Abstract
Bimanual skills are important for goal-oriented activities. Children with unilateral cerebral palsy (UCP) have deficits in unimanual and bimanual motor control and learning. The application of non-invasive brain stimulation with existing motor training may further promote motor learning, however the effects of stimulation on bimanual learning have not been examined. Here, we assessed performance of a novel bimanual skill (modified Speed Stacks task) in eight children with UCP before, during, and after a combined motor training and brain stimulation intervention. Participants received ten days (120 minutes/day) of goal-oriented bimanual therapy combined initially with transcranial direct current stimulation (tDCS, 20 minutes/day). Results showed task improvement tapered (p<0.001) during and after the intervention and task variability decreased in 6/8 participants, indicating the potential impact of novel rehabilitation to improve skill learning in children with UCP. Future work is required to understand how both tDCS and bimanual training contribute to learning bimanual tasks.
Keywords: Bimanual learning, unilateral cerebral palsy, transcranial direct current stimulation
1. Introduction
Bimanual skills are an important aspect of everyday hand use, and involve distinct processing in both primary and supplemental motor areas facilitated by interhemispheric connections to regulate execution of commands.1 Children with unilateral cerebral palsy (UCP) and weakness primarily on one side of the body, have deficits in bimanual motor control but benefit from bimanual training interventions to achieve goals requiring use of both hands.2–7 Therapeutic applications of non-invasive brain stimulation, namely transcranial direct current stimulation (tDCS), have emerged to potentially enhance unimanual function during intensive physical rehabilitation. Prior randomized trials have shown combined tDCS and motor training to be safe and feasible in children with UCP, and begin to demonstrate improved outcomes as well.8–11 Overall, the effects of tDCS on motor skill learning in typically-developed adults show that tDCS enhances bimanual performance and skill learning, however there may be variability depending on the stimulation parameters used.12 While learning new bimanual skills may be slower in children with UCP compared to typically-developing peers,13 the impact of combined tDCS and motor training on bimanual skill learning has not been investigated. The addition of brain stimulation applied to the non-lesioned hemisphere may help re-balance interhemispheric activity to a greater extent than achieved by bimanual training alone. This may lead to enhanced motor learning, and thereby decrease the overall duration and intensity of bimanual motor training. Understanding the added influence of brain stimulation to promote the acquisition and retention of bimanual skills is an important step in optimizing novel rehabilitation interventions for children with UCP. Therefore, we studied the effects of a combined tDCS and bimanual motor training intervention on the acquisition a new bimanual skill in children and young adults with UCP who exhibited intact contralateral corticospinal tract (CST) projections from the lesioned hemisphere. Targeting this specific pattern of brain circuitry, we predicted that cathodal tDCS applied to the non-lesioned hemisphere, combined with bimanual training, would rebalance interhemispheric activity and lead to increases in bimanual motor learning.
2. Materials and Methods
2.1. Participants:
Children and young adults, ages 7–21, with a clinical diagnosis of UCP and imaging-confirmed perinatal stroke were recruited as part of a broader tDCS intervention study using a laboratory database of past study participants and recruitment of new participants through physician referrals. Inclusion criteria required the presence of a contralateral motor evoked potential from both hemispheres as assessed by transcranial magnetic stimulation (TMS). Exclusion criteria were seizures within the past two years, implanted metal or medical devices contraindicated for brain stimulation, co-occurring disorders or medical condition (e.g. brain injury, neoplasm), communication deficits preventing involvement in study procedures, or a history of phenol or botulinum toxin-A injections within the past 6 months. This study was approved by the University of Minnesota’s Institutional Review Board. All participants provided age-appropriate informed consent, or assent with appropriate caregiver consent.
2.2. Study design and intervention:
This was an open-label, multiple baseline study designed to generate hypotheses regarding the effects of tDCS on bimanual motor learning. A full description of the study design can be found in a separate publication.9 Each participant completed four weekly baseline assessments (B1-B4), during which bimanual performance was assessed. B1-B3 were completed via video conferencing, and B4 was completed in-person at a university facility. The intervention then occurred at a university facility in groups of 3–5 children over ten consecutive weekdays, consisting of 120 total minutes of bimanual motor training. For the first 20 minutes of each session, participants received tDCS concurrent with training. Motor training was delivered by trained interventionists, under the supervision of experienced therapists, following a study protocol and focused on participant-selected goals established by use of the Canadian Occupational Performance Measure. Goals consisted of bimanual (76%) and unimanual (24%) tasks, which were incorporated in the context of the training. For example, for the goal of putting on earrings, unimanual (e.g. stabilization of the earring with the more-affected hand) and bimanual (e.g. stabilizing the earring with the more affected hand while inserting the earring backing with the less-affected hand) tasks were incorporated into the intervention. Participants were paired 1:1 with an interventionist and did not practice the assessment-related bimanual task (i.e. modified Speed Stacks task or Assisting Hand Assessment-AHA) during the intervention.14
Stimulation was applied using a 25 cm2 rubber electrode housed in a 5×7 cm medical-grade sponge moistened with 8 mL of saline. The cathode (inhibitory) was positioned on the motor hotspot of the non-lesioned hemisphere, and the anode (reference) on the contralateral supraorbital aspect of the forehead (1 × 1 Limited Total Energy, Soterix Medical Inc. New York, NY). We predicted that the tDCS montage would reduce interhemispheric inhibition from the non-lesioned hemisphere upon the lesioned hemisphere and would be efficacious for our sample with contralateral CST connectivity. Motor hotspots for tDCS electrode placement were determined during Baseline (B4) TMS testing. Stimulation was delivered at 1.5 mA intensity for 20 minutes, with an initial 30 second ramp-up phase. All participants received real stimulation (i.e. no sham condition). Bimanual task performance was assessed once during each of the ten intervention days (I1-I10) when participants were engaged in other bimanual activities but not receiving stimulation. Outcomes were measured within one week following the intervention during a Post-test assessment (Post).
2.3. Bimanual Task:
Participants performed a modified version of the 3–3-3 Speed Stacks task (SST), which requires stacking and un-stacking three sets of three plastic cups (clear plastic, 12.1 cm high, 9.65 base diameter size, 7 cm top diameter) in a pre-specified pattern (Figure 1).13 Each of the three sets was placed on a horizontal surface in front of the participant seated at a comfortable level to the surface. For a complete explanation of the sequence, the reader is directed to the Speed Stacks website (https://www.speedstacks.com/). Time to complete the task was measured beginning when the participant touched the first cup, and ended when the last cup was stacked. Time was not stopped if a cup was dropped, and participants were required to repeat a step if any part of the sequence was not performed correctly. For assessments B1-B4 and Post, three trials were attempted; for assessments I1-I10, a single trial was completed. Additional clinical assessments of function included 1) the AHA (B4 and Post, 1 trial), 2) the Box-and-Blocks test (BBT; B1-B4 and Post, 3 trials), 3) mirror movements (B4 and Post, 3 trials) using the Woods and Teuber scale.15,16 The Manual Ability Classification System (MACS) was used as a classification tool of functional status of our sample.17
Figure 1.
Participant completing Speed Stacks task.
2.4. Analysis:
We examined task performance (completion time) and task variability (absolute standard deviation and the coefficient of variation-CV of repeated trials). Because the data did not meet assumptions for repeated-measures ANOVA, statistical comparisons were made using a non-parametric Friedman’s test. If a main effect was present at the α=0.05 level, post-hoc comparisons between Post and each Baseline time (B1-B4) were made using individual Wilcoxon signed-rank tests, and multiple comparisons corrected by adjusting the α -value for significance based on the number of tests. Performance and variability measures were correlated to changes in hand function as measured by the BBT and AHA using Spearman rank correlations. All analyses were performed in Matlab (Mathworks, Natick, MA).
3. Results and Discussion
Eight participants completed all intervention and assessment sessions. The characteristics of our sample were: mean age = 13 years 3 months ± 3 years 8 months; 5 female; MACS level I (n=1), II (n=5) III (n=2); right hemiparesis (n=6); mean AHA logit scale score = 58.3 ± 15.0, mean more-affected hand BBT = 19 blocks (range 0–35). One child had mirror movements score of 6/12 in the less-affected hand; all other children scored 3/12 or less on this scale. Comparing the averages of Post and Baseline performance, the mean change in the AHA was 4±4.6 logits (t7= −2.14, p=0.07), and for the BBT was 1±6 blocks (t7 = −0.35, p = 0.74) in the more-affected hand. Individual change scores and additional motor performance measures are described in a separate publication.9
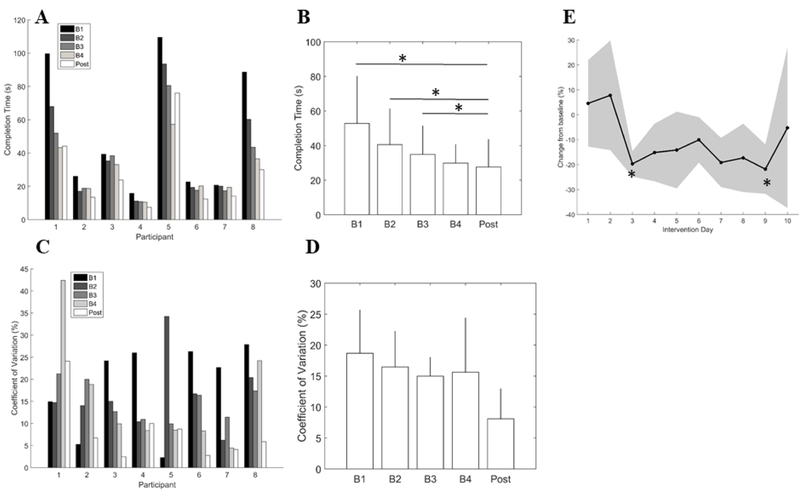
Significant improvements in the SST were observed over time (χ2=25.2, p <0.001). Post-hoc showed borderline significant differences in performance between Post and all but the last Baseline assessment after correction for multiple comparisons (Post-B1: p=0.008, Post-B2: p=0.008, Post-B3: p=0.008, Post-B4: p=0.25). Differences between baseline assessments also neared significance (B1-B2: p=0.008, B1-B3: p=0.008, B1-B3=0.008). Variability as measured with CV was not significantly different across time points. (Figure 2D, p=0.18), however 6/8 (75%) of participants had reduced CV at Post compared to B4. Since task stability (i.e. less variability) is a marker of motor learning, our finding of decreased variability following the intervention in 6 of 8 participants indicates a possible role for tDCS and bimanual training to enhance learning. Improvements in movement coordination and synchrony related to two-handed activities or goals have also been shown to be specific to bimanual training.5,18 Given that there was not a sham tDCS group, we acknowledge that generalization from the bimanual training component of the intervention may explain the improvements in learning the SST.
Figure 2.
(A) Individual task performance, (B) average group change in task performance, (C) Individual task variability (coefficient of variation, %), (D) average group change in task variability, (E) Daily performance during intervention displayed as % change from baseline. Error bars in (B) and (D) are 95% confidence intervals. Shaded region in (E) is 95% confidence region. *p<0.0125. B1-B4: Repeated baseline assessments 1–4, Post: post-testing; s: seconds.
Performance was significantly different across the intervention days I1-I10 (χ2=23.21, p = 0.006; Figure 2E). Post-hoc tests revealed significant differences between I1 and I3 (p<0.01) and I1 and I9 (p<0.01) after multiple comparisons correction. Although an exponential-like learning curve was not observed, a sharp improvement was noted on Day 3 and maintained throughout the duration of training. This plateau in performance after 6 hours of training (Day 3) was earlier than in the study by Hung and Gordon (2014), in which significant changes in performance were not observed until after 24 hours of training (days 4–6).13 While they did not have multiple baseline assessment, participants in that study were also involved in bimanual therapy without receiving tDCS, supporting a potential benefit of tDCS leading to faster skill acquisition in our study. Overall, a direct comparison of skill acquisition using a sham tDCS group would more conclusively answer these questions.
We also found significant correlations between bimanual and unimanual performance in this small sample at the fourth baseline assessment (SST vs. BBT: ρ = −0.81, p = 0.022; SST vs. AHA: ρ = −0.81, p= 0.022), which supports previous findings in children with UCP.19 Significant correlations were not observed between changes in unimanual and bimanual performance from B4 to Post (ΔSST vs. ΔBBT: ρ = −0.10, p = 0.84; ΔSST vs. ΔAHA: ρ = −0.20, p = 0.65), indicating there was not a consistent generalization of the intervention to unimanual skills. Both bimanual and constraint-induced movement therapies have been shown to improve unimanual function when used at higher doses (60–90 hours compared to 20 hours in the present study).20,21 Our study would suggest 20 hours of combined stimulation and motor training may be sufficient to produce positive changes in bimanual but not unimanual function, although the optimal dosing of training and brain stimulation for bimanual motor learning remains to be determined.
The study incorporated a cathodal contralesional tDCS montage designed to reduce exaggerated interhemispheric inhibition from the non-lesioned hemisphere upon the lesioned hemisphere, thereby increasing excitability and potential recovery By decreasing excitability in the non-lesioned hemisphere with cathodal stimulation, the potential exists to negatively influence the stronger, less-affected upper-limb and therefore the ability to execute bimanual actions. Using cathodal stimulation applied to the non-lesioned hemisphere, we found mixed results related to the combination of bimanual motor training and cathodal contralesional tDCS to improve unimanual function.9 A recent study by Kirton et al. showed significant improvement in subjective measures of motor performance, but did not find improvements in a unimanual task (BBT) following 10 days of combined constraint therapy and tDCS.8 Prior adult studies of tDCS and bimanual motor learning have shown stronger effects using anodal (i.e. excitatory) tDCS, but the effects of tDCS montage on bimanual motor learning is still in question.12 It remains to be determined if anodal tDCS is a more effective approach for promoting both unimanual and bimanual upper-limb function and learning in children with UCP.
One important factor when considering these results is that we only included participants with intact contralateral CST connections from both brain hemispheres. Children with this pattern of CST connectivity typically have overall less upper-limb impairments compared to children who lack connectivity from the lesioned hemisphere to the more-affected upper-limb.22 Some of our participants also exhibited prominent ipsilateral projections from the non-lesioned hemisphere to the more-affected upper-limb, illustrating a form of bilateral CST reorganization following early brain injury. Bimanual movement in typically-developing brains arises from balanced contributions from both hemispheres, and the presence of brain injury may disrupt this balance. Thus, the execution, learning and retention of bimanual tasks is likely different when both lesioned and non-lesioned hemispheres contribute to upper-limb movement, as in children with bilateral CST reorganization. Thus, studying bimanual motor learning acquisition in children with UCP different CST reorganization patterns would be a valuable area of future research.
In summary, we found that performance of a novel bimanual task decreased after ten days combined tDCS and bimanual motor training in children and young adults with UCP, which may indicate improved learning of a bimanual skill. These results will inform our understanding of upper-limb rehabilitation in children and young adults with UCP and how brain stimulation may help improve immediate and long-term outcomes.
Acknowledgments
This study was supported the National Institutes of Health (NIH) Eunice Kennedy Shriver National Institutes of Child Health and Development K01 Award (HD078484–01A1), the Cerebral Palsy Foundation, the Foundation for Physical Therapy Magistro Family Grant, the University of Minnesota’s MnDRIVE (Minnesota’s Discovery, Research, and Innovation Economy (MnDRIVE) initiative, and the University of Minnesota Marie Louise Wales Fellowship. The project described was also supported in part by Award UL1 TR000114 and KL2 TR000113. The authors thank the Center for Neurobehavioral Development at the University of Minnesota, the student interventionists and the families, caregivers and participants involved in this study. No potential conflict of interest is disclosed by the authors.
Contributor Information
Samuel T. Nemanich, Department of Rehabilitation Medicine, University of Minnesota, 420 Delaware St SE, MMC 388, Minneapolis, MN 55455.
Tonya L. Rich, Email: rich1038@umn.edu, Department of Rehabilitation Medicine, University of Minnesota, 420 Delaware St SE, MMC 388, Minneapolis, MN 55455.
Andrew M. Gordon, Email: ag275@tc.columbia.edu, Department of Biobehavioral Sciences, Teacher’s College, Columbia University, 1152B Thorndike Hall, New York, NY 10027.
Kathleen M. Friel, Email: kaf3001@med.cornell.edu, Burke Neurological Institute, Weill-Cornell Medicine, White Plains, New York 10605.
Bernadette T. Gillick, Email: gillick@umn.edu, Department of Rehabilitation Medicine, University of Minnesota, 420 Delaware St SE, MMC 388, Minneapolis, MN 55455.
References
- 1.Gerloff C, Andres FG. Bimanual coordination and interhemispheric interaction. Acta Psychol (Amst) 2002;110(2–3):161–86. [DOI] [PubMed] [Google Scholar]
- 2.Hung YC, Charles J, Gordon AM. Bimanual coordination during a goal-directed task in children with hemiplegic cerebral palsy. Dev Med Child Neurol 2004;46(11):746–53. [DOI] [PubMed] [Google Scholar]
- 3.Hung YC, Charles J, Gordon AM. Influence of accuracy constraints on bimanual coordination during a goal-directed task in children with hemiplegic cerebral palsy. Exp Brain Res 2010;201(3):421–8. [DOI] [PubMed] [Google Scholar]
- 4.Gordon AM, Schneider JA, Chinnan A, Charles JR. Efficacy of a hand-arm bimanual intensive therapy (HABIT) in children with hemiplegic cerebral palsy: a randomized control trial. Dev Med Child Neurol 2007;49(11):830–8. [DOI] [PubMed] [Google Scholar]
- 5.Gordon AM, Hung YC, Brandao M, Ferre CL, Kuo HC, Friel K, Petra E, Chinnan A, Charles JR, Schneider JA and others. Bimanual training and constraint-induced movement therapy in children with hemiplegic cerebral palsy: a randomized trial. Neurorehabil Neural Repair 2011;25(8):692–702. [DOI] [PubMed] [Google Scholar]
- 6.Hung YC, Brandao MB, Gordon AM. Structured skill practice during intensive bimanual training leads to better trunk and arm control than unstructured practice in children with unilateral spastic cerebral palsy. Res Dev Disabil 2017;60:65–76. [DOI] [PubMed] [Google Scholar]
- 7.Gordon AM, Bleyenheuft Y, Steenbergen B. Pathophysiology of impaired hand function in children with unilateral cerebral palsy. Dev Med Child Neurol 2013;55 Suppl 4:32–7. [DOI] [PubMed] [Google Scholar]
- 8.Kirton A, Ciechanski P, Zewdie E, Andersen J, Nettel-Aguirre A, Carlson H, Carsolio L, Herrero M, Quigley J, Mineyko A and others. Transcranial direct current stimulation for children with perinatal stroke and hemiparesis. Neurology 2017;88(3):259–267. [DOI] [PubMed] [Google Scholar]
- 9.Rich TL, Nemanich ST, Chen M, Friel KM, Feyma T, Krach L, Nawshin T, Meekins G, Gillick BT. Transcranial direct current stimulation (tDCS) paired with occupation-centered bimanual training in children with unilateral cerebral palsy: A preliminary study. Neural Plasticity 2018;In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gillick BT, Feyma T, Menk J, Usset M, Vaith A, Wood TJ, Worthington R, Krach LE. Safety and feasibility of transcranial direct current stimulation in pediatric hemiparesis: randomized controlled preliminary study. Phys Ther 2015;95(3):337–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gillick B, Rich T, Nemanich S, Chen CY, Menk J, Mueller B, Chen M, Ward M, Meekins G, Feyma T and others. Transcranial direct current stimulation and constraint-induced therapy in cerebral palsy: A randomized, blinded, sham-controlled clinical trial. Eur J Paediatr Neurol 2018;22(3):358–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pixa NH, Pollok B. Effects of tDCS on Bimanual Motor Skills: A Brief Review. Front Behav Neurosci 2018;12:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hung YC, Gordon AM. Motor learning of a bimanual task in children with unilateral cerebral palsy. Res Dev Disabil 2013;34(6):1891–6. [DOI] [PubMed] [Google Scholar]
- 14.Cusick A, Lannin NA, Lowe K. Adapting the Canadian Occupational Performance Measure for use in a paediatric clinical trial. Disabil Rehabil 2007;29(10):761–6. [DOI] [PubMed] [Google Scholar]
- 15.Krumlinde-Sundholm L, Holmefur M, Kottorp A, Eliasson AC. The Assisting Hand Assessment: current evidence of validity, reliability, and responsiveness to change. Dev Med Child Neurol 2007;49(4):259–64. [DOI] [PubMed] [Google Scholar]
- 16.Woods BT, Teuber HL. Mirror movements after childhood hemiparesis. Neurology 1978;28(11):1152–7. [DOI] [PubMed] [Google Scholar]
- 17.Eliasson AC, Krumlinde-Sundholm L, Rosblad B, Beckung E, Arner M, Ohrvall AM, Rosenbaum P, group M. Using the MACS to facilitate comunication about manual abilities of children with cerebral palsy. Dev Med Child Neurol. Volume 49 England2007. p 156–7. [DOI] [PubMed] [Google Scholar]
- 18.Hung YC, Casertano L, Hillman A, Gordon AM. The effect of intensive bimanual training on coordination of the hands in children with congenital hemiplegia. Res Dev Disabil 2011;32(6):2724–31. [DOI] [PubMed] [Google Scholar]
- 19.Sakzewski L, Ziviani J, Boyd R. The relationship between unimanual capacity and bimanual performance in children with congenital hemiplegia. Dev Med Child Neurol 2010;52(9):811–6. [DOI] [PubMed] [Google Scholar]
- 20.Smorenburg AR, Gordon AM, Kuo HC, Ferre CL, Brandao M, Bleyenheuft Y, Carmel JB, Friel KM. Does Corticospinal Tract Connectivity Influence the Response to Intensive Bimanual Therapy in Children With Unilateral Cerebral Palsy? Neurorehabil Neural Repair 2017;31(3):250–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gordon AM, Chinnan A, Gill S, Petra E, Hung YC, Charles J. Both constraint-induced movement therapy and bimanual training lead to improved performance of upper extremity function in children with hemiplegia. Dev Med Child Neurol 2008;50(12):957–8. [DOI] [PubMed] [Google Scholar]
- 22.Holmstrom L, Vollmer B, Tedroff K, Islam M, Persson JK, Kits A, Forssberg H, Eliasson AC. Hand function in relation to brain lesions and corticomotor-projection pattern in children with unilateral cerebral palsy. Dev Med Child Neurol 2010;52(2):145–52. [DOI] [PubMed] [Google Scholar]