Abstract
In the current study, we examined the attunement and transmission of mother-child diurnal cortisol among maltreating (N = 165) and nonmaltreating (N = 83) mothers and their preschool-aged children. Over half of the families had a substantiated child maltreatment case with the mother as the perpetrator. Mothers collected three saliva samples (waking, midday, and bedtime) on themselves and their child on two consecutive days, which were later assayed for cortisol. This design allows for the examination of concurrent attunement, as well as cross-lagged transmission, across the day. Results from actor-partner interdependence models revealed significant differences in mother-child cortisol attunement and transmission between the maltreating and nonmaltreating groups. Specifically, only maltreating mothers transmitted cortisol to their children, and were attuned at first waking; only nonmaltreating dyads were attuned at midday. Implications of these results for sociocultural models of stress physiology and for our understanding of how child maltreatment affects diurnal cortisol regulation are discussed.
Child maltreatment jeopardizes children’s health, and emotional, cognitive, and biobehavioral development (Cicchetti & Valentino, 2006). It is estimated that approximately 750,000 children in the United States are victims of substantiated abuse and neglect each year, with 4 times as many children investigated by the child welfare system (US DHHS, 2018). Among those investigated, approximately two-thirds of preschool-aged children exhibit substantial behavioral, emotional, and/or developmental problems (Stahmer et al., 2005). Despite clear links between maltreatment and poor developmental outcomes, the mechanisms of how experiences get ‘under the skin’ are not fully understood. The hypothalamic-pituitary-adrenal (HPA) axis is thought to be a primary physiological system that translates external experiences into internal functioning. Neglectful and abusive parenting alters HPA activity (Cicchetti, Rogosch, & Oshri, 2011) and individual differences in the responsivity of this system is thought to mediate the relationship between early adversity and the development of pathology (e.g., Loman, Gunnar & The Early Experience, Stress, and Neurobehavioral Development Center, 2009).
When faced with a physical or psychological stressor, the HPA axis activates neurochemical responses initiating with increased corticotropin-releasing factor levels and ending with the release of cortisol (Gunnar, Doom, & Esposito, 2015). The HPA axis is highly sensitive to social interactions, and children rely to a large degree on caregivers to soothe and calm emotional and physiological arousal (Gunnar & Donzella, 2002; Hofer, 2006). For example, sensitive caregiving and higher maternal caregiving quality are associated with overall lower cortisol reactivity (Laurent et al., 2016; Hibel, Granger, Blair, & Cox, 2011), and greater cortisol recovery (Albers, Riksen-Walraven, Sweep, & de Weerth, 2008). In addition to acute release in response to a stressor, healthy cortisol secretion also follows a reliable diurnal pattern marked by high levels at waking, a steep decline across the day, and reaching a nadir around bedtime (Adam & Kumari, 2009; Smyth, Hucklebridge, Thorn, Evans, & Clow 2013). Children’s diurnal patterns are also under social control, with higher maternal parenting quality associated with steeper (i.e., healthier) declines across the day (Pendry & Adam, 2007).
Though an extensive body of work has shown maternal behaviors regulate child physiology, more recent examinations have extended this area of research revealing that maternal physiology is also associated with child physiology. Specifically, maternal cortisol reliably associates or attunes with child cortisol at multiple time points across the day (Hibel, Trumbell, & Mercado, 2014; Middlemiss, Granger, Goldberg, & Nathans, 2012; Papp, Pendry, & Adam, 2009) and across childhood (Hibel, Granger, Blair, & Finegood, 2015; Atkinson et al., 2013; Laurent, Ablow, & Measelle, 2012; Sethre-Hofstad et al., 2002). Attuned cortisol is thought to be a physiological manifestation of shared emotional and behavioral experiences (e.g., Feldman, 2007; Hibel et al., 2014), and dyads who spend more time together have been found to be more strongly attuned (Papp et al., 2009). Thus, mothers who are sensitive to child cues and synchronize their responses to match children’s behaviors and emotions also have stronger adrenocortical attunement (e.g., Atkinson et al., 2013; Hibel et al., 2015). We aim to extend this work to examine attunement across the day, in maltreating and nonmaltreating dyads.
Child maltreatment and adrenocortical activity.
Child maltreatment is a profound stressor, threatening children’s safety and security. Maltreated children are subjected to extreme physical punishment or neglect of the child’s emotional and/or physical needs. These threatening and stressful relationships result in repeated activations of stress responsive physiology, and also fail to provide children with effective means of recovery or regulation (Repetti, Taylor, & Seeman, 2002). Adaptive increases in cortisol allow individuals to behaviorally respond to an immediate challenge; however the allostatic load model suggests that chronic HPA activations may result in physiological dysregulations, and long-term mental and physical health problems (McEwen, 1998; Shonkoff, 2016). Interestingly, chronic stressors have been found to both accentuate and steepen (hypercortisolism: McEwen, 2000; Miller, Chen, & Zhou, 2007) and attenuate and flatten (hypocortisolism: Heim, Ehlert, & Hellhammer, 2000; Miller, Chen, & Zhou, 2007), HPA responses to stress and diurnal rhythms. The direction of the dysregulation (hypo- or hyper-) has been found to depend on characteristics of the individual exposed (e.g., gender, age) and the stressor (e.g., timing, intensity, nature) (Bernard, Frost, Bennett, & Lindhiem, 2017; Miller et al., 2007).
Examining the full breadth of the HPA-maltreatment literature reveals similarly mixed findings of both hyper- and hypo-responses to acute stressors (Heim, Newport, Bonsall, Miller, Nemeroff, 2001; Sumner, McLaughlin, Walsh, Sheridan, & Koenan, 2014). With regard to diurnal cortisol, a recent meta-analysis found a significant pattern of hypocortisolism in agency-referred individuals. Specifically, though there was no evidence of dysregulation in afternoon cortisol slopes, morning cortisol was significantly reduced (Bernard et al., 2017). This dampening of morning cortisol was not dependent on the individual’s age, gender, or type of maltreatment they experienced (i.e., neglect, abuse). Though the authors did examine differences in childhood compared to adulthood, the majority of studies reported included children in middle childhood and adolescence. Studies focusing on just preschool children have also revealed reduced morning cortisol (Bruce, Fisher, Pears, & Levine, 2009; Cicchetti, Rogosch, Toth, & Sturge-Apple, 2011), and overall flatter diurnal slopes (Dozier et al., 2006; Valentino et al., 2015).
It should be noted that some studies with older children (Cicchetti & Rogosch, 2001), or preschoolers who experienced sexual abuse (Tricket et al., 2010) reveal heightened cortisol levels across the day. Studies also suggest that patterns of cortisol regulation among school aged children may be moderated by gender (Doom, Cicchetti, Rogosch, & Dackis, 2013) and internalizing symptoms (Cicchetti, Rogosch, Gunnar & Toth, 2010). Together, these studies highlight the dysregulatory nature of maltreatment, by changing typical diurnal variation (i.e., morning rises and diurnal declines). While these studies provide a foundation to understand the implications of maltreatment on child physiological regulation, they do not provide information on the role of caregiver physiology in regulating child physiology. Given previous findings on attunement and shared physiology between caregiver, assessing attunement might provide critical insight on maltreatment’s dysregulation of child stress physiology (Atkinson, Jamieson, Khoury, Ludmer, & Gonzalez, 2016).
Studies have shown that mothers under duress might share their emotional and physiological state with their child, resulting in the attunement of negative reactivity states. In a naturalistic study of diurnal rhythms, mother-adolescent dyads reported their emotions and collected cortisol multiple times across two days. Findings revealed mothers and adolescents who reported greater daily negative affect also displayed stronger cortisol attunement (Papp et al., 2009). Similarly, mothers who use harsh and punitive parenting techniques have been found to have greater adrenocortical attunement surrounding a stressor, compared to those who do not (Hibel et al., 2009). Notably, a recent study revealed that attunement of morning cortisol was only present when mothers reported a history of their own childhood abuse (Fuchs, Moehler, Resch, & Kaess, 2017). In light of these findings it has been suggested that attunement in the context of maternal dysfunction might serve as a mechanism in the intergenerational transmission of risk (Hibel et al., 2009; Laurent et al., 2011).
Attunement vs. transmission.
According to Butler (2011), attunement is characterized as a coordinated bidirectional exchange. The conceptualization of attunement does not assume causal ordering, but suggests that both members of the dyad influence one another. Interestingly, as mentioned above, most mother-child attunement studies do in fact assume direction, specifically that mothers’ influence their children, and children are externally regulated by their mothers (Hibel et al., 2009; Laurent et al., 2011). This directional understanding of mother-child physiology, however, is more closely aligned with the characterization of transmission. Specifically, Butler (2011) states that physiological transmission requires a temporal ordering in physiological measurements. In other words, if maternal stress and stress physiology is thought impact child regulation, testing this assumption requires temporally spaced assessments.
To date, only one study has examined the potential for cortisol levels to be actively transmitted, as opposed to passively shared via attunement. In that study, Hibel and colleagues utilized an experimental design where mothers were either randomized to have a stressful marital interaction or a positive one, after which mothers interacted with their infants post Lab-TAB challenge. For mothers in the stressed group, cortisol reactivity and recovery predicted their infants’ subsequent cortisol reactivity and recovery. Further, in dyads where mothers exhibited higher levels of intrusive parenting, maternal cortisol reactivity more strongly predicted children’s cortisol reactivity (Hibel & Mercado, 2017). In other words, intrusive parenting facilitated transmission. These findings suggest that physiology can transmit across dyads, and that in dyads with problematic parenting (whereby maltreatment is the most pathological form of problematic parenting) dysregulated maternal stress physiology will prospectively predict dysregulated child adrenocortical functioning.
The current study.
We extend Hibel and Mercado’s work in three important ways: 1) we simultaneously examine attunement and transmission to understand their unique contributions; 2) we examine these processes across two days, as opposed to a short laboratory paradigm; and 3) we examine these processes in maltreating and non-maltreating dyads to clarify the role of relationship functioning in determining attunement and transmission. Specifically, utilizing a dyadic analysis in a sample of maltreating and nonmaltreating mother-child dyads we examined whether attunement and transmission of stress physiology across the day differs in the context of maltreatment. An actor-partner interdependence modeling (APIM) framework allows for simultaneous estimation of the influence family members have on each other without modeling each member as an outcome in separate models (Kenny & Cook, 1999). In the current study, cortisol was sampled across the day in both mothers and children, allowing for the examination of concurrent attunement, as well as cross-lagged transmission, across the day. Further, estimating cross-lagged APIM models in a multigroup framework allows us to test whether attunement and transmission unfolds differently for maltreating dyads compared to dyads from a community sample. Based on previous studies highlighting the co-regulatory nature of attunement (Feldman, 2012), and propensity for sensitive caregiving to accentuate attunement, we hypothesize the mother-child dyads from nonmaltreating families will exhibit greater levels of attunement than the maltreating families. We hypothesize shared cortisol, in the context of stress and high-risk relationships, actually represents a process by which dysregulation “spills over” or transmits from one person to the other, as opposed to a process of co-regulation or attunement. Therefore, we expect that maltreating dyads will only exhibit cross-lagged predictions from previous maternal stress physiology to subsequent child physiology. In sum, we expect nonmaltreating dyads to exhibit cortisol attunement, while maltreating dyads will transmit cortisol.
Method
Participants
The participants included 248 mothers and their children, aged 3 to 6 years from a medium-sized Midwestern city. The maltreatment group included 165 families in which the mother had been named as a perpetrator of substantiated cases of child maltreatment. Families (n = 83) with no child welfare system history were recruited from community agencies such as Head Start, the housing authority, and the Special Supplemental Nutrition Program for Women, Infants, and Children office to be demographically similar to the maltreating families. In all families children were living with their biological mothers. Participants were screened for endocrine disorders or continual corticosteroid use (Granger, Hibel, Fortunato, & Kapelewski, 2009), which affect cortisol levels; however no families were excluded for these reasons. Maltreated and nonmaltreated dyads did not differ on a number of important demographic characteristics, with the exception of marital status (see Table 1).
Table 1.
Sample Characteristics by Maltreatment Group
| Maltreating (n = 165) |
Nonmaltreating (n = 83) |
|||
|---|---|---|---|---|
| Variable | M | SD | M | SD |
| 1. Maternal Age | 29.58 | 5.35 | 30.37 | 6.85 |
| 2. Child Age | 4.93 | 1.15 | 4.86 | 1.13 |
| 3. Maternal Language (PPVT-4) | 84.30 | 12.75 | 86.22 | 12.64 |
| n (%) | n (%) | |||
| 4. Child Sex: Male | 83 (50.3%) | 42 (50.6%) | ||
| 5. Child Race/Ethnicity | ||||
| African American | 66 (40.0%) | 34 (41.0%) | ||
| Caucasian | 48 (29.1%) | 15 (18.1%) | ||
| Hispanic and Other | 51 (30.9%) | 34 (41.0%) | ||
| 6. Maternal Education | ||||
| Some Middle or High School | 56 (33.9%) | 18 (21.7%) | ||
| Completed High School/GED | 55 (33.3%) | 25 (30.1%) | ||
| Some Trade School or College | 38 (23.0%) | 26 (31.3%) | ||
| Completed Trade School or | 15 (9.1%) | 12 (14.5%) | ||
| Bachelor’s/Associate’s | 1 (.6%) | 2 (2.4) | ||
| 7. Income <$12,000 | 95 (57.6%) | 45 (54.2%) | ||
| 8. Married | 20 (12.1%) | 28 (33.7%)* | ||
| 9. Has Partner | 90 (54.5%) | 52 (62.6%) | ||
Note. Independent samples t-tests and chi-square tests assessed differences by maltreatment group.
p < .05;
p < .01.
Maltreating families were recruited through the Department of Child Services (DCS). Eligible participants were provided with an informational flyer by their DCS Family Case Workers, and they were asked whether they would be interested in sharing their contact information with project staff. Project staff subsequently contacted interested families to discuss enrollment. All families provided informed consent and signed release forms granting access to their DCS records. Family maltreatment history was verified through extensive examinations of each family’s case history and supplemented with maternal interview. Only families who have never received child protective services through DCS and indicated no maltreatment on the maternal interview were included in the nonmaltreating comparison sample.
Maltreatment Classifications
DCS records were coded using the Maltreatment Classification System (MCS; Barnett, Manly, & Cicchetti, 1993). The MCS provides operational criteria for determining the occurrence of subtypes of maltreatment which includes sexual abuse, physical abuse, physical neglect, and emotional maltreatment. Sexual abuse is coded when any sexual contact or attempted sexual conduct occurred between the child and an adult. Physical abuse is determined by injuries that had been inflicted upon a child by nonaccidental means. Physical neglect is coded for failure of the primary caregiver to meet a child’s needs for food, clothing, shelter, health care, education, hygiene, or safety. Emotional maltreatment is coded for chronic or extreme neglect or disregard of children’s emotional needs and includes witnessing domestic violence (see Barnett et al., 1993). The severity, chronicity, perpetrator, and the developmental timing of each maltreatment incident were also assessed. MCS ratings were supplemented by information obtained during the Maternal Maltreatment Classification Interview (MMCI; Cicchetti, Toth, & Manly, 2002), a structured interview based on the MCS. Approximately 20% of the maltreated sample was double coded (n = 32) by two coders, and reliability was established (κ = .81–1.0).
Of the maltreated children, 4.3% were sexually abused, 12.4% were physically abused, 80.7% were neglected, and 60.2% were emotionally maltreated. Consistent with previous research (Manly et al., 2001), subtype comorbidity was high such that many maltreated children (60.9%) experienced multiple forms of abuse and neglect. This includes 36.7% who experienced 2 subtypes, 20.2% who experienced 3 subtypes, and 3.7% who experienced 4 subtypes of maltreatment. All families in the maltreating sample had at least one substantiated DCS case in which the mother was named the perpetrator. The average length of time since the last maltreatment incident was just under 1 year (356 days), with a range of 20 to 1704 days.
Procedure
Data for the current study were drawn from the baseline assessment of a longitudinal RCT of an intervention for maltreating mothers and their preschool-aged children and were collected between 2013 and 2017. Before randomization into treatment conditions, all families completed a baseline assessment consisting of one session in the home followed by one in the laboratory. Research staff conducting the assessments were naive to families’ maltreatment status. During the home assessment, mothers engaged in free-play with their children and were then trained to collect three saliva samples (waking, midday, and evening) on themselves and their children for two consecutive weekend days. Mothers and children were compensated for their time. The study protocol was approved by the Institutional Review Board of the University of Notre Dame.
Measures
Diurnal Cortisol.
Saliva collection kits were brought to participants’ homes during their baseline home visit. Collection kits included pre-labeled sample vials, straws (for the mothers), saliva collection sponges (for the children; SalivaBio, LLC), and two collection bottles with MEMS 6 TrackCap Monitors (Medication Event Monitoring System; WestRock Switzerland, Ltd.). One collection bottle was for mothers’ samples and one for children’s samples. Mothers were asked to keep the bottles in the freezer with the caps on, to add each sample to the appropriate bottle as they were collected across the two days, and to close the bottles with the MEMS cap after each opening. Mothers were also asked to report the time of each sample on a written log. Mothers were informed that the MEMS caps would track the date and time of each cap opening, thus providing an objective assessment of their collection times. Mothers were trained to collect saliva via passive drool (Granger et al, 2009) for themselves and via sponge for their children. Research assistants observed mothers collect one practice sample from herself and her child during training, including placing the samples into the MEMS cap sealed bottles, and provided corrective feedback to ensure comprehension of the collection procedures.
Participants provided three salivary samples: immediately upon waking, before lunch, and before bed (Adam & Kumari, 2009) on two consecutive weekend days. Weekends were selected as to ensure that mothers and children would be home together. Participants were instructed to drink water 10 minutes before collection (except at waking), and not to eat, smoke, or drink alcohol or caffeine within 20 minutes of providing salivary samples. Mothers were given a cell phone to enhance adherence to the protocols. For example, mothers were asked to estimate their waking, lunch, and bedtimes for the following day and using this schedule, text-based reminders were sent to the cell phone 20 minutes before each collection time and mothers were asked to respond. If they did not respond within 30 minutes, mothers received a phone call.
Respondents were instructed to keep the saliva samples in the provided bottles in the freezer until their lab assessment. Participants brought their samples to the lab assessment on ice in portable coolers when they attended their lab assessment. Families without freezers were provided a cooler in which samples could be kept; these samples were picked up by staff and transported to the lab each morning. Samples were stored in an ultralow freezer (−80 C) with back-up generator until analysis (Granger et al., 2007).
During the lab session, the MEMS cap times were downloaded by placing the caps on a MEMS reader-device. The dates and times of cap openings were compared with the self-reported log sheets. Data were considered adherent if cap openings and the self-reported log sheets did not differ by more than 30 minutes for the waking sample, or by 60 minutes for the midday and bedtime sample. Families without at least one adherent day of collection were asked to resample. Ultimately, the maltreating and nonmaltreating families did not significantly differ on most indices of adherence to the collection protocol (for additional details see Valentino, De Alba, Hibel, Fondren, & McDonnell, 2017). Cortisol values above 3.00 μg/dl were initially removed as biological outliers. After the removal of biological outliers, cortisol values were log transformed to correct for positive skewness and screened for statistical outliers 3 standard deviations above or below the mean Maternal and child cortisol across the two days were averaged such that mothers and children each had three cortisol values, waking, midday, and bedtime. After averaging across the two days, 96% of mothers had all three cortisol values, 1.2% had only two cortisol values, and .4% had one, similarly, 91% of children had all three cortisol values, 3.2% had two, and 1.6% had one.
Post-hoc Measures of Maternal Characteristics
As previously noted, data for this study were drawn from a large randomized controlled trial that included assessment of additional maternal characteristics. We incorporated several maternal characteristics related to parenting, maternal psychopathology, and trauma history in post-hoc analyses to evaluate how these factors may contribute to attunement and transmission differences as a function of maltreatment group.
Maternal sensitivity.
Maternal sensitivity was coded from twenty minutes of videotaped play, including 10 minutes of free-play in the lab and 10 minutes in the child’s home, using the Mini- Preschool Maternal Behavior Q-sort (Pederson, Moran, & Bento, 2013). Two independent raters double coded 20% of the interactions and established adequate reliability (interrater reliability = .80). The global sensitivity score which compares each individual to the prototypically sensitive caregiver was used in the current analyses.
Questionnaires.
The 20-item Center for Epidemiological Studies Depression Scale (Radloff, 1977) assessed mothers depressive symptomatology and demonstrated acceptable internal consistency (α = .79). The Childhood Trauma Questionnaire assessed childhood maltreatment (Bernstein & Fink, 1998) using a total of 28 items. Subscales showed adequate internal consistency in the current sample (subscale α range = .72–.96, average = .87). The Impact of Events Scale, Revised (Weise & Marmar, 1997) was used to assess current maternal trauma symptoms, i.e., symptoms experienced in the past seven days. Internal consistency was excellent (α = .93).
Analytic Strategy
In order to assess the attunement and transmission of cortisol, we estimated cross-lagged APIM models in Mplus version 7.3 (Muthén & Muthén, 1998–2017). In these path models, the relationship between mother and child cortisol at each of the three time points across the day is examined (i.e., attunement). Further, ‘cross-lagged’ paths where mother’s cortisol at one time point was predicted as a function of her child’s previous cortisol value (i.e., child to mother transmission; Bernard, Kashy, Levendosky, Bogat, & Lonstein, 2017) were also modeled. Similarly, a child’s cortisol at one time point was predicted as a function of their mother’s previous cortisol value (i.e., mother to child transmission). The models also control for the individual’s own cortisol at the previous time point (i.e., actor effects). Given the purposes of the current study are to examine only partner effects (attunement and transmission), actor effects are only presented for descriptive information. All models were estimated using full information maximum likelihood and controlled for differences in sample collection time between mother and child. By utilizing an APIM framework, the models accounted for collinearity between predictors and outcomes (Kenny & Cook, 1999). Goodness of fit was evaluated by using the following cut-offs for model fit indices: a non-significant chi-square test, a root mean square error of estimation (RMSEA) value less than .06, a comparative fit index (CFI) and Tucker-Lewis Index (TLI) value equal or greater than .95, and a standardized root mean square residual (SRMR) value less than .08 (Hu & Bentler, 1999; Ullman, 2013).
To test whether attunement and transmission vary by maltreatment status, we estimated the same cross-lagged APIM model in a multiple-group analysis (Ullman, 2013). In multiple-group analyses, unique cross-lagged models are fit to different subgroups allowing for group differences on estimated parameters. In this study, the two groups examined were a sample of maltreating families and a demographically comparable sample of nonmaltreating families. To test for differences between the two groups we first estimated a baseline model where all path coefficients were allowed to vary across groups. We then compared the baseline model, to a second more restrictive model that statistically constrained all path coefficients to be equal across the two groups. If the more restrictive second model fit the data better, this suggests that maltreatment status does not influence attunement and transmission in the current sample. Differences in model fit were tested using the likelihood ratio test, with a nonsignificant likelihood ratio test suggesting the model with equality constraints fits the data better.
Results
Descriptive Statistics and Preliminary Analyses
The maltreating and nonmaltreating families were similar on several important demographic characteristics (Table 1). However, groups significantly differed on maternal marital status, χ2(1)=16.78, p < .001, with more nonmaltreating mothers reporting being married than maltreating mothers. Interestingly, mothers did not significantly differ in whether they reported having a partner, χ2(1) = 1.25, p = .26, with the majority of the mothers reporting having a partner (62.2% nonmaltreating mothers, 54.6% maltreating mothers). Marital status was initially included as a covariate but did not change the pattern of findings and overall was not significantly related to mother or child cortisol, therefore, marital status was not covaried in subsequent analyses. Similarly, the amount of days since DCS report of maltreatment was not correlated with mother or child’s cortisol levels and therefore not included as a covariate.
Across the entire sample, children declined in cortisol from waking (M = 0.36 μg/ml, SD = 0.29) to midday (M = 0.27 μg /ml, SD = 0.41; t (226) = 12.42, p <.001), and midday to bedtime (M = 0.23 μg /ml, SD = 0.44; t (226) = 8.44, p < .001; see Figure 1). For mothers, cortisol declined from waking (M = 0.31 μg /ml, SD = 0.18) to midday (M = 0.18 μg /ml, SD = 0.12; t (241) = 14.22, p <.001), and midday to bedtime (M = 0.13 μg /ml, SD = 0.18; t (238) = 10.18, p <.001; see Figure 1). Cortisol levels did not differ by maltreatment status except for mother’s morning cortisol. Maltreating mothers had higher cortisol levels upon awakening (M = 0.33 μg /ml, SD = 0.19) than nonmaltreating mothers (M = .28 μg /ml, SD = .14; t (213) = −2.08, p <.05). Further, mother and child cortisol levels did not differ by maltreatment subtype.
Figure 1.
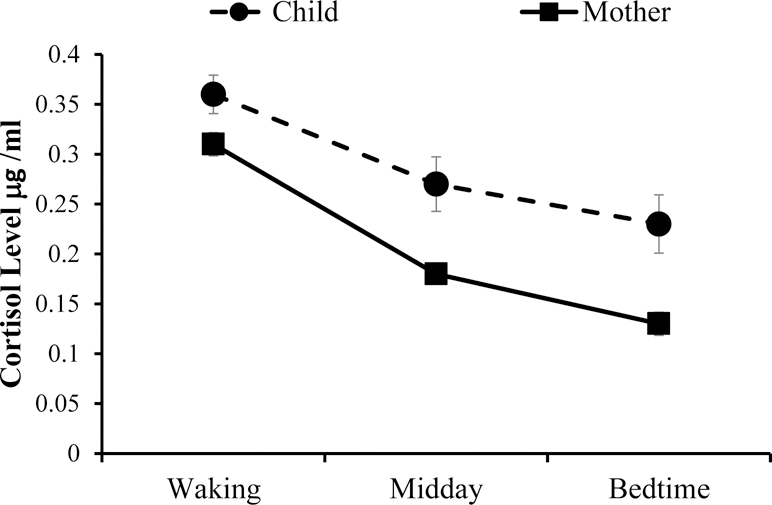
Mother and child cortisol levels decline across the day for the entire sample. Overall there are no mean differences in cortisol levels between the maltreating and nonmaltreating sample except that maltreating mothers had higher morning cortisol levels than nonmaltreating mothers.
Associations between cortisol values and variables known to influence cortisol levels (i.e., wake time, medication use, race) were explored before including these variables as covariates in the models. Wake time was significantly correlated with mother (r = .13, p = .04) and children’s awakening cortisol sample (r = −.17, p = .01). However, there were no mean differences by medication use (0 = no use; 1 = reported use) between groups, for either mother (sample 1: t(239) = .08, p = .94; sample 2: t(240) = .04, p = .97; sample 3: t(236) = .93, p = .35) or child cortisol values (sample 1: t(230) = .03, p = .97; sample 2: t(227) = −1.13, p = .26; sample 3: t(228) = −1.16, p = .25). There were also no mean differences in cortisol values by race (African-American, White, or Hispanic/Other) between the two groups, for either mother (sample 1: F(2, 240) = 1.13, p = .32; sample 2: F(2, 241) = .20, p = .82; sample 3 F(2, 237) = 2.04, p = .13) or child (sample 1: F(2, 231) = .51, p = .60; sample 2: F(2, 229) = .60, p = .55; sample 3 F(2, 230) = .57, p = .56). Based on these preliminary analyses, wake time was included in subsequent models in addition to a dummy coded variable measuring whether participants completed the wake-up sample (based on MEMs cap data) within 15 minutes of their self-reported wake up time.
Autoregressive paths in the cross-lagged model were significant for both mother and child. In other words, mother’s morning cortisol sample was significantly associated with her pre-lunch cortisol sample (β = .49, p < .001), and the pre-lunch sample predicted mother’s bedtime cortisol sample (β = .38, p < .001). The same was true for children, their cortisol sample in the morning predicted their cortisol levels at lunchtime (β = .61, p < .001), and lunchtime cortisol predicted bedtime cortisol levels (β = .59, p < .001). These associations did not differ by maltreatment status but are reported separately by group in Figure 2.
Figure 2.
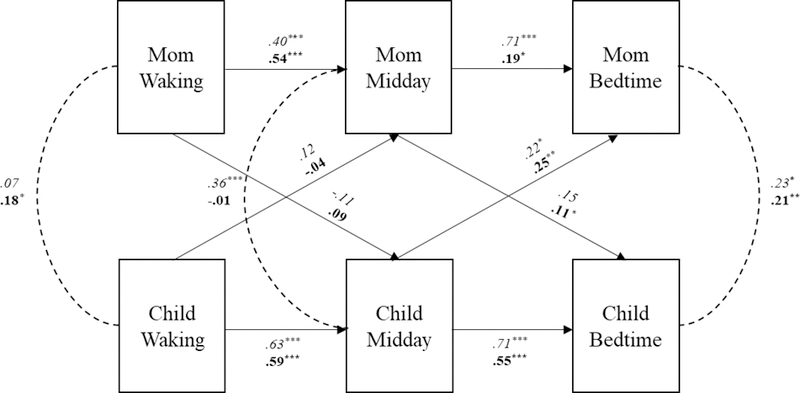
Standardized coefficients for mother and child cortisol associations at waking, midday and bedtime, in the nonmaltreated sample (italicized) and maltreated sample (bolded) from freely estimated multigroup cross-lagged model. Covariates include time of waking and sampling compliance based on MEMS cap data. *p <.05, **p < .01, ***p <.001.
Mother-Child Attunement and Transmission Across Entire Sample
We examined reciprocal (attunement) and lagged (transmission) associations between mother and child cortisol samples across the day: awakening, before lunch, and prior to bedtime. Examining all paths across the entire sample fit the data adequately, χ2 (20) = 20.37, p = .43, CFI = .99, TLI = .99, RMSEA = .01, SRMR = .04. Mother-child cortisol attunement across the day was estimated by covariances at each sampling time point; for ease of interpretation, covariances were converted into correlations. When looking at the entire sample, mother and child cortisol was attuned during the morning (r = .16, p < .01), uncorrelated prior to lunch (r = .10, p = .11), and significantly attuned at bedtime (r = .21, p < .001). To assess transmission of daily cortisol levels we examined cross-lagged partner paths. Across the entire sample, mother’s lunchtime cortisol was found to predict children’s bedtime cortisol (β = .12, p < .01), and children’s lunchtime cortisol significantly predicted mother’s bedtime cortisol (β = .28, p < .001).
Multigroup Analysis of Mother-Child Attunement and Transmission
Next, we explored whether attunement and transmission differed by child maltreatment status by comparing model fit indices from a freely estimated model and a model statistically constraining all paths to be equal between the maltreated and nonmaltreated samples. The freely estimated model fit the data adequately, χ2 (40) = 42.34, p = .37, CFI = .99, TLI = .99, RMSEA = .02, SRMR = .05. In contrast, the model constraining paths to be equal between groups did not fit the data as well, χ2 (60) = 95.76, p < .01, CFI = .92, TLI = .92, RMSEA = .07, SRMR = .14. A likelihood ratio test found imposing equality constraints significantly worsened model fit suggesting a freely estimated model in which path coefficients can differ between groups fit the data better, χ2 (20) = 54.11, p < .001. Therefore, we interpreted the freely estimated multigroup model.
Nonmaltreated group.
Cortisol attunement across the day was estimated by covariances at each sampling time point that were converted into correlations for ease of interpretation. In the nonmaltreated dyads, mother and child cortisol was not attuned during the morning (r = .07, p = .58), attuned at lunchtime (r = .36, p < .01), and attuned at bedtime (r = .23, p <.05). Transmission of cortisol was only found from child to mother, with children’s cortisol prior to lunch predicting mother’s bedtime cortisol (β = .22, p < .05; see Figure 2).
Maltreated group.
Mother and child’s cortisol was attuned during the morning (r = .18, p < .01). Cortisol levels were not attuned at lunchtime (r = −.01, p = .84), and once again attuned at bedtime (r = .21, p < .01). In contrast to the nonmaltreated group, cortisol was transmitted from mother to child and from child to mother, across the day (see Figure 2). Specifically, mother’s cortisol prior to lunch predicted children’s cortisol levels before bedtime (β = .11, p < .05) and in a similar fashion, children’s cortisol prior to lunch predicted mother’s bedtime cortisol (β = .25, p < .001).
Post-hoc analyses.
To highlight the behavioral correlates of relationship functioning that were not examined in the current statistical models but may have influenced group differences in attunement and transmission, we examined group differences in maternal and family characteristics as a post-hoc analysis. Power limitations prohibit the examination of these family process variables in the multigroup dyadic analyses, however, examining group differences between the maltreating and nonmaltreating group illustrates what family characteristics may have facilitated attunement and transmission. Table 2 reveals maltreating mothers report more depressive symptoms t(245) = 3.03 p = .003, more childhood trauma experiences t(222) = 3.23, p = .002, and greater trauma symptoms t(222) = 3.23, p = .001. In addition, maltreating mothers were rated as less sensitive with their children during the laboratory free-play, t(241) = 1.95, p = .05. Finally, maltreating mothers reported spending more hours engaged in caregiving activities with their children than nonmaltreating mothers t(214.5) = 2.75, p = .007. Overall, these maternal characteristics were not related to maternal or child cortisol (p values ranged from .16 to .94) with the exception of maternal depressive symptoms being negatively correlated with the child’s waking cortisol (r = −.15, p = .02). A final post-hoc analysis was run to control for depression in the main model; including this covariate did not substantively change attunement or transmission associations.
Table 2.
Maternal Characteristics by Maltreatment Group
| Maltreating (n = 165) |
Nonmaltreating (n = 83) |
|||
|---|---|---|---|---|
| Variable | M | SD | M | SD |
| 1. Maternal Sensitivity Q-sort | .38 | .42 | .48 | .36* |
| 2. Maternal Depressive Symptoms | 14.86 | 10.07 | 10.95 | 8.58** |
| 3. Maternal Childhood Trauma History | 52.07 | 21.92 | 43.27 | 19.62** |
| 4. Maternal Trauma Symptoms | 22.60 | 14.78 | 16.29 | 11.5** |
| 5. Time spent caring (hr) | 7.37 | 6.10 | 5.49 | 4.45** |
Note. Independent samples t-tests assessed differences by maltreatment group.
p < .05
p < .01.
Discussion
The current study makes an important and novel contribution to the literature by being the first to examine differences in the attunement and transmission of mother-child diurnal cortisol among maltreated and nonmaltreated families. Mothers and children collected cortisol at three time points across two days. From these collections we examined the concurrent (attunement) and cross-lagged (transmission) associations between mother and child cortisol. Findings revealed attunement and transmission differed across the maltreated and nonmaltreated dyads. Specifically, significant mother to child cortisol transmission only occurred in the maltreatment group, mother and child morning cortisol was only attuned in the maltreating group, and mother and child midday cortisol was only attuned in the nonmaltreating group. Together, these findings suggest that the prior experience of maltreatment during early childhood might encourage the spillover of stress across the parent-child dyad, and change the timing/occurrence of attunement.
Attunement.
Mother-child adrenocortical attunement is thought to support the maturation and developmental trajectory of the child’s ability to handle stress, and capacity for social affiliation (Feldman, 2007). In fact, scholars have recently suggested that due to children’s dependence on their caregivers, assessing attunement is critical in understanding child stress physiology (Atkinson et al, 2016). Specifically, mothers’ sensitive and responsive caregiving has been found to facilitate the moment to moment coordination of mother and child physiology. Similarly, as hypothesized, we found that only the nonmaltreated community sample exhibited attunement in mother-child cortisol during the middle of the day.
Previous studies have suggested that greater time spent together increases attunement (Papp et al., 2009), suggesting maltreated dyads are engaged in fewer activities together than nonmaltreated dyads, and thus have fewer opportunities to attune. However, mothers were asked how many hours they spent in activities such as reading, playing, talking, feeding, etc with their child and interestingly, as reported in the post-hoc analyses, maltreating mothers report spending more hours engaged in these caregiving activities than nonmaltreating mothers. This suggests that either these mothers are over-estimating the amount of time they are caring for their child, or it is not the amount of time spent together, but the quality of these interactions that matter for the development of attunement.
Maltreating mothers tend to have trouble regulating their emotions, have disrupted perceptions of their children’s emotional signals, and have controlling or neglectful patterns of parent-child interactions (Cicchetti & Valentino, 2006). Likewise, as seen in Table 2, maltreating mothers were rated as significantly less sensitive during the mother-child free-play, than the nonmaltreating mothers. Numerous disjointed emotional, perceptual, and behavioral interactions might limit the dyad’s ability to effectively and efficiently attune physiologically. Indeed, the majority of the maltreated sample in the current analyses was referred due to neglect. Neglect is characterized by the failure of parents to meet children’s basic needs, including appropriate supervision and interaction, suggesting these mothers may provide minimal emotional and physiological connections with their children during the day. Specifically, withdrawn and disconnected parenting styles might not provide children with needed external regulation (Hibel & Mercado, 2017) nor provide children with opportunities to attune and physiologically connect with their caregivers. Future studies should explore the role of characteristics that differ between the maltreating and nonmaltreating mothers (e.g., domestic violence, trauma symptoms), in potentiating or attenuating mother-child attunement across the day and in explaining associations between maltreatment and mother-child attunement in mediational designs.
Despite no significant attunement during the middle of the day for the maltreating dyads, all families exhibited cortisol attunement at bedtime. This might suggest that even in dyads with histories of neglect, bedtime might provide the chance to engage and connect, at least physiologically. Counter to expectations, the maltreated dyads started their day attuned. Previous research shows genetic factors to be more indicative of morning cortisol, and environmental factors more strongly associated with evening cortisol (Schreiber et al., 2009). Gene by environment interactions might help explain the morning attunement in the maltreating dyads. Specifically, mothers who maltreat their children are likely to have been maltreated themselves (Dixon, Browne, & Hamilton-Giachritsis, 2005) or to have been exposed to significant life trauma (Roberts et al., 2012) compared to mothers who do not maltreat their children. Indeed, these adverse experiences are reflected in Table 2. The combination of shared genetic vulnerabilities and shared traumatic experiences might increase the likelihood of attuned morning cortisol. Interestingly, a recent study also found morning attunement, but only in mother-child dyads where the mother had experienced abuse as a child (Fuchs et al., 2017). Importantly, morning attunement does not reflect co-regulatory processes derived for moment to moment interactions, given that cortisol was collected immediately upon awaking. Yet, as these dyads do engage and spend time together during their first morning hours awake, instead of becoming more strongly attuned (as the nonmatreating dyads do) the maltreating dyads dissociate and physiologically disconnect. Future studies should explore the behavioral dynamics of these dyads to uncover the mechanisms through which mother-child interactions function to (dis)coordinate physiology.
Transmission.
The transmission and reception of emotions across individuals coordinates group behaviors, and creates a shared understanding of group needs (e.g., Butler, 2011). In the context of mother-child relationships, maternal transmission of negative emotions may serve to set children on a developmental course of upregulated stress responsivity. Specifically, it has been suggested that harsh and/or neglectful parenting is a mechanism for the intergenerational transmission of heightened stress reactivity (Champagne & Meaney, 2001). Importantly, results from a recent experiment suggest that transmission of stress physiology from mother to child only occurs in the context of maternal stress. When mothers were randomized to either undergo a marital conflict or positive marital discussion, maternal cortisol reactivity and recovery to the conflict (but not positive) discussion predicted subsequent child cortisol reactivity and recovery to stress (Hibel & Mercado, 2017). In the current analyses we examined the propensity for maternal cortisol to prospectively predict child cortisol later in the day. Similar to Hibel and Mercado’s findings, only in the maltreatment group did maternal adrenocortical activity predict child adrenocortical activity. Maltreating mothers tend to be characterized by greater stress than nonmaltreating mothers. Behaviorally, maltreating mothers tend to display more anger, negativity, and reactivity and less sensitivity compared to nonmaltreating mothers (Cicchetti et al., 2011). Emotionally, maltreating mothers also exhibit higher rates of psychopathology including depression and traumatic stress symptoms (Cicchetti & Valentino, 2006), and as seen in Table 2, mothers in this sample report similar experiences and exhibit similar behaviors. Thus, maternal transmission of stress physiology across the day might be part of the process by which child maltreatment leads to dysregulations in children’s behavior and physiology and subsequent risk for poor mental and physical health throughout the lifespan (Anda et al, 2006; Cicchetti & Tucker, 1994).
The current analyses not only captured mother to child transmission, but also child to mother transmission. Though behavioral synchrony is conceived as a dyadic construct, developmental psychologists place the onus to synchronize on the mother. Sensitive and responsive parenting behavior is child focused such that the mother is responding to her child’s cues (Ainsworth, Blehar, Waters, & Wall, 1978; Sroufe, 1979). Thus, sensitive mothers must be receptive to child signals of distress, responding with empathy and providing a supportive buffer (Spangler et al., 1994; Bowlby, 1969). Likewise, for mothers in the nonmaltreating sample, child stress physiology during the day predicted mothers’ evening cortisol. Whereas maltreating mothers also showed this association, it is important to remember that these mothers are not providing an effective buffer, as they are also transmitting their physiology to their child. In other words, while mothers in the nonmaltreating group receive their child’s stress signal, they do not also transmit their own stress signal. These findings across the day raise important questions regarding how different patterns of dyadic responsivity and transmission may affect the development and maturation of children’s stress response system.
Overall, the results of the current investigation make an important contribution to our understanding of how early adversity affects children’s diurnal cortisol regulation. In particular, our findings highlight the need to frame our understanding of child stress physiology within social ecological contexts (Cicchetti & Valentino, 2006) and suggest that there are important differences between maltreating and nonmaltreating mother-child dyads regarding the extent to which mother-child physiology affects one another. As recommended by Atkinson and colleagues (2016), child stress physiology during early childhood may be best understood in the context of the primary caregiver’s physiological functioning. We expand this recommendation to include the analysis of diurnal cortisol regulation in addition to mother-child cortisol reactivity to laboratory stressors and challenges. Notably, aside from one paper examining maternal diurnal physiology in mothers with a history of abuse (Fuchs et al., 2017); none of the existing literature on child maltreatment and diurnal cortisol to date has incorporated indices of maternal diurnal stress regulation into the study design. Our findings suggest that mother-child physiological attunement and transmission are critical to gaining a more comprehensive understanding of child stress physiology during early childhood. Mother-child attunement and stress transmission may be important moderators or mediators of associations between child maltreatment and subsequent developmental trajectories that could help explain discrepant findings in the literature with regard to how early adversity affects child stress physiology.
The results of the current study should be interpreted within the context of a number of limitations. First, our models are based on two consecutive days of diurnal cortisol collection; as such our results are tentative. Future research should seek to replicate these findings over multiple measurement occasions to better understand mother and child physiological attunement and transmission across the day. It is important to note, however, that maltreating families may become less adherent to cortisol collection procedures over multiple days of sampling, thus including objective measurement of cortisol collection timing is important to establish participant adherence and confirm the validity of the cortisol data (Valentino et al., 2017).
Second, we acknowledge that our maltreated sample was comprised, primarily, of families in which child neglect had occurred. As such, our findings may not generalize to mother-child dyads in which more severe forms of maltreatment such as physical and sexual abuse have occurred. In those more severe instances of maltreatment, children are more likely to be removed from their homes and placed into foster care. Thus, another important future research direction is to learn more regarding possible attunement and transmission of stress physiology among foster parent- foster child dyads. Notably, much of the existing empirical work on diurnal cortisol regulation among maltreated children has included samples of children placed in foster care as opposed to maltreating children who are in the custody of their parents (e.g., Bruce et al., 2009; Dozier et al., 2006). Furthermore the relatively small proportion of children in our sample who experienced abuse precluded our ability to examine effects of maltreatment subtype. It will be important to examine potential differences in attunement and transmission among abusive as compared to neglecting families, as some research examining parasympathetic stress responses has indicated diverging profiles of physiological concordance measured via RSA between mother-child dyads from abusing and neglecting families (Lunkenheimer, Busuito, Brown, & Skowron, 2018). In addition to maltreatment subtypes, future research should also address factors such as chronicity of maltreatment and time since the last occurrence in understanding how maltreatment leads to differences in attunement and transmission. Prior work has also suggested that maternal childhood maltreatment history may be an important moderator of mother-toddler diurnal cortisol attunement (Fuchs et al., 2017). As such, future research should also consider maternal maltreatment history as a moderator of mother-child physiological attunement within maltreating and nonmaltreating families.
Conclusion
Numerous studies have documented the concurrent attunement of mother and child physiology; however this study is one of the first to document the active transmission of physiology across the mother-child dyad. Importantly, without longitudinal studies we are limited in our understanding of how mother-child attunement and transmission affects the development of children’s self-regulatory capacities. For example, though attunement is often conceptualized as part of the underlying process by which maternal behavior organizes the infant’s behavioral and physiological activity (e.g., Feldman, 2007), it may be the case that less (or no) attunement among maltreating dyads is adaptive for young maltreated children. In other words, while humans may have evolved to postnatally develop regulatory abilities that match the harshness or kindness of their mothers’ care, the advantage of a high reactivity profile while living with maltreating parents might be lost once the child leaves the home. In the long term, it might be more adaptive for the child to not attune to their pathological mother nor receive their mothers’ transmission of stress. Given the results of the current study, which establishes that the context of maltreatment is associated with differences in mother-child physiological attunement and transmission, longitudinal studies of these dyadic physiological processes are necessary to determine the mechanisms that explain how child maltreatment leads to such differences and the extent to which they become adaptive or maladaptive over time.
Acknowledgments
The authors wish to thank Heidi Miller and the H2H project staff for their invaluable assistance with this project. Additionally, we are grateful to the children and families that participated in this study and the Department of Child Services of St. Joseph County. This research was supported by grant 5 R01 HD071933-03 to K. Valentino.
References
- Adam EK (2006) Transactions among adolescent trait and state emotion and diurnal and momentary cortisol activity in naturalistic settings. Psychoneuroendocrinology, 31, 664–679 [DOI] [PubMed] [Google Scholar]
- Adam EK, & Kumari M (2009) Assessing salivary cortisol in large-scale, epidemiological research. Psychoneuroendocrinology, 34, 1423–1436 [DOI] [PubMed] [Google Scholar]
- Adam EK, Quinn ME, Tavernier R, Tavernier MT, Dahlke KA, Gilbert KE, (2017). Diurnal cortisol slopes and mental and physical health outcomes: A systematic review and meta-analysis. Psychoneuroendocrinology, 83, 25–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ainsworth MD, Blehar MC Waters E, & Wall (1978). Patterns of attachment: A psychological study of the strange situation Hillsdale, NJ: Erlbaum [Google Scholar]
- Albers EM, Marianne Riksen-Walraven J, Sweep FCGJ, & Weerth C. De. (2008). Maternal behavior predicts infant cortisol recovery from a mild everyday stressor. Journal of Child Psychology and Psychiatry and Allied Disciplines, 49(1), 97–103. 10.1111/j.1469-7610.2007.01818.x [DOI] [PubMed] [Google Scholar]
- Anda R, Felitti V, Bremner J, Walker J, Whitfield C, & Perry B (2006). The enduring effects of abuse and related adverse experiences in childhood: A convergence of evidence from neurobiology and epidemiology. European Archives of Psychiatry and Clinical Neuroscience, 256, 174–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson L, Gonzalez A, Kashy DA, Santo Basile V, Masellis M, Pereira J, Levitan R (2013) Maternal sensitivity and infant and mother adrenocortical function across challenges. Psychoneuroendocrinology 38(12), 2943–2951. 10.1016/j.psyneuen.2013.08.001 [DOI] [PubMed] [Google Scholar]
- Atkinson L, Jamieson B, Khoury J, Ludmer L, Gonzalez A (2016) Stress Physiology in Infancy and Early Childhood: Cortisol Flexibility, Attunement and Coordination. Journal of Neuroendocrinology, 28, 1–12. 10.1111/jne.12408 [DOI] [PubMed] [Google Scholar]
- Barnett D, Manly JT, & Cicchetti D (1993). Defining child maltreatment: The interface between policy and research. In Cicchetti D, & Toth SL (Eds.), Child abuse, child development, and social policy (pp.7–74). Norwood, NJ: Ablex. [Google Scholar]
- Bernard K, Frost A, Bennett CB, & Lindhiem O (2017). Maltreatment and diurnal cortisol regulation: a meta-analysis. Psychoneuroendocrinology, 78, 57–67. [DOI] [PubMed] [Google Scholar]
- Bernard NK, Kashy DA, Levendosky AA, Bogat GA, & Lonstein JS (2017). Do different data analytic approaches generate discrepant findings when measuring mother–infant HPA axis attunement?. Developmental psychobiology, 59(2), 174–184. [DOI] [PubMed] [Google Scholar]
- Bernstein DP, & Fink L (1998). Childhood Trauma Questionnaire: A retrospective self-report manual San Antonio, TX: The Psychological Corporation. [Google Scholar]
- Bowlby J (1969). Attachment and loss: Attachment. Attachment (Vol. 1) 10.1177/000306518403200125 [DOI]
- Butler EA (2011) Temporal Interpersonal Emotion Systems The “TIES” That Form Relationships. Personality and Social Psychology Review 15(4), 367–393. 10.1177/1088868311411164 [DOI] [PubMed] [Google Scholar]
- Bruce J, Fisher PA, Pears KC, & Levine S (2009). Morning cortisol levels in preschool- aged foster children: Differential effects of maltreatment type. Developmental Psychobiology, 51, 14–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champagne F, & Meaney MJ (2001) Like mother, like daughter: evidence for non-genomic transmission of parental behavior and stress responsivity. Progress in Brain Research 133:287–302. [DOI] [PubMed] [Google Scholar]
- Cicchetti D, & Rogosch FA (2001). Diverse patterns of neuroendocrine activity in maltreated children. Development and Psychopathology, 13, 677–694. [DOI] [PubMed] [Google Scholar]
- Cicchetti D, Rogosch FA, Gunnar MR, & Toth SL (2010). The differential impacts of early abuse on internalizing problems and diurnal cortisol activity in school-aged children. Child Development, 25, 252–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicchetti D, Rogosch FA, Oshri A (2011). Interactive effects of CRHR1, 5-HTTLPR, and child maltreatment on diurnal cortisol regulation and internalizing symptomatology. Developmental Psychopathology, 23, 1125–1138. doi: 10.1017/S0954579411000599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicchetti D, Rogosch FA, Toth SL, & Sturge-Apple ML (2011). Normalizing the Development of Cortisol Regulation in Maltreated Infants Through Preventive Interventions. Development and Psychopathology, 23(3), 789–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicchetti D, Toth SL, & Manly JT (2002). Maternal Maltreatment Classification Interview Rochester, NY: Mt. Hope Family Center; Unpublished measure. [Google Scholar]
- Cicchetti D & Tucker D (1994). Development and self-regulatory structures of the mind. Development & Psychopathology, 6, 533–549. [Google Scholar]
- Cicchetti D & Valentino K (2006). An ecological-transactional perspective on child maltreatment: failure of the average expectable environment and its influence on child development. Cicchetti D, & Cohen D (Eds.). Development and Psychopathology, vol. 3, 2nd Ed. (pp 129–201). Hoboken, NJ: John Wiley & Sons. [Google Scholar]
- Cook WL, & Kenny DA (2005). The actor–partner interdependence model: A model of bidirectional effects in developmental studies. International Journal of Behavioral Development, 29(2), 101–109. [Google Scholar]
- Dixon L, Browne K, & Hamilton-Giachritsis C (2005). Risk factors of parents abused as children: A mediational analysis of the intergenerational continuity of child maltreatment (part I). Journal of Child Psychology and Psychiatry and Allied Disciplines, 46(1), 47–57. doi: 10.1111/j.1469-7610.2004.00339.x [DOI] [PubMed] [Google Scholar]
- Doom JR, Cicchetti D, Rogosch FA, & Dackis MN (2013). Child maltreatment and gender interactions as predictors of differential neuroendocrine profiles. Psychoneuroendocrinology, 38(8), 1442–1454. 10.1016/j.psyneuen.2012.12.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dozier M, Manni M, Gordon K, Peloso E, Gunnar MR, Stovall-McClough KC, Eldreth & Levine S, (2006). Foster Children’s Diurnal Production of Cortisol: An Exploratory Study. Child Maltreatment , 11(2), 189–197 [DOI] [PubMed] [Google Scholar]
- Feldman R (2007). Infant Biological Foundations Synchrony and Developmental Outcomes. Current Directions in Psychological Science, 16(6), 340–345. 10.1111/j.1467-8721.2007.00532.x [DOI] [Google Scholar]
- Feldman R (2012) Bio-behavioral synchrony: A model for integrating biological and microsocial behavioral processes in the study of parenting. Parenting 12(2–3), 154–164. 10.1080/15295192.2012.683342 [DOI] [Google Scholar]
- Fuchs A, Moehler E, Resck F, & Kaess M (2017). The effect of a maternal history of childhood abuse on adrenocortical attunement in mothers and their toddlers. Developmental Psychobiology 2017;59:639–652. [DOI] [PubMed] [Google Scholar]
- Granger DA, Blair C, Willoughby M, Kivlighan KT, Hibel LC, Fortunato CK, & Wiegand LE (2007). - Individual differences in salivary cortisol and alpha-amylase in mothers and their infants: relation to tobacco smoke exposure.; - Developmental psychobiology. - Developmental Psychobiology [DOI] [PubMed]
- Granger DA, Hibel LC, & Fortunato CK, Kapelewski CH (2009). Medication effects on salivary cortisol: Mechanisms of action, a “watch list”, and tactics to minimize impact in biobehavioral research. Psychoneuroendocrinology, 34, 1437–48. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Doom JR, & Esposito EA (2015) Psychoneuroendocrinology of stress. Handbook of Child Psychology and Developmental Science 10.1002/9781118963418.childpsy304 [DOI]
- Gunnar MR, & Donzella B (2002). Social regulation of the cortisol levels in early human development. Psychoneuroendocrinology, 27(1–2), 199–220. [DOI] [PubMed] [Google Scholar]
- Heim C, Newport DJ, Heit S, Graham YP, Wilcox M, Bonsall R, … Nemeroff CB (2000). Pituitary-Adrenal and Autonomic Responses to Stress in Women After Sexual. The Journal of American Medical Association, 284(5), 592–597. 10.1001/jama.2013.285447. [DOI] [PubMed] [Google Scholar]
- Heim C, Owens MJ, Plotsky PM, & Nemeroff CB (1997). The role of early adverse life events in the etiology of depression and posttraumatic stress disorder. Annals of the New York Academy of Sciences, 821(404), 194–207. 10.1111/j.1749-6632.1997.tb48279.x [DOI] [PubMed] [Google Scholar]
- Hibel L, Granger D, Blair C, & Cox M (2011). Maternal sensitivity buffers the adrenocortical implications of intimate partner violence exposure during early childhood. Development and Psychopathology, 23(2), 689–701. doi: 10.1017/S0954579411000010 [DOI] [PubMed] [Google Scholar]
- Hibel LC, Granger DA, Blair C, Cox MJ, & The Family Life Project Key Investigators. (2009). Intimate partner violence moderates the association between mother–infant adrenocortical activity across an emotional challenge. Journal of Family Psychology, 23(5), 615–625. 10.1037/a0016323 [DOI] [PubMed] [Google Scholar]
- Hibel LC, Granger DA, Blair C, Finegood E, & The Family Life Project Investigators (2015). Maternal-Child Adrenocortical Attunement in Early Childhood: Continuity and Change. Developmental Psychobiology, 57, 83–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibel LC, Trumbell JM, & Mercado E (2014) Work/non-workday differences in mother, child, and mother–child morning cortisol in working mothers and their children. Early Human Development 90(1), 1–7. 10.1016/j.earlhumdev.2013.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibel LC, & Mercado E (2017) Marital conflict predicts mother-to-infant adrenocortical transmission. Child Development 10.1111/cdev.13010 [DOI] [PMC free article] [PubMed]
- Heim C, Newport DJ, Bonsall R, Miller AH, & Nemeroff CB (2001) Altered pituitary-adrenal axis responses to provocative challenge tests in adult survivors of childhood abuse. Am. J. Psychiatry, 158, 575–581 [DOI] [PubMed] [Google Scholar]
- Hofer MA (2006). Psychobiological roots of early attachment. Current Directions in Psychological Science, 15, 84–88. [Google Scholar]
- Hu L & Bentler PM (1999) Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Structural Equation Modeling: A Multidisciplinary Journal, 6:1, 1–55, DOI: 10.1080/10705519909540118 [DOI] [Google Scholar]
- Kenny DA, & Cook W (1999). Partner effects in relationship research: Conceptual issues, analytic difficulties, and illustrations. Personal Relationships, 6(4), 433–448. 10.1111/j.1475-6811.1999.tb00202.x [DOI] [Google Scholar]
- Kuras YI, Assaf N, Thoma MV, Gianferante D, Hanlin L, Chen X, Fiksdal A, & Rohleder N, (2017). Blunted Diurnal Cortisol Activity in Healthy Adults with Childhood Adversity. Frontiers in Human Neuroscience, 11, 574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurent HK, Ablow JC, & Measelle J (2012) Taking stress response out of the box: Stability, discontinuity, and temperament effects on HPA and SNS across social stressors in mother–infant dyads. Developmental Psychology 48, 35 http://psycnet.apa.org/doi/10.1037/a0025518 [DOI] [PubMed] [Google Scholar]
- Laurent H, Harold G, Leve L, Shelton K, & Van Goozen S (2016). Understanding the unfolding of stress regulation in infants. Development and Psychopathology, 28(4pt2), 1431–1440. doi: 10.1017/S0954579416000171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurent HK, Stevens A, & Ablow JC (2011). Neural Correlates of Hypothalamic-Pituitary-Adrenal Regulation of Mothers with Their Infants. Biological Psychiatry, 70(9), 826–832. 10.1016/J.BIOPSYCH.2011.06.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loman MM, Gunnar MR, & The Early Experience, Stress, and Neurobehavioral Development Center (2009) Developmental determinants of sensitivity and resistance to stress. Neuroscience & Biobehavioral Reviews, 34, 867–87619481109 [Google Scholar]
- Lunkenheimer E, Busuito A, Brown KM, & Skowron EA (2018). Mother–Child Coregulation of Parasympathetic Processes Differs by Child Maltreatment Severity and Subtype. Child Maltreatment Advanced online publication: January 11, 2018: 10.1177/1077559517751672 [DOI] [PMC free article] [PubMed]
- Manly JT, Kim JE, Rogosch FA, & Cicchetti D (2001). Dimensions of child maltreatment and children’s adjustment: contributions of developmental timing and subtype. Development and psychopathology, 13 4, 759–82. [PubMed] [Google Scholar]
- McEwen BS (2000). Effects of adverse experiences for brain structure and function. Biological Psychiatry, 48(8), 721–731. 10.1016/S0006-3223(00)00964-1 [DOI] [PubMed] [Google Scholar]
- Middlemiss W, Granger DA, Goldberg WA, & Nathans L (2012) Asynchrony of mother–infant hypothalamic–pituitary–adrenal axis activity following extinction of infant crying responses induced during the transition to sleep. Early Human Development 88(4), 227–232. 10.1016/j.earlhumdev.2011.08.010 [DOI] [PubMed] [Google Scholar]
- Miller GE, Chen E, & Zhou ES (2007). If it goes up, must it come down? Chronic stress and the hypothalamic-pituitary-adrenocortical axis in humans. Psychological Bulletin, 133(1), 25–45. 10.1037/0033-2909.133.1.25 [DOI] [PubMed] [Google Scholar]
- Muthén LK & Muthén BO (1998–2017). Mplus User’s Guide Eighth Edition. Los Angeles, CA: Muthén & Muthén. [Google Scholar]
- Papp L, M., Pendry P, & Adam E,K (2009) Mother-adolescent physiological synchrony in naturalistic settings: Within-family cortisol associations and moderators. Journal of Family Psychology, 23, 882–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pederson DR, Moran G, & Bento S (2013). The Maternal Behavior Q-sort: Assessing Maternal Sensitivity and the Quality of Mother-Infant Interaction. Unpublished Manual University of Western Ontario. [Google Scholar]
- Pendry P, & Adam EK (2007). Associations between parents’ marital functioning, maternal parenting quality, maternal emotion and child cortisol levels. International Journal of Behavioral Development, 31(3), 218–231. DOI: 10.1177/0165025407074634 [DOI] [Google Scholar]
- Radloff LS (1977). The CES-D scale: A self report depression scale for research in the general population. Applied Psychological Measurements, 1, 385–401. [Google Scholar]
- Repetti RL, Taylor SE, & Seeman TE (2002). Risky families: family social environments and the mental and physical health of offspring. Psychological Bulletin, 128(2), 330–366. [PubMed] [Google Scholar]
- Roberts AL, Galea S, Austin SB, Cerda M, Wright RJ, Rich-Edwards JW, & Koenen KC (2012). Posttraumatic stress disorder across two generations: Concordance and mechanisms in a population-based sample. Biological psychiatry, 72(6), 505–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schonkoff J (2012). The lifelong effects of early childhood adversity and toxic stress. Pediatrics, 129, e232–e246. [DOI] [PubMed] [Google Scholar]
- Schreiber JE, Shirtcliff E, Van Hulle C, Lemery-Chalfant K, Klein MH, Kalin NH, Goldsmith HH (2006). Environmental influences on family similarity in afternoon cortisol levels: Twin and parent-offspring designs. Psychoneuroendocrinology, 31, 1131–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sethre-Hofstad L, Stansbury K, Rice MA, 2002. Attunement of maternal and child adrenocortical response to child challenge. Psychoneuroendocrinology 27, 731–747. [DOI] [PubMed] [Google Scholar]
- Shonkoff JP (2016). Capitalizing on advances in science to reduce the health consequences of early childhood adversity. JAMA Pediatrics, 170(10), 1003–1007. 10.1001/jamapediatrics.2016.1559 [DOI] [PubMed] [Google Scholar]
- Smyth N, Hucklebridge F, Thorn L, Evans P, & Clow A (2013) Salivary Cortisol as a Biomarker in Social Science Research. Social and Personality Psychology Compass 79, 605–625 [Google Scholar]
- Spangler G, Schieche M, Ilg U, Maier U, & Ackermann C (1994). Maternal sensitivity as an external organizer for biobehavioral regulation in infancy. Developmental Psychobiology, 27(7), 425–437. [DOI] [PubMed] [Google Scholar]
- Sroufe LA (1979). The Coherence of Individual Development. American Psychologist, 34(10), 834–841. 10.1037/0003-066X.34.10.834 [DOI] [Google Scholar]
- Stahmer AC, Leslie LK, Hurlburt M, Barth RP, Webb MB, Landsverk J, & Zhang J (2005) Developmental and behavioral needs and service use for young children in child welfare. Pediatrics, 116(4):891–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stawski RG, Cichy KE, Piazza JR, & Almeida DM, (2013). Psychoneuroendocrinology 38, 2654–2665. 10.1016/j.psyneuen.2013.06.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumner JA, McLaughlin KA, Walsh K, Sheridan MA, & Koenen KC (2014). CRHR1 genotype and history of maltreatment predict cortisol reactivity to stress in adolescents. Psychoneuroendocrinology, 43, 71–80. 10.1016/j.psyneuen.2014.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trickett P, Noll J, Susman E, Shenk C, & Putnam F (2010). Attenuation of cortisol across development for victims of sexual abuse. Development and Psychopathology, 22(1), 165–175. doi: 10.1017/S0954579409990332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullman JB (2013). Structural equation modeling. In Tabachnick BG & Fidell LS (Eds.), Using multivariate statistics (6th ed., pp. 676–731). New York, NY: Allyn Bacon. [Google Scholar]
- U.S. Department of Health & Human Services, Administration for Children and Families, Administration on Children, Youth and Families, Children’s Bureau. (2018). Child maltreatment 2016 Available from https://www.acf.hhs.gov/cb/research-data-technology/statistics-research/child-maltreatment.
- Valentino K, De Alba A, Hibel LC, Fondren K, & McDonnell CG (2017). Adherence to Diurnal Cortisol Sampling Among Mother–Child Dyads From Maltreating and Nonmaltreating Families. Child Maltreatment, 22(4), 286–294. 10.1177/1077559517725208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentino K, Hibel LC, Cummings EM, Nuttall A, Comas M, McDonnell CG (2015) Maternal reminiscing mediates the effect of child maltreatment on behavioral and physiological functioning. Special Issue in Development and Psychopathology, Multilevel Developmental Perspectives on Child Maltreatment: Current Research and Future Perspectives, 27, Special Issue 4 pt 2, 1515–1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss DS, & Marmar CR (1997). The Impact of Event Scale-Revised. In Wilson JP & Keane TM (Eds.), Assessing Psychological Trauma and PTSD (pp.399–411). New York: Guilford. [Google Scholar]


