Summary
Cas12a is an RNA-guided endonuclease, which displays great potentials and several advantages over the well-known Cas9 in genome editing and engineering. Here, we established a quantitative kinetic scheme to describe the conformational dynamics of Cas12a/crRNA/dsDNA ternary complexes. The highly dynamic nature of Cas12a complexes, including their reversible formation, disassembly, and transition between different conformational states, is likely to be one of the key aspects contributing to their high specificity. The non-target strand is cleaved when its cleavage sites are released from DNA duplex after DNase activation of Cas12a. Cleaved non-target strand stabilizes target strand pre-cleavage states to permit subsequent cleavage and to ensure two DNA strands cleaved in a well-defined order. The extent of complementarity between crRNA and DNA modulates the relative stabilities of target strand pre-cleavage states targeting different cleavage sites. Our discoveries provide insights to fully elucidate the working mechanisms of Cas12a and to optimize it for genome engineering.
Subject Areas: Biological Sciences, Biochemistry, Molecular Biology, Cell Biology, Structural Biology
Graphical Abstract
Highlights
-
•
Dynamic processes of Cas12a contribute to its high specificity
-
•
Cleavage sites of non-target strand are released from duplex after DNase activation
-
•
Cleaved non-target strand stabilizes target strand pre-cleavage states
-
•
Multiple target strand pre-cleavage states target different cleavage sites
Biological Sciences; Biochemistry; Molecular Biology; Cell Biology; Structural Biology
Introduction
CRISPR (clustered regularly interspersed short palindromic repeats)-Cas (CRISPR-associated) systems found in bacteria and archaea provide adaptive immunity against exogenous genetic elements (Barrangou et al., 2007, Marraffini and Sontheimer, 2008, Horvath and Barrangou, 2010, Marraffini, 2015). Guide RNAs encoded in CRISPR repeat-spacer arrays are transcribed and processed into individual CRISPR RNAs (crRNAs), which guide Cas proteins to recognize and cleave invading DNAs or RNAs in a sequence-dependent manner (Plagens et al., 2015). Based on the number of Cas proteins in the crRNA-effector complex, CRISPR-Cas systems are divided into two classes. Several Cas proteins are needed to form an effector complex in class I CRISPR-Cas systems, whereas a single multiple-domain Cas protein is sufficient for target recognition and cleavage in class II systems (Makarova et al., 2015, Hille and Charpentier, 2016, Wright et al., 2016). The simplicity of class II CRISPR-Cas systems greatly facilitates their application in genome engineering.
Cas9 protein, especially Streptococcus pyogenes Cas9, which belongs to class II type II CRISPR-Cas systems, is most thoroughly investigated and widely used in genome engineering and manipulation (Doudna and Charpentier, 2014, Hsu et al., 2014, Sander and Joung, 2014). Within class II CRISPR-Cas systems, type V Cas12a protein, also known as Cpf1, displays its own unique features and serves as an alternative and complementation to Cas9. Lachnospiraceae bacterium Cas12a (LbCas12a) and Acidaminococcus sp. BV3L6 Cas12a (AsCas12a) exhibited little or no tolerance for mismatches in mammalian gene editing, indicating their higher specificity than Cas9 (Kim et al., 2016, Kleinstiver et al., 2016, Toth et al., 2016, Tu et al., 2017). In addition, Cas12a contains an RNA nuclease domain to process its own precursor crRNA, simplifying its application for multiplexed gene editing (Fonfara et al., 2016, Zetsche et al., 2017). Furthermore, Cas12a exhibits RNA-independent DNase activity and target-binding-induced indiscriminate single-stranded DNase activity, which enable development of novel DNA detection methods with extremely high sensitivity (Sundaresan et al., 2017, Chen et al., 2018, Gootenberg et al., 2018). Therefore, Cas12a has attracted great attention and been widely used (Li et al., 2018, Swarts and Jinek, 2018).
Structural and biochemical studies revealed that Cas12a exhibits a different structural architecture from Cas9, which leads to its distinct molecular mechanisms (Dong et al., 2016, Gao et al., 2016, Yamano et al., 2016, Yamano et al., 2017, Stella et al., 2017, Swarts et al., 2017, Jeon et al., 2018, Singh et al., 2018, Strohkendl et al., 2018). Dual tracrRNA:crRNA or a fused single-guide RNA guides Cas9 to recognize 3′ G-rich protospacer-adjacent motifs (PAMs) on double-stranded DNAs (dsDNAs). Two nuclease domains, HNH and RuvC, are used by Cas9 to cleave dsDNA through their concerted motions to generate blunt ends 3 bp upstream of PAM sites (Jinek et al., 2012, Sternberg et al., 2015). On the other hand, Cas12a is guided by a single crRNA to recognize 5′ T-rich PAMs. Cas12a contains a single RuvC endonuclease domain, which cleaves non-target strand (NTS) and target strand (TS) one by one to create staggered ends (Zetsche et al., 2015, Yamano et al., 2016, Swarts et al., 2017, Jeon et al., 2018, Strohkendl et al., 2018). In addition, evidences showed that Cas12a has multiple cleavage sites on both DNA strands (Stella et al., 2017, Swarts et al., 2017, Strohkendl et al., 2018).
Several single-molecule fluorescence resonance energy transfer (smFRET) assays have been utilized to characterize conformational dynamics of Cas12a (Jeon et al., 2018, Singh et al., 2018, Stella et al., 2018). Singh and coworkers examined how rates of Cas12a/crRNA/dsDNA ternary complex formation and DNA cleavage are modulated by complementarity between crRNA and DNA (Singh et al., 2018). On the other hand, FRET pairs located within Francisella novicida Cas12a (FnCas12a) protein and between crRNA and NTS of AsCas12a complexes both showed that Cas12a ternary complexes display several distinctive conformational states, including PAM recognition, R-loop expansion, and NTS and TS pre-cleavage states (Jeon et al., 2018, Stella et al., 2018). Here, using smFRET between crRNA and TS, we established a quantitative kinetic scheme to quantify conformational dynamics of Cas12a ternary complexes and to describe how the extent of crRNA-TS heteroduplex formation modulates transition rates and reaction pathways among different conformational states. We discovered that 14 base pairs of crRNA-DNA heteroduplex at the PAM-proximal end were sufficient to trigger the nuclease activation of Cas12a, whereas more crRNA-DNA base pairs were required to free NTS cleavage sites from DNA duplex to permit cleavage. After NTS cleavage, TS pre-cleavage states were highly stabilized to enable two DNA strands cleaved subsequently. There were several TS pre-cleavage states producing cleaved fragments of different length, whose relative stabilities were modulated by the extent of crRNA-DNA complementarity. Our new findings shed lights on molecular mechanisms of Cas12a-mediated DNA cleavage.
Results
Conformational Dynamics of Cas12a/crRNA/dsDNA Ternary Complexes
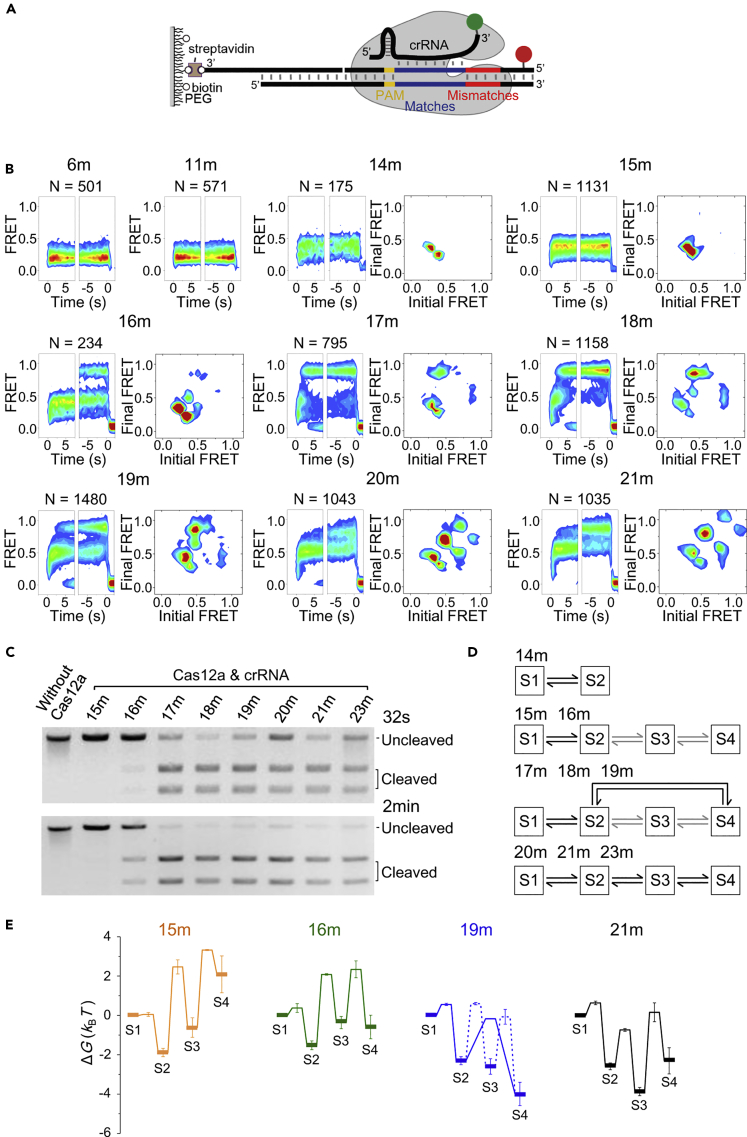
To probe the conformational dynamics of Cas12a ternary complexes from their formation until DNA cleavage, we utilized smFRET between crRNA and dsDNA. As shown in Figure 1A, dsDNAs containing Cy5-labeled TS were immobilized on polyethylene glycol-passivated microscope glass slides. An objective-based total internal reflection fluorescence microscope was used to capture smFRET trajectories, whose recording was started 5 s before the delivery of pre-formed Cas12a/crRNA complexes, containing Cy3 at the 3′ end of crRNA (Table S1), to surface immobilized dsDNA (Table S2). Formation of stable Cas12a/crRNA/dsDNA complexes, indicated by black arrows in Figure 1A, caused simultaneous appearance of both Cy3 and Cy5 signals. The increase of Cy3/Cy5 FRET efficiencies evidenced that Cas12a/crRNA/dsDNA complexes underwent a series of conformational changes to bring Cy3- and Cy5-labeling sites close to each other (Figures 1A and 1B). When a full cognate dsDNA containing 23 matched bases toward crRNA was used, termination of FRET signals was accompanied by disappearance of Cy5 signals in ∼90% of single-molecule events (blue arrows in Figure 1A). We have carefully adjusted the laser power to obtain optimal signal-to-noise ratio and to minimize the contributions of photobleaching (Figure S1A). Therefore, loss of FRET was due to the release of Cy5-labeled DNA fragments from Cas12a ternary complexes after DNA cleavage (Figures S1B and S1C), which agreed with previous reports (Jeon et al., 2018, Singh et al., 2018, Strohkendl et al., 2018).
Figure 1.
Conformational Dynamics of Cas12a Ternary Complexes on Fully Matched dsDNA
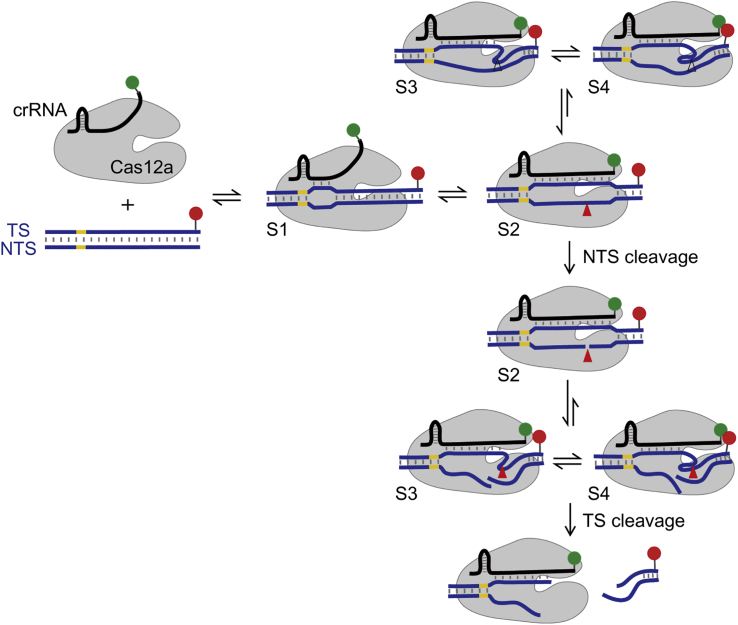
(A) Representative smFRET trajectories and cartoons illustrating corresponding states. Cy3 (green dots) is labeled at 3′ end of crRNA, Cy5 (red dots) is labeled at the TS 28 nucleotides away from the PAM. Black lines are apparent FRET efficiencies, and red lines are hidden Markov modeling of FRET. Immobilized dsDNA contains 23 matched bases toward the crRNA. Under 532 nm excitation, spontaneous appearance of Cy3 and FRET signals represents the formation of Cas12a ternary complexes on immobilized dsDNAs (black arrows), and disappearance of FRET corresponds to dissociation of the cleaved PAM-distal DNA fragments (blue arrows). Four distinctive FRET states are indicated and assigned as S1–S4 from low to high values.
(B) Time-dependent FRET probability density plots synchronized at the appearance of FRET (defined as t = 0 in the left panel) or at the disappearance of FRET (defined as t = 0 in the right panel). From them, evolution of FRET after complex formation or before release of cleaved DNA can be carefully examined. Overall, FRET efficiencies increase over time after complex formation.
(C) A transition density plot reflecting transition frequencies among FRET states, in which initial and final FRET values for each transition event are accumulated into a two-dimensional histogram.
(D) A kinetic scheme displaying transition pathways and rates (s−1) among different FRET states. All experiments were repeated three times, and SEMs were used as error bars.
We identified four distinctive FRET states from single-molecule trajectories of Cas12a ternary complexes using a Hidden Markov Model-based software (McKinney et al., 2006). They were assigned as S1, S2, S3, and S4 states, whose FRET efficiencies were 0.28 ± 0.01, 0.49 ± 0.01, 0.75 ± 0.01, and 0.95 ± 0.01, respectively. Almost all FRET events were initiated at S1, the lowest FRET state. Interestingly, as shown by representative traces, FRET events could terminate from either S3 or S4 state (Figures 1A and S1C), two highest FRET states, after transiting through an intermediate FRET state (S2). Although the overall trend was that FRET efficiencies of ternary complexes increased over time, spontaneous transitions from a high- to a low-FRET state were commonly detected (Figure 1A). FRET transition density plot (Figure 1C) showed that dynamic transitions mostly occurred between two conformational states whose FRET values were close to each other. Dwell times of each state and transition rates among them were extracted and quantified. In all, a quantitative kinetic scheme containing four conformational states was established to describe the conformational dynamics of Cas12a ternary complexes (Figure 1D).
Using FRET pairs located on crRNA and NTS, a similar smFRET study by Jeon and colleagues also discovered that AsCas12a ternary complexes present four distinctive FRET states (Jeon et al., 2018). After formation of ternary complexes, AsCas12a gradually transited from the lowest FRET state to the highest FRET state until release of cleaved DNA. Overall, their discoveries and assignment of FRET states are mostly consistent with ours. However, AsCas12a only displays irreversible transitions from low- to high-FRET states and cleaved DNAs are released from the highest FRET state, whereas LbCas12a clearly displays reversible transitions between different conformational states and release of cleaved DNA fragments can occur from either the S3 or S4 state (Figure 1). LbCas12a still displayed similar behaviors when crRNA-NTS FRET pair was used instead of crRNA-TS FRET pair (Figures 1 and S1D). Therefore, we speculated that different Cas12a orthologs could present quite different dynamic and biochemical behaviors originating from their own intrinsic properties (Singh et al., 2018).
Using FRET pairs located on FnCas12a protein, another smFRET study revealed that FnCas12a/crRNA/dsDNA complexes also spontaneously fluctuated among four FRET states. The fact that both LbCas12a and FnCas12a display highly dynamic and reversible transitions among different conformations indicates that they have lower energy barriers among different states than AsCas12a, which might cause LbCas12a and FnCas12a to have faster rates to transit into pre-cleavage states and to cleave dsDNA than AsCas12a (Figure 2) (Singh et al., 2018).
Figure 2.
Conformational Dynamics of Cas12a Ternary Complexes on Partial Cognate dsDNAs
(A) Cartoons illustrating immobilization, labeling sites of Cy3 (green dot) and Cy5 (red dot), and positions of crRNA/DNA matches and mismatches.
(B) Time-dependent FRET probability density plots and transition density plots (when applicable) of Cas12a on partial cognate dsDNAs. dsDNAs are named after the number of matched bases between crRNA and TS at their PAM-proximal ends.
(C) Cleavage of dsDNAs by Cas12a at 25°C for 32 s or 2 min.
(D) Kinetic schemes describing dynamics of Cas12a complexes.
(E) Four representative energy landscapes of Cas12a complexes. Bold horizontal lines represent four conformational states captured by smFRET, and thin horizontal lines represent energy barriers along transition pathways. For 19m-dsDNA, which contains parallel reaction pathways, the major pathway is shown in solid lines and the minor pathway in dashed lines. For clarity, energy landscapes of Cas12a with other dsDNAs were displayed in Figure S4C. kB is the Boltzmann constant and T is the temperature.
Dynamics of Cas12a Complexes on Partial Cognate dsDNAs and Assignment of Conformational States
Using the smFRET assays described above, we quantified how the conformational dynamics of ternary complexes were modulated when partial cognate dsDNA targets were present (Figures 2 and S2). Here, we used the same crRNA and altered the sequence of dsDNA targets, whose names were based on the number of matched bases between crRNA and TS at PAM-proximal end (Figure 2A and Table S2). Therefore, 23m-dsDNA was the cognate target, whereas 6m- and 21m-dsDNAs contained 6 and 21 consecutive matched bases at their PAM-proximal end, respectively.
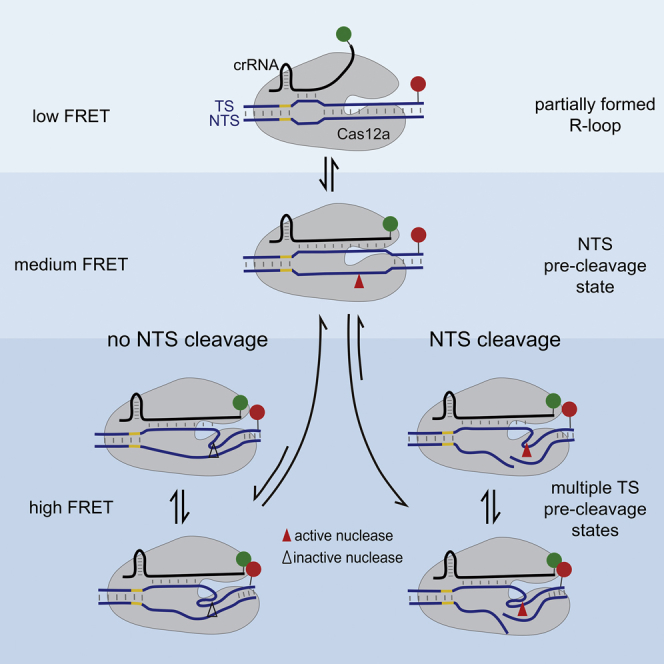
When 6 or 11 mismatched bases were introduced at the PAM-proximal end of 23m-dsDNA, almost no Cas12a/crRNA-binding events or FRET signals were detected. On the other hand, Cas12a complexes containing 6 and 11 matched bases at the PAM-proximal end both exhibited a single FRET state corresponding to the S1 state (Figures 2B and S2). In addition, disappearance of their FRET signals was caused by dissociation of Cy3-labeled Cas12a/crRNA from immobilized dsDNA, because FRET and Cy3 signals disappeared simultaneously in most cases (∼90%, example traces shown in Figure S2). Thus, S1 is an intermediate state formed after PAM recognition and partial R-loop formation.
The S2 state appeared when there were 14 matched bases between crRNA and TS at the PAM-proximal end. Under such condition, Cas12a complexes mainly fluctuated between the S1 and S2 states and barely sampled the S3 or S4 states (Figures 2B and S2). The populations of S3 and S4 increased to 9% ± 1% and 11% ± 4%, respectively, when 16m-dsDNA was used. With dsDNAs containing 17 or more matched bases toward crRNA, Cas12a complexes quickly transited through the low-FRET S1 and S2 states and mainly stayed in one of the high-FRET states, S3 or S4. Their abilities to quickly transit into S3 or S4 strongly correlated with their cleavage activities (Figures 2B and 2C). In addition, dissociation of cleaved DNA fragments, indicated by disappearance of FRET and Cy5 signals, can also occur from either S3 or S4 (Figures 1A, S1C, and S2). These results suggested that S3 and S4 states are likely to fulfill similar functions. Previous results from single-molecule and ensemble biochemical measurements suggested that the cleavage of NTS precedes the cleavage of TS (Jeon et al., 2018, Stella et al., 2018, Strohkendl et al., 2018). Using Cy3-labeled NTS and Cy5-labeled TS, we also discovered that cleaved NTS fragments were released before cleaved TS in most cases (Figure S3). Together, we proposed that S2 is the NTS pre-cleavage state formed when the R-loop is extended to the PAM-distal end. Both S3 and S4 represent TS pre-cleavage states, whose difference will be described below.
Energy Landscapes of Cas12a Complexes
Cas12a complexes containing 14 or more matched bases between crRNA and TS displayed transitions among different conformational states (Figures 2B and 2D). Based on their major transition pathways, quantitative kinetic schemes containing transition rates extracted from single-molecule trajectories were established (Figures S4A and S4B). The differences in free energies between conformational states and the energy barriers along reaction pathways were quantified and used to plot energy landscapes of Cas12a complexes with partial cognate and full cognate dsDNAs (Figures 2E and S4C).
Several features of conformational dynamics of Cas12a complexes could be summarized from their FRET patterns and energy landscapes. (1) Once Cas12a ternary complexes were formed, they were initiated at the S1 state, an intermediate state containing partially formed R-loop. In our experiments, Cas12a complexes remained in S1 when matched bases between crRNA and TS were 11 or less, whereas Cas12a could advance to other states when matched bases were 14 or more. (2) When dsDNA contained 14 or more matched bases toward crRNA, transition rates from S1 to S2 were fast (0.48–1.00 s−1, Figure S4B). However, biochemical assays revealed that 14m-dsDNA could not be cleaved and cleavage of 15m-dsDNA and 16m-dsDNA required several minutes or more (Figures 2C and S5A) (Stella et al., 2018). These results suggested that Cas12a complexes containing intact dsDNAs are able to quickly access S2, the NTS pre-cleavage state, from the initial S1 state after further extension of the R-loop. (3) The S3 state was the most stable state when there were 20 or more matched bases between crRNA and TS. On the other hand, the S4 state became the most stable state and Cas12a displayed significant transition from S2 to S4 when there were 17–19 matched bases. Our observation that dsDNAs containing 17–23 matches were all cleaved by Cas12a at similar rates supports our assignment that both S3 and S4 are TS pre-cleavage states (Figure 2C). In the presence of 15 or 16 matched bases, cleavage rate was significantly slower because S3 and S4 became less stable than S2 and were sampled by Cas12a complexes at low frequencies (Figures 2B and 2E).
S3 and S4 States Are TS Pre-cleavage States Targeting Different Cleavage Sites
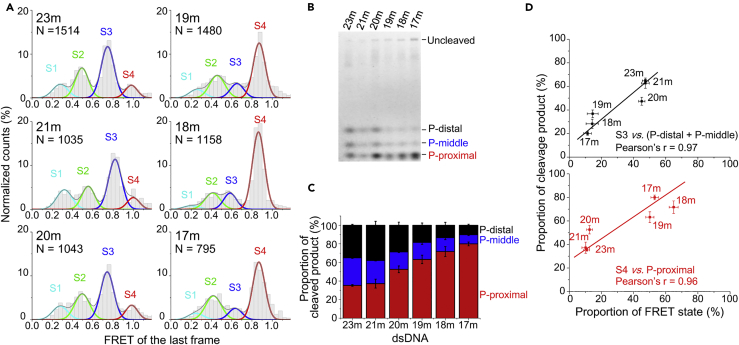
Recent reports (Stella et al., 2017, Swarts et al., 2017, Strohkendl et al., 2018) indicated that Cas12a could cleave TS at different sites and produce fragments with different lengths. We hypothesized that the S3 and S4 states target different cleavage sites. The proportion of Cas12a complexes staying in S4 as the last state before disappearance of FRET increased from 11% ± 2% with 23m-dsDNA to 53% ± 2% with 17m-dsDNA (Figure 3A). Using Cy5-labeled TS, we discovered that there were three cleaved TS fragments of different lengths (Figures 3B and S5B). The proportion of cleaved product P-proximal increased from 35% ± 1% with 23m-dsDNA to 80% ± 2% with 17m-dsDNA (Figure 3C). Thus the proportion of the S4 FRET state strongly correlated with the proportion of cleaved product P-proximal, whereas the proportion of the S3 FRET state strongly correlated with the proportion of cleaved products P-distal and P-middle (Figure 3D). Together, our results supported the model that both S3 and S4 states are pre-cleavage states targeting different TS cleavage sites to produce fragments of different lengths.
Figure 3.
Correlation between FRET Pattern and TS Cleavage Pattern of Cas12a
(A) FRET distributions of Cas12a complexes in the last frame before disappearance of FRET.
(B) Cleavage of TS. dsDNAs are labeled at the PAM-distal end of TSs. Three cleaved fragments of different lengths are separated on 15% urea-PAGE. They are assigned as P-distal, P-middle, and P-proximal, whose cleavage sites on TS are about +26, +25, and +24, respectively (Figure S5B).
(C) Proportion of cleaved TS fragments of different lengths.
(D) Linear correlation between proportion of FRET states and proportion of TS fragments. They display a strong positive correlation with Pearson's r ≥ 0.96. All experiments were repeated three times, and SEMs were used as error bars.
NTS Cleavage Site Is Freed from DNA Duplex after DNase Activation of Cas12a
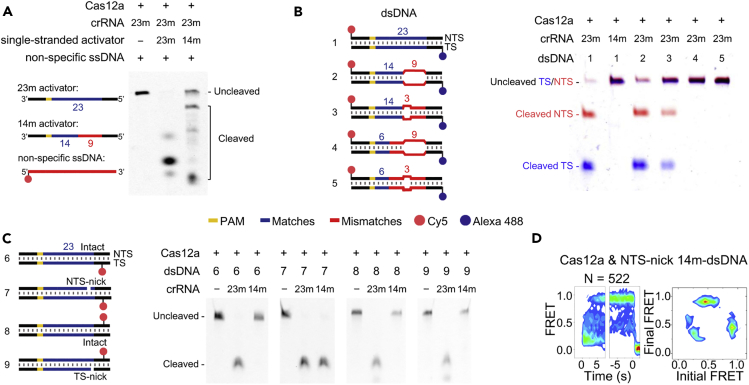
Although Cas12a displayed no cleavage activities toward 14m-dsDNA within 30 min (Figure S5A), the TS of 14m-dsDNA in its single-stranded form was able to induce indiscriminate single-stranded DNase activity of Cas12a (Figure 4A). In addition, pre-unwound 14m-dsDNAs containing mismatches between TS and NTS from +15 to +17 or from +15 to +23 can be cleaved by Cas12a, whereas pre-unwound 6m-dsDNAs containing mismatches at the same sites cannot be cleaved (Figure 4B). Together, these results suggested that 14 base pairs between crRNA and TS at the PAM-proximal end were sufficient to trigger the nuclease activation of Cas12a. The NTS cleavage site located around +18 is freed in pre-unwound dsDNAs to permit its accessibility and cleavage, whereas the NTS cleavage site in 14m-dsDNA remains in its duplex form and thus prevents further cleavage. Therefore, we speculated that freeing single-stranded NTS from DNA duplex is an important checkpoint after nuclease activation and 17 or more base pairs between crRNA and TS are needed to fully release NTS cleavage site to enable quick cleavage (Figure 2C).
Figure 4.
Cleavage and smFRET of Pre-unwound or Pre-cleaved DNAs
(A) Indiscriminate single-stranded DNA cleavage activated by 23m- or 14m- single-stranded activators, which contain 23 and 14 matched bases toward 23m-crRNA at the PAM-proximal end, respectively. Matched bases toward 23m-crRNA are shown in blue, whereas mismatched bases toward 23m-crRNA are shown in red. Non-specific ssDNA was labeled with Cy5 at the 5′ end.
(B) Cleavage of pre-unwound dsDNAs. Cas12a was guided by 23m-crRNA or 14m-crRNA, which contain 23 or 14 matched bases at their PAM-proximal ends toward 23m-dsDNA, respectively. Pre-unwound segments are plotted as bulges. Labeling sites are shown in cartoons.
(C) Cleavage of 23m-dsDNA containing pre-cleaved NTS or TS. Locations of the pre-cleaved and labeling sites are shown in cartoons. In the presence of 14m-crRNA, pre-cleaved NTS (NTS-nick) caused cleavage of TS (dsDNA #7), whereas pre-cleaved TS (TS-nick) did not lead to cleavage of NTS (dsDNA #9).
(D) Time-dependent FRET probability density and transition density plots of Cas12a complexes on NTS-nick 14m-dsDNAs.
NTS Cleavage Governs TS Cleavage
Next, we introduced nicks at the cleavage sites of the NTS (termed NTS-nick) or the TS (termed TS-nick) to generate dsDNAs containing pre-cleaved NTS or pre-cleaved TS. When there were only 14 matched bases between crRNA and TS at the PAM-proximal end, pre-cleaved NTS led to significant cleavage of TS, whereas no cleavage of NTS was detected in the presence of pre-cleaved TS (Figure 4C). smFRET measurements also showed that Cas12a with NTS-nick 14m-dsDNA can quickly transit to TS pre-cleavage states (Figure 4D), whereas Cas12a with intact 14m-dsDNA barely samples TS pre-cleavage states (Figure 2B). Together, our observation that pre-cleaved NTS permitted cleavage of TS and cleavage of NTS was not affected by pre-cleaved TS suggested that NTS cleavage precedes and governs TS cleavage.
TS Pre-cleavage States Are Stabilized after NTS Cleavage
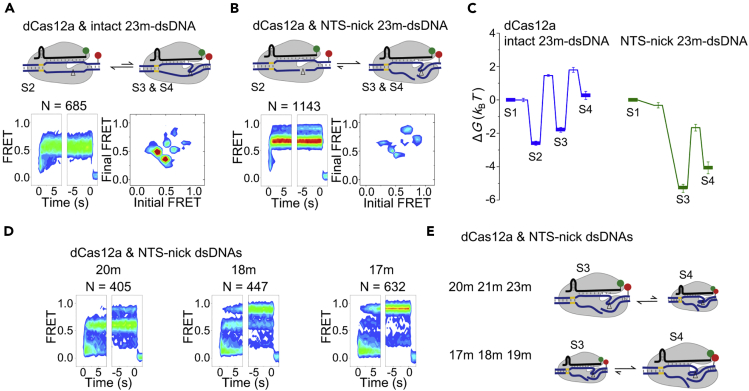
To examine how cleavage of NTS affects the dynamics of Cas12a complexes, we used dCas12a whose nuclease activity is completely abolished by introducing the E925A point mutation (Yamano et al., 2017). Interestingly, dCas12a ternary complexes formed with intact 23m-dsDNA also displayed all four FRET states as we described above (Figure 5A). However, dCas12a complexes mainly stayed in the NTS pre-cleavage state (S2 state) and occasionally sampled the TS pre-cleavage states (S3 and S4 states, Figures 5A and S6). From energy landscape, we can clearly visualize that S3 and S4 were less stable than S2 for dCas12a (Figure 5C), whereas S3 and S4 were stabilized and at least one of them became more stable than S2 for active Cas12a complexes (Figures 2E and S4C). With dsDNA containing pre-cleaved NTS (NTS-nick), dCas12a complexes quickly transited into and mainly stayed in the TS pre-cleavage states, S3 and S4 (Figures 5B and S6). We quantified that the presence of pre-cleaved NTS in dCas12a/23m-dsDNA complexes stabilized the S3 and S4 states (ΔG = −3.5 ± 0.3 and −4.4 ± 0.4 kBT, respectively) and destabilized the S2 state (ΔG = 1.2 ± 0.3 kBT) (Figure 5C). Together, our results suggested that Cas12a complexes containing intact NTS can only transiently sample TS pre-cleavage states, whereas cleaved NTS stabilized TS pre-cleavage states to facilitate subsequent cleavage step.
Figure 5.
Conformational Dynamics of dCas12a Complexes
(A and B) Time-dependent FRET probability density plots and transition density plots of dCas12a complexes on intact (A) or NTS-nick (B) 23m-dsDNAs.
(C) Energy landscapes of dCas12a complexes on intact or NTS-nick 23m-dsDNAs.
(D) Time-dependent FRET probability density plots of dCas12a complexes on NTS-nick 20m-, 18m-, and 17m-dsDNAs.
(E) Cartoons demonstrating how transition rates between TS-pre-cleavage states S3 and S4 were modulated by the extent of crRNA-DNA complementarity.
When pre-cleaved NTS was introduced into 20m-, 18m-, and 17m-dsDNAs, proportion of S4 increased when the number of matched bases decreased (Figures 5D and 5E). Similar behaviors were also captured using Cas12a complexes (Figures 2B and 3A). These results indicated that the relative stabilities of TS pre-cleavage states, S3 and S4, were modulated by the extent of complementarity between crRNA and TS.
Discussion
Previous structural and biochemical studies (Stella et al., 2017, Stella et al., 2018, Jeon et al., 2018, Strohkendl et al., 2018) indicated that Cas12a ternary complexes went through a highly dynamic process to permit dsDNA cleavage, whose main steps included crRNA invasion, R-loop formation, R-loop expansion, and conformational changes to allow two DNA strands to be cleaved by the RuvC nuclease domain sequentially. Here, we utilized FRET pair located on the crRNA and TS to capture the conformational dynamics of individual Cas12a complexes from their formation until release of cleaved DNA fragments in detail. Four distinctive conformational states distinguished by their FRET efficiencies were identified by our smFRET measurements. In addition to previous discoveries, we established the following quantitative kinetic scheme to describe the reaction pathways of Cas12a ternary complexes (Figure 6). When the Cas12a/crRNA binary complex recognizes its target, ternary complex is formed in the S1 FRET state, an intermediate state containing partially formed R-loop. Extension of the R-loop to the PAM-distal end causes further interactions between Cas12a and the heteroduplex, which trigger conformational changes of Cas12a to activate its nuclease center and to adopt the NTS pre-cleavage conformational state identified as the S2 FRET state. Cas12a complex containing intact NTS can only transiently sample S3 and S4 states, TS pre-cleavage states targeting different cleavage sites. Once NTS is cleaved, TS pre-cleavage states are greatly stabilized and become long-lived states. The extent of complementarity between crRNA and TS modulates the relative stabilities of S3 and S4, which eventually causes different patterns of cleaved TS fragments (Figure 3B). Last, the PAM-distal DNA fragments quickly dissociate from the complexes, whereas the PAM-proximal DNA fragments remain bound with Cas12a/crRNA.
Figure 6.
A Kinetic Scheme Describing Reaction Pathways of Cas12a/crRNA/dsDNA Ternary Complexes from Formation Until DNA Cleavage
Cas12a proteins are shown in gray, crRNAs are shown in black, and dsDNAs are shown in purple. Cy3 and Cy5 are labeled at crRNAs and TSs of dsDNAs, respectively. Two-way and one-way arrows indicate reversible and irreversible reactions, respectively. Once Cas12a/crRNA binary complexes recognize their target sites and initiate the R-loop formation, ternary complexes are formed in the S1 state. Cas12a complexes transit into the S2 state when the R-loop is extended to contain 14 or more base pairs. Cas12a complexes containing intact NTS can only transiently sample S3 and S4 states from S2 without carrying out TS cleavage. After irreversible cleavage of NTS, S3 and S4 states are stabilized, in which the nuclease activation centers target different cleavage sites on TS. Cleavage of TS occurring from S3 and S4 generates cleaved TS fragments of different lengths, and PAM-distal fragments are released from the complexes.
Previous studies showed that the length and composition of the 5′ end of crRNA affected the nuclease activities of Cas12a (Park et al., 2018, Singh et al., 2018). Here, we used a crRNA, which contained four G nucleotides at its 5′ end and displayed slower cleavage rate than the crRNA containing a single U at its 5′ end, to repeat several experiments described above (Figure S7). In general, stabilities of different conformational states and transition rates among them were moderately affected by G nucleotides at the 5′ end of the crRNA, whereas the overall reaction pathways and kinetic schemes remained the same.
Unlike Cas9, which can form stable binding on off-target sites containing eight or more base pairs at the PAM-proximal end without cleaving dsDNA (Singh et al., 2016), Cas12a has to cleave dsDNA to remain stably bound (Singh et al., 2018). Our kinetic scheme provides an explanation toward such phenomena (Figure 6). Cas12a complexes containing intact dsDNAs could disassemble quickly through a series of reversible steps, whereas irreversible cleavage steps of DNA prevent disassembly process. Furthermore, in the presence of partial cognate dsDNA targets containing 15 or 16 matched bases, competition between the disassembly and cleavage steps causes a lot of unproductive formation of Cas12a ternary complexes (Jeon et al., 2018, Singh et al., 2018), which reduces effective cleavage rates sharply (Figures 2C and S5A). Similarly, the ribosome utilizes a series of reversible steps to compete with forward reactions to achieve high specificity in tRNA selection (Pape et al., 1998, Geggier et al., 2010). Therefore the highly dynamic nature of Cas12a ternary complexes is likely to be one of the key mechanisms contributing to their higher specificity than Cas9 (Kim et al., 2016, Kleinstiver et al., 2016, Toth et al., 2016, Tu et al., 2017).
Conformational changes of Cas12a protein, including movements of the REC linker, lid, and finger to interact with heteroduplex at several locations, serve as the checkpoints to activate its nuclease catalytic center (Stella et al., 2018). Here, we discovered that 14 matched bases between crRNA and TS at the PAM-proximal end are sufficient to trigger nuclease activation of Cas12a, including its indiscriminate nuclease activity (Figures 4A–4C). Our observation, that Cas12a cannot cleave intact 14m-dsDNA but can cleave 14m-dsDNA containing pre-cleaved NTS or pre-unwound NTS, indicated that 14 base pairs between crRNA and TS are insufficient to release NTS cleavage sites (around +18) from dsDNA. On the other hand, in the presence of 17 or more base pairs between crRNA and TS or pre-unwound dsDNA targets, NTS cleavage sites are fully released. Therefore, NTS cleavage and subsequent TS cleavage are carried out quickly once the nuclease center is activated (Figure 2C). Together, we proposed that, after Cas12a nuclease activation, the R-loop has to extend further to free NTS cleavage sites from the DNA duplex, which is another important checkpoint to permit its accessibility and cleavage.
Although dCas12a complexes containing intact 23m-dsDNA can transiently sample TS pre-cleavage states S3 and S4, they are less stable than S3 and S4 states of active Cas12a or dCas12a containing pre-cleaved NTS (Figures 5C and S4C). In addition, FRET efficiencies of S3 and S4 states of dCas12a (0.65 ± 0.04 and 0.89 ± 0.03 for intact 23m-dsDNA) were significantly smaller than the corresponding values of Cas12a (0.75 ± 0.01 and 0.95 ± 0.01), which indicated that their conformational structures are similar but not exactly the same. Thus the presence of intact NTS protects TS from cleavage by destabilizing TS pre-cleavage states and preventing the correct positioning of Cas12a catalytic center toward cleavage sites on TS. Once NTS is cleaved, TS pre-cleavage states are stabilized in the correct conformations to license subsequent cleavage of TS. As a result, two DNA strands are cleaved in a sequentially coordinated fashion.
Until now, there is no direct structural information regarding how Cas12a cuts its TS. It is proposed that Cas12a and Cas12b may utilize similar mechanisms to cleave TS. In structures of AacCas12b complexes, TS adopts a sharp bending to bind the RuvC catalytic center in the same polarity as NTS does (Yang et al., 2016). To achieve such conformation after NTS cleavage, additional conformational rearrangements of Cas12a complexes in combination with shortening the crRNA-TS heteroduplex are required to correctly target TS cleavage sites (Stella et al., 2018). Here, we proposed that TS pre-cleavage states, S3 and S4, contain different bending conformations of TS caused by different extent of heteroduplex unwinding. According to our proposed model (Figure 6), Cas12a complexes with more PAM-distal-end mismatches are more likely to further unwind the R-loop, to cause further bending of TS, and to cleave at sites closer to the PAM, than the ones containing fewer or no mismatches. Such speculation is fully consistent with our observation (Figures 3 and S7). Overall, our smFRET measurements provided direct evidences that Cas12a complexes can access multiple conformations in their TS pre-cleavage states.
Based on our established kinetic scheme, we speculated that one of the key aspects leading to high specificity of Cas12a is the reversible formation of Cas12a/crRNA/dsDNA complexes before DNA cleavage, which reduces effective cleavage rates toward off-target DNAs sharply. Release of NTS cleavage sites from DNA duplex serves as another important checkpoint after nuclease activation to initiate cleavage steps. NTS cleavage precedes and licenses TS cleavage to ensure that Cas12a cleaves both DNA strands in a well-ordered coordinated fashion. Cas12a complexes in TS pre-cleavage states adopt several alternative conformations targeting and cleaving different sites on TS. Thus, our findings provide insights to elucidate the working mechanisms of Cas12a and to optimize and engineer Cas12a as an effective and multipurpose tool in genome editing and manipulation.
Limitations of the Study
We do want to point out that LbCas12a generates three cleaved TS fragments of different lengths, which indicates that there should be three TS pre-cleavage conformational states targeting different sites. However, different conformational states might present similar FRET efficiencies and be assigned as a single FRET state, when their differences cannot be resolved by the distance between FRET-labeling sites (Stella et al., 2018). As a result, we only captured two FRET states, S3 and S4, as TS pre-cleavage states.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
This project was supported by funds from the National Natural Science Foundation of China (21877069 and 31570754), Tsinghua-Peking Joint Center for Life Sciences, Beijing Advanced Innovation Center for Structural Biology, and Beijing Frontier Research Center for Biological Structure to C.C. The authors would like to thank Dr. Zhiwei Huang from Harbin Institute of Technology for providing plasmid to express LbCas12a.
Author Contributions
L.Z. and C.C. designed the experiments; L.Z., R.S., M.Y., S.P., and Y.C. prepared materials and reagents; L.Z. and R.S. performed experiments; L.Z. and C.C. analyzed the data and wrote the paper.
Declaration of Interests
The authors declare no competing interests.
Published: September 27, 2019
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2019.08.005.
Supplemental Information
References
- Barrangou R., Fremaux C., Deveau H., Richards M., Boyaval P., Moineau S., Romero D.A., Horvath P. CRISPR provides acquired resistance against viruses in prokaryotes. Science. 2007;315:1709–1712. doi: 10.1126/science.1138140. [DOI] [PubMed] [Google Scholar]
- Chen J.S., Ma E., Harrington L.B., Da Costa M., Tian X., Palefsky J.M., Doudna J.A. CRISPR-Cas12a target binding unleashes indiscriminate single-stranded DNase activity. Science. 2018;360:436–439. doi: 10.1126/science.aar6245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong D., Ren K., Qiu X., Zheng J., Guo M., Guan X., Liu H., Li N., Zhang B., Yang D. The crystal structure of Cpf1 in complex with CRISPR RNA. Nature. 2016;532:522–526. doi: 10.1038/nature17944. [DOI] [PubMed] [Google Scholar]
- Doudna J.A., Charpentier E. Genome editing. The new frontier of genome engineering with CRISPR-Cas9. Science. 2014;346:1258096. doi: 10.1126/science.1258096. [DOI] [PubMed] [Google Scholar]
- Fonfara I., Richter H., Bratovic M., Le Rhun A., Charpentier E. The CRISPR-associated DNA-cleaving enzyme Cpf1 also processes precursor CRISPR RNA. Nature. 2016;532:517–521. doi: 10.1038/nature17945. [DOI] [PubMed] [Google Scholar]
- Gao P., Yang H., Rajashankar K.R., Huang Z., Patel D.J. Type V CRISPR-Cas Cpf1 endonuclease employs a unique mechanism for crRNA-mediated target DNA recognition. Cell Res. 2016;26:901–913. doi: 10.1038/cr.2016.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geggier P., Dave R., Feldman M.B., Terry D.S., Altman R.B., Munro J.B., Blanchard S.C. Conformational sampling of aminoacyl-tRNA during selection on the bacterial ribosome. J. Mol. Biol. 2010;399:576–595. doi: 10.1016/j.jmb.2010.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gootenberg J.S., Abudayyeh O.O., Kellner M.J., Joung J., Collins J.J., Zhang F. Multiplexed and portable nucleic acid detection platform with Cas13, Cas12a, and Csm6. Science. 2018;360:439–444. doi: 10.1126/science.aaq0179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille F., Charpentier E. CRISPR-Cas: biology, mechanisms and relevance. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2016;371 doi: 10.1098/rstb.2015.0496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath P., Barrangou R. CRISPR/Cas, the immune system of bacteria and archaea. Science. 2010;327:167–170. doi: 10.1126/science.1179555. [DOI] [PubMed] [Google Scholar]
- Hsu P.D., Lander E.S., Zhang F. Development and applications of CRISPR-Cas9 for genome engineering. Cell. 2014;157:1262–1278. doi: 10.1016/j.cell.2014.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon Y., Choi Y.H., Jang Y., Yu J., Goo J., Lee G., Jeong Y.K., Lee S.H., Kim I.S., Kim J.S. Direct observation of DNA target searching and cleavage by CRISPR-Cas12a. Nat. Commun. 2018;9:2777. doi: 10.1038/s41467-018-05245-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jinek M., Chylinski K., Fonfara I., Hauer M., Doudna J.A., Charpentier E. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science. 2012;337:816–821. doi: 10.1126/science.1225829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D., Kim J., Hur J.K., Been K.W., Yoon S.H., Kim J.S. Genome-wide analysis reveals specificities of Cpf1 endonucleases in human cells. Nat. Biotechnol. 2016;34:863–868. doi: 10.1038/nbt.3609. [DOI] [PubMed] [Google Scholar]
- Kleinstiver B.P., Tsai S.Q., Prew M.S., Nguyen N.T., Welch M.M., Lopez J.M., McCaw Z.R., Aryee M.J., Joung J.K. Genome-wide specificities of CRISPR-Cas Cpf1 nucleases in human cells. Nat. Biotechnol. 2016;34:869–874. doi: 10.1038/nbt.3620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T., Zhu L., Xiao B., Gong Z., Liao Q., Guo J. CRISPR-Cpf1-mediated genome editing and gene regulation in human cells. Biotechnol. Adv. 2018 doi: 10.1016/j.biotechadv.2018.10.013. [DOI] [PubMed] [Google Scholar]
- Makarova K.S., Wolf Y.I., Alkhnbashi O.S., Costa F., Shah S.A., Saunders S.J., Barrangou R., Brouns S.J., Charpentier E., Haft D.H. An updated evolutionary classification of CRISPR-Cas systems. Nat. Rev. Microbiol. 2015;13:722–736. doi: 10.1038/nrmicro3569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marraffini L.A. CRISPR-Cas immunity in prokaryotes. Nature. 2015;526:55. doi: 10.1038/nature15386. [DOI] [PubMed] [Google Scholar]
- Marraffini L.A., Sontheimer E.J. CRISPR interference limits horizontal gene transfer in staphylococci by targeting DNA. Science. 2008;322:1843–1845. doi: 10.1126/science.1165771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinney S.A., Joo C., Ha T. Analysis of single-molecule FRET trajectories using hidden Markov modeling. Biophys. J. 2006;91:1941–1951. doi: 10.1529/biophysj.106.082487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pape T., Wintermeyer W., Rodnina M.V. Complete kinetic mechanism of elongation factor Tu-dependent binding of aminoacyl-tRNA to the A site of the E. coli ribosome. EMBO J. 1998;17:7490–7497. doi: 10.1093/emboj/17.24.7490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H.M., Liu H., Wu J., Chong A., Mackley V., Fellmann C., Rao A., Jiang F., Chu H., Murthy N. Extension of the crRNA enhances Cpf1 gene editing in vitro and in vivo. Nat. Commun. 2018;9:3313. doi: 10.1038/s41467-018-05641-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plagens A., Richter H., Charpentier E., Randau L. DNA and RNA interference mechanisms by CRISPR-Cas surveillance complexes. FEMS Microbiol. Rev. 2015;39:442–463. doi: 10.1093/femsre/fuv019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sander J.D., Joung J.K. CRISPR-Cas systems for editing, regulating and targeting genomes. Nat. Biotechnol. 2014;32:347–355. doi: 10.1038/nbt.2842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh D., Mallon J., Poddar A., Wang Y., Tippana R., Yang O., Bailey S., Ha T. Real-time observation of DNA target interrogation and product release by the RNA-guided endonuclease CRISPR Cpf1 (Cas12a) Proc. Natl. Acad. Sci. U S A. 2018;115:5444–5449. doi: 10.1073/pnas.1718686115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh D., Sternberg S.H., Fei J., Doudna J.A., Ha T. Real-time observation of DNA recognition and rejection by the RNA-guided endonuclease Cas9. Nat. Commun. 2016;7:12778. doi: 10.1038/ncomms12778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stella S., Alcon P., Montoya G. Structure of the Cpf1 endonuclease R-loop complex after target DNA cleavage. Nature. 2017;546:559–563. doi: 10.1038/nature22398. [DOI] [PubMed] [Google Scholar]
- Stella S., Mesa P., Thomsen J., Paul B., Alcón P., Jensen S.B., Saligram B., Moses M.E., Hatzakis N.S., Montoya G. Conformational activation promotes CRISPR-Cas12a catalysis and resetting of the endonuclease activity. Cell. 2018;175:1856–1871.e21. doi: 10.1016/j.cell.2018.10.045. [DOI] [PubMed] [Google Scholar]
- Sternberg S.H., LaFrance B., Kaplan M., Doudna J.A. Conformational control of DNA target cleavage by CRISPR-Cas9. Nature. 2015;527:110–113. doi: 10.1038/nature15544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strohkendl I., Saifuddin F.A., Rybarski J.R., Finkelstein I.J., Russell R. Kinetic basis for DNA target specificity of CRISPR-Cas12a. Mol. Cell. 2018;71:816–824.e3. doi: 10.1016/j.molcel.2018.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundaresan R., Parameshwaran H.P., Yogesha S.D., Keilbarth M.W., Rajan R. RNA-independent DNA cleavage activities of Cas9 and Cas12a. Cell Rep. 2017;21:3728–3739. doi: 10.1016/j.celrep.2017.11.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swarts D.C., Jinek M. Cas9 versus Cas12a/Cpf1: structure-function comparisons and implications for genome editing. Wiley Interdiscip. Rev. RNA. 2018:e1481. doi: 10.1002/wrna.1481. [DOI] [PubMed] [Google Scholar]
- Swarts D.C., van der Oost J., Jinek M. Structural basis for guide RNA processing and seed-dependent DNA targeting by CRISPR-Cas12a. Mol. Cell. 2017;66:221–233.e4. doi: 10.1016/j.molcel.2017.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth E., Weinhardt N., Bencsura P., Huszar K., Kulcsar P.I., Talas A., Fodor E., Welker E. Cpf1 nucleases demonstrate robust activity to induce DNA modification by exploiting homology directed repair pathways in mammalian cells. Biol. Direct. 2016;11:46. doi: 10.1186/s13062-016-0147-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu M., Lin L., Cheng Y., He X., Sun H., Xie H., Fu J., Liu C., Li J., Chen D. A ‘new lease of life’: FnCpf1 possesses DNA cleavage activity for genome editing in human cells. Nucleic Acids Res. 2017;45:11295–11304. doi: 10.1093/nar/gkx783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright A.V., Nunez J.K., Doudna J.A. Biology and applications of CRISPR systems: harnessing nature's toolbox for genome engineering. Cell. 2016;164:29–44. doi: 10.1016/j.cell.2015.12.035. [DOI] [PubMed] [Google Scholar]
- Yamano T., Nishimasu H., Zetsche B., Hirano H., Slaymaker I.M., Li Y., Fedorova I., Nakane T., Makarova K.S., Koonin E.V. Crystal structure of Cpf1 in complex with guide RNA and target DNA. Cell. 2016;165:949–962. doi: 10.1016/j.cell.2016.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamano T., Zetsche B., Ishitani R., Zhang F., Nishimasu H., Nureki O. Structural basis for the canonical and non-canonical PAM recognition by CRISPR-Cpf1. Mol. Cell. 2017;67:633–645.e3. doi: 10.1016/j.molcel.2017.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H., Gao P., Rajashankar K.R., Patel D.J. PAM-dependent target DNA recognition and cleavage by C2c1 CRISPR-Cas endonuclease. Cell. 2016;167:1814–1828.e12. doi: 10.1016/j.cell.2016.11.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zetsche B., Gootenberg J.S., Abudayyeh O.O., Slaymaker I.M., Makarova K.S., Essletzbichler P., Volz S.E., Joung J., van der Oost J., Regev A. Cpf1 is a single RNA-guided endonuclease of a class 2 CRISPR-Cas system. Cell. 2015;163:759–771. doi: 10.1016/j.cell.2015.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zetsche B., Heidenreich M., Mohanraju P., Fedorova I., Kneppers J., DeGennaro E.M., Winblad N., Choudhury S.R., Abudayyeh O.O., Gootenberg J.S. Multiplex gene editing by CRISPR-Cpf1 using a single crRNA array. Nat. Biotechnol. 2017;35:31–34. doi: 10.1038/nbt.3737. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.