Abstract
Studying neuronal circuits at cellular resolution is very challenging in vertebrates due to the size and optical turbidity of their brains. Danionella translucida, a close relative of zebrafish, was recently introduced as a model organism for investigating neural network interactions in adult individuals. Danionella remains transparent throughout its life, has the smallest known vertebrate brain and possesses a rich repertoire of complex behaviours. Here we sequenced, assembled and annotated the Danionella translucida genome employing a hybrid Illumina/Nanopore read library as well as RNA-seq of embryonic, larval and adult mRNA. We achieved high assembly continuity using low-coverage long-read data and annotated a large fraction of the transcriptome. This dataset will pave the way for molecular research and targeted genetic manipulation of this novel model organism.
Subject terms: Neuroscience, Model vertebrates, Next-generation sequencing, Sequence annotation
| Design Type(s) | sequence assembly objective • sequence annotation objective • transcription profiling design |
| Measurement Type(s) | transcription profiling assay • genome |
| Technology Type(s) | RNA sequencing • DNA sequencing |
| Factor Type(s) | age |
| Sample Characteristic(s) | Danionella translucida • whole body |
Machine-accessible metadata file describing the reported data (ISA-Tab format)
Background & Summary
The size and opacity of vertebrate tissues limit optical access to the brain and hinder investigations of intact neuronal networks in vivo. As a result, many scientists focus on small, superficial brain areas, such as parts of the cerebral cortex in rodents, or on early developmental stages of small transparent organisms, like zebrafish larvae. In order to overcome these limitations, Danionella translucida (DT), a transparent cyprinid fish1,2 with the smallest known vertebrate brain, was recently developed as a novel model organism for the optical investigation of neuronal circuit activity in vertebrates3,4. The majority of DT tissues remain transparent throughout its life (Fig. 1). DT displays a variety of social behaviours, such as schooling and vocal communication, and is amenable to genetic manipulation using genetic tools that are already established in zebrafish. As such, this species is a promising model organism for studying the function of neuronal circuits across the entire brain. Yet, a continuous annotated genome reference is still needed to enable targeted genetic and transgenic studies and facilitate the adoption of DT as a model organism.
Fig. 1.

Male adult Danionella translucida showing transparency.
Next-generation short-read sequencing advances steadily decreased the price of the whole-genome sequencing and enabled a variety of genomic and metagenomic studies. However, short-read-only assemblies often struggle with repetitive and intergenic regions, resulting in fragmented assembly and poor access to regulatory and promoter sequences5,6. Long-read techniques, such as PacBio and Nanopore, can generate reads up to 2 Mb7, but they are prone to errors, including frequent indels, which can lead to artefacts in long-read-only assemblies6. Combining short- and long-read sequencing technologies in hybrid assemblies recently produced high-quality genomes in fish8,9.
Here we report the hybrid Illumina/Nanopore-based assembly of the Danionella translucida genome. A combination of deep-coverage Illumina sequencing with a single Nanopore sequencing run produced an assembly with scaffold N50 of 340 kb and Benchmarking Universal Single-Copy Orthologs (BUSCO) genome completeness score of 92%. Short- and long-read RNA sequencing data used together with other fish species annotated proteomes produced an annotation dataset with BUSCO transcriptome completeness score of 86%.
Methods
Genomic sequencing libraries
For genomic DNA sequencing we generated paired-end and mate-pair Illumina sequencing libraries and one Nanopore library. We extracted DNA from fresh DT tissues with phenol-chloroform-isoamyl alcohol. For Illumina sequencing, we used 5 days post fertilisation (dpf) old larvae. A shotgun paired-end library with 500 bp insert size was prepared with TruSeq DNA PCR-Free kit (Illumina). Sequencing on HiSeq 4000 generated 1.347 billion paired-end reads. A long ~10 kb mate-pair library was prepared using the Nextera Mate Pair Sample Prep Kit and sequenced on HiSeq 4000, resulting in 554 million paired-end reads. Raw read library quality was assessed using FastQC v0.11.810.
A Nanopore sequencing high-molecular-weight gDNA library was prepared from 3 months post fertilisation (mpf) DT tails. We used 400 ng of DNA with the 1D Rapid Sequencing Kit (SQK-RAD004) according to manufacturer’s instructions to produce the longest possible reads. This library was sequenced with the MinION sequencer on a single R9.4 flowcell using MinKNOW v1.11.5 software for sequencing and base-calling, producing a total of 4.3 Gb sequence over 825k reads. The read library N50 was 11.6 kb with the longest read being approximately 200 kb. Sequencing data statistics are summarised in Table 1.
Table 1.
Sequencing library statistics.
| Illumina paired-end gDNA | |
| Number of reads | 1.347 × 109 |
| Total library size | 136.047 Gb |
| Insert size | 500 bp |
| Read length | 2 × 101 bp |
| Estimated coverage | 186× |
| Illumina mate-pair gDNA | |
| Number of reads | 554.134 × 106 |
| Total library size | 55.968 Gb |
| Insert size | 10 kb |
| Read length | 2 × 101 bp |
| Estimated coverage | 77× |
| Nanopore gDNA | |
| Number of reads | 824.880 × 103 |
| Total library size | 4.288 Gb |
| Read length N50 | 11.653 kb |
| Estimated coverage | 5.8× |
| Nanopore cDNA | |
| Number of reads | 208.822 × 103 |
| Total library size | 279.584 Mb |
| Read length N50 | 1.812 kb |
| BGI 3 dpf larvae mRNA | |
| Number of reads | 130.768 × 106 |
| Total library size | 13.077 Gb |
| Read length | 2 × 100 bp |
| BGI adult mRNA | |
| Number of reads | 128.546 × 106 |
| Total library size | 12.855 Gb |
| Read length | 2 × 100 bp |
gDNA stands for genomic DNA sequencing, cDNA for reverse-transcribed complementary DNA, mRNA for poly-A tailed RNA sequencing.
Genome assembly
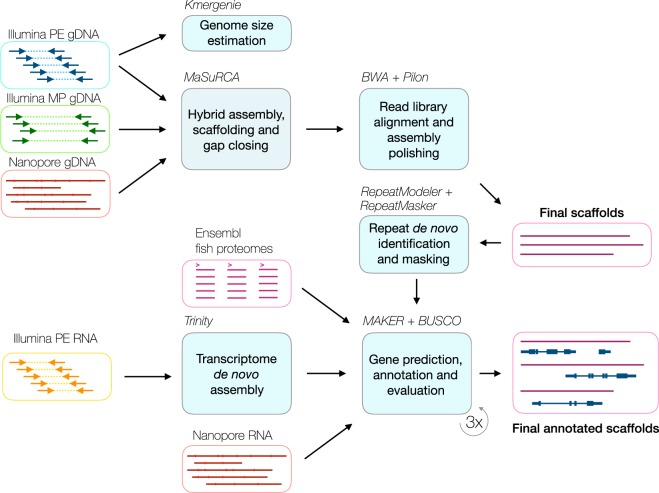
The genome assembly and annotation pipeline is shown in Fig. 2. We estimated the genome size using the k-mer histogram method with Kmergenie v1.7016 on the paired-end Illumina library preprocessed with fast-mcf v1.04.80711,12, which produced a putative assembly size of approximately 744 Mb. This translates into 186-fold Illumina and 5.8-fold Nanopore sequencing depths.
Fig. 2.
DT genome assembly and annotation pipeline. PE, paired-end; MP, mate-pair.
Multiple published assembly pipelines utilise a combination of short- and long-read sequencing. Our assembler of choice was MaSuRCA v3.2.613, since it has already been used to generate high-quality assemblies of fish genomes8,9, providing a large continuity boost even with low amount of input long reads14. Briefly, Illumina paired-end shotgun reads were non-ambiguously extended into the superreads, which were mapped to Nanopore reads for error correction, resulting in megareads. These megareads were then fed to the modified CABOG assembler that assembles them into contigs and, ultimately, mate-pair reads were used to do scaffolding and gap repair.
Following MaSuRCA author’s recommendation8, we have turned off the frgcorr module and provided raw paired-end and mate-pair read libraries for in-built preprocessing with the QuorUM error corrector13,15. The initial genome assembly size estimated with the Jellyfish assembler module was 938 Mb. After the MaSuRCA pipeline processing we have polished the assembly with one round of Pilon v1.22, which attempts to resolve assembly errors and fill scaffold gaps using preprocessed reads mapped to the assembly16. Leftover contaminants were filtered during the processing of the genome submission to the NCBI database. Statistics of the resulting assembly were generated using bbmap stats toolkit v37.3217 and are presented in Table 2.
Table 2.
DT genome assembly statistics and completeness.
| Genome assembly statistics | |
| Total scaffolds | 27,639 |
| Total contigs | 36,005 |
| Total scaffold sequence | 735.303 Mb |
| Total contig sequence | 725.703 Mb |
| Gap sequences | 1.306% |
| Scaffold N50 | 340.819 kb |
| Contig N50 | 133.131 kb |
| Longest scaffold | 3.085 Mb |
| Longest contig | 995.155 kb |
| Fraction of genome in >50 kb scaffolds | 88.3% |
| BUSCO genome completeness score | |
| Complete | 91.5% |
| Single | 87.0% |
| Duplicated | 4.5% |
| Fragmented | 3.6% |
| Missing | 4.9% |
| Total number of Actinopterygii orthologs | 4,584 |
The resulting 735 Mb assembly had a scaffold N50 of 341 kb, the longest scaffold being more than 3 Mb. To assess the completeness of the assembly we used BUSCO v318 with the Actinopterygii ortholog dataset. In total, 91.5% of the orthologs were found in the assembly.
Transcriptome sequencing and annotation
We used three sources of transcriptome evidence for the DT genome annotation: (i) assembled poly-A-tailed short-read and raw Nanopore cDNA sequencing libraries, (ii) protein databases from sequenced and annotated fish species and (iii) trained gene prediction software. For Nanopore cDNA sequencing we extracted total nucleic acids from 1–2 dpf embryos using phenol-chloroform-isoamyl alcohol extraction followed by DNA digestion with DNAse I. The resulting total RNA was converted to double-stranded cDNA using poly-A selection at the reverse transcription step with the Maxima H Minus Double-Stranded cDNA Synthesis Kit (ThermoFisher). The double-stranded cDNA sequencing library was prepared and sequenced in the same way as the genomic DNA with MinKNOW v1.13.1, resulting in 190 Mb sequence data distributed over 209k reads. These reads were filtered to remove 10% of the shortest ones. For short-read RNA-sequencing, we have extracted total RNA with the TRIzol reagent (Invitrogen) from 3 dpf larvae and from adult fish. RNA was poly-A enriched and sequenced as 100 bp paired-end reads on the BGISEQ-500 platform. After preprocessing the library sizes were 65.4 million read pairs for 3 dpf larvae and 64.3 million read pairs for adult fish specimens (Table 1). We first assembled the 100 bp paired-end RNA-seq reads de novo using Trinity v2.8.4 assembler19. This produced 222448 contigs with an N50 length of 3586 bp, clustered into 146103 “genes”. BUSCO transcriptome analysis revealed 96% of complete Actinopterygii orthologs in the Trinity assembly. These contigs, together with the Nanopore cDNA reads and proteomes of 11 fish species from Ensembl20 were used as the transcript evidence in MAKER v2.31.10 annotation pipeline21. Repetitive regions were masked using a de novo generated DT repeat library (RepeatModeler v1.0.11)22. The highest quality annotations with average annotation distance (AED) < 0.25 were used to train SNAP23 and Augustus24 gene predictors. Gene models were then polished over two additional rounds of re-training and re-annotation. The final set of annotations consisted of 24,097 protein-coding gene models with an average length of 13.4 kb and an average AED of 0.18 (Table 3). We added putative protein functions using MAKER from the UniProt database25 and protein domains from the interproscan v5.30–69.0 database26. tRNAs were searched for and annotated using tRNAscan-SE v1.427. The BUSCO transcriptome completeness search found 86% of complete Actinopterygii orthologs in the annotation set. An example Interactive Genomics Viewer (IGV) v2.4.328 window with the dnmt1 gene is shown on Fig. 3, demonstrating the annotation and RNA-seq coverage.
Table 3.
DT transcriptome annotation statistics.
| Total protein-coding gene models | 24,097 |
| Total functionally annotated gene models | 21,491 |
| Gene models with AED <0.5 | 95% |
| Mean AED | 0.18 |
| BUSCO annotation completeness score | |
| Complete | 86.3% |
| Single | 80.6% |
| Duplicated | 5.7% |
| Fragmented | 7.1% |
| Missing | 6.6% |
| Total number of Actinopterygii orthologs | 4,584 |
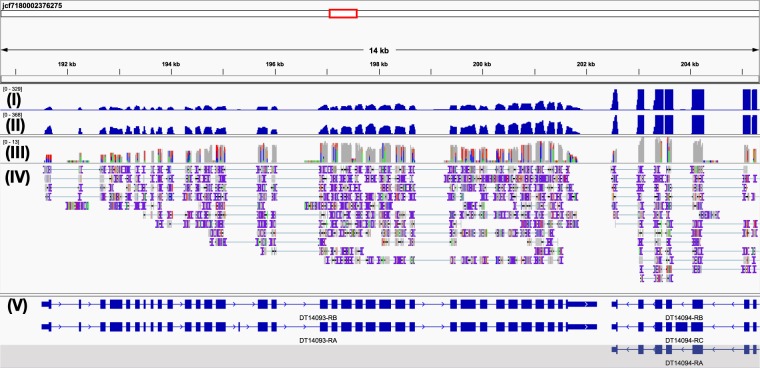
Fig. 3.
IGV screenshot of the dnmt1 locus in the DT genome assembly, with short-read RNA coverage, mapped Nanopore cDNA-seq reads and alternative splicing annotation. Tracks from top to bottom: (I) adult RNA-seq coverage, (II) 3 dpf RNA-seq coverage, (III) Nanopore cDNA-seq coverage, (IV) Nanopore cDNA-seq read mapping and (V) annotation with alternative splicing isoforms.
Data Records
Raw sequencing libraries and genome and transcriptome assemblies are deposited to NCBI SRA as part of the BioProject SRP13659429.
The genome assembly with gene and transcript annotations has been deposited at GenBank under the accession number SRMA0000000030 (the version described in this paper is SRMA01000000), as well as on figshare in FASTA/GFF3 format31. The Trinity transcriptome assembly has been deposited at NCBI TSA under accession number GHNV0000000032 (the version described in this paper is GHNV01000000), as well as on figshare31.
Kmergenie-generated kmer abundance histograms and a summary report together with the genome size estimation are deposited at figshare31.
MAKER pipeline annotation output GFF3 file containing evidence mapping, identified repetitive elements and gene models, MAKER-predicted transcripts and proteins, IGV-compatible short-read and long-read RNA-seq coverage, raw sequencing read library FASTQC quality analysis report and intron orthology data together with their custom analysis code are available on figshare31.
Technical Validation
DT and zebrafish intron size distributions
The predicted genome size of DT is around one half of the zebrafish reference genome33. Danionella dracula, a close relative of DT, possesses a unique developmentally truncated morphology34 and has a genome of a similar size (ENA Accession Number GCA_900490495.1). In order to validate our genome assembly, we set out to compare the compact genome of DT to the zebrafish reference genome.
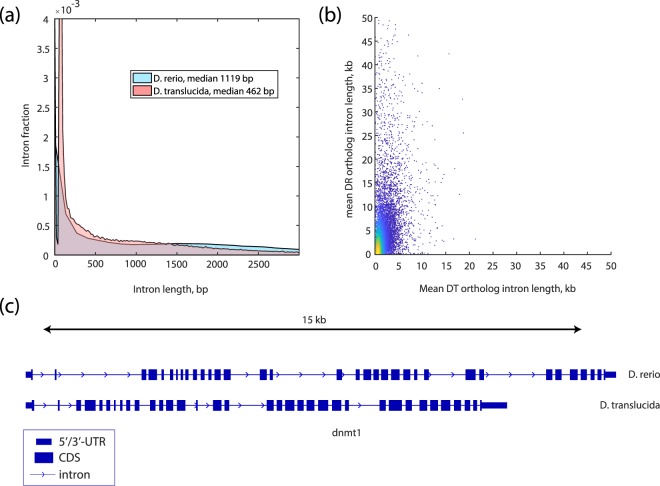
Changes in the intron lengths have been shown to be a significant part of genomic truncations and expansions, such as a severe intron shortening in another miniature fish species, Paedocypris35, or an intron expansion in zebrafish36. We therefore compared the distribution of total intron sizes from the combined Ensembl/Havana zebrafish annotation20 to the MAKER-produced DT annotation (Fig. 4a). We found that the DT intron size distribution is similar to other fish species investigated in ref.35 which stands in stark contrast to the large tail of long introns in zebrafish. Median intron length values are in the range of the observed genome size difference (462 bp in DT as compared to 1,119 bp in zebrafish).
Fig. 4.
Intron size distribution in DT (red) in comparison to zebrafish (DR, blue). (a) Intron size distribution of all transcripts in DR and DT. (b) Intron size relationship for identified DR-DT orthologous proteins. (c) A comparison of dnmt1 orthologous loci in both fish. 5′/3′ UTR, untranslated regions; CDS, coding sequence.
To investigate the difference in intron sizes on the transcript level, we compared average intron sizes for orthologous protein-coding transcripts in DT and zebrafish. We have identified orthologs in DT and zebrafish protein databases with the help of the conditional reciprocal best BLAST hit algorithm (CRB-BLAST)37. In total, we have identified 19,192 unique orthologous protein pairs. For 16,751 of those orthologs with complete protein-coding transcript exon annotation in both fish we calculated their respective average intron lengths (Fig. 4b). The distribution was again skewed towards long zebrafish introns in comparison to DT. As an example, Fig. 4c shows dnmt1 locus for the zebrafish and DT orthologs.
ISA-Tab metadata file
Acknowledgements
We would like to thank Jörg Henninger for helpful discussions and critical reading of this manuscript. This work was funded by the NeuroCure Cluster of Excellence (DFG, project EXC-2049-390688087) to M.S. and B.J. B.J. is a recipient of a Starting Grant by the European Research Council (ERC-2016-StG-714560) and the Alfried Krupp Prize for Young University Teachers, awarded by the Alfried Krupp von Bohlen und Halbach-Stiftung.
Author Contributions
M.K. collected samples, conducted sequencing, genome assembly and annotation and conducted data analysis. L.S. collected samples and participated in data analysis. M.S. and B.J. conceived and supervised the study.
Code Availability
Software used for read preprocessing, genome and transcriptome assembly and annotation is described in the Methods section together with the versions used. Custom MATLAB code used for orthology analysis is deposited on figshare31.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Markus Schuelke, Email: markus.schuelke@charite.de.
Benjamin Judkewitz, Email: benjamin.judkewitz@charite.de.
ISA-Tab metadata
is available for this paper at 10.1038/s41597-019-0161-z.
References
- 1.Roberts TR. Danionella translucida, a new genus and species of cyprinid fish from Burma, one of the smallest living vertebrates. Environ. Biol. Fishes. 1986;16:231–241. doi: 10.1007/BF00842977. [DOI] [Google Scholar]
- 2.Britz R, Conway KW, Rüber L. Spectacular morphological novelty in a miniature cyprinid fish, danionella dracula n. sp. Proc. Biol. Sci. 2009;276:2179–2186. doi: 10.1098/rspb.2009.0141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schulze L, et al. Transparent danionella translucida as a genetically tractable vertebrate brain model. Nat. Methods. 2018;15:977–983. doi: 10.1038/s41592-018-0144-6. [DOI] [PubMed] [Google Scholar]
- 4.Penalva, A. et al. Establishment of the miniature fish species Danionella translucida as a genetically and optically tractable neuroscience model. Preprint at 10.1101/444026v1.full (2018).
- 5.Shendure J, Ji H. Next-generation DNA sequencing. Nat. Biotechnol. 2008;26:1135–1145. doi: 10.1038/nbt1486. [DOI] [PubMed] [Google Scholar]
- 6.Watson, M. Mind the gaps - ignoring errors in long read assemblies critically affects protein prediction. Preprint at 10.1101/285049v1 (2018).
- 7.Payne, A., Holmes, N., Rakyan, V. & Loose, M. Whale watching with BulkVis: A graphical viewer for Oxford Nanopore bulk fast 5 files. Preprint at 10.1101/312256v1.full (2018).
- 8.Tan MH, et al. Finding Nemo: hybrid assembly with Oxford Nanopore and Illumina reads greatly improves the Clownfish (Amphiprion ocellaris) genome assembly. GigaScience. 2018;7:1–6. doi: 10.1093/gigascience/gix137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tørrensen OK, et al. An improved genome assembly uncovers prolific tandem repeats in Atlantic cod. BMC Genomics. 2017;18:1–23. doi: 10.1186/s12864-016-3406-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Andrews, S. FastQC: a quality control tool for high throughput sequence data, http://www.bioinformatics.babraham.ac.uk/projects/fastqc (2010).
- 11.Aronesty E. Comparison of Sequencing Utility Programs. Open Bioinforma J. 2013;7:1–8. doi: 10.2174/1875036201307010001. [DOI] [Google Scholar]
- 12.Chikhi R, Medvedev P. Informed and automated k-mer size selection for genome assembly. Bioinformatics. 2014;30:31–37. doi: 10.1093/bioinformatics/btt310. [DOI] [PubMed] [Google Scholar]
- 13.Zimin AV, et al. The MaSuRCA genome assembler. Bioinformatics. 2013;29:2669–2677. doi: 10.1093/bioinformatics/btt476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tan MH, et al. A hybrid de novo assembly of the sea pansy (Renilla muelleri) genome. GigaScience. 2019;8:1–7. doi: 10.1093/gigascience/giz026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marçais G, Yorke JA, Zimin A. QuorUM: An Error Corrector for Illumina Reads. PLoS One. 2015;10:1–13. doi: 10.1371/journal.pone.0130821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Walker BJ, et al. Pilon: An integrated tool for comprehensive microbial variant detection and genome assembly improvement. PLoS One. 2014;9:1–14. doi: 10.1371/journal.pone.0112963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bushnell, B. BBmap short-read aligner, and other bioinformatics tools, http://sourceforge.net/projects/bbmap/ (2016).
- 18.Simao FA, Waterhouse RM, Ioannidis P, Kriventseva EV, Zdobnov EM. BUSCO: assessing genome assembly and annotation completeness with single-copy orthologs. Bioinformatics. 2015;31:3210–3212. doi: 10.1093/bioinformatics/btv351. [DOI] [PubMed] [Google Scholar]
- 19.Grabherr MG, et al. Full-length transcriptome assembly from RNA-seq data without a reference genome. Nat. Biotechnol. 2011;29:644–652. doi: 10.1038/nbt.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zerbino DR, et al. Ensembl 2018. Nucleic Acids Res. 2018;46:D754–D761. doi: 10.1093/nar/gkx1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cantarel BL, et al. MAKER: An easy-to-use annotation pipeline designed for emerging model organism genomes. Genome Res. 2008;18:188–196. doi: 10.1101/gr.6743907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smit, A. F. A. & Hubley, R. Repeat Modeler Open-1.0, http://www.repeatmasker.org (2008).
- 23.Korf, I. Gene finding in novel genomes. BMC Bioinformatics5, 1–9 (2004). [DOI] [PMC free article] [PubMed]
- 24.Stanke M, et al. AUGUSTUS: ab initio prediction of alternative transcripts. Nucleic Acids Res. 2006;34:W435–W439. doi: 10.1093/nar/gkl200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.The UniProt Consortium UniProt: the universal protein knowledgebase. Nucleic Acids Res. 2017;45:D158–D169. doi: 10.1093/nar/gkw1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jones P, et al. InterProScan 5: genome-scale protein function classification. Bioinformatics. 2014;30:1236–1240. doi: 10.1093/bioinformatics/btu031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lowe TM, Eddy SR. tRNAscan-SE: A program for improved detection of transfer RNA genes in genomic sequence. Nucleic Acids Res. 1997;25:955–964. doi: 10.1093/nar/25.5.0955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thorvaldsdóttir H, Robinson JT, Mesirov JP. Integrative Genomics Viewer (IGV): high-performance genomics data visualization and exploration. Brief. Bioinformatics. 2013;14:178–192. doi: 10.1093/bib/bbs017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.2019. NCBI Sequence Read Archive. SRP136594
- 30.2019. GenBank. SRMA00000000
- 31.Kadobianskyi M, Schulze L, Schuelke M, Judkewitz B. 2019. Hybrid genome assembly and annotation of Danionella translucida. figshare. [DOI] [PMC free article] [PubMed]
- 32.2019. GenBank. GHNV00000000
- 33.Howe K, et al. The zebrafish reference genome sequence and its relationship to the human genome. Nat. Commun. 2013;496:498–503. doi: 10.1038/nature12111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Britz R, Conway KW. Danionella dracula, an escape from the cypriniform Bauplan via developmental truncation? J. Morphol. 2016;277:147–166. doi: 10.1002/jmor.20486. [DOI] [PubMed] [Google Scholar]
- 35.Malmstrøm M, et al. The most developmentally truncated fishes show extensive hox gene loss and miniaturized genomes. Genome Biol. Evol. 2018;10:1088–1103. doi: 10.1093/gbe/evy058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moss SP, Joyce DA, Humphries S, Tindall KJ, Lunt DH. Comparative analysis of teleost genome sequences reveals an ancient intron size expansion in the zebrafish lineage. Genome Biol. Evol. 2011;3:1187–1196. doi: 10.1093/gbe/evr090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aubry S, Kelly S, Kümpers BMC, Smith-Unna RD, Hibberd JM. Deep evolutionary comparison of gene expression identifies parallel recruitment of trans-factors in two independent origins of C4 photosynthesis. PLoS Genet. 2014;10:1–16. doi: 10.1371/journal.pgen.1004365. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- 2019. NCBI Sequence Read Archive. SRP136594
- 2019. GenBank. SRMA00000000
- Kadobianskyi M, Schulze L, Schuelke M, Judkewitz B. 2019. Hybrid genome assembly and annotation of Danionella translucida. figshare. [DOI] [PMC free article] [PubMed]
- 2019. GenBank. GHNV00000000
Supplementary Materials
Data Availability Statement
Software used for read preprocessing, genome and transcriptome assembly and annotation is described in the Methods section together with the versions used. Custom MATLAB code used for orthology analysis is deposited on figshare31.