Abstract
After exposure to a traumatic event, a subset of people develop posttraumatic stress disorder (PTSD). One of the key deficits in PTSD is regulation of fear, and impaired inhibition of fear-potentiated startle has been identified as a potential physiological biomarker specific to PTSD. As part of a larger clinical trial, this study investigated the effects of a CRF receptor 1 antagonist, GSK561679, on inhibition of fear-potentiated startle during a conditional discrimination fear-conditioning paradigm, termed AX+/BX-. Prior research using this paradigm has demonstrated deficits in inhibition of conditioned fear in several PTSD populations. The randomized, double-blind, placebo-controlled clinical trial compared fear inhibition between female PTSD participants taking 350 mg/day GSK561679 (n = 47 pre, and 29 post treatment) and patients taking a placebo pill (n = 52 pre, and 30 post treatment) daily for six weeks. There was no significant difference between the two groups in their acquisition of fear or discrimination between threat and safety cues, and no pre-post treatment effect on these measures. However, there was a significant effect of treatment on inhibition of FPS during the AB trials in the AX+/BX- transfer test (p < 0.05). While all PTSD participants showed typical impairments in fear inhibition prior to treatment, GSK561679 enhanced fear inhibition post-treatment, independent of clinical effects. The current study suggests that CRF receptor 1 antagonism may have specific effects within neural circuitry mediating fear inhibition responses, but not overall symptom presentation, in PTSD.
1. Introduction
Posttraumatic stress disorder (PTSD) is a debilitating psychiatric disorder that can develop in individuals who experience a severe or life-threatening event, such as combat, assault, or a natural disaster. PTSD symptoms include re-experiencing the event, avoidance of reminders, negative affect and cognitions, and increased reactivity and irritability. While this is a heterogeneous clinical disorder, it has been shown to be associated with physiological measures of fear expression and fear inhibition (Jovanovic & Norrholm, 2016). These measures can be captured experimentally, providing intermediate phenotypes which can serve as biomarkers of treatment effects. Psychophysiological measures such as skin conductance, heart rate, and startle response have been successfully used as measures of treatment effects for psychotherapy (Robison-Andrew et al., 2014; Rothbaum et al., 2014; Wangelin & Tuerk, 2015). However, fear inhibition has not previously been used to assess the biological effects of treatment.
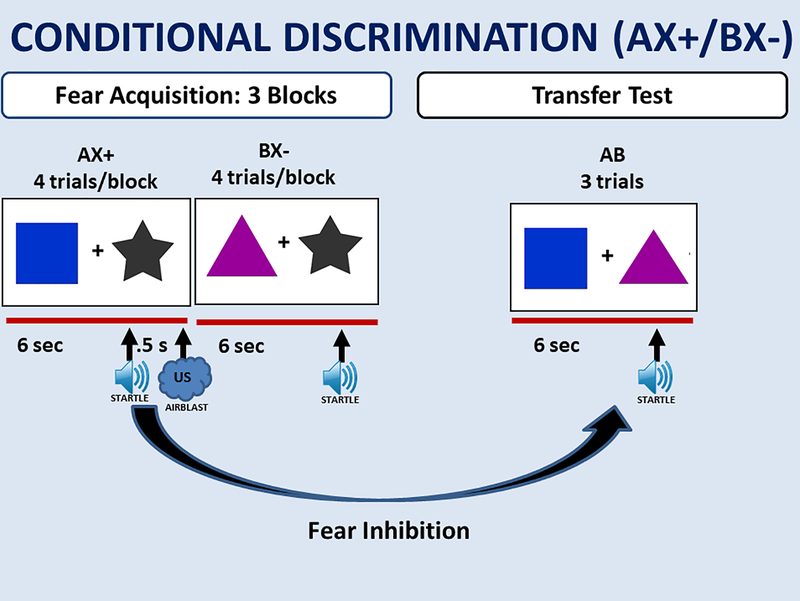
Fear and anxiety can easily be measured in humans using psychophysiological tools. The present study utilized the acoustic startle response during fear conditioning to assess fear-related phenotypes. The startle response is increased in the presence of fear eliciting stimuli, known as fear-potentiated startle, which can be measured across mammalian species (Myers et al., 2009). Fear-potentiated startle (FPS) has been shown to be associated with PTSD in a number of fear conditioning paradigms (Grillon & Morgan, 1999; Jovanovic, Norrholm, Blanding, Davis, et al., 2010; Jovanovic et al., 2009; Morgan, Grillon, Southwick, Davis, & Charney, 1995). FPS can be used in Pavlovian fear conditioning in which a conditioned stimulus (CS) comes to predict the occurrence of an aversive unconditioned stimulus (US). This conditioned fear response can be inhibited in paradigms involving safety signal learning, discrimination between cues predicting threat and safety, or fear extinction; these processes are often impaired in anxiety disorders, including PTSD (Duits et al., 2015; Jovanovic & Ressler, 2010; Norrholm et al., 2011). The ability to inhibit FPS in the presence of safety can be tested using a conditional discrimination paradigm, termed AX+/BX-, in which safety (B) transfers inhibitory properties to a threatening cue (A), reducing fear to a compound of the two stimuli (AB), Figure 1. This paradigm is adapted from an animal model of fear inhibition and attempts to disentangle fear potentiation and fear inhibition (Myers & Davis, 2004), and has pointed to impaired fear inhibition as a potential physiological biomarker specific to PTSD, relative to comorbid depression, in combat and civilian cohorts (Jovanovic, Kazama, Bachevalier, & Davis, 2012; Sijbrandij, Engelhard, Lommen, Leer, & Baas, 2013). A study using this paradigm found a positive correlation between levels of hypothalamus-pituitary-adrenal (HPA) axis hormones and FPS in PTSD patients (Jovanovic, Norrholm, Blanding, Phifer, et al., 2010). These data indicate a direct relationship between these neuroendocrine factors and startle, such that manipulation of the HPA axis could alter these psychophysiological responses. As such, fear inhibition could be mediated by PTSD treatments targeting the HPA axis.
Figure 1.
AX+/BX- Schematic representation of experimental design. Fear inhibition is tested by comparing the AB test trials to AX+ trials during acquisition. Inter-trial and inter-block intervals were randomized between 9 – 22 seconds as in previous work (e.g., Jovanovic et al., 2005).
Currently, pharmacological treatment for PTSD is limited, and very little progress has been made over the last decade as no new medications have shown convincing efficacy based on the most recent Institute of Medicine report (Medicine, 2014). According to published guidelines, selective serotonin reuptake inhibitors (SSRIs) are the first-line pharmacological agents used to treat PTSD, but the magnitude of the treatment effect of SSRIs for PTSD is debated (Forbes et al., 2010). Possible new alternatives to current medications include drugs that target components of the HPA axis, particularly corticotropin releasing factor (CRF), which could also impact fear inhibition. PTSD patients have higher levels of CRF, both in blood plasma and in cerebrospinal fluid (Baker et al., 1999; Bremner et al., 1997; De Kloet et al., 2007). Animal studies have shown that administration of a CRF receptor antagonist reduces freezing in rodents after a fear-conditioning paradigm (Deak et al., 1999). More specifically, mice with a silenced CRF receptor 1 gene display a decreased biological and physiological anxiety response during a physical-restraint stressor (Smith et al., 1998). A single nucleotide polymorphism (SNP) in same CRFR1 gene in humans, rs110402, has been implicated in patients with PTSD who have been exposed to childhood trauma (Bradley et al., 2008). Suppression of the HPA axis with a synthetic glucocorticoid, dexamethasone, also reduces exaggerated FPS (Jovanovic et al., 2011) and facilitates fear extinction (Michopoulos et al., 2017) in PTSD. Further, successful response to the administration of hydrocortisone to veterans suffering from PTSD has been related to glucocorticoid sensitivity (Yehuda et al., 2015). Collectively, these studies suggest a specific role of the CRF system in regulation of fear and anxiety related symptoms of PTSD.
A novel CRF1 receptor 1 antagonist, GSK561679, likely affects HPA axis, fear, and anxiety. Recent clinical trial data suggest that it may not be effective in targeting PTSD symptoms in all patients; however, in a subset of patients carrying the risk SNP in CRFR1 rs110402 gene who also had high levels of childhood trauma, GSK561679 significantly reduced PTSD symptoms compared to placebo (Dunlop et al., 2017). Therefore, it is possible that this drug has beneficial effects on trauma-related neurobiology, but it is unclear whether it alters psychophysiological measures of fear in PTSD. The purpose of the current study was to examine if the administration of GSK561679 during a six-week clinical trial in adults with PTSD would improve inhibition of FPS to safety signals, given that this is a frequently observed deficit in PTSD (Duits et al., 2015; Jovanovic & Ressler, 2010), and most likely treatment target. In addition, the mediating role of CRF receptor 1 antagonism in fear inhibition was examined using GSK561679. Utilizing the above task which is designed to specifically test fear inhibition, it was hypothesized that GSK561679 would increase inhibition from pre- to post-treatment compared to placebo.
2. Method
This experiment was part of a larger double-blind, randomized, placebo-controlled clinical trial of the investigational CRF1 receptor 1 antagonist GSK561679 (Clinicaltrials.gov: NCT01018992) that performed at four academic sites (Dunlop et al., 2017; Dunlop et al., 2014). The present study examined the FPS data from a pre-treatment baseline and post-treatment assessment of the six-week clinical trial. The clinical trial was comprised of a screening phase, a six-week double-blind treatment phase of 350 mg per os nightly of GSK561679 or matching placebo, and a one-month follow-up phase. During the treatment phase, participants completed four post-randomization visits at weeks 1, 2, 4, and 6 that included clinician-administered assessments of symptom severity and assessment of adverse events. Participants underwent the AX+/BX- conditional discrimination paradigm pre- and post-treatment, using different colored stimuli at each visit. All participants provided written informed consent approved by the Institutional Review Board (IRB) of each participating institution.
2.1. Participants
Startle data were available for 99 participants (from the overall parent study sample of 128) and were included in this study (n=47 were in the drug condition and n=52 were in the placebo condition). All participants were female, aged 18 to 65, with chronic PTSD. Men were excluded due to concerns about potential toxicity of GSK561679 to the male reproductive tract. Chronic PTSD was defined by meeting the Diagnostic and Statistical Manual of Mental Disorders, 4th Edition (DSM-IV-TR) criteria for current PTSD for a duration of at least three months as assessed via the Structured Clinical Interview for DSM-IV (First, Spitzer, Gibbon, & Williams, 1995). Participants were recruited and tested at Emory University, Baylor College of Medicine, Icahn School of Medicine at Mount Sinai, and the University of California San Francisco/San Francisco Veterans Affairs Medical Center. Detailed study design, including the full inclusion and exclusion criteria have been previously published (Dunlop et al., 2014). To be eligible for the trial, patients had to demonstrate at least moderate severity of PTSD, assessed as a score of ≥50 on the Clinician Administered PTSD Scale for DSM-IV (CAPS, Blake et al., 1995) at both the screening and baseline visits. Important exclusion criteria included current or past psychotic disorder, bipolar disorder, or obsessive compulsive disorder; current substance abuse or dependence; current use of a psychotropic or a steroid medication; current clinically significant suicidal or homicidal ideation; or any current or planned litigation regarding the traumatic event. In addition, participants were screened for hearing loss using an audiometer and excluded from the startle procedure if they did not detect pure tones ranging from 500–4000 Hz at a 35 dB threshold in each ear.
2.2. Drug Treatment
Participants were randomized to GSK561679 or placebo in a 1:1 ratio. The randomization sequences were separate for each site, and generated by a statistician not involved in the data analysis. Participants were instructed to take 350mg/day or matched pill placebo each evening between 6pm and 8pm and make a record in their daily dosing diary. All participants assigned to the GSK561679 arm included in the current study demonstrated compliance with the study medication based on detectable serum levels at week 6.
2.3. Experimental Design
The AX+/BX- conditional discrimination paradigm was used to assess FPS and fear inhibition (Jovanovic et al., 2005), see Figure 1. During the fear acquisition phase of the paradigm, the CS was a simultaneous presentation of two shapes on a computer monitor, one of the shapes was either A or B, and the other was X. The contingency of the X shape was conditional on the presence of A or B in the compound. The AX+ compound was the threatening conditioned stimulus (CS+), and was always reinforced with the aversive US, which was a 250ms airblast with an intensity of 80 psi directed to the larynx. The BX- shape combination was never paired with the US, making it the non-reinforced CS-. The color and shape of A and B differed, but the “X” cue remained the same across both presentations. The acquisition phase consisted of three blocks of 4 trials of AX+, BX-, and noise alone (NA) trials. Immediately after the acquisition phase, fear inhibition was assessed during a transfer test, during which the previously conditioned A and B cues were presented together to as an AB compound. The transfer test included only three AB trials, in order to minimize extinction effects, and capture immediate transfer of safety. The stimuli were presented on the screen for 6 seconds and the inter-trial intervals (ITIs) as well as inter-block intervals were randomized in duration ranging from 9 to 22 seconds.
2.4. Fear-Potentiated Startle Data Acquisition
As in our previous studies of FPS, the startle response was measured using electromyogram (EMG) of the right orbicularis oculi (eyeblink) muscle with two 5-mm Ag/AgCl electrodes filled with electrolyte gel. One electrode was placed 1 cm below the pupil and the second one was placed immediately lateral to the first one. A ground electrode was placed on the mastoid bone. All impedances were measured using a checktrode meter, and were ≤6 kilo ohms. The startle response data was acquired using Biopac MP150 for Windows (Biopac Systems, Inc., Goleta, CA) as described in our previous studies. All data was sampled at 1000 Hz and amplified with a gain of 5000 using the EMG module of the Biopac system. The acquired data were rectified and filtered between 28 Hz and 500 Hz, with a 60 Hz notch filter, using MindWare software (MindWare Technologies, Ltd., Gahanna, OH) and exported for statistical analyses. Startle magnitude of the eyeblink muscle contraction 20–200 ms after presentation of the startle probe was used as a measure of the acoustic startle response. The startle probe was a 108-dB (A) SPL, 40ms burst of broadband noise with near instantaneous rise time, delivered binaurally through headphones. The A, B, and X shapes were presented on a computer monitor 3 feet in front of the participant using Superlab 4.0 presentation software (Cedrus, Inc., San Pedro, CA). A response pad (RB730, Cedrus, Inc.) was included to assess contingency awareness during the experiment.
2.5. Data Analysis
Fear acquisition was examined using a repeated-measures analysis of variance (RM-ANOVA) comparing the average startle magnitude on NA trials to the average startle magnitude to AX+ trials during the last block of the acquisition phase. To further assess fear conditioning, discrimination was analyzed using a RM-ANOVA comparing AX+ and BX- in the last block of acquisition. 2 × 2 RM-ANOVAs tested a within-subjects factor of treatment (pre and post), a between groups factor of condition (GSK561679 and placebo), and covariates of site and NA startle response averaged across all noise alone trials in acquisition pre and post treatment.
Fear inhibition was tested by comparing startle magnitude to AX+ trials during the acquisition phase to startle magnitude to AB trials in the transfer test. We used a RM-ANOVA, with a within-subjects factor of treatment (pre and post), a between groups factors of condition (GSK561679 and placebo), and covariates of site and NA startle response across all trials during pre and post treatment in order to control for individual variability in startle response at each visit. All analyses were conducted in SPSS 24 for Windows (IBM, Armonk, NY), with alpha set at 0.05.
3. Results
Data from 59 participants, obtained from the parent study sample of 96 completers (Dunlop et al., 2017), were available post treatment (N = 29 drug condition and N = 30 placebo) and were included in the final sample. Demographic data from participants are shown in Table 1. The GSK561679 and placebo group did not differ in age, race, degree of childhood trauma (as measured by Childhood Trauma Questionnaire), depression, or PTSD symptom severity on the CAPS pre or post treatment.
Table 1.
Demographic and clinical data for the sample
| GSK (n=29) | PLACEBO (n=30) | p | |
|---|---|---|---|
| Age (M, SD) | 40.75 (11.68) | 41.87 (11.99) | NS |
| Race (%) | NS | ||
| African American/Black | 34.50% | 46.70% | |
| White | 58.60% | 46.70% | |
| Other | 6.90% | 6.60% | |
| PTSD severity pre-treatment (M, SD) | 77.00 (14.08) | 75.46 (20.22) | NS |
| PTSD severity post-treatment (M, SD) | 63.26 (19.77) | 50.76 (27.75) | NS |
| Depression severity pre-treatment (M, SD) | 26.67 (6.81) | 25.75 (8.39) | NS |
| Depression severity post-treatment (M, SD) | 20.23 (9.73) | 19.34 (8.35) | NS |
| Comorbid diagnosis of major depressive disorder (%) | 68.09% | 73.08% | NS |
| Comorbid anxiety disorder diagnosis (%) | 31.91% | 42.31% | NS |
| Childhood trauma (M, SD) | 79.11 (25.17) | 77.29 (23.39) | NS |
Note. PTSD severity assessed via the CAPS for DSM-IV. Depression severity assessed via Montgomery Asperg Depression Rating Scale (Montgomery & Asperg, 1979). Depression and anxiety disorder diagnoses assessed via the SCID-IV. Childhood trauma assessed via Childhood Trauma Questionnaire (Bernstein et al., 2003).
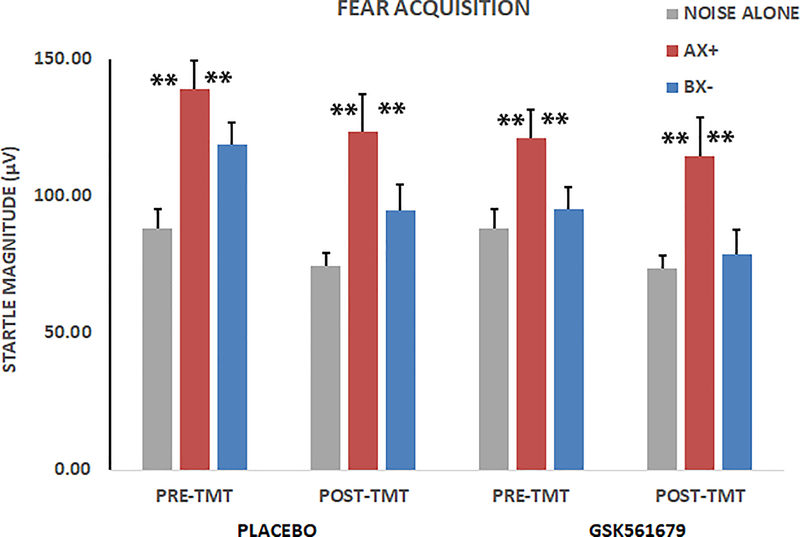
The 2 X 3 X 2 RM-ANOVA of fear acquisition with within-subjects variables of treatment (pre, post) and trial type (NA, AX+, BX-) and between-groups variable of condition (GSK561679, placebo) showed a significant main effect of trial type (F(2,108) = 7.16, p = 0.001, ƞp2 = 0.12; Figure 2), with no main or interaction effects of treatment. There was also no main or interaction effect of condition on acquisition, and no three-way effect of trial type, treatment and condition. Planned contrasts within trial type showed higher startle magnitude to AX+ compared to NA (F(1,54) = 10.77, p = 0.002, ƞp2 = 0.17; Figure 2) and higher startle magnitude to AX+ than BX- (F(1,54) = 6.97, p =0.01, ƞp2 = 0.11; Figure 2).
Figure 2.
Average startle magnitude to NA, AX+ and BX- trials during acquisition. There was a significant increase in magnitude to the AX+ trials across all groups, F(1,54) = 10.77, p < 0.01. There was less startle to BX- compared to AX+, F(1,54) = 6.97, p = 0.01, demonstrating discrimination between threat and safety signals. There were no effects of treatment. **p ≤ 0.01
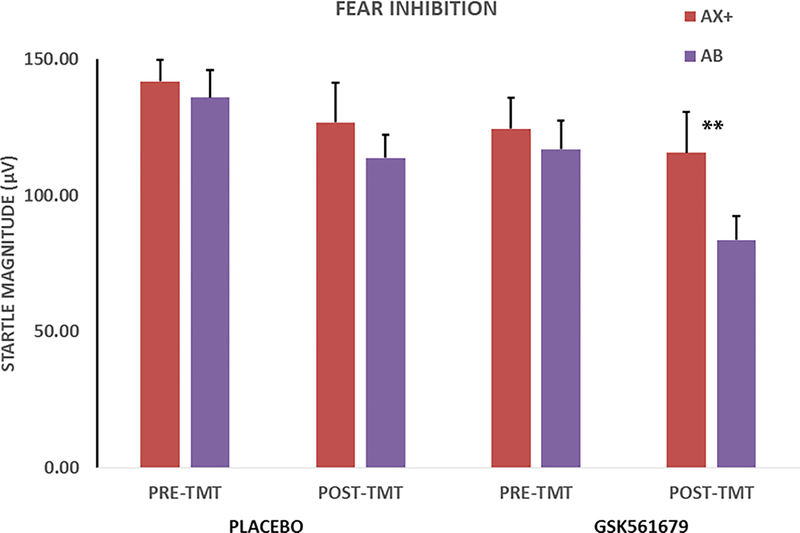
The 2 X 2 X 2 RM-ANOVA of fear inhibition with within-subjects variables of treatment (pre, post) and trial type (AX+, AB) and between-groups variable of condition (GSK561679, placebo) revealed an interaction of trial type with treatment (F(1,52) = 5.02, p = 0.03, ƞp2 = 0.09), but no main or interaction effects of condition. We followed up the interaction of trial type and Treatment with one-way RM-ANOVA of trial type separately before and after treatment. Prior to treatment, the patients did not show fear inhibition to AB trials compared to AX+ (F(1,51) = 0.05, p = 0.83, ƞp2 = 0.001), whereas startle magnitude to AB trials was significantly reduced compared to AX+ after treatment (F(1,51) = 8.01, p = 0.007, ƞp2 = 0.14).
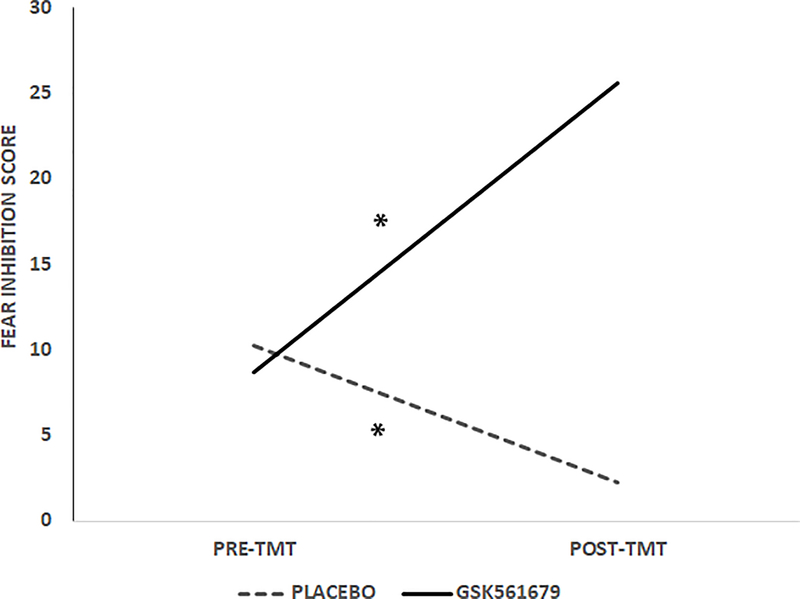
Given our hypothesis that GSK561679 would increase fear inhibition, we conducted an RM-ANOVA of trial type post treatment separately for drug and placebo groups. Analysis of fear inhibition separately within each condition revealed that the trial type effect post treatment was only significant in the GSK561679 group, which showed less startle magnitude to AB than to AX+ trials (F(1,26) = 7.50, p = 0.01, ƞp2 = 0.22; Figure 3), while there was no significant difference in startle magnitude in the placebo group (F(1,28) = 0.61, p = 0.44, ƞp2 = 0.02; Figure 3). We derived an inhibition score by subtracting startle magnitude to AB from AX+ (a larger score indicated better inhibition). This inhibition score was compared across treatment within each condition while controlling for the level of startle magnitude to AX+ during fear acquisition, given that individual levels of fear conditioning varied. This analysis also showed that the fear inhibition score increased pre- to post-treatment in the GSK561679 group (F(1,25) = 5.20, p = 0.03, ƞp2 = 0.17; Figure 4)) while it actually decreased in the placebo group (F(1,26) = 4.60, p = 0.04, ƞp2 = 0.15; Figure 4).
Figure 3.
Average startle magnitude to AX+ and AB trials during transfer test. There was a significant effect of treatment, in that the drug group demonstrated fear inhibition at post treatment, F(1,26) = 7.50, p = 0.01, but not prior to treatment. The placebo group showed impaired fear inhibition pre and post-treatment. **p ≤ 0.01
Figure 4.
Average inhibition score (AX+ minus AB) across Treatment and Condition. This analysis also showed that the fear inhibition score increased pre- to post-treatment in the drug group, F(1,25) = 5.20, p < 0.05, and decreased in the placebo group (F(1,26) = 4.60, p < 0.05. Larger score indicates better fear inhibition. * p ≤ 0.05
3. Discussion
The current study examined the effect of GSK561679, a CRF1 receptor antagonist, in increasing fear inhibition in PTSD patients. Fear inhibition was assessed using the AX+/BX- conditional discrimination paradigm with FPS, which specifically allowed for the analysis of fear acquisition, discrimination, and inhibition. Both the GSK561679 and placebo groups showed normal acquisition of FPS and impaired inhibition to a safety cue prior to treatment, consistent with our previous studies using this paradigm in PTSD (Jovanovic et al., 2012; Jovanovic & Norrholm, 2011). The active drug and placebo groups did not differ on fear acquisition or discrimination, nor did these measures change with treatment. However, inhibition of fear improved during the safety transfer test in the GSK561679 group, but not the placebo group. Further, analysis of the drug group showed that the PTSD-related deficits in fear inhibition were normalized after treatment, and FPS to the AB trials was significantly lower than to the AX+ trials as we have observed in our prior research with healthy volunteers (Jovanovic et al., 2005).
It is important to note that the drug did not affect the fear learning per se, or discrimination learning. Therefore, the CRF receptor 1 antagonist did not negatively impact learning mechanisms; rather, it specifically enhanced the degree to which learned safety cues inhibited fear responses to threatening stimuli. In our previous studies, we have found that this specific deficit, rather than a more broad learning deficit, is associated with higher PTSD symptoms (Jovanovic et al., 2009). The larger clinical trial of GSK561679 did not find therapeutic benefits of the drug in reducing PTSD symptoms (Dunlop et al., 2017). One of the possible interpretations of the negative results is that the effects of CRF receptor 1 antagonism may vary based on the degree of receptor binding in different brain regions. CRF 1 receptors are expressed in various brain regions, including the hypothalamus, amygdala, bed nucleus of the stria terminalis, hippocampus, cortex, and cerebellum (Steckler & Holsboer, 1999). It may be that in some regions, such as the central amygdala, a principal anatomical substrate underlying the expression of learned fear, CRF receptor 1 antagonism may act to increase fear and anxiety (Dunlop et al., 2017). This interpretation is consistent with another study using the same GSK561679 drug at a single higher dose (400mg) in healthy subjects, which found that the drug increased FPS to a threat cue, while also reducing noise alone startle during inter-trial intervals (Grillon et al., 2015). In contrast, CRF receptor 1 antagonism in the prefrontal cortex may differ in function (Magalhaes et al., 2010), which may specifically act to enhance fear inhibition (Jovanovic et al., 2013).
It is important to note that future studies should further address the discordance observed in the current study as it relates to: (1) psychophysiological outcomes as compared to clinical indices and (2) altered fear inhibition coupled with the lack of effects on fear acquisition or discrimination. With regard to the former, we have previously reported drug-specific psychophysiological effects that were inconsistent with clinical findings (e.g., Rothbaum et al., 2014; Norrholm et al., 2016) and the disparity likely reflects divergent underlying neurobiological mechanisms. It is possible that CRFR1 antagonism has circuit-level effects within limbic inhibition pathways that do not generalize to the expression of larger scale, phenotypic posttraumatic stress reactions. With regard to the latter, anatomical dissociations have been observed in recent rodent studies of fear acquisition, discrimination, and inhibition. Inactivation of the posterior insular cortex, for example, has been shown to attenuate conditioned inhibition responses specifically while sparing discrimination and retention of learned fear (Foilb et al., 2016).
According to a recent report, PTSD can manifest diagnostically in over 600,000 possible clinical presentations (Galatzer-Levy & Bryant, 2013) with patients potentially exhibiting more robust symptoms in one or more symptom clusters as part of the disease process (Jovanovic & Norrholm, 2011). It may be that clinical presentations of PTSD that include significant fear-related symptoms (e.g., emotional and physiological reactivity to trauma-related cues) as predominant features are more directly associated with HPA axis/CRF system dysregulation and, as such, more receptive to pharmacological interventions that target this dysregulation such as CRFR1 antagonism.
Genetic data suggest that GSK561679 may be effective in individuals with the CRFR1 GG genotype and high levels of childhood trauma (Bradley et al., 2008; Dunlop et al., 2017). In the present study, we were not adequately powered to examine the effects of CRFR1 GG genotype and childhood trauma on fear inhibition, though we have seen genetic effects on inhibition of FPS in larger PTSD samples (Norrholm et al., 2013). The effect of genotype suggests that the CRF receptor 1 antagonist may have very specific neurobiological effects that are not robust in a general PTSD population, or in broad fear or anxiety phenotypes. Our previous studies showing associations between HPA axis dysregulation and fear inhibition (Jovanovic et al., 2011) suggest common pathways affected by CRFR1. Although we were not powered to examine specific genetic effects in the current study, it is possible that the CRFR1 GG genotype represents a risk factor that reduces inhibition of fear, which can then be compensated for pharmacologically. A recent study of GSK561679’s effects in healthy volunteers found increased conditioned fear (Grillon et al., 2015), however, we did not see the same effect in PTSD patients. However, in our study, we controlled for noise alone startle levels, which were reduced in the study with healthy volunteers. In testing of inhibition, we controlled for individual variability in fear conditioning, in order to account for any individual differences that were not evident in the group analyses.
A limitation of the current study is that only women were included (Dunlop et al., 2017). Additionally, the conditional discrimination paradigm is sensitive to cycle effects and estradiol level (Glover et al., 2013), which were not controlled in the current study. Oral contraceptive use, which likely reduces circulating estradiol levels, was not an exclusion for the study. Nevertheless, only a small number of women reported using oral contraceptives (3 women in each group); therefore, lack of cycling is unlikely to explain the observed drug effects on fear inhibition. Future studies should also examine estradiol levels during treatment, especially if fear inhibition paradigms are used as treatment outcomes.
The study had additional limitations, such as a high discontinuation rate and resulting small sample size at post-treatment. It is important to note that drop-out rates did not differ by treatment condition (Dunlop et al., 2017). Further, the FPS data collected post-treatment at week six represent effects of circulating drug, as serum levels indicated presence of GSK561679 at this time point. As such, it is unclear whether the drug would have any lasting effects on inhibition via neural plasticity or as a result of the acute effects of drug. Finally, the discrepancies between the current FPS results and the negative clinical trial result (Dunlop et al., 2017), and with the FPS data in healthy volunteers (Grillon et al., 2015), should be further explored to examine potential mechanisms of action for the CRF receptor 1 antagonist. Specifically, since trauma exposure and PTSD are likely to alter the HPA axis and CRF receptor function (de Kloet et al., 2007; Yehuda et al., 2015), our results indicate that inhibition of FPS may be used as a tool to investigate these impacts in traumatized individuals relative to healthy unaffected individuals.
In conclusion, the current study suggests that fear inhibition using FPS in a fear conditioning paradigm can serve as an intermediate phenotype of PTSD treatment effects. This measure has been repeatedly associated with PTSD diagnosis, and shows plasticity and change with treatment. However, the FPS measures did not correspond to clinical outcomes in the current trial. Research Domain Criteria (RDoC) approaches in clinical research advocate the use of neuroscience-based measures across different units of analyses, including psychophysiology (Cuthbert, 2014). FPS is a potentially useful tool in an RDoC approach that can index treatment-related changes in fear-related domains.
Acknowledgements
This work was supported by National Institute of Mental Health (NIMH) Grant Nos. U19 MH069056 (to BWD, HM) and K23 MH086690 (to BWD) and Veterans Affairs Clinical Science Research and Development project 09SNIMH-002 (to TCN). This research was conducted with resources and the use of facilities at the Michael E. DeBakey Veterans Affairs Medical Center, Houston, TX. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the United States government. The GSK561679 compound is licensed by Neurocrine Biosciences (San Diego, California). GlaxoSmithKline contributed the study medication, the matching placebo, and the funds to support subject recruitment and laboratory testing, and Neurocrine Biosciences conducted the pharmacokinetic analyses. GlaxoSmithKline and Neurocrine Biosciences were not involved in the data collection, data analysis, or interpretation of findings.
TJ has received funding from NIMH and Brain and Behavior Research Foundation. EJD, SDN, and SSI have funding from VA Merit. SJM has received research funding from the NIH, Department of Veterans Affairs, Johnson Family Chair, and Janssen Research and Development. He has served as a consultant to Acadia, Alkermes, Cerecor, Otsuka, and Valeant, and serves on an advisory board for VistaGen Therapeutics. TCN has received research support from the NIMH, Department of Defense, and Department of Veterans Affairs. In the past 3 years, he has served as a consultant to Resilience Therapeutics and Insys Therapeutics. In the past 5 years, DI has consulted for Avanir, Axome, CNS Response, INSYS Therapeutics, Lundbeck, Otsuka, Servier, and Sunovion, and has received grant/research support through the Icahn School of Medicine at Mount Sinai from Alkermes, Astra Zeneca, Brainsway, Euthymics, Neosync, Roche, and Shire. BOR received funding from the Wounded Warrior Project, Department of Defense, the Brain and Behavior Research Foundation, NIMH, and McCormick Foundation. BOR receives royalties from Oxford University Press, Guilford, APPI, and Emory University, and has received one advisory board payment from Genentech. BWD has received research support from Acadia, Assurex, Axsome, Bristol-Myers Squibb, Janssen, GlaxoSmithKline, NIMH, Otsuka, Pfizer, and Takeda. He has served as a consultant to Pfizer and Medavante.
Footnotes
JK, KG, and HM report no biomedical financial interests or potential conflicts of interest. ClinicialTrials.gov: NCT01018992
The study is registered at Clinicaltrials.gov: NCT01018992.
References
- Baker DG, West SA, Nicholson WE, Ekhator NN, Kasckow JW, Hill KK, Bruce AB, Orth DN & Geracioti TD Jr (1999). Serial CSF corticotropin-releasing hormone levels and adrenocortical activity in combat veterans with posttraumatic stress disorder. American Journal of Psychiatry, 156(4):585–588. [DOI] [PubMed] [Google Scholar]
- Bernstein DP, Stein JA, Newcomb MD, Walker E, Pogge D, Ahluvalia T, … & Zule W (2003). Development and validation of a brief screening version of the Childhood Trauma Questionnaire. Child Abuse & Neglect, 27(2), 169–190. 10.1016/S0145-2134(02)00541-0 [DOI] [PubMed] [Google Scholar]
- Blake DD, Weathers FW, Nagy LM, Kaloupek DG, Gusman FD, Charney DS, & Keane TM (1995). The development of a clinician-administered PTSD scale. J Trauma Stress, 8(1), 75–90. 10.1002/jts.2490080106 [DOI] [PubMed] [Google Scholar]
- Bradley RG, Binder EB, Epstein MP, Tang Y, Nair HP, Liu W, Gillespie CF, Berg T, Evces M, Newport DJ, Stowe ZN, Heim CM, Nemeroff CB, Schwartz A, Cubells JF, & Ressler KJ (2008). Influence of child abuse on adult depression: Moderation by the corticotropin-releasing hormone receptor gene. Arch Gen Psychiatry, 65(2), 190–200. 10.1001/archgenpsychiatry.2007.26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner JD, Licinio J, Darnell A, Krystal JH, Owens MJ, Southwick SM, Nemeroff CB, & Charney DS (1997). Elevated CSF corticotropin-releasing factor concentrations in posttraumatic stress disorder. American Journal of Psychiatry, 154(5), 624–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuthbert BN (2014). Research Domain Criteria: Toward future psychiatric nosology. Asian Journal of Psychiatry, 7, 4–5. 10.1016/j.ajp.2013.12.007 [DOI] [PubMed] [Google Scholar]
- De Kloet C, Vermetten E, Geuze E, Lentjes E, Heijnen C, Stalla G, & Westenberg H (2007). Elevated plasma corticotrophin-releasing hormone levels in veterans with posttraumatic stress disorder. Progress in Brain Research, 167, 287–291. 10.1016/S0079-6123(07)67025-3 [DOI] [PubMed] [Google Scholar]
- Deak T, Nguyen KT, Ehrlich AL, Watkins LR, Spencer RL, Maier SF, … Webster E (1999). The impact of the nonpeptide corticotropin-releasing hormone antagonist antalarmin on behavioral and endocrine responses to stress. Endocrinology, 140(1), 79–86. [DOI] [PubMed] [Google Scholar]
- Duits P, Cath DC, Lissek S, Hox JJ, Hamm AO, Engelhard IM, van den Hout MA, & Baas JMP (2015). Updated meta-analysis of classical fear conditionoing in the anxiety disorders. Depression and Anxiety, 32(4), 239–253. 10.1002/da.22353 [DOI] [PubMed] [Google Scholar]
- Dunlop BW, Binder EB, Iosifescu D, Mathew SJ, Neylan TC, Pape JC, Carrillo-Roa T, Green C, Kinkead B, Grigoriadis D, Rothbaum BO, Nemeroff CB, Mayberg HS (2017). Corticotropin-releasing factor receptor 1 antagonism is ineffective for women with posttraumatic Stress Disorder. Biological Psychiatry, 82(12); 866–874. 10.1016/j.biopsych.2017.06.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlop BW, Rothbaum BO, Binder EB, Duncan EJ, Harvey PD, Jovanovic T, Kelley ME, Kinkead B, Kutner M, Iosifescu DV, Mathew SJ, Neylan TC, Kilts CD, Nemeroff CB, Mayberg HS (2014). Evaluation of a corticotropin releasing hormone type 1 receptor antagonist in women with posttraumatic stress disorder: Study protocol for a randomized controlled trial. Trials, 15, 240 10.1186/1745-6215-15-240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foilb AR, Flyer-Adams JG, Maier SF, & Christianson JP (2016). Posterior insular cortex is necessary for conditioned inhibition of fear. Neurobiol Learn Mem 134 (B):317–27. 10.1016/j.nlm.2016.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, & Williams JB (1995). Structured Clinical Interview for DSM-IV Axis I Disorders-Patient. SCID-I/P, Version 20th edition. New York: Biometrics Research Department, New York State Psychiatric Institute. [Google Scholar]
- Forbes D, Creamer M, Bisson JI, Cohen JA, Crow BE, Foa EB, Friedman MJ, Keane TM, Kudler HS, & Ursano RJ (2010). A guide to guidelines for the treatment of PTSD and related conditions. Journal of Traumatic Stress, 23(5), 537–552. 10.1002/jts.20565 [DOI] [PubMed] [Google Scholar]
- Galatzer-Levy IR & Bryant RA (2013). 636,120 ways to have posttraumatic stress disorder. Perspect Psychol Sci, 8(6):651–62. 10.1177/1745691613504115 [DOI] [PubMed] [Google Scholar]
- Glover EM, Mercer KB, Norrholm SD, Davis M, Duncan E, Bradley B, Ressler KJ, & Jovanovic T (2013). Inhibition of fear is differentially associated with cycling estrogen levels in women. Journal of Psychiatry & Neuroscience : JPN, 38(5), 341–348. 10.1503/jpn.120129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillon C, Hale E, Lieberman L, Davis A, Pine DS, & Ernst M (2015). The CRH 1 antagonist GSK561679 increases human fear but not anxiety as assessed by startle. Neuropsychopharmacology, 40(5), 1064–1071. 10.1038/npp.2014.316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillon C, & Morgan CA 3rd. (1999). Fear-potentiated startle conditioning to explicit and contextual cues in Gulf War veterans with posttraumatic stress disorder. Journal of Abnormal Psychology, 108(1), 134–142. 10.1037/0021-843X.108.1.134 [DOI] [PubMed] [Google Scholar]
- Jovanovic T, Ely T, Fani N, Glover EM, Gutman D, Tone EB, Norrholm SD, Bradley B, & Ressler KJ (2013). Reduced neural activation during an inhibition task is associated with impaired fear inhibition in a traumatized civilian sample. Cortex, 49(7), 1884–1891. 10.1016/j.cortex.2012.08.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanovic T, Kazama A, Bachevalier J, & Davis M (2012). Impaired safety signal learning may be a biomarker of PTSD. Neuropharmacology, 62(2), 695–704. 10.1016/j.neuropharm.2011.02.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanovic T, Keyes M, Fiallos A, Myers KM, Davis M, & Duncan E (2005). Fear potentiation and fear inhibition in a human fear-potentiated startle paradigm. Biological Psychiatry, 57(12), 1559–1564. 10.1016/j.biopsych.2005.02.025 [DOI] [PubMed] [Google Scholar]
- Jovanovic T, & Norrholm SD (2011). Neural mechanisms of impaired fear inhibition in posttraumatic stress disorder. Frontiers in Behavioral Neuroscience, 5 10.3389/fnbeh.2011.00044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanovic T, & Norrholm SD (2016). Human psychophysiology and PTSD In Liberzon I & Ressler KJ (Eds.), Neurobiology of PTSD: From Brain to Mind (pp. 292–316). New York, NY: Oxford University Press. [Google Scholar]
- Jovanovic T, Norrholm SD, Blanding NQ, Davis M, Duncan E, Bradley B, & Ressler KJ (2010). Impaired fear inhibition is a biomarker of PTSD but not depression. Depression and Anxiety, 27(3), 244–251. 10.1002/da.20663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanovic T, Norrholm SD, Blanding NQ, Phifer JE, Weiss T, Davis M, Duncan EJ, Bradley B, & Ressler KJ (2010). Fear potentiation is associated with hypothalamic-pituitary-adrenal axis function in PTSD. Psychoneuroendocrinology, 35, 846–857. 10.1016/j.psyneuen.2009.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanovic T, Norrholm SD, Fennell JE, Keyes M, Fiallos AM, Myers KM, Davis M, & Duncan EJ (2009). Posttraumatic stress disorder may be associated with impaired fear inhibition: Relation to symptom severity. Psychiatry Research, 167(1–2), 151–160. 10.1016/j.psychres.2007.12.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanovic T, Phifer JE, Sicking K, Weiss T, Norrholm SD, Bradley B, & Ressler KJ (2011). Cortisol suppression by dexamethasone reduces exaggerated fear responses in posttraumatic stress disorder. Psychoneuroendocrinology. 36(10):1540–52. 10.1016/j.psyneuen.2011.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanovic T, & Ressler KJ (2010). How the neurocircuitry and genetics of fear inhibition may inform our understanding of PTSD. American J Psychiatry, 167, 648–662. 10.1176/appi.ajp.2009.09071074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magalhaes AC, Holmes KD, Dale LB, Comps-Agrar L, Lee D, Yadav PN, Drysdale L, Poulter MO, Roth BL, Pin JP, Anisman H, & Ferguson SSG (2010). CRF receptor 1 regulates anxiety behavior via sensitization of 5-HT2 receptor signaling. Nature Neuroscience, 13, 622 10.1038/nn.2529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medicine I. o. (2014). Treatment for Posttraumatic Stress Disorder in Military and Veteran Populations: Final Assessment. Washington, DC: : The National Academies Press. [PubMed] [Google Scholar]
- Michopoulos V, Norrholm SD, Stevens JS, Glover EM, Rothbaum BO, Gillespie CF, Schwartz AC, Ressler KJ, & Jovanovic T (2017). Dexamethasone facilitates fear extinction and safety discrimination in PTSD: A placebo-controlled, double-blind study. Psychoneuroendocrinology, 83, 65–71. 10.1016/j.psyneuen.2017.05.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery SA, & Asberg. (1979): A new depression scale designed to be sensitive to change. British Journal of Psychiatr, 134: 382–389. 10.1192/bjp.134.4.382 [DOI] [PubMed] [Google Scholar]
- Morgan CA 3rd, Grillon C, Southwick SM, Davis M, & Charney DS (1995). Fear-potentiated startle in posttraumatic stress disorder. Biological Psychiatry, 38(6), 378–385. 10.1016/0006-3223(94)00321-S [DOI] [PubMed] [Google Scholar]
- Myers KM, & Davis M (2004). AX+, BX- discrimination learning in the fear-potentiated startle paradigm: Possible relevance to inhibitory fear learning in extinction. Learn Mem, 11(4), 464–475. 10.1101/lm.74704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers KM, Toufexis DJ, Winslow JT, Jovanovic T, Norrholm SD, Duncan E, & Davis M (Eds.). (2009). Measurement of fear inhibition in rats, monkeys, and humans with and without posttraumatic stress disorder, using the AX+, BX- paradigm. New York City: The Guilford Press. [Google Scholar]
- Norrholm SD, Jovanovic T, Olin IW, Sands LA, Karapanou I, Bradley B, & Ressler KJ (2011). Fear extinction in traumatized civilians with posttraumatic stress disorder: Relation to symptom severity. Biol Psychiatry, 69(6), 556–563. 10.1016/j.biopsych.2010.09.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norrholm SD, Jovanovic T, Gerardi M, Breazeale KG, Davis M, Duncan EJ, Ressler KJ, Bradley B, Rizzo A, & Rothbaum BO (2016). Baseline Psychophysiological and Cortisol Reactivity as a Predictor of PTSD Treatment Outcome in Virtual Reality Exposure Therapy. Behaviour Research and Therapy. 82:28–37. 10.1016/j.brat.2016.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robison-Andrew EJ, Duval ER, Nelson CB, Echiverri-Cohen A, Giardino N, Defever A, … Rauch SAM (2014). Changes in trauma-potentiated startle with treatment of posttraumatic stress disorder in combat veterans. Journal of Anxiety Disorders, 28(4), 358–362. 10.1016/j.janxdis.2014.04.002 [DOI] [PubMed] [Google Scholar]
- Rothbaum BO, Price M, Jovanovic T, Norrholm SD, Gerardi M, Dunlop BW, … Ressler KJ (2014). A randomized, double-blind evaluation of d-cycloserine or alprazolam combined with virtual reality exposure therapy for posttraumatic stress disorder in Iraq and Afghanistan war veterans. American Journal of Psychiatry, 171(6), 640–648. 10.1176/appi.ajp.2014.13121625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sijbrandij M, Engelhard IM, Lommen MJJ, Leer A, & Baas JMP (2013). Impaired fear inhibition learning predicts the persistence of symptoms of posttraumatic stress disorder (PTSD). Journal of Psychiatric Research, 47(12), 1991–1997. 10.1016/j.jpsychires.2013.09.008 [DOI] [PubMed] [Google Scholar]
- Smith GW, Aubry J-M, Dellu F, Contarino A, Bilezikjian LM, Gold LH, … Bentley CA (1998). Corticotropin releasing factor receptor 1–deficient mice display decreased anxiety, impaired stress response, and aberrant neuroendocrine development. Neuron, 20(6), 1093–1102. 10.1016/S0896-6273(00)80491-2 [DOI] [PubMed] [Google Scholar]
- Steckler T, & Holsboer F (1999). Corticotropin-releasing hormone receptor subtypes and emotion. Biological Psychiatry, 46(11), 1480–1508. 10.1016/S0006-3223(99)00170-5 [DOI] [PubMed] [Google Scholar]
- Wangelin BC, & Tuerk PW (2015). Taking the pulse of prolonged exposure therapy: physiological reactivity to trauma imagery as an objective measure of treatment response. Depression and Anxiety, 32(12), 927–934. 10.1002/da.22449 [DOI] [PubMed] [Google Scholar]
- Yehuda R, Bierer LM, Pratchett LC, Lehrner A, Koch EC, Van Manen JA, … Hildebrandt T (2015). Cortisol augmentation of a psychological treatment for warfighters with posttraumatic stress disorder: Randomized trial showing improved treatment retention and outcome. Psychoneuroendocrinology, 51, 589–597. 10.1016/j.psyneuen.2014.08.004 [DOI] [PubMed] [Google Scholar]