Abstract
Disseminated intravascular coagulation (DIC) is an acquired clinicobiological syndrome characterized by widespread activation of coagulation leading to fibrin deposition in the vasculature, organ dysfunction, consumption of clotting factors and platelets, and life-threatening hemorrhage. Disseminated intravascular coagulation is provoked by several underlying disorders (sepsis, cancer, trauma, and pregnancy complicated with eclampsia or other calamities). Treatment of the underlying disease and elimination of the trigger mechanism are the cornerstone therapeutic approaches. Therapeutic strategies specific for DIC aim to control activation of blood coagulation and bleeding risk. The clinical trials using DIC as entry criterion are limited. Large randomized, phase III clinical trials have investigated the efficacy of antithrombin (AT), activated protein C (APC), tissue factor pathway inhibitor (TFPI), and thrombomodulin (TM) in patients with sepsis, but the diagnosis of DIC was not part of the inclusion criteria. Treatment with APC reduced 28-day mortality of patients with severe sepsis, including patients retrospectively assigned to a subgroup with sepsis-associated DIC. Treatment with APC did not have any positive effects in other patient groups. The APC treatment increased the bleeding risk in patients with sepsis, which led to the withdrawal of this drug from the market. Treatment with AT failed to reduce 28-day mortality in patients with severe sepsis, but a retrospective subgroup analysis suggested possible efficacy in patients with DIC. Clinical studies with recombinant TFPI or TM have been carried out showing promising results. The efficacy and safety of other anticoagulants (ie, unfractionated heparin, low-molecular-weight heparin) or transfusion of platelet concentrates or clotting factor concentrates have not been objectively assessed.
Keywords: disseminated intravascular coagulation, sepsis, antithrombin, activated protein C, thrombomodulin, tissue factor pathway inhibitor
Introduction
Disseminated intravascular coagulation (DIC) is a life-threatening syndrome characterized by disseminated and often uncontrolled activation of coagulation. This syndrome is associated with a high risk of macro- and microvascular thrombosis and progressive consumption coagulopathy, which leads to an increased bleeding risk. Several pathological conditions may trigger DIC (Table 1), with sepsis, cancer, trauma, and obstetric calamity ranking among the most frequent triggering factors.1 The risk of DIC is particularly high in patients with sepsis. Indeed, DIC occurs in 30% to 50% of them, whereas the frequency of DIC is approximately 10% in patients with solid tumors, trauma, or obstetric calamities.1–3 The overall prevalence of DIC in hospitalized patients is unknown because the established clinical scoring systems for DIC are not systematically employed in clinical practice. Furthermore, the prevalence of DIC depends on the context of hospitalization and is higher in critically ill patients hospitalized in intensive care units (ICUs). In this subset of patients, the prevalence of DIC ranges from 8.5% to 34% depending on the underlying diagnoses and the diagnostic scoring system used.3–7
Table 1.
Clinical Conditions Associated With DIC.
| Clinical Conditions Triggering DIC | Causes of DIC |
|---|---|
| Sepsis or severe infection |
|
| Trauma |
|
| Organ destruction |
|
| Malignancy |
|
| Obstetrical calamities |
|
| Vascular abnormalities |
|
| Liver disease |
|
| Severe toxic or immunological reactions |
|
Abbreviations: DIC, disseminated intravascular coagulation; HELLP, hemolysis, elevated liver enzyme levels, and low platelet levels.
In critically ill patients with DIC, the 28-day mortality rate is 20% to 50%, significantly higher than that of critically ill patients who do not fulfill the DIC criteria.3 Early diagnosis and appropriate treatment of DIC is of critical importance to favorable patient outcomes.
Definition of DIC
Disseminated intravascular coagulation is an acquired syndrome characterized by dispersed and uncontrolled activation of blood coagulation leading to intravascular fibrin formation. The signature feature of DIC is the loss of localized activation of coagulation and the inefficiency of natural coagulation inhibitors to downregulate thrombin generation. According to the definition of the International Society of Thrombosis and Haemostasis scientific subcommittee, DIC is an acquired syndrome characterized by the intravascular activation of coagulation with loss of localization arising from different causes. It can originate from and cause damage to the microvasculature, which if sufficiently severe, can produce organ dysfunction.8
Pathogenesis of DIC
Although the first clinical and pathological observations related to DIC were made in the 19th century,9 detailed knowledge on the pathogenesis of the syndrome has only been attained in past decades. In recent years, some of the mechanisms involved in pathological microvascular fibrin deposition in DIC have been clarified10,11:
excessive thrombin generation triggered by tissue factor (TF) overexpression related to the underlying disease process
platelet activation
defective natural anticoagulant pathways, including tissue factor pathway inhibitor (TFPI), antithrombin (AT), and protein C (PC)
insufficient fibrin degradation due to impaired fibrinolysis or exaggerated fibrin and fibrinogen degradation
concomitant activation of the inflammatory process
Fibrin formation is accompanied by activation of fibrinolysis, the extent of which depends on plasminogen activator inhibitor 1 (PAI-1), thrombin-activatable fibrinolysis inhibitor (TAFI), and other factors related to the underlying disease and the capacity of regulatory mechanisms. If not adequately counteracted by fibrinolysis, fibrin deposition may cause diffuse obstruction of the microvasculature.
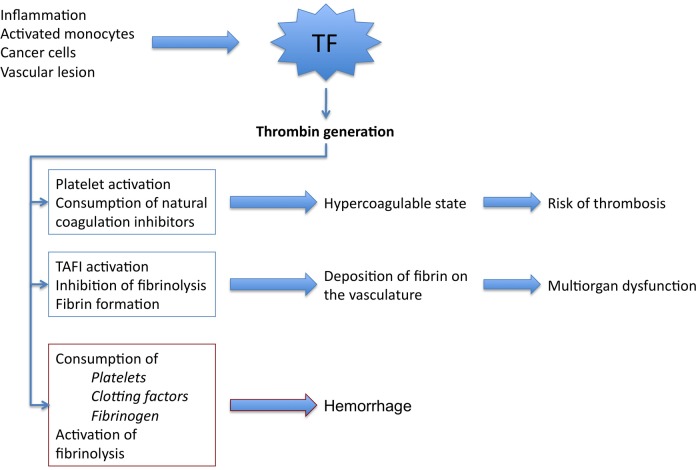
Microvascular thrombosis in conjunction with hemodynamic and metabolic derangements contributes to multi-organ dysfunction. Signs of organ dysfunction include renal insufficiency, respiratory failure, hypotension, and circulatory failure or impaired cerebral function. In addition, intravascular coagulation activation may contribute to the formation of macrovascular thrombi, resulting in venous or arterial obstruction and embolization. These mechanisms and the associated clinical manifestations are schematically presented in Figure 1.
Figure 1.
Pathogenetic pathways in DIC. Activation of coagulation is driven by TF overexpression leading to explosive and disseminated thrombin generation, which results in consumption of natural coagulation inhibitors (mainly AT and PC) and in a hypercoagulable state. Thrombin, among other inducers, enhances platelet activation. Activated platelets amplify hypercoagulable state. Inhibition of fibrinolysis, through TAFI activation, increases fibrin formation and deposition in the microvasculature. This mechanism—among others—is implicated in the pathogenesis of organ dysfunction and multi-organ failure. Sustained thrombin generation has, as a consequence, the consumption of clotting factors, platelets, and fibrinogen. Severe clotting factor and fibrinogen deficiency together with severe thrombocytopenia are in the origin of the hemorrhagic syndrome in DIC. AT indicates antithrombin; DIC, disseminated intravascular coagulation; PC, protein C; TF, tissue factor.
Tissue Factor Triggered Initiation of Thrombin Generation in DIC
Tissue factor is a major trigger of the coagulation process. The TF expression on monocytes and endothelial cells is induced by inflammatory mediators, predominantly through transcriptional mechanisms.12,13 Overexpression of TF by activated monocytes and endothelial cells triggers activation of coagulation.14,15 It is also overexpressed by some types of cancer cells.16 Phospholipid microparticles (MPs) originating from active monocytes and platelet–monocyte complexes are another source of TF. The TF-bearing MPs have been detected in plasma of patients with meningococcal sepsis.17,18 Increased exposure of negatively charged phospholipid surfaces further facilitates the assembly of prothrombinase (factor Xa [FXa]/FVa/phospholipids/Ca++)] and intrinsic tenase (FIXa/FVIIIa/phospholipids/Ca++) leading to the propagation of thrombin generation.19 Cell membrane procoagulant surfaces, rich in phosphatidylserine, are provided by externalization of the inner leaflet of cell membranes on activation and apoptosis.20 Additionally, very-low-density lipoprotein (VLDL) particles, specifically VLDL complexes with C-reactive protein, are found at increased levels in severe sepsis and may further enhance and sustain thrombin generation.21
In addition, thrombin activates platelets, leading to P-selectin translocation and the subsequent upregulation of TF expression. This indicates that P-selectin may play a significant role in the initiation of thrombin generation in DIC. In addition, P-selectin is positively correlated with DIC score, fibrinogen consumption, fibrinolysis, thrombin activation markers, and TFPI.22
Platelets
Disseminated intravascular coagulation is often associated with a decline in platelet count due to the consumption of platelets in the course of coagulation activation. Platelet consumption is a process that implicates platelet activation. Thus, the expression of procoagulant phospholipids at the external side of platelet membrane, the release of procoagulant and pro-inflammatory proteins and vasoactive molecules (ie, FV, platelet factor 4, serotonin, adrenaline, prostaglandins) during platelet activation, and the formation and release of procoagulant MPs enhance the hypercoagulable state and contribute to the dissemination of thrombin generation. Platelets are also actively involved in the pathogenesis of DIC. Increased expression of membrane glycoprotein IIb/IIIa in its activated form and increased expression of P-selectin may contribute to the microvascular occlusions observed in DIC.23
Microparticle Formation and Sepsis
The formation of procoagulant MPs, originating from platelets, endothelial cells, erythrocytes, and leukocytes, is enhanced in sepsis. Leukocyte and platelet-derived MPs induce the release of endothelial (interleukin [IL]-1β, IL-6, IL-8, monocyte chemoattractant protein 1) and monocytic (IL-1β, tumor necrosis factor [TNF] α, IL-8) cytokines and enhance the level of oxidized phospholipids, which activate endothelial cells and leukocytes in return. Accordingly, MPs have a significant role in sepsis-induced inflammation.
The role of platelet-derived MP in procoagulant activity was illustrated by Sinauridze and colleagues who demonstrated that platelet-derived MPs are 50- to 100-fold more procoagulant than activated platelets.24 The procoagulant properties of platelet-derived MP stem from the combined presence of phosphatidylserine—a procoagulant aminophospholipid exposed after stimulation that supports the assembly of the blood clotting enzyme complexes—and TF.
Role of Calpains and Calpastatin in Inflammation, Sepsis, and DIC
The calcium-activated cysteine proteases, calpains, play a central role in MP release from cells, especially platelets. The Ca21-activated calpains degrade cytoskeletal proteins such as talin or vinculin and directly induce MP release into the extracellular microenvironment. In addition, calpains degrade the nuclear factor (NF) κB inhibitor I-κB and thereby induce the nuclear translocation of NF-κB, an important mediator of IL-6, IL-1, and TNF-α gene expression. The inflammatory cytokine IL-6 is one of the major mediators involved in coagulation activation and is correlated with the formation of platelet-derived MP in humans.
Calpastatin, a calpain-specific physiological inhibitor, may cause selective reduction in inflammation, DIC, lymphocyte apoptosis, and later immunodepression, without impairing critical innate immune functions such as neutrophil infiltration or interfering with the basal calpain activity required for cellular homeostasis. Calpastatin increases survival and reduces multi-organ dysfunction during sepsis, mainly through the decrease of procoagulant MP release.25
Natural Anticoagulant Pathways in DIC
Under normal conditions, blood coagulation is downregulated by the stoichiometric inhibitors TFPI and AT and the dynamic PC anticoagulant pathway. During the evolution of DIC, however, the natural anticoagulant pathways of TFPI, AT, and PC are severely compromised. Studies in animal and human models of sepsis have provided important insight on the role of natural anticoagulant pathway deficiencies in the pathogenesis of sepsis-related DIC.26
Tissue factor pathway inhibitor
Tissue factor pathway inhibitor is a plasma Kunitz-type serine protease inhibitor that inhibits the initiation phase of thrombin generation induced by TF. The TFPI stoichiometrically inhibits TF-FVIIa complex in the presence of FXa.27 In vivo administration of recombinant TFPI (rTFPI) in experimental animal models prevents thrombosis and fibrin deposition on subendothelial human matrix, reduces mortality from Escherichia coli-induced septic shock, and protects against DIC development.28 Elevated levels of TFPI have been found in patients with sepsis-induced DIC in conjunction with elevated TF levels, suggesting a relative deficiency of TFPI to neutralize TF pathway activation.29–31
Antithrombin
Antithrombin is a serine protease inhibitor that stoichiometrically inhibits thrombin (FIIa), FXa, FVIIa, FIXa, and FXIa. Low levels of AT in the plasma of patients with sepsis-associated DIC are correlated with increased mortality.32 Low AT levels may lead to diminished inhibition of thrombin, FXa, and other procoagulant serine proteases. The decrease in AT during DIC is not exclusively related to the hypercoagulable state.33,34 It has been proposed that the AT deficiency in DIC is attributable to rapid consumption after the formation of thrombin–antithrombin (TAT) complexes due to high rate of thrombin generation and the presence of other coagulation cascade serine proteases.35 However, recent studies have shown that AT deficiency in sepsis is more closely related to capillary leak, volume expansion, and reduced synthesis. It has been suggested that inactivation and degradation of AT by neutrophil elastase and other enzymes may also contribute to AT deficiency in DIC but have failed to prove the presence of proteolytic fragments of AT in the plasma of patients with sepsis-induced DIC.36–38
The dynamic PC anticoagulant pathway
Protein C
Thrombin binds to thrombomodulin (TM) expressed on vascular endothelial surfaces in the presence of the endothelial PC receptor and acquires anticoagulant properties by activating PC.39 In the presence of protein S (PS), activated protein C (APC) inhibits FVa and FVIIIa, resulting in degradation of prothrombinase and intrinsic tenase enzymatic complexes. This leads to downregulation of thrombin generation processes.40 The role of the PC anticoagulant pathway in the pathogenesis of DIC has been studied in experimental models of sepsis-induced DIC and in patients with sepsis. Protein C deficiency is a frequent finding in patients with sepsis due mainly to enhanced consumption, impaired liver synthesis, and vascular leakage.41 The acquired PC deficiency in sepsis-induced DIC is related to hypercoagulable state and increased mortality.42
Thrombomodulin
Reduced TM expression on endothelial cells due to inflammatory mediators, such as TNF-α, is thought to explain the impaired activation of PC in patients with DIC.43,44 Although soluble TM levels are typically elevated in the plasma of patients with DIC,45 this likely does not represent enhanced TM production or secretion but rather is indicative of increased cellular damage and shedding of TM from the vascular endothelium.46 Additionally, these soluble forms of TM are incapable of efficiently activating PC. The combination of low levels of TM on the endothelial surface with reduced availability of intact endothelial surface due to impaired microvascular perfusion compromises the anticoagulant function of the APC system.
Protein S
In plasma from healthy donors, approximately 60% of the cofactor PS is bound to a complement regulatory protein, C4-binding protein (C4BP), and thus cannot exert its role as cofactor for activated PC. The increased C4BP levels in plasma as a consequence of the acute-phase reaction in sepsis may result in a further decrease in active (unbound) PS levels and leads to further impairment of the PC system.47
Fibrinolysis
Impaired fibrin degradation due to inhibition of the fibrinolytic system serves as an additional mechanism in the pathogenesis of some forms of DIC. The initial response to coagulation activation in bacteremia and endotoxemia is an increase in fibrinolytic capacity due to enhanced release of tissue plasminogen activator (tPA) from the endothelium and acceleration of tPA-induced plasminogen activation by fibrin, a cofactor in this reaction. Levels of PAI-1, the main inhibitor of fibrinolysis, increase during the course of an inflammatory reaction. Accordingly, severe sepsis, a condition associated with a pronounced acute inflammatory reaction, can often lead to hypofibrinolytic DIC and subsequent microvascular thrombosis and organ dysfunction. The PAI-1 is also released from activated platelets. Studies in patients with DIC have shown that high plasma levels of PAI-1 were among the strongest predictors of mortality.8 Additionally, patients with genetic variants leading to increased levels of PAI-1 have a lower survival rate in severe sepsis. The lack of microvascular thrombi formation in the kidneys of PAI-1-knockout mice challenged with endotoxin further demonstrates the important role of PAI-1 in the inhibition of fibrinolysis in DIC.48
High concentrations of thrombin lead to the activation of TAFI. Activated TAFI is a carboxypeptidase that cleaves lysine residues essential for the binding of tPA, plasminogen, and plasmin to fibrin, rendering fibrin more resistant to fibrinolysis. Similar to PC activation, TAFI activation is TM dependent, but the activation of TAFI by thrombin-TM complexes is favored over the activation of PC, particularly at low TM concentrations.49
The role of TAFI in the pathogenesis of the thrombohemorrhagic syndrome in patients with DIC has not been fully investigated. Reduced activity of TAFI in patients with overt DIC is a biological evidence of enhanced fibrinolysis. Low TAFI activity is an independent predictor of patient death and multiple organ dysfunction syndrome. Although the relationship between TAFI activity and the phases of DIC requires further investigation, elucidation of the activation of fibrinolysis during the course of DIC might allow targeted therapeutic interventions to optimize fibrinolysis in order to overcome fibrinolytic shutdown.50
Nucleosomes
Elevated levels of extracellular DNA in plasma, also known as cell-free DNA, have been reported in diverse pathologies including cancer, trauma, thrombosis, sepsis, and DIC. Nucleosomes are involved in epigenetic regulation and consist of DNA wound around a histone protein core. Nucleosomes can be released into the extracellular environment through apoptosis, necrosis, and NETosis. Neutrophil extracellular trap (NETs) formation (NETosis) is a unique form of cell death; it is an active process whereby neutrophils release NETs in response to inflammatory stimuli. The NETs are comprised of a meshwork of DNA, histones, bactericidal factors, and neutrophil granule enzymes that function to trap and contain pathogens. NETosis can be triggered not only by infectious pathogens and their components but also by activated platelets, reactive oxygen species, antibodies, and inflammatory cytokines in a process called “immunothrombosis.” Although immunothrombosis plays a role in host defense by trapping and killing bacteria, fungi, parasites, and viruses, this protective mechanism can lead to DIC if left unregulated. The histones in NETs can activate platelets, induce endothelial cell damage, enhance thrombin generation, and inhibit anticoagulant PC activation.
The NETs facilitate local activation of coagulation to form fibrin clots that entrap pathogens, enhance pathogen clearance, and stimulate inflammation to recruit leukocytes. Histones have been shown to activate coagulation in a FXII-dependent manner in in vitro coagulation assays, and histone–DNA complexes have been shown to correlate significantly with the FXIIa level in the plasma of patients with suspected DIC.51 Extracellular DNA can have procoagulant, antifibrinolytic, and pro-inflammatory effects.
Nucleosomes, histones, and DNA are detectable in the blood of patients with DIC. In a cohort of 199 patients with suspected DIC, nucleosome levels were elevated compared to healthy controls. Furthermore, patients with higher levels of DNA–histone complexes had poorer survival than those with lower levels.52 Histones were also found to be elevated in patients with sepsis compared to nonseptic patients in a cohort of 165 patients admitted to medical and surgical ICUs.53 Plasma nucleosome and DNA levels were also found to be elevated in patients with DIC associated with hematologic malignancy.54
Clinical Manifestations of DIC
Depending on the underlying disease, the intensity of coagulation activation, and the deficiency of the natural anticoagulant pathways, DIC may present as either:8,55
Latent and compensated activation of coagulation with subtle hemostatic dysfunction and potential increase in thrombotic risk without obvious clinical symptoms. This phase is characterized by an imbalance between activation and inhibition of the coagulation system. Rapid restoration of normal hemostatic functions can be achieved through a combination of reduction in procoagulant stimulus and enhancement of inhibitory mechanisms on the coagulation system. This classification of DIC is often seen in cases involving obstetrics or severe immunologic reactions.
Overt DIC with significantly reduced hemostatic potential. This phase is characterized by the absence of normal regulatory mechanisms. Overt DIC is associated with both bleeding and thrombotic manifestations. This may include both microvascular thrombosis and thrombosis of larger vessels. In most cases, the bleeding is due to reduced hemostatic capacity, a side effect of excessive consumption of clotting factors and platelets during coagulation activation. This condition is also known as “consumption coagulopathy.” Latent, compensated DIC may progress to overt DIC or become a chronic condition.
Observed clinical manifestations mainly depend on the nature and the severity of the underlying disease. Clinical manifestations include multi-organ dysfunction due to microvascular thrombosis and bleeding.
Diagnostic Scores for DIC
The diagnosis of DIC is based on the clinical suspicion related to the underlying disorder and the abnormalities of blood coagulation tests, including prothrombin time (PT), activated partial thromboplastin time (aPTT), platelet count, and fibrinogen levels. There is no gold standard diagnostic test for DIC; instead, diagnosis is based on diagnostic scoring systems that evaluate clinical and laboratory parameters.8,55
Today, there are 5 different diagnostic scoring systems for DIC established by the ISTH, the Japanese Ministry Health and Welfare (JMHW), the Japanese Association for Acute Medicine (JAAM), the British Committee for Standards in Haematology (BCSH), and the Italian Society of thrombosis and Hemostasis (SISET).8,56–59 A scoring system for the diagnosis of DIC was first sanctioned by the JMHW and is summarized in Table 2 .
Table 2.
Scoring System for DIC Established by the JMHW.56 a
| Score | |||
|---|---|---|---|
| Criteria | Points | ||
| 1. Underlying disease | (+) | 1 | |
| (−) | 0 | ||
| 2. Symptoms | (1) Bleeding tendency | (+) | 1 |
| (−) | 0 | ||
| (2) Symptom caused by organ dysfunction | (+) | 1 | |
| (−) | 0 | ||
| 3. Laboratory data | (1) Plasma FDP (µg/mL) | ≥40 | 3 |
| 20-40 | 2 | ||
| 10-20 | 1 | ||
| <10 | 0 | ||
| (2) Platelet count (× 109/L) | ≤50 | 3 | |
| 50-80 | 2 | ||
| 80-120 | 1 | ||
| >120 | 0 | ||
| (3) Plasma fibrinogen (mg/dL) | ≤100 | 2 | |
| 100-150 | 1 | ||
| >150 | 0 | ||
| (4) Prothrombin time ratio (PT of patient/PT of control) | ≥1.67 | 2 | |
| 1.25-1.67 | 1 | ||
| <1.25 | 0 | ||
| Supplemental Data | |||
| (1) Detection of soluble fibrin monomer | |||
| (2) Increase in d-dimers | |||
| (3) Increase in thrombin–antithrombin complex | |||
| (4) Increase plasmin–antiplasmin complex | |||
| (5) Exacerbation of FDP, platelet, fibrinogen within several days | |||
| (6) Improvement of data with anticoagulant therapy | |||
| Interpretation | |||
| 1. Definite DIC | (1) Patients who do not have leukemia, pernicious anemia, liver cirrhosis, or who are not under cancer chemotherapy | More than 7 or 6 points with more than 2 of supplemental data | |
| (2) Patients who have leukemia, pernicious anemia, or who are under cancer chemotherapy points for bleeding tendency and platelet are not included | More than 4 or 3 points with more than 2 of supplemental data | ||
| (3) Patients who have liver cirrhosis | More than 10 or 9 points with more than 2 of supplemental data | ||
| 2. Probable DIC | (1) Patients who do not have leukemia, pernicious anemia, liver cirrhosis, or who are not under cancer chemotherapy | 6 points | |
| (2) Patients who have leukemia, pernicious anemia, or who are under cancer chemotherapy points for bleeding tendency and platelet are not included | 3 points | ||
| (3) Patients who have liver cirrhosis | 9 points | ||
The diagnostic criteria include:
the stratifying question: “Does the underlying disease predispose to DIC?”
the evaluation of typical clinical manifestations (ie, bleeding or organ dysfunction potentially related to microvascular thrombosis),
the assessment of 4 laboratory parameters (platelet count, PT, fibrinogen and fibrin/fibrinogen degradation products [FDPs])
The overt DIC scoring system endorsed by the DIC subcommittee of the ISTH, summarized in Table 3, is based on the JMHW DIC score. The question regarding the presence of a typical underlying disease predisposing to DIC is the prerequisite for the use of the score. The term “fibrin-related marker (FRM)” which encompasses soluble fibrin (SF) and d-dimers is used instead of “fibrinogen/fibrin degradation products (FDP).” This approach is preferable to the evaluation of FDP as FDP assays are not currently widely available outside Japan. Currently, the most prevalent FRM is d-dimers antigen.60
Table 3.
Scoring System for Overt and Nonovert DIC Endorsed by International Society on Thrombosis and Haemostasis.55
| Score | |||
|---|---|---|---|
| Fibrin-related marker | Strong increase | 3 | |
| Moderate increase | 2 | ||
| No increase | 0 | ||
| Platelet count (× 109/L) | <50 | 2 | |
| 50-100 | 1 | ||
| >100 | 0 | ||
| Fibrinogen level (mg/dL) | <1.0 | 1 | |
| ≥1.0 | 0 | ||
| Prothrombin time (second) | >6 | 2 | |
| 3-6 | 1 | ||
| <3 | 0 | ||
| Diagnosis of DIC If Score is ≥5 Points | |||
Abbreviations: DIC, disseminated intravascular coagulation; PT, prothrombin time.
An additional version of the ISTH DIC score for the diagnosis of “nonovert DIC” has been introduced including the same parameters, but with an added kinetic component that compares the results attained at 2 subsequent measurements.8,55 Deterioration of the parameters increases the score, whereas improvement reduces the score. In addition, results of AT and/or PC measurement may be included. The use of the ISTH nonovert DIC score has been shown to identify additional high-risk patients.
The JAAM DIC scoring criteria, summarized in Table 4, includes platelet count, PT, fibrin/FDPs, and specific criteria of systemic inflammatory response syndrome. This provides the JAAM DIC scoring system with an acceptable validity for the diagnosis of trauma-related DIC with the fibrinolytic phenotype and a higher sensitivity than the ISTH overt DIC criteria in trauma patients. However, while the JAAM diagnostic criteria displayed a high sensitivity for DIC, the ISTH overt DIC diagnostic criteria displayed a high specificity for DIC.59 The Italian clinical practice guidelines for the diagnosis and treatment of DIC suggest the use of the diagnostic scores ISTH, JMHW, or JAAM over standalone tests.59 Recently, a new set of criteria based on the JAAM DIC criteria has been proposed, including PC activity and PAI-1 levels as additional parameters.61
Table 4.
| Score | |
|---|---|
| Systemic inflammatory response syndrome criteria | |
| ≥3 | 1 |
| 0-2 | 0 |
| Platelet counts (×106/L) | |
| <80 or more than 50% decrease within 24 hours | 3 |
| 80-120 or more than 30% decrease within 24 hours | 1 |
| ≥120 | 0 |
| Prothrombin time (value of patient/normal value) | |
| ≥1.2 | 1 |
| <1.2 | 0 |
| Fibrin/fibrinogen degradation products (mg/L) | |
| ≥25 | 3 |
| 10-25 | 1 |
| ≤10 | 0 |
| Diagnosis | |
| 4 points or more | DIC |
| Criteria for systematic inflammatory response syndrome | |
| Temperature <36°C or >38°C | |
| Heart rate >90 beats/min | |
| Respiratory rate >20 breath/min or PaCO2 >32 torr (<4.3 kPa) | |
| White cell blood counts >12 000/mm3, <4000 cells/mm3 or 10% immature (band) forms | |
Abbreviations: DIC, disseminated intravascular coagulation; Paco 2, partial pressure of carbon dioxide in arterial blood.
Several authors have questioned the inclusion of fibrinogen as an indispensable component of the DIC scoring systems. In patients with sepsis, plasma fibrinogen levels are generally elevated due to the acute-phase reaction. Thus, low levels of fibrinogen are rarely encountered in patients with overt DIC. On the other hand, low fibrinogen levels are a common and prognostically important feature of conditions associated with hyperfibrinolytic DIC, such as amniotic fluid embolism, DIC associated with hematogical malignancies, or transfusion reactions. The only marker included in the scores that directly relates to coagulation activation is the FRM. In Japan, most laboratories use fibrinogen/FDP as the FRM of choice, whereas D-dimers is the only FRM available in most laboratories worldwide.
Biomarkers for the Diagnosis and Follow-Up of DIC
Although numerous biomarkers have been studied for their relevance to the diagnosis and prognosis of sepsis and sepsis-associated DIC, there is currently no single biomarker or biomarker panel accepted for use in this application. Levels of endogenous anticoagulants, including AT, PC, and TM, are among the most promising candidates as biomarkers for use in sepsis and DIC.62 In a large cohort of patients with sepsis and suspected DIC, reduced AT and PC levels were predictive of mortality, regardless of whether the patient met the ISTH criteria for overt DIC.63 Other markers of various aspects of coagulation and fibrinolysis have also been studied, with promising results, including TAT, SF, TFPI, PAI-1, von Willebrand factor, and Adamts-13.62 D-dimers, a component of the ISTH DIC scoring algorithm, and fibrin monomer have been proposed as individual predictive markers of DIC.64 Other studies have focused on markers of endothelial activation, on markers of inflammation, or on novel markers, including extracellular nucleosomes and histones. Rotational thromboelastometry has also been explored as a potential point-of-care test for the diagnosis of sepsis-associated DIC, with promising results in a small study.65
Therapeutic Strategies for DIC
The basis of DIC treatment is the removal of the underlying causative factor. In some cases, particularly those associated with placental abruption and intrauterine death, DIC will resolve completely within hours of the resolution of the underlying condition. However, DIC will most often progress even after appropriate treatment of the underlying disease, as in cases of patients with sepsis-induced DIC.
Ideally, an effective treatment for DIC would distinguish between hyperfibrinolytic and hypofibrinolytic DIC and between overrun-type and degradation-type DIC. However, laboratory diagnostic means for such distinctions are not universally available. In most cases, treatment decisions will be based solely on routine laboratory tests, such as PT, platelet count, fibrinogen level, and an FRM (D-dimers, FDP, or SF). Repeated analyses are helpful to monitor the kinetics of the reaction and distinguish between rapidly progressing and chronic forms of DIC.
In most cases, the hallmark clinical indicator of DIC is overt bleeding, which may be related to consumption of coagulation factors. Bleeding may also be attributable to a variety of other factors, including dilution, impaired synthesis of coagulation factors, vitamin K deficiency, activation of fibrinolysis, or administration of anticoagulant or antiplatelet drugs. The obvious treatment for bleeding is replenishment of the procoagulant components, but this may have detrimental effects under circumstances of uncontrolled coagulation activation.
Organ dysfunction due to microvascular thrombosis is a less obvious sign of DIC, as it may also be attributable to various other pathophysiological mechanisms in severely ill patients. A rational treatment for microvascular thrombosis would be the administration of coagulation inhibitors profibrinolytic agents, which may have the adverse effect of enhanced bleeding. A recombinant form of soluble human TM has been approved in Japan for the management of DIC in various conditions.
In the next part of this review, we discuss the common treatment options in DIC and analyze the clinical trials that assessed the efficacy and safety of administration of natural coagulation inhibitors in patients with DIC.
Restoration of Deficient Natural Anticoagulant Pathways
Antithrombin concentrates
Since AT is one of the most important physiological inhibitors of coagulation, its role in the treatment of DIC was supported by favorable results from phase II clinical trials66–68 that led to a large, double-blind, placebo-controlled, multicenter phase III clinical trial in patients with severe sepsis (the KyberSept Trial).32 In all, 2314 patients were randomized to receive either intravenous (IV) high-dose concentrate of AT (30 000 IU IV infusion in total over 4 days) or placebo (1% human albumin). Heparin in prophylactic doses (<10 000 IU daily) as well as heparin flush for catheter patency were allowed in the study protocol. The study aimed to attain supratherapeutic AT levels. Despite the fact that high AT levels (180% of normal) were attained in AT-treated patients, the 28-day mortality rate was not significantly different between the 2 groups (37.8% in AT group and 43.6% in placebo group, P = .08). In patients receiving AT and concomitant heparin, a significantly increased rate of bleeding was observed (23.8% for AT group vs 13.5% for the placebo group, P < .001; Table 5).
Table 5.
Clinical Trials Assessing the Efficacy and Safety of Treatment With Natural Coagulation Inhibitors (Antithrombin, Activated Protein C, or Thrombomodulin) Concentrates in Patients With Sepsis and/or DIC.
| Clinical Trial | Clinical Context | Natural Coagulation Inhibitor (NCI) Versus Comparator | 28-Day Mortality | Bleeding | ||
|---|---|---|---|---|---|---|
| NCI (%) | Comparator (%) | NCI (%) | Comparator (%) | |||
| Warren et al32
KyberSept N = 2314 |
Severe sepsis | AT (3000 IU IV infusion over 96 hours) vs placebo | 37.8 | 43.6 | 23.8 | 13.5 |
| Kienast et al69
Post hoc analysis of KyberSept N = 563 |
Severe sepsis | AT (3000 IU IV over 96 hours without concomitant use of heparin) vs placebo | 25.4 | 40a | 7 | 5.2 |
| Iba et al72
N = 729 |
Sepsis + DIC | AT (3000 IU/d for 3 consecutive days) vs AT (1500 IU/d for 3 consecutive days) | 25.3 | 34.8a | 4.17 | 1.40 |
| Bernard et al78
PROWESS N = 1520 |
Severe sepsis | DAA (0.024 mg /kg/h over 96 hours) vs placebo | 24.7 | 30.8a | 3.5 | 2 |
| Aoki et al79
N = 132 |
Severe sepsis | rAPC (12.5 IU/kg/h for 6 days) vs UFH (IV dose adjusted according to aPTT for 6 days) | 20.4 | 40a | Higher in heparin groupa | |
| Dhainaut et al80
Post hoc analysis of PROWESS |
Severe sepsis + overt DIC | DAA (0.024 mg/kg/h over 96 hours) vs placebo | 30.5 | 43a | 4.72 | 2.71 |
| Abraham et al81
ADDRESS N = 2613 |
Sepsis | DAA (0.024 mg/kg/h for 96 hours) vs placebo | 18.5 | 17 | 2.2 | 3.9a |
| Nadel et al82
RESOLVE N = 477 |
Severe sepsis | DAA (0.024 mg/kg/h for 96 hours) vs placebo | 17.2 | 17.5 | 6.7 | 6.8 |
| Bernard et al83
ENHANCE N = 2375 |
Severe sepsis | DAA (0.024 mg/kg/h for 96 hours) vs placebo | 25.3 | 24.7 | 6.5 | 3.5 |
| Ranieri et al84
PROWESS-SHOCK N = 1696 |
Severe sepsis | APC (0.024 mg/kg/h over 96 hours) vs placebo | 26.4 | 24.2 | 1.2 | 1 |
| Levi et al99
XPRESS N = 1994 |
Severe sepsis | APC + UFH or LMWH vs APC (0.024 mg/kg/h) | 28.3 | 31.9 | 10.8 | 8.1 |
| Abraham et al89
OPTIMIST INR >1.2; N = 1754 INR <1.2; N = 201 |
Severe sepsis | rTFPI (0.025 mg/kg/h for 96 hours) vs placebo | 34.2 | 33.9 | 6.5 | 4.8 |
| 12 | 22.9 | 6.0 | 3.3 | |||
| Saito et al91
N = 234 |
Infection, malignancy | TM (0.06 mg/kg once per day for 6 days) vs UFH (8 IU/kg/h for 6 days) | 28 | 34.6 | 43.1 | 56.5 |
| Kawano et al92
N = 35 |
DIC with infectious disease, hematological disease | rTM (380 IU/kg/d for 6 consecutive days. In patients with renal insufficiency, the dose was 130 IU/kg/d | 20 | |||
Abbreviations: aPTT, activated partial thromboplastin time; AT, antithrombin; DAA, drotrecogin alfa activated; DIC, disseminated intravascular coagulation; INR, international normalized ratio; IV, intravenous; LMWH, low-molecular-weight heparin; rAPC, recombinant activated protein C; rTFPI, recombinant tissue factor pathway inhibitor; TM, thrombomodulin; UFH, unfractionated heparin.
a P < .05.
In a post hoc subgroup analysis of the KyberSept trial, patients with severe sepsis who did not receive concomitant heparin were analyzed.69 A group of 563 patients who did not receive concomitant heparin were identified, and 40.7% of them (229/563) had DIC. In AT-treated patients with DIC, the 28-day mortality rate of 25.4% was significantly lower compared to placebo-treated patients (40.0%; P = .02). In contrast, patients with DIC without concomitant heparin administration AT did not show any favorable effect on 28-day mortality compared to the placebo group. The frequency of bleeding was similar in the 2 groups (Table 5).
Another post hoc subgroup analysis of the data from the KyberSept Trial focused on the influence of heparin coadministration with AT on long-term mortality, adverse events, and thromboembolic events.70 The 28-day mortality in patients who received only AT (n = 698) was 37.8% compared to 43.6% in the placebo group and was not statistically significant. Combination of AT and heparin treatment resulted in a significantly increased risk of bleeding that was related to increased mortality. Moreover, the incidence of serious thromboembolic events was not increased in patients who received only AT compared to those who received AT and heparin. Interestingly, mortality was lowest (36.3%) in the patients receiving heparin without AT concentrate. Since heparin administration was given based on the discretion of the treating physician, and patients were only randomized to receive either AT concentrate or placebo, no conclusions can be drawn concerning the effect of heparin alone.
A retrospective study of 435 critically ill patients treated with AT (30 IU/kg/h) showed that on the first day of the treatment, 50% of the patients responded to the treatment and had AT plasma levels higher than 60%. Patients who responded to AT on the first day of the treatment showed resolution of DIC (according to the ISTH DIC score) and improved mortality.71
A prospective nonrandomized survey was conducted on patients with sepsis-induced DIC with AT activity levels less than or equal to 70%. Patients were treated with AT at 1500 IU/d (n = 650) or 3000 IU/d (n = 79) for 3 consecutive days. Bleeding events occurred in 6.52% of patients (severe bleeding, 1.71%). A significant decrease in initial AT level (below 50%) was observed in 69.6% of patients in the AT3000 group and 48.2% in the AT1500 group (P < .01). A logistic regression analysis conducted using age, gender, body weight, initial AT activity, and supplemented AT dose revealed that higher initial AT activity (odds ratio [OR], 1.032; P < .001), AT dose of 3000 IU/d (OR, 1.912; P = .026), and age (OR, 0.985; P = .023) were significant factors for improved survival. The risk of severe bleeding was less than 2%, and concomitant administration of heparin did not increase the risk. The survival in AT1500 group was 65.2%, while that in AT3000 group was 74.7% (Table 5).72 An additional study examined infected patients meeting JAAM DIC criteria and with an AT level of ≤70% who received AT at 1500 or 3000 IU/d and determined that survivors exhibited significantly higher peak levels of AT at day 4 than nonsurvivors (65.0% in nonsurvivors vs 85.1% in survivors, P < .001).73
A retrospective study of patients mechanically ventilated with septic shock and confirmed DIC by the JAAM criteria following emergency surgery for lower intestinal tract perforation was performed in Japan. This study showed no overall difference in mortality between patients receiving and not receiving AT. However, when propensity score matching was used to create matched groups of 518 patients receiving AT and 518 patients not receiving AT, a reduction in 28-day mortality was observed (27.6% for AT vs 19.9% for control; difference, 7.7%; 95% confidence interval [CI], 2.5-12.9).74
These results must be interpreted with caution, as they stem from subgroup or retrospective analyses and not from randomized controlled clinical trials. However, they suggest that AT treatment might be effective and safe in patients with sepsis-associated DIC, but not in patients without DIC. The post hoc analysis of the data from the KyberSept trial only apply to high-dose AT treatment, resulting in supranormal AT levels, and prospective data using the diagnosis of DIC as entry criterion are not available. A recent systematic review and meta-analysis of trials of AT in critically ill patients concluded that the administration of AT is not supported in critically ill patients, including patients with sepsis and DIC, as no overall impact is seen on mortality and a significant increase in bleeding is observed.75 There is currently no data supporting the administration of AT concentrate to attain normal AT levels in patients with DIC and no evidence to support or discourage the concomitant administration of heparins for prophylaxis of venous thromboembolism (VTE). Currently, treatment with AT concentrate is only mandated in patients with congenital AT deficiency, or as a supplement to heparin therapy in selected patients with acquired AT deficiency, such as in extracorporeal circulation.
Protein C and APC concentrates
The dynamic natural anticoagulant pathway of PC is of major importance in the inhibition of thrombin generation. In addition, APC—drotrecogin alfa activated (DAA)—exerts anti-inflammatory activity by inhibiting cytokine production, preventing neutrophil activation, and inhibiting leukocyte adhesion and rolling.76 In patients with sepsis-induced DIC, plasma levels of PC are decreased. Thus, supplementation treatment with APC concentrates is a rational therapeutic approach. The PC concentrates have been used for treatment of patients with sepsis-associated purpura fulminans, a form of DIC typically observed in patients with meningococcal and pneumococcal sepsis. Purpura fulminans is characterized by microvascular thrombosis in the skin due to a strongly hypercoagulant and hypofibrinolytic condition, ultimately leading to tissue necrosis and secondary hemorrhage.
A beneficial effect of DAA was seen in an early dose-finding randomized placebo-controlled clinical trial including 131 patients with sepsis. This study showed that administration of DAA caused a dose-dependent decrease in the levels of D-dimers and IL-6 with an acceptable safety profile.77 The dose of 24 µg/kg/h for 96 hours was considered optimal and was assessed in a phase III randomized, double-blind, placebo-controlled multicenter trial (Recombinant Human Activated Protein C Worldwide Evaluation in Severe Sepsis [PROWESS] trial).78
The PROWESS trial planned to enroll 1690 patients with severe sepsis associated with organ dysfunction and randomized them to receive recombinant human APC (rhAPC; 24 µg/kg/h over 96 hours) or placebo. Reduction of 28-day mortality was defined as the primary end point of the study. After the second interim analysis of 1520 patients, it was found that treatment with DAA leads to significant reduction of 28-day mortality. For this reason, the trial was stopped before complete enrollment of the initially planned number of patients. Mortality was 24.7% in the DAA group as compared to 30.8% in the placebo group (relative risk [RR] reduction, 19.4%; 95% CI, 6.6-30.5; P < .05). In patients receiving DAA, D-dimer levels in plasma dropped by 25% after 2 days of DAA administration, whereas they increased by 10% in the placebo group, indicating an inhibition of coagulation activation by DAA treatment. The levels of IL-6 decreased more rapidly in patients receiving DAA, as compared to placebo group, showing that DAA treatment has some anti-inflammatory effects in patients with sepsis. In the DAA-treated group, an increased rate of bleeding complications was observed compared to the placebo group, particularly following invasive procedures. The incidence of severe hemorrhage was 3.5% in the DAA group and 2% in the placebo group (P = .06; Table 5).
A smaller randomized double-blind prospective trial was performed to evaluate the efficacy and safety of APC (not DAA) and unfractionated heparin (UFH) in 132 patients with sepsis-associated DIC.79 Patients were randomized to receive APC (12.5 U/kg/h IV) and/or UFH (8 IU/kg/h IV) by continuous IV infusion for 6 days. The 28-day mortality rate tended to be lower in APC group than in UFH group (20.4% in APC group vs 40% in heparin group; P < .05). These results suggest that low doses of DAA can improve DIC more effectively than heparin, although the study was too small to demonstrate effects on organ failure and mortality (Table 5).
A post hoc analysis of the data from the PROWESS trial (using DAA) showed that 29% of the patients (454/1568 patients) were classified as having overt DIC (as defined by the ISTH score).80 In the placebo group, the 28-day mortality in patients with overt DIC was 43%, compared to 27% in patients without DIC. Thus, the presence of overt DIC is a significant predictor of mortality, independently of the Acute Physiology and Chronic Health Evaluation score (APACHE II) and age. Treatment with DAA tended to improve mortality in patients without DIC (22.1%; RR, 0.81; 95% CI, 0.66-1) and in patients with overt DIC (30.5%; RR, 0.71; 95% CI, 0.55-0.9); however, the difference did not reach statistical significance compared to placebo (P > .05). The rate of serious bleeding during DAA treatment in patients with overt DIC was 4.7% compared to the placebo group (2.7%), but was not statistically significant (P > .05). These data support the idea that DAA is particularly beneficial in patients with DIC and that uncontrolled activation of blood coagulation is one of the main contributors to mortality in severe sepsis (Table 5).
In another randomized, double-blind, placebo-controlled trial (Administration of Drotrecogin Alfa (Activated) in Early Stage Severe Sepsis [ADDRESS]), 2613 patients with sepsis were randomized to receive DAA or placebo.81 These patients were deemed low mortality risk because they had an APACHE score lower than 25 or only suffered a single-organ failure. The 28-day mortality, the in-hospital mortality, and the incidence of serious bleeding were defined as composite efficacy and safety primary end points of the study. The 28-day mortality was 18.5% in DAA group and 17% in placebo group, (P = .34) and the in-hospital mortality was 20.6% in DAA group and 20.5% in the placebo group (P = .98). In contrast, severe hemorrhagic episodes were significantly more frequent in DAA group (3.9%) compared to the placebo group (2.2%; P = .01).
In pediatric patients, the REsearching severe Sepsis and Organ dysfunction in children: a gLobal perspectiVE (RESOLVE) study was performed to evaluate the use of DAA in children with severe sepsis (n = 477). The patients were randomized into 2 groups, 237 received placebo and 240 patients received treatment with DAA (24 μg/kg/h for 96 hours). This study also showed no clinical benefit of DAA treatment in 28-day mortality (17.5% in placebo group vs 17.2% in DAA group). In contrast, an increased incidence of intracranial bleeding was observed (4.6% in DAA group vs 2.1% in the placebo group), but the difference was not statistically significant (P = .13).82
An increased bleeding risk was also observed in the Extended Evaluation of Recombinant human Activated Protein C trial, an open-label study where the incidence of serious bleeding at 28 days was 6.5% and that of intracranial hemorrhage at 28 days was 1.5%.83
The Italian guidelines for the treatment of DIC suggest the use of rhAPC (grade D) but not in obstetric or pediatric patients with sepsis-associated DIC.59 The BCSH guideline recommended against DAA administration in thrombocytopenic patients and those at high risk of bleeding.58
With the increasing uncertainty about efficacy and the concerns about bleeding risk, the Committee for Medicinal Products for Human Use section of the European Medicines Agency (EMA) called for reexamination of the risk/benefit profile of DAA. An industry-sponsored placebo-controlled trial, named PROWESS-SHOCK, was initiated. The primary objective of the PROWESS-SHOCK trial was all-cause mortality at 28 days.83 At the recent completion of the PROWESS-SHOCK trial involving 1697 patients, preliminary analyses showed a 28-day all-cause mortality rate of 26.4% in DAA-treated patients compared to 24.2% in placebo-treated patients with an RR of 1.09 (95% CI, 0.92-1.28; P = .31). The study also failed in its secondary end point: the reduction of mortality in the population of patients with severe PC deficiency. The risk of severe bleeding was 1.2% in the DAA group and 1% in the placebo group, suggesting there was no increased harm (Table 5). Following these results, the manufacturer decided to withdraw the product from the market worldwide, and both the EMA and the US Federal Drug Administration Agency have advised physicians not to initiate treatment with DAA in new patients and to stop ongoing treatment. In this context, the Activated Protein C and Corticosteroids for Human Septic Shock trial started in 2010 to evaluate DAA in combination with low-dose corticosteroids for the management of septic shock. In 2011, the trial was suspended after the withdrawal of DAA from the market. The Scientific Committee continued the trial according to a 2-parallel group design comparing low-dose steroids with placebo and analyzed the effects of DAA on patients included before trial suspension.84 At the time of trial suspension, 411 patients had been recruited, 208 had received DAA, and 203 had received placebo. On day 90, there were 99 deaths (47.6%) among the 208 patients receiving DAA and 94 deaths (46.3%) among the 203 patients receiving placebo (P = .79). There was no evidence of a difference between DAA and placebo for any secondary outcomes or serious adverse events.85 In view of the above, Thachil et al withdrew the recommendations given by the BCSH guideline on the use of APC in DIC.86
Tissue factor pathway inhibitor
Since coagulation activation in sepsis-induced DIC is mediated primarily through the TF/FVIIa pathway and the increase in TF expression compared to TFPI, substitution of rTFPI is a rational treatment approach. A pilot randomized, double-blind, placebo-controlled study was performed in 16 healthy volunteers who received IV bacterial lipopolysaccharide. Intravenous infusion of TFPI induced a dose-dependent inhibition of thrombin generation but had no influence on the fibrinolytic and cytokine response.87
A randomized, placebo-controlled phase II clinical trial assessed the efficacy and safety of rTFPI administration in 210 patients with severe sepsis.88 Patients were randomized to receive a continuous infusion of rTFPI (0.025 or 0.05 mg/kg/h for 4 days) or placebo. Administration of rTFPI led to inhibition of coagulation activation and suppressed the inflammatory response (shown by reduction in TAT complexes and IL-6 levels in plasma). Although the trial was not powered to show efficacy, a trend toward reduction of the 28-day mortality was found, and the treatment was found to be safe with both studied doses.
Based on these results, a large randomized, double-blind, placebo-controlled phase III trial (OPTIMIST) was conducted to evaluate the efficacy and safety of rTFPI (tifacogin) in severe sepsis.89 The studied population consisted of 1754 patients with severe sepsis having international normalized ratio (INR) greater than or equal to 1.2 and 201 patients with INR lower than 1.2. Patients from each group randomly received either rTFPI (0.025 mg/kg/g for 96 hours) or placebo. The 28-day mortality in patients with high INR who received rTFPI was 34.2%, similar to that observed in the placebo group (33.9%; P = .88). In contrast, patients with low INR who received rTFPI had a lower 28-day mortality of 12% compared to 22.9% in the placebo group. However, the difference was not statistically significant (P > .05). Moreover, in both cohorts, there was a nonsignificant increase in the overall incidence of serious bleeding (6.5% in rTFPI group and 4.8% in the placebo group with high INR and 6% in rTFPI group and 3.3% in the placebo group with low INR; P > .05 in both INR groups; Table 5).
Recombinant Human Soluble TM
Recombinant human soluble TM (ART-123) has a high affinity for thrombin. Τhe thrombin–ART-123 complex activates PC, which in the presence of PS inactivates FVIIIa and FVa, thereby inhibiting the propagation of thrombin generation. Initial studies have shown that TM plays a central role in inhibiting coagulation activation and the inflammatory response.90 Based on these observations, TM is studied as an antithrombotic treatment in patients with DIC.
A multicenter, double-blind, randomized, parallel-group, phase III trial compared the efficacy and safety of ART-123 to that of low-dose UFH for the treatment of DIC.91 Two hundred thirty-four patients with DIC caused by infection or hematological malignancy were randomized to receive ART-123 (0.06 mg/kg once daily) or UFH (8 IU/kg/h for 24 hours continuous IV infusion) for 6 days. The primary efficacy end point was DIC resolution. Resolution of DIC was observed in 66.1% of patients treated with ART-123 compared to 49.9% of the patients treated with UFH (absolute difference, 16.2%; 95% CI, 3.3-29.1). Moreover, patients in the ART-123 group showed greater improvement in clinical course of bleeding symptoms (P = .027). The 28-day mortality rate in patients with infection treated with ART-123 was 28% versus 35% in the placebo group (absolute difference, −6.6%; 95% CI, −24.6 to 11.3, P > .05), whereas in patients with malignancy, it was 17.2% in ART group versus 18% in heparin group (absolute difference, −0.8%; 95% CI, −14.2 to 12.5; P > .05). The incidence of bleeding during 7 days after the start of treatment was significantly lower in the ART group as compared to the heparin group (43.1% in ART group vs 56.5% respectively, P = .048; Table 5).
To elucidate the clinical characteristics and outcomes of DIC, a retrospective study analyzed 35 patients with DIC treated with recombinant TM (rTM). In addition to the treatment of underlying diseases, patients received rTM for 6 consecutive days. Disseminated intravascular coagulation was resolved in 21 (60%) of the patients at 7 days after administration. Furthermore, 7 of the remaining 14 patients with DIC who did not attain resolution at 7 days attained resolution at an average of 12.1 days. Consequently, 28 (80%) of 35 patients were alive with resolution of DIC after a 28-day observation period.92
The rTM is currently approved for use in Japan, and several retrospective analyses of the safety and efficacy of rTM have been performed on Japanese patient cohorts. A retrospective analysis was performed on 452 propensity-matched pairs of patients (with and without rTM administration) admitted to ICU with sepsis and DIC, diagnosed according to the JAAM criteria. Patients treated with rTM demonstrated a significantly lower rate of in-hospital mortality as compared to those who did not receive this treatment (OR, 0.757; 95% CI, 0.576-0.999, P = .049). Furthermore, no increase in bleeding was observed in patients treated with rTM.93 A retrospective study of the use of rTM in obstetric DIC demonstrated improvement in laboratory parameters, including platelets, d-dimers, fibrinogen, and INR, compared to the control group. However, no significant differences were observed for any measurement of organ damage or failure.94 A post hoc subgroup analysis of a retrospective analysis of patients with sepsis-associated DIC treated with or without rTM aimed to identify subgroups of patients with DIC who may benefit the most from this treatment. This analysis showed that treatment with rTM was associated with reduced mortality only in the highest risk patient group, as defined by an APACHE II score of 24 to 29 (adjusted hazard ratio, 0.281; 95% CI, 0.093-0.850; P = .025).95
Successful use of rTM has also been documented in small analyses of patients with DIC secondary to hematological malignancy. A retrospective analysis including 47 patients with DIC, diagnosed according the JAAM criteria, secondary to acute myeloid leukemia, demonstrated greater survival among patients treated with rTM than patients treated with the low-molecular-weight heparin (LMWH) dalteparin (P = .016), with fewer bleeding-associated deaths and a higher rate of DIC resolution also seen in the rTM treatment group.96
In a recent study, administration of ART-123 at a dosage of 0.06 mg/kg/d for 6 days was associated with decreased generation of thrombin and its markers in critically ill patients with sepsis-associated coagulopathy. This study demonstrated that ART-123 is a safe intervention in critically ill patients with coagulopathy due to sepsis. These results of this study support the hypothesis that TM exerts therapeutic actions at multiple sites in sepsis-associated coagulopathy and warranted further studies.97 A phase III Safety and Efficacy Study of ART-123 in Subjects With Severe Sepsis and Coagulopathy is currently undergoing and is expected to complete by May 2018.
Antithrombotic Drugs
Administration of rapidly acting anticoagulant agents to increase the inhibition of upregulated thrombin generation is a possible therapeutic approach for patients with DIC. Both UFH and LMWH are the most widely used rapidly acting antithrombotic drugs. A small randomized controlled trial showed that LMWH is superior to UFH for treating DIC.98
In a randomized, double-blind phase IV trial, LMWH, UFH, or placebo was administrated every 12 hours during DAA (24 IU/kg/h for 96 hours) infusion in patients with severe sepsis (n = 1994).99 This trial showed that heparin administration concomitant with DAA slightly decreased the 28-day mortality (28.3%) compared to the placebo group (31.9%; P = .08). During the first 6 days of treatment, there was a significantly higher frequency of bleeding in patients treated with heparin (10.8%) compared to placebo (8.1%; P = .049). The incidence of ischemic stroke during the first 6 days was significantly higher in the group treated with DAA alone (1.3%) than in the group treated with DAA and heparin (0.3%; P = .02). The incidence of VTE was not significantly different between the 2 groups (5.7% in DAA and heparin group vs 7% in DAA group, P > .05; Table 5).
Another randomized clinical trial assessed the efficacy and safety of UFH in patients with sepsis (n = 319). The patients were randomized to receive placebo or UFH (500 IU/h IV for 7 days), and the end points were the median length of stay in the ICU and the 28-day mortality. This trial failed to demonstrate a beneficial effect either in the hospitalization (12.5 days for placebo group and 12 days for UFH group; P = .97) or in the 28-day mortality rate (16% in the placebo group vs 14% in UFH group; P = .65).100
A meta-analysis of trials of anticoagulant therapy in patients with sepsis analyzed 24 randomized controlled trials that assessed the efficacy and safety of anticoagulant therapies including AT, APC, TFPI, UFH, LMWH, rTM, gabexate mesilate, and TF antagonist in patients with DIC.101 Pooled analysis of these trials demonstrated no reduction in overall mortality for patients with sepsis-induced coagulopathy, defined as changes in INR, platelet count, AT activity, and D-dimer levels. However, a significant reduction in mortality was observed overall in the population with sepsis-associated DIC as defined by either the ISTH or JAAM criteria who received any of the examined treatments as compared to those who did not receive any anticoagulant treatment (RR, 0.72, 95% CI, 0.62-0.85, P < .01). Pooled analysis demonstrated a significant increase in bleeding associated with the anticoagulant treatment (RR, 1.33, 95% CI, 1.12-1.57, P < .01). Based on this analysis, the authors concluded that anticoagulant therapy might be appropriate in patients with sepsis-associated DIC, but not necessarily in patients with less severe coagulation dysfunction.
Currently, UFH and LMWH are recommended for the prophylaxis against VTE in severely ill patients. Moreover, according to current guidelines, LMWH is preferable over UFH due to safety advantages, such as lower risk of heparin-induced thrombocytopenia, more predictable and stable anticoagulant effect, and lower risk of dosing errors.
Beyond heparins, some limited experimental evidence suggests that the direct inhibitors of thrombin, such as lepirudin, might downregulate coagulation activation in DIC.102 The role of specific AT-dependent inhibitors of FXa, such as fondaparinux, and direct inhibitors of FXa, such as rivaroxaban or apixaban, in the management of hypercoagulability in patients with DIC has not been investigated.
Plasma and Platelet Substitution Therapy
Replacement of coagulation factors and platelets may be necessary in patients with DIC presenting with consumption coagulopathy, especially in those with active bleeding or those who need an invasive procedure. The theory that administration of blood components might exacerbate disseminated coagulation activation has never been proven in clinical or experimental studies.
In patients with DIC with active bleeding or those at high risk of bleeding, the administration of platelet concentrate and fresh-frozen plasma (FFP) is recommended, without high-quality evidence. The threshold for transfusion platelets depends on the clinical state of patients with DIC. Platelet concentrate usually is administered in patients with DIC with active bleeding and a platelet count lower than 50 × 109/L or in nonbleeding patients undergoing chemotherapy who develop DIC and a platelet count less than 20 × 109/L.103
Another strategy for coagulation factor replacement is the use of specific concentrates. Fibrinogen is the final substrate of blood coagulation activation and thrombin generation and is also required for effective platelet aggregation. In patients with fibrinogen levels lower than 1.5 g/dL, treatment with fibrinogen concentrate may considerably reverse the hemorrhagic diathesis. However, the recommendations on the use of fibrinogen concentrates in the management of bleeding related to DIC are based mainly on case reports.
For replacement of vitamin K–dependent coagulation factors, prothrombin complex concentrates (PCCs) may be used. These concentrates provide coagulation FII, FVII, FIX, and FX in a low volume and can be rapidly administered. In coagulation factor deficiency due to liver dysfunction, PCCs combined with FFP as a source of FV can be administered (typically 2000 units of PCC plus 2 units of FFP), although there are no clinical trials to assess the efficacy of this therapeutic option.
Patients with severe overt DIC often display low FVII levels or hemorrhage refractory to transfusion with FFP, PCCs, and platelet concentrates. Treatment with rFVIIa (Eptacog alfa activated) may be helpful to alleviate severe bleeding in these patients.104,105 Treatment with rFVIIa might be beneficial in patients with impaired platelet function, a possible side effect, potentially due to DIC-associated platelet exhaustion or treatment with various drugs, including nonsteroid anti-inflammatory drugs and antibiotics. Recombinant FVIIa has been shown to of interest in the management of obstetrical DIC secondary to placental abruption or amniotic fluid embolism and other conditions associated with severe peripartum hemorrhage.106 However, this treatment should be used with caution because of the increased thromboembolic risk associated with the administration for rFVIIa.
The FVIII or von Willebrand factor concentrates are rarely used in patients with sepsis-associated DIC, since these factors are already typically elevated due to the acute-phase reaction. Repeated measurement of global clotting tests might be useful to monitor the progression of coagulation defects. Vitamin K deficiency may be distinguished from global coagulation factor deficits by comparing the levels of the vitamin K–dependent coagulation FII, FVII, or FX, and the non-vitamin K-dependent FV. In the case of a vitamin K deficiency, administration of vitamin K by IV route is helpful.
Recommendations for the Treatment of DIC
So far, no universal consensus has been reached for the treatment of DIC. Although less than half of all DIC cases are caused by sepsis, the majority of existing reviews, including guidelines in Europe and North America on the treatment of DIC, concern only septic DIC. Three guidelines for diagnosis and treatment of DIC have been published in the literature by the Japanese Society of Thrombosis Hemostasis/DIC Subcommittee (JSTH),57 the BCSH,58 and the SISET.59 The JSTH has established an expert consensus for DIC treatment that has been approved by its inside evaluation committee and outside evaluation committees of the JAAM, the Japanese Society of Intensive Care Medicine, and the Japanese Association for Infectious Diseases. The subcommittee for DIC of the Scientific and Standardization Committee (SSC/ISTH) harmonized these guidelines in a report entitled “Guidance for the diagnosis and treatment of DIC from harmonization of the recommendations from three guidelines.”56
Disseminated intravascular coagulation is, therefore, classified as follows: asymptomatic type (marked clinical symptoms of DIC not observed but laboratory findings confirm DIC), marked bleeding type, and organ failure type. The appropriate treatment differs and is based on the type of DIC. These treatments are summarized in Table 6.
Table 6.
| Classification (Symptom) | Treatment for Underlying Disease | Anticoagulation Therapy | Antifibrinolytic Therapy | Fibrinolytic Therapy | Blood Transfusion | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| UFH | LMWH | DS | SPI | AT | FFP | Platelet Concentrates | ||||||
| General | O | C | B2 | C | B2 | B1 b | D | D | Oc | Oc | ||
| Asymptomatic | Blood transfusion | Not necessary | O | C | B2 | C | B2 | B2 b | D | D | ||
| Necessary | O | C | B2 | C | B2 | B2 b | D | D | B2 c | B2 c | ||
| Bleeding | Minor | O | C | B2 | C | B2 | B2 b | D | D | |||
| Severe | O | D | D | D | B1 | B2 b | Ce | D | Oc | Oc | ||
| Organ failure | O | C | B2 | C | B2 | B1 b | D | D | ||||
| Complication | Major thrombosis | O | B2 | B1 | B2 | C | B2 b | D | d | |||
| TTP | O | C | B2 | C | B2 | B2 b | D | D | O | D | ||
| HIT | O | D | D | D | B2 | B2 b | D | D | D | |||
| Recommendation Levels (Modified Kish Guide)g | ||||||||||||
| Recommendation | ||||||||||||
| Consensus | Treatment does not have a high quality of evidence, but it should be carried out as common sense | |||||||||||
| A | Treatment has high quality of evidence, and the clinical usefulness is clear | |||||||||||
| B1 | Treatment has moderately high quality of evidence, or it has high quality of evidence but the clinical usefulness is not significant | |||||||||||
| B2 | Treatment does not have a high quality of evidence, but it has few deleterious effects and it is carried out clinically | |||||||||||
| C | Treatment does not have a high quality of evidence or the clinical usefulness is not clear | |||||||||||
| D | Treatment has high quality of evidence, and it has deleterious effects | |||||||||||
Abbreviations: AT, antithrombin; DIC, disseminated intravascular coagulation; DS, danaparoid sodium; FFP, fresh-frozen plasma; HIT, heparin-induced thrombocytopenia; LMWH, low-molecular-weight heparin; SPI, serine protease inhibitor; TTP, thrombotic thrombocytopenic purpura; UFH, unfractionated heparin.
a O denotes consensus.
b Limited in patients with less than 70% of AT.
c According to the guideline for blood transfusion.
d Consultation with specialist for fibrinolytic therapy.
f Consultation with specialist for antifibrinolytic therapy.
g Recommendation levels determined according to “the guidelines of DIC treatment preparation committee” according to the evidence level and medical care of Japan. Physician should decide on the adequate treatment, not only according to the guidelines but also according to the condition of each patient and institute.
An international group of experts published recommendations for the supportive treatment of DIC based on results from clinical trials and expert opinion. Overall, for treatment of patients with nonovert DIC in the absence of bleeding or thrombosis, recommendations are based on the treatment of the underlying triggering pathology.107 For sepsis-associated DIC, most of the experts recommend monitoring accompanied by prophylactic LMWH for thromboprophylaxis. In patients with overt DIC, platelet transfusion is recommended to maintain a platelet count of at least 50 × 109/L for patients with active bleeding or 20 × 109/L for patients with no bleeding signs.
For patients with acute deep vein thrombosis or pulmonary embolism with overt DIC and bleeding, the use of an inferior vena cava filter was recommended, with anticoagulant therapy started after the cessation of bleeding.
Recommendations have also been made for DIC associated with specific conditions, including cancer108 and postpartum hemorrhage.109 For patients with cancer-associated DIC, the Scientific and Standardization Committee of the ISTH has laid out recommendations for treatment with UFH, LMWH, platelet concentrates, and FFP. This contrasts with the Japanese recommendations, which include the use of rTM, AT, gabexate mesilate, and nafamostat mesilate.108 For women with coagulopathy associated with postpartum hemorrhage, the Scientific and Standardization Committee of the ISTH recommends monitoring of hemostasis through PT, aPTT, Clauss fibrinogen testing, or thromboelastometry. For ongoing postpartum hemorrhage with prolonged PT or aPTT, the administration of FFP is recommended, particularly if the PT or aPTT is greater than 1.5 times normal. For ongoing postpartum hemorrhage resistant to other treatments, tranexamic acid or activated FVII can be considered.109
Discussion
Disseminated intravascular coagulation is a life-threatening disorder of hemostasis, occurring in about 10% of acutely ill patients. Although different underlying pathologies can induce DIC, there is usually a common pathogenic mechanism observed. The main trigger of DIC stems from widespread overexpression of TF in blood circulation, which leads to excessive thrombin generation, low levels of natural coagulation inhibitors (mainly AT and PC), imbalance of the equilibrium between TFPI and TF, fibrinolysis impairment or activation, fibrin deposition in microvasculature, and consumption of platelets and clotting factors. The clinical manifestations of DIC depend on the underlying disease, individual predisposing factors, and the phase of the degradation of defense mechanisms. The inefficiency of natural anticoagulant pathways in general precedes the profound deficiency of clotting factors. The high mortality rate of 20% to 50% in DIC drives the need for early diagnosis of DIC and aggressive treatment of both the underlying disease and the coagulation abnormalities. Early diagnosis of DIC is of major importance for restoration of blood coagulation abnormalities and patient’s survival. From the beginning of the 21st century, the ISTH launched a concreted action for the systematization of a scoring system for the diagnosis of DIC.55 Following comprehensive work from several national and international expert consensus statements on diagnosis of DIC,56–58 the ISTH endorsed integral diagnostic scoring systems that evaluate clinical and laboratory parameters for diagnosis of DIC.8
Rational therapeutic strategies include supplementation with natural coagulation inhibitors, such as AT, PC, APC, rTFPI, or agents that enhance activation of PC in plasma such as rTM. However, as of yet, these therapies have shown no demonstrable beneficial effects on mortality rate of patients with severe sepsis, with or without DIC. It is noteworthy that these treatments have not been tested in a prospective randomized clinical trial using DIC as an entry criterion. The proper management of patients with DIC remains controversial, and the clinical trials assessing the efficacy and safety of the different therapeutic strategies in DIC are yet to be done.
The KyberSept,32 PROWESS,78 and OPTIMIST89 trials are 3 large-scale phase III placebo-controlled randomized clinical trials that assessed the efficacy and safety of treatment with natural coagulation inhibitors (AT, activated PC, and rTFPI, respectively) in patients with sepsis. In these trials, 28-day mortality was defined as the primary efficacy end point. Among the 3 trials, only the PROWESS showed a clear beneficial effect of treatment with APC with an absolute risk reduction of 6% in 28-day mortality in patients with severe sepsis. In contrast, further studies failed to demonstrate a clear beneficial effect of APC in the treatment of DIC. Results of these studies suggest that the 3 studied natural coagulation inhibitors do not have similar importance in the progression of sepsis. However, the results of these trials are not directly comparable due to the heterogeneity of disease severity between trial populations.
A common characteristic of trials of natural coagulation inhibitors is the enrollment of patients with sepsis, rather than patients diagnosed specifically with DIC. Sepsis is one of the most frequent causes of DIC. However, less than 50% of patients with sepsis fulfill the diagnostic criteria of DIC.32,78 Mortality in sepsis is a multifactorial event and is not caused exclusively by DIC. In the absence of DIC, clinical progression in sepsis is dominated by the consequences of the inflammatory response.110,111 The results of the PROWESS trial support the concept that the potential anti-inflammatory activity of DAA likely has some beneficial effect in patients with severe sepsis. This is in accordance with experimental studies on animal models, which showed that the PC anticoagulant pathway interferes closely with inflammation process.41,42 However, this favorable effect was not confirmed by the PROWESS-SHOCK study.84 In addition, the ADDRESS trial showed that in patients with less severe sepsis, DAA has no beneficial effect on 28-day mortality compared to placebo, and it increases the bleeding risk compared to placebo.81 Although results of subgroup analysis of phase III clinical trials have major limitations, they indicate that treatment with natural coagulation inhibitors might have a favorable effect on mortality in patients with sepsis-associated DIC, leading to the hypothesis that a better definition of inclusion criteria regarding the presence of DIC is needed in order to demonstrate the efficacy of these treatments. In accordance with this hypothesis, the results of the study with rTM conducted in patients with DIC induced by infection or hematological malignancy showed a benefit concerning 28-day mortality compared with treatment with UFH. Treatment with rTM appeared to be superior to UFH concerning mortality of patients with DIC.91 In order to clarify the benefits of natural coagulation inhibitors in the treatment of DIC, future clinical trials must target homogenous groups of patients with DIC induced by sepsis, cancer or other relevant causes, and use DIC as the primary entry criterion.
Since patients with DIC present with a hypercoagulable state, administration of rapidly acting antithrombotic agents in patients is a rational therapeutic approach. Among them, heparins (UFH and LMWHs) appear to be the most widely used antithrombotic drug in DIC, although this is not supported by sufficient clinical data. A consistent finding of post hoc analysis of the trials with natural coagulation inhibitors is that coadministration of heparin has a marginal beneficial effect on the efficacy but substantially increases the bleeding risk. Nonspecific binding of heparin chains with plasma proteins other than AT and the risk of heparin-induced thrombocytopenia are potential limitations in the use of heparin in patients with DIC. Specific direct inhibitors of FXa (rivaroxaban, apixaban, edoxaban) or thrombin (hirudin, argatroban, or dabigatran) might have some place in the antithrombotic treatment in patients with DIC due to their predictable antithrombotic activity and lack of risk of heparin-induced thrombocytopenia.112,113 However, the renal elimination of these agents is a major limitation in their use in patients hospitalized in ICU. Fondaparinux could also have a place in the treatment of DIC-associated hypercoagulability since its high affinity for AT could optimize the residual activity of this important natural coagulation inhibitor if renal function is normal. The efficacy and safety of these drugs in the treatment of DIC should be assessed in future clinical studies.
Although there is very limited data to support the efficacy of FFP, fibrinogen concentrate, PCCs, and platelet transfusion in controlling DIC, these are still the standard of care in patients with severe DIC-associated hemorrhage. The controversy of this therapeutic approach stems from the presence of a hypercoagulable state. The FFP, fibrinogen concentrates, PCCs, and platelet concentrates are routinely given when severe clotting factor deficiencies, severe thrombocytopenia, or active bleeding are present. Transfusions are generally monitored by routine clotting tests such as PT, aPTT, and fibrinogen. Assays based on thromboelastography may aid in the identification of patients with hyperfibrinolysis as well as early diagnosis of hypofibrinogenemia and severe clotting factor deficiency.114 The impact on the clinical outcome and the resolution of DIC is unknown. Case reports show that administration of rFVIIa might have some power to control refractory and life-threatening bleeding, but the risk of thrombosis should be considered in light of systemic coagulation activation and possible hypofibrinolysis. Recombinant FVIIa could be used in life-threatening hemorrhage associated with hyperfibrinolytic DIC, as typically observed in obstetrical calamities. In patients with DIC of other causes than sepsis, the level of evidence is even lower. Clinicians have to rely on a small number of case reports or case series, as well as “expert opinions” expressed in various review articles. A frequent reaction is to ignore DIC and concentrate on treatment of bleeding by replacement of coagulation factors and platelets.
Conclusion
The rationale of DIC treatment is based on the pathogenic mechanism and the phase of the syndrome but currently lacks substantial evidence from clinical trials. Although data from phase III clinical trials on the treatment of patients with DIC are not available, the subgroup analysis of the studies presented in this review propose a rational therapeutic strategy for DIC following these principles:
- Downregulation of the hypercoagulable state
- via administration of rapidly acting antithrombotic drugs mainly LMWH and use of UFH when the renal function is compromised
- via administration of concentrates of natural coagulation inhibitors (AT, rhAPC, rTFPI, and rTM) in order to restore the efficiency of the natural anticoagulant pathways when there is documented deficiency.
Restoration of clotting factor deficiencies when plasma levels are very low as confirmed by specific laboratory assessment and the bleeding risk is high or active bleeding is present.
Administration of rapidly acting hemostatic agents or antifibrinolytic drugs to control active major bleeding.
The analysis of clinical trials and the pathophysiologic mechanism show that DIC is a heterogeneous condition requiring individualized treatment. Optimal treatment is based on close laboratory monitoring and new global coagulation assays such as whole blood thromboelastography/thromboelastometry or other global assays for thrombin generation.
The use of new specific antithrombotic agents to downregulate the hypercoagulable state in DIC, the identification of the specific coagulation abnormalities, and the adaptation of the therapeutic intervention are approaches for DIC treatment that still require further exploration.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Okabayashi K, Wada H, Ohta S, Shiku H, Nobori T, Maruyama K. Hemostatic markers and the sepsis-related organ failure assessment score in patients with disseminated intravascular coagulation in an intensive care unit. Am J Hematol. 2004;76(3):225–229. [DOI] [PubMed] [Google Scholar]
- 2. Sallah S, Wan JY, Nguyen NP, Hanrahan LR, Sigounas G. Disseminated intravascular coagulation in solid tumors: clinical and pathologic study. Thromb Haemost. 2001;86(3):828–833. [PubMed] [Google Scholar]
- 3. Gando S, Saitoh D, Ogura H, et al. ; Japanese Association for Acute Medicine Disseminated Intravascular Coagulation (JAAM DIC) Study Group, Japanese Association for Acute Medicine Disseminated Intravascular Coagulation (JAAM DIC) Study Group. Natural history of disseminated intravascular coagulation diagnosed based on the newly established diagnostic criteria for critically ill patients: results of a multicenter, prospective survey. Crit Care Med. 2008;36(1):145–150. [DOI] [PubMed] [Google Scholar]
- 4. Angstwurm MW, Dempfle CE, Spannagl M. New disseminated intravascular coagulation score: a useful tool to predict mortality in comparison with Acute Physiology and Chronic Health Evaluation II and Logistic Organ Dysfunction Scores. Crit Care Med. 2006;34(2):314–320. [DOI] [PubMed] [Google Scholar]
- 5. Toh CH, Downey C. Performance and prognostic importance of a new clinical and laboratory scoring system for identifying non-overt disseminated intravascular coagulation. Blood Coagul Fibrin. 2005;16(1):69–74. [DOI] [PubMed] [Google Scholar]
- 6. Sivula M, Tallgren M, Pettilä V. Modified scores for disseminated intravascular coagulation in the critically ill. Intensive Care Med. 2005;31(9):1209–1214. [DOI] [PubMed] [Google Scholar]
- 7. Bakhtiari K, Meijers JC, de Jonge E, Levi M. Prospective validation of the International Society of Thrombosis and Haemostasis scoring system for disseminated intravascular coagulation. Crit Care Med. 2004;32(12):2416–2421. [DOI] [PubMed] [Google Scholar]
- 8. Wada H, Trachil J, Di Nisio M, et al. The Scientific Standardisation Committee on DIC of the International Society on Thrombosis Haemostasis. Guidance for diagnosis and treatment in DIC from harmonization of the recommendations from three guidelines. J Thromb Haemost. 2013;11(11):761–767. [Google Scholar]
- 9. Dupuy M. Injections de matiere celebrale dans les veines. Gaz Med Paris. 1834;2:524. [Google Scholar]
- 10. Giles AR, Nesheim ME, Mann KG. Studies of factors V and VIII: C in an animal model of disseminated intravascular coagulation. J Clin Invest. 1984;74(6):2219–2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gando S, Nanzaki S, Sasaki S, Kemmotsu O. Significant correlation between tissue factor and thrombin markers in trauma and septic patients with disseminated intravascular coagulation. Thromb Haemost. 1998;79(6):1111–1115. [PubMed] [Google Scholar]
- 12. Ahamed J, Niessen F, Kurokawa T, et al. Regulation of macrophage procoagulant responses by the tissue factor cytoplasmic domain in endotoxemia. Blood. 2007;109(12):5251–5259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mackman N. Role of tissue factor in hemostasis and thrombosis. Blood Cells Mol Dis. 2006;36(2):104–107. [DOI] [PubMed] [Google Scholar]
- 14. Solovey A, Gui L, Key NS, Hebbel RP. Tissue factor expression by endothelial cells in sickle cell anemia. J Clin Invest. 1998;101(9):1899–1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Franco RF, de Jonge E, Dekkers PE, et al. The in vivo kinetics of tissue factor messenger RNA expression during human endotoxemia: relationship with activation of coagulation. Blood. 2000;96(2):554–559. [PubMed] [Google Scholar]
- 16. Rak J, Milsom C, May L, Klement P, Yu J. Tissue factor in cancer and angiogenesis: the molecular link between genetic tumor progression, tumor neovascularization, and cancer coagulopathy. Semin Thromb Hemost. 2006;32(1):54–70. [DOI] [PubMed] [Google Scholar]
- 17. Nieuwland R, Berckemans RJ, McGregor S, et al. Cellular origin and procoagulant properties of microparticles in meningococcal sepsis. Blood. 2000;95(3):930–935. [PubMed] [Google Scholar]
- 18. Satta N, Toti F, Feugeas O, et al. Monocyte vesiculation is a possible mechanism for dissemination of membrane-associated procoagulant activities and adhesion molecules after stimulation by lipopolysaccharide. J Immunol. 1994;153(7):3245–3245. [PubMed] [Google Scholar]
- 19. Kalafatis M, Swords NA, Rand MD, Mann KG. Membrane-dependent reactions in blood coagulation: role of the vitamin K-dependent enzyme complexes. Biochim Biophys Acta. 1994;1227(3):113–129. [DOI] [PubMed] [Google Scholar]
- 20. Østerud B, Bjorklid E. The tissue factor pathway in disseminated intravascular coagulation. Semin Thromb Hemost. 2001;27(6):605–617. [DOI] [PubMed] [Google Scholar]
- 21. Toh CH, Samis J, Downey C, et al. Biphasic transmittance waveform in the APTT coagulation assay is due to the formation of a calcium dependent complex of C-reactive protein with very low density lipoprotein and is a novel marker of impending disseminated intravascular coagulation. Blood. 2002;100(7):2522–2529. [DOI] [PubMed] [Google Scholar]
- 22. Mosad E, Elsayh KI, Eltayeb AA. Tissue factor pathway inhibitor and P-selectin as markers of sepsis-induced non-overt disseminated intravascular coagulopathy. Clin Appl Thromb Haemostas. 2011;17(1):80–87. [DOI] [PubMed] [Google Scholar]
- 23. Geeaerts T, Haïk W, Tremey B, et al. Trouble de la coagulation lors du traumatisme cranio-encéphalique: physiopathologie et conséquences thérapeutiques. Ann Fr Anesth Réanim. 2010;29(9):e177–e181. [DOI] [PubMed] [Google Scholar]
- 24. Sinauridze EI, Kireev DA, Popenko NY, et al. Platelet microparticle membranes have 50- to 100-fold higher specific procoagulant activity than activated platelets. Thromb Haemost. 2007;97(3):425–434. [PubMed] [Google Scholar]
- 25. Zafrani l, Gerotziafas GT, Byrnes C, et al. Calpastatin controls polymicrobial sepsis by limiting procoagulant microparticles release. Am J Respir Crit Care Med. 2012;185(7):744–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Levi M, de Jonge E, van der Poll T, ten Cate H. Advances in the understanding of the pathogenetic pathways of disseminated intravascular coagulation result in more insight in the clinical picture and better management strategies. Semin Thromb Hemost. 2001;27(6):569–575. [DOI] [PubMed] [Google Scholar]
- 27. Broze GJ., Jr Tissue factor pathway inhibitor. Thromb Haemost. 1995;74(1):90–93. [PubMed] [Google Scholar]
- 28. Asakura H, Ontachi Y, Mizutani T, et al. Elevated levels of free tissue factor pathway inhibitor antigen in cases of disseminated intravascular coagulation caused by various underlying diseases. Blood Coagul Fibrin. 2001;12(1):1–8. [DOI] [PubMed] [Google Scholar]
- 29. Gando S, Nanzaki S, Morimoto Y, Ishitani T, Kemmotsu O. Tissue factor pathway inhibitor response does not correlate with tissue factor-induced disseminated intravascular coagulation and multiple organ dysfunction syndrome in trauma patients. Crit Care Med. 2001;29(2):262–266. [DOI] [PubMed] [Google Scholar]
- 30. Gando S, Kameue T, Morimoto Y, Matsuda N, Hayakawa M, Kemmotsu O. Tissue factor production not balanced by tissue factor pathway inhibitor in sepsis promotes poor prognosis. Crit Care Med. 2002;30(8):1729–1734. [DOI] [PubMed] [Google Scholar]
- 31. Lwaleed BA, Bass PS. Tissue factor pathway inhibitor: structure, biology and involvement in disease. J Pathol. 2006;208(3):327–339. [DOI] [PubMed] [Google Scholar]
- 32. Warren BL, Eid A, Singer P, et al. ; KyberSept Trial Study Group. KyberSept Trial Study Group. Caring for the critically ill patient. High dose antithrombin III in severe sepsis: a randomized controlled trial. JAMA. 2001;286(15):1869–1878. [DOI] [PubMed] [Google Scholar]
- 33. Mesters RM, Mannucci PM, Coppola R, Keller T, Ostermann H, Kienast J. Factor VIIa and antithrombin III activity during severe sepsis and septic shock in neutropenic patients. Blood. 1996;88(3):881–886. [PubMed] [Google Scholar]
- 34. Niessen RW, Lamping RJ, Jansen PM, et al. Antithrombin acts as a negative acute phase protein as established with studies on HepG2 cells and in baboons. Thomb Haemost. 1997;78(3):1088–1092. [PubMed] [Google Scholar]
- 35. Emerson TE., Jr Antithrombin III replacement in animal models of acquired antithrombin III deficiency. Blood Coagul Fibrin. 1994;5(1):S37–S45. [DOI] [PubMed] [Google Scholar]
- 36. Asakura H, Ontachi Y, Mizutani T, et al. Decreased plasma activity of antithrombin or protein C is not due to consumption coagulopathy in septic patients with disseminated intravascular coagulation. Eur J Haematol. 2001;67(3):170–175. [DOI] [PubMed] [Google Scholar]
- 37. Opal SM. Therapeutic rationale for antithrombin III in sepsis. Crit Care Med. 2000;28(9):S34–S37. [DOI] [PubMed] [Google Scholar]
- 38. Aibiki M, Fukuoka N, Umakoshi K, Ohtsubo S, Kikuchi S. Serum albumin levels anticipate antithrombin III activities before and after antithrombin III agent in critical patients with disseminated intravascular coagulation. Shock. 2007;28(2):139–144. [DOI] [PubMed] [Google Scholar]
- 39. Esmon CT. The roles of protein C and thrombomodulin in the regulation of blood coagulation. J Biol Chem. 1989;264(9):4743–4746. [PubMed] [Google Scholar]
- 40. Kisiel W. Human plasma protein C: isolation, characterization and mechanism of activation by alpha-thrombin. J Clin Invest. 1979;64(3):761–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. ten Cate H. Pathophysiology of disseminated intravascular coagulation in sepsis. Crit Care Med. 2000;28(9):S9–S11. [DOI] [PubMed] [Google Scholar]
- 42. Levi M, de Jonge E, van der Poll T. Rationale for restoration of physiological anticoagulant pathways in patients with sepsis and disseminated intravascular coagulation. Crit Care Med. 2001;29(7):S90–S94. [DOI] [PubMed] [Google Scholar]
- 43. Moore KL, Andreoli SP, Esmon NL, Esmon CT, Bang NU. Endotoxin enhances tissue factor and suppresses thrombomodulin expression of human vascular endothelium in vitro. J Clin Invest. 1987;79(1):124–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Moore KL, Esmon CT, Esmon NL. Tumor necrosis factor leads to the internalization and degradation of thrombomodulin from the surface of bovine aortic endothelial cells and culture. Blood. 1989;73(1):159–165. [PubMed] [Google Scholar]
- 45. Gando S, Kameue T, Nanzaki S, Nakanishi Y. Cytokines, soluble thrombomodulin and disseminated intravascular coagulation in patients with systemic inflammatory response syndrome. Thromb Res. 1995;80(6):519–526. [DOI] [PubMed] [Google Scholar]
- 46. Ishii H, Uchiyama H, Kazama M. Soluble thrombomodulin antigen in conditioned medium is increased by damage of endothelial cells. Thromb Haemost. 1991;65(5):618–623. [PubMed] [Google Scholar]
- 47. Taylor FB, Jr, Dahlback B, Chang AC, et al. Role of free protein S and C4b binding protein in regulating the coagulation response to Escherichia coli. Blood. 1995;86(7):2642–2652. [PubMed] [Google Scholar]
- 48. Yamamoto K, Loskutoff DJ. Fibrin deposition in tissues from endotoxin-treated mice correlates with decreases in the expression of urokinase-type but not tissue-type plasminogen activator. J Clin Invest. 1996;97(11):2440–2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Mosnier LO, Meijers JC, Bouma BN. Regulation of fibrinolysis in plasma by TAFI and protein C is dependent on the concentration of thrombomodulin. Thromb Haemost. 2001;85(1):5–11. [PubMed] [Google Scholar]
- 50. Hayakawa M, Sawamura A, Gando S, Jesmin S, Naito S, Ieko M. A low TAFI activity and insufficient activation of fibrinolysis by both plasmin and neutrophil elastase promote organ dysfunction in disseminated intravascular coagulation associated with sepsis. Thromb Res. 2012;130(6):906–913. [DOI] [PubMed] [Google Scholar]
- 51. Park HS, Gu JY, You HJ, Kim JE, Kim HK. Factor XII-mediated contact activation related to poor prognosis in disseminated intravascular coagulation. Thromb Res. 2016;138:103–107. [DOI] [PubMed] [Google Scholar]
- 52. Kim JE, Lee N, Gu JY, Yoo HJ, Kim HK. Circulating levels of DNA-histone complex and dsDNA are independent prognostic factors of disseminated intravascular coagulation. Thromb Res. 2015;135(6):1064–1069. [DOI] [PubMed] [Google Scholar]
- 53. Chen Q, Ye L, Jin Y, et al. Circulating nucleosomes as a predictor of sepsis and organ dysfunction in critically ill patients. Int J Dis. 2012;16(7):558–564. [DOI] [PubMed] [Google Scholar]
- 54. Hell L, Thaler J, Martinod K, et al. OC-16 neutrophil extracellular traps and tissue factor-bearing microvesicles: a liaison danger use causing over DIV in cancer patients? Thromb Res. 2016;140(1):174–175. [DOI] [PubMed] [Google Scholar]
- 55. Taylor FB, Toh CH, Hoots WK, Wada H, Levi M; Scientific Subcommittee on Disseminated Intravascular Coagulation (DIC) of the International Society on Thrombosis and Haemostasis (ISTH). Towards definition, clinical and laboratory criteria, and a scoring system for disseminated intravascular coagulation. Thromb Haemost. 2001;86(5):1327–1330. [PubMed] [Google Scholar]
- 56. Wada H, Gabazza EC, Asakura H, et al. Comparison of diagnostic criteria for disseminated intravascular coagulation (DIC): diagnostic criteria of the International Society of Thrombosis and Hemostasis and of the Japanese Ministry of Health and Welfare for overt DIC. Am J Hematol. 2003;74(1):17–22. [DOI] [PubMed] [Google Scholar]
- 57. Wada H, Asakura H, Okamoto K, et al. Japanese Society of Thrombosis Hemostasis/DIC Subcommittee: expert consensus for the treatment of DIC in Japan. Thromb Res. 2010;125(1):6–11. [DOI] [PubMed] [Google Scholar]
- 58. Levi M, Toh CH, Thachil J, Watson HG. Guidelines for the diagnosis and management of disseminated intravascular coagulation. British Committee for Standards in Haematology. Br J Haematol. 2009;145(1):24–33. [DOI] [PubMed] [Google Scholar]
- 59. Di Nisio M, Baudo F, Cosmi B, et al. ; on behalf of the Italian Society for Thrombosis and Haemostasis. Diagnosis and treatment of disseminated intravascular coagulation: guidelines of the Italian Society for Haemostasis and Thrombosis (SISET). Thromb Res. 2012;129(5):177–184. [DOI] [PubMed] [Google Scholar]
- 60. Dempfle CE, Wurst M, Smolinski M, et al. Use of soluble fibrin antigen instead of d-dimers as fibrin related marker may enhance the prognostic power of the ISTH overt DIC score. Thromb Haemost. 2004;91(4):812–818. [DOI] [PubMed] [Google Scholar]
- 61. Umemura Y, Yamakawa K, Kiguchi T, et al. Design and evaluation of new unified criteria for disseminated intravascular coagulation based on the Japanese Association for Acute Medicine Criteria. Clin Appl Thromb Hemost. 2016;22(2):153–160. [DOI] [PubMed] [Google Scholar]
- 62. Iba T, Ito T, Maruyama I, et al. Potential diagnostic markers for disseminated intravascular coagulation of sepsis. Blood Rev. 2016;30(2):149–155. [DOI] [PubMed] [Google Scholar]
- 63. Hjorleifsson E, Sigurdsson MI, Gudmundsdottir BR, Sigurdsson GH, Onundarson PT. Prediction of survival in patients suspected of disseminated intravascular coagulation. Acta Anaesthesiolog Scand. 2015;59(7):870–880. [DOI] [PubMed] [Google Scholar]
- 64. Singh N, Mishra P, Tyagi S, Upadhyay AD, Saxena R. Evaluation of the diagnostic performance of fibrin monomer in comparison to d-dimer in patients with overt and nonovert disseminated intravascular coagulation. Clin Appl Thromb Hemost. 2017;23(5):460–465. [DOI] [PubMed] [Google Scholar]
- 65. Koami H, Sakamoto Y, Ohta M, et al. Can rotational thromboelastometry predict septic disseminated intravascular coagulation? Blood Coagul Fibrin. 2015;26(7):778–783. [DOI] [PubMed] [Google Scholar]
- 66. Inthorn D, Hoffmann JN, Hartl WH, Mühlbayer D, Jochum M. Effect of antithrombin III supplementation on inflammatory response in patients with severe sepsis. Shock. 1998;10(2):90–96. [DOI] [PubMed] [Google Scholar]
- 67. Eisele B, Lamy M, Thijs LG, et al. Antithrombin III in patients with severe sepsis: a randomized, placebo-controlled, double-blind multicenter trial plus meta-analysis on all randomized, placebo-controlled, double-blind trials with AT III in severe sepsis. Intensive Care Med. 1998;24(7):663–672. [DOI] [PubMed] [Google Scholar]
- 68. Baudo F, Caimi TM, de Cataldo F, et al. Antithrombin III replacement therapy in patients with sepsis and/or postsurgical complications: a controlled double-blind, randomized multicenter study. Intensive Care Med. 1998;24(4):336–342. [DOI] [PubMed] [Google Scholar]
- 69. Kienast J, Juers M, Wiedermann CJ, et al. Treatment effects of high-dose antithrombin without concomitant heparin in patients with severe sepsis with or without DIC. J Thromb Haemost. 2006;4(1):90–97. [DOI] [PubMed] [Google Scholar]
- 70. Hoffmann JN, Wiedermann CJ, Juers M, et al. ; KyberSept Investigators. Benefit/risk profile of high-dose antithrombin in patients with severe sepsis treated with or without concomitant heparin. Thromb Haemost. 2006;95(5); 850–856. [PubMed] [Google Scholar]
- 71. Gando S, Sawamura A, Hayakawa M, et al. First day dynamic changes in antithrombin III activity after supplementation have a predictive value in critically ill patients. Am J Hematol. 2006;81(12):907–914. [DOI] [PubMed] [Google Scholar]
- 72. Iba T, Saito D, Wada H, et al. Efficacy and bleeding risk of antithrombin supplementation in septic disseminated intravascular coagulation: a prospective multicenter survey. Thromb Res. 2012;130(3):129–133. [DOI] [PubMed] [Google Scholar]
- 73. Iba T, Gando S, Saitoh D, et al. Efficacy and bleeding risk of antithrombin supplementation in patients with septic disseminated intravascular coagulation: a third survey. Clin Appl Thromb Hemost. 2017;23(5):422–428. [DOI] [PubMed] [Google Scholar]
- 74. Tagami T, Matsui H, Fushimi K, Yasunaga H. Supplemental dose of antithrombin use in disseminated intravascular coagulation patients after abdominal sepsis. Thromb Haemost. 2015;114(3):537–545. [DOI] [PubMed] [Google Scholar]
- 75. Allingstrup M, Wetterslev J, Ravn FB, Møller AM, Afshari A. Antithrombin III for critically ill patients: a systematic review with meta-analysis and trial sequential analysis. Intensive Care Med. 2016;42(4):505–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Esmon CT. The endothelial cell protein C receptor. Thromb Haemost. 2000;83(5):639–643. [PubMed] [Google Scholar]
- 77. Bernard GR, Ely EW, Wright TJ, et al. Safety and dose relationship of recombinant human activated protein C for coagulopathy in severe sepsis. Crit Care Med. 2001;29(11):2051–2059. [DOI] [PubMed] [Google Scholar]
- 78. Bernard GR, Vincent JL, Laterre PF, et al. Recombinant Human Protein C Worldwide Evaluation in Severe Sepsis (PROWESS) Study Group. Efficacy and safety of recombinant human activated protein C for severe sepsis. N Engl J Med. 2001;344(10):699–709. [DOI] [PubMed] [Google Scholar]
- 79. Aoki N, Matsuda T, Saito H, et al. ; CTC-111-IM Clinical Research Group. A comparative double-blinded randomized trial of activated protein C and unfractionated heparin in treatment of disseminated intravascular coagulation. Int J Hematol. 2002;75(5):540–547. [DOI] [PubMed] [Google Scholar]
- 80. Dhainaut JF, Yan SB, Joyce DE, et al. Treatment effects of drotrecogin alfa (activated) in patients with severe sepsis with or without overt disseminated intravascular coagulation. J Throm Haemost. 2004;2(11):1924–1933. [DOI] [PubMed] [Google Scholar]
- 81. Abraham E, Laterre PF, Garg R, et al. Administration of Drotrecogin Alfa (Activated) in Early Stage Severe Sepsis (address) Study Group. Drotrecogin alfa (activated) for adults with severe sepsis and low risk of death. N Engl J Med. 2005;353(13):1332–1341. [DOI] [PubMed] [Google Scholar]
- 82. Nadel S, Goldstein B, Williams MD, et al. REsearching Severe Sepsis and Organ Dysfunction in Children: A Global Perspective (RESOLVE) Study Group. Drotrecogin alfa (activated) in children with severe sepsis: a multicentre phase III randomised controlled trial. Lancet. 2007;369(9564):836–843. [DOI] [PubMed] [Google Scholar]
- 83. Bernard GR, Margolis BD, Shanies HM, et al. Extended Evaluation of Recombinant Human Activated Protein C United States Investigators. Extended evaluation of recombinant human activated protein C United States Trial (ENHANCE US): a single-arm, phase 3B, multicenter study of drotrecogin alfa (activated) in severe sepsis. Chest. 2004;125(6):2206–2216. [DOI] [PubMed] [Google Scholar]
- 84. Ranieri VM, Thompson BT, Barie PS, et al. ; PROWESS-SHOCK Study Group. Drotrecogin alfa (activated) in adults with septic shock. N Engl J Med. 2012;366(22):2055–2064. [DOI] [PubMed] [Google Scholar]
- 85. Annane D, Timsit JF, Megarbane B, et al. APROCCHSS Trial Investigators. Recombinant human activated protein C for adults with septic shock: a randomized controlled trial. Am J Respir Crit Care Med. 2013;187(10):1091–1097. [DOI] [PubMed] [Google Scholar]
- 86. Thachil J, Toh CH, Levi M, Watson HG. The withdrawal of activated protein C from the use in patients with severe sepsis and DIC (amendment to the BCSH guideline on disseminated intravascular coagulation). Br J Haematol. 2012;157(4):493–516. [DOI] [PubMed] [Google Scholar]
- 87. de Jonge E, Dekkers PE, Creasey AA, et al. Tissue factor pathway inhibitor dose-dependently inhibits coagulation activation without influencing the fibrinolytic and cytokine response during human endotoxemia. Blood. 2000;95(4):1124–1129. [PubMed] [Google Scholar]
- 88. Abraham E, Reinhart K, Svoboda P, et al. Assessment of the safety of recombinant tissue factor pathway inhibitor in patients with severe sepsis: a multicenter, randomized, placebo-controlled, single-blind, dose escalation study. Crit Care Med. 2001;29(11):2081–2089. [DOI] [PubMed] [Google Scholar]
- 89. Abraham E, Reinhart K, Opal S, et al. ; OPTIMIST Trial Study Group. Efficacy and safety of tifacogin (recombinant tissue factor pathway inhibitor) in severe sepsis: a randomized controlled trial. JAMA. 2003;290(2):238–247. [DOI] [PubMed] [Google Scholar]
- 90. Ikeguchi H, Maruyama S, Morita Y, et al. Effects of human soluble thrombomodulin on experimental glomerulonephritis. Kidney Int. 2002;61(12):490–501. [DOI] [PubMed] [Google Scholar]
- 91. Saito H, Maruyama I, Shimazaki S, et al. Efficacy and safety of recombinant human soluble thrombomodulin (ART-123) in disseminated intravascular coagulation: results of a phase III, randomized, double-blind clinical trial. J Thromb Haemost. 2007;5(1):31–41. [DOI] [PubMed] [Google Scholar]
- 92. Kawano N, Yoshida S, Ono N, et al. Clinical features and outcomes of 35 disseminated intravascular coagulation cases treated with recombinant human soluble thrombomodulin at a single institution. J Clin Exp Hematop. 2011;51(2):101–107. [DOI] [PubMed] [Google Scholar]
- 93. Hayakawa M, Yamakawa K, Saito S, et al. for the Japan Septic Disseminated Intravascular Coagulation (JSEPTIC DIC) Study Group. Recombinant human soluble thrombomodulin and mortality in sepsis-induced disseminated intravascular coagulation: a multicentre retrospective study. Thromb Haemost. 2016;115(6):1157–1166. [DOI] [PubMed] [Google Scholar]
- 94. Yoshihara M, Uno K, Tano S, et al. The efficacy of recombinant human soluble thrombomodulin for obstetric disseminated intravascular coagulation: a retrospective study. Crit Care. 2015;19:369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Yoshimura J, Yamakawa K, Ogura H, et al. Benefit profile of recombinant human soluble thrombomodulin in sepsis-induced disseminated intravascular coagulation: a multicentre propensity score analysis. Crit Care. 2015;19:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Takezako N, Sekiguchi N, Nagata A, et al. Recombinant human thrombomodulin in the treatment of acute myeloid leukemia patients complicated by disseminated intravascular coagulation: retrospective analysis of outcomes between patients treated with heparin and recombinant human thrombomodulin therapy. Thromb Res. 2015;136(1):20–23. [DOI] [PubMed] [Google Scholar]
- 97. Vincent JL, Ramesh MK, Ernest D, et al. A randomized, double-blind, placebo-controlled, phase 2B study to evaluate the safety and efficacy of recombinant human soluble thrombomodulin, ART-123, in patients with sepsis and suspected disseminated intravascular coagulation. Crit Care Med. 2013;41(9):2069–2079. [DOI] [PubMed] [Google Scholar]
- 98. Sakugawa N, Hasegawa H, Maki M, Nakagawa M, Nakashima M. Clinical evaluation of low molecular weight heparin on disseminated intravascular coagulation: a multicenter co-operative double-blind trial in comparison with heparin. Thromb Res. 1993;72(6):475–500. [DOI] [PubMed] [Google Scholar]
- 99. Levi M, Levy M, Williams MD, et al. ; Xigris and Prophylactic HepaRin Evaluation in Severe Sepsis (XPRESS) Study Group. Prophylactic heparin in patients with severe sepsis treated with drotrecogin alfa (activated). Am J Respir Crit Care Med. 2007;176(5):483–490. [DOI] [PubMed] [Google Scholar]
- 100. Jaimes F, De la Rosa G, Morales C, et al. Unfractioned heparin for treatment of sepsis: a randomised clinical trial (the HETRASE STUDY). Crit Care Med. 2009;37(4):1185–1196. [DOI] [PubMed] [Google Scholar]
- 101. Umemura Y, Yamakawa K, Ogura H, Yuhara H, Fujimi S. Efficacy and safety of anticoagulant therapy in three specific populations with sepsis: a meta-analysis of randomized controlled trials. J Thromb Haemost. 2016;14(3):518–530. [DOI] [PubMed] [Google Scholar]
- 102. Pernerstorfer T, Hollenstein U, Hansen JB, et al. Lepirudin blunts endotoxin-induced coagulation activation. Blood. 2000;95(5):1729–1734. [PubMed] [Google Scholar]
- 103. Levi M, Opal SM. Coagulation abnormalities in critically ill patients. Crit Care. 2006;10(4):222–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Schmid S, Friesenecker B, Lorenz I, et al. Administration of recombinant activated factor VII (NovoSeven) in three cases of uncontrolled bleeding caused by disseminated intravascular coagulopathy. Clin Appl Thromb Hemost. 2007;13(3):313–317. [DOI] [PubMed] [Google Scholar]
- 105. Franchini M, Manzato F, Salvagno GL, Lippi G. Potential role of recombinant activated factor VII for the treatment of severe bleeding associated with disseminated intravascular coagulation: a systematic review. Blood Coagul Fibrin. 2007;18(7):589–593. [DOI] [PubMed] [Google Scholar]
- 106. Wang CY, Chen YC, Lin CH, Hwang KS, Su HY. Successful treatment with recombinant blood factor VIIa in severe postpartum hemorrhage-induced disseminated intravascular coagulation. Taiwan J Obstet Gynecol. 2016;55(2);301–302. [DOI] [PubMed] [Google Scholar]
- 107. Squizzato A, Hunt BJ, Kinasewitz GT, et al. Supportive management strategies for disseminated intravascular coagulation: an international consensus. Thromb Haemost. 2016;115(5):896–904. [DOI] [PubMed] [Google Scholar]
- 108. Wada H, Matsumoto T, Aota T, Yamashita Y, Suzuki K, Katayama N. Management of cancer-associated disseminated intravascular coagulation: guidance from the SSC of the ISTH: comment. J Thromb Haemost. 2016;14(6):1314–1315. [DOI] [PubMed] [Google Scholar]
- 109. Collins P, Abdul-Kadir R, Thachil J. Management of coagulopathy associated with postpartum haemorrhage: guidance from the SSC of the ISTH. J Thromb Haemost. 2016;14(1):205–210. [DOI] [PubMed] [Google Scholar]
- 110. Neurath MF. New therapies for sepsis: focus on the interleukin (IL)12 family member IL27. Ann Rheum Dis. 2007;66(suppl 3):ii29–ii31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Matsuda N, Hattori Y. Vascular biology in sepsis: pathophysiological and therapeutic significance of vascular dysfunction. J Smooth Muscle Res. 2007;43(4):117–137. [DOI] [PubMed] [Google Scholar]
- 112. Gerotziafas GT, Samama MM. Heterogeneity of synthetic factor Xa inhibitors. Curr Pharm Des. 2005;11(30):3855–3876. [DOI] [PubMed] [Google Scholar]
- 113. Weitz JI, Bates SM. New anticoagulants. J Thromb Haemost. 2005;3(8):1843–1853. [DOI] [PubMed] [Google Scholar]
- 114. Hoffmann JN, Schick K. Antithrombin and hypercoagulability in sepsis: insights from thrombelastography? Crit Care. 2007;11 (1):115–127. [DOI] [PMC free article] [PubMed] [Google Scholar]