Abstract
Introduction
In this study, the effects of omega‐3 fatty acids were examined in a rat model of spinal cord injury.
Methods
The rats were classified into sham, control, spinal cord injury plus 50 mg/kg Omega‐3 fatty acids and spinal cord injury plus 100 mg/kg Omega‐3 fatty acids. The levels of oxidative, apoptotic, and inflammatory markers were examined in each of these groups.
Results
Altered lipid peroxidation, reduced glutathione (GSH), superoxide dismutase (SOD), glutathione peroxidase (Gpx), and catalase were normalized. Omega‐3 fatty acid supplementation decreased tumor necrosis factor‐alpha (TNF‐α) and interleukin‐6 (IL‐6) levels by >50%. TNF‐α and IL‐6 mRNA expression were reduced. Caspase‐3, p53, bax, and pro‐NGF mRNA expression levels were increased by 1.3‐, 1.4‐, 1.2‐, and 0.9‐fold, respectively, whereas bcl‐2 mRNA expression was decreased by 0.77‐fold in control rats. Omega‐3 fatty acid supplementation decreased p53, caspase‐3, bax, and pro‐NGF mRNA expression by >40%, while the level of bcl‐2 mRNA expression was increased by 286.9%. Omega‐3 fatty acid supplementation decreased caspase‐3 and p53 protein expression by >30%.
Conclusion
Taken together, our results suggested that omega‐3 fatty acid supplementation reduced oxidative stress, apoptosis, and the levels of inflammatory markers in ischemia‐reperfusion‐induced rats.
Keywords: apoptosis, inflammation, omega‐3 fatty acid, oxidative stress, rat
1. INTRODUCTION
Omega‐3 fatty acids are important polyunsaturated fatty acids with some roles in normal cellular metabolism (Scorletti & Byrne, 2013). Essential fatty acids have been reported to be important in membrane fluidity, inflammatory eicosanoids, and neural membrane oxidation (Salvati, Attorri, DiBenedetto, DiBiase, & Leonardi, 2006). The daily recommended level of omega‐3 fatty acid is 300 mg for healthy adults. Leukotriene‐5, thromboxane‐3, and prostaglandin‐3 are derived from essential fatty acids, and are known to be therapeutically important in inflammatory conditions as well as for mental health (de Batlle et al., 2012; Huan et al., 2004; Kiecolt‐Glaser, Belury, Andridge, Malarkey, & Glaser, 2011; Peet, Brind, Ramchand, Shah, & Vankar, 2001; Perica & Delas, 2011). The omega‐6 fatty acid has been reported to have a beneficial effect on osteoporosis (Maggio et al., 2009). Ahmed, Abd, and Samad (2013) reported the therapeutic importance of omega‐3 fatty acid in protecting against bone modification in salt‐loaded rats.
Spinal cord injury leads to functional changes in autonomic function, loss of sensation, and muscle wasting (Krucoff, Rahimpour, Slutzky, Edgerton, & Turner, 2016). The induction in apoptosis in neurons and oligodendrocytes leads to axonal degeneration, demyelination, and dysfunction (Lee et al., 2003; Yune et al., 2008). In spinal cord injury, apoptosis of oligodendrocytes and neurons leads to increased oxidative stress and inflammation (Bao & Liu, 2002; Yune et al., 2008). Reactive oxygen species (ROS) and proinflammatory cytokines are produced in inflammatory and neurodegenerative states (Block & Hong, 2005; Min et al., 2004; Qin et al., 2004). Hussein, El‐Banna, Razik, and El‐Naggar (2018) reported that interleukins (IL) are essential cytokines involved in several immunological processes. The microglia release pro‐nerve growth factor (pro‐NGF), which leads to cell death (Yune et al., 2007). Therefore, novel therapeutic agents with efficacy against inflammation and oxidative stress are required. This study investigates the effects of omega‐3 fatty acids against spinal cord injury.
2. MATERIALS AND METHODS
2.1. Rats
Male albino rats were purchased from the Academy of Medical Sciences and Peking Union Medical College, Beijing, China. The rats weighed 180–210 g and were maintained in standard rat polypropylene cages and kept under standard environmental conditions of 12 hr of light and 12 hr of the dark with a relative humidity of 60% ± 5% and temperature of 25°C ± 0.5°C. All the animal experiment was carried out under the guidelines of the Department of Orthopedic Surgery, Peking Union Medical College Hospital. Ethical committees of Peking Union Medical College Hospital approved all animal care and experimental protocols. All animal surgery was conducted under anesthesia, and maximum effort was made to reduce animal suffering.
2.2. An experimental model of spinal cord injury
An experimental model of spinal cord injury was established in rats as described previously (Naruo et al., 2003; Sakanaka et al., 2007). Briefly, Spinal cord injury was established by longitudinal surgical incision following exposure of 1.5% halothane. The surgical incision was formed from the lower to mid thoracic vertebrae on the backside of the rats. The weight (20 g) was kept on the exposed Th12 surface for 30 min to induce injury. Then, weight was removed from the Th12 surface and muscles, and skin was sutured. Rats were kept under appropriate laboratory conditions for 24 hr.
2.3. Treatments
The rats were classified into four groups: group I, sham operation plus normal saline only; group II, control with spinal cord injury plus normal saline only; group III, spinal cord injury plus 50 mg/kg Omega‐3 fatty acids; and group IV, spinal cord injury plus 100 mg/kg Omega‐3 fatty acids. Normal saline was used to dissolve fatty acids. Control and sham rats were administered normal saline. Omega‐3 fatty acid solution or normal saline was administered orally to rats for 30 days.
2.4. Oxidative markers
Lipid peroxidation was measured as malondialdehyde (MDA) content through the measurement of thiobarbituric acid reactive species (TBARS). The final lipid peroxidation product was measured at 534 nm. Catalase, GSH, Gpx, and SOD levels were observed as described previously (Kaddour et al., 2016).
2.5. Inflammatory markers
Serum levels of interleukin (IL)‐6 and tumor necrosis factor‐alpha (TNF‐α) were determined as described previously (Hend, Omnia, Hekmat, & Amira, 2014). The levels of TNF‐α and IL‐6 mRNA expression were quantified as described previously (Moon et al., 2012). Following treatment, total RNA was isolated from the rat brain and converted into cDNA by oligo (dt) primers. The mRNA expression was determined by RT‐PCR. The relative ratios of mRNA expression were determined according to the 2‐∆∆ C T method. The primers used for mRNA amplification are shown in Table 1.
Table 1.
List of primers used in RT‐PCR reaction for the amplification of following markers
| S.No. | Markers | Forward primer | Reverse primer |
|---|---|---|---|
| 1 | TNF‐α | 5′‐CCCAGACCCTCACACTCAGAT−3′ | 5′‐TTG TCC CTTGAA GAG AAC CTG−3′ |
| 2 | IL−6 | 5′‐AAGTTTCTCTCCGCAAGATAC TTCCAGCCA−3′ | 5′‐AGG CAAATTTCCTGGTTATATCCA GTTT−3′ |
| 3 | p53 | 5′‐TAACAGTTCCTGCATGGGCGGC−3′ | 5′‐AGGACAGGCACAAACACGCACC−3′ |
| 4 | Bax | 5′‐TGG AGCTGCAGAGGATGATTG−3′ | 5′‐GAAGTTGCCGTCAGAAAACATG−3′ |
| 5 | Caspase−3 | 5′‐TTAATAAAGGTATCCATGGAGAACACT−3′ | 5′‐TTAGTGATAAAAATAGAGTTCTTTTGTGAG−3' |
| 6 | Bcl−2 | 5′‐CAC CCC TGG CAT CTT CTC CTT−3′ | 5′‐AGC GTC TTC AGA GAC AGC CAG−3′ |
| 7 | proNGF | 5′‐CTTCAGCATTCCCTTGACAC−3′ | 5′‐TGAGCACACACACGCAGGC−3′ |
| 8 | GAPDH | 5′‐TCCCTCAAGATTGTCAGCAA−3′ | 5′‐AGATCCACAACGGATACATT−3′ |
2.6. Apoptotic markers
The levels of p53, bcl‐2, bax, caspase‐3, and pro‐NGF mRNA expression were determined as described previously (Moon et al., 2012). Following treatment, total RNA was isolated from the rat brain and converted into cDNA by oligo (dt) primers. The mRNA expression was determined by RT‐PCR. The relative ratios of mRNA expression were determined according to the 2‐∆∆ C T method. The primers used for mRNA amplification are shown in Table 1. The protein in the animal cell homogenate was run on sodium dodecyl sulfate‐polyacrylamide gel electrophoresis (SDS‐PAGE). Polyvinylidene fluoride membrane was used for transferring the proteins. Nonspecific proteins were blocked by Tris‐buffered saline (TBST). Then, the membranes were incubated with primary and secondary antibodies, and protein levels were quantified (Moon et al., 2012).
2.7. Statistical analyses
Values are given as the means and standard deviation (SD). Analysis of variance (ANOVA) was used to analyze the data, and Turkey's post hoc test was carried out for comparisons. In all analyses, p < 0.05 was taken to indicate statistical significance.
3. RESULTS
In this study, the altered lipid peroxidation, GSH, SOD, Gpx, and catalase were normalized by omega‐3 fatty acid treatment (Table 2, p < 0.042). MDA content was enhanced by 1.14 nmol/ml in control rats. However, omega‐3 fatty acid supplementation reduced MDA content by >50% (p < 0.031, Table 2). In the control group, catalase, SOD, Gpx, and GSH levels were reduced by 83%, 77.6%, 74.6%, and 69.7%, respectively (Table 2, p < 0.023). However, omega‐3 fatty acid supplementation normalized these levels (Table 2, p < 0.035).
Table 2.
Effect of omega‐3 fatty acid supplementation on oxidative markers in spinal cord injury induced rats
| Markers | Group I (Sham) | Group II (Control) | Group III (50 mg/kg of omega−3 fatty acids) | Group IV (100 mg/kg of omega−3 fatty acids) |
|---|---|---|---|---|
| MDA (nmol/ml) | 0.25 ± 0.011 | 1.14 ± 0.12* | 0.86 ± 0.06# | 0.41 ± 0.03# |
| Catalase (U/ml) | 14.1 ± 0.6 | 2.4 ± 0.1* | 6.3 ± 0.31# | 12.1 ± 0.31# |
| SOD (U/ml) | 319.4 ± 19.6 | 71.5 ± 5.8* | 161.2 ± 11.4# | 293.2 ± 16.1# |
| Gpx (U/ml) | 0.63 ± 0.01 | 0.16 ± 0.01* | 0.31 ± 0.01# | 0.56 ± 0.03# |
| GSH (nmol/ml) | 0.66 ± 0.02 | 0.20 ± 0.01* | 0.33 ± 0.03# | 0.59 ± 0.03# |
p < 0.05,
p < 0.05.
The levels of TNF‐α and IL‐6 (8.9 and 11.4 U/ml, respectively) were substantially enhanced in group II. However, omega‐3 fatty acid supplementation decreased TNF‐α and IL‐6 by >50% (Figures 1 and 2, p < 0.042). The levels of TNF‐α and IL‐6 mRNA expression were increased by 1.3‐fold and 1.1‐fold, respectively, in the control group. However, omega‐3 fatty acid treatment significantly reduced TNF‐α mRNA expression by 0.26‐fold and 0.48‐fold in groups III and IV, respectively (Figure 3, p < 0.037). Omega‐3 fatty acid supplementation significantly decreased IL‐6 mRNA expression by 0.18‐fold and 0.41‐fold in groups III and IV, respectively (Figure 3, p < 0.046).
Figure 1.
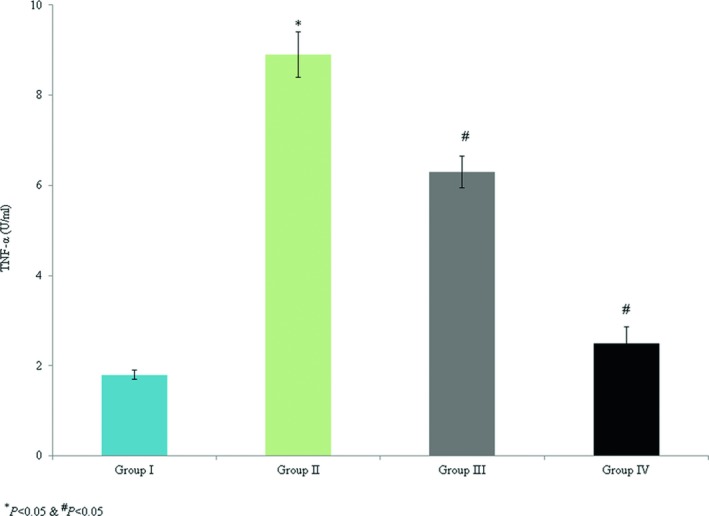
Protective effect of omega‐3 fatty acid treatment on serum TNF‐α in an experimental model of ischemia‐reperfusion‐induced rats. *p < 0.05 versus sham (group I) and # p < 0.05 versus control (group II, spinal cord injury plus normal saline only)
Figure 2.
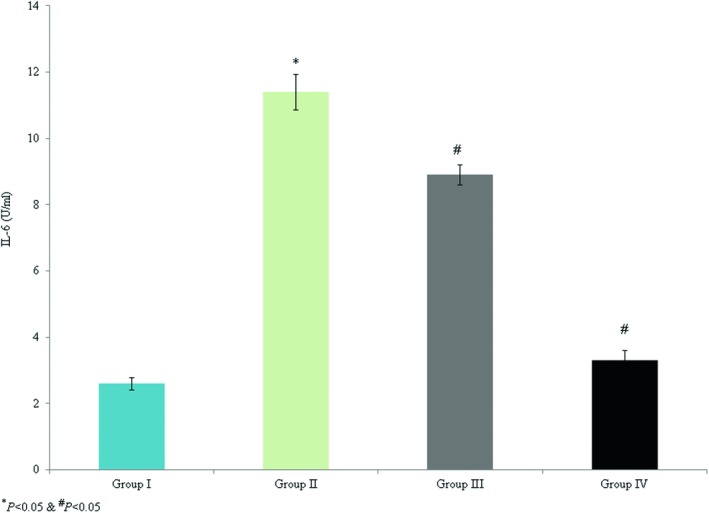
Protective effect of omega‐3 fatty acid supplementation on serum IL‐6 in an experimental model of ischemia‐reperfusion‐induced rats. *p < 0.05 versus sham (group I) and # p < 0.05 versus control (group II, spinal cord injury plus normal saline only)
Figure 3.
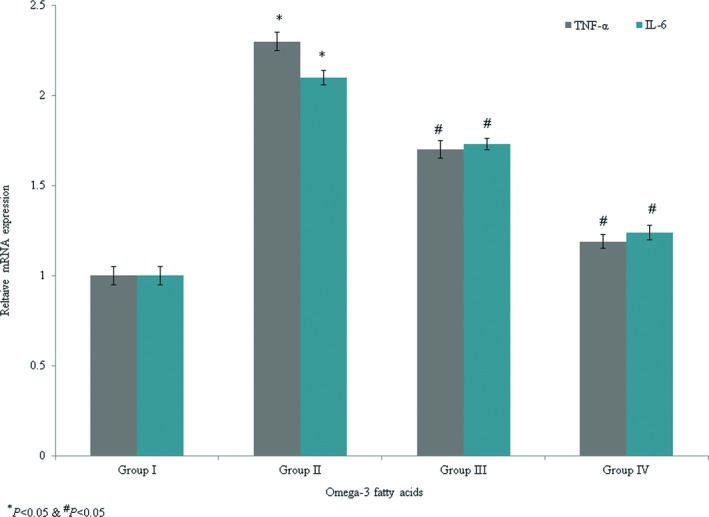
Protective efficacy of omega‐3 fatty acid supplementation on mRNA levels of TNF‐α and IL‐6 in an experimental model of ischemia‐reperfusion‐induced rats. *p < 0.05 versus sham (group I) and # p < 0.05 versus control (group II, spinal cord injury plus normal saline only)
The levels of p53, bcl‐2, bax, caspase‐3, and pro‐NGF mRNA expression were determined. Caspase‐3, p53, bax, and pro‐NGF mRNA expression were enhanced by 1.3‐, 1.4‐, 1.2‐, and 0.9‐fold, respectively, while bcl‐2 mRNA expression was decreased by 0.77‐fold in the control rats. Omega‐3 fatty acid supplementation significantly reduced caspase‐3, p53, bax, and pro‐NGF mRNA expression by >40%, while the level of bcl‐2 mRNA expression was enhanced by 286.9% (Figure 4, p < 0.033). Immunohistochemical analyses indicated that caspase‐3 and p53 protein expression levels were increased by 1.1‐fold and 1.06‐fold, respectively, in the control rats (Figures 5, 6, p < 0.021). Omega‐3 fatty acid treatment reduced the levels of caspase‐3 and p53 protein expression by >30% (Figures 5, 6, p < 0.036).
Figure 4.
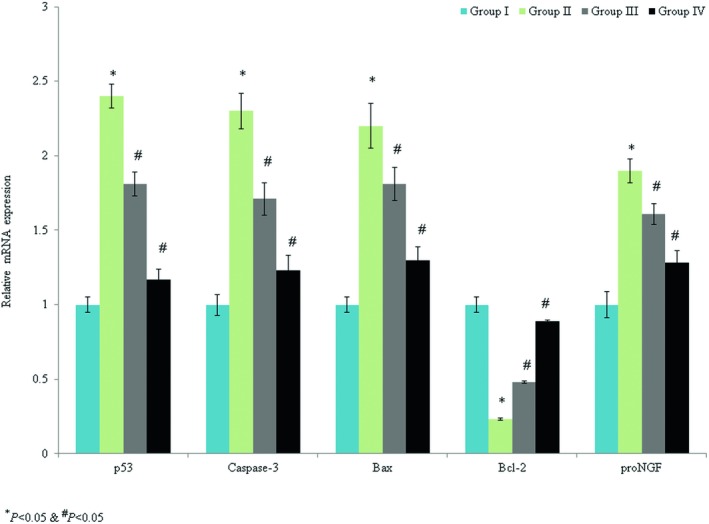
Protective effects of omega‐3 fatty acid supplementation on caspase‐3, p53, bax, bcl‐2, and pro‐NGF mRNA expression in an experimental model of ischemia‐reperfusion‐induced rats. *p < 0.05 versus sham (group I) and # p < 0.05 versus control (group II, spinal cord injury plus normal saline only)
Figure 5.
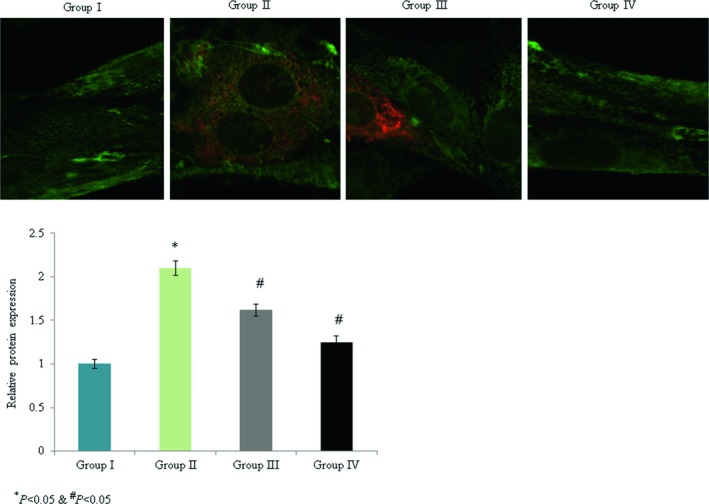
Protective effects of omega‐3 fatty acid supplementation on caspase‐3 protein expression in an experimental model of ischemia‐reperfusion‐induced rats. *p < 0.05 versus sham (group I) and # p < 0.05 versus control (group II, spinal cord injury plus normal saline only). Scale bar is 100 µm
Figure 6.
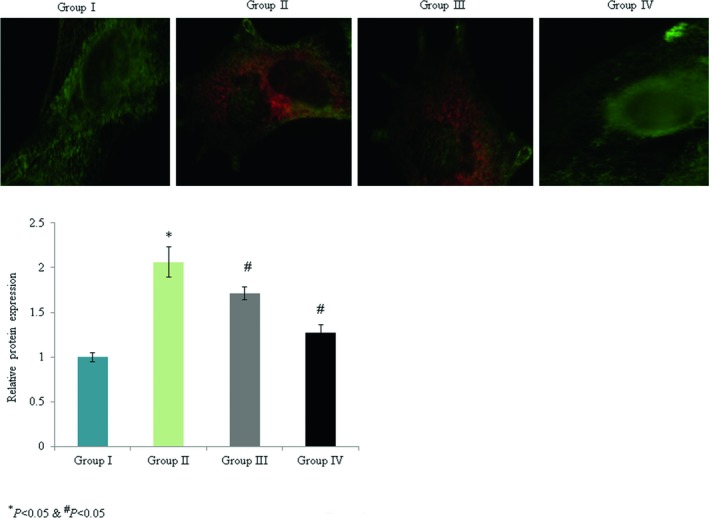
Protective effects of omega‐3 fatty acid supplementation on p53 protein expression in an experimental model of ischemia‐reperfusion‐induced rats. *p < 0.05 versus sham (group I) and # p < 0.05 versus control (group II, spinal cord injury plus normal saline only). Scale bar is 100 µm
4. DISCUSSION
Omega‐3 fatty acids, which are essential polyunsaturated fatty acids with some roles in normal cellular metabolism (Scorletti & Byrne, 2013), have been reported to be necessary for membrane fluidity, inflammatory eicosanoids, and neural membrane oxidation (Salvati et al., 2006). Coqueiro, Bueno, and Simões (2011) reported that omega‐3 fatty acid supplementation significantly reduced acute inflammatory marker and muscle lesion marker levels. Researchers have reported the neuroprotective effect of omega‐3 polyunsaturated fatty acids against PD (Mori et al., 2018). The possible neuroprotective effect of omega‐3 fatty acids against ischemia‐induced rats (Nobre et al., 2016). Georgiou et al. (2017) have reported the neuroprotective effects of omega‐3 fatty acids against ischemic optic neuropathy induced rats. Researchers have reported the neuroprotective effect of omega‐3 fatty acids to increase neurogenesis in the culture model of hypoxic condition (Lo Van et al., 2019).
Leukotriene‐5, thromboxane‐3, and prostaglandin‐3 are derived from essential fatty acids, and are known to be therapeutically valuable in inflammation and mental health conditions (de Batlle et al., 2012; Huan et al., 2004; Kiecolt‐Glaser et al., 2011; Peet et al., 2001; Perica & Delas, 2011). Hussein et al. (2018) reported that ILs are essential cytokines involved in several immunological processes. Supplementation with omega‐3 fatty acids has been shown to reduce levels of inflammatory markers and lipid peroxidation in chronic cardiomyopathy patients (Silva et al., 2017). Hu et al. (2018) reported the beneficial effects of omega‐3 fatty acid supplementation on inflammatory markers in chronic kidney disease. Omega‐3 fatty acids reduce lipid peroxidation and increase SOD activity (Avramovic et al., 2012). The observations of reduced lipid peroxidation and elevated antioxidant marker levels observed in this study with omega‐3 fatty acid supplementation were consistent with the above findings.
Supplementation with omega‐3 fatty acid significantly inhibited oxidative stress in rat liver regeneration (Ozgur et al., 2017). Spinal cord injury leads to functional changes in autonomic function, loss of sensation, and muscle wasting (Krucoff et al., 2016). The induction in apoptosis in neurons and oligodendrocytes has been reported to lead to axonal degeneration, demyelination, and dysfunction (Lee et al., 2003; Yune et al., 2008). In spinal cord injury, apoptosis of oligodendrocytes and neurons leads to increased oxidative stress and inflammation (Bao & Liu, 2002; Yune et al., 2008). Proinflammatory cytokines and ROS are produced during inflammatory and neurodegenerative states (Block & Hong, 2005; Min et al., 2004; Qin et al., 2004). The microglia release pro‐NGF, which leads to cell death (Yune et al., 2007). Sinha et al. (2009) reported that omega‐3‐fatty acids had an antiapoptotic effect in the developing rat brain. Omega‐3 fatty acids reduced oxidative stress and apoptosis in a rat model of doxorubicin‐induced testicular damage (Uygur et al., 2014). These results were consistent with our results, indicating the inhibition of apoptosis by omega‐3 fatty acid supplementation.
5. CONCLUSION
In summary, the results of the study suggested that omega‐3 fatty acid supplementation significantly reduced oxidative stress, apoptosis, and the levels of inflammatory markers in ischemia‐reperfusion‐induced rats.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Bi J, Chen C, Sun P, Tan H, Feng F, Shen J. Neuroprotective effect of omega‐3 fatty acids on spinal cord injury induced rats. Brain Behav. 2019;9:e01339 10.1002/brb3.1339
Funding information
This work was supported by the Natural Science Foundation of China (No: 81330044 and No: 81772424).
REFERENCES
- Ahmed, M. A. , Abd, E. L. , & Samad, A. A. (2013). Benefits of omega‐3 fatty acid against bone changes in salt‐loaded rats: Possible role of the kidney. Physiological Reports, 1(5), e00106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avramovic, N. , Dragutinovic, V. , Krstic, D. , Colovic, M. , Trbovic, A. , de Luka, S. , … Popovic, T. (2012). The effects of omega 3 fatty acid supplementation on brain tissue oxidative status in aged wistar rats. Hippokratia, 16(3), 241–245. [PMC free article] [PubMed] [Google Scholar]
- Bao, F. , & Liu, D. (2002). Peroxynitrite generated in the rat spinal cord induces neuron death and neurological deficits. Neuroscience, 115, 839–849. 10.1016/S0306-4522(02)00506-7 [DOI] [PubMed] [Google Scholar]
- Block, M. L. , & Hong, J. S. (2005). Microglia and inflammation‐mediated neurodegeneration: Multiple triggers with a common mechanism. Progress in Neurobiology, 76, 77–98. 10.1016/j.pneurobio.2005.06.004 [DOI] [PubMed] [Google Scholar]
- Coqueiro, D. , Bueno, P. , & Simões, M. (2011). Acute effect of omega‐3 polyunsaturated fatty acids on muscle lesion and inflammation markers in rats subjected to resistive exercises. Gazzetta Medica Italiana Archivio per Le Scienze Mediche, 170, 397–403. [Google Scholar]
- de Batlle, J. , Sauleda, J. , Balcells, E. , Gómez, F. P. , Méndez, M. , Rodriguez, E. , … Garcia‐Aymerich, J. (2012). Association between Omega3 and Omega6 fatty acid intakes and serum inflammatory markers in COPD. Journal of Nutritional Biochemistry, 23, 817–821. [DOI] [PubMed] [Google Scholar]
- Firat, O. , Makay, O. , Yeniay, L. , Gokce, G. , Yenisey, C. , & Coker, A. (2017). Omega‐3 fatty acids inhibit oxidative stress in a rat model of liver regeneration. Annals of Surgical Treatment and Research, 93(1), 1–10. 10.4174/astr.2017.93.1.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgiou, T. , Wen, Y. T. , Chang, C. H. , Kolovos, P. , Kalogerou, M. , Prokopiou, E. , … Tsai, R. K. (2017). Neuroprotective effects of omega‐3 polyunsaturated fatty acids in a rat model of anterior ischemic optic neuropathy. Investigative Ophthalmology & Visual Science, 58(3), 1603–1611. 10.1167/iovs.16-20979 [DOI] [PubMed] [Google Scholar]
- Hend, M. T. , Omnia, E. K. , Hekmat, M. T. , & Amira, A. F. (2014). Potential anti‐inflammatory effect of lemon and hot pepper extracts on adjuvant‐induced arthritis in mice. The Journal of Basic & Applied Zoology, 67, 149–157. [Google Scholar]
- Hu, C. , Yang, M. , Zhu, X. , Gao, P. , Yang, S. , Han, Y. , … Sun, L. (2018). Effects of omega‐3 fatty acids on markers of inflammation in patients with chronic kidney disease: A controversial issue. Therapeutic Apheresis and Dialysis, 22(2), 124–132. 10.1111/1744-9987.12611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huan, M. , Hamazaki, K. , Sun, Y. , Itomura, M. , Liu, H. , Kang, W. , … Hamazaki, T. (2004). Kang, Suicide attempt and n‐3 fatty acid levels in red blood cells: A case‐control study in China. Biological Psychiatry, 56, 490–496. 10.1016/j.biopsych.2004.06.028 [DOI] [PubMed] [Google Scholar]
- Hussein, J. , El‐Banna, M. , Razik, T. A. , & El‐Naggar, M. E. (2018). Biocompatible zinc oxide nanocrystals stabilized via hydroxyethyl cellulose for mitigation of diabetic complications. International Journal of Biological Macromolecules, 107 (Pt A), 748–754. 10.1016/j.ijbiomac.2017.09.056 [DOI] [PubMed] [Google Scholar]
- Kaddour, T. , Omar, K. , Oussama, A. T. , Nouria, H. , Iméne, B. , & Abdelkader, A. (2016). Aluminium-induced acute neurotoxicity in rats: Treatment with aqueous extract of Arthrophytum (Hammada scoparia). Journal of Acute Disease, 5, 470–482. [Google Scholar]
- Kiecolt‐Glaser, J. K. , Belury, M. A. , Andridge, R. , Malarkey, W. B. , & Glaser, R. (2011). Omega‐3 supplementation lowers inflammation and anxiety in medical students: A randomized controlled trial. Brain, Behavior, and Immunity, 25, 1725–1734. 10.1016/j.bbi.2011.07.229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krucoff, M. O. , Rahimpour, S. , Slutzky, M. W. , Edgerton, V. R. , & Turner, D. A. (2016). Enhancing nervous system recovery through neurobiologics, neural interface training, and neurorehabilitation. Frontiers in Neuroscience, 10, 584 10.3389/fnins.2016.00584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, S. M. , Yune, T. Y. , Kim, S. J. , Park, D. W. , Lee, Y. K. , Kim, Y. C. , … Oh, T. H. (2003). Minocycline reduces cell death and improves functional recovery after traumatic spinal cord injury in the rat. Journal of Neurotrauma, 20, 1017–1027. 10.1089/089771503770195867 [DOI] [PubMed] [Google Scholar]
- Lo Van, A. , Sakayori, N. , Hachem, M. , Belkouch, M. , Picq, M. , Fourmaux, B. , … Bernoud‐Hubac, N. (2019). Targeting the brain with a neuroprotective omega‐3 fatty acid to enhance neurogenesis in hypoxic condition in culture. Molecular Neurobiology, 56(2), 986–999. 10.1007/s12035-018-1139-0 [DOI] [PubMed] [Google Scholar]
- Maggio, M. , Artoni, A. , Lauretani, F. , Borghi, L. , Nouvenne, A. , Valenti, G. , & Ceda, G. P. (2009). The impact of omega‐3 fatty acids on osteoporosis. Current Pharmaceutical Design, 15, 4157–4164. [DOI] [PubMed] [Google Scholar]
- Min, K. J. , Pyo, H. K. , Yang, M. S. , Ji, K. A. , Jou, I. , & Joe, E. H. (2004). Gangliosides activate microglia via protein kinase C and NADPH oxidase. Glia, 48, 197–206. 10.1002/glia.20069 [DOI] [PubMed] [Google Scholar]
- Moon, Y. J. , Lee, J. Y. , Oh, M. S. , Pak, Y. K. , Park, K. S. , Oh, T. H. , & Yune, T. Y. (2012). Inhibition of inflammation and oxidative stress by Angelica dahuricae radix extract decreases apoptotic cell death and improves functional recovery after spinal cord injury. Journal of Neuroscience Research, 90(1), 243–256. 10.1002/jnr.22734 [DOI] [PubMed] [Google Scholar]
- Mori, M. A. , Delattre, A. M. , Carabelli, B. , Pudell, C. , Bortolanza, M. , Staziaki, P. V. , … Ferraz, A. C. (2018). Neuroprotective effect of omega‐3 polyunsaturated fatty acids in the 6‐OHDA model of Parkinson's disease is mediated by a reduction of inducible nitric oxide synthase. Nutritional Neuroscience, 21(5), 341–351. 10.1080/1028415X.2017.1290928 [DOI] [PubMed] [Google Scholar]
- Naruo, S. , Okajima, K. , Taoka, Y. , Uchiba, M. , Nakamura, T. , Okabe, H. , & Takagi, K. (2003). Prostaglandin E1 reduces compression trauma‐induced spinal cord injury in rats mainly by inhibiting neutrophil activation. Journal of Neurotrauma, 20(2), 221–228. [DOI] [PubMed] [Google Scholar]
- Nobre, M. E. , Correia, A. O. , Mendonça, F. N. , Uchoa, L. R. , Vasconcelos, J. T. , de Araújo, C. N. , … Viana, G. S. (2016). Omega‐3 fatty acids: Possible neuroprotective mechanisms in the model of global ischemia in rats. Journal of Nutrition and Metabolism, 2016, 6462120 10.1155/2016/6462120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozgur, F. , Ozer, M. , Levent, Y. , Goksel, G. , Cigdem, Y. , & Ahmet, C. (2017). Omega-3 fatty acids inhibit oxidative stress in a rat model of liver regeneration. Annals of Surgical Treatment and Research, 93(1), 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peet, M. , Brind, J. , Ramchand, C. N. , Shah, S. , & Vankar, G. K. (2001). Two double‐blind placebo‐controlled pilot studies of eicosapentaenoic acid in the treatment of schizophrenia. Schizophrenia Research, 49, 243–251. [DOI] [PubMed] [Google Scholar]
- Perica, M. M. , & Delas, I. (2011). Essential fatty acids and psychiatric disorders. Nutrition in Clinical Practice, 26, 409–425. 10.1177/0884533611411306 [DOI] [PubMed] [Google Scholar]
- Qin, L. , Liu, Y. , Wang, T. , Wei, S. J. , Block, M. L. , Wilson, B. , … Hong, J. S. (2004). NADPH oxidase mediates lipopolysaccharide‐induced neurotoxicity and proinflammatory gene expression in activated microglia. Journal of Biological Chemistry, 279, 1415–1421. 10.1074/jbc.M307657200 [DOI] [PubMed] [Google Scholar]
- Sakanaka, M. , Zhu, P. , Zhang, B. , Wen, T. C. , Cao, F. , Ma, Y. J. , … Hata, R. (2007). Intravenous infusion of dihydroginsenoside Rb1 prevents compressive spinal cord injury and ischemic brain damage through upregulation of VEGF and Bcl‐xL. Journal of Neurotrauma, 24, 1037–1054. [DOI] [PubMed] [Google Scholar]
- Salvati, S. , Attorri, L. , DiBenedetto, R. , DiBiase, A. , & Leonardi, F. (2006). Polyunsaturated fatty acids and neurological diseases. Mini Reviews in Medicinal Chemistry, 6, 1201–1211. [DOI] [PubMed] [Google Scholar]
- Scorletti, E. , & Byrne, C. D. (2013). Omega‐3 fatty acids, hepatic lipid metabolism, and nonalcoholic fatty liver disease. Annual Review of Nutrition, 33, 231–248. 10.1146/annurev-nutr-071812-161230 [DOI] [PubMed] [Google Scholar]
- Silva, P. S. D. , Mediano, M. F. F. , Silva, G. M. S. D. , Brito, P. D. , Cardoso, C. S. A. , Almeida, C. F. , … Sousa, A. S. (2017). Omega‐3 supplementation on inflammatory markers in patients with chronic Chagas cardiomyopathy: A randomized clinical study. Nutrition Journal, 16(1), 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha, R. A. , Khare, P. , Rai, A. , Maurya, S. K. , Pathak, A. , Mohan, V. , … Bandyopadhyay, S. (2009). Anti‐apoptotic role of omega‐3‐fatty acids in developing brain: Perinatal hypothyroid rat cerebellum as apoptotic model. International Journal of Developmental Neuroscience, 27, 377–383. 10.1016/j.ijdevneu.2009.02.003 [DOI] [PubMed] [Google Scholar]
- Uygur, R. , Aktas, C. , Tulubas, F. , Uygur, E. , Kanter, M. , Erboga, M. , … Ozen, O. A. (2014). Protective effects of fish omega‐3 fatty acids on doxorubicin‐induced testicular apoptosis and oxidative damage in rats. Andrologia, 46(8), 917–926. 10.1111/and.12173 [DOI] [PubMed] [Google Scholar]
- Yune, T. Y. , Lee, J. Y. , Jiang, M. H. , Kim, D. W. , Choi, S. Y. , & Oh, T. H. (2008). Systemic administration of PEP‐1‐SOD1 fusion protein improves functional recovery by inhibition of neuronal cell death after spinal cord injury. Free Radical Biology and Medicine, 45, 1190–1200. 10.1016/j.freeradbiomed.2008.07.016 [DOI] [PubMed] [Google Scholar]
- Yune, T. Y. , Lee, J. Y. , Jung, G. Y. , Kim, S. J. , Jiang, M. H. , Kim, Y. C. , … Oh, T. H. (2007). Minocycline alleviates death of oligodendrocytes by inhibiting pro‐nerve growth factor production in microglia after spinal cord injury. Journal of Neuroscience, 27, 7751–7761. 10.1523/JNEUROSCI.1661-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


