Abstract
Introduction
The corpus callosum serves the essential role of relaying cognitive information between the homologous regions in the left and the right hemispheres of the brain. Cognitive impairment is a core dysfunction of schizophrenia, but much of its pathophysiology is unknown. The aim of this study was to elucidate the association between microstructural abnormalities of the corpus callosum and cognitive dysfunction in schizophrenia.
Methods
We examined stepwise multiple regression analysis to investigate the relationship of the fractional anisotropy (FA) of callosal fibers in each segment with z‐scores of each brief assessment of cognition in schizophrenia subtest and cognitive composite score in all subjects (19 patients with schizophrenia [SZ group] and 19 healthy controls [HC group]). Callosal fibers were separated into seven segments based on their cortical projection using tract‐specific analysis of diffusion tensor imaging.
Results
The FA of callosal fibers in the temporal segment was significantly associated with z‐scores of token motor test, Tower of London test, and the composite score. In the SZ group, the FA of callosal fibers in the temporal segment was significantly associated with the z‐score of the Tower of London test. In addition, the FA of callosal fibers in temporal segment showed significant negative association with the positive and negative syndrome scale negative score in the SZ group. Compared to the HC group, the FA in temporal segment was significantly decreased in the SZ group.
Conclusion
Our results suggest that microstructural abnormalities in the callosal white matter fibers connecting bilateral temporal lobe cortices contribute to poor executive function and severe negative symptom in patients with schizophrenia.
Keywords: cognition, corpus callosum, diffusion tensor imaging, magnetic resonance imaging, schizophrenia
1. INTRODUCTION
The disconnection hypothesis in schizophrenia (Friston, 1999) is supported by repeated reports of abnormalities of white matter (WM) fibers that connect brain regions (Kubicki & Shenton, 2014; Samartzis, Dima, Fusar‐Poli, & Kyriakopoulos, 2014; Wheeler & Voineskos, 2014). The corpus callosum (CC), the largest commissural fiber bundle in the brain, connects the left and the right hemispheres and serves an essential role of relaying sensory, motor, and cognitive information between the homologous regions (Huang et al., 2005; Ribolsi, Daskalakis, Siracusano, & Koch, 2014). Many studies have investigated abnormalities of the CC in schizophrenia with an aim of studying the disconnection between the two hemispheres (Isobe et al., 2016; Ribolsi et al., 2014).
Advances in diffusion tensor imaging (DTI) have enabled capture of microstructural WM abnormality. Techniques to build three‐dimensional fiber tracts based on DTI provide opportunities to investigate how specific fiber tracts may affect disorders, by visualizing the trajectories of specific WM fiber bundles and by quantitatively characterizing each fiber tract (Lee et al., 2005; Wakana et al., 2007).
Cognitive impairment is a core dysfunction of schizophrenia that is associated with functional prognosis (Green & Harvey, 2014), but much of its pathophysiology is unknown. Cognitive performance is strongly associated with communication between multiple brain regions (Fox et al., 2005). Development of CC in childhood is correlated with intelligence, processing speed, and problem‐solving ability, and is thought to play a fundamental role in cognitive functioning (Hinkley et al., 2012). However, few studies have performed detailed investigation of the relationship between microstructural abnormalities of CC and cognitive functioning in schizophrenia.
In the current study, we examined the association between microstructural abnormalities of CC fibers and cognitive dysfunction in schizophrenia by segmenting the CC fibers based on their cortical projection regions using DTI tract‐specific analysis (TSA). We hypothesized that patients with schizophrenia show microstructural abnormalities in CC fibers and these abnormalities are related to their cognitive impairment.
2. METHODS
2.1. Subjects
The subjects were 19 patients with schizophrenia (SZ group) and 19 healthy controls (HC group; Table 1). The subjects were diagnosed by two independent well‐trained psychiatrists based on the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (APA, 1994), and were recruited from Wakayama Medical University Hospital. Patients with comorbid psychiatric, neurological, or medical illness, or those with substance or alcohol abuse were excluded from the study. All patients were on antipsychotic medication. Equivalent doses of antipsychotics were calculated using the equivalent conversion table originally reported by Inagaki and Inada (Inada & Inagaki, 2015). This study was approved by the Ethics Committee of Wakayama Medical University, and written informed consent was obtained from all subjects.
Table 1.
Demographic and clinical characteristics
| HC group (n = 19) | SZ group (n = 19) | Statistics | ||||
|---|---|---|---|---|---|---|
| Mean ± SD | Range | Mean ± SD | Range | p | ||
| Gender, male/femalea, n | 7/12 | 9/10 | χ 2 = 0.43 | 0.45 | ||
| Ageb | 41.89 ± 10.25 | 30–60 | 44.16 ± 7.98 | 34–60 | t = −0.76 | 0.74 |
| Duration of illness, years | 18.58 ± 10.12 | |||||
| PANSS positive | 14.05 ± 5.78 | |||||
| PANSS negative | 18.16 ± 5.70 | |||||
| PANSS general psychopathology | 32.53 ± 10.36 | |||||
| PANSS total | 64.74 ± 20.11 | |||||
| Medication, CPZ equivalent (mg/day) | 642.26 ± 330.66 | |||||
| Verbal memoryb, z‐score | −0.04 ± 0.12 | −2.17 ± 1.30 | t = 5.25 | 0.00 | ||
| Digit sequencingb, z‐score | 0.21 ± 0.94 | −1.52 ± 0.95 | t = 5.63 | 0.00 | ||
| Token motor taskb, z‐score | 0.19 ± 0.90 | −1.88 ± 1.62 | t = 4.89 | 0.00 | ||
| Verbal fluencyb, z‐score | 0.37 ± 1.00 | −1.31 ± 1.11 | t = 4.88 | 0.00 | ||
| Symbol coding taskb, z‐score | 0.92 ± 1.10 | −1.86 ± 1.97 | t = 5.38 | 0.00 | ||
| Tower of Londonb, z‐score | 0.04 ± 0.94 | −1.72 ± 2.19 | t = 3.22 | 0.00 | ||
| Composite scoreb, z‐score | 0.28 ± 0.78 | −1.74 ± 1.17 | t = 6.25 | 0.00 | ||
Abbreviations: CPZ, chlorpromazine; HC, healthy controls; n, number; PANSS, Positive and Negative Syndrome Scale; SD, standard deviation; SZ, schizophrenia.
Chi‐square test.
Independent‐samples t test.
2.2. Neuropsychological measurements
The severity of clinical symptoms was assessed using the Positive and Negative Syndrome Scale (PANSS). Neurocognitive function was tested by experienced psychologists using the Brief Assessment of Cognition in Schizophrenia (BACS) Japanese version (Kaneda et al., 2007), which is widely used in Japan (Ikebuchi et al., 2017; Itakura et al., 2017; Satogami, Takahashi, Yamada, Ukai, & Shinosaki, 2017; Sawada et al., 2017; Takahashi et al., 2013). This battery includes six subtests: list learning test (verbal memory), digit sequencing test (working memory), token motor test, verbal fluency test, symbol coding test (attention), and the Tower of London test (executive function). In the BACS, z‐scores were calculated for each subcomponent score using means and standard deviations based on the dataset of healthy Japanese populations (Kaneda et al., 2013). The composite score was calculated by averaging all z‐scores of six subcomponents.
2.3. MRI data acquisition
We acquired anatomical MRI and DTI data on a 3.0T MR scanner (Achieva TX 3.0T; Philips Medical Systems) using a 32‐element sensitivity‐encoding head coil. A 3D fast field echo T1‐weighted sequence was used for anatomical MRI (TR/TE = 7.0/3.3 ms, FOV = 220 mm, 210 slices, acquisition voxel size = 0.86 × 0.86 × 0.9 mm, and a slice thickness = 0.9 mm). DTI images were acquired using a single‐shot spin‐echo echoplanar imaging diffusion sequence with fifty‐five 2.5‐mm slices (no interslice gap), TR/TE = 6,421/69 ms, FOV = 224 mm, acquisition voxel size = 2.0 × 2.0 × 2.5 mm, and 2b values of 0 and 1,000, 15 directions.
2.4. DTI data processing
Some DTI‐derived data, such as fractional anisotropy (FA) and the apparent diffusion coefficient (ADC), provide information on WM diffusion (Basser & Pierpaoli, 1996). FA is a composite measure of three eigenvalues (λ 1, λ 2, and λ 3). λ 1, the largest in three, which is called the axial diffusivity (AD), is the component parallel to, and λ 2 and λ 3, whose average is called the radial diffusivity (RD), are components perpendicular to the axonal fibers (Basser, 1995; Wozniak & Lim, 2006). We selected FA as a main index because it measures the degree of water diffusion anisotropy on a scale from zero to one and characterizes WM microstructural abnormalities (Basser & Pierpaoli, 1996). In addition, FA is the most widely used anisotropy measure (O'Donnell & Westin, 2011). We used Philips Extended Workspace (EWS, Release 2.6.3.1; Philips) to analyze DTI data. FA threshold for line tracking was set to 0.2. The maximum angle threshold was 50°. We performed tractography using the two‐regions‐of‐interest (ROIs) approach. In recent years, analyses have been carried out by segmenting CC fibers using a two‐ROIs approach into multiple regions based on the cortical regions that the CC projects to (Huang et al., 2005; Lebel, Caverhill‐Godkewitsch, & Beaulieu, 2010). One of our previous studies applied that technique in mood disorder investigations (Yamada et al., 2015). The first reference ROI was focused on the CC in a midsagittal slice, and secondary ROI was seven separate cortices spanning both sides of the midline (Appendix S1). As seen in Figure 1, callosal fibers were separated into seven segments based on their cortical projection zones. Ordered from front to back, the seven sections were as follows: orbital frontal, anterior frontal, superior frontal, superior parietal, posterior parietal, temporal, and occipital. All ROIs were drawn in accordance with specific anatomical landmarks and guidelines that were followed carefully and consistently for all patients (Figure 2). Same as the previous studies (Brandstack, Kurki, Laalo, Kauko, & Tenovuo, 2016; Huang et al., 2005; Lebel et al., 2010), fibers that were clearly not part of the anatomical connectivity of the tracking were manually removed with exclusion ROIs to include only fibers within the desired tract. Calculation of FA was made by averaging all voxels for each region over the entire tracking. Tractography was performed by one operator (Y.O.). In order to assess validity of ROI procedure, another operator (K.T.) who was blinded subjects' diagnosis, age, gender, and handedness analyzed five subjects in the SZ group and five subjects in the HC group, and interoperator reliabilities for FA values were examined. Intraclass correlation coefficients of FA value of seven segments (orbital frontal, anterior frontal, superior frontal, superior parietal, posterior parietal, temporal, and occipital) were 0.903, 0.956, 0.976, 0.976, 0.726, 0.953, and 0.740, respectively.
Figure 1.
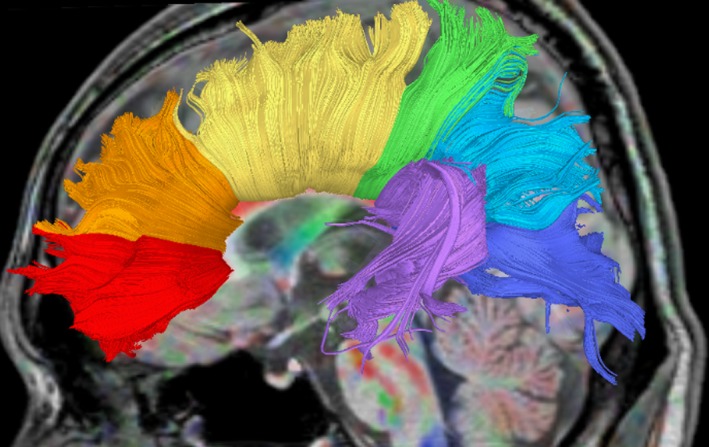
Segmentation of corpus callosum by tractography. The corpus callosum was subdivided into seven separate segments using a two‐regions‐of‐interest (ROIs) fiber tracking approach in accordance with a determined rule and specific anatomical landmarks. The seven segments are, in order from most front to most back, as follows: orbital frontal (OF), anterior frontal (AF), superior frontal (SF), superior parietal (SP), posterior parietal (PP), temporal (Temp), and occipital (Occ)
Figure 2.
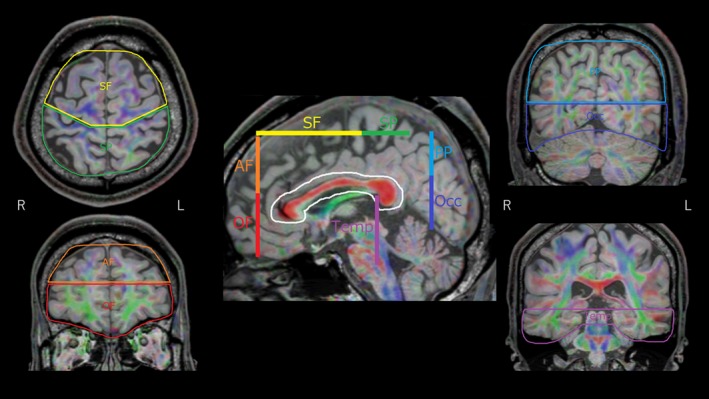
Locations of secondary regions‐of‐interest (ROI) to separate fibers projecting to different cortical areas
2.5. Statistics
The differences between the SZ and HC groups in age and z‐scores of each neurocognitive test were examined by independent‐samples t test. Gender difference between the groups was assessed using the chi‐square test. Using Pearson's correlation test, correlation of FA of each callosal segment with age was assessed in the HC groups, and correlation of FA of each callosal segment with age and equivalent doses of antipsychotics was assessed in the SZ group. Correlation of the z‐score of neuropsychological tests with equivalent doses of antipsychotics was also assessed using Pearson's correlation test in the SZ group. In the above analyses, the statistical significance level was set at p < 0.05. Stepwise multiple regression analysis was performed, with the z‐scores of list learning test, digit sequencing test, token motor test, verbal fluency test, symbol coding test, the Tower of London test, and the composite score as dependent variables, and the FA of callosal fibers in each segment as independent variables to investigate the relationship between the FA and cognitive function in all subjects. The statistical significance level was set at p < 0.0071 (adjusted for the Bonferroni correction; 0.05/7 cognitive scores) for cognitive tests. If we find significant relationship between FA of callosal fibers and z‐scores of cognitive subtests in all subjects, subsequent stepwise multiple regression analysis in same relation was performed in the SZ group. The statistical significance level was set at p < 0.05/number of tests (adjusted for the Bonferroni correction). Stepwise multiple regression analysis was also performed to investigate the relationship between the FA of callosal fibers in each segment and PANSS scores in the SZ group. The statistical significance level was set at p < 0.0125 (adjusted for the Bonferroni correction; 0.05/4 PANSS subtests). Independent‐samples t test was used to examine differences between the SZ and HC groups in the FA of callosal fiber in the segment which identified the significant relation in regression analysis in the SZ group. The statistical significance level was set at p < 0.05/number of tests (adjusted for the Bonferroni correction). In the same approach, stepwise multiple regression analysis was also performed with other DTI index, which includes the ADC, AD, and RD of callosal fibers in each segment as independent variables. All statistical analyses were performed using the IBM SPSS Statistics for Windows (IBM Japan, Ltd.).
3. RESULTS
3.1. Demographic and clinical characteristics
There were no differences in age and gender between the SZ and HC groups (Table 1). In the BACS, the SZ group showed significantly lower z‐scores in six subtests and composite scores when compared to those in the HC groups (Table 1).
3.2. Correlation of the FA of callosal fibers with age and equivalent dose of antipsychotics
The FA of callosal fibers significantly correlated with age in the superior frontal segment (r = −0.604, p = 0.006) and posterior parietal segment (r = 0.506, p = 0.027) in the HC group and in the anterior frontal segment (r = −0.481, p = 0.037) in the SZ group. There were no significant correlations between the FA of callosal fibers and equivalent dose of antipsychotics in the SZ group.
3.3. Correlation of the z‐score of neuropsychological tests and equivalent doses of antipsychotics
There were no significant correlations between the z‐score of neuropsychological tests and equivalent dose of antipsychotics in the SZ group (verbal memory, r = −0.079, p = 0.749; working memory, r = 0.238, p = 0.326; token motor test, r = −0.151, p = 0.536; verbal fluency test, r = 0.155, p = 0.525; attention, r = 0.077, p = 0.754; executive function, r = −0.150, p = 0.541; composite score, r = −0.017, p = 0.943).
3.4. Stepwise multiple regression analysis of the FA
In all subjects, the FA of callosal fibers in the temporal segment was significantly associated with the z‐scores of token motor test, Tower of London test, and the composite score. The regression for token motor test identified the FA of temporal segment as predictive variable accounting for 19.1% of the variance (Β = 46.2; F = 8.52; p = 0.006). The regression for the Tower of London test identified the FA of temporal segment as predictive variable accounting for 24.0% of the variance (B = 58.6; F = 11.3; p = 0.002). The regression for the composite score identified the FA of temporal segment as predictive variable accounting for 18.5% of the variance (B = 38.8; F = 8.14; p = 0.007). In the SZ group, the FA of callosal fibers in the temporal segment was significantly associated with the z‐score of the Tower of London test. The regression for the Tower of London test identified the FA of temporal segment as predictive variable accounting for 29.5% of the variance (B = 73.2; F = 7.12; p = 0.016; Figure 3). In the SZ group, the FA of callosal fibers in the posterior parietal segment was significantly associated with the scores of the PANSS positive, PANSS negative, PANSS general psychopathology, and PANSS total. The FA of callosal fibers in the temporal segment was significantly associated with the score of the PANSS negative. The regression for the PANSS positive identified the FA of posterior parietal segment as predictive variable accounting for 39.8% of the variance (B = 275.84; F = 11.26; p = 0.004). The regression for the PANSS negative identified the FA of posterior parietal segment (B = 396.79; p < 0.001) and temporal segment (B = −197.60; p = 0.004) as predictive variable accounting for 65.9% of the variance (F = 15.45; p < 0.001). The regression for the PANSS general psychopathology identified the FA of posterior parietal segment as predictive variable accounting for 52.4% of the variance (B = 567.38; F = 18.72; p < 0.001). The regression for the PANSS total identified the FA of posterior parietal segment as predictive variable accounting for 54.4% of the variance (B = 1,121.63; F = 20.26; p < 0.001).
Figure 3.
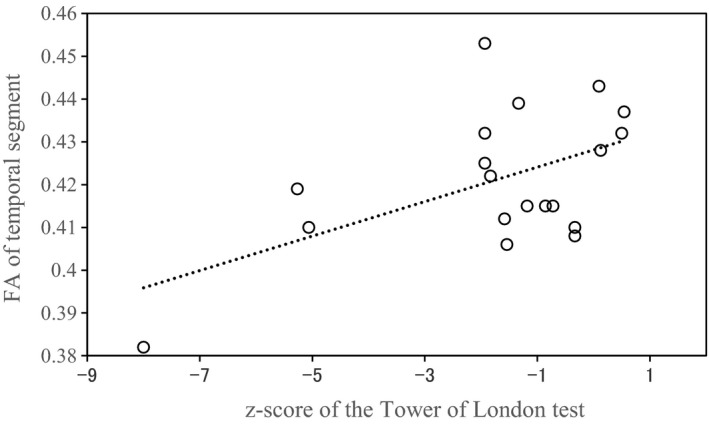
Scattergram for the association between fractional anisotropy (FA) of callosal fibers in the temporal segment and score of Tower of London test in the schizophrenia (SZ) group
3.5. Differences in the FA of callosal fiber in the segment which identified the regression in the SZ group between the SZ and HC groups
Independent‐samples t test revealed significant differences in FA between the SZ and HC groups in temporal segment (t = 2.80, p = 0.008) but not in posterior parietal segments (t = 1.75, p = 0.088; Figure 4 and Table 2).
Figure 4.
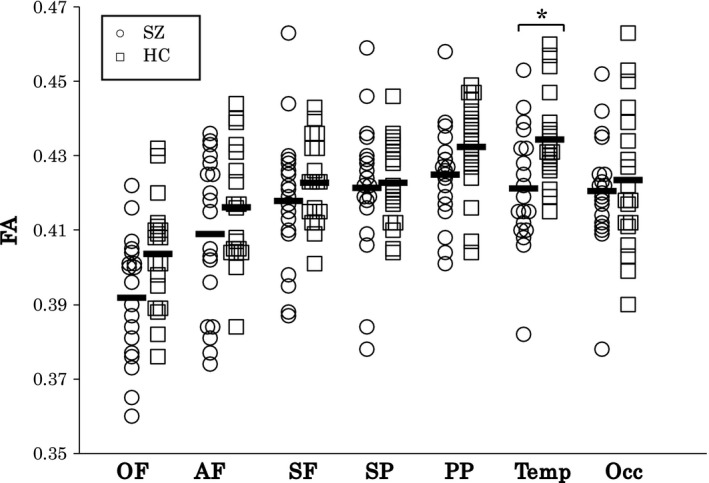
Scattergram for the FA of callosal fibers in the OF, AF, SF, SP, PP, Temp, and Occ segments in HC and SZ groups. The black bars represent means of each segment. Data marked * are significant at p < 0.025. AF, anterior frontal; FA, fractional anisotropy; HC, healthy controls; Occ, occipital; OF, orbital frontal; PP, posterior parietal; SF, superior frontal; SP, superior parietal; SZ, schizophrenia; Temp, temporal
Table 2.
FA and callosal fibers in the OF, AF, SF, SP, PP, Temp, and Occ segments in the HC and SZ groups
| HC group | SZ group | Statistics | ||
|---|---|---|---|---|
| Mean ± SD | Mean ± SD | t | p | |
| OF | 0.404 ± 0.015 | 0.392 ± 0.017 | ||
| AF | 0.416 ± 0.016 | 0.409 ± 0.021 | ||
| SF | 0.423 ± 0.011 | 0.418 ± 0.019 | ||
| SP | 0.423 ± 0.011 | 0.421 ± 0.019 | ||
| PP | 0.432 ± 0.013 | 0.425 ± 0.013 | 1.75 | 0.088 |
| Temp | 0.434 ± 0.012 | 0.421 ± 0.016 | 2.80 | 0.008 |
| Occ | 0.423 ± 0.020 | 0.421 ± 0.015 | ||
Abbreviations: AF, anterior frontal; FA, fractional anisotropy; HC, healthy controls; Occ, occipital; OF, orbital frontal; PP, posterior parietal; SD, standard deviation; SF, superior frontal; SP, superior parietal; SZ, schizophrenia; Temp, temporal.
3.6. Statistical analysis on the ADC, AD, and RD
In all subjects, the regression for verbal memory identified the ADC of orbital frontal segment (B = −24.58; p = 0.004) and posterior parietal segment (B = 10.88; p = 0.02) as predictive variable accounting for 25.5% of the variance (F = 6.00; p = 0.006). The regression for symbol coding test identified the ADC of posterior parietal segment (B = 13.01; p = 0.027) and occipital segment (B = −19.13; p = 0.003) as predictive variable accounting for 26.6% of the variance (F = 6.33; p = 0.005). The regression for the composite score identified the ADC of orbital frontal segment (B = −15.46; p = 0.037), posterior parietal segment (B = 10.47; p = 0.008), and occipital segment (B = −9.10; p = 0.035) as predictive variable accounting for 34.2% of the variance (F = 5.89; p = 0.002). In the SZ group, the ADC of callosal fibers in the occipital segment was significantly associated with the z‐score of verbal memory and the composite score. The regression for verbal memory identified the ADC of occipital segment as predictive variable accounting for 36.2% of the variance (B = −12.98; F = 9.64; p = 0.006), and the regression for the composite score identified the ADC of occipital segment as predictive variable accounting for 37.4% of the variance (B = −11.91; F = 10.16; p = 0.005).
In all subjects, the regression for symbol coding test identified the AD of temporal segment (B = 10.08; p = 0.015) and occipital segment (B = −13.01; p = 0.007) as predictive variable accounting for 25.3% of the variance (F = 5.92; p = 0.006). The regression for the Tower of London identified the AD of anterior frontal segment (B = −12.44; p = 0.020) and temporal segment (B = 11.55; p = 0.003) as predictive variable accounting for 25.7% of the variance (F = 6.04; p = 0.006). In the SZ group, the AD of callosal fibers in the orbital frontal and occipital segment was significantly associated with the z‐score of symbol coding test. The regression for symbol coding test identified the AD of orbital frontal segment (B = 28.84; p = 0.024) and occipital segment (B = −20.66; p = 0.001) as predictive variable accounting for 50.5% of the variance (F = 8.16; p = 0.004).
In all subjects, the regression for verbal memory identified the RD of the orbital frontal segment (B = −21.08; p = 0.013), the temporal segment (B = 13.97; p = 0.012), and the occipital segment (B = −10.96; p = 0.041) as predictive variable accounting for 34.5% of the variance (F = 5.96; p = 0.002). In the SZ group, the RD of callosal fibers in the occipital segment was significantly associated with the z‐score of verbal memory. The regression for verbal memory identified the RD of occipital segment as predictive variable accounting for 33.9% of the variance (B = −13.10; F = 8.74; p = 0.009). In the SZ group, the RD of callosal fibers in the posterior parietal and occipital segment was significantly associated with the scores of the PANSS general psychopathology and PANSS total. The regression for the PANSS general psychopathology identified the RD of posterior parietal segment (B = −398.75; p = 0.001) and occipital segment (B = 109.26; p = 0.010) as predictive variable accounting for 54% of the variance (F = 9.40; p = 0.002). The regression for the PANSS total identified the RD of posterior parietal segment (B = −751.67; p = 0.001) and occipital segment (B = 227.06; p = 0.007) as predictive variable accounting for 52.3% of the variance (F = 8.78; p = 0.003).
In the AD, independent‐samples t test revealed significant differences in orbital frontal segment (t = −3.06, p = 0.004) but not in occipital segment (t = −1.05, p = 0.300). In the ADC and RD, independent‐samples t test revealed no significance.
4. DISCUSSION
In the current study, we extracted and divided the CC fibers into seven regions based on the cortical projection regions, and investigated the relationship between the FA values of the CC fibers and cognitive function in schizophrenia. In the SZ group, FA values of the CC that connects the bilateral temporal lobe cortices associated with executive function scores, and FA value of this tract was significantly decreased compared to the HC group. These results indicate an association between microstructural abnormalities of the CC white matter and cognitive dysfunction in schizophrenia.
In the current study, we found a statistically significant association between white matter abnormalities and executive function impairment. Impairment in executive function is one of the most common dysfunctions observed in disease courses of schizophrenia (Orellana & Slachevsky, 2013). We assessed executive function using the Tower of London test. The Tower of London test requires several cognitive processes including working memory (Elliott, 2003), processing speed, response inhibition (Asato, Sweeney, & Luna, 2006; Zook, Davalos, Delosh, & Davis, 2004), and visuospatial processing (Newman, Carpenter, Varma, & Just, 2003), necessitating functional coordination among multiple cortical and subcortical regions (Unterrainer & Owen, 2006). As white matter fibers connect brain regions, many studies have reported a relationship between white matter abnormalities and cognitive function in schizophrenia (Canu, Agosta, & Filippi, 2015). Executive dysfunction of schizophrenia has been reported to be associated with white matter abnormalities in major fiber bundles that connect frontal and temporal lobes, such as superior longitudinal fasciculus and uncinate fasciculus (Kubicki et al., 2002, 2003; Nestor et al., 2004; Pérez‐Iglesias et al., 2010). In schizophrenia, associations also have been reported between superior longitudinal fasciculus and working memory (Karlsgodt et al., 2008), uncinate fasciculus and verbal memory (Nestor et al., 2004; Szeszko et al., 2008), inferior longitudinal and inferior frontooccipital fasciculi and processing speed, verbal learning, and visual learning (Liu et al., 2013), diffuse white matter abnormalities and processing speed (Karbasforoushan, Duffy, Blackford, & Woodward, 2015; Rigucci et al., 2013), visual memory (Rigucci et al., 2013), and social cognition (Rigucci et al., 2013). In the current study, we found the significant association between FA values of the CC fibers connecting bilateral temporal lobe cortices and executive function scores. Studies reported association between bilateral cortical thickness reductions in the temporal lobe and executive dysfunction in schizophrenia (Hartberg et al., 2010), as well as an association between white matter volume reductions in the temporal lobe and verbal memory, attention, problem solving, and working memory dysfunctions in a follow‐up study of early‐onset schizophrenia (Andreasen et al., 2011). The current results suggest that the disconnection between bilateral temporal lobe cortices contributes to poor executive function in schizophrenia.
The SZ group demonstrated decreased FA values in the temporal segments of CC white matter fibers relative to the HC group. Impairment in the temporal lobe with schizophrenia was reported repeatedly. Ellison‐Wright and Bullmore carried out meta‐analysis of the coordinates of fractional anisotropy differences (Ellison‐Wright & Bullmore, 2009). This meta‐analysis of 15 studies (including a total of 407 patients with schizophrenia and 383 comparison subjects) found that significant reductions were present in the left frontal deep white matter and the left temporal deep white matter (Ellison‐Wright & Bullmore, 2009). The second region, in the temporal lobe, is traversed by WM tracts interconnecting the frontal lobe, insula, hippocampus–amygdala, and temporal and occipital lobe. This suggests that WM tracts in the temporal lobe may affect in schizophrenia, with the disconnectivity of the gray matter regions which they link. Decreased FA values of the CC on the DTI whole‐brain analysis in schizophrenia have been repeatedly reported in the rostrum (Ellison‐Wright et al., 2014; Fujino et al., 2014; Gu et al., 2016; Hummer et al., 2016; Kochunov et al., 2014; Kong et al., 2011; Lener et al., 2015; Melicher et al., 2015; Pérez‐Iglesias et al., 2010; Pomarol‐Clotet et al., 2010; Roalf et al., 2013; Spalletta et al., 2015; Zhang et al., 2014, 2016), body (Fujino et al., 2014; Melicher et al., 2015; Pérez‐Iglesias et al., 2010; Roalf et al., 2013; Zhang et al., 2014, 2016), and splenium of the CC (Cheung et al., 2008; Ellison‐Wright et al., 2014; Fujino et al., 2014; Gasparotti et al., 2009; Melicher et al., 2015; Zhang et al., 2014). A meta‐analysis of 22 studies found decreased FA values in the genu and splenium of CC in schizophrenia (Zhuo, Liu, Wang, Tian, & Tang, 2016). These previous studies suggest the validity of segmenting the CC fibers in an anatomically accurate manner when comparing the FA values between patients with schizophrenia and healthy individuals. Several studies have examined decreased FA values in schizophrenia by segmenting the CC in the sagittal slices (Balevich et al., 2015; Knöchel et al., 2012; Li et al., 2014; Rotarska‐Jagiela et al., 2008). Balevich et al. (2015) divided the CC into five anteroposterior segments, but did not find statistically significant decrease of FA values in any specific segments. In studies with segmentation of the CC into nine regions, statistically significant FA reductions were observed in the inferior and superior genu, isthmus (Knöchel et al., 2012), anterior, middle, posterior genu, posterior body, anterior splenium (Li et al., 2014), inferior and superior genu, and splenium (Rotarska‐Jagiela et al., 2008). Whitford et al. parcellated the CC fibers into six segments based on the cortical regions they projected, and examined the difference between patients with schizophrenia and healthy participants (Whitford et al., 2010). They found a statistically significant decrease in FA of the frontal fibers in the patient group. FA values of the temporal fibers were reduced in the schizophrenia group but not statistically significantly so. The discrepancy between the current study and Whitford's study may be partially explained by differences in the DTI methods of analysis and in the study participants. In the current study, we divided the CC fibers based on their cortical projection regions using the two‐ROIs approach. We followed the procedures used in relevant previous studies to determine the location of ROIs and exclusion criteria (Huang et al., 2005; Lebel et al., 2010; Yamada et al., 2015), and we achieved tractography with high anatomical accuracy in each region. On the other hand, Whitford et al. segmented the CC into clusters after whole‐brain tractography. Our study participants included both men and women; however, Whitford's study included only men. TSA is useful as it delineates how fiber tracts connect functional brain regions, providing information on structural connectivity. Our results suggest microstructural abnormalities in the temporal regions of the CC white matter fibers connecting the two hemispheres.
In our SZ group, we observed statistically significant relation between FA values and PANSS scores in the temporal and posterior parietal segments of CC white matter fibers connecting the two hemispheres. The FA of callosal fibers in the temporal segment was significantly negatively associated with the score of the PANSS negative. Several studies have reported that executive function in schizophrenia was associated with the PANSS negative score (Clark, Warman, & Lysaker, 2010; Kishi et al., 2010; Rodriguez‐Jimenez et al., 2010). Microstructural abnormalities of callosal fiber in the temporal segment may be associated with negative symptom in schizophrenia. On the other hand, the FA of callosal fibers in the posterior parietal segment was significantly positively associated with all scores of the PANSS, indicating higher FA related to the higher severity of the symptoms. These results suggest paradoxical effect of anisotropy of callosal fibers in the posterior parietal segment on psychiatric symptom. However, these results should be interpreted cautiously because there was no significant difference in FA of callosal fibers in the posterior parietal segment between the SZ and HC groups. There has been no consensus on the relationship between white matter abnormalities and psychiatric symptoms of schizophrenia. With regard to FA values of the CC and psychiatric symptoms, study results are inconsistent. They reported FA values of the anterior CC and negative correlation with both negative symptoms (Gu et al., 2016; Kubicki et al., 2008; Nakamura et al., 2012) and positive symptoms (Knöchel et al., 2012; Kubicki et al., 2008). FA values of the posterior CC were negatively correlated with negative symptoms (Rigucci et al., 2013), negatively correlated with positive symptoms (Kubicki et al., 2008), but positively correlated with positive symptoms (Rotarska‐Jagiela et al., 2009). FA values of other white matter fibers have also been reported to have inconsistent directions of association, with studies reporting positive correlations with both positive (Andreasen et al., 2011; Chan et al., 2010; Cheung et al., 2011; Choi et al., 2011; Lee et al., 2013; Moriya et al., 2010; Psomiades et al., 2016; Rotarska‐Jagiela et al., 2009, 2008; Seok et al., 2007; Szeszko et al., 2008; Whitford et al., 2010) and negative symptoms (Lener et al., 2015; Mendelsohn, Strous, Bleich, Assaf, & Hendler, 2006; Michael, Calhoun, Pearlson, Baum, & Caprihan, 2008) and negative correlations both with positive (Lee et al., 2013; Skelly et al., 2008) and with negative symptoms (Balevich et al., 2015; Gu et al., 2016; Luck et al., 2011; Mendelsohn et al., 2006; Michael et al., 2008; Moriya et al., 2010; Rigucci et al., 2013; Szeszko et al., 2008; Wolkin et al., 2003). Many studies reported no relationship between FA values and psychiatric symptoms using whole‐brain analyses (Chen et al., 2013; Kyriakopoulos et al., 2009; Kyriakopoulos, Vyas, Barker, Chitnis, & Frangou, 2008; Liu et al., 2013; Melicher et al., 2015; Sugranyes et al., 2012; Wang et al., 2013) and more specific region‐of‐interest analyses including the CC (Li et al., 2014) and other regions (Kawashima et al., 2009; Kitamura et al., 2005; Kitis et al., 2012; Kumra et al., 2004; Price et al., 2008). The mixed findings in past studies regarding FA values and psychiatric symptoms may be partially explained by the differences in the number of participants, severity of the symptoms of the participants, and their treatment histories.
In our SZ group, the AD value of callosal fibers in the orbital frontal segment significantly associated with attention scores, and the AD value of this tract was significantly increased compared to the HC group. Histological information indicates the AD assesses axonal function (Mac Donald, Dikranian, Bayly, Holtzman, & Brody, 2007). Some previous studies reported no significant difference in AD value of the CC between patients with schizophrenia and healthy controls (Hummer et al., 2016; Kochunov et al., 2014; Liu et al., 2013; Spalletta et al., 2015; Whitford et al., 2010). The largest coordinated meta‐analysis on DTI data showed significantly higher AD value in schizophrenia patients compared with healthy controls in the fornix but showed no significant differences in the AD value in the CC (Kelly et al., 2018). To our knowledge, there is no study reported relationship of AD value with cognitive function in schizophrenia. The studies on patients with essential tremor showed positive correlation between the AD value and cognitive function (Julian et al., 2017; Bhalsing et al., 2015). Same as these previous studies on essential tremor, our SZ group showed significant relationship of increased AD with better attention scores, while AD value in the SZ group was significantly increased compared to the HC group. The further studies are needed to interpret this paradoxical relation of AD value with cognitive function in the SZ group.
The current study has some limitations. First, the gender composition of our HC and SZ groups was not fully matched. All participants in the SZ group were taking antipsychotic medications at the time of the MRI examination. Gender ratios were not statistically different between the two groups, and no correlation was found between FA values and antipsychotic medication dosage in the SZ group. However, gender difference and antipsychotic medication use are potential confounding factors for diffusion changes. Second, the sample size was relatively small. Last, no distortion correction was performed in DTI analysis. We offered new insight into relationship between microstructural abnormalities in callosal fibers and cognitive function in schizophrenia, but the results of our study should be confirmed in future studies using more subject with an appropriate control over FA value‐affecting factors.
In summary, we found microstructural abnormalities in the CC white matter fibers connecting bilateral temporal lobe cortices contribute to poor executive function and severe negative symptom in patients with schizophrenia.
CONFLICT OF INTEREST
None of the authors have potential conflict of interests to be disclosed.
DATA AVAILABILITY STATEMENT
Research data are not shared.
Supporting information
ACKNOWLEDGMENT
This work was supported by JSPS KAKENHI (Grant Number 26461754; K.S). The authors would like to thank Yuka Sakamoto, Masahiro Yamamoto, Takeshi Samizo, Mika Taketomo, and Yuji Nakao for their contributions to this study.
Ohoshi Y, Takahashi S, Yamada S, et al. Microstructural abnormalities in callosal fibers and their relationship with cognitive function in schizophrenia: A tract‐specific analysis study. Brain Behav. 2019;9:e01357 10.1002/brb3.1357
REFERENCES
- American Psychiatric Association . (1994). Diagnostic and statistical manual of mental disorders, 4th ed. Washington, DC: American Psychiatric Association. [Google Scholar]
- Andreasen, N. C. , Nopoulos, P. , Magnotta, V. , Pierson, R. , Ziebell, S. , & Ho, B. C. (2011). Progressive brain change in schizophrenia: A prospective longitudinal study of first‐episode schizophrenia. Biological Psychiatry, 70, 672–679. 10.1016/j.biopsych.2011.05.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asato, M. R. , Sweeney, J. A. , & Luna, B. (2006). Cognitive processes in the development of TOL performance. Neuropsychologia, 44, 2259–2269. 10.1016/j.neuropsychologia.2006.05.010 [DOI] [PubMed] [Google Scholar]
- Balevich, E. C. , Haznedar, M. M. , Wang, E. , Newmark, R. E. , Bloom, R. , Schneiderman, J. S. , … Hazlett, E. A. (2015). Corpus callosum size and diffusion tensor anisotropy in adolescents and adults with schizophrenia. Psychiatry Research, 231, 244–251. 10.1016/j.pscychresns.2014.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basser, P. J. (1995). Inferring microstructural features and the physiological state of tissues from diffusion‐weighted images. NMR in Biomedicine, 8, 333–344. 10.1002/nbm.1940080707 [DOI] [PubMed] [Google Scholar]
- Basser, P. J. , & Pierpaoli, C. (1996). Microstructural and physiological features of tissues elucidated by quantitative‐diffusion‐tensor MRI. Journal of Magnetic Resonance, Series B, 111, 209–219. 10.1006/jmrb.1996.0086 [DOI] [PubMed] [Google Scholar]
- Benito‐León, J. , Mato‐Abad, V. , Louis, E. D. , Hernández‐Tamames, J. A. , Álvarez‐Linera, J. , Bermejo‐Pareja, F. , … Romero, J. P. (2017). White matter microstructural changes are related to cognitive dysfunction in essential tremor. Scientific Reports, 7, 2978 10.1038/s41598-017-02596-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhalsing, K. S. , Kumar, K. J. , Saini, J. , Yadav, R. , Gupta, A. K. , & Pal, P. K. (2015). White matter correlates of cognitive impairment in essential tremor. American Journal of Neuroradiology, 36, 448–453. 10.3174/ajnr.A4138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandstack, N. , Kurki, T. , Laalo, J. , Kauko, T. , & Tenovuo, O. (2016). Reproducibility of tract‐based and region‐of‐interest DTI analysis of long association tracts. Clinical Neuroradiology, 26, 199–208. 10.1007/s00062-014-0349-8 [DOI] [PubMed] [Google Scholar]
- Canu, E. , Agosta, F. , & Filippi, M. (2015). A selective review of structural connectivity abnormalities of schizophrenic patients at different stages of the disease. Schizophrenia Research, 161, 19–28. 10.1016/j.schres.2014.05.020 [DOI] [PubMed] [Google Scholar]
- Chan, W.‐Y. , Yang, G.‐L. , Chia, M.‐Y. , Lau, I.‐Y. , Sitoh, Y.‐Y. , Nowinski, W. L. , & Sim, K. (2010). White matter abnormalities in first‐episode schizophrenia: A combined structural MRI and DTI study. Schizophrenia Research, 119, 52–60. 10.1016/j.schres.2009.12.012 [DOI] [PubMed] [Google Scholar]
- Chen, L. , Chen, X. , Liu, W. , Wang, Q. , Jiang, T. , Wang, J. , … Tang, J. (2013). White matter microstructural abnormalities in patients with late‐onset schizophrenia identified by a voxel‐based diffusion tensor imaging. Psychiatry Research, 212, 201–207. 10.1016/j.pscychresns.2012.05.009 [DOI] [PubMed] [Google Scholar]
- Cheung, V. , Cheung, C. , McAlonan, G. M. , Deng, Y. , Wong, J. G. , Yip, L. , … Chua, S. E. (2008). A diffusion tensor imaging study of structural dysconnectivity in never‐medicated, first‐episode schizophrenia. Psychological Medicine, 38, 877–885. 10.1017/S0033291707001808 [DOI] [PubMed] [Google Scholar]
- Cheung, V. , Chiu, C. P. Y. , Law, C. W. , Cheung, C. , Hui, C. L. M. , Chan, K. K. S. , … Chen, E. (2011). Positive symptoms and white matter microstructure in never‐medicated first episode schizophrenia. Psychological Medicine, 41, 1709–1719. 10.1017/S003329171000156X [DOI] [PubMed] [Google Scholar]
- Choi, H. , Kubicki, M. , Whitford, T. J. , Alvarado, J. L. , Terry, D. P. , Niznikiewicz, M. , … Shenton, M. E. (2011). Diffusion tensor imaging of anterior commissural fibers in patients with schizophrenia. Schizophrenia Research, 130, 78–85. 10.1016/j.schres.2011.04.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark, L. K. , Warman, D. , & Lysaker, P. H. (2010). The relationships between schizophrenia symptom dimensions and executive functioning components. Schizophrenia Research, 124, 169–175. 10.1016/j.schres.2010.08.004 [DOI] [PubMed] [Google Scholar]
- Elliott, R. (2003). Executive functions and their disorders. British Medical Bulletin, 65, 49–59. 10.1093/bmb/65.1.49 [DOI] [PubMed] [Google Scholar]
- Ellison‐Wright, I. , & Bullmore, E. (2009). Meta‐analysis of diffusion tensor imaging studies in schizophrenia. Schizophrenia Research, 108, 3–10. 10.1016/j.schres.2008.11.021 [DOI] [PubMed] [Google Scholar]
- Ellison‐Wright, I. , Nathan, P. J. , Bullmore, E. T. , Zaman, R. , Dudas, R. B. , Agius, M. , … Cannon, D. M. (2014). Distribution of tract deficits in schizophrenia. BMC Psychiatry, 14, 99 10.1186/1471-244X-14-99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox, M. D. , Snyder, A. Z. , Vincent, J. L. , Corbetta, M. , Van Essen, D. C. , & Raichle, M. E. (2005). The human brain is intrinsically organized into dynamic, anticor‐related functional networks. Proceedings of the National Academy of Sciences of the United States of America, 102, 9673–9678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston, K. J. (1999). Schizophrenia and the disconnection hypothesis. Acta Psychiatrica Scandinavica. Supplementum, 395, 68–79. [DOI] [PubMed] [Google Scholar]
- Fujino, J. , Takahashi, H. , Miyata, J. , Sugihara, G. , Kubota, M. , Sasamoto, A. , … Murai, T. (2014). Impaired empathic abilities and reduced white matter integrity in schizophrenia. Progress in Neuro‐Psychopharmacology & Biological Psychiatry, 48, 117–123. 10.1016/j.pnpbp.2013.09.018 [DOI] [PubMed] [Google Scholar]
- Gasparotti, R. , Valsecchi, P. , Carletti, F. , Galluzzo, A. , Liserre, R. , Cesana, B. , … Sacchetti, E. (2009). Reduced fractional anisotropy of corpus callosum in first‐contact, antipsychotic drug‐naive patients with schizophrenia. Schizophrenia Research, 108, 41–48. 10.1016/j.schres.2008.11.015 [DOI] [PubMed] [Google Scholar]
- Green, M. F. , & Harvey, P. D. (2014). Cognition in schizophrenia: Past, present, and future. Schizophrenia Research: Cognition, 1, e1–e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu, C. , Zhang, Y. , Wei, F. , Cheng, Y. , Cao, Y. , & Hou, H. (2016). Magnetic resonance imaging DTI‐FT study on schizophrenic patients with typical negative first symptoms. Experimental and Therapeutic Medicine, 12, 1450–1454. 10.3892/etm.2016.3469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartberg, C. B. , Lawyer, G. , Nyman, H. , Jönsson, E. G. , Haukvik, U. K. , Saetre, P. , … Agartz, I. (2010). Investigating relationships between cortical thickness and cognitive performance in patients with schizophrenia and healthy adults. Psychiatry Research, 182, 123–133. 10.1016/j.pscychresns.2010.01.001 [DOI] [PubMed] [Google Scholar]
- Hinkley, L. B. N. , Marco, E. J. , Findlay, A. M. , Honma, S. , Jeremy, R. J. , Strominger, Z. , … Sherr, E. H. (2012). The role of corpus callosum development in functional connectivity and cognitive processing. PLoS ONE, 7, e39804 10.1371/journal.pone.0039804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, H. , Zhang, J. , Jiang, H. , Wakana, S. , Poetscher, L. , Miller, M. I. , … Mori, S. (2005). DTI tractography based parcellation of white matter: Application to the mid‐sagittal morphology of corpus callosum. NeuroImage, 26, 195–205. 10.1016/j.neuroimage.2005.01.019 [DOI] [PubMed] [Google Scholar]
- Hummer, T. A. , Francis, M. M. , Vohs, J. L. , Liffick, E. , Mehdiyoun, N. F. , & Breier, A. (2016). Characterization of white matter abnormalities in early‐stage schizophrenia. Early Intervention in Psychiatry, 13, 660–668. 10.1111/eip.12359 [DOI] [PubMed] [Google Scholar]
- Ikebuchi, E. , Sato, S. , Yamaguchi, S. , Shimodaira, M. , Taneda, A. , Hatsuse, N. , … Ito, J.‐I. (2017). Does improvement of cognitive functioning by cognitive remediation therapy effect work outcomes in severe mental illness? A secondary analysis of a randomized controlled trial. Psychiatry and Clinical Neurosciences, 71, 301–308. 10.1111/pcn.12486 [DOI] [PubMed] [Google Scholar]
- Inada, T. , & Inagaki, A. (2015). Psychotropic dose equivalence in Japan. Psychiatry and Clinical Neurosciences, 69, 440–447. 10.1111/pcn.12275 [DOI] [PubMed] [Google Scholar]
- Isobe, M. , Miyata, J. , Hazama, M. , Fukuyama, H. , Murai, T. , & Takahashi, H. (2016). Multimodal neuroimaging as a window into the pathological physiology of schizophrenia: Current trends and issues. Neuroscience Research, 102, 29–38. 10.1016/j.neures.2015.07.009 [DOI] [PubMed] [Google Scholar]
- Itakura, M. , Pu, S. , Ohdachi, H. , Matsumura, H. , Yokoyama, K. , Nagata, I. , … Kaneko, K. (2017). Association between social functioning and prefrontal cortex function during a verbal fluency task in schizophrenia: A near‐infrared spectroscopic study. Psychiatry and Clinical Neurosciences, 71, 769–779. 10.1111/pcn.12548 [DOI] [PubMed] [Google Scholar]
- Kaneda, Y. , Sumiyosh, T. , Keefe, R. , Ishimoto, Y. , Numata, S. , & Ohmori, T. (2007). Brief assessment of cognition in schizophrenia: Validation of the Japanese version. Psychiatry and Clinical Neurosciences, 61, 602–609. 10.1111/j.1440-1819.2007.01725.x [DOI] [PubMed] [Google Scholar]
- Kaneda, Y. , Sumiyoshi, T. , Nakagome, K. , Ikezawa, S. , Omori, T. , Furukoori, N. , … Matsumoto, K. (2013). Evaluation of cognitive functions in a normal population in Japan using the Brief assessment of cognition in schizophrenia Japanese version (BACS‐J). Seishin Igaku, 55, 167–175. (in Japanese). [Google Scholar]
- Karbasforoushan, H. , Duffy, B. , Blackford, J. U. , & Woodward, N. D. (2015). Processing speed impairment in schizophrenia is mediated by white matter integrity. Psychological Medicine, 45, 109–120. 10.1017/S0033291714001111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsgodt, K. H. , van Erp, T. G. M. , Poldrack, R. A. , Bearden, C. E. , Nuechterlein, K. H. , & Cannon, T. D. (2008). Diffusion tensor imaging of the superior longitudinal fasciculus and working memory in recent‐onset schizophrenia. Biological Psychiatry, 63, 512–518. 10.1016/j.biopsych.2007.06.017 [DOI] [PubMed] [Google Scholar]
- Kawashima, T. , Nakamura, M. , Bouix, S. , Kubicki, M. , Salisbury, D. F. , Westin, C.‐F. , … Shenton, M. E. (2009). Uncinate fasciculus abnormalities in recent onset schizophrenia and affective psychosis: A diffusion tensor imaging study. Schizophrenia Research, 110, 119–126. 10.1016/j.schres.2009.01.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly, S. , Jahanshad, N. , Zalesky, A. , Kochunov, P. , Agartz, I. , Alloza, C. , … Donohoe, G. (2018). Widespread white matter microstructural differences in schizophrenia across 4322 individuals: Results from the ENIGMA Schizophrenia DTI Working Group. Molecular Psychiatry, 23, 1261–1269. 10.1038/mp.2017.170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishi, T. , Moriwaki, M. , Kawashima, K. , Okochi, T. , Fukuo, Y. , Kitajima, T. , … Iwata, N. (2010). Investigation of clinical factors influencing cognitive function in Japanese schizophrenia. Neuroscience Research, 66, 340–344. 10.1016/j.neures.2009.12.007 [DOI] [PubMed] [Google Scholar]
- Kitamura, H. , Matsuzawa, H. , Shioiri, T. , Someya, T. , Kwee, I. L. , & Nakada, T. (2005). Diffusion tensor analysis in chronic schizophrenia. A preliminary study on a high‐field (3.0T) system. European Archives of Psychiatry and Clinical Neurosciences, 255, 313–318. [DOI] [PubMed] [Google Scholar]
- Kitis, O. , Ozalay, O. , Zengin, E. B. , Haznedaroglu, D. , Eker, M. C. , Yalvac, D. , … Gonul, A. S. (2012). Reduced left uncinate fasciculus fractional anisotropy in deficit schizophrenia but not in non‐deficit schizophrenia. Psychiatry and Clinical Neurosciences, 66, 34–43. 10.1111/j.1440-1819.2011.02293.x [DOI] [PubMed] [Google Scholar]
- Knöchel, C. , Oertel‐Knöchel, V. , Schönmeyer, R. , Rotarska‐Jagiela, A. , van de Ven, V. , Prvulovic, D. , … Linden, D. E. J. (2012). Interhemispheric hypoconnectivity in schizophrenia: Fiber integrity and volume differences of the corpus callosum in patients and unaffected relatives. NeuroImage, 59, 926–934. 10.1016/j.neuroimage.2011.07.088 [DOI] [PubMed] [Google Scholar]
- Kochunov, P. , Chiappelli, J. , Wright, S. N. , Rowland, L. M. , Patel, B. , Wijtenburg, S. A. , … Elliot Hong, L. (2014). Multimodal white matter imaging to investigate reduced fractional anisotropy and its age‐related decline in schizophrenia. Psychiatry Research, 223, 148–156. 10.1016/j.pscychresns.2014.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong, X. , Ouyang, X. , Tao, H. , Liu, H. , Li, L. I. , Zhao, J. , … Liu, Z. (2011). Complementary diffusion tensor imaging study of the corpus callosum in patients with first‐episode and chronic schizophrenia. Journal of Psychiatry & Neuroscience, 36, 120–125. 10.1503/jpn.100041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubicki, M. , & Shenton, M. E. (2014). Diffusion tensor imaging findings and their implications in schizophrenia. Current Opinion in Psychiatry, 27, 179–184. 10.1097/YCO.0000000000000053 [DOI] [PubMed] [Google Scholar]
- Kubicki, M. , Styner, M. , Bouix, S. , Gerig, G. , Markant, D. , Smith, K. , … Shenton, M. (2008). Reduced interhemispheric connectivity in schizophrenia‐tractography based segmentation of the corpus callosum. Schizophrenia Research, 106, 125–131. 10.1016/j.schres.2008.08.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubicki, M. , Westin, C.‐F. , Maier, S. E. , Frumin, M. , Nestor, P. G. , Salisbury, D. F. , … Shenton, M. E. (2002). Uncinate fasciculus findings in schizophrenia: A magnetic resonance diffusion tensor imaging study. American Journal of Psychiatry, 159, 813–820. 10.1176/appi.ajp.159.5.813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubicki, M. , Westin, C.‐F. , Nestor, P. G. , Wible, C. G. , Frumin, M. , Maier, S. E. , … Shenton, M. E. (2003). Cingulate fasciculus integrity disruption in schizophrenia: A magnetic resonance diffusion tensor imaging study. Biological Psychiatry, 54, 1171–1180. 10.1016/S0006-3223(03)00419-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumra, S. , Ashtari, M. , McMeniman, M. , Vogel, J. , Augustin, R. , Becker, D. E. , … Szeszko, P. (2004). Reduced frontal white matter integrity in early‐onset schizophrenia: A preliminary study. Biological Psychiatry, 55, 1138–1145. 10.1016/j.biopsych.2004.02.025 [DOI] [PubMed] [Google Scholar]
- Kyriakopoulos, M. , Perez‐Iglesias, R. , Woolley, J. B. , Kanaan, R. A. A. , Vyas, N. S. , Barker, G. J. , … McGuire, P. K. (2009). Effect of age at onset of schizophrenia on white matter abnormalities. The British Journal of Psychiatry, 195, 346–353. 10.1192/bjp.bp.108.055376 [DOI] [PubMed] [Google Scholar]
- Kyriakopoulos, M. , Vyas, N. S. , Barker, G. J. , Chitnis, X. A. , & Frangou, S. (2008). A diffusion tensor imaging study of white matter in early‐onset schizophrenia. Biological Psychiatry, 63, 519–523. 10.1016/j.biopsych.2007.05.021 [DOI] [PubMed] [Google Scholar]
- Lebel, C. , Caverhill‐Godkewitsch, S. , & Beaulieu, C. (2010). Age‐related regional variations of the corpus callosum identified by diffusion tensor tractography. NeuroImage, 52, 20–31. 10.1016/j.neuroimage.2010.03.072 [DOI] [PubMed] [Google Scholar]
- Lee, S. K. , Kim, D. I. , Kim, J. , Kim, D. J. , Kim, H. D. , Kim, D. S. , … Mori, S. (2005). Diffusion‐tensor MR imaging and fiber tractography: A new method of describing aberrant fiber connections in developmental CNS anomalies. Radiographics, 25, 53–65. discussion 66–68. 10.1148/rg.251045085 [DOI] [PubMed] [Google Scholar]
- Lee, S.‐H. , Kubicki, M. , Asami, T. , Seidman, L. J. , Goldstein, J. M. , Mesholam‐Gately, R. I. , … Shenton, M. E. (2013). Extensive white matter abnormalities in patients with first‐episode schizophrenia: A diffusion tensor imaging (DTI) study. Schizophrenia Research, 143, 231–238. 10.1016/j.schres.2012.11.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lener, M. S. , Wong, E. , Tang, C. Y. , Byne, W. , Goldstein, K. E. , Blair, N. J. , … Hazlett, E. A. (2015). White matter abnormalities in schizophrenia and schizotypal personality disorder. Schizophrenia Bulletin, 41, 300–310. 10.1093/schbul/sbu093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, J. , Kale Edmiston, E. , Chen, K. , Tang, Y. , Ouyang, X. , Jiang, Y. , … Wang, F. (2014). A comparative diffusion tensor imaging study of corpus callosum subregion integrity in bipolar disorder and schizophrenia. Psychiatry Research, 221, 58–62. 10.1016/j.pscychresns.2013.10.007 [DOI] [PubMed] [Google Scholar]
- Liu, X. , Lai, Y. , Wang, X. , Hao, C. , Chen, L. , Zhou, Z. , … Hong, N. (2013). Reduced white matter integrity and cognitive deficit in never‐medicated chronic schizophrenia: A diffusion tensor study using TBSS. Behavioural Brain Research, 252, 157–163. 10.1016/j.bbr.2013.05.061 [DOI] [PubMed] [Google Scholar]
- Luck, D. , Buchy, L. , Czechowska, Y. , Bodnar, M. , Pike, G. B. , Campbell, J. S. W. , … Lepage, M. (2011). Fronto‐temporal disconnectivity and clinical short‐term outcome in first episode psychosis: A DTI‐tractography study. Journal of Psychiatric Research, 45, 369–377. 10.1016/j.jpsychires.2010.07.007 [DOI] [PubMed] [Google Scholar]
- Mac Donald, C. L. , Dikranian, K. , Bayly, P. , Holtzman, D. , & Brody, D. (2007). Diffusion tensor imaging reliably detects experimental traumatic axonal injury and indicates approximate time of injury. Journal of Neuroscience, 27, 11869–11876. 10.1523/JNEUROSCI.3647-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melicher, T. , Horacek, J. , Hlinka, J. , Spaniel, F. , Tintera, J. , Ibrahim, I. , … Hoschl, C. (2015). White matter changes in first episode psychosis and their relation to the size of sample studied: A DTI study. Schizophrenia Research, 162, 22–28. 10.1016/j.schres.2015.01.029 [DOI] [PubMed] [Google Scholar]
- Mendelsohn, A. , Strous, R. D. , Bleich, M. , Assaf, Y. , & Hendler, T. (2006). Regional axonal abnormalities in first episode schizophrenia: Preliminary evidence based on high b‐value diffusion‐weighted imaging. Psychiatric Research, 146, 223–229. 10.1016/j.pscychresns.2005.12.010 [DOI] [PubMed] [Google Scholar]
- Michael, A. M. , Calhoun, V. D. , Pearlson, G. D. , Baum, S. A. , & Caprihan, A. (2008). Correlations of diffusion tensor imaging values and symptom scores in patients with schizophrenia. Conference Proceedings IEEE Engineering in Medicine and Biology Society, 2008, 5494–5497. 10.1109/IEMBS.2008.4650458 [DOI] [PubMed] [Google Scholar]
- Moriya, J. , Kakeda, S. , Abe, O. , Goto, N. , Yoshimura, R. , Hori, H. , … Korogi, Y. (2010). Gray and white matter volumetric and diffusion tensor imaging (DTI) analyses in the early stage of first‐episode schizophrenia. Schizophrenia Research, 116, 196–203. 10.1016/j.schres.2009.10.002 [DOI] [PubMed] [Google Scholar]
- Nakamura, K. , Kawasaki, Y. , Takahashi, T. , Furuichi, A. , Noguchi, K. , Seto, H. , … Suzuki, M. (2012). Reduced white matter fractional anisotropy and clinical symptoms in schizophrenia: A voxel‐based diffusion tensor imaging study. Psychiatry Research, 202, 233–238. 10.1016/j.pscychresns.2011.09.006 [DOI] [PubMed] [Google Scholar]
- Nestor, P. G. , Kubicki, M. , Gurrera, R. J. , Niznikiewicz, M. , Frumin, M. , McCarley, R. W. , … Shenton, M. E. (2004). Neuropsychological correlates of diffusion tensor imaging in schizophrenia. Neuropsychology, 18, 629–637. 10.1037/0894-4105.18.4.629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman, S. D. , Carpenter, P. , Varma, S. , & Just, M. A. (2003). Frontal and parietal participation in problem solving in the Tower of London: fMRI and computational modeling of planning and high‐level perception. Neuropsychologia, 41, 1668–1682. 10.1016/S0028-3932(03)00091-5 [DOI] [PubMed] [Google Scholar]
- O'Donnell, L. J. , & Westin, C.‐F. (2011). An introduction to diffusion tensor image analysis. Neurosurgery Clinics of North America, 22(2), 185–196. 10.1016/j.nec.2010.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orellana, G. , & Slachevsky, A. (2013). Executive functioning in schizophrenia. Frontiers in Psychiatry, 4, 35 10.3389/fpsyt.2013.00035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez‐Iglesias, R. , Tordesillas‐Gutiérrez, D. , Barker, G. J. , McGuire, P. K. , Roiz‐Santiañez, R. , Mata, I. , … Crespo‐Facorro, B. (2010). White matter defects in first episode psychosis patients: A voxelwise analysis of diffusion tensor imaging. NeuroImage, 49, 199–204. 10.1016/j.neuroimage.2009.07.016 [DOI] [PubMed] [Google Scholar]
- Pomarol‐Clotet, E. , Canales‐Rodríguez, E. J. , Salvador, R. , Sarró, S. , Gomar, J. J. , Vila, F. , … McKenna, P. J. (2010). Medial prefrontal cortex pathology in schizophrenia as revealed by convergent findings from multimodal imaging. Molecular Psychiatry, 15, 823–830. 10.1038/mp.2009.146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price, G. , Cercignani, M. , Parker, G. J. M. , Altmann, D. R. , Barnes, T. R. E. , Barker, G. J. , … Ron, M. A. (2008). White matter tracts in first‐episode psychosis: A DTI tractography study of the uncinate fasciculus. NeuroImage, 39, 949–955. 10.1016/j.neuroimage.2007.09.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Psomiades, M. , Fonteneau, C. , Mondino, M. , Luck, D. , Haesebaert, F. , Suaud‐Chagny, M. F. , … Brunelin, J. (2016). Integrity of the arcuate fasciculus in patients with schizophrenia with auditory verbal hallucinations: A DTI‐tractography study. NeuroImage: Clinical, 12, 970–975. 10.1016/j.nicl.2016.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribolsi, M. , Daskalakis, Z. J. , Siracusano, A. , & Koch, G. (2014). Abnormal asymmetry of brain connectivity in schizophrenia. Frontiers in Human Neuroscience, 8, 1010 10.3389/fnhum.2014.01010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigucci, S. , Rossi‐Espagnet, C. , Ferracuti, S. , De Carolis, A. , Corigliano, V. , Carducci, F. , … Comparelli, A. (2013). Anatomical substrates of cognitive and clinical dimensions in first episode schizophrenia. Acta Psychiatrica Scandinavica, 128, 261–270. 10.1111/acps.12051 [DOI] [PubMed] [Google Scholar]
- Roalf, D. R. , Ruparel, K. , Verma, R. , Elliott, M. A. , Gur, R. E. , & Gur, R. C. (2013). White matter organization and neurocognitive performance variability in schizophrenia. Schizophrenia Research, 143, 172–178. 10.1016/j.schres.2012.10.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez‐Jimenez, R. , Bagney, A. , Martinez‐Gras, I. , Ponce, G. , Sanchez‐Morla, E. M. , Aragües, M. , … Palomo, T. (2010). Executive function in schizophrenia: Influence of substance use disorder history. Schizophrenia Research, 118, 34–40. 10.1016/j.schres.2009.09.025 [DOI] [PubMed] [Google Scholar]
- Rotarska‐Jagiela, A. , Oertel‐Knoechel, V. , DeMartino, F. , van de Ven, V. , Formisano, E. , Roebroeck, A. , … Linden, D. E. J. (2009). Anatomical brain connectivity and positive symptoms of schizophrenia: A diffusion tensor imaging study. Psychiatry Research, 174, 9–16. 10.1016/j.pscychresns.2009.03.002 [DOI] [PubMed] [Google Scholar]
- Rotarska‐Jagiela, A. , Schönmeyer, R. , Oertel, V. , Haenschel, C. , Vogeley, K. , & Linden, D. E. (2008). The corpus callosum in schizophrenia‐volume and connectivity changes affect specific regions. NeuroImage, 39, 1522–1532. 10.1016/j.neuroimage.2007.10.063 [DOI] [PubMed] [Google Scholar]
- Samartzis, L. , Dima, D. , Fusar‐Poli, P. , & Kyriakopoulos, M. (2014). White matter alterations in early stages of schizophrenia: A systematic review of diffusion tensor imaging studies. Journal of Neuroimaging, 24, 101–110. 10.1111/j.1552-6569.2012.00779.x [DOI] [PubMed] [Google Scholar]
- Satogami, K. , Takahashi, S. , Yamada, S. , Ukai, S. , & Shinosaki, K. (2017). Omega‐3 fatty acids related to cognitive impairment in patients with schizophrenia. Schizophrenia Research: Cognition, 9, 8–12. 10.1016/j.scog.2017.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawada, K. , Kanehara, A. , Sakakibara, E. , Eguchi, S. , Tada, M. , Satomura, Y. , … Kasai, K. (2017). Identifying neurocognitive markers for outcome prediction of global functioning in individuals with first‐episode and ultra‐high‐risk for psychosis. Psychiatry and Clinical Neurosciences, 71, 318–327. 10.1111/pcn.12522 [DOI] [PubMed] [Google Scholar]
- Seok, J. H. , Park, H. J. , Chun, J. W. , Lee, S. K. , Cho, H. S. , Kwon, J. S. , … Kim, J. J. (2007). White matter abnormalities associated with auditory hallucinations in schizophrenia: A combined study of voxel‐based analyses of diffusion tensor imaging and structural magnetic resonance imaging. Psychiatry Research, 156, 93–104. 10.1016/j.pscychresns.2007.02.002 [DOI] [PubMed] [Google Scholar]
- Skelly, L. R. , Calhoun, V. , Meda, S. A. , Kim, J. , Mathalon, D. H. , & Pearlson, G. D. (2008). Diffusion tensor imaging in schizophrenia: Relationship to symptoms. Schizophrenia Research, 98, 157–162. 10.1016/j.schres.2007.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spalletta, G. , De Rossi, P. , Piras, F. , Iorio, M. , Dacquino, C. , Scanu, F. , … Chiapponi, C. (2015). Brain white matter microstructure in deficit and non‐deficit subtypes of schizophrenia. Psychiatry Research, 231, 252–261. 10.1016/j.pscychresns.2014.12.006 [DOI] [PubMed] [Google Scholar]
- Sugranyes, G. , Kyriakopoulos, M. , Dima, D. , O'Muircheartaigh, J. , Corrigall, R. , Pendelbury, G. , … Frangou, S. (2012). Multimodal analyses identify linked functional and white matter abnormalities within the working memory network in schizophrenia. Schizophrenia Research, 138, 136–142. 10.1016/j.schres.2012.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szeszko, P. R. , Robinson, D. G. , Ashtari, M. , Vogel, J. , Betensky, J. , Sevy, S. , … Bilder, R. M. (2008). Clinical and neuropsychological correlates of white matter abnormalities in recent onset schizophrenia. Neuropsychopharmacology, 33, 976–984. 10.1038/sj.npp.1301480 [DOI] [PubMed] [Google Scholar]
- Takahashi, S. , Ukai, S. , Kose, A. , Hashimoto, T. , Iwatani, J. , Okumura, M. , … Shinosaki, K. (2013). Reduction of cortical GABAergic inhibition correlates with working memory impairment in recent onset schizophrenia. Schizophrenia Research, 146, 238–243. 10.1016/j.schres.2013.02.033 [DOI] [PubMed] [Google Scholar]
- Unterrainer, J. M. , & Owen, A. M. (2006). Planning and problem solving: From neuropsychology to functional neuroimaging. Journal of Physiology ‐ Paris, 99, 308–317. 10.1016/j.jphysparis.2006.03.014 [DOI] [PubMed] [Google Scholar]
- Wakana, S. , Caprihan, A. , Panzenboeck, M. M. , Fallon, J. H. , Perry, M. , Gollub, R. L. , … Mori, S. (2007). Reproducibility of quantitative tractography methods applied to cerebral white matter. NeuroImage, 36, 630–644. 10.1016/j.neuroimage.2007.02.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Q. , Cheung, C. , Deng, W. , Li, M. , Huang, C. , Ma, X. , … Li, T. (2013). Fronto‐parietal white matter microstructural deficits are linked to performance IQ in a first‐episode schizophrenia Han Chinese sample. Psychological Medicine, 43, 2047–2056. 10.1017/S0033291712002905 [DOI] [PubMed] [Google Scholar]
- Wheeler, A. L. , & Voineskos, A. N. (2014). A review of structural neuroimaging in schizophrenia: From connectivity to connectomics. Frontiers in Human Neuroscience, 8, 653 10.3389/fnhum.2014.00653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitford, T. J. , Kubicki, M. , Schneiderman, J. S. , O'Donnell, L. J. , King, R. , Alvarado, J. L. , … Shenton, M. E. (2010). Corpus callosum abnormalities and their association with psychotic symptoms in patients with schizophrenia. Biological Psychiatry, 68, 70–77. 10.1016/j.biopsych.2010.03.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolkin, A. , Choi, S. J. , Szilagyi, S. , Sanfilipo, M. , Rotrosen, J. P. , & Lim, K. O. (2003). Inferior frontal white matter anisotropy and negative symptoms of schizophrenia: A diffusion tensor imaging study. American Journal of Psychiatry, 160, 572–574. 10.1176/appi.ajp.160.3.572 [DOI] [PubMed] [Google Scholar]
- Wozniak, J. R. , & Lim, K. O. (2006). Advances in white matter imaging: A review of in vivo magnetic resonance methodologies and their applicability to the study of development and aging. Neuroscience & Biobehavioral Reviews, 30, 762–774. 10.1016/j.neubiorev.2006.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada, S. , Takahashi, S. , Ukai, S. , Tsuji, T. , Iwatani, J. , Tsuda, K. , … Shinosaki, K. (2015). Microstructural abnormalities in anterior callosal fibers and their relationship with cognitive function in major depressive disorder and bipolar disorder: A tract‐specific analysis study. Journal of Affective Disorders, 174, 542–548. 10.1016/j.jad.2014.12.022 [DOI] [PubMed] [Google Scholar]
- Zhang, F. , Qiu, L. , Yuan, L. , Ma, H. , Ye, R. , Yu, F. , … Wang, K. (2014). Evidence for progressive brain abnormalities in early schizophrenia: A cross‐sectional structural and functional connectivity study. Schizophrenia Research, 159, 31–35. 10.1016/j.schres.2014.07.050 [DOI] [PubMed] [Google Scholar]
- Zhang, X. Y. , Fan, F.‐M. , Chen, D.‐C. , Tan, Y.‐L. , Tan, S.‐P. , Hu, K. , … Soares, J. C. (2016). Extensive white matter abnormalities and clinical symptoms in drug‐naive patients with first‐episode schizophrenia: A voxel‐based diffusion tensor imaging study. Journal of Clinical Psychiatry, 77, 205–211. 10.4088/JCP.14m09374 [DOI] [PubMed] [Google Scholar]
- Zhuo, C. , Liu, M. , Wang, L. , Tian, H. , & Tang, J. (2016). Diffusion tensor MR imaging evaluation of callosal abnormalities in schizophrenia: A meta‐analysis. PLoS ONE, 11, e0161406 10.1371/journal.pone.0161406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zook, N. A. , Davalos, D. B. , Delosh, E. L. , & Davis, H. P. (2004). Working memory, inhibition, and fluid intelligence as predictors of performance on Tower of Hanoi and London tasks. Brain and Cognition, 56, 286–292. 10.1016/j.bandc.2004.07.003 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Research data are not shared.


