Abstract
Background
Depressed individuals often perceive neutral facial expressions as emotional. Neurobiological underpinnings of this effect remain unclear. We investigated the differences in prefrontal cortical (PFC) activation in depressed individuals vs. healthy controls (HC) during recognition of emotional and neutral facial expressions using functional near infrared spectroscopy (fNIRS).
Method
In Experiment 1, 33 depressed individuals and 20 HC performed the Emotion Intensity Rating task in which they rated intensity of facial emotional expressions. In Experiment 2, a different set of participants (18 depressed individuals and 16 HC) performed the same task while their PFC activation was measured using fNIRS.
Results
Both experiments showed that depressed individuals were slower and less accurate in recognizing neutral, but not happy or fearful, facial emotional expressions. Experiment 2 revealed that lower accuracy for neutral facial emotional expressions was associated with lower right PFC activation in depressed individuals, but not HC. In addition, depressed individuals, compared to HC, had lower right PFC activation during recognition of happy facial expressions.
Limitations
Relatively small sample size
Conclusions
Recognition of neutral facial expressions is impaired in depressed individuals. Greater impairment corresponds to lower right PFC activation during neutral face processing. Recognition of happy facial expressions is comparable for depressed individuals and HC, but the former have significantly lower right PFC activation. Taken together, these findings suggest that the ability of depressed individuals to discriminate neutral and emotional signals in the environment may be affected by aberrant functioning of right PFC.
Keywords: Depression, functional near-infrared spectroscopy, prefrontal cortex, recognition of facial emotional expressions
1. INTRODUCTION
Mood disorders such as major depressive and bipolar disorders affect approximately 10% of population in the world (Steel et al., 2014). Depressed individuals suffer from perceptual, cognitive and behavioral dysfunction that becomes more severe when symptom severity increases (Judd et al., 2005, 2000). One of these dysfunctions is impaired recognition of emotional and neutral facial expressions (Derntl et al., 2009; Gur et al., 1992; Persad and Polivy, 1993; Rubinow and Post, 1992; Drevets, 2001). Studies have shown that depressed individuals recognized neutral facial expressions less accurately than happy or sad, while healthy controls (HC) recognized neutral, happy and sad facial expressions equally accurately (Leppänen et al., 2004). Some studies demonstrated that depressed individuals misinterpreted happy faces as neutral and neutral faces as sad (e.g., Gur et al., 1992), showing mood congruent bias in processing of positive and negative emotions (Gray et al., 2006) with negative perceptual bias increasing along with the increases in depression severity (Bilderbeck et al., 2017; Münkler et al., 2015; Surguladze et al., 2004). Understanding neurobiological underpinnings of this phenomenon may facilitate development of new treatment strategies to help affected individuals cope with situations that are emotionally neutral in nature but perceived as, for example, threatening or sad.
Neuroimaging studies using functional magnetic resonance imaging (fMRI) have implicated lateral and medial prefrontal cortices (PFC), as well as the insula, amygdala, anterior cingulate, fusiform and parietal cortices in emotional face processing (see Fusar-Poli et al., 2009 for a meta-analysis). The PFC regions such as dorsolateral prefrontal cortex (DLPFC) and frontopolar regions are involved in emotion regulation and cognitive control (e.g., Ochsner and Gross, 2005) with greater activation in these regions observed for more unpleasant stimuli (Colibazzi et al., 2010). Mood disordered individuals, compared with HC, show increased DLPFC activation during processing of happy faces (Demenescu et al., 2011) and decreased activation during processing of fearful or angry faces (Zhong et al., 2011). Depressed individuals with greater longitudinal increases in DLPFC and frontopolar activation during negative affect regulation showed greater improvement of depressive symptoms over time, compared with depressed individuals who showed lower longitudinal increases in these regions (Heller et al., 2013). These findings suggest that impaired processing of emotional faces in individuals with mood disorders may be related to emotion dysregulation and aberrant functioning of the PFC. Neural correlates of diminished ability to recognize neutral facial expressions in depressed individuals remain unclear, however.
Most previous neuroimaging studies of emotional face processing used functional magnetic resonance imaging (fMRI). fMRI provides good spatial resolution, but is expensive, not portable, sensitive to subject’s motion and has many contraindications that limit subject participation. Functional near infrared spectroscopy (fNIRS) is a type of optical imaging used to measure brain activation during task performance (e.g., Bendall et al., 2016; Obrig, 2014). While both fMRI and fNIRS methods measure blood oxygenation level-dependent (BOLD) signal related to the changes in the concentration of deoxygenated hemoglobin (hbr) (Kim and Ogawa, 2012), fNIRS also allows to measure oxy-hemoglobin (hbo) and total hemoglobin (Huppert et al., 2006). Increases in brain activation in the fNIRS studies are indicated by increases in hbo and decreases in hbr (e.g., Obrig et al., 2000). Findings from fMRI and fNIRS studies are correlated (Cui et al., 2011), but compared with fMRI, fNIRS has lower spatial resolution and limited measurement depth of about 1–1.5 cm (Boas et al., 2014; Strangman et al., 2002), which renders brain structures such as anterior cingulate cortex, medial temporal cortex or striatum inaccessible. However, compared to fMRI, fNIRS is less expensive, portable, less affected by subject motion and permits examination of vulnerable populations, especially those who cannot tolerate the MRI environment due to anxiety, claustrophobia, obesity, metal in the body or other issues. This makes fNIRS especially appealing for clinical neuroimaging research.
Only few previous neuroimaging studies used fNIRS in mood disorders research (Adorni et al., 2016; Zhang et al., 2015). For example, one fNIRS study showed that the changes in depression severity over time negatively correlated with changes in left prefrontal cortical activation during a verbal working memory task (Sato et al., 2011). Another fNIRS study showed that both individuals with bipolar disorder and those with major depression, compared to HC, had lower prefrontal cortical activation during working memory tasks (Schecklmann et al., 2011). No previous fNIRS study examined emotion processing in mood disordered individuals.
Our study is the first to employ fNIRS to examine the differences in PFC activation in depressed individuals vs. HC during recognition of emotional and neutral facial expressions. In Experiment 1, we designed and piloted the experimental paradigm to examine the hypothesis that depressed individuals make more errors than HC when judging neutral facial expressions (e.g., Gur et al., 1992; Leppänen et al., 2004; Surguladze et al., 2004). In Experiment 2, we sought to replicate behavioral findings from Experiment 1 and to examine the relationship between the behavioral findings and PFC activation as measured by the fNIRS method. Given previous findings that PFC is involved in recognition of emotional expressions (Fusar-Poli et al., 2009; Perry et al., 2017) and that depressed individuals showed both impaired emotion processing (Derntl et al., 2009; Gur et al., 1992; Persad and Polivy, 1993; Rubinow and Post, 1992; Drevets, 2001) and aberrant functioning of PFC (Fitzgerald et al., 2008; Hamilton et al., 2012), we hypothesized that lower neutral face recognition accuracy maybe related to aberrant PFC activation patterns in depressed individuals, compared to HC.
2. METHOD
2.1. Participants
The study was approved by the University of Pittsburgh Institutional Review Board. Written informed consent was obtained from all participants. HC without personal or family history of psychiatric disorders and depressed individuals suffering from major depressive or bipolar I or II disorder were recruited from local and university communities, counseling and medical centers, outpatient clinics, and from other research studies through advertisements and referrals. The participants were right handed, fluent in English, and were matched on age, gender and IQ. A total of 53 participants (33 depressed individuals diagnosed with either major depressive or bipolar disorders, 20 HC) participated in Experiment 1 (behavioral study) and 35 different participants (19 depressed individuals diagnosed with either major depressive or bipolar disorders, 16 HC) participated in Experiment 2 (fNIRS study). One depressed individual in Experiment 2 misunderstood instructions and was excluded from the dataset, leaving 34 participants in the fNIRS data analysis.
2.2. Clinical assessment
All diagnoses were made by a trained clinician and confirmed by a psychiatrist according to DSM-5 criteria using M.I.N.I.7.0 (Sheehan, 1998; Sheehan et al., 1997). Current depression symptoms measured by the Hamilton Rating Scale for Depression (HDRS-25; Hamilton, 1960), current mania symptoms measured by the Young Mania Rating Scale (YMRS; Young et al., 1978). Lifetime depression and hypo/mania spectrum symptomatology were measured using the mood spectrum self-report questionnaire (Dell’Osso et al., 2002). Exclusion criteria for both experiments included history of head injury, neurodevelopmental disorders, systemic medical illness, premorbid IQ<85 measured by the National Adult Reading Test (Blair and Spreen, 1989) current alcohol/drug abuse, YMRS scores >10 on the day of the experiment. A total psychotropic medication load was calculated for each participant, with greater numbers and doses of medications corresponding to a greater medication load (as described in Hassel et al., 2008; Manelis et al., 2016). Table 1 reports means and standard deviations of participants’ demographic and clinical characteristics for both experiments. The ratio of male/female for depressed individuals and HC were compared using a chi-square test. When appropriate, other demographic and clinical characteristics were compared in depressed individuals and HC using a t-test. These results are reported in Table 1.
Table 1.
Demographic and clinical characteristic of participants in Experiments 1 and 2
| Experiment 1 | Experiment 2 | |||||
|---|---|---|---|---|---|---|
| HC (n=20) Mean(SD) | Depressed (n=33) Mean(SD) | Statistics HC vs. Depressed | HC (n=16) Mean(SD) | Depressed (n=18) Mean(SD) | Statistics HC vs. Depressed | |
| Age | 25.72(6.85) | 23.57(5.27) | ns | 23.69(3.45) | 23.63(3.7) | ns |
| Gender (female/male) | 14/6 | 23/10 | ns | 14/2 | 16/2 | ns |
| IQ | 108.62(6.96) | 111.56(5.83) | ns | 107.57(4.33) | 109.49(7.49) | ns |
| Diagnosis (MDD/BD-I/BD-II) | na | 19/1/13 | na | 13/0/5 | ||
| Number of participants taking psychotropic medications: | na | 23 | na | 7 | ||
| 1 medication | na | 10 | na | 4 | ||
| 2 medications | na | 12 | na | 3 | ||
| >2 medications | na | 1 | na | 0 | ||
| Number of participants taking Antidepressants | na | 21 | na | 6 | ||
| Number of participants taking Antipsychotics | na | 0 | na | 0 | ||
| Number of participants taking Mood stabilizers | na | 3 | na | 2 | ||
| Number of participants taking Benzodiazepines | na | 5 | na | 1 | ||
| Number of participants taking Stimulants | na | 1 | na | 0 | ||
| Total medications load | na | 1.64(1.5) | na | 0.6(0.85) | ||
| Current depression (HDRS-25) | 1.3(1.26) | 24.18(6.74) | t(51)=−14.9, p<0.001 | 1.62(1.5) | 24.11 (6.2) | t(32)=−14.2, p<0.001 |
| Current mania (YMRS) | 0.35(0.75) | 2.33(2.3) | t(51)=−3.7, p<0.001 | 0.25(0.45) | 2.67(2.47) | t(32)=−3.8, p<0.001 |
| Lifetime depression (MOODs-SR) | 1(1.45) | 21.15(2.94) | t(51)=−28.6, p<0.001 | 0.69(0.87) | 20.67(2.97) | t(32)=−25.9, p<0.001 |
| Lifetime mania (MOODs-SR) | 4.55(4.15) | 12.42(6.89) | t(51)=−4.6, p<0.001 | 1.88(1.59) | 13.2(6.45) | t(32)=−6.8, p<0.001 |
Note. ns - not significant. SD - standard deviation
2.3. Behavioral paradigm
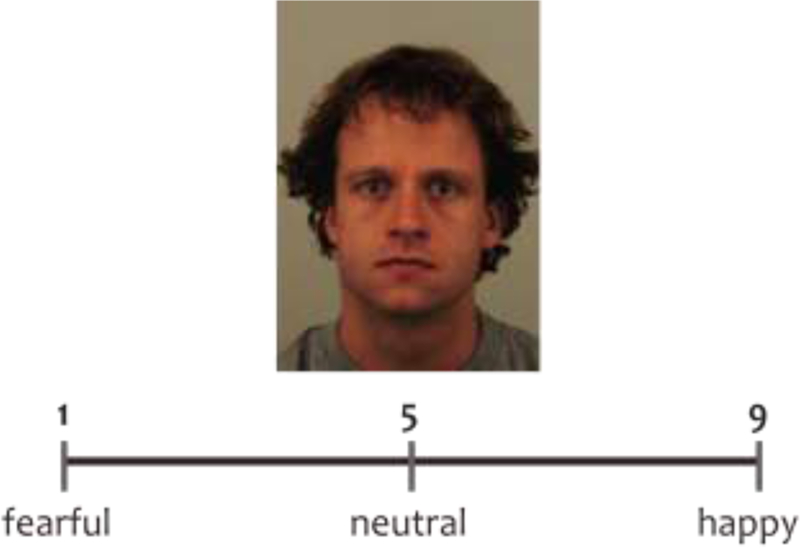
Participants performed the Emotion Intensity Rating task modeled after Erwin et al. (1992), in which they were shown happy, neutral and fearful faces taken from the Karolinska Directed Emotional Faces (KDEF) database (Goeleven et al., 2008). Participants were asked to rate the intensity of each face’s emotional expression on the scale 1 to 9 by pressing the corresponding buttons on the PC keyboard (Fig. 1). They were informed that there was no right or wrong answer, so all judgments had to be made based on a subject’s personal perception of each emotional face. Participants were instructed to give the face the score of 5 if they believed that the face was neutral, give the face the score between 6 and 9 if they believed that the face was happy (6 would be given to slightly happy faces and 9 would be given to very happy faces), and give the scores between 1 and 4 if they believed that the face was fearful (4 would be given to slightly fearful faces and 1 would be given to very fearful faces). We believe that these instructions made the task more sensitive to individual differences in perception of facial emotions because they did not require subjects to look for a ground truth for emotional categories (e.g., “I think most people would consider this face neutral, so I should respond it is neutral even though it seems slightly fearful to me”).
Fig 1.
Example of a Neutral trial in the Emotion Intensity Rating task.
There were 48 faces to judge (16 happy, 16 neutral, 16 fearful). The faces were shown in the center of the screen one at a time. The response options were shown on the scale 1 to 9. The scale was located below the face (Fig. 1). The task was self-paced. We asked participants to respond as quickly as possible but did not limit the duration of a trial. In Experiment 1, there was a 500 msec inter-trial interval (ITI). In Experiment 2, there was a 4500 msec ITI to better separate neural responses on each trial. The E-Prime program for Experiment 2 also incorporated triggers to mark the onset and offset of happy, neutral and fearful face stimuli.
2.4. fNIRS data acquisition
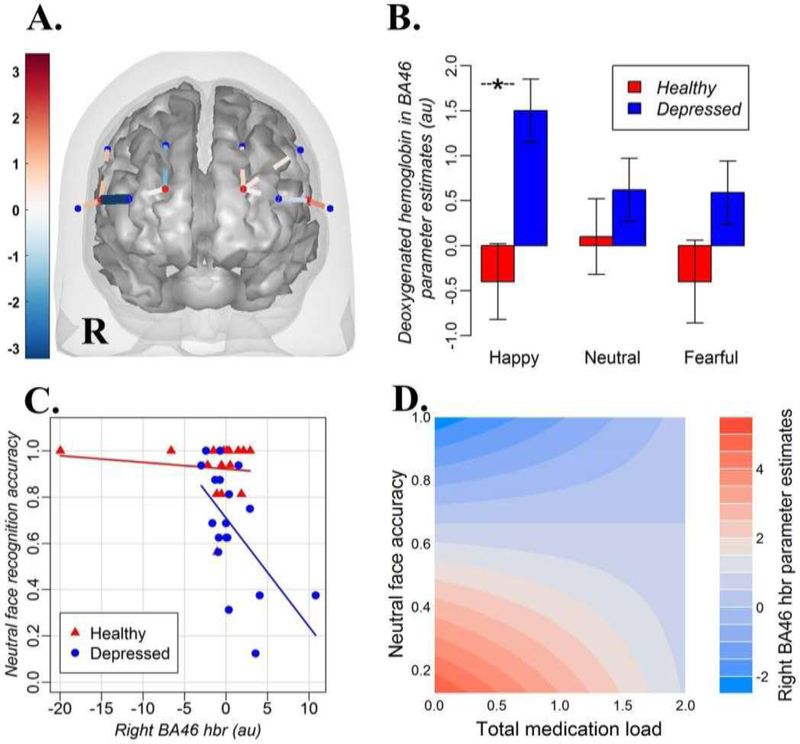
Optical imaging data were collected from bilateral frontal cortices using a CW6 fNIRS system (Techen, Inc., Milford, MA, USA) with laser optodes. The fNIRS probe consisted of a total of 4 light sources emitting light at two wavelengths (690-nm and 830-nm) and 8 light detectors. Including the two wavelengths allowed us to examine the concentration of both oxygenated (hbo) and deoxygenated (hbr) hemoglobin using the modified Beer-Lambert law (Delpy and Cope, 1997). In each hemisphere, the optodes in the probe were located 3 cm apart and were installed in a neoprene cap. The probe was built according to the 10/20 system and covered left and right BA10, BA 45 and BA46 (Fig 3). The center of the lower edge of the probe was aligned with FpZ.
Fig 3.
(A) A brain mesh with the probe and significant channels (source-detector pairs) showing the deoxygenated hemoglobin differences between the groups for the brain activation x neutral face recognition accuracy interaction. The dark blue channel corresponds to right BA46. The location of fNIRS sensors are shown overlaid on the Colin27 atlas template for display purposes. R = right hemisphere. The blue dots on the probe are detectors, the red dots are light sources. The color bar shows t-statistics for the differences between depressed individuals vs. HC in accuracy x activation slopes. (B) Parameter estimates (betas) for deoxygenated hemoglobin (hbr) in BA46 during recognition of happy, neutral and fearful emotional expression in depressed individuals and HC. The star (-*-) denotes significantly different comparisons. (C) Between-group differences in the interaction effect between brain activation in BA46 as reflected by the measures of deoxygenated hemoglobin (hbr) and accuracy for neutral faces in depressed individuals and HC. (D) The illustration of the medication load x accuracy for neutral faces interaction on activation in right BA46 hbr. Blue color stands for lower hbr values and higher brain activation, while red color stands for higher hbr values and lower brain activation
The fNIRS data were collected using a custom-built data acquisition interface (Abdelnour and Huppert, 2009). Data were collected at 20Hz. The stimuli were presented using E-Prime (Psychology Software Tools, Sharpsburg, PA, USA) software that was synchronized with the fNIRS data acquisition in Experiment 2. During the fNIRS experiment, participants were comfortably seated at the desk in front of the PC computer with the probe located on their head.
2.5. Data analyses
Behavioral and neuroimaging data analyses were conducted using linear mixed effects models that use explanatory variables and random effects to estimate group-level effects. It was shown that linear mixed effect models outperform traditionally used repeated measures ANOVA (Kristensen & Hansen, 2004) by taking into account the subject’s level variance. Given that mixed effects models take into account multiple sources of variance, degrees of freedom for mixed models can be only roughly estimated. For example, a different heuristics is applied for degrees of freedom calculation in a contrast analysis based on estimated marginal means from a mixed effects model than that in a traditional t-test.
2.5.1. Behavioral data analysis
The responses were considered accurate when participants assigned ‘5’ to neutral faces, scores between 6 and 9 to happy faces and scores between 1 and 4 to fearful faces. All behavioral data in Experiments 1 and 2 were analyzed using R (https://www.r-project.org/). Response accuracy and reaction time (RT) in both experiments were analyzed using linear mixed effects models implemented through the lmerTest package in R (Kuznetsova, Brockhoff, & Christensen, 2014) using the Satterthwaite’s degrees of freedom approach (Satterthwaite, 1946) to estimate denominator degrees of freedom for F or t statistic. Group (HC/ Depressed) and emotions (positive/neutral/negative) were explanatory variables, and subject was a random effect variable.
A contrast analysis to examine the group differences for each emotional condition was based on estimated marginal means from a mixed model and was implemented using the ‘psycho’ package in R (Makowski, 2018). The p-values were adjusted for dependent multiple comparisons at a false discovery rate (FDR) with the significance level set at 0.05 (q ≤ 0.05) (Yekutieli & Benjamini, 1999).
2.5.2. fNIRS data analysis
The fNIRS data were analyzed using the NIRS toolbox (Santosa et al., 2018). The data were resampled from 20Hz to 4Hz, converted to the changes in optical density over time and then converted to oxygenated hemoglobin (hbo) and deoxygenated hemoglobin (hbr) estimates using the modified Beer-Lambert law with a partial pathlength correction factor of 0.1 (Strangman et al., 2003).
The first-level (or subject-level) analyses used the statistical autoregressively whitened weighted least-squares (general linear) regression model (Barker et al., 2013) with happy, neutral, and fearful faces as explanatory variables. This model statistically addresses both increased false-discovery rates introduced by serially-correlated noise due to physiology in fNIRS and outliers related to motion artifacts (for the review see Huppert, 2016). This model has been previously validated and compared with other linear regression approaches and showed better sensitivity-specificity characteristics (Santosa et al., 2018). The canonical HRF function was used to model a hemodynamic response with an undershoot period (Santosa et al., 2018).
The first-level models were incorporated into group-level analyses for which the model was whitened using the error-covariance of the first level GLM model (Santosa et al., 2018). To control for multiple comparisons (including the number of comparison conditions and NIRS channels), a false discovery rate (FDR) correction was used with the significance level set at 0.05 (q ≤ 0.05) (Benjamini and Hochberg, 1995).
The first group-level analysis used mixed effects models with group (HC/depressed) and emotion (happy/neutral/fearful) as explanatory variables, and subject as a random effect to identify the effects of group and emotional condition, as well as the interaction between the two on brain activation in the right and left PFC. A subsequent contrast analysis compared PFC activation in depressed individuals vs. HC for each emotional condition. The second group-level analysis used mixed effects models with group (HC/depressed), condition (happy/neutral/fearful) and accuracy for neutral face recognition as explanatory variables, and subject as a random effect to identify the interaction between these variables on brain activation in the right and left PFC. The latter analysis was conducted only for neutral faces because there was no sufficient variability in recognition accuracy for fearful and happy faces (Fig.1). A subsequent contrast analysis compared the slopes for the relationship between PFC activation and accuracy for neutral face recognition in depressed individuals vs. that in HC. Given that the groups did not differ in mean age, IQ or gender composition, these variables were not entered into the model.
2.5.3. Exploratory analysis: Effect of Medications
Exploratory analyses examined a possibility that the effects observed in the analyses described above were due to the effect of psychotropic medications that some depressed individuals had been taken. The effect of medications was examined in the group of depressed individuals using two strategies: (1) by comparing the effects identified by the mixed effects models for depressed individuals ON vs. those OFF psychotropic medications, and (2) by using a psychotropic medication load as a covariate in the group-level mixed effect models.
3. RESULTS
3.1. Experiment 1
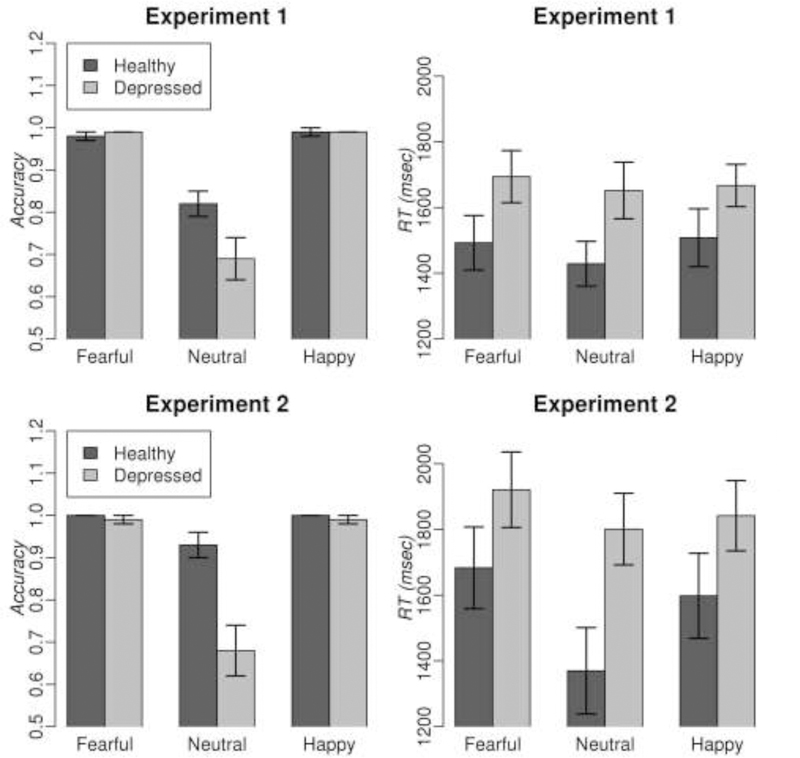
The mixed effects models revealed a significant effect of condition (F(2,153)= 52.2, p<0.001) and group*condition interaction (F(2, 153)= 4.25, p<0.05) on participants’ accuracy to recognize emotional and neutral facial expressions, but no main effect of group. HC were significantly more accurate than depressed individuals during recognition of neutral facial expressions (t=3.35, q=0.002, df=153). The groups were not different in accuracy for happy and fearful faces. The same analysis of RT revealed a significant main effect of group (F(1,51)= 4.03, p<0.05) on RT, but no effect of condition, or group*condition interaction. There were no significant between-group differences in RT for any specific emotion. The results are illustrated in Fig. 2. An exploratory analysis showed that response accuracy and RT in depressed individuals did not depend either on whether depressed individuals were ON or OFF psychotropic medications, or on a total medication load.
Fig 2.
Mean accuracy and reaction time (RT) during rating of intensity of facial emotional expressions in depressed individuals and healthy controls in Experiments 1 and 2. Error bars show standard errors of mean.
3.2. Experiment 2
The mixed effects models revealed significant effects of condition (F(2,64)= 26.5, p<0.001), group (F(1,32)= 12.8, p <0.001) and group*condition interaction (F(2,64)= 11.9, p<0.001) on participants’ accuracy to recognize emotional and neutral facial expressions. As in Experiment 1, the only significant difference between HC and depressed individuals was observed for neutral facial expression recognition accuracy (t=6.1, q<0.001, df=96) with HC been more accurate than depressed individuals. The analysis of RT revealed significant effects of condition (F(2,64)= 8.4, p <0.001) and group (F(1,32)= 4.2, p <0.05), but no significant group*condition interaction effect). HC were faster than depressed individuals during recognition of neutral facial expression (t=6.1, q<0.001, df=46). An exploratory analysis showed that response accuracy and RT in depressed individuals did not depend either on whether depressed individuals were ON or OFF psychotropic medications, or on a total medication load.
3.2.2. fNIRS results
The mixed effects models revealed no significant effects of Emotion or Group, or Group x Emotion interaction on PFC activation. A contrast analysis based on estimated marginal means from a mixed model revealed no significant group effect on brain activation for neutral and fearful faces. The same contrast analysis, however, revealed lower PFC activation in depressed individuals relative to HC as indicated by significantly higher concentration of deoxygenated hemoglobin (hbr) in right BA45 and BA46 in depressed individuals vs. in HC (BA45: t=3.64, q=0.005, df=96, power=0.97; BA46: t=3.45, q=0.005, df=96, power=0.96) to happy faces (Fig. 3B). As the exploratory analyses showed, brain activation in right BA45 and BA46 for happy faces in depressed individuals did not depend either on whether depressed individuals were ON or OFF psychotropic medications, or on a total medication load.
The mixed effects model and a follow-up contrast analysis that examined whether the relationship between PFC activation during recognition of neutral facial expressions and corresponding accuracy for recognition of neutral facial expressions differed between depressed individuals and HC revealed significant between-group differences in right BA46 (t=−3.0, q=0.036, df=96, power=0.92) with a steeper slope observed for depressed individuals vs. HC. A follow-up analysis showed that this effect was explained by the fact that depressed individuals, but not HC, had a significant negative relationship between neutral face recognition accuracy and concentration of deoxygenated hemoglobin in right BA46 (t=−4.5, q=0.0008, df=96, power=0.99; Fig. 3A and 3C), thus suggesting that those depressed individuals who were more accurate on the task also had higher activation in right BA46. An exploratory analysis showed that the effect of accuracy on brain activation for neutral faces in depressed individuals did not depend on whether depressed individuals were ON or OFF psychotropic medications. There was the interaction effect of a total medication load and accuracy for neutral faces on right BA46 activation (BA46 hbr: t=3.8, q=0.009, df=51, power=0.98), however (Fig. 3D). The latter indicated that the relationship between accuracy and BA46 activation was more pronounced for depressed individuals with low medication load, than in depressed individuals with higher medication load.
4. DISCUSSION
To our knowledge, this study is the first to examine recognition of facial emotional expressions in depressed individuals with mood disorders using fNIRS. In this study, we examined behavioral and neural correlates of facial emotion recognition in depressed individuals across mood disorders vs. HC. Consistent with previous findings (Gur et al., 1992; Leppänen et al., 2004; Surguladze et al., 2005) and our hypothesis, both experiments demonstrated that depressed individuals were significantly slower and less accurate than HC in recognizing neutral facial expressions, thus showing impairment in recognition of neutral facial expression. fNIRS revealed that the impairment in distinguishing neutral facial expressions from emotional ones in depressed individuals might be related to aberrant decreases in DLPFC activation during face processing.
The DLPFC is involved in emotion regulation and cognitive control (Ochsner and Gross, 2005), as well as in goal planning (Kaller et al., 2011), dealing with ill-structured problems that have no unique correct solution (Gilbert et al., 2010), and increase in behavioral inhibition (Shackman et al., 2009) among other cognitive processes. In this study, we showed that greater behavioral impairment in depressed individuals corresponded to lower right BA46 (or DLPFC) activation during neutral face processing. These effects did not depend on whether depressed individuals were ON or OFF psychotropic medications, but depended on a total medication load. It is noteworthy that the most pronounced effect was observed in less medicated or unmedicated depressed individuals, while the least pronounced effect was observed in those who were most medicated. This might suggest that medications mitigate the effect of depression on recognition of neutral faces. The fact that there was no significant effect of a total medication load on recognition accuracy in depressed individuals does not support that hypothesis, however. Alternatively, higher load of psychotropic medications may hinder the detection of the effects that would be otherwise observed in depressed individuals.
It is also noteworthy that despite comparable recognition accuracy, depressed individuals showed lower activation than HC in the right DLPFC during processing of happy faces. One explanation for this effect is that happy faces are highly salient (e.g., a smile can automatically attract attention) and, consequently, recognizing happy facial expression may be much easier than recognizing neutral facial expressions so lower PFC activation did not affect recognition accuracy on an easy task.
Emotion regulation can rely on top-down or bottom-up strategies (Ochsner and Gross, 2005). The automatic bottom-up emotion regulation is likely disrupted in depressed individuals due to aberrant functioning of the amygdala and other brain regions (Peluso et al., 2009; Surguladze et al., 2005; Suslow et al., 2010) involved in processing of emotional faces (Fusar-Poli et al., 2009). Given that aberrant increases in amygdala activation can result in interpreting emotionally neutral stimuli as emotionally meaningful (Drevets, 2001), the top-down regulatory signal from the PFC might be necessary in order to change the mental representation of a stimulus in the amygdala (Ochsner et al., 2012) and help depressed individuals distinguish emotional and non-emotional stimuli without relying on automatic processing of salient information. Reduced PFC activation may affect the ability of depressed individuals to discriminate between neutral and emotionally-salient facial stimuli.
Multiple previous studies used emotionally neutral stimuli as a comparison baseline for emotional (e.g., happy, fearful, sad) stimuli. Our neuroimaging findings support a recent conclusion (Filkowski and Haas, 2017) that using neutral stimuli as a reference baseline in the studies of emotion processing and regulation is not always appropriate as processing of neutral faces might be impaired in individuals with psychiatric disorders. Using the neutral face baseline to examine PFC activation in fearful and happy emotional conditions would differentially affect depressed individuals and HC, and would also bias within-group comparisons as a function of participants’ ability to accurately recognize neutral facial expressions.
Our study demonstrated that that it was feasible to study the processing of emotional information in PFC in mood disordered individuals using fNIRS. The fNIRS method opens new perspectives in clinical neuroimaging. First, it allows conducting neuroimaging research on patients who would not otherwise meet the inclusion criteria for fMRI due to claustrophobia, excessive weight, metal in the body, etc. Second, it extends the application of neuroimaging to ‘field’ studies that can be conducted, for example, in the doctor’s office or during a therapy session.
Limitations and future directions
While we replicated behavioral findings in two experiments, replicating our fNIRS results in a larger sample would ensure their validity and reliability. Due to small sample sizes, our exploratory analyses regarding the effect of psychotropic medications should be interpreted with caution. Small sample sizes also did not allow us to compare individuals with major depressive vs. those with bipolar I and vs. those with bipolar II disorders. Future studies should examine the differences between individuals with these mood disorder subtypes. Despite small sample sizes, however, all fNIRS analyses had high power (>0.9), suggesting that the sample size was adequate. Our patient sample only included depressed individuals, which did not allow us to make conclusions about how the absence of depressive symptoms (or presence of hypo/manic symptoms) affects patients’ ability to process neutral faces. Future cross-sectional and longitudinal studies should examine how changes in mood state (improvement/worsening of depression and/or mania symptoms) affect participants’ ability to identify neutral facial expressions and how these changes affect PFC activation. Our findings point to the right DLPFC as a potential target of neuromodulation to improve the ability of depressed individuals to discriminate neutral and emotional signals in the environment. Future studies should examine how modulation of the right DLPFC affects processing of neutral and emotional stimuli in depressed individuals and HC.
Highlights.
The differences in DLPFC activation in depressed individuals vs. healthy controls during recognition of emotional and neutral facial expressions was examined using functional near-infrared spectroscopy.
Depressed individuals, compared to healthy controls, were slower and less accurate in recognizing neutral, but not happy or fearful, facial emotional expressions.
Depressed individuals, but not healthy controls, rely on functioning of the right DLPFC during recognition of neutral and happy facial expressions
Depressed individuals who had lower activation in the right DLPFC during recognition of neutral facial expression also had lower recognition accuracy for neutral facial expressions compared to depressed individuals who had greater right DLPFC activation.
Acknowledgments
This work was supported by the grants from the National Institute of Health K01MH104348 to A.M. The authors thank participants for taking part in this research study.
Role of the Funding Source
The funder had no role in study design, data collection, analysis, or interpretation, the manuscript writing or the decision to submit it for publication.
Author Statement
We wish to confirm that there are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome.
We confirm that the manuscript has been read and approved by all named authors and that there are no other persons who satisfied the criteria for authorship but are not listed. We further confirm that the order of authors listed in the manuscript has been approved by all of us.
We confirm that we have given due consideration to the protection of intellectual property associated with this work and that there are no impediments to publication, including the timing of publication, with respect to intellectual property. In so doing we confirm that we have followed the regulations of our institutions concerning intellectual property.
We further confirm that any aspect of the work covered in this manuscript that has involved human subjects has been conducted with the ethical approval of all relevant bodies and that such approvals are acknowledged within the manuscript.
We understand that the Corresponding Author is the sole contact for the Editorial process (including Editorial Manager and direct communications with the office). She is responsible for communicating with the other authors about progress, submissions of revisions and final approval of proofs. We confirm that we have provided a current, correct email address which is accessible by the Corresponding Author and which has been configured to accept email from the Journal of Affective Disorders.
Footnotes
Declarations of interest: none
Conflict of Interest
The authors report no conflict of interest and no financial relationships with commercial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abdelnour AF, Huppert T, 2009. Real-time imaging of human brain function by near-infrared spectroscopy using an adaptive general linear model. Neuroimage 46, 133–143. 10.1016/j.neuroimage.2009.01.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adorni R, Gatti A, Brugnera A, Sakatani K, Compare A, 2016. Could fNIRS Promote Neuroscience Approach in Clinical Psychology? Front. Psychol 7, 456 10.3389/fpsyg.2016.00456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker JW, Aarabi A, Huppert TJ, 2013. Autoregressive model based algorithm for correcting motion and serially correlated errors in fNIRS. Biomed. Opt. Express 4, 1366–1379. 10.1364/BOE.4.001366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendall RCA, Eachus P, Thompson C, 2016. A Brief Review of Research Using Near-Infrared Spectroscopy to Measure Activation of the Prefrontal Cortex during Emotional Processing: The Importance of Experimental Design. Front. Hum. Neurosci 10, 529 10.3389/fnhum.2016.00529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y, 1995. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B. Methodol 1, 289–300. [Google Scholar]
- Bilderbeck AC, Atkinson LZ, Geddes JR, Goodwin GM, Harmer CJ, 2017. The effects of medication and current mood upon facial emotion recognition: findings from a large bipolar disorder cohort study. J. Psychopharmacol 31, 320–326. 10.1177/0269881116668594 [DOI] [PubMed] [Google Scholar]
- Blair JR, Spreen O, 1989. Predicting premorbid IQ: a revision of the National Adult Reading Test. Clin. Neuropsychol 3, 129–136. [Google Scholar]
- Boas DA, Elwell CE, Ferrari M, Taga G, 2014. Twenty years of functional near-infrared spectroscopy: introduction for the special issue. Neuroimage 85 Pt 1, 1–5. 10.1016/j.neuroimage.2013.11.033 [DOI] [PubMed] [Google Scholar]
- Colibazzi T, Posner J, Wang Z, Gorman D, Gerber A, Yu S, Zhu H, Kangarlu A, Duan Y, Russell JA, Peterson BS, 2010. Neural systems subserving valence and arousal during the experience of induced emotions. Emotion 10, 377–389. 10.1037/a0018484 [DOI] [PubMed] [Google Scholar]
- Cui X, Bray S, Bryant DM, Glover GH, Reiss AL, 2010. A quantitative comparison of NIRS and fMRI across multiple cognitive tasks. NeuroImage 54, 2808–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dell’Osso L, Armani A, Rucci P, Frank E, Fagiolini A, Corretti G, Shear MK, Grochocinski VJ, Maser JD, Endicott J, Cassano GB, 2002. Measuring mood spectrum: comparison of interview (SCI-MOODS) and self-report (MOODS-SR) instruments. Compr. Psychiatry 43, 69–73. [DOI] [PubMed] [Google Scholar]
- Delpy DT, Cope M, 1997. Quantification in tissue near-infrared spectroscopy. Philos. Trans. R. Soc. B Biol. Sci 352, 649. [Google Scholar]
- Demenescu LR, Renken R, Kortekaas R, van Tol M-J, Marsman JBC, van Buchem MA, van der Wee NJA, Veltman DJ, den Boer JA, Aleman A, 2011. Neural correlates of perception of emotional facial expressions in out-patients with mild-to-moderate depression and anxiety. A multicenter fMRI study. Psychol. Med 41, 2253–2264. 10.1017/S0033291711000596 [DOI] [PubMed] [Google Scholar]
- Derntl B, Seidel E-M, Kryspin-Exner I, Hasmann A, Dobmeier M, 2009. Facial emotion recognition in patients with bipolar I and bipolar II disorder. Br. J. Clin. Psychol 48, 363–375. 10.1348/014466509X404845 [DOI] [PubMed] [Google Scholar]
- Drevets WC, 2001. Neuroimaging and neuropathological studies of depression: implications for the cognitive-emotional features of mood disorders. Curr. Opin. Neurobiol 11, 240–249. [DOI] [PubMed] [Google Scholar]
- Erwin RJ, Gur RC, Gur RE, Skolnick B, Mawhinney-Hee M, Smailis J, 1992. Facial emotion discrimination: I. Task construction and behavioral findings in normal subjects. Psychiatry Res 42, 231–240. [DOI] [PubMed] [Google Scholar]
- Filkowski MM, Haas BW, 2017. Rethinking the Use of Neutral Faces as a Baseline in fMRI Neuroimaging Studies of Axis-I Psychiatric Disorders. J. Neuroimaging 27, 281–291. 10.1111/jon.12403 [DOI] [PubMed] [Google Scholar]
- Fitzgerald PB, Laird AR, Maller J, Daskalakis ZJ, 2008. A meta-analytic study of changes in brain activation in depression. Hum. Brain Mapp 29, 683–695. 10.1002/hbm.20426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fusar-Poli P, Placentino A, Carletti F, Landi P, Allen P, Surguladze S, Benedetti F, Abbamonte M, Gasparotti R, Barale F, Perez J, McGuire P, Politi P, 2009. Functional atlas of emotional faces processing: a voxel-based meta-analysis of 105 functional magnetic resonance imaging studies. J. Psychiatry Neurosci 34, 418–432. [PMC free article] [PubMed] [Google Scholar]
- Gilbert SJ, Zamenopoulos T, Alexiou K, Johnson JH, 2010. Involvement of right dorsolateral prefrontal cortex in ill-structured design cognition: an fMRI study. Brain Res 1312, 79–88. 10.1016/j.brainres.2009.11.045 [DOI] [PubMed] [Google Scholar]
- Goeleven E, De Raedt R, Leyman L, Verschuere B, 2008. The Karolinska directed emotional faces: a validation study. Cogn. Emot 22, 1094–1118. [Google Scholar]
- Gray J, Venn H, Montagne B, Murray L, Burt M, Frigerio E, Perrett D, Young AH, 2006. Bipolar patients show mood-congruent biases in sensitivity to facial expressions of emotion when exhibiting depressed symptoms, but not when exhibiting manic symptoms. Cogn. Neuropsychiatry 11, 505–520. [DOI] [PubMed] [Google Scholar]
- Gur RC, Erwin RJ, Gur RE, Zwil AS, Heimberg C, Kraemer HC, 1992. Facial emotion discrimination: II. Behavioral findings in depression. Psychiatry Res 42, 241–251. [DOI] [PubMed] [Google Scholar]
- Hamilton JP, Etkin A, Furman DJ, Lemus MG, Johnson RF, Gotlib IH, 2012. Functional neuroimaging of major depressive disorder: a meta-analysis and new integration of baseline activation and neural response data. Am. J. Psychiatry 169, 693–703. [DOI] [PubMed] [Google Scholar]
- Hamilton M, 1960. A rating scale for depression. J. Neurol. Neurosurg. Psychiatry 23, 56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassel S, Almeida JR, Kerr N, Nau S, Ladouceur CD, Fissell K, et al. , 2008. Elevated striatal and decreased dorsolateral prefrontal cortical activity in response to emotional stimuli in euthymic bipolar disorder: no associations with psychotropic medication load. Bipolar Disord 10, 916–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller AS, Johnstone T, Peterson MJ, Kolden GG, Kalin NH, Davidson RJ, 2013. Increased prefrontal cortex activity during negative emotion regulation as a predictor of depression symptom severity trajectory over 6 months. JAMA psychiatry 70, 1181–1189. 10.1001/jamapsychiatry.2013.2430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huppert TJ, 2016. Commentary on the statistical properties of noise and its implication on general linear models in functional near-infrared spectroscopy. Neurophotonics 3, 10401 10.1117/1.NPh.3.1.010401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judd LL, Akiskal HS, Schettler PJ, Endicott J, Leon AC, Solomon DA, Coryell W, Maser JD, Keller MB, 2005. Psychosocial disability in the course of bipolar I and II disorders: a prospective, comparative, longitudinal study. Arch. Gen. Psychiatry 62, 1322–1330. 10.1001/archpsyc.62.12.1322 [DOI] [PubMed] [Google Scholar]
- Judd LL, Akiskal HS, Zeller PJ, Paulus M, Leon AC, Maser JD, Endicott J, Coryell W, Kunovac JL, Mueller TI, Rice JP, Keller MB, 2000. Psychosocial disability during the long-term course of unipolar major depressive disorder. Arch. Gen. Psychiatry 57, 375–380. [DOI] [PubMed] [Google Scholar]
- Kaller CP, Rahm B, Spreer J, Weiller C, Unterrainer JM, 2011. Dissociable contributions of left and right dorsolateral prefrontal cortex in planning. Cereb. Cortex 21, 307–317. 10.1093/cercor/bhq096 [DOI] [PubMed] [Google Scholar]
- Kim S-G, Ogawa S, 2012. Biophysical and physiological origins of blood oxygenation level-dependent fMRI signals. J. Cereb. Blood Flow Metab 32, 1188–1206. 10.1038/jcbfm.2012.23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuznetsova A, Brockhoff PB, Christensen RHB, 2017. lmerTest Package: Tests in Linear Mixed Effects Models. Journal of Statistical Software 82(13), 1–26. https://doi:10.18637/jss.v082.i13 [Google Scholar]
- Leppänen JM, Milders M, Bell JS, Terriere E, Hietanen JK, 2004. Depression biases the recognition of emotionally neutral faces. Psychiatry Res 128, 123–133. 10.1016/j.psychres.2004.05.020 [DOI] [PubMed] [Google Scholar]
- Makowski D, 2018. The Psycho Package: An Efficient and Publishing-Oriented Workflow for Psychological Science. Journal of Open Source Software 3(22), 470 https://github.com/neuropsychology/psycho.R [Google Scholar]
- Manelis A, Almeida JR, Stiffler R, Lockovich JC, Aslam HA, & Phillips ML, 2016. Anticipation-related brain connectivity in bipolar and unipolar depression: a graph theory approach. Brain 139(Pt 9), 2554–2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Münkler P, Rothkirch M, Dalati Y, Schmack K, Sterzer P, 2015. Biased recognition of facial affect in patients with major depressive disorder reflects clinical state. PLoS One 10, e0129863 10.1371/journal.pone.0129863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obrig H, 2014. NIRS in clinical neurology - a “promising” tool? Neuroimage 85 Pt 1, 535–546. 10.1016/j.neuroimage.2013.03.045 [DOI] [PubMed] [Google Scholar]
- Obrig H, Neufang M, Wenzel R, Kohl M, Steinbrink J, Einhäupl K, Villringer A, 2000. Spontaneous low frequency oscillations of cerebral hemodynamics and metabolism in human adults. Neuroimage 10.1006/nimg.2000.0657 [DOI] [PubMed]
- Ochsner KN, Gross JJ, 2005. The cognitive control of emotion. Trends Cogn. Sci 9, 242–249. 10.1016/j.tics.2005.03.010 [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Silvers JA, Buhle JT, 2012. Functional imaging studies of emotion regulation: a synthetic review and evolving model of the cognitive control of emotion. Ann. N. Y. Acad. Sci 1251, E1--24 10.1111/j.1749-6632.2012.06751.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peluso MAM, Glahn DC, Matsuo K, Monkul ES, Najt P, Zamarripa F, Li J, Lancaster JL, Fox PT, Gao J-H, Soares JC, 2009. Amygdala hyperactivation in untreated depressed individuals. Psychiatry Res 173, 158–161. 10.1016/j.pscychresns.2009.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry A, Saunders SN, Stiso J, Dewar C, Lubell J, Meling TR, Solbakk AK, Endestad T, … Knight RT, 2017. Effects of prefrontal cortex damage on emotion understanding: EEG and behavioural evidence. Brain 140, 1086–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persad SM, Polivy J, 1993. Differences between depressed and nondepressed individuals in the recognition of and response to facial emotional cues. J. Abnorm. Psychol 102, 358–368. [DOI] [PubMed] [Google Scholar]
- Rubinow DR, Post RM, 1992. Impaired recognition of affect in facial expression in depressed patients. Biol. Psychiatry 31, 947–953. [DOI] [PubMed] [Google Scholar]
- Santosa H, Zhai X, Fishburn F, Huppert T, 2018. The NIRS Brain AnalyzIR Toolbox. Algorithms 11, 73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato H, Katura T, Koizumi H, Aoki R, Matsuda R, 2011. Correlation of within-individual fluctuation of depressed mood with prefrontal cortex activity during verbal working memory task: optical topography study. J. Biomed. Opt 16, 126007. [DOI] [PubMed] [Google Scholar]
- Satterthwaite FE, 1946. An approximate distribution of estimates of variance components. Biom Bull 2, 110–114. doi: 10.2307/3002019. [DOI] [PubMed] [Google Scholar]
- Schecklmann M, Dresler T, Beck S, Jay JT, Febres R, Haeusler J, Jarczok TA, Reif A, Plichta MM, Ehlis A-C, others, 2011. Reduced prefrontal oxygenation during object and spatial visual working memory in unpolar and bipolar depression. Psychiatry Res. Neuroimaging 194, 378–384. [DOI] [PubMed] [Google Scholar]
- Shackman AJ, McMenamin BW, Maxwell JS, Greischar LL, Davidson RJ, 2009. Right dorsolateral prefrontal cortical activity and behavioral inhibition. Psychol. Sci 20, 1500–1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan DV, 1998. MINI-Mini International neuropsychiatric interview-english version 5.0. 0-DSM-IV. J Clin Psychiatry 59, 34–57. [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Janavs J, Weiller E, Keskiner A, Schinka J, Knapp E, Sheehan MF, Dunbar GC, 1997. The validity of the Mini International Neuropsychiatric Interview (MINI) according to the SCID-P and its reliability. Eur. Psychiatry 12, 232–241. [Google Scholar]
- Steel Z, Marnane C, Iranpour C, Chey T, Jackson JW, Patel V, Silove D, 2014. The global prevalence of common mental disorders: a systematic review and meta-analysis 1980--2013. Int. J. Epidemiol 43, 476–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strangman G, Culver JP, Thompson JH, Boas DA, 2002. A quantitative comparison of simultaneous BOLD fMRI and NIRS recordings during functional brain activation. Neuroimage 17, 719–731. [PubMed] [Google Scholar]
- Strangman G, Franceschini MA, Boas DA, 2003. Factors affecting the accuracy of near-infrared spectroscopy concentration calculations for focal changes in oxygenation parameters. Neuroimage 18, 865–879. [DOI] [PubMed] [Google Scholar]
- Surguladze S, Brammer MJ, Keedwell P, Giampietro V, Young AW, Travis MJ, Williams SCR, Phillips ML, 2005. A differential pattern of neural response toward sad versus happy facial expressions in major depressive disorder. Biol. Psychiatry 57, 201–209. 10.1016/j.biopsych.2004.10.028 [DOI] [PubMed] [Google Scholar]
- Surguladze SA, Young AW, Senior C, Brébion G, Travis MJ, Phillips ML, 2004. Recognition accuracy and response bias to happy and sad facial expressions in patients with major depression. Neuropsychology 18, 212–218. 10.1037/0894-4105.18.2.212 [DOI] [PubMed] [Google Scholar]
- Suslow T, Konrad C, Kugel H, Rumstadt D, Zwitserlood P, Schöning S, Ohrmann P, Bauer J, Pyka M, Kersting A, Arolt V, Heindel W, Dannlowski U, 2010. Automatic mood-congruent amygdala responses to masked facial expressions in major depression. Biol. Psychiatry 67, 155–160. 10.1016/j.biopsych.2009.07.023 [DOI] [PubMed] [Google Scholar]
- Young RC, Biggs JT, Ziegler VE, Meyer DA, 1978. A rating scale for mania: reliability, validity and sensitivity. Br. J. Psychiatry 133, 429–435. [DOI] [PubMed] [Google Scholar]
- Yekutieli D, Benjamini Y, 1999. Resampling-based false discovery rate controlling multiple test procedures for correlated test statistics. J. Stat. Plan. Inference 82, 171–196. [Google Scholar]
- Zhang H, Dong W, Dang W, Quan W, Tian J, Chen R, Zhan S, Yu X, 2015. Near-infrared spectroscopy for examination of prefrontal activation during cognitive tasks in patients with major depressive disorder: a meta-analysis of observational studies. Psychiatry Clin. Neurosci 69, 22–33. 10.1111/pcn.12209 [DOI] [PubMed] [Google Scholar]
- Zhong M, Wang X, Xiao J, Yi J, Zhu X, Liao J, Wang W, Yao S, 2011. Amygdala hyperactivation and prefrontal hypoactivation in subjects with cognitive vulnerability to depression. Biol. Psychol 88, 233–242. 10.1016/j.biopsycho.2011.08.007 [DOI] [PubMed] [Google Scholar]