8 [IQR 5–9], p<0.001), and radiation exposure (clinicians 3 [IQR 2–5] vs patients 7 [IQR 5–8], p<0.001).
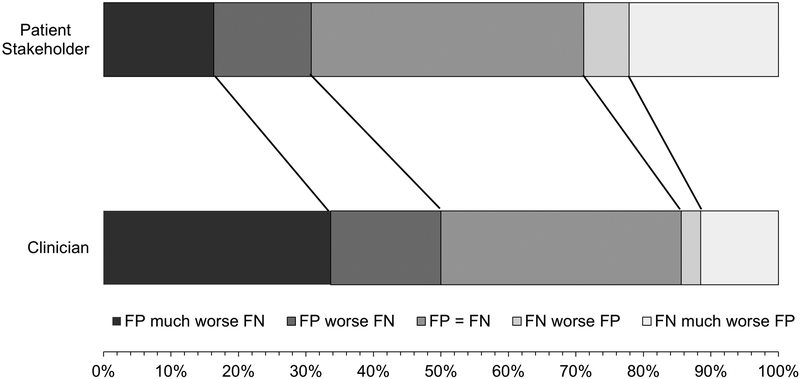
Clinicians and patient stakeholders generally rated harms related to amyloid PET results and their disclosure as critically important (Table 4). Clinicians expressed greater concern pertaining to harms associated with a false positive test: i.e., a test result suggesting that a person would develop symptomatic AD in the next 3 years, when they would/did not (Table 4, question 3). This was the only question where clinicians rated importance higher than patient stakeholders. For the single multiple-choice question asking respondents to identify which was worse, a false positive or negative test result, more clinicians were worried about false positive results, whereas more patient stakeholders were worried about false negative test results (p=0.02) (Figure 1).
Table 4.
Importance of Harms Related to Individual Testing Results
| Clinicians (n=104) |
Patient Stakeholders (n=104) |
P-value | |
|---|---|---|---|
| 1: How harmful would it be if testing suggests someone has Alzheimer disease when they do not? (This is called a “false positive” diagnosis.) | 8 (IQR 7–9) Range 4–9 |
8 (IQR 7–9) Range 1–9 |
0.952 |
| 2: How harmful would it be if testing suggests someone does not have Alzheimer disease when they do? (This is called a “false negative” diagnosis.) | 7 (IQR 6–8) Range 1–9 |
8 (IQR 7–9) Range 1–9 |
0.001 |
| 3: How harmful would it be if a test suggests someone will develop Alzheimer disease dementia within the next 3 years but they do not? | 8 (IQR 7–9) Range 1–9 |
7 (IQR 7–8) Range 1–9 |
0.001 |
| 4: How harmful would it be if a test is positive for Alzheimer disease and doctors stop looking for other possible explanations for a patient’s problems? | 7 (IQR 5–8) Range 1–9 |
8 (IQR 6–9) Range 3–9 |
<0.001 |
| 5. How harmful is depression resulting from receiving a correct Alzheimer disease diagnosis? | 6 (IQR 4–7) Range 1–9 |
7 (IQR 6–9) Range 1–9 |
<0.001 |
| 6: How harmful is suicide or a suicide attempt due to receiving a correct Alzheimer disease diagnosis? | 9 (IQR 7–9) Range 1–9 |
9 (IQR 7–9) Range 1–9 |
0.751 |
| 7: How harmful is discrimination based on the diagnosis of Alzheimer disease? | 8 (IQR 7–9) Range 1–9 |
8 (IQR 7–9) Range 1–9 |
0.366 |
| 8: How harmful is an inability to get long-term care insurance due to a diagnosis of Alzheimer disease? | 8 (IQR 7–9)* Range 1–9 |
8 (IQR 7–9) Range 1–9 |
0.688 |
| 9: How harmful is the loss of job/volunteer opportunities due to a diagnosis of Alzheimer disease? | 8 (IQR 6–9) Range 1–9 |
8 (IQR 6–9) Range 1–9 |
0.505 |
| 10. How harmful is the loss of driving due to a diagnosis of Alzheimer disease? | 6 (IQR 5–8) Range 1–9 |
8 (IQR 6–9) Range 1–9 |
0.006 |
Responses: A little harmful (limited importance) 1–3, harmful (important) 4–6, extremely harmful (critical importance 7–9). Data presented as median (interquartile range, IQR).
n=103 for this question
Figure 1. Patient Representative and Clinician Views Regarding False Negative vs False Positive Dementia Diagnosis.
After an explanation of the meaning of “false negative” and “false positive” test results, patient stakeholders and clinicians were asked how they felt these two possible testing errors compared. More clinicians worried about false positives (FP) and more patient representatives worried about false negatives (FNs) (p=0.02).
Subgroup analyses
The findings observed in the full population were also seen in subgroup analyses comparing responses between clinicians and patient stakeholders stratified by recruitment group (guideline panelists, public comment respondents, and clinicians from the AAN versus the general public). However, no differences were statistically significant after correcting for multiple comparisons. Similarly, no significant differences were observed between patients and caregivers (Table e-2, Supplemental Digital Content 3) or dementia specialists and other neurologists (Table e-3, Supplemental Digital Content 3) after adjusting for multiple comparisons.
DISCUSSION
This survey investigated clinician and patient stakeholder importance ratings for populations, outcomes and harms derived from proposed questions for an evidence-based guideline relating to amyloid PET use. Although patient stakeholders consistently assigned higher median importance ratings than clinicians, two main patterns emerged for the most prominent differences between groups: 1) patient stakeholders placed greater value on testing and outcome prediction in asymptomatic individuals; and, 2) patient stakeholders were generally more concerned about false negative than false positive diagnoses, whereas the opposite was true for clinicians.
Biomarker testing in cognitively normal subjects
Appropriate use criteria for amyloid imaging dictates that asymptomatic individuals should not receive amyloid imaging.12 However, patient stakeholders in this study indicated that including asymptomatic individuals in the guideline population was critically important, as was including outcomes for asymptomatic populations. This is consistent with a population-based survey, which reported that 12.5% of general public respondents (174/1389) would be “extremely interested” in knowing their amyloid status, even in the absence of effective disease-modifying treatments. Interest was highest in respondents expressing interest in longitudinal AD study participation (71/205, 34.6%) and those with a relative with dementia (40/92, 43.5%).20
These results suggest that clinicians are likely to receive requests for amyloid imaging from asymptomatic individuals should CMS approve amyloid PET coverage. Research to date finds low risks of psychological harm after asymptomatic individuals receive amyloid PET results,21–24 but these results may not be generalizable. Individuals enrolled in biomarker studies tend to have high educational attainment and low baseline anxiety and depression. In addition, results are disclosed through carefully designed counseling and educational sessions—approaches that may not be readily translated to clinical environments. Even with such strategies, 38% (19/50) of highly educated asymptomatic individuals receiving elevated amyloid PET scan results misinterpreted results as suggesting either equivocal risk of developing AD or implying an AD diagnosis.25 These results underscore the importance of developing and testing tools to support amyloid PET result disclosure, and careful consideration of necessary expertise for ordering amyloid PET and accurately disclosing results. Clinicians and patient stakeholders in the current study agreed that inappropriate ordering was a critically important concern.
While this study focused on amyloid PET, discussion of appropriate use in asymptomatic individuals transcends biomarker type. Currently biomarker use in asymptomatic individuals occurs primarily in research contexts. Researchers disclose results when biomarkers are screening tools for clinical trial inclusion or out of respect for participants’ autonomy. Although autonomy is commonly cited as the reason for providing results to asymptomatic individuals, ethicists debate the validity of such an approach.26,27 Healthy research participants perceive biomarker results as valuable when they have personal utility and when results are accompanied by information regarding the risk level and suggestions for action.28 With challenges in quantifying the degree of risk and the lack of disease-modifying approaches, the personal utility of biomarker results for asymptomatic individuals in clinical settings is uncertain. This highlights the need for careful pretest counseling. Studies of response to amyloid PET disclosure in routine clinical use will be critical to identify risks and benefits outside research contexts.
Emphasis on having a diagnosis
Responses to multiple survey questions suggest that patient stakeholders want a diagnosis, even if the diagnosis is incorrect (i.e., “false positive”), while clinicians are more concerned about the detrimental effects of delivering an incorrect diagnosis. Physician reluctance to make or disclose dementia diagnoses relates to low confidence in one’s ability to diagnose dementia,29,30 not wanting to give a diagnosis that isn’t definite,30 perceived lack of benefit of early diagnoses,29,31 concerns regarding emotional distress,31,32 the perception that the patient does not want the diagnosis,30,31 belief that costs outweigh benefits,31,32 concerns that the patient will be unable to understand the diagnosis,31,32 therapeutic nihilism,29,31,32 concerns about dementia stigma,29–32 lack of training,29 limited access to specialists or support services,31 and belief that diagnosis will strain medical systems.29,31 Ethicists also note potential negative effects of both correct (stigma, despair, implications for family members) and incorrect (inappropriate treatment, cost) dementia diagnoses.33
By contrast, the pattern of patient stakeholder responses emphasizes the high value patients and caregivers place on having “answers” in the form of a specific diagnosis. This is consistent with patient and caregiver reports that convey perceived advantages of dementia diagnoses including improved understanding of what is happening,5 improved family patience with the person with dementia,34 validation that something is wrong,34,35 establishing a treatment plan and connecting to resources,5,35 and improved decision-making and future planning.5,34,36 Individuals also report a “right to know.”5,36 Dementia diagnosis disclosure reduces anxiety for some patients and caregivers37 and may associate with higher patient quality of life.38 Patients and caregivers report diagnostic benefits after amyloid PET including improved recognition of the need to plan for the future, a sense that the diagnosis is more definitive, and relief (with negative and positive scans).39 Using amyloid PET to aid diagnosis in atypical dementia syndromes resulted in reduced caregiver anxiety and depression, improved disease understanding, better future planning, enhanced quality of life, increased confidence in next steps, better disease acceptance, and an improved appreciation of time with loved ones.40 While few studies have considered the consequences of false positive versus false negative outcomes in dementia testing, a forced-choice experiment performed with individuals without cognitive impairment found that one driver of interest in diagnostic testing in asymptomatic individuals was the view that false negative results would be devastating.7 This aligns with the views of over 20% of patient stakeholders in our study who indicated that a false negative result would be much worse than a false positive one (Figure 1).
Strengths and Limitations
Strengths of this study include respondents representing multiple stakeholder types, incorporation of GRADE methodology,17 and real-life implications for a guideline in development. The design informing guideline question development is also a limitation, as other interesting questions regarding amyloid PET use and use of other biomarkers were not explored. Some survey questions surrounded the use of amyloid PET in populations without cognitive impairment, but these populations were not represented in survey respondents. Other limitations include the reliance on neurology providers with U.S.-based practices and the recruitment of patient stakeholders who self-reported cognitive impairment and may therefore represent a more engaged/interested subset of individuals. Both factors may limit study generalizability. Sample size was insufficient to investigate the effect of certain demographics, including age and race. While the survey repeatedly encouraged respondents to use the full response range, patient stakeholders consistently rated items in the “extremely important” range. Finally, it is unknown whether after further thought (with or without counseling), the pattern of responses related to diagnostic testing of asymptomatic individuals or the balance of false positive and negative responses would remain the same.
Conclusions
Biomarkers such as amyloid PET play an increasing role in the etiologic diagnosis of MCI and dementia.1–3 With recent research showing that amyloid PET use affects diagnosis and treatment in a memory clinic41 (and results of the IDEAS study pending), amyloid PET use is likely to increase in clinical practice. Understanding patient stakeholder and clinician views in this setting is critical. The current survey identified that patient stakeholders place a greater value than clinicians on amyloid PET testing and outcomes for asymptomatic populations. These findings underscore the importance of research considering the potential benefits and harms associated with disclosure of amyloid PET results to asymptomatic individuals, and the need to develop standards for amyloid PET ordering and result disclosure. Survey results also demonstrate that patients and caregivers are highly invested in obtaining an etiologic dementia diagnosis, to the degree that they are more concerned about going undiagnosed than receiving an incorrect diagnosis. These results emphasize the importance of identifying patients’ and caregivers’ personal priorities and developing practical approaches that address these needs when evaluating an individual for dementia.
Supplementary Material
(1) Supplemental Digital Content 1: survey instruments
(2) Supplemental Digital Content 2: Table e-1 (subgroup demographics)
(3) Supplemental Digital Content 3: Tables e-2, e-3 (sub-analyses)
ACKNOWLEDGEMENTS
This work was funded by the Agency for Healthcare Research and Quality (AHRQ K08HS24159). AHRQ played no role in study design, the collection, analysis, or interpretation of data, or writing the manuscript. The content is solely the responsibility of the authors and does not necessarily represent the official views of ARHQ. AHRQ shares the public access policy of the National Institutes of Health.
Conflicts of Interest and Source of Funding:
M.J. Armstrong is supported by an ARHQ K08 career development award (K08HS24159) through which the current study was performed. She also receives research support from a 1Florida ADRC (AG047266) pilot grant and as the local PI of a Lewy Body Dementia Association Research Center of Excellence. She receives compensation from the American Academy of Neurology for work as an evidence-based medicine methodology consultant. G.S. Day is involved in research supported by an in-kind gift of radiopharmaceuticals from Avid Radiopharmaceuticals and is currently participating in clinical trials of anti-dementia drugs sponsored by Eli Lilly and Biogen. He holds stocks (>$10,000) in ANI Pharmaceuticals (a generic pharmaceutical company). C. D. Mullins receives consulting income from Bayer, Boehringer-Ingelheim, Illumina, Pfizer, Sanofi, and Regeneron. For the remaining authors none were declared.
REFERENCES
- 1.McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:263–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Albert MS, DeKosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:270–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jack CR Jr., Bennett DA, Blennow K, et al. NIA-AA Research Framework: Toward a biological definition of Alzheimer’s disease. Alzheimers Dement. 2018;14:535–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alzheimer’s Association. 2015 Alzheimer’s disease facts and figures Alzheimers Dement. 2015;11:332–384. [DOI] [PubMed] [Google Scholar]
- 5.van den Dungen P, van Kuijk L, van Marwijk H, et al. Preferences regarding disclosure of a diagnosis of dementia: a systematic review. Int Psychogeriatr. 2014;26:1603–1618. [DOI] [PubMed] [Google Scholar]
- 6.Martin S, Kelly S, Khan A, et al. Attitudes and preferences towards screening for dementia: a systematic review of the literature. BMC Geriatr. 2015;15:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mühlbacher A, Johnson FR, Yang JC, et al. Do you want to hear the bad news? The value of diagnostic tests for Alzheimer’s disease. Value Health. 2016;19:66–74. [DOI] [PubMed] [Google Scholar]
- 8.Robinson SM, Canavan M, O’Keeffe ST. Preferences of older people for early diagnosis and disclosure of Alzheimer’s disease (AD) before and after considering potential risks and benefits. Arch Gerontol Geriatr. 2014;59:607–612. [DOI] [PubMed] [Google Scholar]
- 9.Martin S, Fleming J, Cullum S, et al. Exploring attitudes and preferences for dementia screening in Britain: contributions from carers and the general public. BMC Geriatr. 2015;15:110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Qaseem A, Forland F, Macbeth F, et al. Guidelines International Network: toward international standards for clinical practice guidelines. Ann Intern Med. 2012;156:525–531. [DOI] [PubMed] [Google Scholar]
- 11.Committee on Standards for Developing Trustworthy Clinical Practice Guidelines, Graham R, Mancher M, Miller Wolman D, et al. , eds. Clinical Practice Guidelines We Can Trust. Washington, DC: The National Academies Press; 2011. Available at: http://www.nationalacademies.org/hmd/Reports/2011/Clinical-Practice-Guidelines-We-Can-Trust.aspx. Accessed August 18, 2017. [PubMed] [Google Scholar]
- 12.Johnson KA, Minoshima S, Bohnen NI, et al. Appropriate use criteria for amyloid PET: a report of the Amyloid Imaging Task Force, the Society of Nuclear Medicine and Molecular Imaging, and the Alzheimer’s Association. Alzheimers Dement. 2013;9:e-1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frey KA, Lodge MA, Meltzer CC, et al. ACR-ASNR practice parameter for brain PET/CT imaging dementia. Clin Nucl Med. 2016;41:118–125. [DOI] [PubMed] [Google Scholar]
- 14.Minoshima S, Drzezga AE, Barthel H, et al. SNMMI procedure standard/EANM practice guideline for amyloid PET imaging of the brain 1.0. J Nucl Med. 2016;57:1316–1322. [DOI] [PubMed] [Google Scholar]
- 15.Jacques L, Jensen TS, Rollins J, et al. Final decision memorandum for CAG-00431N beta amyloid positron emission tomography in dementia and neurodegenerative disease. Center for Medicare and Medicaid Services; 2013. Available at: https://www.cms.gov/medicare-coverage-database/details/nca-decision-memo.aspx?NCAId=265. Accessed 5 Jan 2018. [Google Scholar]
- 16.Armstrong MJ, Mullins CD, Gronseth GS, et al. Impact of patient involvement on clinical practice guideline development: a parallel group study. Implement Sci. 2018;13:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guyatt GH, Oxman AD, Kunz R, et al. GRADE guidelines: 2. Framing the question and deciding on important outcomes. J Clin Epidemiol. 2011;64:395–400. [DOI] [PubMed] [Google Scholar]
- 18.Gronseth GS, Cox J, Gloss D, et al. Clinical Practice Guideline Process Manual, 2017 ed. Minneapolis MN: The American Academy of Neurology; 2017. [Google Scholar]
- 19.Noether GE. Sample size determination for some common nonparametric tests. J Am Stat Assoc. 1987;82:645–647. [Google Scholar]
- 20.Gooblar J, Roe CM, Selsor NJ, et al. Attitudes of research participants and the general public regarding disclosure of Alzheimer disease research results. JAMA Neurol. 2015;72:1484–1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lim YY, Maruff P, Getter C, et al. Disclosure of positron emission tomography amyloid imaging results: a preliminary study of safety and tolerability. Alzheimers Dement. 2016;12:454–458. [DOI] [PubMed] [Google Scholar]
- 22.Burns JM, Johnson DK, Liebmann EP, et al. Safety of disclosing amyloid status in cognitively normal older adults. Alzheimers Dement. 2017;13:1024–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de Wilde A, van Buchem MM, Otten RHJ, et al. Disclosure of amyloid positron emission tomography results to individuals without dementia: a systematic review. Alzheimers Res Ther. 2018;10:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wake T, Tabuchi H, Funaki K, et al. The psychological impact of disclosing amyloid status to Japanese elderly: a preliminary study on asymptomatic patients with subjective cognitive decline. Int Psychogeriatr. 2018;30:635–639. [DOI] [PubMed] [Google Scholar]
- 25.Mozersky J, Sankar P, Harkins K, et al. Comprehension of an elevated amyloid positron emission tomography biomarker result by cognitively normal older adults. JAMA Neurol. 2018;75:44–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bunnik EM, Richard E, Milne R, et al. On the personal utility of Alzheimer’s disease-related biomarker testing in the research context. J Med Ethics. 2018;44:830–834. [DOI] [PubMed] [Google Scholar]
- 27.Molinuevo JL, Cami J, Carné X, et al. Ethical challenges in preclinical Alzheimer’s disease observational studies and trials: Results of the Barcelona summit. Alzheimers Dement. 2016;12:614–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Milne R, Bunnik E, Diaz A, et al. Perspectives on communicating biomarker-based assessments of Alzheimer’s disease to cognitively healthy individuals. J Alzheimers Dis. 2018;62:487–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aminzadeh F, Molnar FJ, Dalziel WB, et al. A review of barriers and enablers to diagnosis and management of persons with dementia in primary care. Can Geriatr J. 2012;15:85–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Phillips J, Pond CD, Paterson NE, et al. Difficulties in disclosing the diagnosis of dementia: a qualitative study in general practice. Br J Gen Pract. 2012;62:e546–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dhedhi SA, Swinglehurst D, Russell J. ‘Timely’ diagnosis of dementia: what does it mean? A narrative analysis of GPs’ accounts. BMJ Open. 2014;4:e004439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bamford C, Lamont S, Eccles M, et al. Disclosing a diagnosis of dementia: a systematic review. Int J Geriatr Psychiatry. 2004;19:151–169. [DOI] [PubMed] [Google Scholar]
- 33.Mattsson N, Brax D, Zetterberg H. To know or not to know: ethical issues related to early diagnosis of Alzheimer’s disease. Int J Alzheimers Dis. 2010;2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Connell CM, Boise L, Stuckey JC, et al. Attitudes toward the diagnosis and disclosure of dementia among family caregivers and primary care physicians. Gerontologist. 2004;44:500–507. [DOI] [PubMed] [Google Scholar]
- 35.Karnieli-Miller O, Werner P, Aharon-Peretz J, et al. Expectations, experiences, and tensions in the memory clinic: the process of diagnosis disclosure of dementia within a triad. Int Psychogeriatr. 2012;24:1756–1770. [DOI] [PubMed] [Google Scholar]
- 36.Riva M, Caratozzolo S, Cerea E, et al. Diagnosis disclosure and advance care planning in Alzheimer disease: opinions of a sample of Italian citizens. Aging Clin Exp Res. 2014;26:427–434. [DOI] [PubMed] [Google Scholar]
- 37.Carpenter BD, Kissel EC, Lee MM. Preferences and life evaluations of older adults with and without dementia: reliability, stability, and proxy knowledge. Psychol Aging. 2007;22:650–655. [DOI] [PubMed] [Google Scholar]
- 38.Mate KE, Pond CD, Magin PJ, et al. Diagnosis and disclosure of a memory problem is associated with quality of life in community based older Australians with dementia. Int Psychogeriatr. 2012;24:1962–1971. [DOI] [PubMed] [Google Scholar]
- 39.Grill JD, Cox CG, Kremen S, et al. Patient and caregiver reactions to clinical amyloid imaging. Alzheimers Dement. 2017;13:924–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bensaïdane MR, Beauregard JM, Poulin S, et al. Clinical utility of amyloid PET imaging in the differential diagnosis of atypical dementias and its impact on caregivers. J Alzheimers Dis. 2016;52:1251–1262. [DOI] [PubMed] [Google Scholar]
- 41.de Wilde A, van der Flier WM, Pelkmans W, et al. Association of amyloid positron emission tomography with changes in diagnosis and patient treatment in an unselected memory clinic cohort: the ABIDE project. JAMA Neurol. 2018;75:1062–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(1) Supplemental Digital Content 1: survey instruments
(2) Supplemental Digital Content 2: Table e-1 (subgroup demographics)
(3) Supplemental Digital Content 3: Tables e-2, e-3 (sub-analyses)