Abstract
Methylmercury (MeHg) is a ubiquitous environmental toxicant that leads to long-lasting neurological deficits in animals and humans. Curcumin, a polyphenol obtained from the rhizome of turmeric, has well-known antioxidant functions. Here, we evaluated curcumin’s efficacy in mitigating MeHg-induced cytotoxicity and further investigated the underlying mechanism of this neuroprotection in primary rat astrocytes. Pretreatment with curcumin (2, 5, 10 and 20 μM for 3, 6, 12 and 24 h) protected against MeHg-induced (5 μM for 6 h) cell death in a time and dose-dependent manner. Curcumin (2, 5 10 or 20 μM) pretreatment for 12 h significantly ameliorated the MeHg-induced astrocyte injury and oxidative stress, as evidenced by morphological findings, lactate dehydrogenase (LDH) release, reactive oxygen species (ROS) generation, and glutathione (GSH) and catalase (CAT) levels. Moreover, curcumin pretreatment increased Nrf2 nuclear translocation and downstream enzyme expression, heme oxygenase-1 (HO-1) and NADPH quinone reductase-1 (NQO1). Knockdown of Nrf2 with siRNA attenuated the protective effect of curcumin against MeHg-induced cell death. However, both the pan-protein kinase C (PKC) inhibitor, Ro 31–8220, and the selective PKCδ inhibitor, rottlerin, failed to suppress the curcumin-activated Nrf2/ARE pathway and attenuate the protection exerted by curcumin. Taken together, these findings confirm that curcumin protects against MeHg-induced neurotoxicity by activating the Nrf2/ARE pathway and this protection is independent of PKCδ activation. More studies are needed to understand the mechanisms of curcumin cytoprotection.
Keywords: curcumin, methylmercury, Nrf2, astrocytes, PKCδ, PKC
Graphical Abstract
1. Introduction
Mercury (Hg) is a ubiquitous environmental heavy metal with potential human toxicity, notably neurotoxicity. Methylmercury (CH3Hg, MeHg), which is formed in the aquatic environment by sulfate-reducing bacteria and enters the food chain, was responsible for the devastating and irreversible neurodevelopmental effects referred to as “Minamata Disease” (Dórea, 2008). Chronic exposure to MeHg via ingestion of seafood remains a major concern for human health, especially for women and infants living in low- and middle-income countries (Fukuda et al., 1999; Hong et al., 2012; Sheehan et al., 2014).
In children and adults, MeHg targets the peripheral and then the central nervous system (CNS), leading to sensory disorders, cerebellar dysfunction, hearing and visual impairment, and ultimately potential paralysis and death (Farina et al., 2011). In the mammalian CNS, astrocytes are the preferential site of MeHg accumulation and the main target of cytotoxicity (Aschner and Kimelberg 1991; Dave et al., 1993; Aschner et al., 1994; Charleston et al., 1995), but its injurious mechanisms have yet to be fully delineated. Mechanisms advanced to date include, but are not limited to disruption of the oxidative stress response (Farina et al., 2011), mitochondrial dysfunction (Polunas et al., 2011), neurotransmitter alterations (Yin et al., 2007), calcium dyshomeostasis (Yang et al., 2016) and/or glutamate (Glu) uptake/metabolism disorders (Franco et al., 2009).
Oxidative stress is one of the major mechanisms implicated in MeHg-induced CNS injury (Farina and Aschner 2017). As an electrophile with high affinity for thiol (-SH) and/or selenol (-SeH) groups of endogenous molecules (Verkhratsky 2010), MeHg can disrupt key cellular antioxidants and thereby impair antioxidant capacity. In addition, MeHg triggers the formation of reactive oxygen species (ROS), which further accelerate the MeHg-induced imbalance in redox homeostasis. Nuclear factor-E2-related factor 2 (Nrf2), bound to Kelch-like ECH-associated protein 1 (Keap1) in the cytoplasm in the absence of stimuli, is a transcription factor that mediates the induction of antioxidant genes encoding cytoprotective proteins. Upon stimulation, Nrf2 is released from Keap1 and enters the nucleus (Itoh et al., 1999; Li and Kong 2009), where it interacts with an antioxidant response element (ARE) to initiate the transcription of target genes coding for proteins responsible for detoxication and antioxidation, such as glutathione (GSH), catalase (CAT), heme oxygenase-1 (HO-1), NADPH quinone reductase-1 (NQO1) and glutamylcysteine (Prestera et al., 1993; Prestera and Talalay 1995). The Nrf2/ARE pathway is a master regulator of oxidative stress, and its activation is well established in suppressing MeHg-induced neurotoxicity (Toyama et al., 2007; Wang et al., 2009; Ni et al., 2010).
Curcumin, a polyphenol used in curry spice, is obtained from the rhizome of turmeric (Curcuma longa), and turmeric has long been used as a traditional medicinal, especially in China, India and other Asian countries (Anand et al., 2008). Previous studies have shown that curcumin possesses versatile biological properties, including: antioxidant, anti-inflammatory, antifibrotic, anticancer, antimicrobial and immunotherapeutic activities (Prasad et al., 2014). Several studies have corroborated the efficacy of curcumin as a powerful activator of the Nrf2/ARE pathway. For example, curcumin protects against mercuric chloride (HgCl2)-induced hepatic injury by antagonizing oxidative stress and activating the Nrf2/ARE pathway in rats (Liu et al., 2017). Similarly, curcumin attenuates lipopolysaccharide/D-galactosamine-induced acute liver injury, partly through the activation of Nrf2 nuclear translocation (Xie et al., 2017). In the CNS, curcumin acts as a neuroprotectant in a rotenone-induced Parkinson’s disease-like model, again via Nrf2 activation (Cui et al., 2016). Thus, it is essential to examine whether curcumin protects against MeHg-induced oxidative stress and cytotoxicity in astrocytes via Nrf2/ARE signaling.
Furthermore, the underlying mechanism responsible for Nrf2 activation remains controversial. Two primary pathways for the uncoupling of Nrf2 and Keap1, referred to as the Keap1-dependent and Keap1-independent mechanisms, have been reported. The former, involves cysteine residues in Keap1 that are modified by electrophilic substances, resulting in Keap1 conformational changes that lead to the dissociation of Nrf2 (Eggler et al., 2005). In the Keap1-independent Nrf2 activation mechanism, Nrf2 protein is phosphorylated by several signal transduction pathways, including the mitogen-activated protein kinases (MAPKs), protein kinase C (PKC), pancreatic endoplasmic reticulum kinase (PERK) and phosphatidylinositol 3-kinase (PI3K)/Akt signaling pathways (Cullinan et al., 2003; Bak et al; 2012; Fão et al., 2019). Notably, PKC may be a particularly important mediator of Nrf2 activation (Huang et al., 2002; Bloom and Jaiswal 2003). PKC consists of a family of at least 10 mammalian serine/threonine kinases that are subdivided into three classes based on their functions and activation requirements. These include the classical (α, β1, β2 and γ), novel (δ, ε, η and θ) and atypical (ξ and ι) isoforms (Parker and Murrayrust 2004). PKC has been reported to directly phosphorylate the Ser40 site of Nrf2 to facilitate dissociation from Keap1 (Huang et al., 2002) and curcumin exerts its antioxidant property by activating the PKCδ/Nrf2/ARE pathway in human monocytes (Rushworth et al., 2006). However, both the role of PKC, especially the PKCδ isoform, in the activation of Nrf2/ARE pathway by curcumin in primary astrocytes and potential neuroprotection against MeHg have yet to be delineated.
Previous studies on MeHg-induced CNS damage have focused on its effects on neurons (Tamm et al., 2006; Wei et al., 2014; Shao et al., 2015), and to a lesser extent on glial cells (Yin et al., 2011; Pieper et al., 2014). Astrocytes are the most abundant cell type in the brain and are involved in a variety of important neuronal functions. Ample evidence corroborates MeHg’s propensity to interfere with homeostatic functions in astrocytes, amongst them the inhibition of uptake of the GSH precursor, cystine (Allen et al., 2001). Taken into account the above information, we focused herein on the underlying mechanisms of curcumin afforded neuroprotection within the context of MeHg-induced cytotoxicity in primary rat astrocytes.
2. Materials and methods
2.1. Chemicals and reagents
MeHg, curcumin, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl diphenyltetrazolium bromide (MTT), rottlerin and Ro 31–8220, were purchased from Sigma-Aldrich (St. Louis, MO, USA). Antibodies for detecting PKCδ were purchased from Abcam (Cambridge, UK). Antibodies for HO-1 and Nrf2 were obtained from Santa Cruz (Santa Cruz, CA, USA), and for NQO-1 and Keap1 from Immunoway (Plano, TX, USA). Dulbecco’s modified Eagle’s medium (DMEM)/F12 was purchased from Hyclone (Logan, UT, USA). Penicillin, streptomycin and fetal bovine serum (FBS) were purchased from Gibco (Carlsbad, CA, USA). The lactate dehydrogenase (LDH) release assay, GSH assay kit and ROS assay kit were obtained from Jiancheng Bioengineering Institute of Nanjing (Nanjing, Jiangsu, PRC). The catalase (CAT) assay kit, and nuclear and cytoplasmic protein extraction kit were purchased from Beyotime Institute of Biotechnology (Shanghai, PRC).
2.2. Primary rat astrocyte culture and identification
Postnatal 1–2-day Sprague-Dawley (SD) rats were provided by the Laboratory Animal Center of Jiangsu University. Protocols were approved by the animal ethics committee of Jiangsu University and carried out in accordance with established Guiding Principles for Animal Research.
Primary astrocyte cultures were prepared as described previously (Bai et al., 2015; Bai et al., 2016; Fang et al., 2016; Yang et al., 2018). Briefly, cerebral cortices were harvested and separated from the meninges under sterile conditions. The tissues were dissociated with trypsin for 15 min. Cells were suspended in fresh DMEM/F-12 supplemented with 10% FBS and 1% penicillin/streptomycin. After centrifugation at 1000 × g for 5 min, the cell pellets were resuspended and seeded on dishes. The cultures were maintained at 37°C in a humidified 5% CO2–95% air atmosphere. Culture medium was changed after 24 h, and then replaced every 3 days. At confluence (10–14 days), cells that remained adhered to the flasks were astrocytes. The purity of the astrocyte cultures, ascertained by immunocytochemical staining with glial fibrillary acidic protein (GFAP), was > 95% (data not shown).
2.3. Cell treatment
Rat primary astrocytes were plated onto different culture plates based on the assay requirements, and cultured for 24 – 48 h. Next, the medium was replaced with medium containing various concentrations of curcumin (0, 2, 5 10 and 20 μM in DMSO). After the indicated incubation time (3, 6, 12 or 24 h), cells were washed twice with phosphate-buffered saline (PBS) and then incubated in medium containing MeHg (5 μM) for 6 h. Ro 31–8220 (1 and 2 μM) or rottlerin (2 and 4 μM) was added to the curcumin treatment for 12 h. The transient transfection of Nrf2-targeted siRNA was performed before curcumin treatment.
2.4. Cell viability assay
The colorimetric 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay was performed to detect mitochondrial reductase activity and thereby evaluate cell viability. Briefly, the yellow tetrazole MTT was added to the culture medium to a final concentration of 0.5 mg/mL. After the cells were incubated at 37°C for 4 h, the culture medium containing MTT was removed. Dimethyl sulfoxide was added to solubilize the dark-blue formazan reaction product in intact cells. Absorbance was measured at 570 nm with a Bio-Rad 680 microplate reader (Bio-Rad Laboratories Inc., Hercules, CA, USA). Cell viability was expressed as the percentage of viability of untreated cells, which served as the control group and was designated as 100%. The concentration and exposure times to curcumin (2, 5, 10, and 20 μM for 3, 6, 12 and 24 h) and/or MeHg (1, 2, 5 and 10 μM for 3, 6, 12 and 24 h) were based on our previous study as well as literature reports (Bai et al., 2015; Parada et al., 2015; Fang et al., 2016; Pierozan et al., 2017).
2.5. LDH release assay
The activity of lactate dehydrogenase (LDH) released by damaged cells into the medium was determined as described by Decker and Lohmann-Mahhers (Decker and Lohmannmatthes 1988). At the end of treatment, the cell culture medium was transferred into wells in 96-well plates and assayed for LDH activity following the manufacturer’s instructions. Absorbance was measured at 490 nm with a Bio-Rad 680 microplate reader. The relative LDH release rate was calculated as U/mL media in the experimental group divided by U/mL media in the control group ×100%.
2.6. Detection of intracellular ROS formation
Levels of intracellular reactive oxygen species (ROS) were assessed using the oxidative conversion of cell-permeable 2ʹ,7ʹ-dichlorofluorescein diacetate (DCFH-DA) to fluorescent 2ʹ,7ʹ-dichlorodihydrofluoroscein (DCFH). After the indicated treatment, cells were incubated with 10 μmol/L DCFH-DA in serum-free medium for 30 min at 37°C. Fluorescence was measured at 480/530 nm (excitation/emission) by Cytation™ 5 (Biotek, Winooski, VT, USA).
2.7. GSH content assay
Glutathione (GSH) levels were determined by oxidizing GSH with 5,5-dithiobis(2-nitrobenzoic acid) (DTNB) and measuring the yellow derivative 5’-thio-2-nitrobenzoic acid spectrophotometrically at 405 nm (Ellman 1959). GSH assay was performed according to the instructions of the assay kit. Results are expressed as GSH (μmol)/g of cell protein.
2.8. CAT activity assay
Catalase (CAT) activity was measured by the ammonium molybdate spectrophotometric method (Goth 1991), which was performed according to the instructions of the assay kit. Absorbance was measured at 520 nm with a Bio-Rad 680 microplate reader. The results are expressed as units of CAT activity per mg of cell protein (U/mg protein), where 1 U of CAT is defined as the amount of enzyme that decomposes 1 μmol of H2O2 per s.
2.9. Preparation of nuclear and cytoplasmic proteins
Nuclear and cytoplasmic proteins were prepared with a Nuclear and Cytoplasmic Protein Extraction Kit (Beyotime, Shanghai, PRC). Briefly, cell plasma membranes were disrupted by hypotonic lysis buffer and after centrifugation at 12,000–16,000g for 5 min, the supernatant was collected as cytoplasmic proteins. The pellet, which contained the nuclear proteins, was further extracted with a high-salt nuclear protein extraction reagent. All protein fractions were stored at −70°C until use.
2.10. Immunofluorescence staining for Nrf2 localization
Astrocytes were seeded at 3 × 104 cells per well in 24-well plates. After treatment with MeHg (5 μM for 6 h) with or without the pretreatment with curcumin (10 μM for 12 h), cells were rinsed with PBS and fixed with 4% paraformaldehyde for 15 min at room temperature (RT). The astrocytes were then permeabilized with 0.1% Triton X-100 for 30 min, washed with PBS three times for 3 min, and blocked in 5% bovine serum albumen (BSA) in PBS for 2 h at RT. The cells were next incubated with primary anti-Nrf2 antibody (1:200 dilution in PBS containing 5% BSA) at 4°C overnight. After that, cells were washed with PBS three times and incubated with fluorescein isothiocyanate (FITC)-labeled secondary antibodies (1:100 dilution in PBS) in dark at 37° for 1 h. After three rinses in PBS, the nuclei were stained with 4’,6-diamidino-2-phenylindole (DAPI) for 10 min. Finally, cells were examined with a fluorescence microscope (Zeiss Axio Observer, Oberkochen, Germany).
2.11. Western blot analysis
Cells were washed twice with PBS after treatment and solubilized with radioimmunoprecipitation assay (RIPA) lysis buffer containing 1% phenylmethanesulfonylfluoride (PMSF) (Beyotime). The protein concentration of the supernatant was determined using a BCA protein assay kit (Beyotime). Aliquots (30–40 μg) of total protein or nuclear proteins were separated by 8–12% SDS-PAGE, transferred onto polyvinylidene difluoride (PVDF) membranes (Millipore, Merk, German), and then blocked with 5% skim milk in TBST (10 mM Tris-HCl, 120 mM NaCl, 0.1% Tween 20, pH 7.4) for 2 h at RT. Next, the membranes were incubated with the appropriate primary antibodies at 4°C overnight. Membranes were washed three times with TBST, incubated with the corresponding secondary antibodies for 1 h at RT, and then washed three times with TBST. Visualization was carried out using the ECL+TM method and scanning with a MiniChemi Mini Size Chemiluminescent Imaging System (Beijing Sage Creation Science, Beijing, PRC). The images were analyzed with Image J software (National Institutes of Health, Bethesda, MD, USA).
2.12. Transient transfection of Nrf2-targeted siRNA
The Nrf2-targeted small interfering RNA (siRNA): 5’-GCAUGUUACGUGAUGAGGAUGGAAA-3’ was designed and purchased from Invitrogen (Thermo Fisher, Waltham, MA, USA). Before transfection, astrocytes were seeded at 5 × 104 cells per well in 24-well plates with complete culture medium in the absence of antibiotics and cultured to confluency at 50–70%. Transfection of cells with siRNA was performed using Lipofectamine ®RNAiMAX transfection reagent (Invitrogen) according to the manufacturer’s instructions. Briefly, Nrf2-targeted siRNA (10 pmol) was diluted in 50 μL of serum-free Opti-MEM® I Medium (Gibco), mixed with an equal volume of Lipofectamine® RNAiMAX Reagent (3 μL) and incubated for 5 min at RT. Then, the medium was removed from the cells and the siRNA/Lipofectamine solution added directly to the cells with 450 μL of Opti-MEM® I Medium. After incubation for 24 h, the siRNA/Lipofectamine solution was removed and the cells were washed three times with PBS. BLOCK-iT™ Alexa Fluor ® Red Fluorescent Oligo (Thermo Fisher, Waltham, MA, USA) was used to assess the transfection efficiency of the Nrf2-targeted siRNA. Alternatively, complete medium in the absence of antibiotics was added to the cells for 48 h. Western blot analysis and cell viability assay were performed as previously described. Before the cell viability assay, the astrocytes were pretreated with 5 μM curcumin for 12 h, followed by treatment with 5 μM MeHg for 6 h.
2.13. Statistical analysis
Data are presented as mean ± SD. Multiple comparisons were carried out using one-way analysis of variance (ANOVA) followed by Dunnett’s test or Student-Newman-Keuls test as a post hoc test depending on the test purpose. All assays were repeated independently at least three times (n≥3). Each repeated experiment was performed with independently derived astrocyte cultures. All statistical analyses were performed using SPSS 19.0 (Chicago, IL, USA). P < 0.05 was considered statistically significant.
3. Results
3.1. Curcumin pretreatment protects against MeHg-induced acute astrocyte damage and oxidative stress
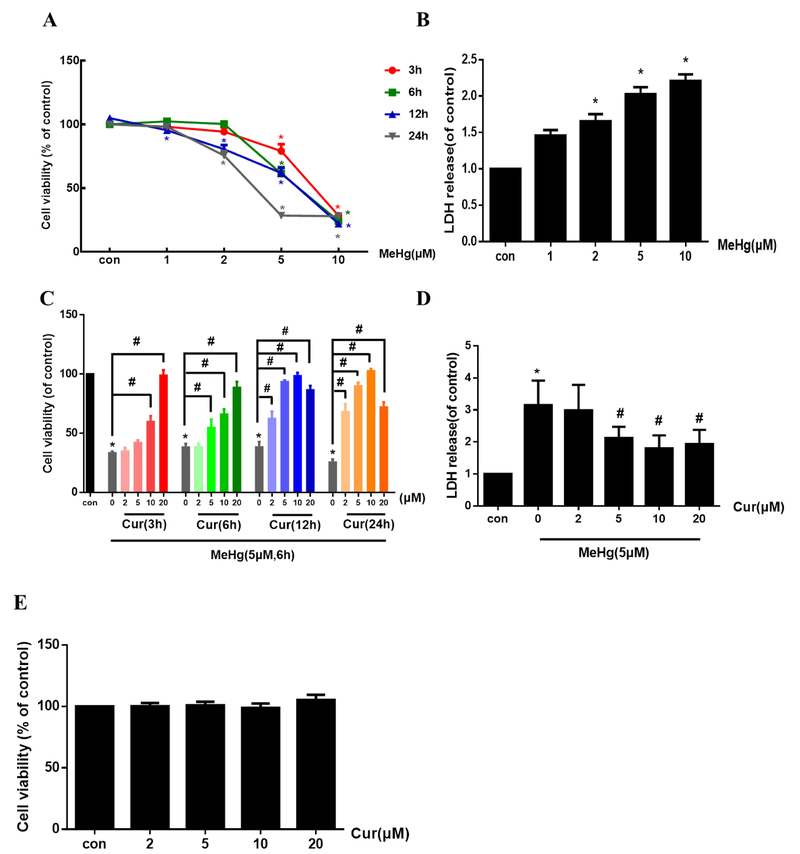
The MTT and LDH release assays were used to investigate the effects of MeHg on viability and cytotoxicity, respectively, in primary rat astrocytes. As shown in Fig. 1A, MeHg (0, 1, 2, 5 and 10 μM) treatment for the indicated times (3, 6, 12 and 24 h) led to reduced cell viability in a concentration- and time-dependent manner. When the cells were treated with 5 μM MeHg for 6 h, cell viability was decreased to ~60% compared with controls (P < 0.05). Under the same conditions, LDH leakage into the culture medium was also significantly increased compared with controls (P < 0.05, Fig. 1B). Based on these observations, in subsequent experiments treatment was carried out with 5 μM MeHg for 6 h.
Fig. 1.
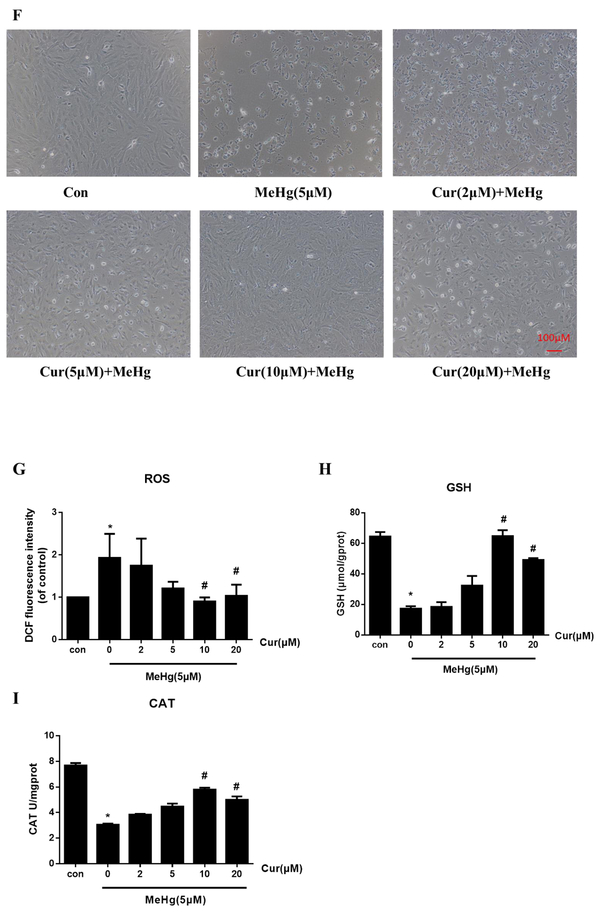
Curcumin protects against MeHg-induced acute astrocytic injury and oxidative stress. (A) Astrocytes were treated with MeHg at the indicated concentrations for up to 24 h, and cell viability evaluated by the MTT assay. (B) MeHg-induced cytotoxicity was measured by the LDH release assay. Cells were treated with MeHg at the indicated concentration for 6 h. (C) Astrocytes were treated with 5 μM MeHg after pretreatment with 0, 2, 5, 10 or 20 μM curcumin for 3, 6, 12 and 24 h, and cell viability was determined with the MTT assay. (D) Effects of curcumin pretreatment (2, 5, 10 and 20 μM for 12 h) on MeHg-induced cytotoxicity, as measured by the LDH release assay. (E) Effects of curcumin (2, 5, 10 and 20 μM for 24 h) on astrocyte viability, as determined by the MTT assay. (F–I) Effects of curcumin pretreatment (2, 5, 10 and 20 μM for 12 h) on MeHg-induced astrocyte morphology changes, ROS production, GSH content and CAT activity. Scale bar: 100 μm. Data are expressed as mean ± SD of at least three independent experiments (*P < 0.05 vs. control. #P < 0.05 vs. MeHg alone group. Cur, curcumin; MeHg, methylmercury.
Next, we tested whether curcumin protects astrocytes against MeHg-induced cytotoxicity. We treated cells with 5 μM MeHg for 6 h with/without different concentrations of curcumin pretreatment (2, 5, 10 and 20 μM) for different time (3, 6, 12 and 24 h). As shown in Fig. 1C, pretreatment with curcumin (2, 5 and 10 μM for 3, 6, 12 and 24 h) attenuated the MeHg-induced cell death in a concentration- and time-dependent manner. When cells were pretreated with 10 μM curcumin for 24 h, cell viability were increased to nearly 100%, achieving the best protective effect against MeHg. However, the highest concentration of curcumin (20 μM) led to a decrease in its protective effect with the increasing pretreatment time (Fig. 1C), while 20 μM curcumin for 3, 6, 12 and 24 h still significantly reversed the decreased cell viability induced by MeHg (5 μM for 6 h). Moreover, MeHg-induced cytotoxicity, as assessed by LDH leakage, was significantly decreased by curcumin treatment (5, 10 and 20 μM) for 12 h compared with the MeHg alone treated group (P < 0.05, Fig. 1D). Treatment with curcumin alone at concentrations of 2–20 μM for 24 h (the longest time of treatment used in this study) did not cause any apparent cytotoxicity as measured by the MTT assay (Fig. 1E), and 0.5‰ DMSO (the maximum concentration for dissolving curcumin) for 3, 6, 12 and 24 h also did not caused significant effects on the cell viability of astrocytes (supplementary material, Fig. S1). Additionally, pretreatment with curcumin (2, 5, 10 and 20 μM for 12 h) reversed the MeHg-induced morphological alterations, including cell detachment and shrinkage of cell bodies (Fig. 1F). In the following experiments, 2, 5, 10 or 20 μM curcumin treatments for 12 h were consistently used.
Next, we examined whether curcumin pretreatment suppresses MeHg-induced oxidative stress by measuring ROS generation (Fig. 1G), GSH content (Fig. 1H) and CAT activity (Fig. 1I). Treatment with MeHg (5 μM for 6 h) caused a dramatic increase in astrocytic ROS-induced 2’,7’-dichlorofluorescein (DCF) fluorescence intensity (P < 0.05), corroborated by a significant concomitant decrease in GSH content and CAT activity (P < 0.05). In contrast, ROS production in the curcumin-pretreated group (10 and 20 μM for 12 h) was significantly decreased (53.1% and 46.3%, respectively), compared with the MeHg alone treated group (P < 0.05). Furthermore, 10 and 20 μM curcumin pretreatment significantly increased GSH levels (73.3% and 64.8%, respectively) and CAT activity (47.2% and 38.8%, respectively), compared with the MeHg alone treatment (P < 0.05). Taken together, these data established the efficacy of curcumin pretreatment in protecting astrocytes from MeHg-induced oxidative stress.
3.2. Curcumin pretreatment induces Nrf2 nuclear translocation and the expression of its downstream genes, HO-1 and NQO1.
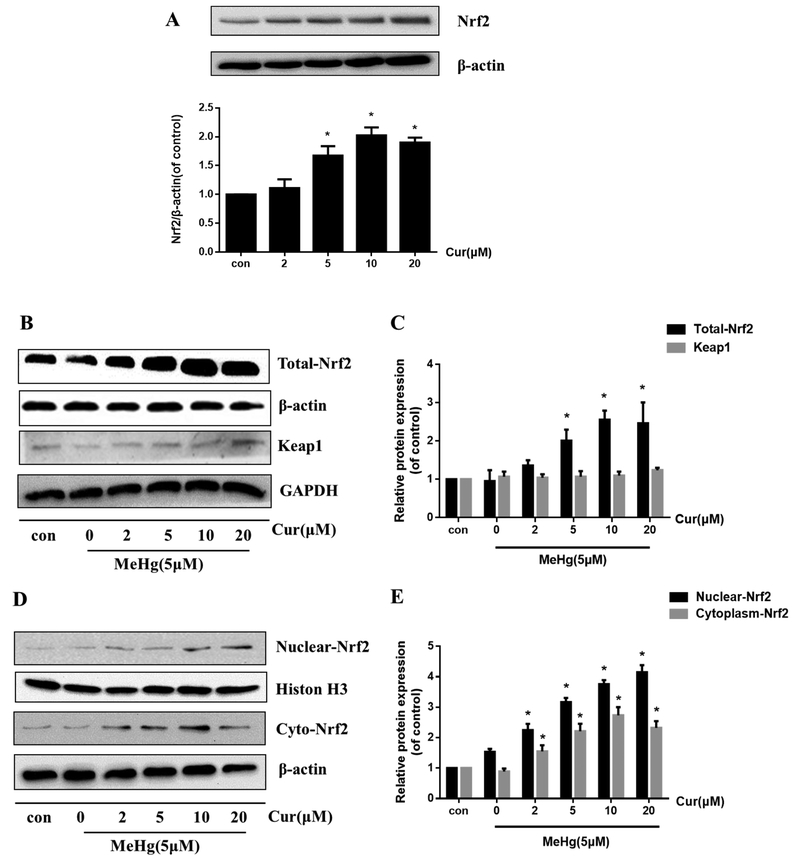
The Nrf2 signaling pathway is a master regulator of oxidative stress. Once activated, Nrf2 dissociates from Keap1, followed by its translocation into the nucleus, where it interacts with the antioxidant response element (ARE) to initiate the transcription of target genes.
To examine whether curcumin activated the Nrf2 pathway, we first determined its effect on total Nrf2 expression. As is shown in Fig. 2A, total Nrf2 protein expression in astrocytes was increased by curcumin (2, 5, 10 and 20 μM for 12 h) in a concentration-dependent manner. Next, we determined the effect of curcumin in cells treated with 5 μM MeHg for 6 h (Fig. 2B and C). Results showed that 5, 10 and 20 μM curcumin pretreatment significantly increased the expression of total Nrf2 protein compared with MeHg alone (P < 0.05). In addition, subcellular fractions of astrocytes revealed that the expression of nuclear Nrf2 was significantly increased in a concentration-dependent manner by curcumin pretreatment compared with the MeHg alone treated group (P < 0.05; Fig. 2D and E). Interestingly, the amount of Nrf2 in the cytoplasm showed an analogous, though smaller, upward trend compared with that seen in the nucleus (Fig. 2D and E), likely indicating that curcumin increased total astrocytic Nrf2 expression (Fig. 2B and C). However, the level of Keap1 showed no significant change upon pretreatment with curcumin compared with the MeHg alone treated group (Fig. 2B and C).
Fig. 2.
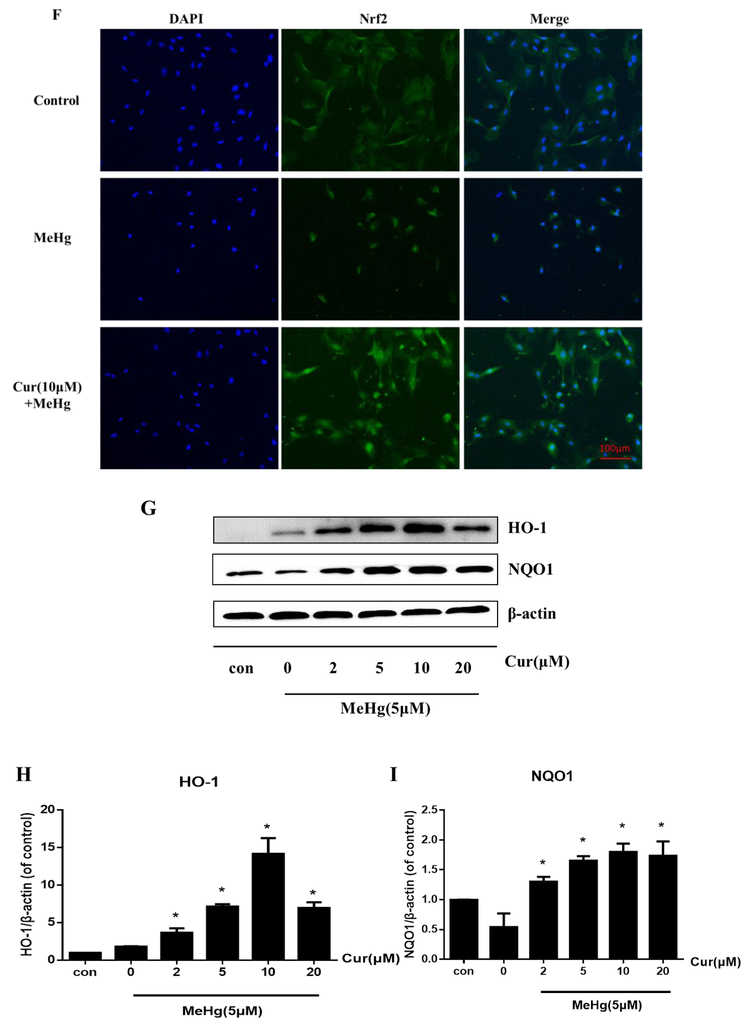
Curcumin pretreatment activates Nrf2/ARE signaling. (A) Effects of curcumin treatment alone at the indicated concentrations for 12 h on total Nrf2 levels. In the following experiments, primary rat astrocytes were treated with MeHg (5 μM for 6 h) in the absence or presence of curcumin pretreatment (2, 5, 10 or 20 μM for 12 h). (B, C) Effects of curcumin pretreatment on the expression level of total Nrf2 and Keap1. (D, E) Effects of curcumin pretreatment on the expression level of nuclear and cytoplasmic Nrf2. Histone H3 and β-actin were used as the internal control of the nucleus and cytoplasm, respectively. (F) Representative images of Nrf2 localiztion by immunofluorescence staining. Green fluorescence (FITC) indicates Nrf2 and blue (DAPI) indicates the nucleus. Scale bar: 100 μm. (G–I) Effects of curcumin pretreatment on the protein expression level of the Nrf2 target genes, HO-1 and NQO1. Data are expressed as mean ± SD of at least three independent experiments. *P < 0.05 vs. MeHg group. Cur, curcumin; MeHg, methylmercury.
Immunofluorescence staining was also used to dissect out the activation of the Nrf2 pathway by curcumin pretreatment. As shown in Fig. 2F, nuclear Nrf2 staining (green) was more abundant in the curcumin-pretreatment (10 μM, 12 h) group than in the MeHg alone treated group, where Nrf2 appeared to be mostly localized to the cytoplasm, indicating that curcumin pretreatment promoted Nrf2 translocation to the nucleus. To corroborate the role of curcumin in activating the Nrf2/ARE pathway, the downstream phase II detoxifying genes, HO-1 and NQO1, were analyzed. As shown in Fig. 2G–I, the expression of these proteins was significantly elevated in the curcumin-pretreated cells (2, 5, 10 and 20μM) compared with the MeHg alone treated group (P < 0.05).
3.3. Nrf2/ARE signaling is involved in the protection afforded by curcumin against MeHg-induced cytotoxicity in primary rat astrocytes
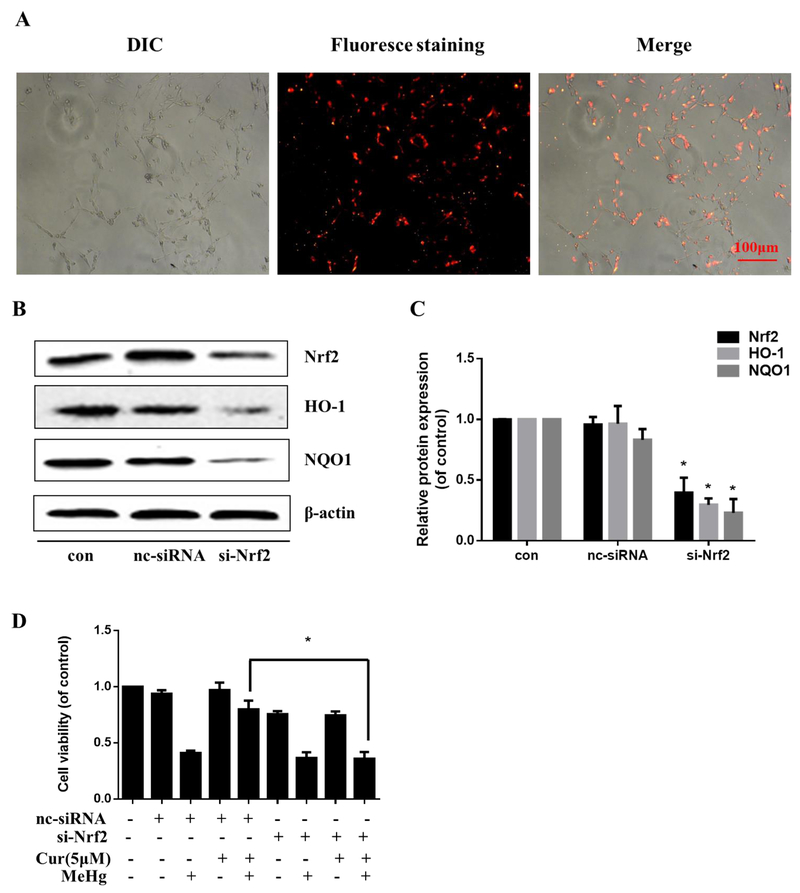
Knockdown of Nrf2 with siRNA was performed to assess the role of Nrf2 in protection of curcumin from MeHg toxicity. BLOCK-iT™ Alexa Fluor ® Red Fluorescent Oligo was used to assess siRNA uptake. As shown in Fig. 3A, most cell nuclei were stained red, indicating optimal transfection efficiency. Western blotting showed that the siRNA targeting Nrf2 significantly decreased Nrf2 protein levels, and its downstream genes, HO-1 and NQO1, compared with the nc-siRNA (P < 0.05; Fig. 3B and C). Notably, as is shown in Fig. 3D, cell viability in si-Nrf2+Cur (5 μM)+MeHg group significantly decreased compared with that of nc-siRNA+Cur (5 μM)+MeHg group. The results established that knockdown of Nrf2 blocked the cytoprotective effect of curcumin (5 μM for 12 h) against the MeHg-induced decrease in astrocyte viability, suggesting that activation of the Nrf2/ARE pathway is involved in the protective effect of curcumin against MeHg-induced neurotoxicity in these cells.
Fig. 3.
Nrf2/ARE signaling mediates the protection of curcumin against MeHg-induced cytotoxicity in primary rat astrocytes. (A) Representative images of the BLOCK-iT™ Alexa Fluor ® Red Fluorescent Oligo assessment of the cationic lipid-mediated delivery of siRNA. The dye used was Alexa Fluor® 555 (red). Scale bar: 100 μM. (B, C) The protein expression level of Nrf2, HO-1 and NQO1 after siRNA transfection. *P < 0.05 vs. nc-siRNA. (D) Effect of Nrf2 knockdown by siRNA on the protective effect of curcumin (5 μM, 12 h) against MeHg-induced cytotoxicity (5 μM, 6 h) as assessed by the MTT assay. *P<0.05 vs. nc-siRNA+Cur+MeHg group. Data are expressed as mean ± SD of at least three independent experiments. Cur, curcumin; MeHg, methylmercury.
3.4. PKCδ is not involved in Nrf2/ARE activation by curcumin and the protection against MeHg-induced cytotoxicity in primary rat astrocytes
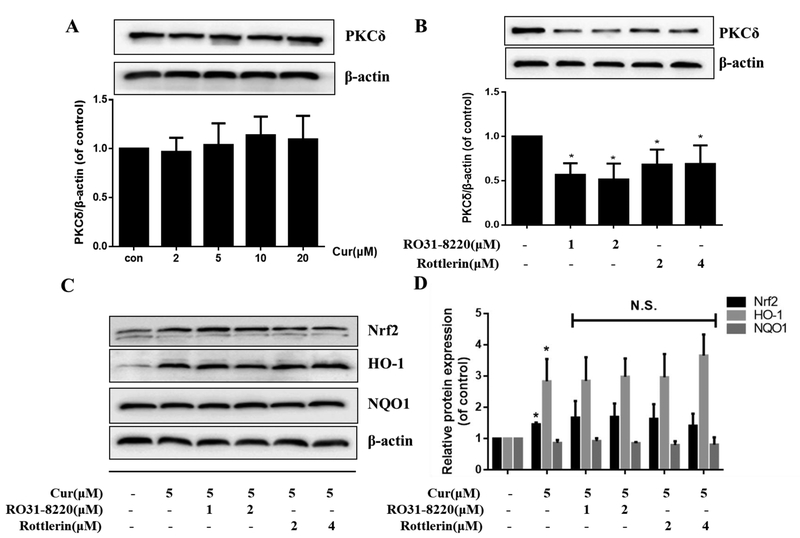
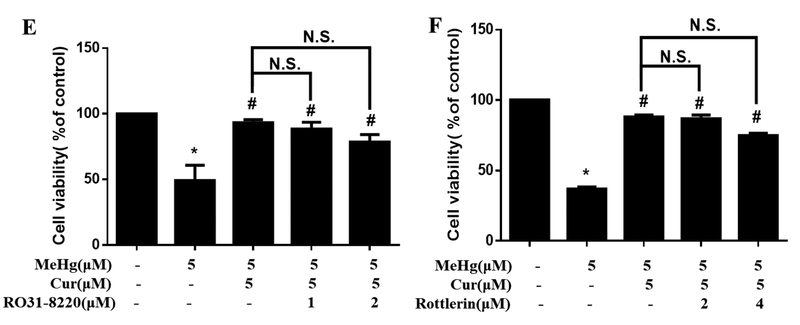
Protein Kinase C (PKC) has been reported to mediate the activation of Nrf2 by phosphorylating Nrf2 at Ser40, thus stimulating its release from Keap1 and translocation to the nucleus. Therefore, we examined the role of the PKCδ isoform in the curcumin-mediated neuroprotection against MeHg in primary rat astrocytes. First, treatment for 12 h at the indicated concentrations established that curcumin did not significantly change the expression level of PKCδ (Fig. 4A). To further address the role of PKCδ in curcumin-induced activation of Nrf2/ARE pathway and protection against MeHg in primary rat astrocytes, two PKCδ inhibitors, Ro 31–8220 and rottlerin, were used. Ro 31–8220 is a pan-PKC inhibitor, while rottlerin is a selective PKCδ inhibitor. Results showed that treatment with Ro 31–8220 (1 and 2 μM for 12 h) or rottlerin (2 and 4 μM for 12 h) significantly blocked the expression of PKCδ (Fig. 4B), and a negligible effect on astrocyte viability (at these concentrations and time points), as measured by the MTT assay (data not shown). Next, we examined the effects of the two PKC inhibitors on the curcumin-induced Nrf2 activation. As is shown in Fig. 4C and D, the both PKC inhibitors did not attenuate the curcumin-induced increase in the expression of Nrf2 and HO-1 compared with the curcumin alone group. Interestingly, treatment with curcumin alone (5 μM for 12 h) did not significantly increase the expression of NQO1, which appears inconsistent with the results noted in the curcumin pretreatment experiment (Fig. 2G). This suggests that curcumin pretreatment followed by MeHg treatment has a more pronounced effect on the activation of the Nrf2 pathway, including NQO1 expression, compared with the cells treated with curcumin alone. More importantly, both Ro 31–82201 and rottlerin did not affect the protective efficacy of curcumin (5 μM for 12 h) against MeHg (Fig. 4E and F). These results imply that the protective effects of curcumin against MeHg-induced neurotoxicity in primary rat astrocytes are independent of PKCδ activation.
Fig. 4.
The protection of curcumin against MeHg-induced toxicity in primary rat astrocytes independently of PKCδ. (A) Effects of curcumin on the expression of PKCδ. (B) Effect of Ro 31–8220 and rottlerin on the expression of PKCδ. *P < 0.05 vs. control group. (C, D) Effect of co-treatment with Ro 31–8220 or rottlerin and curcumin (5 μM, 12 h) on the expression of Nrf2, HO-1 and NQO1. *P < 0.05 vs. Blank control group. N.S. (P > 0.05) vs. curcumin alone group. (E, F) Effect of Ro 31–8220 or rottlerin on the protective effects of curcumin against MeHg-induced astrocyte death. *P < 0.05 vs. control group, #P < 0.05 vs. MeHg alone treatment group. N.S. (P > 0.05) vs. Cur+MeHg treatment group. Data are expressed as mean ± SD of at least three independent experiments. Cur, curcumin; MeHg, methylmercury. N.S., not significant.
4. Discussion
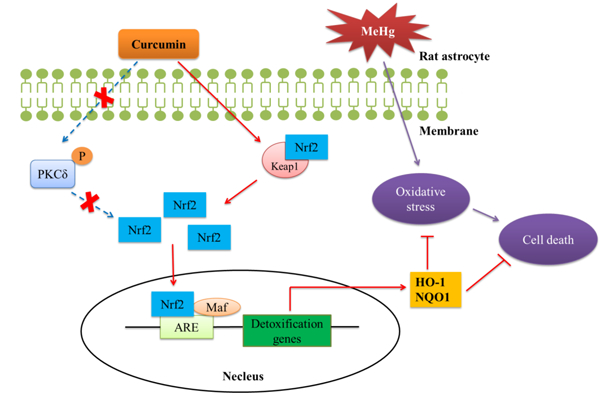
Although various mechanisms underlie MeHg’s neurotoxicity, the most important one appears to be oxidative stress. Curcumin, a natural phytochemical with a medicinal history of millennia, exerts antioxidative effects against numerous diseases. In the present study, we report that curcumin suppressed MeHg-induced cytotoxicity and ameliorated oxidative stress in primary rat astrocytes. Furthermore, our results demonstrated that the neuroprotection afforded by curcumin was associated with the activation of the Nrf2/ARE pathway, and was independent of phosphorylation of Nrf2 by PKCδ.
MeHg predominantly targets the CNS, eliciting a plethora of cytotoxic events, including in glial cells, which preferentially accumulate the highest concentrations of this metal (Morken et al., 2005). The mechanism of MeHg-induced neurotoxicity is considered to be associated with oxidative damage by either overproduction of ROS and/or by inactivation of the antioxidant defense system (Aschner et al., 2007; Kaur et al., 2009). Our results corroborate the involvement of oxidative stress in MeHg-induced neurotoxicity, as evidenced by the increased generation of ROS, decreased GSH levels and decreased CAT activity, all of which may be attributed to the electrophilic properties of MeHg. MeHg covalently binds to cellular proteins through their reactive thiols, or non-protein sulfhydryl or disulfide isomerase (PDI), referred to as S-mercuration, thus damaging biomolecules (Kanda et al., 2014; Makino et al., 2015; Thaigarajan et al., 2018).
Curcumin, the bioactive component of turmeric and curry, is a lipophilic compound with a long history of medicinal use that has been widely applied in various fields (Anand et al., 2008), including metal overdose (García-Niño et al., 2014), HIV infection (Kumari et al., 2015), cancer (Cianciosi et al., 2018), diabetes (Nabavi et al., 2015) and wound healing (Akbik et al., 2014). The antioxidant efficacy represents the most prominent property of curcumin, and it has been found to be effective in treatment of nervous system injuries induced by neurotoxic agents, including formaldehyde (Ciftci et al., 2015), rotenone (Cui et al., 2016) and 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (Jayaraj et al., 2014). Recent evidence also suggests a reduction in the toxicity of MeHg by plant components (Chang et al., 2019), and it has also been reported that curcumin attenuates MeHg-induced neurotoxicity in SH-SY5Y cells (Petroni et al., 2010; meeting abstract) as well MeHg-induced pancreatic damage in mice (Sudjarwo et al., 2017). However, there is no study on the antagonistic effect of curcumin against MeHg-induced astrocytic cytotoxicity and its specific mechanism.
Our results demonstrate the ability of curcumin to protect astrocytes from MeHg-induced oxidative injury and cellular death (Fig.1C–I). In addition, we found that curcumin pretreatment activates the Nrf2/ARE pathway in astrocytes, evidenced by the increase in the nuclear translocation of Nrf2 and the expression of the downstream proteins, HO-1 and NQO1 (Fig. 2G–I). Interestingly, apart from the increase in nuclear Nrf2, cytoplasmic Nrf2 was also upregulated, indicating that the increased overall Nrf2 expression was induced by curcumin (Fig. 2A–C) or that Nrf2 degradation was inhibited (Nguyen et al., 2003; Stewart et al., 2003). In addition, knockdown of Nrf2 suppresses both the expression of downstream proteins and the protective effect of curcumin pretreatment on MeHg-induced astrocytic death (Fig. 3). Taken together, these results corroborate our hypothesis that curcumin antagonizes MeHg-induced cytotoxicity by promoting the Nrf2/ARE signaling pathway in primary rat astrocytes. Hence, curcumin is a potential neuroprotective agent for astrocytes in MeHg-induced neurotoxicity.
Numerous studies have shown that administering a natural compound to induce phase II enzymes is sufficient to promote antioxidant and protective activities. HO-1 has been demonstrated to protect against lead-induced neurotoxicity (Li et al., 2016), and represents a novel target for therapeutic intervention against neurodegeneration (Zhang et al., 2012). NQO1 is a cytosolic flavoprotein that prevents ROS generation and scavenges oxygen radicals, thereby showing a direct role in protection against oxidative stress (Kenjiro et al., 2015). It has been reported that HO-1 and NQO1 are involved in the protection offered by sulforaphane against MeHg overdose in rat cerebral cortex (Feng et al., 2017). Consistent with these observations, we found that curcumin pretreatment induced HO-1 and NQO1 expression in astrocytes. More importantly, the protective effect of curcumin against MeHg injury was attenuated by Nrf2 silencing, along with a decrease in HO-1 and NQO1 expression. This suggests curcumin-induced upregulation of HO-1 and NQO1 depends on the nuclear expression of Nrf2. However, it should be noted that although HO-1 is often chosen as a marker of Nrf2 activation, it can also be upregulated by hypoxia-inducible factor-1 (HIF-1) (Jazwa et al., 2015; Yan et al., 2016) and by other regulating factors (Chien et al., 2015). Therefore, we cannot exclude the role of other signaling pathways in these effects.
The underlying mechanism of Nrf2 activation by curcumin pretreatment is theoretically mediated by two different modes of action, Keap1-dependent or Keap1-independent (Bryan et al., 2013). Keap1 forms a homodimer responsible for sequestering Nrf2 in the cytosol and facilitates the Cullin 3 (Cul3)-mediated polyubiquitination of Nrf2, leading to its proteasomal degradation, thereby rendering it inactive (Kang et al., 2004). When cells experience oxidative stress or levels of electrophiles increase, a subset of cysteine residues in Keap1 is modified. These modifications result in a conformational change in the protein, which causes the release of Nrf2 and disruption of ubiquitin transfer, thus leading to the activation of Nrf2 translocation (Eggler et al., 2005). Qin et al. (2014) have reported that baicalein reduced the steady-state level of Keap1 by increasing its ubiquitination and modifications, which led to Nrf2 escaping from ubiquitination. Here, the expression of Keap1 in response to curcumin pretreatment was unchanged (Fig. 2B). This suggests that the curcumin-induced Nrf2 activation may not involve Keap1. However, at this point, we cannot exclude the possibility of conformational changes in the Keap1 protein.
Apart from the Keap1-dependent mechanism, several studies have suggested that post-translational modifications of Nrf2, mainly phosphorylation, may contribute to its nuclear translocation, thus regulating the activation of the Nrf2/ARE pathway. PKCδ, one of the novel PKC classes, has been shown to play a significant role in Nrf2 phosphorylation, thereby disrupting the association between Nrf2 and Keap1 (Huang et al., 2002; Bloom and Jaiswal 2003; Parker and Murrayrust 2004). Interestingly, we found no positive relationship between PKCδ and the Nrf2/ARE-regulated detoxifying genes. As shown in Fig. 4A, treatment with curcumin alone had no significant effect on the expression of PKCδ. More importantly, the pan-PKC inhibitor, Ro 31–8220, and the selective PKCδ inhibitor, rottlerin, did not significantly decrease the expression of Nrf2 and the detoxifying proteins (Fig. 4C and D). Furthermore, these PKCδ inhibitors did not significantly change the protective effects of curcumin against MeHg-induced astrocyte death (Fig. 4E and F). These results suggest that PKCδ is not involved in the curcumin-induced Nrf2/ARE pathway activation and neuroprotection against MeHg in primary rat astrocytes. Similarly, Zhang et al.(2016) have reported that PKC was not involved in Nrf2 activation by Tempol, which protects against renal damage after I/R injury. Indeed, regulation of PKC by polyphenols, including curcumin, is isoform-dependent. The activation or inhibition of PKC depends on membrane, Ca2+ ions, cofactors and the cell or tissue type (Das et al., 2016). Different membrane translocation machinery present in diverse cellular systems may lead to the different roles of PKC in various pathological models (Kim et al., 2016). In addition, PKCε-induced Nrf2 activation is an important contributor in the maintenance of endothelial homeostasis and resistance to injury (Mylroie et al., 2015). Therefore, we cannot exclude at this point the involvement of other PKC isoforms in the protective effect of curcumin against MeHg-induced astrocyte toxicity.
Since various pathways may contribute to Nrf2 activation, it is possible that a singular inhibition, such as PKCδ, may fail to reverse the overall Nrf2 activation effect induced by curcumin. This may afford an alternative explanation for the lack of effect of the PKCδ inhibitors on the activation of the Nrf2/ARE pathway and the protection afforded by curcumin against MeHg-induced astrocytic toxicity.
In conclusion, we show that pretreatment with curcumin protects against MeHg-induced acute cellular injury and oxidative stress in primary rat astrocytes. The protective effects of curcumin were attributed to Nrf2 nuclear translocation and the expression of the phase II detoxifying enzymes, HO-1 and NQO1. Additionally, the neuroprotection afforded by curcumin was PKCδ-independent.
Supplementary Material
Acknowledgements
This work was supported in part by the Natural Science Foundation of China (No. 30872139, No. 81273124, No. 81302459 and No. 81400911), the Natural Science Foundation of Jiangsu Province (No. BK20140573) and by grants from the National Institute of Environmental Health Sciences (NIEHS R01ES07331, NIEHS R01ES10563 and NIEHS R01ES020852). We thank Michael Bell, PhD, from Liwen Bianji, Edanz Editing China (www.liwenbianji.cn/ac), for editing the English text of a draft of this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure Statement
The authors declare no conflict of interest regarding the publication of this study.
References
- Akbik D, Ghadiri M, Chrzanowski W, Rohanizadeh R, 2014. Curcumin as a wound healing agent. Life. Sci 116(1), 1–7. [DOI] [PubMed] [Google Scholar]
- Allen JW, Shanker G, Aschner M, 2001. Methylmercury inhibits the in vitro uptake of the glutathione precursor, cystine, in astrocytes, but not in neurons. Brain, Res. 894(1), 131–140. [DOI] [PubMed] [Google Scholar]
- Anand P, Thomas SG, Kunnumakkara AB, Sundaram C, Harikumar KB, Sung B, Tharakan ST, Misra K, Priyadarsini IK, Rajasekharan KN, 2008. Biological activities of curcumin and its analogues (congeners) made by man and mother nature. Biochem, Pharmacol. 76(11), 1590–1611. [DOI] [PubMed] [Google Scholar]
- Aschner M, Kimelberg HK, 1991. The use of astrocytes in culture as model systems for evaluating neurotoxic-induced-injury. Neurotoxicology. 12(3), 505–517. [PubMed] [Google Scholar]
- Aschner M, Mullaney KJ, Wagoner D, Lash LH, Kimelberg HK, 1994. Intracellular glutathione (GSH) levels modulate mercuric chloride (MC)- and methylmercuric chloride (MeHgCl)-induced amino acid release from neonatal rat primary astrocytes cultures. Brain, Res. 664(1–2), 133–140. [DOI] [PubMed] [Google Scholar]
- Aschner M, Syversen T, Souza DO, Rocha JBT, Farina M, 2007. Involvement of glutamate and reactive oxygen species in methylmercury neurotoxicity. Braz, J, Med, Biol, Res. 40(3), 285–291. [DOI] [PubMed] [Google Scholar]
- Bai Y, Yin C, Zhao W, Li B, Qian H, Ma J, Xing G, Wang S, Li F, Aschner M, Lu R, 2015. Differential protection of pre- versus post-treatment with curcumin, Trolox, and N-acetylcysteine against acrylonitrile-induced cytotoxicity in primary rat astrocytes. Neurotoxicology. 51, 58–66. [DOI] [PubMed] [Google Scholar]
- Bai Y, Zhao W, Yin C, Fang Y, Ma J, Li B, Qian H, Xing G, Wang S, Li F, Aschner M, Lu R, 2016. Preconditioning of endoplasmic reticulum stress protects against acrylonitrile-induced cytotoxicity in primary rat astrocytes: The role of autophagy. Neurotoxicology. 55, 112–121. [DOI] [PubMed] [Google Scholar]
- Bak MJ, Jun M, Jeong WS, 2012. Procyanidins from wild grape (vitis amurensis) seeds regulate ARE-mediated enzyme expression via Nrf2 coupled with p38 and PI3K/Akt pathway in HepG2 cells. Int, J, Mol, Sci. 13(12), 801–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom DA, Jaiswal AK, 2003. Phosphorylation of Nrf2 at Ser40 by protein kinase C in response to antioxidants leads to the release of Nrf2 from iNrf2, but is not required for Nrf2 stabilization/accumulation in the nucleus and transcriptional activation of antioxidant response element-mediated NAD(P)H:Quinone oxidoreductase-1 gene expression. J, Biol, Chem. 278(45), 44675–44682. [DOI] [PubMed] [Google Scholar]
- Bryan HK, Olayanju A, Goldring CE, Park BK, 2013. The Nrf2 cell defence pathway: Keap1-dependent and -independent mechanisms of regulation. Biochem, Pharmacol. 85(6), 705–717. [DOI] [PubMed] [Google Scholar]
- Chang J, Zhou Y, Wang Q, Aschner M, Lu R, 2019. Plant components can reduce methylmercury toxication: A mini-review. Biochim, Biophys, Acta, Gen, Subj. pii: S0304–4165(19)30018–2. [DOI] [PubMed] [Google Scholar]
- Charleston JS, Body RL, Mottet NK, Vahter ME, Burbacher TM, 1995. Autometallographic determination of inorganic mercury distribution in the cortex of the calcarine sulcus of the monkey Macaca fascicularis following long-term subclinical exposure to methylmercury and mercuric chloride. Toxicol, Appl, Pharmacol. 132(2), 325–333. [DOI] [PubMed] [Google Scholar]
- Chen W, Sun Z, Wang XJ, Jiang T, Huang Z, Fang D, Zhang DD, 2009. Direct interaction between Nrf2 and p21(Cip1/WAF1) upregulates the Nrf2-mediated antioxidant response. Mol, Cell. 34(6), 663–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chien PT, Lin CC, Hsiao LD, Yang CM, 2015. Induction of HO-1 by carbon monoxide releasing molecule-2 attenuates thrombin-induced COX-2 expression and hypertrophy in primary human cardiomyocytes. Toxicol, Appl, Pharmacol. 289(2), 349–359. [DOI] [PubMed] [Google Scholar]
- Cianciosi D, Varela-Lopez A, Forbes-Hernandez TY, Gasparrini M, Afrin S, Reboredo-Rodriguez P, Zhang J, Quiles JL, Nabavi SF, Battino M, Giampieri F, 2018. Targeting molecular pathways in cancer stem cells by natural bioactive compounds. Pharmacol, Res. 135, 150–165. [DOI] [PubMed] [Google Scholar]
- Ciftci G, Aksoy A, Cenesiz S, Sogut MU, Yarim GF, Nisbet C, Guvenc D, Ertekin A, 2015. Therapeutic role of curcumin in oxidative DNA damage caused by formaldehyde. Microsc, Res, Tech, 78(5), 391–395. [DOI] [PubMed] [Google Scholar]
- Cui Q, Li X, Zhu H, 2016. Curcumin ameliorates dopaminergic neuronal oxidative damage via activation of the Akt/Nrf2 pathway. Mol, Med, Rep. 13(2), 1381. [DOI] [PubMed] [Google Scholar]
- Culbreth M, Zhang Z, Aschner M, 2017. Methylmercury augments Nrf2 activity by downregulation of the Src family kinase Fyn. Neurotoxicology. 62, 200–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullinan SB, Zhang D, Hannink M, Arvisais E, Kaufman RJ, Diehl JA, 2003. Nrf2 is a direct PERK substrate and effector of PERK-dependent cell survival. Mol, Cell, Biol. 23(20), 7198–7209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das J, Ramani R, Suraju MO, 2016. Polyphenol compounds and PKC signaling. Biochim, Biophys, Acta. 1860(10), 2107–2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dave V, Mullaney KJ, Goderie S, Kimelberg HK, Aschner M, 1993. Astrocytes as mediators of methylmercury neurotoxicity: Effects on D-aspartate and serotonin uptake. Dev, Neurosci. 16(3–4), 222–231. [DOI] [PubMed] [Google Scholar]
- Decker T, Lohmannmatthes ML, 1988. A quick and simple method for the quantitation of lactate dehydrogenase release in measurements of cellular cytotoxicity and tumor necrosis factor (TNF) activity. J, Immunol, Methods. 115, 61–69. [DOI] [PubMed] [Google Scholar]
- Dórea JG, 2008. Persistent, bioaccumulative and toxic substances in fish: Human health considerations. Sci, Total, Environ. 400(1–3), 93–114. [DOI] [PubMed] [Google Scholar]
- Eades G, Yang M, Yao Y, Zhang Y, Zhou Q, 2011. MiR-200a regulates Nrf2 activation by targeting Keap1 mRNA in breast cancer cells. J, Biol, Chem. 286(47), 40725–40733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggler AL, Liu G, Pezzuto JM, van Breemen RB, Mesecar AD, 2005. Modifying specific cysteines of the electrophile-sensing human keap1 protein is insufficient to disrupt binding to the Nrf2 domain Neh2. Proc Natl Acad Sci U S A 102(29), 10070–10075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellman GL, 1959. Tissue sulfhydryl groups. Arch, Biochem, Biophys. 82, 70–77. [DOI] [PubMed] [Google Scholar]
- Fang Y, Guo C, Zhang P, Zhao W, Wang S, Xing G, Shi H, Peng W, Aschner M, Lu R, 2016. Role of autophagy in methylmercury-induced neurotoxicity in rat primary astrocytes. Arch, Toxicol. 90(2), 333–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fão L, Mota SI, Rego AC, 2019. c-Src regulates Nrf2 activity through PKCδ after oxidant stimulus. Biochim, Biophys, Acta, Mol, Cell, Res. 1866(4), 686–698 [DOI] [PubMed] [Google Scholar]
- Farina M, Aschner M, 2017. Methylmercury-induced neurotoxicity: Focus on prooxidative events and related consequences. Adv, Neurobiol. 18, 267–286. [DOI] [PubMed] [Google Scholar]
- Farina M, Aschner M, Rocha JB, 2011. Oxidative stress in MeHg-induced neurotoxicity. Toxicol, Appl, Pharmacol. 256(3), 405–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng S, Xu Z, Wang F, Yang T, Liu W, Deng Y, Xu B, 2017. Sulforaphane prevents methylmercury-induced oxidative damage and excitotoxicity through activation of the Nrf2-ARE pathway. Mol, Neurobiol. 54(1), 375–391. [DOI] [PubMed] [Google Scholar]
- Fukuda Y, Ushijima K, Kitano T, Sakamoto M, Futatsuka M, 1999. An analysis of subjective complaints in a population living in a methylmercury-polluted area. Environ, Res. 81(2), 100. [DOI] [PubMed] [Google Scholar]
- Franco JL, Posser T, Dunkley PR, Dickson PW, Mattos JJ, Martins R, Bainy ACD, Marques MR, Dafre AL, Farina M, 2009. Methylmercury neurotoxicity is associated with inhibition of the antioxidant enzyme glutathione peroxidase. Free, Radic, Biol, Med. 47(4), 449. [DOI] [PubMed] [Google Scholar]
- García-Niño WR, Pedraza-Chaverrí J, 2014. Protective effect of curcumin against heavy metals-induced liver damage. Food, Chem, Toxicol. 69, 182–201. [DOI] [PubMed] [Google Scholar]
- Goth L, 1991. A simple method for determination of serum catalase activity and revision of reference range. Clin, Chim, Acta. 196, 143–151. [DOI] [PubMed] [Google Scholar]
- Guo Y, Yu S, Zhang C, Kong AN, 2015. Epigenetic regulation of Keap1-Nrf2 signaling. Free, Radic, Biol, Med. 88, 337–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong YS, Ym K, Lee K-E, 2012. Methylmercury exposure and health effects. J, Prev, Med, Public, Health. 45(6), 353–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang HC, Nguyen T, Pickett CB, 2002. Phosphorylation of Nrf2 at Ser-40 by protein kinase C regulates antioxidant response element-mediated transcription. J, Biol, Chem. 277(45), 42769–42774. [DOI] [PubMed] [Google Scholar]
- Huang T, Zhao J, Guo D, Pang H, Zhao Y, Song J, 2018. Curcumin mitigates axonal injury and neuronal cell apoptosis through the PERK/Nrf2 signaling pathway following diffuse axonal injury. Neuroreport. 29(8), 661–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh K, Wakabayashi N, Katoh Y, Ishii T, Igarashi K, Engel JD, Yamamoto M, 1999. Keap1 represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the amino-terminal Neh2 domain. Genes, Dev. 13(1), 76–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayaraj RL, Elangovan N, Manigandan K, Singh S, Shukla S, 2014. CNB-001 a novel curcumin derivative, guards dopamine neurons in MPTP model of Parkinson’s disease. Biomed, Res, Int. 2014, 236182–236182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jazwa A, Stoszko M, Tomczyk M, Bukowska-Strakova K, Pichon C, Jozkowicz A, Dulak J, 2015. HIF-regulated HO-1 gene transfer improves the post-ischemic limb recovery and diminishes TLR-triggered immune responses - effects modified by concomitant VEGF overexpression. Vascul, Pharmacol. 71, 127–138. [DOI] [PubMed] [Google Scholar]
- Kanda H, Shinkai Y, Kumagai Y, 2014. S-mercuration of cellular proteins by methylmercury and its toxicological implications. J, Toxicol, Sci. 39(5), 687–700. [DOI] [PubMed] [Google Scholar]
- Kang MI, Kobayashi A, Wakabayashi N, Kim SG, Yamamoto M, 2004. Scaffolding of Keap1 to the actin cytoskeleton controls the function of Nrf2 as key regulator of cytoprotective phase 2 genes. Proc, Natl, Acad, Sci, U, S, A. 101(7), 2046–2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur P, Evje L, Aschner M, Syversen T, 2009. The in vitro effects of selenomethionine on methylmercury-induced neurotoxicity. Toxicol, In, Vitro. 23(3), 378–385. [DOI] [PubMed] [Google Scholar]
- Kenjiro T, Akiko HH, Masakazu T, Gaku T, Takeshi N, Masutaka F, 2015. Cynaropicrin attenuates UVB-induced oxidative stress via the AhR-Nrf2-Nqo1 pathway. Toxicol, Lett. 234(2), 74–80. [DOI] [PubMed] [Google Scholar]
- Kim KM, Heo DR, Kim YA, Lee J, Kim NS, Bang OS, 2016. Coniferaldehyde inhibits lps-induced apoptosis through the PKC alpha/beta II /Nrf-2/Ho-1 dependent pathway in RAW264.7 macrophage cells. Environ, Toxicol, Pharmacol. 48, 85–93. [DOI] [PubMed] [Google Scholar]
- Kumari N, Kulkarni AA, Lin X, McLean C, Ammosova T, Ivanov A, Hipolito M, Nekhai S, Nwulia E, 2015. Inhibition of HIV-1 by curcumin a, a novel curcumin analog. Drug, Des, Devel, Ther. 9, 5051–5060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Kong AN, 2009. Molecular mechanisms of Nrf2-mediated antioxidant response. Mol, Carcinog. 48(2), 91–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Ye F, Li L, Chang W, Wu X, Chen J, 2016. The role of HO-1 in protection against lead-induced neurotoxicity. Neurotoxicology. 52(2), 1–11. [DOI] [PubMed] [Google Scholar]
- Liu W, Xu Z, Li H, Guo M, Yang T, Feng S, Xu B, Deng Y, 2017. Protective effects of curcumin against mercury-induced hepatic injuries in rats, involvement of oxidative stress antagonism, and Nrf2-ARE pathway activation. Hum, Exp, Toxicol. 36(9), 949–966. [DOI] [PubMed] [Google Scholar]
- Makino K, Okuda K, Sugino E, Nishiya T, Toyama T, Iwawaki T, Fujimura M, Kumagai Y, Uehara T, 2015. Correlation between attenuation of protein disulfide isomerase activity through S-mercuration and neurotoxicity induced by methylmercury. Neurotox, Res. 27(2), 99–105. [DOI] [PubMed] [Google Scholar]
- Morken TS, Sonnewald U, Aschner M, Syversen T, 2005. Effects of methylmercury on primary brain cells in mono- and co-culture. Toxicol, Sci. 87(1), 169–175. [DOI] [PubMed] [Google Scholar]
- Mylroie H, Dumont O, Bauer A, Thornton CC, Mackey J, Calay D, Hamdulay SS, Choo JR, Boyle JJ, Samarel AM, Randi AM, Evans PC, Mason JC, 2015. PKCepsilon-CREB-Nrf2 signalling induces HO-1 in the vascular endothelium and enhances resistance to inflammation and apoptosis. Cardiovasc, Res. 106(3), 509–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabavi SF, Thiagarajan R, Rastrelli L, Daglia M, Sobarzosanchez E, Alinezhad H, Nabavi SM, 2015. Curcumin: A natural product for diabetes and its complications. Curr, Top, Med, Chem. 15(23), 2445–2455. [DOI] [PubMed] [Google Scholar]
- Nguyen T, Sherratt PJ, Huang HC, Yang CS, Pickett CB, 2003. Increased protein stability as a mechanism that enhances Nrf2-mediated transcriptional activation of the antioxidant response element. Degradation of Nrf2 by the 26 s proteasome. J, Biol, Chem. 278(7), 4536–4541. [DOI] [PubMed] [Google Scholar]
- Ni M, Li X, Yin Z, Jiang H, Sidoryk-Wegrzynowicz M, Milatovic D, Cai J, Aschner M, 2010. Methylmercury induces acute oxidative stress, altering Nrf2 protein level in primary microglial cells. Toxicol, Sci. 116(2), 590–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parada E, Buendia I, Navarro E, Avendaño C, Egea J, López MG, 2015. Microglial HO-1 induction by curcumin provides antioxidant, antineuroinflammatory, and glioprotective effects. Mol, Nutr, Food, Res. 59(9), 1690–700. [DOI] [PubMed] [Google Scholar]
- Parker PJ, Murrayrust J, 2004. PKC at a glance. J, Cell, Sci. 117, 131. [DOI] [PubMed] [Google Scholar]
- Petroni DH, Debasis M, Tsai J, George WJ, 2010. Examination of the neuroprotective effects of liposome encapsulated curcumin against methylmercury induced neurotoxicity in SH-SY5Y cells. Faseb, J. 24, 759–4. (meeting abstract). [Google Scholar]
- Pieper I, Ca W, Bornhorst J, Ebert F, Leffers L, Holtkamp M, Hoseler P, Weber T, Mangerich A, Burkle A, Karst U, Schwerdtle T, 2014. Mechanisms of Hg species induced toxicity in cultured human astrocytes: Genotoxicity and DNA-damage response. Metallomics. 6(3), 662–671. [DOI] [PubMed] [Google Scholar]
- Pierozan P, Biasibetti H, Schmitz F, Ávila H, Fernandes CG, Pessoa-Pureur R, Wyse ATS, 2017. Neurotoxicity of Methylmercury in Isolated Astrocytes and Neurons: the Cytoskeleton as a Main Target. Mol, Neurobiol. 54(8), 5752–5767. [DOI] [PubMed] [Google Scholar]
- Polunas M, Halladay A, Tjalkens R, Philbert M, Lowndes H, Reuhl K, 2011. Role of oxidative stress and the mitochondrial permeability transition in methylmercury cytotoxicity. Neurotoxicology. 32(5), 526–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad S, Gupta SC, Tyagi AK, Aggarwal BB, 2014. Curcumin, a component of golden spice: From bedside to bench and back. Biotechnol, Adv. 32(6), 1053–1064. [DOI] [PubMed] [Google Scholar]
- Prestera T, Holtzclaw WD, Zhang Y, Talalay P, 1993. Chemical and molecular regulation of enzymes that detoxify carcinogens. Proc, Natl, Acad, Sci, U, S, A. 90(7), 2965–2969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prestera T, Talalay P, 1995. Electrophile and antioxidant regulation of enzymes that detoxify carcinogens. Proc, Natl, Acad, Sci, U, S, A. 92(19), 8965–8969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin S, Deng F, Wu W, Jiang L, Yamashiro T, Yano S, Hou DX, 2014. Baicalein modulates Nrf2/Keap1 system in both Keap1-dependent and Keap1-independent mechanisms. Arch, Biochem, Biophys. 559, 53–61. [DOI] [PubMed] [Google Scholar]
- Rushworth SA, Ogborne RM, Charalambos CA, O’Connell MA, 2006. Role of protein kinase C delta in curcumin-induced antioxidant response element-mediated gene expression in human monocytes. Biochem, Biophys, Res, Commun. 341(4), 1007–1016. [DOI] [PubMed] [Google Scholar]
- Sangokoya C, Telen MJ, Chi JT, 2010. Microrna miR-144 modulates oxidative stress tolerance and associates with anemia severity in sickle cell disease. Blood. 116(20), 4338–4348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao Y, Daniel F, Ning Z, Mailloux R, Chan HM, 2015. Methylmercury can induce Parkinson’s-like neurotoxicity similar to 1-methyl-4- phenylpyridinium: A genomic and proteomic analysis on MN9D dopaminergic neuron cells. J, Toxicol, Sci. 40(6), 817–828. [DOI] [PubMed] [Google Scholar]
- Sheehan MC, Burke TA, Navas-Acien A, Breysse PN, McGready J, Fox MA, 2014. Global methylmercury exposure from seafood consumption and risk of developmental neurotoxicity: A systematic review. Bull, World, Health, Organ. 92(4), 254–269F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart D, Killeen E, Naquin R, Alam S, Alam J, 2003. Degradation of transcription factor Nrf2 via the ubiquitin-proteasome pathway and stabilization by cadmium. J, Biol, Chem. 278(4), 2396–2402. [DOI] [PubMed] [Google Scholar]
- Sudjarwo SA, Eraiko K, Sudjarwo GW, Koerniasari., 2017. Antioxidant activity of curcumine as protector on methylmercury induced pancreas damage in mice. Journal of Chinese Pharmaceutical Sciences. 26(3), 196–201. [Google Scholar]
- Tamm C, Duckworth J, Hermanson O, Ceccatelli S, 2006. High susceptibility of neural stem cells to methylmercury toxicity: Effects on cell survival and neuronal differentiation. J, Neurochem. 97(1), 69–78. [DOI] [PubMed] [Google Scholar]
- Thiagarajan K, Gamit N, Mandal S, Ayyathan DM, Chandrasekaran R, 2018. Amelioration of methylmercury induced neural damage by essential oil of Selinum vaginatum (Edgew) c. B. Clarke. Pak, J, Pharm, Sci. 31(2), 399–404. [PubMed] [Google Scholar]
- Toyama T, Shinkai Y, Yasutake A, Taguchi K, Tong KI, Yamamoto M, Kumagai Y, Kumagai Y, 2007. Cytoprotective role of Nrf2/Keap1 system in methylmercury toxicity. Biochem, Biophys, Res, Commun. 363(3), 645–650. [DOI] [PubMed] [Google Scholar]
- Verkhratsky A, 2010. Physiology of neuronal–glial networking. Neurochem, Int. 57(4), 332–343. [DOI] [PubMed] [Google Scholar]
- Wang L, Jiang H, Yin Z, Aschner M, Cai J, 2009. Methylmercury toxicity and Nrf2-dependent detoxification in astrocytes. Toxicol, Sci. 107(1), 135–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei L, Sweeney AJ, Sheng L, Fang Y, Kindy MS, Xi T, Gao BZ, 2014. Single-neuron axonal pathfinding under geometric guidance: Low-dose-methylmercury developmental neurotoxicity test. Lab, Chip. 14(18), 3564–3571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie YL, Chu JG, Jian XM, Dong JZ, Wang LP, Li GX, Yang NB, 2017. Curcumin attenuates lipopolysaccharide/ d -galactosamine-induced acute liver injury by activating Nrf2 nuclear translocation and inhibiting NF-κB activation. Biomed, Pharmacother. 91, 70–77. [DOI] [PubMed] [Google Scholar]
- Yan L, Cao X, Zeng S, Li Z, Lian Z, Wang J, Lv F, Wang Y, Li Y, 2016. Associations of proteins relevant to MAPK signaling pathway (p38MAPK-1,HIF-1 and HO-1) with coronary lesion characteristics and prognosis of peri-menopausal women. Lipids, Health, Dis. 15(1), 187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang B, Bai Y, Yin C, Qian H, Xing G, Wang S, Li F, Bian J, Aschner M, Lu R, 2018. Activation of autophagic flux and the Nrf2/ARE signaling pathway by hydrogen sulfide protects against acrylonitrile-induced neurotoxicity in primary rat astrocytes. Arch, Toxicol. 92(3), 2093–2108. [DOI] [PubMed] [Google Scholar]
- Yang M, Yao Y, Eades G, Zhang Y, Zhou Q, 2011. MiR-28 regulates Nrf2 expression through a Keap1-independent mechanism. Breast, Cancer, Res, Treat. 129(3), 983–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang T, Xu Z, Liu W, Xu B, Deng Y, 2016. Protective effects of alpha-lipoic acid on MeHg-induced oxidative damage and intracellular Ca(2+) dyshomeostasis in primary cultured neurons. Free, Radic, Res. 50(5), 542–556. [DOI] [PubMed] [Google Scholar]
- Yin Z, Milatovic D, Aschner JL, Syversen T, Rocha JBT, Souza DO, Sidoryk M, Albrecht J, Aschner M, 2007. Methylmercury induces oxidative injury, alterations in permeability and glutamine transport in cultured astrocytes. Brain, Res. 1131(1), 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Z, Lee E, Ni M, Jiang H, Milatovic D, Rongzhu L, Farina M, Rocha JB, Aschner M, 2011. Methylmercury-induced alterations in astrocyte functions are attenuated by ebselen. Neurotoxicology. 32(3), 291–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan X, Xu C, Pan Z, Keum YS, Kim JH, Shen G, Yu S, Oo KT, Ma J, Kong AN, 2006. Butylated hydroxyanisole regulates ARE-mediated gene expression via Nrf2 coupled with ERK and JNK signaling pathway in HepG2 cells. Mol, Carcinog. 45(11), 841–850. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Cui W, Li G, Yuan S, Xu D, Hoi MP, Lin Z, Dou J, Han Y, Lee SM, 2012. Baicalein protects against 6-OHDA-induced neurotoxicity through activation of Keap1/Nrf2/HO-1 and involving PKCalpha and PI3K/AKT signaling pathways. J, Agric, Food, Chem. 60(33), 8171–8182. [DOI] [PubMed] [Google Scholar]
- Zhang G, Wang Q, Zhou Q, Wang R, Xu M, Wang H, Wang L, Wilcox CS, Liu R, Lai EY, 2016. Protective effect of tempol on acute kidney injury through PI3K/Akt/Nrf2 signaling pathway. Kidney, Blood, Press, Res. 41(2), 129–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y, Morris A, Sunkara M, Layne J, Toborek M, Hennig B, 2012. Epigallocatechin-gallate stimulates NF-E2-related factor and heme oxygenase-1 via caveolin-1 displacement. J, Nutr, Biochem. 23(2), 163–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.