Abstract
The purpose of this work is to investigate whether imaging sequences of flash-replenishment contrast enhanced ultrasound (CEUS) of the kidney result in chronic or acute bioeffects. Kidneys of female Fischer 344 rats were imaged using the flash-replenishment technique. Animals were separated into four groups (N=31). Imaging was conducted with a 4C1 probe, driven by an Acuson Sequoia system with Definity microbubbles as the ultrasound contrast agent. During the flash phase of the imaging sequence, one kidney in each animal was exposed to either a mechanical index (MI) of 1.0 or 1.9. For each MI, half of the animals were sacrificed shortly after imaging (4 hours) or after 2 weeks. A blinded veterinary nephropathologist reviewed the histopathology of both the imaged and control (non-imaged) kidney. Blood urea nitrogen (BUN) was measured for each animal prior to imaging and at the time of necropsy. Histopathology assessments in both the 1.0 and 1.9 MI groups revealed no signs of hemorrhage at either the 4-hour or 2-week time point. BUN showed minor but statistically significant elevations in both the 1.0 and 1.9 MI groups, but no significant difference was present at the 2-week time point in the 1.0 MI group. All BUN levels (at both time points) remained in the normal range. In conclusion, CEUS with flash-replenishment imaging sequences did not result in kidney bioeffects observable with histology at early or late time points. Increases in BUN levels were observed after imaging, but were minimized when using a moderate MI (1.0).
Keywords: Contrast enhanced ultrasound, ultrasound contrast agents, microbubble, bioeffects
1. Introduction
Contrast-enhanced ultrasound (CEUS) with microbubble contrast agents has been in use for decades outside the United States. However, use of FDA approved CEUS contrast agents has been limited to echocardiography in the United States until April 2016, when Lumason (Bracco Diagnostics, Inc., Monroe Township, NJ, USA) was approved for adult and pediatric imaging of the liver. There are several advantages of CEUS over contrast-enhanced computed tomography (CT) or magnetic resonance imaging (MRI), including increased enhancement sensitivity due to lack of contrast extravasation outside of the vasculature, lack of contrast nephrotoxicity, lack of ionizing radiation, and the ability to capture real-time imaging[1], [2]. Of these, the lack of nephrotoxicity has made CEUS of particular interest for kidney imaging since CT and MRI contrast agents are sometimes contraindicated in patients with compromised kidney function [3].
During a low mechanical-index CEUS study, wash-in of a bolus of microbubbles into the area of interest is captured and saved as a cine loop [4]. However, this type of non-destructive microbubble imaging only permits visualization of the target area once during contrast wash in. Visualization of another region of interest or a repeat study requires waiting for several minutes until the contrast has cleared circulation and can be re-administered. Alternatively, CEUS studies can also be performed using a continuous infusion of microbubbles and delivering moderate to high MI pulses (0.7–1.9) [5], [6][5], [6], to disrupt the microbubbles and clear the microbubble signal. This technique is referred to as destruction-reperfusion or flash-replenishment. It allows for multiple cycles of contrast wash-in to be achieved in rapid succession, providing the potential to image multiple planes of interest or to repeat visualization of flow dynamics in a target region [5].
As with any pharmaceutical agent or medical device, ensuring safety of the contrast agent is necessary in addition to determining clinical efficacy. In the case of CEUS imaging, there are years of clinical experience with hundreds of thousands of patients that support a negligible amount of severe adverse effects after administration [7]–[10]. However, the scope of clinical data is limited becausehuman histology samples are often not available since biopsies are usually not performed at the time of imaging. Moreover, long-term clinical follow-up has not been collected. In contrast, preclinical studies have investigated bioeffects of CEUS extensively. Previous studies have observed bioeffects in organs like the lungs, liver, intestines, and heart [11]–[15], but for this study, we will limit our focus to the kidney. Prior reports provide evidence that microbubbles exposed to high MI pulses in the kidney, such as those used in flash-replenishment imaging, can lead to ruptured capillaries, particularly the glomerular capillaries. In a series of animal studies in small rodents, published from 2002–2017, Miller et al. and Wible et al. [16]–[23], demonstrated external bruising and glomerular capillary hemorrhage (GCH) in kidneys exposed to a range of frequencies (0.4 to 7.5 MHz), high frame rate (24+ Hz, [18], [20]), and high MI imaging (MI of 1.9) [20], [21].
In contrast, other researchers have provided evidence showing high MI CEUS imaging in the kidney can be performed without evidence of bioeffects. Johnson et al. performed flash-replenishment CEUS in rats at 7.0 MHz and MI of 1.9 [24], and found no GCH. Jimenez et al. [25] investigated CEUS imaging in a porcine model at a mechanical index of 1.9 and a frequency of 1.5 MHz and observed no GCH in this larger kidney model.
Church and Miller [22] proposed that a combination of factors, specifically microbubble size at a certain frequency and pressure threshold, is necessary for the onset of bioeffects, such as GCH and petechial hemorrhage. Their kidney bioeffect model was tested by Miller et al. (2017) and shown to be valid at the frequencies tested—3.6 MHz and 5.5 MHz [23]. Variations in acoustic parameters, including the frequency, pulse repetition frequency of high MI pulses, acoustic focus, contrast dose, and exposure time, may be key factors resulting in the disparate degree of kidney injury in the different studies. In addition, protocol differences, including animal model, total acoustic exposure volume, and time of pathologic assessment make direct comparison of the model developed in Church and Miller [22] to models used in previous studies challenging [24], [25].
The success of CEUS safety in human patients can be attributed to the safety guidelines that are used both in the United States and internationally by leading institutions in ultrasound imaging [13]–[15], [26], which are largely founded on preclinical research. Generally, in regards to concern for mechanical bioeffects from administered contrast agent cavitation, it is recommended to maintain an MI below 0.4 during imaging [13]–[15]. In the Definity package insert[27], which includes FDA approved protocols, Lantheus recommends imaging at an MI of 0.8 or below. It is also noted that factors, such as contrast agent dose, transmit center frequency, peak negative pressure, acoustic exposure time, and transmit pulse repetition frequency, can alter the level of bioeffects observed when imaging above an MI of 0.4 [13], [15].
In light of these recommendations, we believe it is important to conduct bioeffect experimentation to assure safety, when imaging outside of clinical recommendations, taking into account specific imaging acoustic parameters (i.e. transmit frequency, MI, pulse duration, etc.) and accompanying contrast agent parameters (i.e. the formulation and the dosage). We therefore sought to replicate parameters similar to those used in high MI flash-replenishment imaging of humans to determine if these parameters would generate similar injury as seen in preclinical studies, i.e. GCH. Specifically, bioeffects were measured after an imaging sequence that included flash pulses, above an MI of 0.8 and limited to one second in exposure, followed by contrast imaging pulses (MI<0.4). Though the experiment included a pre-clinical dose regimen of contrast agents (much higher dose than is recommended for human use), the study was intended to assess the combination of the flash-sequence and the following CPS imaging. These sequences were repeated across the kidney volume to minimize risk of missing evidence of injury on histology due to sampling error.
We compared flash pulses at two MIs (both destructive to microbubbles): the FDA approved clinical maximum MI for non-contrast ultrasound of 1.9, the acoustic level at which previous studies demonstrated GCH [20], [21], and also a lower MI of 1.0, which is still sufficient to disrupt microbubbles and can therefore be used for flash-replenishment imaging. We hypothesized that injury might be inducible, but that it would be transient and would not cause long-lasting bioeffects.
This study used female Fischer 344 rat kidneys as a model for potential kidney bioeffects induced by imaging sequences that included MI pulses of 1.0 and 1.9 and assessed at 4-hours and 2-weeks post-imaging. Histologic examination of the kidney (gold standard of assessment) was performed by a veterinary expert in nephropathology. Blood urea nitrogen (BUN), obtained prior to CEUS and at time of necropsy, was assessed in combination with histology to determine kidney health.
2. Materials and Methods
2.1. Animal Preparation
All animal procedures were certified by the University of North Carolina at Chapel Hill Animal Care and Use Committee. A total of 31 adult female Fischer 344 rats (150–250g) were used for the study (Charles River Laboratories International, Wilmington, MA, USA). During imaging, animals were anesthetized via nose cone administration of 2% isoflurane (Piramal Enterprises Limited, Mumbai, India) mixed with pure oxygen, flowing at a volumetric rate of 1.0 L/min. A 24-gauge tail vein catheter was inserted, which was used for blood collection and contrast agent injection. Animals were kept warm while under isoflurane anesthesia with a heating pad, and breathing was monitored for the duration of the imaging procedure. For imaging preparation, hair was removed on the right flank of the animal with electric clippers (Pocket Pro Universal Trimmer, Wahl Clipper Corporation, Sterling, IL USA; 2.4oz and 4×2×1 inch in dimension) and hair depilation cream (Nair Hair Removal Lotion, Church & Dwight, Ewing Township, NJ, USA). Ultrasound gel was later applied to couple the transducer to the animal. The right kidney received CEUS imaging, while the contralateral kidney was only exposed to microbubbles. Further details on the imaging procedure can be found in the Imaging Procedure section.
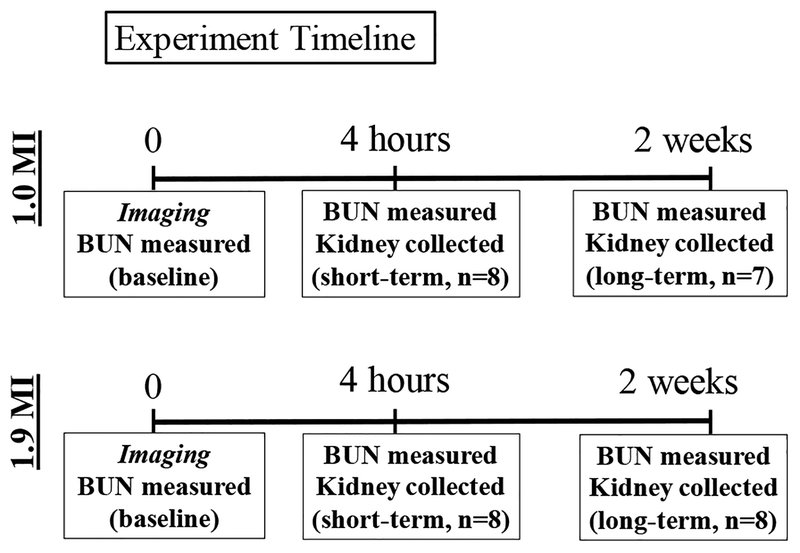
A total of 31 animals was included in the study results. Animals were separated into four groups based on MI exposure and time of assessment of kidney histopathology. All animals were imaged once at either an MI of 1.0 (n=15) or 1.9 (n=16) at time 0. The study consisted of two time points within each MI exposure group—short and long term evaluations. Short term bioeffects were assessed 4 hours post imaging (n=16), while long term bioeffects were assessed 2 weeks post imaging (n=15). Figure 1 depicts a timeline for the study.
Figure 1. Experiment Timeline.
On the first day, animal BUN levels were measured prior to being imaged (t=0). For short term bioeffect assessment, blood and kidneys were collected for BUN and histopathology four hours post imaging. For long term bioeffect assessment, blood and kidneys were collected two weeks after imaging. This timeline was followed for animals exposed to 1.0 MI and 1.9 MI microbubble destructive pulses.
2.2. Microbubble Contrast Agent
FDA approved Definity (Lantheus, North Billerica, MA, USA) was used as the microbubble contrast agent in this study. The vials were activated in a vial shaker for 45 sec prior to use in imaging (Vialmix Shaker, Bristol-Myers Squibb, New York, NY, USA). As per the Definity package insert, activated Definity possesses a mean diameter range from 1.1 μm to 3.3 μm and a maximum concentration of 1.2×1010 particles/mL. The contrast injection was prepared by diluting 200 μL of Definity into 400 μL of 0.9% saline. Prior studies have suggested that increased microbubble concentration may increase the likelihood of bioeffects [12], [18], [19][12], [18], [19]. The dose we used is over 10 times higher than the clinically recommended infusion concentration (1.3 mL of Definity in 50 mL of saline), and over 60 times higher than the recommended double bolus injection (single bolus is 10 μL/kg). We chose this higher concentration in order to test the limits of the parameter range which might be experienced in clinical use.
2.3. Ultrasound System
All imaging was performed on a Siemens/Acuson Sequoia 512 (Mountain View, CA, USA) using a 1–4 MHz 4C1 curvilinear transducer. The 4C1 transducer was chosen for this study as it is a common transducer used in human kidney imaging. B-mode imaging was performed at an MI of 1.5 at a frequency of 3 MHz and a frame rate of 21 Hz. Cadence pulse sequence (CPS) software was utilized for performing CEUS imaging at an MI of 0.21, a frequency of 1.5 MHz, and a frame rate of 14 Hz. The microbubble destruction (MBD) or flash pulse was set on the system and performed at an MI of 1.0 or 1.9, a frequency of 3 MHz, and a frame rate of 10 Hz. The duration of the pulse was set to 1 second. Reperfusion of contrast was captured for 5 seconds before the transducer was stepped to the next plane.
2.4. Full Volume Imaging Procedure
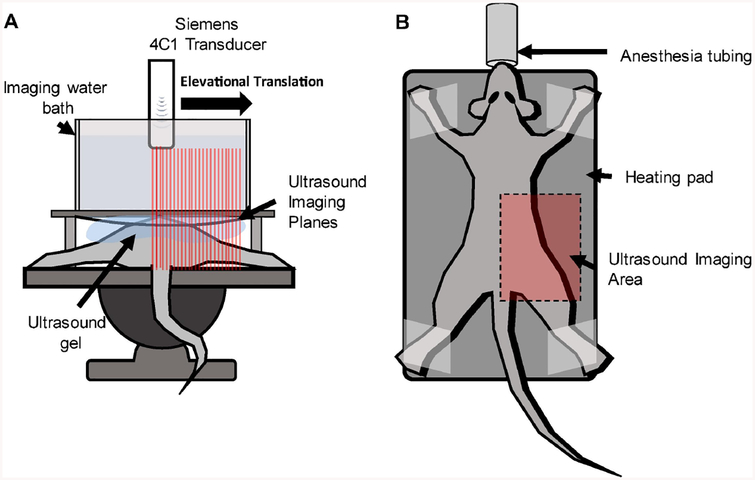
Rats were placed in the prone position and imaged in the sagittal plane (see Figure 2). Ultrasound gel was applied to the imaging area. A custom designed water bath, with an acoustically transparent bottom, was placed on top of the rat so that the ultrasound gel coupled with the bottom of the bath. The bath was filled with water to a height that allowed the focus of the transducer to be set at 60 mm, which is a clinically relevant depth of penetration. As a result of the 4C1 transducer design, and the size of the animal model used, the focus of the transducer covered a majority of the animal’s abdominal depth. The 4C1 transducer was clamped to a 3-axis motion stage and lowered into the water bath.
Figure 2. Imaging Setup.
Animals were positioned such that only one kidney would be exposed to the ultrasound field. A) A water bath was placed above the animal so that the focus of the transducer would align with the kidney. B) The transducer was steered across one side of the animal, while the animal was administered isoflurane through a nose cone and a heating pad maintained a healthy body temperature (35.9–37.5°C).
B-mode imaging was performed and the motion stage was manually manipulated to locate the center of the kidney. Once located, a custom LabVIEW (National Instruments, Austin, TX, USA) program which was synchronized with the ultrasound scanner was used to scan a 1.5 cm region in order to expose the entire kidney volume to the peak ultrasound field. The scan region was restricted to the right side of the rat, and did not pass over the mid-line of the animal, in order to prevent exposure to the left kidney. Once the positioning of the rat and transducer was complete, microbubble injection began in 1.0 mL syringe (Norm-Ject®, Henke-sass Wolf of America, Dudley, MA, USA) at a rate of 40 μL/min (Definity recommends 4.0 mL/min in humans [27]), which was kept constant with a syringe pump (Pump11, Harvard Apparatus, Houston, MA, USA). After contrast arrival was detected in CPS mode, the 3D flash-reperfusion sequence was initiated.
The custom LabVIEW program (Texas Instruments, Dallas, TX, USA) was designed to mechanically move the transducer in the elevational dimension at a step size of 1 mm. A 1 mm step size was chosen to ensure that every region of the kidney was exposed to the MBD pulse at least twice. The elevational beam width of the transducer is ~2mm, so there was significant overlap of the high energy pulse at each location. At each position, the scanner was triggered by the LabVIEW program to output the MBD pulse, and then wait 5 seconds for sufficient contrast reperfusion. Each 3D scan lasted approximately 2 minutes, with a total infused volume of approximately 80–100 μL. Once complete, animals were continually monitored and kept warm until awake from anesthesia, at which point they were placed back into their respective cages.
2.5. Kidney Blood Urea Nitrogen Chemistry Analysis
Serum BUN levels were used to observe kidney function prior to imaging and at the final time point. Blood preparation included separating the serum using a centrifuge at 1,000–2,000 × g for ~10 mins. Serum was stored in a −25°C freezer, and submitted to the UNC Animal Clinical Chemistry and Genetic Expression Laboratory for BUN analysis. Changes in BUN levels for each experimental group were assessed using paired T-test through MATLAB (MathWorks, Natick, MA, USA).
2.6. Histology
At the designated time point, animals were humanely euthanized, and both kidneys were immediately collected. Kidneys from the animals were fixed using formalin for 4–5 days (Azer Scientific, Morgantown, PA, USA) and then stored in 70% ethanol (Decon Labs, King of Prussia, PA, USA). The kidneys were routinely embedded in paraffin, sliced to 5 μm, and stained with hematoxylin and eosin (H&E) by the UNC Animal Histopathology Core. Three slices were chosen randomly by professionals in the histopathology core, and placed on a glass slide. Bioeffects evidenced in the kidney slices were blindly assessed by a veterinary nephropathologist (REC). Quantitative analysis included counting the total red blood cell (RBC) cast score. In this assessment, the number of RBC casts (including fragmented and intact RBCs in tubular lumens) were counted in ten sequential fields of view, across all three slices, at 10× magnification which allowed examination of the entire kidney cortex. The presence of these elements was taken as a sign of hemorrhage in the tissue prior to necropsy. The total value across the kidney sections is referred to as the total RBC cast score, and the average for each group is presented. Statistical assessment was also conducted in MATLAB. Additionally, 100 glomeruli were evaluated per kidney and any glomerulus with GCH (red blood cells within Bowman’s space) was counted.
3. Results
3.1. Kidney Clinical Chemistry at 4-hours and 2 weeks post imaging
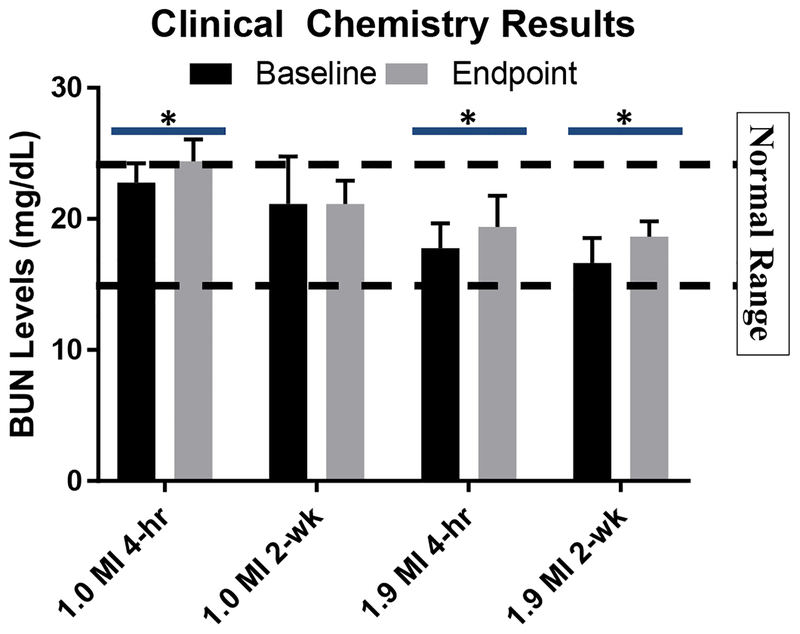
BUN levels 4 hours post imaging, in both the 1.0 and 1.9 MI groups, were significantly different from their baseline measurements (see Figure 3). Normal range of BUN levels for female Fischer rats is 19.18 ± 2.39 mg/dL (95% confidence internal of ~15–24 mg/dL) [28]. The 1.0 MI group increased from 23 ± 1 mg/dL to 24 ± 1 mg/dL (P=0.01), which is at the upper limit of the normal range. This reflects a mean percent change of 7%. The 1.9 MI group increased from 18 ± 1 mg/dL to 19 ± 1 mg/dL (P=0.02). Though this group began with a lower BUN than the animals in the 1.0 MI group, the mean percent change for the 1.9 MI group was 9%, similar to the 1.0 MI group.
Figure 3. Clinical Chemistry Results.
Average baseline and endpoint data collected for each group were analyzed via paired T-test. The BUN levels after 2 weeks in the 1.9 MI pulse group were significantly higher than baseline levels. In addition, BUN levels of both groups analyzed at 4 hours were also found to be statically significant. P-values between 0.01 and 0.05 are indicated with the symbol ‘*’. Normal BUN range is indicated with dashed lines (15 to 24 mg/dL).
BUN levels 2 weeks post imaging in the 1.0 MI group showed no significant change. Average BUN levels in the 1.9 MI group 2 weeks post imaging increased from 17 ± 1 mg/dL to 19 ± 1 mg/dL (P=0.04), which reached statistical significance. This reflects a mean percent change of 12%. Except for the 1.0 MI 4-hour group, BUN levels remained well within normal range.
3.2. Histopathology at 4-hours and 2-weeks
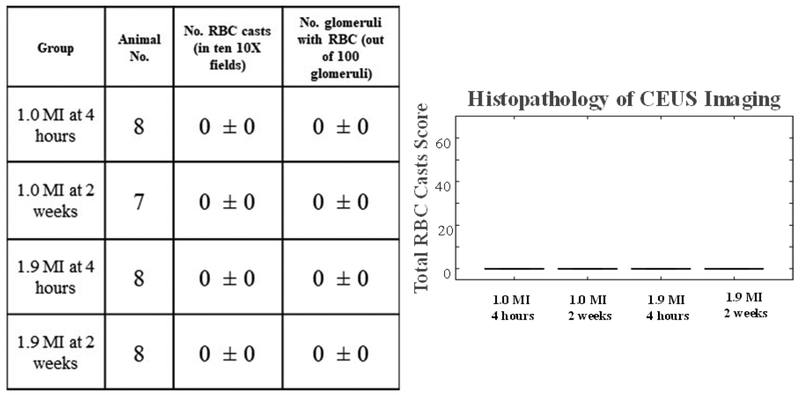
Histopathologic review of the kidneys at their final time points revealed no evidence of hemorrhage with any type of pulse transmitted at any time point (see Figure 4).
Figure 4. Histopathology Results.
No bioeffects were observed after 1.0 and 1.9 MI pulse or at either time point.
4. Discussion
In this study, we utilized clinically relevant acoustic parameters for CEUS imaging in order to investigate potential in vivo bioeffects. Given our results, it is important to consider variations in the protocol used to arrive at our findings, compared to previously published work. First, our experimental methods involved a volumetric scan of the kidney with 1mm steps such that each plane of imaging received at most 2 seconds of flash pulses as opposed to singular plane assessment used by Miller et al. [16], [18], [20], [21], [23] and Jimenez et al.(2008) [25], in which all flashes were administered in the same plane. The imaging parameters used in this study (center frequency, frame rate, pulse repetition frequency, number of flash pulses per imaging plane, etc.) are more relevant for assessing what is clinically performed, as opposed to multiple (>5) seconds of flash pulses in a single plane which is more useful for determining the minimal threshold for bioeffects to occur. Contrary to this idea, we chose several imaging parameters with the intention of increasing the probability of inducing bioeffects, including increased microbubble dose and flow rate (compared to the clinical recommendation), and a 5 second wait time following flash pulses to increase contrast presence. This was done to focus observations on the clinical acoustic parameters, by testing them in an environment likely to develop bioeffects, as evidenced by previously cited preclinical kidney investigations, where Miller et al. showed that increasing the contrast agent concentration increases the presence of bioeffects [18].
Second, histopathologic assessment was conducted on a subset of kidney tissue, rather than the entire kidney, for each subject. Even though we only examined a subset of kidney tissue, because we performed a volumetric scan, any subset of tissue assessed would have been exposed to flash-perfusion imaging. To provide another measure of kidney function, we also quantified BUN, as a surrogate marker of whole kidney function at the same time points.
In regards to our findings, we observed some similarities to previous work. At the shortest time point, 4 hours post imaging, we observed no tubular injury or hemorrhage whether the kidney received an MI of 1.9 or 1.0, consistent with Jimenez et al. and Johnson et al. [24], [25]. However, we observed a mild elevation in BUN levels for both groups. Although BUN levels remained in the normal range, the statistically significant increase in BUN levels suggest the possibility of some bioeffects not associated with visible hemorrhage. BUN is a non-specific marker of kidney injury and can be elevated due to a number of factors including high-protein diet, liver disease, and gastrointestinal bleed. In addition, we used the 4C1 probe, which is designed for human imaging, and given our model was a rat, we were unable to limit CEUS imaging to just the rat kidney so adjacent abdominal organs likely received the high MI pulses, which may also have contributed to the small increase in BUN. Histopathologic assessments for this study were conducted in the kidney only. It is also plausible that the elevation in BUN at 4 hours might have been a product of dehydration while recovering from the anesthesia during imaging or a direct result of anesthesia itself which can cause vasodilation and low blood pressures with resultant temporary decrease in organ perfusion.
A key difference between our study and many other previous publications is the inclusion of the longer time point. Miller et al. (2009) showed a decrease in histologic injury from 4 hours to 2 days post exposure to 1.9 MI pulses. By weeks 1 and 4, the location of imaging was difficult to find, indicating some recovery of the earlier seen injury. Histology did indicate signs of inflammation at the 1 week time point, and at 4 weeks, signs of fibrosis were found [20]. Similarly, at 2 weeks, we observed a statistically significant elevation in BUN levels, although still in the normal range, for the 1.9 MI but not the 1.0 MI group. Despite the increase in BUN in the 1.9 MI group, we found no evidence of RBC casts or other findings consistent with acute or persistent chronic injury via histologic examination. Based on these findings, we are unable to identify the cause of the increase in BUN in the absence of hemorrhage and cannot definitively state that the rise in BUN is kidney related. In a pilot study conducted by the authors in order to determine experimental parameters, RBC casts were found in the tubules of only one group of the pilot study (n=4), the group receiving 1.9 MI pulses assessed 24-hours post imaging (see Supplemental Information published in Data in Brief). The pilot differed from the study presented in three ways: (1) contrast imaging following flash pulses lasted for 1 sec, instead of 5 sec, (2) animals were positioned on their side, exposing both the experimental and control kidneys to the CEUS imaging, and (3) short term bioeffects were assessed 24 hours post-imaging. After observing hemorrhaging in the 1.9 MI group at 24 hours time point was changed to 4 hours to assess if the RBC casts orginated in the tubules, or formed in the glomeruli and migrated to the tubules over time. Additoinally, with more contrast imaging time, bubbles circulating would have more time to fill the field of view before transmitting the next flash pulse.
In this pilot study, the hemorrhage was not accompanied by a significant increase in BUN. This could be explained by a low experimental population. It is important to note that both studies demonstrated an increase in BUN levels 2 weeks post imaging. This could be indicative of a potential bioeffect in the clinical chemistry assessment 2 weeks post imaging that the histology assessment did not demonstrate 4 hours post imaging. One explanation for histology differences in the two studies may be a sampling error. A small subset of kidney tissue is being analyzed, in comparison to the total kidney volume. In anticipation of this fact, clinical chemisty was conducted to gather global kidney fuction. When combining the results of both the main and pilot study, it can be concluded that both studies show some sort of bioeffect (chemical or histologic) after exposure to 1.9 MI flashes. In the case of 1.0 MI, no histological evidence was ever found in either study. There was only the potential for kidney-related bioeffects shown in the BUN increase at 4 hours. Regardless of the study, no indications of bioeffects were found in the 1.0 MI group being assessed 2 weeks after imaging. This indicates an acute but transient nature to microbubble-mediated CEUS bioeffects, and if there is low level injury occurring at an MI of 1.0 shortly after imaging, it can be avoided by implementing even lower MI MBD pulses during CEUS imaging (0.7 to <1.0).
We believe these findings can be used to guide clinical imaging parameter decisions in order to minimize potential kidney bioeffects. Using a clinical system and probe, clinically-approved microbubble agents, and imaging parameters similar to those that would be used clinically, we were able to assess the potential for bioeffects resulting from flash-replenishment CEUS of the rodent kidney. When considering translation of CEUS imaging parameters to human subjects, it is important to consider that although we focused the transducer at a clinically relevant depth of 6 cm, the ultrasound beam only traveled through less than 1 cm of attenuating tissue, in contrast to a clinical scenario where the ultrasound beam is attenuated by 6 cm or more of abdominal tissue. The MI value we used was based on the derated peak-negative-pressure transmitted to the focus of the transducer. It is likely that in a human patient, even less pressure will reach the focus, compared to the experimented rodent kidney models. Potentially, a given transmitted MI will generate lower levels of injury in human patients than what was observed in the presented study.
5. Conclusions
Microbubble destruction induced bioeffects during CEUS imaging were observed to be associated with mild elevations in BUN levels and, in the case of our pilot study, histologic indications of hemorrhaging in kidney tubules. The histologic signs of bioeffects were transient, and the BUN levels remained predominantly within the normal range for the female Fischer rat. Most notably, both of these indications were manageable by implementing lower MI pulses (1.0). By maintaining an MI of 1.0 for MBD pulsing, we were able to eliminate short-term serum elevations in BUN after 2 weeks of recovery. Because there were mild but significant increases in BUN at the 2-week time point in the 1.9 MI group, and kidney injury is one of several potential causes, we suggest that until the possibility of persistent kidney effects can be completely eliminated, this be avoided by operating at a lower MI (such as 1.0 or less) when using MBD pulses for flash-replenishment imaging.
Highlights.
The effects of contrast enhanced ultrasound imaging on the kidney were assessed
Kidney was exposed to microbubble-destructive pulses across the volume of the kidney
Effects on the kidney were measured by blood urea nitrogen and histopathology
No histologic evidence of bioeffects were observed
Insignificant changes in blood urea nitrogen were observed after 2 weeks when an MI of 1.0 was used.
Acknowledgements
The authors would like to thank the staff of the UNC Animal Histopathology Core and UNC Animal Clinical Chemistry Core for their work with this project. Animal histopathology was performed in the LCCC Animal Histopathology Core Facility at the University of North Carolina at Chapel Hill with special assistance from Traci Raley and Amanda Brown. The LCCC Animal Histopathology Core is supported in part by an NCI Center Core Support Grant (2P30CA016086–40) to the UNC Lineberger Comprehensive Cancer Center. Gloria Nyankima was supported by the NIH F31 grant (5 F31 CA206602–03) during the time of the study. This work was partially funded by the North Carolina Translational & Clinical Sciences Institute via the NC TraCs grant IHHAR11503. The project described was also supported by the National Center for Advancing Translational Sciences (NCATS), National Institutes of Health, through Grant Award Number TraCs UL1TR002489. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Conflict of Interest
E. Chang is supported by a research grant (CG#16013) sponsored by Lantheus Medical Imaging, the distributor of the Definity microbubbles used in this study, though she was not supported during the time of the study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could appect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Tenant SC and Gutteridge CM, “The clinical use of contrast-enhanced ultrasound in the kidney,” Ultrasound, vol. 24, no. 2. pp. 94–103, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Wilson SR, Greenbaum LD, and Goldberg BB, “Contrast-enhanced ultrasound: What is the evidence and what are the obstacles?,” American Journal of Roentgenology, vol. 193, no. 1 pp. 55–60, 2009. [DOI] [PubMed] [Google Scholar]
- [3].Chang EH, Chong WK, Kasoji SK, Dayton PA, and Rathmell WK, “Management of Indeterminate Cystic Kidney Lesions: Review of Contrast-enhanced Ultrasound as a Diagnostic Tool,” Urology, vol. 87 pp. 1–10, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Kasoji SK, Chang EH, Mullin LB, Chong WK, Rathmell WK, and Dayton PA, “A Pilot Clinical Study in Characterization of Malignant Renal Cell Carcinoma Subtype with Contrast-Enhanced Ultrasound.,” Ultrason. Imaging, pp. 1–11, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Feingold S, Gessner R, Guracar IM, and Dayton PA, “Quantitative volumetric perfusion mapping of the microvasculature using contrast ultrasound,” Invest. Radiol, vol. 45, no. 10, pp. 669–674, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Wei K, Le E, Bin J-P, Coggins M, Thorpe J, and Kaul S, “Quantification of renal blood flow with contrast-enhanced ultrasound,” J. Am. Coll. Cardiol, vol. 37, no. 4, pp. 1135–1140, 2001. [DOI] [PubMed] [Google Scholar]
- [7].Piscaglia F et al. , “The safety of Sonovue® in abdominal applications: Retrospective analysis of 23188 investigations,” Ultrasound Med. Biol, vol. 32, no. 9, pp. 1369–1375, 2006. [DOI] [PubMed] [Google Scholar]
- [8].Feinstein SB et al. , “Contrast enhanced ultrasound imaging,” J. Nucl. Cardiol, vol. 17, no. 1, pp. 106–115, 2010. [DOI] [PubMed] [Google Scholar]
- [9].Wei K et al. , “The Safety of Definity and Optison for Ultrasound Image Enhancement: A Retrospective Analysis of 78,383 Administered Contrast Doses,” J. Am. Soc. Echocardiogr, vol. 21, no. 11, pp. 1202–1206, 2008. [DOI] [PubMed] [Google Scholar]
- [10].Abdelmoneim SS et al. , “Safety of Contrast Agent Use During Stress Echocardiography. A 4-Year Experience From a Single-Center Cohort Study of 26,774 Patients,” JACC Cardiovasc. Imaging, vol. 2, no. 9, pp. 1048–1056, 2009. [DOI] [PubMed] [Google Scholar]
- [11].Miller DL and Gies RA, “Gas-body-based contrast agent enhances vascular bioeffects of 1.09 MHz ultrasound on mouse intestine,” Ultrasound Med. Biol, vol. 24, no. 8, pp. 1201–1208, 1998. [DOI] [PubMed] [Google Scholar]
- [12].Miller DL, Gies RA, and Chrisler WB, “Ultrasonically induced hemolysis at high cell and gas body concentrations in a thin-disc exposure chamber,” Ultrasound Med. Biol, vol. 23, no. 4, pp. 625–633, 1997. [DOI] [PubMed] [Google Scholar]
- [13].Fowlkes JB, “American Institute of ultrasound in medicine consensus report on potential bioeffects of diagnostic ultrasound: Executive summary,” J. Diagnostic Med. Sonogr, 2011. [DOI] [PubMed] [Google Scholar]
- [14].ter Haar G, “Safety and bio-effects of ultrasound contrast agents,” Med Biol Eng Comput, vol. 47, no. 8, p. 893–900. Epub 2009 Jul 14., 2009. [DOI] [PubMed] [Google Scholar]
- [15].Dalecki D, “WFUMB safety symposium on echo-contrast agents: In vitro bioeffects,” Ultrasound Med. Biol, 2007. [DOI] [PubMed] [Google Scholar]
- [16].Wible JH, Galen KP, Wojdyla JK, Hughes MS, Klibanov AL, and Brandenburger GH, “Microbubbles induce renal hemorrhage when exposed to diagnostic ultrasound in anesthetized rats,” Ultrasound Med. Biol, vol. 28, no. 11–12, pp. 1535–1546, 2002. [DOI] [PubMed] [Google Scholar]
- [17].Miller DL, “Overview of experimental studies of biological effects of medical ultrasound caused by gas body activation and inertial cavitation,” Prog. Biophys. Mol. Biol, vol. 93, no. 1–3, pp. 314–330, 2007. [DOI] [PubMed] [Google Scholar]
- [18].Miller DL, Dou C, Wiggins RC, Wharram BL, Goyal M, and Williams AR, “An in vivo rat model simulating imaging of human kidney by diagnostic ultrasound with gas-body contrast agent.,” Ultrasound Med. Biol, vol. 33, no. 1, pp. 129–35, 2007. [DOI] [PubMed] [Google Scholar]
- [19].Miller DL, Averkiou MA, Brayman AA, Everbach EC, Holland CK, and Wu J, “Bioeffects considerations for diagnostic ultrasound contrast agents,” J. Ultrasound Med, vol. 27, no. 4, pp. 611–632, 2008. [DOI] [PubMed] [Google Scholar]
- [20].Miller DL, Dou C, and Wiggins RC, “Glomerular Capillary Hemorrhage Induced In Rats By Diagnostic Ultrasound With Gas-Body contrast Agent Produces Intratubular Obstruction,” Ultrasound Med. Biol, vol. 35, no. 5, pp. 869–877, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Miller DL, Dou C, and Wiggins RC, “In vivo gas body efficacy for glomerular capillary hemorrhage induced by diagnostic ultrasound in rats,” IEEE Trans. Biomed. Eng, vol. 57, no. 1, pp. 167–174, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Church CC and Miller DL, “A Two-Criterion Model for Microvascular Bio-Effects Induced In Vivo by Contrast Microbubbles Exposed to Medical Ultrasound,” Ultrasound Med. Biol, vol. 42, no. 6, pp. 1385–1398, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Miller DL, Lu X, Dou C, Fabiilli ML, and Church CC, “The Dependence of Glomerular Capillary Hemorrhage Induced by Contrast Enhanced Diagnostic Ultrasound on Microbubble Diameter,” Ultrasound Med. Biol, no. 1998, pp. 1–9, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Johnson K, Cianciolo R, Gessner RC, and Dayton P. a., “A Pilot Study to Assess Markers of Renal Damage in the Rodent Kidney After Exposure to 7 MHz Ultrasound Pulse Sequences Designed to Cause Microbubble Translation and Disruption,” Ultrasound Med. Biol, vol. 38, no. 1, pp. 168–172, Jan. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Jiménez C et al. , “In situ kidney insonation with microbubble contrast agents does not cause renal tissue damage in a porcine model.,” J. Ultrasound Med, vol. 27, no. 11, pp. 1607–15, 2008. [DOI] [PubMed] [Google Scholar]
- [26].Blomley M, Claudon M, and Cosgrove D, “WFUMB safety symposium on ultrasound contrast agents: Clinical applications and safety concerns,” Ultrasound Med. Biol, 2007. [DOI] [PubMed] [Google Scholar]
- [27].Lantheus Medical Imaging, “DEFINITY,” 2015. [Google Scholar]
- [28].Derelanko MJ and Hollinger M. a, Handbook of Toxicology, vol. 124, no. 14 2002. [Google Scholar]